Abstract
The right temporo-parietal junction (RTPJ) is consistently implicated in two cognitive domains, attention and social cognitions. We conducted multi-modal connectivity-based parcellation to investigate potentially separate functional modules within RTPJ implementing this cognitive dualism. Both task-constrained meta-analytic coactivation mapping and task-free resting-state connectivity analysis independently identified two distinct clusters within RTPJ, subsequently characterized by network mapping and functional forward/reverse inference. Coactivation mapping and resting-state correlations revealed that the anterior cluster increased neural activity concomitantly with a midcingulate–motor–insular network, functionally associated with attention, and decreased neural activity concomitantly with a parietal network, functionally associated with social cognition and memory retrieval. The posterior cluster showed the exact opposite association pattern. Our data thus suggest that RTPJ links two antagonistic brain networks processing external versus internal information.
Keywords: Anti-correlation, Attention, Connectivity-based parcellation, Social cognition, Temporo-parietal junction
Introduction
The human right temporo-parietal junction (RTPJ) is a supramodal association area located at the border between the temporal and parietal lobes surrounding the posterior end of the Sylvian fissure. It is at times referred to as posterior inferior parietal lobule, angular gyrus, Brodmann area 39, or posterior superior temporal sulcus. The highly inconsistent neuroanatomical labeling epitomizes the lacking consensus on coordinates, micro- or macroanatomical landmarks that would topographically define RTPJ (cf. Brodmann, 1909; Déjerine, 1895; Mars et al., 2011). Put differently, “temporo-parietal junction” is a vaguely defined term that is frequently used within various cognitive disciplines to refer to a certain functional cortical module.
While the left TPJ is specifically related to processing language and semantics (Binder et al., 2009), an interesting discrepancy emerges when reviewing the literature on functions of the right TPJ: An extensive body of work implies selectivity of the RTPJ for low-level attentional processes, while a similarly extensive body of literature claims selectivity of the RTPJ for the higher-level processing of social information. More specifically, neuroimaging studies in cognitive neuroscience linked RTPJ activity to spatial reorienting (Corbetta et al., 2000), visuo-proprioceptive conflict (Balslev et al., 2005), and multi-modal detection of sensory changes (Downar et al., 2000). Congruently, direct electrical stimulation of the RTPJ during neurosurgery was associated with altered perception and stimulus awareness (Blanke et al., 2002). Finally, RTPJ lesions in humans are associated with hemi-neglect (Corbetta et al., 2000), i.e. failure to orient visual attention to the contra-lesional side.
On the other hand, neuroimaging research in social neuroscience suggests that the RTPJ encodes imagined goals of others’ actions (Hamilton and Grafton, 2008) and contributes to social cognition by specifically representing others’ mental states such as thoughts and intentions (i.e., theory of mind, Saxe and Wexler, 2005). Indeed, transient RTPJ disruption by transcranial magnetic stimulation significantly reduced relying on an agent’s intentions when judging moral scenarios (Young et al., 2010) and resulted in impaired self–other distinction (Uddin et al., 2006). Taken together, one line of research provides converging evidence for a key role of the RTPJ in attentional processes, while another line of research associates this region with social-cognitive processes.
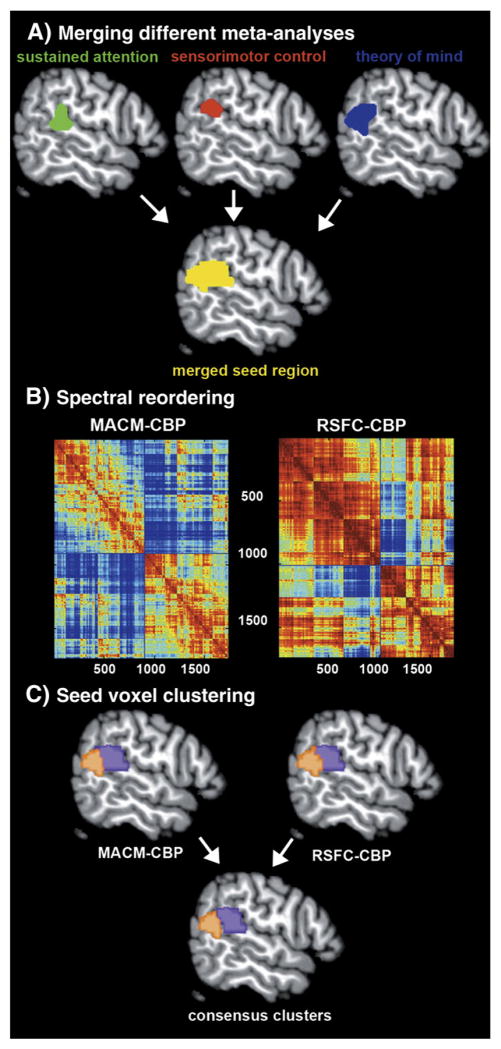
Conceivably, this apparent contradiction may be explained by an interaction of distinct parts of the RTPJ with different brain networks reflecting functional heterogeneity within this region. The combination of connectivity-based parcellation (CBP), mapping task-constrained/-unconstrained connectivity, and large-scale functional inference represents an ideally suited toolbox for this question. In particular, CBP exploits the unique set of input and output connections of any particular functional cortical module (Passingham et al., 2002; Saygin et al., 2012) to “blindly” infer functional parcellations from connectivity data (Johansen-Berg et al., 2004). To both accommodate lacking neuroanatomical consensus (cf. Mars et al., 2011) and acknowledge the diverse functions ascribed to the RTPJ, the volume of interest for CBP (Fig. 1A) was constructed by merging results of three meta-analyses of neuroimaging data on sustained attention (Langner and Eickhoff, in press), sensorimotor control (Jakobs et al., 2012), and theory of mind (Bzdok et al., 2012b).
Fig. 1.
Workflow of the parcellation analysis. (A) A seed region objectively capturing RTPJ’s functional diversity was obtained by combining clusters of converging brain activity from three independent quantitative meta-analyses (Bzdok et al., 2012b; Jakobs et al., 2012; Langner and Eickhoff, in press). (B) The cross-correlation matrix (1736 × 1736 voxels) illustrates the similarity between the whole-brain connectivity profiles of any two seed voxels, computed separately based on voxel-wise MACM and RSFC analysis. Spectral reordering yielded sets of seed voxels that were strongly correlated with each other and weakly correlated with the rest of the matrix. (C) Quantitative CBP was performed on the combined seed region by hierarchical cluster analysis, yielding two distinct clusters of homogeneous connectivity. Importantly, those clusters were highly congruent between MACM and RSFC. All subsequent analyses were conducted on the intersection between anterior respectively posterior clusters derived from MACM- and RSFC-CBP.
First, we thus conducted CBP of the seed region, that is the volume of interest here formed by merging three independent, previously published quantitative meta-analyses. Importantly, the parcellation procedure was performed once for each of two distinct measures of functional connectivity, task-related meta-analytic connectivity modeling (MACM) and task-unrelated resting-state functional connectivity (RSFC). Second, the connectivity-derived sub-regions were characterized by determining their brain-wise connectivity profiles based on the complementary measures of functional connectivity (i.e., MACM and RSFC). Third, we delineated the sub-regions’ functional profiles from above-chance taxonomic associations with meta-data archived in the BrainMap database.
Material and methods
Definition of the RTPJ seed region
We conducted connectivity-based parcellation (CBP) on a volume of interest (VOI) that was derived from three individual quantitative meta-analyses. Please note that we opted for a meta-analytic VOI definition because of the absence of commonly accepted neuroanatomical landmarks to define the location of this functional region (see introduction). Rather than deriving a VOI from single fMRI contrasts, we used quantitative meta-analysis results as they overcome several shortcomings of neuroimaging studies, including small sample sizes and dependence on experimental context (Eickhoff and Bzdok, 2012b). More precisely, we merged three activation clusters located in the RTPJ resulting from three recent meta-analyses of neuroimaging findings on psychological tasks commonly associated with this region: The first, most anterior, cluster (Fig. 1, step 1) resulted from a quantitative meta-analysis of neuroimaging experiments on sustained attention (Langner and Eickhoff, in press), that is the capacity to stay focused on a particular task for extended time periods. A second cluster resulted from a quantitative meta-analysis of neuroimaging studies on sensorimotor control (Jakobs et al., 2012), that is the capacity to integrate exogenous stimuli and contextual information for behavioral response formation. The third, most posterior, cluster resulted from an ALE meta-analysis of neuroimaging experiments on theory of mind (Bzdok et al., 2012b), that is the capacity to model others’ thoughts, beliefs, and behavioral dispositions by abstract inference. Merging these activation clusters yielded a single seed region (1736 voxels) that captures the various functional roles conventionally ascribed to the RTPJ in the neuroscientific literature.
Please note that only the meta-analysis on theory of mind, but not those on sustained attention and sensorimotor control, revealed converging activation in the left TPJ. Although technically feasible, in principle, profound neurobiological differences between the right and left TPJ preclude reiteration of the procedure for the left TPJ given this area’s known hemispheric asymmetry regarding functional specialization (Seghier, 2013), neurological lesion effects (Corbetta et al., 2000), functional (Uddin et al., 2010) and anatomical (Caspers et al., 2011) connectivity, as well as cytoarchitectonic borders and gyral pattern (Caspers et al., 2006, 2008).
The composite VOI was then submitted to a CBP procedure that grouped seed voxels as a function of their similarities in whole-brain connectivity patterns (Eickhoff et al., 2011). Importantly, CBP was performed independently on two approaches for measuring functional connectivity, task-based meta-analytic connectivity modeling (MACM) and task-free resting-state (RSFC) connectivity.
Task-dependent functional connectivity: meta-analytic connectivity modeling
Delineation of whole-brain coactivation maps for each voxel of the RTPJ seed region was performed based on the BrainMap database (www.brainmap.org; Fox and Lancaster, 2002; Laird et al., 2011). We constrained our analysis to fMRI and PET experiments from “normal mapping” neuroimaging studies (no interventions, no group comparisons) in healthy participants, which report results as coordinates in stereotaxic space. These inclusion criteria yielded ~6500 eligible experiments at the time of analysis. Note that we considered all eligible BrainMap experiments because any pre-selection based on taxonomic categories would have constituted a strong a-priori hypothesis about how brain networks are organized. However, it remains elusive how well psychological constructs, such as emotion and cognition, map on regional brain responses (Laird et al., 2009a; Mesulam, 1998; Poldrack, 2006).
A challenge in constructing co-activation maps is the limited number of experiments activating precisely at a particular seed voxel. Hence, pooling across the close spatial neighborhood has become the dominant approach in MACM analysis (Cauda et al., 2011; Eickhoff et al., 2011). In the present study, we realized such pooling across a closely adjacent neighborhood, as needed to reliably determine the co-activation patterns of a given seed voxel, by identifying those among the ~6500 eligible experiments in BrainMap that reported closest activation to that voxel. That is, the experiments associated with each seed voxel were defined by activation at or in the immediate vicinity of this specific seed voxel. In particular, we calculated the respective Euclidean distances between the current seed voxel and individual foci of all databased experiments to identify the 25 up to 100 experiments in steps of five (i.e., closest 25, 30, 35…, 100 experiments) that feature the closest foci. The ensuing 16 experiment sets were then individually submitted to ALE meta-analysis to yield co-activation maps for the current seed voxel. A final co-activation map for each seed voxel was subsequently computed by their voxel-wise median. The seed voxels’ final co-activation map indicates how likely voxels/areas throughout the brain are to increase metabolic activity concomitantly with that seed voxel. This approach allows a robust and unbiased definition of co-activation patterns in spite of the variable and often rather low number of foci at each particular voxel.
More specifically, the key rationale behind using experiments in the close vicinity of a particular seed voxel is to provide a more robust computation of coactivation patterns given the limited number of experiments activating precisely at each voxel. It is noteworthy that the actual spatial dispersion, i.e., induced smoothness, is very small. In particular, the mean distance of the foci, whose experiments were included in the computation of a particular coactivation map, ranged from 1.25 voxels (closest 25 experiments) to 5.1 voxels (closest 100 experiments). This confirms that, indeed, only BrainMap experiments activating in the immediate neighborhood of the respective seed voxel contributed to its coactivation map. The brain-wide coactivation pattern for each seed voxel was then computed by ALE meta-analysis over (all foci reported in) the experiments that were associated with that particular voxel (Eickhoff et al., 2009; Laird et al., 2009a; Turkeltaub et al., 2002).
The key idea behind ALE is to treat the foci reported in the associated experiments not as single points, but as centers for 3D Gaussian probability distributions that reflect the spatial uncertainty associated with neuroimaging results. Using the latest ALE implementation (Eickhoff et al., 2009, 2012; Turkeltaub et al., 2011), the spatial extent of those Gaussian probability distributions was based on empirical estimates of between-subject and between-template variance of neuroimaging foci (Eickhoff et al., 2009). For each experiment, the probability distributions of all reported foci were then combined into a modeled activation (MA) map by the recently introduced “non-additive” approach that prevents local summation effects (Turkeltaub et al., 2011). The voxel-wise union across the MA maps of all experiments associated with a particular seed voxel then yielded an ALE score for each voxel of the brain that describes the coactivation probability of that particular location with the current seed voxel. The ALE scores of all voxels within gray matter (based on 10% probability according to the ICBM [International Consortium on Brain Mapping] tissue probability maps) were then recorded before moving to the next voxel of the seed region.
In sum, quantitative meta-analysis over all foci reported in the experiments associated with the current seed voxel determined how likely any other voxel throughout the brain was to coactivate with that particular seed voxel. Note that no threshold was applied to the ensuing coactivation maps at this point of analysis to retain the complete pattern of coactivation likelihood.
Task-independent functional connectivity: resting-state correlations
Seed-voxel-wise whole-brain connectivity was likewise assessed using resting-state correlations as an independent modality of functional connectivity for cross-validation across different brain states. RSFC fMRI images were acquired from 100 healthy volunteers (50 female, mean age 45.2 years) without any record of neurological or psychiatric disorders. All participants gave written informed consent prior to entering the study, which had been approved by the ethics committee of the University of Bonn. Prior to the imaging session, participants were instructed to keep their eyes closed and just let their mind wander without thinking of anything in particular but not to fall asleep (which was confirmed in post-scan debriefing). For each participant, 300 RSFC EPI images were acquired using blood-oxygen-level-dependent (BOLD) contrast [gradient-echo EPI pulse sequence, TR = 2.2 s, TE = 30 ms, flip angle = 90°, in-plane resolution = 3.1 × 3.1 mm2, 36 axial slices (3.1 mm thickness) covering the entire brain]. The first four scans served as dummy images allowing for magnetic field saturation and were discarded prior to further processing using SPM8 (www.fil.ion.ucl.ac.uk/spm). The EPI images were first corrected for head movement by affine registration using a two-pass procedure. The mean EPI image for each participant was then spatially normalized to the MNI single subject template (Holmes et al., 1998) using the ‘unified segmentation’ approach (Ashburner and Friston, 2005) and the ensuing deformation was applied to the individual EPI volumes. Finally, images were smoothed by a 5-mm FWHM Gaussian kernel to improve signal-to-noise ratio and compensate for residual anatomical variations.
The time-series data of each individual seed voxel were processed as follows (Fox et al., 2009; Weissenbacher et al., 2009): In order to reduce spurious correlations, variance that could be explained by the following nuisance variables was removed: (i) The six motion parameters derived from the image realignment, (ii) the first derivative of the realignment parameters, (iii) mean gray matter, white matter and CSF signal per time-point as obtained by averaging across voxels attributed to the respective tissue class in the SPM 8 segmentation and (iv) coherent signal changes across the whole brain as reflected by the first five components of a principal component analysis (PCA) decomposition of the whole-brain time-series (previously described by Behzadi et al. (2007) and demonstrated by Chai et al. (2012) to increase specificity and sensitivity of the analyses). All of these nuisance variables entered the model as first-order and – except for the PCA components – also as second-order terms (Jakobs et al., 2012). Data were then band-pass filtered preserving frequencies between 0.01 and 0.08 Hz since meaningful resting-state correlations will predominantly be found in these frequencies given that the BOLD-response acts as a low-pass filter (Biswal et al., 1995; Fox and Raichle, 2007).
After temporal preprocessing, we correlated the times-series of each individual seed voxel with those of any other brain voxel. The ensuing correlation values were transformed into Fisher’s Z-scores and recorded in a connectivity matrix. In sum, correlations between spontaneous metabolic fluctuations throughout the brain during mind-wandering in the absence of an externally structured task allowed quantifying the connectivity strength of the current seed voxel with any other voxel.
Connectivity-based parcellation: topographical segregation based on functional connectivity
To identify possibly distinct RTPJ sub-regions with unique connectivity patterns we performed CBP based on MACM (Eickhoff et al., 2011) and RSFC (Kim et al., 2010) analyses. Independent for each modality, the brain-wide connectivity profiles for all seed voxels were combined into a NS × NB coactivation matrix, where NS denotes the number of seed voxels and NB the number of voxels in the reference brain volume.
The most appropriate number of clusters in RTPJ was then, analogous to previous CBP approaches (Johansen-Berg et al., 2004; Kim et al., 2010), determined in a NS × NS cross-correlation matrix. This matrix reflected how strongly the connectivity profiles of each pair of seed voxels correlated with each other. In particular, this matrix was spectrally reordered to minimize the cross-correlation values off the diagonal, hereby forcing voxels whose connectivity profiles are highly correlated close to each other. In doing so, sets of seed voxels emerged that were strongly correlated with each other and weakly correlated with the rest of the matrix. It was this spectrally reordered correlation matrix that favored parcellation into a specific number of clusters.
Further quantitative identification of these distinct clusters that feature similar brain-wide coactivation profiles was performed by hierarchical cluster analysis (Eickhoff et al., 2007; Timm, 2002). In this approach, individual voxels initially form separate clusters which are then successively included into a growing hierarchy by merging the most similar clusters into progressively larger sets of voxels. Correlation between the brain-wide connectivity profiles of seed voxels was used as a similarity measure and average linkage criterion for cluster merging (Timm, 2002). In sum, the individual seed voxels were thus merged depending on the correspondence of their connectivity profiles to identify clusters within the VOI that feature similar functional connectivity (Eickhoff and Bzdok, 2012a; Eickhoff et al., 2011).
The resulting clusters were then anatomically localized by cytoarchitectonic assignment according to maximum probability maps from the Jülich brain atlas (Amunts et al., 1999; Caspers et al., 2006; Geyer, 2004; Gitelman et al., 1999) using the SPM Anatomy toolbox (Eickhoff et al., 2005).
Taken together, the just described first part of our study was concerned with testing for distinct cortical modules in the RTPJ. Performing CBP separately using MACM and RSFC on the meta-analytically defined seed region potentially indicates the presence of biologically meaningful sub-regions in this area. The resulting sub-regions from the independent MACM- and RSFC-CBP analyses are then merged by conjunction analysis to yield consensus clusters composed of seed voxels that were consistently assigned across both approaches. It is important to appreciate that these consensus clusters constitute the basis for all subsequent analyses. In the following we outline the second and third part of our study – determining the connectional and functional profile of each consensus cluster.
Characterization of the CBP-derived clusters: connectivity
Following parcellation of the seed region based on regional heterogeneity in functional connectivity, additional MACM and RSFC analyses were performed on each of the ensuing clusters to characterize their whole-brain connectivity patterns. It is important to note that the above MACM and RSFC analyses assessed seed-voxel-wise connectivity patterns of individual seed voxels, while we here assessed the overall connectivity pattern of a set of seed voxels, i.e., the connectivity of the entire cluster.
For the MACM analyses on the derived clusters, we performed an ALE meta-analysis across all BrainMap experiments featuring at least one focus of activation within the cluster using otherwise the same approach as described above. However, statistical inference was sought at this point, in contrast to the above MACM analysis. To establish which regions were significantly coactivated with a particular cluster of voxels, ALE scores for the MACM analysis of this cluster were compared to a null-distribution that reflects a random spatial association between experiments, but regards the within-experiment distribution of foci as fixed (Eickhoff et al., 2009). This random-effects inference assesses above-chance convergence between experiments. The observed ALE scores from the actual meta-analysis of experiments activating within a particular cluster were then tested against the ALE scores obtained under this null-distribution yielding a p-value based on the proportion of equal or higher random values. The resulting p-values were then thresholded at p < 0.05 following cluster-level family-wise error correction for multiple comparisons (cluster-forming threshold at voxel-level: p < 0.001).
In addition to MACM analyses, RSFC analysis was also performed on the derived clusters. Time courses were extracted for all gray-matter voxels of a given cluster of the individual participant (Ashburner and Friston, 2005). The cluster time course was then expressed as the first eigenvariate of these voxels’ time courses. Pearson correlation coefficients between the time series of the CBP-derived RTPJ clusters and all other gray-matter voxels in the brain were computed to quantify RSFC. These voxel-wise correlation coefficients were then transformed into Fisher’s Z-scores and tested for consistency across participants by a one-sample t-test. The results of this random-effects analysis were then thresholded at p < 0.05 following cluster-level family-wise error correction for multiple comparisons (cluster-forming threshold at voxel-level: p < 0.001), analogous to MACM-derived cluster connectivity.
Characterization of the CBP-derived clusters: conjunction across MACM & RSFC results
To delineate areas showing task-dependent and task-independent functional connectivity with the derived sub-regions in the RTPJ, we performed a conjunction analysis of the MACM and RSFC results using the strict minimum statistics (Nichols et al., 2005). In practice, regions connected with the seed in both connectivity modalities were delineated by computing the intersection of the (cluster-level family-wise-error-corrected) connectivity maps from the two analyses detailed above. In this way, each RTPJ cluster was associated with a network of areas that are congruently connected to that cluster across disparate (i.e., task-focused and mind-wandering) brain states.
Characterization of the CBP-derived clusters: function
After the first part (connectivity-derived identification of distinct clusters in the RTPJ) and second part (delineation of each clusters’ convergent connectivity profile across MACM and RSFC) of our study, the clusters and their thus determined networks were individually submitted to functional profiling, as the third and last part. Please note that this functional characterization constitutes a post-hoc procedure that is subsequent to and independent of the connectivity analyses. The functional characterization was based on the BrainMap meta-data that describe each neuroimaging experiment included in the database. Behavioral domains code the mental processes isolated by the statistical contrasts (Fox et al., 2005b) and comprise the main categories cognition, action, perception, emotion, and interoception, as well as their related sub-categories. Paradigm classes categorize the specific task employed (see http://brainmap.org/scribe/ for the complete BrainMap taxonomy).
Forward inference on the functional characterization then tests the probability of observing activity in a brain region given knowledge of the psychological process, whereas reverse inference tests the probability of a psychological process being present given knowledge of activation in a particular brain region. In the forward inference approach, a cluster’s functional profile was determined by identifying taxonomic labels for which the probability of finding activation in the respective cluster was significantly higher than the overall chance (across the entire database) of finding activation in that particular cluster. Significance was established using a binomial test (p < 0.001; Eickhoff et al., 2011; Laird et al., 2009b). That is, we tested whether the conditional probability of activation given a particular label [P(Activation|Task)] was higher than the baseline probability of activating the region in question per se [P(Activation)]. This base rate thus denotes the probability of finding a (random) activation from BrainMap in the cluster. In the reverse inference approach, a cluster’s functional profile was determined by identifying the most likely behavioral domains and paradigm classes given activation in a particular cluster. This likelihood P(Task|Activation) can be derived from P(Activation|Task) as well as P(Task) and P(Activation) using Bayes’ rule. Significance was then assessed by means of a chi-square test (p < 0.001). In sum, forward inference assessed the probability of activation given a psychological term, while reverse inference assessed the probability of a psychological term given activation.
The contrast analyses between the two clusters’ functional profiles, in turn, were constrained to those experiments in BrainMap activating either cluster. That is, the task associations of experiments in this composite pool were quantified in comparison between the respective clusters. Forward inference here compared the activation probabilities between the two clusters given a particular psychological term, while reverse inference compared the probabilities of a particular psychological term being present given activation in one or the other cluster.
It is important to appreciate that this approach aims at relating defined psychological tasks to the examined brain regions instead of claiming “a unique role” of a brain region for any psychological task (Poldrack, 2006; Yarkoni et al., 2011). Put differently, an association of task X to brain region Y obtained in these analyses does not necessarily imply that neural activity in region Y is limited to task X.
Results
Functional modules in the RTPJ
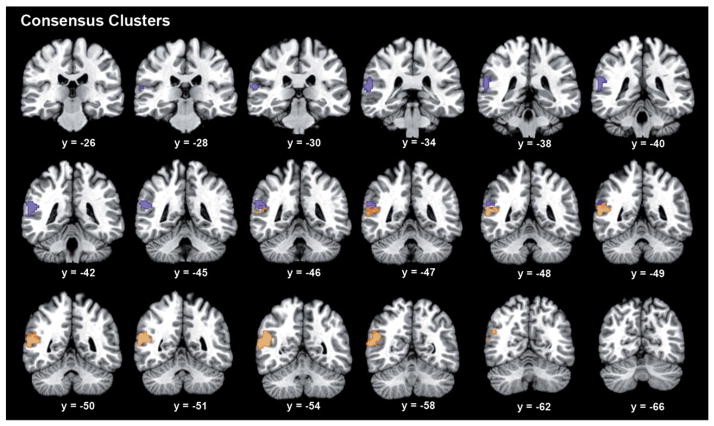
Whole-brain task-dependent (MACM; based on coactivation patterns across a large number of databased neuroimaging experiments (Robinson et al., 2010)) and task-independent (RSFC; based on correlations of slow [<0.1 Hz] fluctuations of fMRI activity during task-free mind-wandering (Biswal et al., 1995)) connectivity analyses were computed for every single voxel within the seed region. For each of these two modalities, the connectivity profiles of all seed voxels were correlated with each other, yielding a symmetric matrix that indicated the similarity in whole-brain connectivity (for the respective modality) across any pair of seed voxels. Spectral reordering (Fig. 1B) of these two (MACM- and RSFC-derived) similarity matrices congruently indicated the presence of two distinct clusters within RTPJ. Hierarchical cluster analysis also provided strong evidence for the same two-cluster solution given congruent clustering of 90% of all seed voxels across both MACM and RSFC analyses (Fig. 1C, Supplementary Fig. 1). The close similarity of these two clustering schemes is further illustrated by virtually identical whole-brain connectivity profiles of the anterior and posterior clusters as derived from either the MACM- or RSFC-CBP analyses (Supplementary Fig. 2). Thus, two independent modalities of functional connectivity, task-dependent MACM and task-independent RSFC, provided highly convergent two-cluster solutions (see Fig. 2 for location; the centers of mass of the anterior and posterior cluster are 58.5/−39/16.5 and 54/−54/16.5, respectively, in MNI space). In contrast, these two modalities diverged strongly when attempting a more fine-grained clustering. In the three-cluster solution, only 38% of the seed voxels were assigned congruently across both analyses. In particular, MACM-and RSFC-based CBP disagreed in further subdividing either the anterior (aRTPJ) or posterior (pRTPJ) cluster, respectively (Supplementary Figs. 3–4). However, the connectivity patterns of the most anterior and posterior cluster identified in either of these three-cluster solutions concurred with the two-cluster solution, whereas the newly formed middle cluster behaved more variably. The distinction into two clusters, therefore, represents the most robust regional differentiation within RTPJ.
Fig. 2.
Anatomy of the anterior and posterior RTPJ cluster. Serial coronal slices of the connectivity-derived clusters in the RTPJ. Coordinates in MNI space.
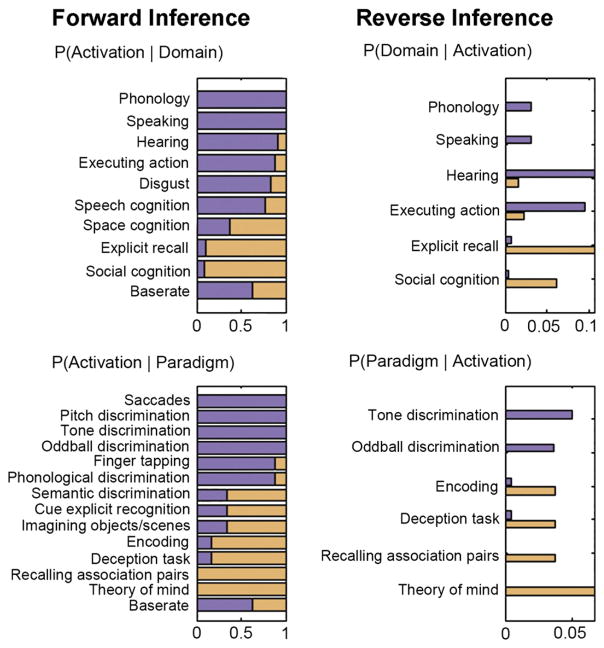
Functional characterization of the two clusters was performed by two quantitative approaches capitalizing on the meta-data of experiments stored in the BrainMap database (Fox and Lancaster, 2002). Forward inference assessed above-chance brain activity in a given cluster based on a particular label of the BrainMap taxonomy (task, behavioral domain), while reverse inference assessed the above-chance occurrence of labels given activation in a particular cluster. Across both approaches, the aRTPJ was congruently associated with auditory, visual, and speech discrimination tasks as well as action execution (Fig. 3). Contrarily, pRTPJ was congruently associated with social-cognitive, theory-of-mind, and deception tasks as well as memory encoding and explicit retrieval. In summary, functional forward and reverse inference linked aRTPJ to attentional-perceptual and action-related processes, while pRTPJ was linked to social-cognitive and memory-related processes.
Fig. 3.
Domain and paradigm associations of the anterior versus posterior RTPJ cluster. BrainMap meta-data were used to perform functional forward (left column) and reverse (right column) inference for the anterior (purple) and posterior (orange) RTPJ cluster. Forward inference determines above-chance brain activity given the presence of a term, while reverse inference determines the above-chance probability of a term given observed brain activity. Base rate denotes the general probability of BrainMap activation in the cluster. The scale indicates to what extent a specific significant term is more associated with the anterior or posterior cluster.
This structural–functional segregation is mirrored by the three quantitative meta-analyses from which the seed was originally formed. The convergent activations for sustained attention and sensorimotor control were entirely located in the aRTPJ, while the convergent activations for theory-of-mind tasks were located predominantly in the pRTPJ. Both clusters were moreover assigned to separate cytoarchitectonic brain areas. The aRTPJ cluster overlapped with areas PF (32%) and PFm (32%), while pRTPJ overlapped with areas PGa (41%) and PGp (10%) (Caspers et al., 2006). Thus, our connectivity-derived discrimination of two sub-units in the RTPJ was corroborated by their disparate functional, and microstructrual, properties.
Functional networks related to the identified clusters
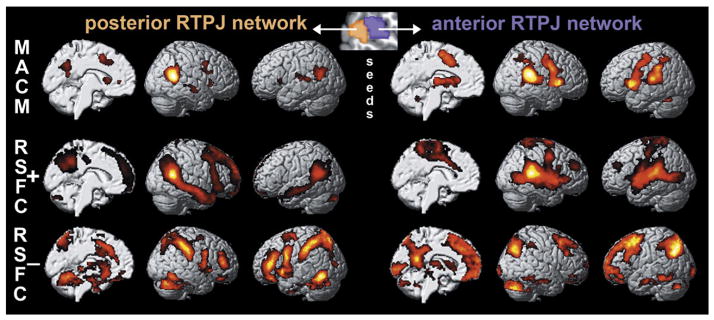
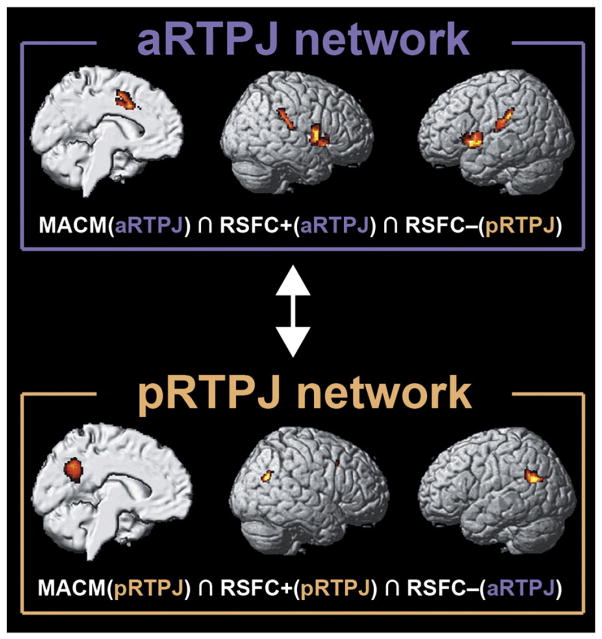
Task-constrained coactivations (MACM) and task-unconstrained time-series correlations (RSFC) congruently showed functional connectivity of the aRTPJ with the bilateral primary motor cortex (cytoarchitectonically assigned to area 4; Geyer et al., 1996), midcingulate cortex/supplementary motor area (MCC/SMA, cytoarchitectonically assigned to cingulate areas a24′/p24′ and premotor area 6; Geyer, 2004) and anterior insula/inferior frontal gyrus (AI/IFG, cytoarchitectonically assigned to areas OP5-7 and 44 (Amunts et al., 1999) but also located in the anterior insula) (Fig. 4, right panel). This set of areas resulting from congruent connectivity to the aRTPJ cluster across both connectivity approaches will subsequently be referred to as “aRTPJ network.” Functional profiling of this network revealed association with pain perception and tactile-attentive tasks as well as action execution and motor control (Supplementary Fig. 5). The pRTPJ, in turn, featured congruent (across MACM and RSFC) functional connectivity with the bilateral inferior parietal cortex (IPC, cytoarchitectonically assigned to areas PGa/p; Caspers et al., 2006, 2008), precuneus, and right middle temporal gyrus (Fig. 4, left panel). Functional profiling of this “pRTPJ network” revealed association with explicit (especially episodic) memory retrieval and semantic discrimination as well as social-cognition and theory-of-mind tasks (Supplementary Fig. 6).
Fig. 4.
Functional connectivity of the parcellation-derived anterior and posterior RTPJ cluster. For each cluster, we assessed the whole-brain functional connectivity profile under task constraints (MACM analysis) and in the unconstrained, “resting” (RSFC analysis) brain, as well as its negative resting-state time-series correlations.
Importantly, we found a reciprocal relationship between the brain networks linked to aRTPJ and pRTPJ, respectively, (Fig. 5) by assessing each cluster’s negative time-series correlations. In particular, bilateral MCC/SMA and AI/IFG were not only positively coupled with the aRTPJ across MACM and RSFC but were also negatively coupled with the pRTPJ in the RSFC analysis. Conversely, bilateral IPC and precuneus (not including the posterior cingulate cortex; cf. Margulies et al., 2009) were positively coupled with the pRTPJ and negatively coupled with the aRTPJ. From a neurophysiological perspective, one set of brain areas thus probably increases metabolic activity together with the aRTPJ and decreases activity with the pRTPJ, while another set of brain areas shows the opposite pattern. These findings suggest a functional anti-correlation between aRTPJ and pRTPJ.
Fig. 5.
Functional anti-correlation between the brain networks connected to the anterior and posterior RTPJ cluster. The MCC/SMA and AI/IFG (upper box) demonstrated a positive functional relationship in MACM and RSFC analysis with aRTPJ as well as a negative one (RSFC−) with pRTPJ. Conversely, bilateral TPJ and precuneus (lower box) were positively related (MACM, RSFC+) to pRTPJ and negatively related (RSFC−) to aRTPJ.
Discussion
The functional role of the RTPJ has long remained enigmatic given implication in very heterogeneous mental functions, especially lower-order attention-/action-related cognition and higher-order social cognition. To reflect this functional spectrum, a functional seed region was constructed by merging quantitative meta-analysis results on sustained attention, sensorimotor control, and theory of mind. We then employed connectivity-based-parcellation (CBP) techniques, bimodal network mapping, and forward/reverse large-scale functional inference to investigate a possible internal differentiation within RTPJ. Application of this methodological toolbox suggested the presence of an anterior and posterior sub-region with antagonistic connectivity and functions.
It is widely assumed that microanatomical and connectional properties constrain the brain’s functional compartments (Campbell, 1905; Felleman and Van Essen, 1991; Passingham et al., 2002). This notion has prompted the development of several connectivity-based parcellation approaches. CBP has previously been performed by exploiting first DTI (Johansen-Berg et al., 2004), later RSFC (Kim et al., 2010), and most recently MACM (Eickhoff et al., 2011). DTI-CBP is based on the estimation of the course and strength of white-matter tracts originating from each particular seed voxel (Jones, 2008). RSFC-CBP, in turn, is based on correlations between spontaneous fluctuations of brain activity in the absence of an externally structured task (Biswal et al., 1995). Finally, MACM-CBP is based on assessing the brain-wide co-activation patterns of each individual seed voxel across a large number of databased neuroimaging results (Eickhoff et al., 2011). Notably, these different approaches to the connectivity-driven identification of biological modules in the human brain have different advantages and disadvantages.
Most importantly, neurobiological knowledge derived from MACM is task-dependent or interventional (i.e., obtained under defined experimental settings), whereas RSFC and DTI data are task-independent or observational (i.e., obtained disregarding the current cognitive set) (Eickhoff and Grefkes, 2011). Consequently, only MACM-CBP can functionally characterize the ensuing clusters by correspondence with task properties, psychological concepts, and taxonomic terms (Bzdok et al., in press). This advantage of MACM-CBP permits formulating novel hypotheses about functional contributions of the parcellation clusters that can subsequently be tested in targeted neuroimaging experiments (Eickhoff et al., 2011). Apart from that DTI-CBP relies on neurophysiologically well characterized phenomena in estimating the non-isotropic, diffusion of water molecules constrained by white-matter anatomy, i.e., axons (Jones, 2008). Contrarily, RSFC- and MACM-CBP both rely on the insufficiently understood blood oxygen level dependent (BOLD) signal resulting from neurovascular coupling (Logothetis and Wandell, 2004), although measured under resting and task conditions, respectively. Moreover, only DTI- and RSFC-CBP can be performed on a single-subject basis (Anwander et al., 2007; Kim et al., 2010), which, in turn, enables investigating the inter-individual differences in regional functional specialization. MACM-CBP, however, may provide parcellation results that generalize across inter-individual neuroanatomical differences and, conceivably, geographic genetic trends given its synthesis of an enormous, database-provided sample size. Taken together, there is currently no gold standard for connectivity-based parcellation of seed regions, as each of the compared approaches inherits different limitations and promises from the underlying connectivity measure. Conjoint application of several CBP approaches on a same brain region might therefore reveal complementary neurobiological properties that are otherwise inaccessible.
This study corroborated and extended the recently proposed differentiation of the right TPJ into an anterior and a posterior RTPJ clusters as based on structural-connectivity-derived parcellation of a hand-drawn seed region (Mars et al., 2011). In contrast, we obtained the same topographical segregation of the RTPJ using a meta-analytically defined, thus objective and topographically specific, seed region. While Mars and colleagues used probabilistic tracing of fiber patterns, the present parcellation approach was based on two diverging modalities of functional connectivity, namely, co-activations with ~6.500 BrainMap experiments (MACM) and resting-state correlations in 100 participants (RSFC). Beyond replication using other modalities, a different methodological approach and independent data, we furthermore characterized the two RTPJ clusters by delineating the complementary task-constrained (MACM) and task-unconstrained (RSFC) connectional properties as well as the relation between the ensuing cluster networks. The clusters’ topographical and connectional features were then complemented by functional decoding using forward/reverse inference (Bzdok et al., in press; Rottschy et al., in press).
The present study thus extends previous methodological techniques and neurobiological knowledge. Methodologically, we employed several novel approaches, including the use of a meta-analytically defined, composite seed region acknowledging the heterogeneous literature, multi-modal CBP allowing for validation of the derived clusters across brain states, as well as forward and reverse functional decoding of both clusters and their associated networks. Neurobiologically, this study is, as far as we know, the first to demonstrate the reciprocity within the RTPJ as indicated by anti-correlated connectivity patterns as well as antagonistic functional profiles of the two clusters and their networks.
The description of the here derived anterior and posterior RTPJ cluster in neuroanatomical terms is not straightforward, reflecting the inconsistent neuroanatomical labeling for this area (see Introduction) and the fact that “temporo-parietal junction” denotes a functional rather than anatomical region (cf. Mars et al., 2011; Mitchell, 2008). Microanatomically, aRTPJ and pRTPJ were here assigned to the cyto-architectonic areas PF/PFm and PGa/PGp, respectively (Caspers et al., 2006). Yet, these overlaps ranged only from 10 to 41% and were probably overestimated due to current lack of surrounding cytoarchitectonic maps (Eickhoff et al., 2005). Macroanatomically, aRTPJ and pRTPJ are both located at the posterior end of the superior temporal gyrus, while pRTPJ additionally extends into adjacent parts of the superior temporal sulcus, ventral aspects of the supramarginal gyrus (around the end of the Sylvian fissure) and rostral aspects of the angular gyrus (around the end of the posterior superior temporal sulcus) of the interior parietal lobule (Nieuwenhuys et al., 2007). Comparatively, existence of a non-human primate homologue of the human RTPJ is uncertain (Geschwind, 1965; Seghier, 2013; Zilles and Palomero-Gallagher, 2001), which hinders systematic discussion of the two clusters’ axonal connections in monkeys. Axonal fiber tracing in humans using diffusion tensor imaging (DTI) tractography was often based on cytoarchitectonic seed regions (e.g., Caspers et al., 2011; Uddin et al., 2010) and consequently has limited value in describing the differential axonal connections of the two RTPJ clusters.
Forward and reverse functional decoding congruently associated the anterior RTPJ cluster with visual, auditory, and speech discrimination tasks as well as action execution, while its network was congruently associated with painful corporal stimulation and tactile-attentive tasks as well as action execution and motor control. In short, aRTPJ and the functionally connected (across MACM and RSFC) bilateral primary motor cortex, MCC/SMA, and AI/IFG (these congruent network areas will henceforth be collectively called “aRTPJ network”) were intimately related to attending to heterogeneous environmental stimuli of multiple modalities as well as controlling motor execution. In fact, attending to various stimuli and indicating responses to those by hand movement is the essence of most neuroimaging paradigms. The present functional profiling results thus predict that the aRTPJ network should increase activity in most neuroimaging studies regardless of experimental variables, such as stimuli and paradigm. Indeed, the brain areas comprising the aRTPJ network were shown to have the highest probability of activation in neuroimaging studies across all cognitive disciplines by large-scale meta-analyses of two separate datasets of more than 1000 studies each (Nelson et al., 2010; Yarkoni et al., 2011). Regarding stimulus processing on the one hand, brain areas connected to aRTPJ further responded to all changes of visual, auditory, or tactile stimulation in a multi-modal fMRI study (Downar et al., 2000). Regarding motor response on the other hand, neural activity in these brain areas was linked to trial-by-trial reaction time variability of the participants’ responses across diverse cognitive tasks in a multi-study analysis (Yarkoni et al., 2009). These previous findings consolidate the broad recruitment of the aRTPJ network during modality-independent external attention and motor response processes.
More specifically, the bilateral MCC/SMA and AI/IFG consistently connected to aRTPJ have been proposed to compose a “saliency network” that detects relevant stimuli to guide behavior (Sridharan et al., 2008). In line with this, the evolutionarily recent von Economo neurons, a specialized cell type exclusively localized in these areas (von Economo, 1926), are believed to rapidly relay bottom-up environmental information to executive and higher associative regions (Allman et al., 2005). Circumscribed MCC lesions in humans indeed entail dysfunctional task-set-related processing (Williams et al., 2004), while circumscribed AI lesions can decrease alertness (Manes et al., 1999). Consistently, comprehensive across-study analyses (Dosenbach et al., 2006; Kurth et al., 2010) attested a role of this network in tonically maintaining the task-imposed cognitive set or the task plan (cf. Duncan, 2010). The synopsis of our and others’ evidence thus suggests that the aRTPJ network is central for sensorimotor control by integrating supramodal stimulus-guided attention and action initiation during externally structured tasks.
In contrast to aRTPJ, functional decoding congruently associated both the posterior RTPJ cluster and its network with social cognition and theory of mind, as well as memory encoding and (episodic) memory retrieval. The pRTPJ cluster was also functionally associated with deception tasks. In short, pRTPJ, functionally connected (across MACM and RSFC) to the bilateral IPC, precuneus, and right middle temporal gyrus, was intimately related to social and memory processes. On the one hand, prior research frequently implicated these network areas here consistently connected to the pRTPJ (collectively “pRTPJ network”) in higher social processes, including perspective-taking (Mar, 2011), social judgments (Bzdok et al., 2012a; Freeman et al., 2010), imagination-driven empathy (Lamm et al., 2011), and moral decisions (Bzdok et al., 2012b). On the other hand, further research also frequently implicated the pRTPJ network in memory processes, including autobiographical/episodic memory retrieval (Spreng et al., 2009) and semantic processing (Binder et al., 2009). The present results thus tie these two largely independent literature streams and suggest a possible neural relationship between social-cognitive and episodic-memory-related processes. It has indeed been hypothesized that higher social cognition might intrinsically draw on building blocks of experience-derived regularities from memory (Bar, 2007; Buckner and Carroll, 2007; Bzdok et al., 2012b; Schacter et al., 2008).
More generally, both higher social-cognitive and episodic memory processing, which was related to pRTPJ, is largely stimulus-independent in nature. Consistently, the topography of the pRTPJ network corresponds to previous meta-analytic definitions of the well known “default-mode network” (Laird et al., 2009b; Schilbach et al., 2012), a set of areas that consistently decrease their activity during experimental paradigms requiring stimulus-oriented processing (Gusnard et al., 2001; Shulman et al., 1997). In line with the functional association with largely stimulus-independent social and memory processes, the default-mode network (Fox et al., 2005a) connected to pRTPJ has previously been closely related to a range of introspective mental tasks, including self-focused reflection (Andrews-Hanna et al., 2010), self–other distinction (Ruby and Decety, 2001), the contemplation of others’ (Mar, 2011) and one’s own (Lombardo et al., 2009) mind states, as well as scene construction processes when envisioning past, fictitious, and future events (Hassabis et al., 2007; Spreng et al., 2009). This invigorates the increasingly recognized relationship between the physiological baseline of the human brain and an introspective psychological baseline implicated in continuous self-related social cognition and memory retrieval (Schilbach et al., 2008, 2012; Timmermans et al., 2012).
In sum, aRTPJ may thus be considered part of an externally oriented, stimulus-driven network that probably controls attention to salient events in our environment and reactions towards these. Conversely, pRTPJ appears to be part of an internally oriented, stimulus-independent network involved in continuous memory-informed mental imagery to potentially predict plausible social events related to self. Although aRTPJ and pRTPJ were each implicated in many seemingly unrelated tasks by functional profiling (Fig. 3, Supplementary Figs. 5–6), the difference between the two task groups could be parsimoniously explained by the required attention to either the external world or self.
This functional segregation is underlined by the here observed anti-correlation between aRTPJ and pRTPJ. Please note that the repeatedly shown anti-correlation between the (aRTPJ-related) saliency and (pRTPJ-related) default-mode network (Fox et al., 2005a; Kelly et al., 2008) was here derived from, and is thus directly related to, different functional modules within RTPJ. Indeed, goal-directed task performance improves with increased activity in saliency-related areas and decreased activity in default-mode areas (Weissman et al., 2006). Conversely, increased activity in areas of the here delineated pRTPJ network were linked to increased occurrence of task-independent thoughts (i.e., mind-wandering) during task execution (Mason et al., 2007). Two fMRI studies employing Granger causality analysis further corroborated the anti-correlation by indicating negative influence of the default-mode on the saliency network (Pisapia et al., 2012) and vice versa (Sridharan et al., 2008). Taken together, present and previous findings converge on the tentative notion that the human RTPJ contains two functionally complementary modules that belong to antagonistic networks potentially underlying externally versus internally oriented processing.
Importantly, we do not mean to propose that attentional versus social processes are uniquely resulting from external versus internal events. On the one hand, attentional shifts can obviously also be endogenously modulated in a constant external environment (Cavanna and Trimble, 2006; Corbetta et al., 2008). On the other hand, social processes are certainly related to information gleaned from the external environment, such as facial cues (eye gaze, emotional expression), gestures, and voices (Berry, 1992; Bzdok et al., 2012a; Lamm et al., 2011; Tamsin et al., 2009). Nevertheless, attentional processes are “often” a result of external events, while social cognitive processes draw “to a large extent” on information that is retrieved from internal information rather than the external environment. We would thus argue that increased aRTPJ activity may be predominantly related to processing external information, whereas pRTPJ activity may be particularly increased when accessing internal information (such as autobiographical memory), while noting that both classes of neural processes are intimately depending on and influenced by each other.
Prima facie, it may seem unsurprising that a seed region defined by consistent neuroimaging activation associated with theory of mind, sensorimotor control, and sustained attention was shown to contain separate cortical modules related to attentional and social processes. The performed approach was however not biased towards this result for the following reasons (cf. Kriegeskorte et al., 2009). First, we opted for a functional seed region definition because the term “temporo-parietal junction” enjoys eager employment despite loose definition, reflected by the missing consensus on its neuroanatomical borders (cf. Mars et al., 2011). The starting point of this study was hence derived verging activation in independent, previously published meta-analytic syntheses of three (and not two) RTPJ functions frequently promoted in largely parallel research lines. Computing individual connectivity maps for each voxel in this composite seed region then enabled testing for subregional heterogeneity. Second, the MACM- and RSFC-derived two-cluster solutions converged in 90%, while the finer-grained three-cluster solutions converged only in 38%, thus indicating the biologically most meaningful RTPJ segregation into two clusters. Conceivably, the bimodal seed region parcellation analysis using connectional heterogeneity could also have determined one or three and more clusters as the best solution. Third, the yet rather limited pool of domain-overarching neuroimaging studies on the RTPJ mainly advocated the view of a single “monolithic” functional module. In particular, a quantitative meta-analysis found a substantial overlap in the RTPJ between lower attentional (reorienting, agency) and higher social (theory of mind, empathy) processes (Decety and Lamm, 2007), interpreted by the authors to reflect an unidentified neural process shared by both these task groups. In line with this conclusion, an fMRI study (Mitchell, 2008) showed overlapping RTPJ activity when participants responded to visual stimuli miscuing locations of target stimuli (Posner task) and inferred mind states of characters from pictorial stories (theory of mind task). Rather than an unknown common computational algorithm, our analyses suggested two distinct cortical modules underlying attentional and social processing. Taken together, our unsupervised approach parcellated the seed region in the RTPJ derived from three quantitative meta-analyses into two clusters in disagreement with the previously assumed single cortical module.
From a larger perspective, the reciprocity of the two adjacent modules in RTPJ becomes even more intriguing considering that topographical proximity is usually found between functionally related brain areas to reduce wiring and transmission costs (Braitenberg and Schüz, 1998; Cherniak, 1994; Klyachko and Stevens, 2003). It is therefore tempting to speculate that aRTPJ and pRTPJ might be part of antagonistic but interrelated neural networks. The connectional and functional properties of the delineated sub-regions may thus qualify RTPJ as a potential switch between exteroceptive and interoceptive mind sets implemented by their networks. The reciprocal nature of those mind sets is well illustrated by consistent reports of continuous shifting between externally (i.e., sensory) and internally (i.e., self- and presumably social/memory-) oriented processing in controlled laboratory settings and daily routine (Smallwood et al., 2007). In fact, it was observed that the more external stimuli are predictable, the more reflection processes become detached from the actual sensory environment and the more stimulus-independent, self-focused thoughts occur (Mason et al., 2007).
This suggests prediction processing (Friston, 2010; von Helmholtz, 1909) as a possible domain-spanning role of the RTPJ, which could readily be related back to its domain-specific involvements in attention and social cognition. More specifically, the allocation of attentional resources is generally guided by the violation of a-priori predictions about external events. Contemplating other individuals’ behavior, in turn, might imply testing memory-informed predictions about forthcoming actions and the agent’s time-invariant traits (Fogassi et al., 2005; Hamilton and Grafton, 2008). Ultimately, the human RTPJ might subserve predictive processes in environment- and introspection-driven cognition to govern the interplay between perception–action cycles and mental imagery.
Supplementary Material
Acknowledgments
This study was supported by the Human Brain Project (R01-MH074457-01A1, PTF, ARL, SBE), the Helmholtz Initiative on Systems-Biology “The Human Brain Model” (K Z, SBE), and the German National Academic Foundation (DB).
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.neuroimage.2013.05.046.
Footnotes
Conflict of interest
The authors declare no conflict of interest. con-
References
- Allman JM, Watson KK, Tetreault NA, Hakeem AY. Intuition and autism: a possible role for Von Economo neurons. Trends Cogn Sci. 2005;9:367–373. doi: 10.1016/j.tics.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K. Broca’s region revisited: cytoarchitecture and intersubject variability. J Comp Neurol. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwander A, Tittgemeyer M, von Cramon DY, Friederici AD, Knosche TR. Connectivity-based parcellation of Broca’s area. Cereb Cortex. 2007;17:816–825. doi: 10.1093/cercor/bhk034. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Balslev D, Nielsen FA, Paulson OB, Law I. Right temporoparietal cortex activation during visuo-proprioceptive conflict. Cereb Cortex. 2005;15:166–169. doi: 10.1093/cercor/bhh119. [DOI] [PubMed] [Google Scholar]
- Bar M. The proactive brain: using analogies and associations to generate predictions. Trends Cogn Sci. 2007;11:280–289. doi: 10.1016/j.tics.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DS. Vocal types and stereotypes: joint effects of vocal attractiveness and vocal maturity on person perception. J Nonverbal Behav. 1992;16 [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Blanke O, Ortigue S, Landis T, Seeck M. Stimulating illusory own-body perceptions. Nature. 2002;419:269–270. doi: 10.1038/419269a. [DOI] [PubMed] [Google Scholar]
- Braitenberg V, Schüz A. Statistics and Geometry of Neuronal Connectivity. Springer; Berlin: 1998. [Google Scholar]
- Brodmann K. Vergleichende Lokalisationslehre der Großhirnrinde. Barth; Leipzig: 1909. [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Laird A, Zilles K, Fox PT, Eickhoff SB. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Hum Brain Mapp. 2012;(Jul 17) doi: 10.1002/hbm.22138. http://dx.doi.org/10.1002/hbm.22138. in press. [DOI] [PMC free article] [PubMed]
- Bzdok D, Langner R, Hoffstaedter F, Turetsky BI, Zilles K, Eickhoff SB. The modular neuroarchitecture of social judgments on faces. Cereb Cortex. 2012;22:951–961. doi: 10.1093/cercor/bhr166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Schilbach L, Vogeley K, Schneider K, Laird AR, Langner R, Eickhoff SB. Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Struct Funct. 2012;217:783–796. doi: 10.1007/s00429-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell AW. Histological Studies on the Localisation of Cerebral Function. Cambridge Univ. Press; Cambridge, U.K: 1905. [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. NeuroImage. 2006;33:430–448. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Caspers S, Eickhoff SB, Geyer S, Scheperjans F, Mohlberg H, Zilles K, Amunts K. The human inferior parietal lobule in stereotaxic space. Brain Struct Funct. 2008;212:481–495. doi: 10.1007/s00429-008-0195-z. [DOI] [PubMed] [Google Scholar]
- Caspers S, Eickhoff SB, Rick T, von Kapri A, Kuhlen T, Huang R, Shah NJ, Zilles K. Probabilistic fibre tract analysis of cytoarchitectonically defined human inferior parietal lobule areas reveals similarities to macaques. Neuroimage. 2011;58(2):362–380. doi: 10.1016/j.neuroimage.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, Cavanna AE, D’Agata F, Sacco K, Duca S, Geminiani GC. Functional connectivity and coactivation of the nucleus accumbens: a combined functional connectivity and structure-based meta-analysis. J Cogn Neurosci. 2011;23(10):2864–2877. doi: 10.1162/jocn.2011.21624. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Castanon AN, Ongur D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. NeuroImage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherniak C. Component placement optimization in the brain. J Neurosci. 1994;14:2418–2427. doi: 10.1523/JNEUROSCI.14-04-02418.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist. 2007;13:580–593. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- Déjerine J. Anatomie des centres nerveux. Rueff; Paris: 1895. [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A multimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci. 2000;3:277–283. doi: 10.1038/72991. [DOI] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci. 2010;14:172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D. Database-driven identification of functional modules in the cerebral cortex. In: Geyer S, Turner R, editors. Microstructural Parcellation of the Human Cerebral Cortex. Springer; Heidelberg: 2012. [Google Scholar]
- Eickhoff SB, Bzdok D. Meta-analyses in basic and clinical neuroscience: state of the art and perspective. In: Ulmer S, Jansen O, editors. fMRI — Basics and Clinical Applications. Springer; Heidelberg: 2012. [Google Scholar]
- Eickhoff SB, Grefkes C. Approaches for the integrated analysis of structure, function and connectivity of the human brain. Clin EEG Neurosci. 2011;42:107–121. doi: 10.1177/155005941104200211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Rottschy C, Zilles K. Laminar distribution and co-distribution of neurotransmitter receptors in early human visual cortex. Brain Struct Funct. 2007;212:255–267. doi: 10.1007/s00429-007-0156-y. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Roski C, Caspers S, Zilles K, Fox PT. Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. NeuroImage. 2011;57:938–949. doi: 10.1016/j.neuroimage.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. NeuroImage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science. 2005;308:662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Fox PT, Lancaster JL. Opinion: mapping context and content: the BrainMap model. Nat Rev Neurosci. 2002;3:319–321. doi: 10.1038/nrn789. [DOI] [PubMed] [Google Scholar]
- Fox DF, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Laird AR, Fox SP, Fox PM, Uecker AM, Crank M, Koenig SF, Lancaster JL. BrainMap taxonomy of experimental design: description and evaluation. Hum Brain Mapp. 2005;25:185–198. doi: 10.1002/hbm.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JB, Schiller D, Rule NO, Ambady N. The neural origins of superficial and individuated judgments about ingroup and outgroup members. Hum Brain Mapp. 2010;31:150–159. doi: 10.1002/hbm.20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K. The free-energy principle: a unified brain theory? Nat. Rev Neurosci. 2010;11:127–138. doi: 10.1038/nrn2787. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. I. Brain. 1965;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Geyer S. The microstructural border between the motor and the cognitive domain in the human cerebral cortex. Adv Anat Embryol Cell Biol. 2004;174(I–VIII):1–89. doi: 10.1007/978-3-642-18910-4. [DOI] [PubMed] [Google Scholar]
- Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Burgel U, Klingberg T, Larsson J, Zilles K, Roland PE. Two different areas within the primary motor cortex of man. Nature. 1996;382:805–807. doi: 10.1038/382805a0. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim YH, Meyer JR, Mesulam M. A large-scale distributed network for covert spatial attention: further anatomical delineation based on stringent behavioural and cognitive controls. Brain. 1999;122(Pt 6):1093–1106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AF, Grafton ST. Action outcomes are represented in human inferior frontoparietal cortex. Cereb Cortex. 2008;18:1160–1168. doi: 10.1093/cercor/bhm150. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Maguire EA. Using imagination to understand the neural basis of episodic memory. J Neurosci. 2007;27:14365–14374. doi: 10.1523/JNEUROSCI.4549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Jakobs O, Langner R, Caspers S, Roski C, Cieslik EC, Zilles K, Laird AR, Fox PT, Eickhoff SB. Across-study and within-subject functional connectivity of a right temporo-parietal junction subregion involved in stimulus-context integration. NeuroImage. 2012;60:2389–2398. doi: 10.1016/j.neuroimage.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens TE, Robson MD, Drobnjak I, Rushworth MF, Brady JM, Smith SM, Higham DJ, Matthews PM. Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci U S A. 2004;101:13335–13340. doi: 10.1073/pnas.0403743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK. Studying connections in the living human brain with diffusion MRI. Cortex. 2008;44:936–952. doi: 10.1016/j.cortex.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. NeuroImage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee JM, Jo HJ, Kim SH, Lee JH, Kim ST, Seo SW, Cox RW, Na DL, Kim SI, Saad ZS. Defining functional SMA and pre-SMA subregions in human MFC using resting state fMRI: functional connectivity-based parcellation method. NeuroImage. 2010;49:2375–2386. doi: 10.1016/j.neuroimage.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyachko VA, Stevens CF. Connectivity optimization and the positioning of cortical areas. Proc Natl Acad Sci U S A. 2003;100:7937–7941. doi: 10.1073/pnas.0932745100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214:519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Kurth F, Fox PM, Uecker AM, Turner JA, Robinson JL, Lancaster JL, Fox PT. ALE meta-analysis workflows via the Brainmap database: progress towards a probabilistic functional brain atlas. Front Neuroinformatics. 2009;3:23. doi: 10.3389/neuro.11.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J Neurosci. 2009;29:14496–14505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Fox PM, Uecker AM, Ray KL, Saenz JJ, Jr, McKay DR, Bzdok D, Laird RW, Robinson JL, Turner JA, Turkeltaub PE, Lancaster JL, Fox PT. The BrainMap strategy for standardization, sharing, and meta-analysis of neuroimaging data. BMC Res Notes. 2011;4:349. doi: 10.1186/1756-0500-4-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Langner R, Eickhoff S. A meta-analytic review of the neural mechanisms of vigilant attention. Psychol Bull. 2013 doi: 10.1037/a0030694. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu Rev Physiol. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Wheelwright SJ, Sadek SA, Suckling J, Baron-Cohen S. Shared neural circuits for mentalizing about the self and others. J Cogn Neurosci. 2009;22:1623–1635. doi: 10.1162/jocn.2009.21287. [DOI] [PubMed] [Google Scholar]
- Manes F, Paradiso S, Robinson RG. Neuropsychiatric effects of insular stroke. J Nerv Ment Dis. 1999;187:707–712. doi: 10.1097/00005053-199912000-00001. [DOI] [PubMed] [Google Scholar]
- Mar RA. The neural bases of social cognition and story comprehension. Annu Rev Psychol. 2011;62:103–134. doi: 10.1146/annurev-psych-120709-145406. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, Villringer A, Castellanos FX, Milham MP, Petrides M. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci U S A. 2009;106:20069–20074. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Sallet J, Schuffelgen U, Jbabdi S, Toni I, Rushworth MF. Connectivity-based subdivisions of the human right “Temporoparietal junction area”: evidence for different areas participating in different cortical networks. Cereb Cortex. 2012;22(8):1894–1903. doi: 10.1093/cercor/bhr268. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Activity in right temporo-parietal junction is not selective for theory-of-mind. Cereb Cortex. 2008;18:262–271. doi: 10.1093/cercor/bhm051. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Dosenbach NU, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE. Role of the anterior insula in task-level control and focal attention. Brain Struct Funct. 2010;214:669–680. doi: 10.1007/s00429-010-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Voogd J, van Huijzen C. The Human Central Nervous System: A Synopsis and Atlas. 4. Steinkopff; 2007. [Google Scholar]
- Passingham RE, Stephan KE, Kotter R. The anatomical basis of functional localization in the cortex. Nat Rev Neurosci. 2002;3:606–616. doi: 10.1038/nrn893. [DOI] [PubMed] [Google Scholar]
- Pisapia N, Turatto M, Lin P, Jovicich J, Caramazza A. Unconscious priming instructions modulate activity in default and executive networks of the human brain. Cereb Cortex. 2012;22:639–649. doi: 10.1093/cercor/bhr146. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends Cogn. Sci. 2006;10:59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT. Metaanalytic connectivity modeling: delineating the functional connectivity of the human amygdala. Hum Brain Mapp. 2010;31:173–184. doi: 10.1002/hbm.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottschy C, Caspers S, Roski C, Reetz K, Dogan I, Schulz JB, Zilles K, Laird AR, Fox PT, Eickhoff SB. Differentiated parietal connectivity of frontal regions for “what” and “where” memory. Brain Struct Funct. 2013 doi: 10.1007/s00429-012-0476-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby P, Decety J. Effect of subjective perspective taking during simulation of action: a PET investigation of agency. Nat Neurosci. 2001;4:546–550. doi: 10.1038/87510. [DOI] [PubMed] [Google Scholar]
- Saxe R, Wexler A. Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia. 2005;43:1391–1399. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Saygin ZM, Osher DE, Koldewyn K, Reynolds G, Gabrieli JD, Saxe RR. Anatomical connectivity patterns predict face selectivity in the fusiform gyrus. Nat Neurosci. 2012;15:321–327. doi: 10.1038/nn.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Episodic simulation of future events: concepts, data, and applications. Ann N Y Acad Sci. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, Vogeley K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious Cogn. 2008;17:457–467. doi: 10.1016/j.concog.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Bzdok D, Timmermans B, Fox PT, Laird AR, Vogeley K, Eickhoff SB. Introspective minds: using ALE meta-analyses to study commonalities in the neural correlates of emotional processing, social & unconstrained cognition. PLoS One. 2012;7:e30920. doi: 10.1371/journal.pone.0030920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist. 2013:43–61. doi: 10.1177/1073858412440596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks.2. Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Smallwood J, McSpadden M, Schooler JW. The lights are on but no one’s home: meta-awareness and the decoupling of attention when the mind wanders. Psychon Bull Rev. 2007;14:527–533. doi: 10.3758/bf03194102. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamsin KS, Robert PB, Alice KM, Hannah MR, Roberts SC. Face, body and speech cues independently predict judgments of attractiveness. J Evol Psychol. 2009:23–35. [Google Scholar]
- Timm NH. Applied Multivariate Analysis. Springer; New York: 2002. [Google Scholar]
- Timmermans B, Schilbach L, Pasquali A, Cleeremans A. Higher order thoughts in action: consciousness as an unconscious re-description process. Philos Trans R Soc Lond B Biol Sci. 2012;367:1412–1423. doi: 10.1098/rstb.2011.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. NeuroImage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum Brain Mapp. 2012;33(1):1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Molnar-Szakacs I, Zaidel E, Iacoboni M. rTMS to the right inferior parietal lobule disrupts self-other discrimination. Soc Cogn Affect Neurosci. 2006;1:65–71. doi: 10.1093/scan/nsl003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Amin H, Rykhlevskaia E, Nguyen DA, Greicius MD, Menon V. Dissociable connectivity within human angular gyrus and intraparietal sulcus: evidence from functional and structural connectivity. Cereb Cortex. 2010;20:2636–2646. doi: 10.1093/cercor/bhq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Economo C. Eine neue Art Spezialzellen des Lobus cinguli und Lobus insulae. Z Ges Neurol Psychiatr. 1926;100:706–712. [Google Scholar]
- von Helmholtz H. Treatise on Physiological Optics. 3. Voss; Hamburg: 1909. [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. NeuroImage. 2009;47:1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Williams ZM, Bush G, Rauch SL, Cosgrove GR, Eskandar EN. Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat Neurosci. 2004;7:1370–1375. doi: 10.1038/nn1354. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Barch DM, Gray JR, Conturo TE, Braver TS. BOLD correlates of trial-by-trial reaction time variability in gray and white matter: a multi-study fMRI analysis. PLoS One. 2009;4:e4257. doi: 10.1371/journal.pone.0004257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Camprodon JA, Hauser M, Pascual-Leone A, Saxe R. Disruption of the right temporoparietal junction with transcranial magnetic stimulation reduces the role of beliefs in moral judgments. Proc Natl Acad Sci U S A. 2010;107:6753–6758. doi: 10.1073/pnas.0914826107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Palomero-Gallagher N. Cyto-, myelo-, and receptor architectonics of the human parietal cortex. NeuroImage. 2001;14:S8–S20. doi: 10.1006/nimg.2001.0823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.