Abstract
Robust successes have been achieved in recent years in conquering the acutely lethal manifestations of heart disease. Many patients who previously would have died now survive to enjoy happy and productive lives. Nevertheless, the devastating impact of heart disease continues unabated, as the spectrum of disease has evolved with new manifestations. In light of this ever-evolving challenge, insights that culminate in novel therapeutic targets are urgently needed. Here, we review fundamental mechanisms of heart failure, both with reduced (HFrEF) and preserved (HFpEF) ejection fraction. We discuss pathways that regulate cardiomyocyte remodeling and turnover, focusing on Ca2+ signaling, autophagy, and apoptosis. In particular, we highlight recent insights pointing to novel connections among these events. We also explore mechanisms whereby potential therapeutic approaches targeting these processes may improve morbidity and mortality in the devastating syndrome of heart failure.
Keywords: Heart failure, Remodeling, Calcium homeostasis, Autophagy, Apoptosis
1. Introduction
Age-adjusted mortality from cardiovascular disease has declined a remarkable 75% over the past 50 years [1]. As a result, many individuals, who previously would have succumbed to heart disease now survive to enjoy happy and productive lives. However, many live with a heart that has been injured. This fact, coupled with deterioration in lifestyle-related issues and the pandemic of obesity, has culminated in dramatic increases in the prevalence of heart failure (HF). By 2030, more than 40% of the US population is projected to suffer from HF or other cardiovascular disease, with estimated costs, direct plus indirect, exceeding $1 trillion [2].
HF is a syndrome that arises from multiple diseases. Myocardial infarction, hypertension, valvular disease, genetic disorders, and many more funnel ultimately into a state in which the heart is no longer able to pump blood adequately to meet the metabolic demands of the body. HF is categorized based on the mechanism of pump dysfunction: diminished ability to contract forcefully during systole to pump blood forward is typically marked by a reduced ejection fraction (HFrEF). In up to half of cases of HF, ejection fraction is preserved (HFpEF) and the major limitation in performance lies in restricted filling during diastole.
Acute HF by definition is the sudden occurrence of signs or symptoms of congestion and diminished forward flow (e.g. extremity swelling, paroxysmal nocturnal dyspnea, breathlessness) requiring medical attention [3,4]. However, if the heart is persistently unable to deliver adequate circulation to the body, then the condition is categorized as chronic HF [4,5]. Aside from differences in presentation, acute and chronic HF have distinct and very significant impact on our healthcare system [6,7]. Central to each of these syndromes is pathological remodeling of the myocardium [8].
Another major mechanism central to both HFrEF and HFpEF is altered excitation–contraction (EC) coupling. This altered EC coupling derives, in part, from changes in the architecture of the cell membrane, altered expression and function of Ca2+-handling proteins, and mal-adaptive redistribution of intracellular Ca2+. Importantly, remodeling entails numerous other changes, including a wide range of transcriptional, signaling, metabolic, and electrophysiological events. Finally, myocyte death is a critical part of many forms of HF.
Mechanisms of HF pathogenesis have been extensively reviewed [9–11] and are summarized (Table 1). Here, we focus on Ca2+ dysfunction and its relation with autophagy and apoptosis within failing cardiomyocytes.
Table 1.
Summary of current hypotheses explaining HF progression.
| Hypothesis | Description | Reviewed in |
|---|---|---|
| Impaired EC coupling | Alterations in Ca2+-handling and cardiomyocyte ultrastructure lead to impaired Ca2+ cycling that impinge on both systolic and diastolic function. Ca2+ overload might trigger mitochondrial dysfunction and cell death | [13,14,18,209] |
| Alterations in sarcomeric proteins |
Switch in myosin, actin, troponin, tropomyosin, and titin isoforms | [292,293] |
| Adrenergic system | Increased adrenergic signaling in HF | [294] |
| Renin-angiotensin-aldosterone axis |
Increased activity of this axis leads to maladaptive hypertension and HF development | [295–297] |
| Epigenetic modification and noncoding RNAs |
Modifications in gene expression associated with cardiac remodeling and HF progression | [298,299] |
| Innate immunity | Increased inflammation in HF patients related to left ventricular remodeling and dysfunction | [300] |
| Reactive oxygen species | Activation of unfolded protein response, apoptosis and senescence | [301] |
| Nitric oxide (NO) | NO insensitivity. Increased ROS leads to peroxynitrite formation and deficient NO-cGMP-PKG activity. Impairment in LV function and endothelial dysfunction |
[302] |
| Changes in autophagic flux | Increased autophagic activity correlates with hypertrophic and HF progression | [192] |
| Mitochondrial dysfunction | Reduced mitochondrial function, increased ROS production and diminished ATP production | [85,291] |
| Increased cell death | Necrosis, apoptosis and autophagy contribute to progressive reduction and remodeling in cardiomyocytes in failing hearts | [116,303] |
2. EC coupling and HF
In order to accomplish fast and powerful muscle contraction, both cardiac and skeletal muscle have evolved a complex membrane architecture to facilitate EC coupling. Electrical depolarization of the myocyte membrane travels rapidly toward the center of the cell via membrane invaginations termed the transverse tubular (t-tubule) network. T-tubules dive into the cell, terminating close to the sarcoplasmic reticulum (SR) to form structures, termed dyads, which are situated at the Z-line regions of the sarcomere. Within the dyad, the t-tubule pit is separated from the SR by a 12 nm gap, a short distance which facilitates rapid diffusion of Ca2+ from the extracellular space to the SR [12]. Membrane depolarization within the t-tubule triggers Ca2+ entry mainly through L-type Ca2+ channels (LTCC), eliciting Ca2+-induced calcium release (CICR) from the SR through the type 2 ryanodine receptor (RyR2). Ca2+ pouring onto the sarcomere relieves actin from troponin C-dependent inhibition, activating cross-bridge cycling and myocyte contraction. Subsequently, Ca2+ is pumped back into the SR by the SR Ca2+ ATPase 2a (SERCA2a) [13]. It is generally accepted that alterations in Ca2+-handling proteins, coupled with remodeling of the t-tubule architecture, contribute to HF pathogenesis, promoting impaired contraction and reductions in cardiac output (Fig. 1).
Fig. 1.
Disruption of t-tubule network in HF. In failing cardiomyocytes, there is progressive deterioration of t-tubule architecture, impeding the normal propagation of action potentials into the cardiomyocyte core. It has been proposed that “orphan” RyR2 are located outside the junctional cleft, triggering a second wave of CICR that perturbs synchronicity during systole and diastole. As these orphan RyR2 are located distant from the dyads, they are activated late, contributing to impaired contractile performance. Distorted t-tubule architecture may also impair signaling elicited by catecholamines and growth factors.
2.1. Ca2+ homeostasis
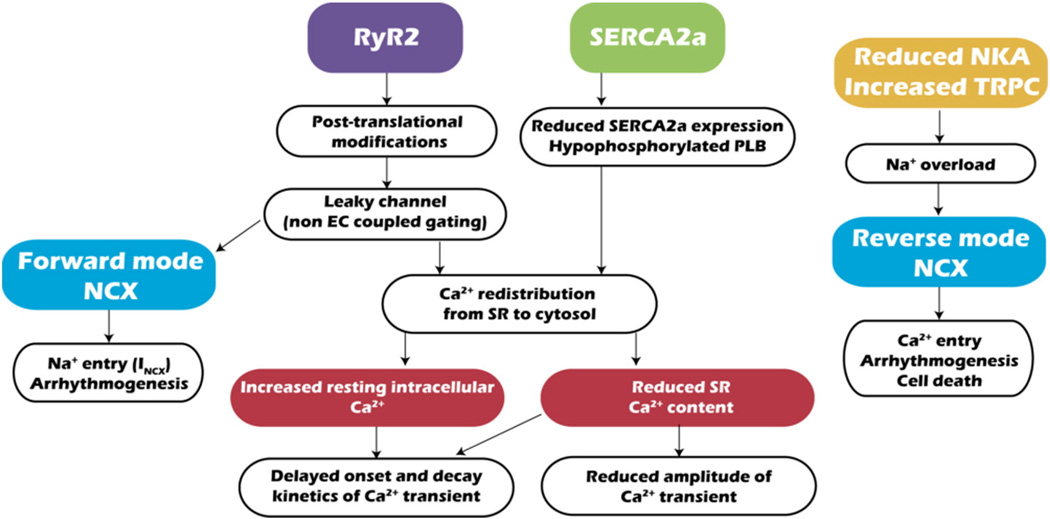
A hallmark of failing cardiomyocytes is altered EC-coupling, including reduced Ca2+ transients (Ca2+ released from SR stores during excitation), as well as delayed onset and decay of those transients. Together, these changes culminate in reduced force of contraction, delayed onset of contraction, and slowed relaxation [14]. In addition, spontaneous Ca2+ release events, normally rare, occur more frequently [13]. Alterations in Ca2+ handling have been ascribed to impaired function of the RyR2, SERCA2a, Na+–Ca2+ exchanger (NCX), and transient receptor potential cation (TRPC) channels (Fig. 2).
Fig. 2.
Ca2+ mishandling in HF. Alterations in RyR2, SERCA2a, NCX, Na+/K+ ATPase (NKA) and TRPC channels lead to impaired Ca2+ and Na+ handling, which negatively impacts the contractibility of failing cardiomyocytes. NKA: Na+/K+ ATPase.
2.1.1. RyR2
Multiple lines of evidence point to relative redistribution of intracellular Ca2+ from SR stores to the cytosol in HF caused by impaired RyR2/SERCA2a function. This redistribution reduces the electrochemical gradient for Ca2+, reduces the amplitude of systolic Ca2+ levels, slows contraction kinetics (promoting systolic dysfunction), and elevates resting Ca2+ (promoting diastolic dysfunction).
It is generally accepted that HF is characterized by an increase in Ca2+ leak from the SR via RyR2, but there exists controversy regarding molecular mechanisms underlying this leak. Numerous factors modulate RyR2 function (reviewed in [15]), such as cytosolic and SR Ca2+, ATP, and Mg2+ concentrations. In addition, a number of signaling pathways govern RyR2 function. Some evidence points to PKA-dependent hyperphosphorylation of RyR2 (at S2808) in the context of the chronic hyperadrenergic activation typical of HF, provoking FKBP12.6 (aka calstabin 2) dissociation from the channel [16]. Dissociation of FKBP12.6 from RyR2, in turn, increases SR Ca2+ leak, which consequently depletes SR Ca2+ stores partially and diminishes Ca2+ transients after membrane depolarization. These events likely contribute to reduced contractibility in failing hearts. More recently, this Ca2+ leak has been associated with mitochondrial Ca2+ overload and dysfunction [17]. Some evidence suggests that stabilization of RyR2-FKBP12.6 with small molecules (“rycals”, S107) protects against post-myocardial infarction HF and reduces arrhythmias in mouse models of Duchenne muscular dystrophy and catecholaminergic polymorphic ventricular tachycardia [18]. A small molecule “rycal” (ARM136) is presently in phase 2 trials for HF and for catecholaminergic polymorphic ventricular tachycardia. Also, adenovirus-mediated over-expression of S100A1, a regulator of both RyR2 and SERCA2, improves heart function in rodent and swine models of HF [19,20].
On the other hand, some data suggest that PKA phosphorylation of RyR2 (S2808) is not a major mechanism in cardiac failure [13,21–23]. Some work has suggested that FKBP12.6 does not modify RyR2 gating properties and that PKA phosphorylation of RyR2 does not displace FKBP12.6 (reviewed in [13,22]). RyR2 can be phosphorylated by CaMKII, rather than by PKA, at a different site. Additional mechanisms including direct RyR2 oxidation, reduced calmodulin binding to RyR2, and reduced S100A1 have been described [13]. In any case, controversy persists, as counter-arguments have been marshaled supporting PKA-induced phosphorylation of RyR2 in HF [18].
Regardless of the specific molecular events leading to SR Ca2+ leak, it is generally accepted that RyR2-mediated Ca2+ leak induces: 1) Ca2+ redistribution from SR to cytosol, reducing the electrochemical gradient and diminishing systolic contractile performance; 2) an increase in basal, cytosolic Ca2+ concentration impairing diastolic function; 3) spontaneous Ca2+ release events (“sparks”); 4) membrane depolarization through the forward mode of NCX (Ca2+ out/Na+ in), triggering delayed afterdepolarizations; and 5) excessive energy consumption to restore Ca2+ gradients.
2.1.2. SERCA
SERCA2a drives ATP-dependent re-uptake of Ca2+ into the SR following mechanical contraction. In some species, e.g. mouse and rat, SERCA2a activity accounts for ≈93% of Ca2+ removal from the cytosol [13]. In other species, e.g. human and rabbit, it represents closer to 74% of Ca2+ removal, with a relatively greater proportion of Ca2+ extrusion occurring to the extracellular space via NCX [13].
SERCA is negatively regulated by several small proteins, including phospholamban (PLB) [24] and sarcolipin [25]. In response to adrenergic drive, PLB is phosphorylated by PKA, releasing SERCA from PLB-mediated inhibition. Similarly, CaMKII can phosphorylate PLB and suppress its inhibitory actions on SERCA [26]. PLB dephosphorylation is mediated by protein phosphatase 1 [27].
Multiple lines of evidence demonstrate that SERCA function is reduced in HF due to reduced expression [28] and/or hypophosphorylation of PLB [29]. Reduced SERCA activity plus increased SR Ca2+ leak synergize to promote systolic and diastolic dysfunction. Supporting this model, cardiomyocyte-restricted silencing of SERCA2a triggers HF [30]. Conversely, SERCA2a over-expression improves myocardial contractility in both human and animal models of HF [31,32]. Recently, a small phase 2 clinical trial (CUPID1) demonstrated beneficial effects of SERCA2a over-expression in patients with NYHA class III/IV HF [33]. Improvements in several parameters were noted, including NYHA functional class, 6-min walk test, peak maximum oxygen consumption, and left ventricular end-systolic volume [33], improvements which persisted at 3 years [34]. However, recently released findings from a phase 2b trial, CUPID2 (ClinicalTrials.gov. Identifier: NCT01643330) [35] showed no improvement in clinical endpoints in patients with HF (http://ir.celladon.net/releasedetail.cfm?ReleaseID=908592). Additional trials are underway testing the effects of SERCA over-expression delivered via intracoronary infusion (ClinicalTrials.gov. Identifier: Phase 2 NCT01966887, NCT00534703, NCT02346422). A possible explanation for failure in the most recent SERCA trial is the complexity of fine-tuning Ca2+ cycling in HF; derangement in cardiomyocyte t-tubule ultrastructure coupled with alterations in Ca2+-handling proteins make it challenging to normalize EC coupling in failing hearts.
2.1.3. NCX
NCX is a bidirectional, electrogenic cation cotransporter that exchanges a single Ca2+ ion for three Na+ ions. As such, each cycle of NCX action generates a current (net transfer of one positive charge). NCX function is governed by the transmembrane gradients of both Na+and Ca2+ and by transmembrane potential. NCX can operate in forward mode (Ca2+ efflux) or reverse mode (Ca2+ influx) [36]. During depolarization, increases in intracellular Na+ mediated by voltage-dependent Na+ channels lessen NCX-dependent Ca2+ extrusion; in extreme circumstances, it can lead to reverse-mode NCX activity, where NCX pumps Ca2+ into the cell. As a result of this mechanism, the junctional cleft is primed with Ca2+, facilitating EC-coupling. During diastole, cytosolic Ca2+ increases from RyR2 activation promote Ca2+ extrusion through NCX activity [37].
It has been proposed that NCX activity is up-regulated in failing heart [38], but divergent evidence also exists [37]. SR Ca2+ leak increases cytosolic Ca2+ and forward mode-dependent Ca2+ extrusion, exacerbating declines in SR Ca2+ and promoting Na+ entry [13]. In addition, due to reduced Na+/K+ ATPase, and possibly increased TRPC channel function in failing hearts [37,39], cytosolic Na+ concentrations are elevated. Na+ accumulation diminishes the transmembrane Na+ gradient, suppressing NCX-dependent Ca2+ extrusion. This can culminate in arrhythmia or even cell death due to Ca2+ overload.
The dual function of NCX (forward and reverse) makes it difficult to target this mechanism pharmacologically for HF treatment. Moreover, lack of selectivity of several NCX blockers, such as KB-R7943 (targeting also RyR1/2 and TRP channels) [40,41], renders clinical translation challenging.
Targeting Na+ overload might be an effective treatment to prevent NCX-mediated Ca2+ overload and alterations in cellular excitability. Na+/K+ ATPase over-expression has been reported to reduce cardiac remodeling after pressure overload [42]. Similarly, inhibition of the late Na+ current using ranolazine improves some hemodynamic parameters, although improvement in relaxation was not observed in patients with HFpEF [43]. Recently, increased late Na+ current has been reported to induce diastolic arrhythmogenesis mediated by CaMKII-dependent SR Ca2+ leak, and inhibition of late Na+ current blunted arrhythmias in failing human cardiomyocytes [44].
2.1.4. TRPC channels
TRPC channels participate in store-operated Ca2+ entry (SOCE), serving to refill depleted SR Ca2+ stores. TRPC channel expression increases during pathological hypertrophy and HF [39], possibly as an adaptive mechanism to compensate for SR Ca2+ leak and consequent store depletion. TRPC channels conduct Ca2+ and Na+ [45], and their activation can contribute to Ca2+ and Na+ overload in failing cardiomyocytes. Inhibition of TRPC subfamilies represses both pressure overload- and angiotensin II-induced cardiac hypertrophy [46]. Furthermore, TRPC channels are implicated in ventricular pathological remodeling and increases in RyR2 leak [47]. Moreover, increased TRPC3 expression increases ischemia/reperfusion (I/R)-induced apoptosis [48], and reduction in TRPV1 levels following cold exposure is associated with cardiac hypertrophy and reduced cardiac function [49]. Together, these lines of evidence suggest that TRPC channels play a deleterious role in HF.
2.2. T-tubule network
Intricately organized dyads along the myocyte promote coordinated Ca2+ release responses and synchronous activation of the sarcomeres. In addition, numerous signaling proteins reside within the t-tubules, facilitating localized signaling. Thus, these structures play a vital role in orchestrating cardiomyocyte function. Ca2+ entry through LTCC increases the local Ca2+ concentration at the dyad, promoting RyR2-dependent Ca2+-induced Ca2+ release. HF-associated disruption in dyad organization can have a negative impact on cardiac performance due to heterogeneous Ca2+ gradients and delayed responses. Indeed, HF is marked by distorted organization of the t-tubule network, a decrease in both transverse and longitudinal elements, and increased t-tubule diameter (reviewed in [12,14]). Whereas most work in this area has centered on ventricular myocytes, alterations in t-tubule organization in atrial cardiomyocytes have been described [50].
Recently, it has been proposed that t-tubule network remodeling is a progressive process that commences early and contributes to HF pathogenesis [51]. Distortions of the t-tubule network are associated with alterations in both electrical activity and Ca2+ release. Recently, simultaneous recording of action potentials and Ca2+ transients by random access multiphoton microscopy demonstrated impaired action potential propagation, along with delayed and reduced Ca2+ rises, in adult cardiomyocytes isolated from failing hearts [52]. Moreover, an increase in sparks and spontaneous action potentials was observed [52].
As mentioned previously, cardiomyocytes from failing hearts are marked by reduced contractile force and prolonged relaxation times [53]. Exposure to formamide, an experimental means of detubulating adult cardiomyocytes, elicits reduced force generation with slower kinetics of contraction/relaxation, a process which can be reversed by RyR2-sensitizing agonists [54]. Together, these findings suggest that cardiomyocytes from failing hearts manifest asynchronous Ca2+ release, leading to slowed contraction and relaxation kinetics, as well as arrhythmogenesis, and that distortion of the t-tubule network is a contributing factor (Fig. 1).
In addition, it has been proposed that t-tubule remodeling generates “orphan RyR2s”, i.e. SR Ca2+-release channels localized relatively distant from the dyad. Activation of these isolated RyR2s can generate a second wave of Ca2+ release triggered by delayed arrival of Ca2+ diffusing from normal RyR2 channels localized appropriately at the dyad. This, in turn, elicits asynchronicity in EC coupling, perturbing both systolic and diastolic function and promoting arrhythmia [55].
Disruption of the t-tubule architecture, as observed in failing hearts, is involved not only in Ca2+ mishandling but may also disrupt cell signaling. In HF, β2-adrenergic receptors are redistributed from deep within the t-tubules to the cell surface, disrupting localized β2-adrenergic signaling [56]. In addition, precise organization of the t-tubule network is required for IGF-1 (insulin-like growth factor-1) signaling in cardiomyocytes [57]. In some cases, a t-tubule may extend to reach the nuclear envelope, allowing IGF-1 or other ligands to signal directly into the nucleus to induce nuclear Ca2+ signals that modulate gene expression [57,58]. IGF-1 is notable for its anti-apoptotic and pro-survival effects in cardiomyocytes and has been proposed as a therapeutic approach for HF [59,60].
2.2.1. Therapeutic manipulation?
In light of the fundamental role of t-tubule architecture in cardiomyocyte function, it is intriguing to speculate that slowing or arresting the progressive deterioration of t-tubule architecture is a target worthy of consideration. Would it even be possible to restore that architecture? Several approaches have been proposed. For one, there is a correlation between the rigidity of the microtubule network and cardiac pathology. Microtubule-stabilizing drugs (e.g. taxol) can cause cardiotoxicity [61, 62], whereas microtubule-destabilizing drugs (e.g. colchicine) are associated with reduced myocardial infarction size [63]. In the setting of cardiomyocyte stretch, activation of NOX2 leads to ROS production and RyR2 sensitization (“X-ROS signaling”) [64]. However, enhanced signaling due to excessive microtubule stabilization can provoke excessive Ca2+ sparks and arrhythmogenesis.
Microtubule depolymerization with colchicine attenuates pressure overload-induced t-tubule remodeling and improves survival and cardiac function, whereas taxol (microtubule-stabilizing drugs) amplify cardiac damage [65]. Microtubule depolymerization is associated with increases in Ca2+ transient amplitude, accelerated transient upstroke, and reduced Ca2+ spark frequency [65]. These findings suggest that t-tubule architecture can be remodeled in injured hearts and raise the intriguing prospect that these events could be targeted therapeutically in established HF. Colchicine has demonstrable benefits in several cardiovascular disorders, including pericarditis, atrial fibrillation, ischemia, and chronic HF [66].
Many proteins contribute to the maintenance of the t-tubule network in both skeletal and cardiac muscle, including junctophilin-2 (JP-2), amphyphisin-2 (BIN-1), telethonin, and caveolin [14]. JP-2 spans the gap between the t-tubule and SR membranes, maintaining dyad structure [12]; it is also required for proper pre- and post-natal development of the t-tubule network [67,68]. Reduced JP-2 levels correlate with HF, and silencing of JP-2 impairs EC-coupling and induces cardiomyocyte hypertrophy [69]. Conversely, JP-2 over-expression reduces the incidence of HF and preserves t-tubule network integrity in mice after pressure overload [70]. It has been proposed that either de-stabilization of microtubules or suppression of Gαq-dependent signaling are related to JP-2 and t-tubule network remodeling [65,71]. Furthermore, reduced abundance of amphiphysin-2 (protein associated with vesicle trafficking) is associated with HF and t-tubule remodeling, likely contributing to Ca2+ mishandling [72].
2.3. Mitochondrial Ca2+
In addition to their vital role as fuel burning sources of energy, mitochondria function as sinks for intracellular Ca2+. Indeed, mitochondrial Ca2+ is required for normal functioning of metabolic enzymes, serving as a cofactor for several of them. In HF, however, elevated cytosolic Ca2+ facilitates accumulation of Ca2+ within mitochondria, which promotes ROS generation, impairs mitochondrial function, and can activate cell death pathways. ROS generation induced by mitochondrial Ca2+ overload may increase RyR2 oxidation, thereby exacerbating cytosolic Ca2+ accumulation and mitochondrial Ca2+ overload [17].
Anatomical and functional coupling between mitochondria and ER has been proposed in several cell types, including cardiomyocytes [73,74]. Whereas definitive studies are lacking, so-called mitochondria-associated ER membranes (MAMs) may play a role in HF pathogenesis. Cardiomyocyte hypertrophy induced by norepinephrine is associated with reduced SR-mitochondria coupling and mitochondrial dysfunction, suggesting disrupted communication between these organelles [75]. More recently, it has been reported that glycogen synthase kinase-3β (GSK-3β) is an essential regulator of MAMs in cardiomyocytes, acting by regulating inositol triphosphate receptor (IP3R) function. Increased GSK-3β activity during hypoxia/reoxygenation increases IP3R phosphorylation and Ca2+ transfer to mitochondria, promoting cell death [76].
3. Cardiomyocyte death in HF
3.1.Apoptosis
Apoptosis is a mechanism of programmed cell death driven primarily by caspases, particularly the final common “executioner” caspases 3, 6, and 7 [77]. Inactive cytosolic caspase isoforms are cleaved in response to cell death signals, triggering their activation and consequent degradation of essential cardiac proteins, including multiple sarcomeric proteins [77–79].
It is well recognized that failing heart is often marked by apoptotic loss of cardiomyocytes. Patients with severe HF exhibit a low, but definitively increased, rate of cardiomyocyte apoptosis of 0.1 −0.25% [80]. This low level activation nevertheless represents 100-fold greater activation relative to basal rates in normal heart and if persistent for a year could result in greater than 30% loss of cardiac mass [81,82].
3.1.1. Mechanisms
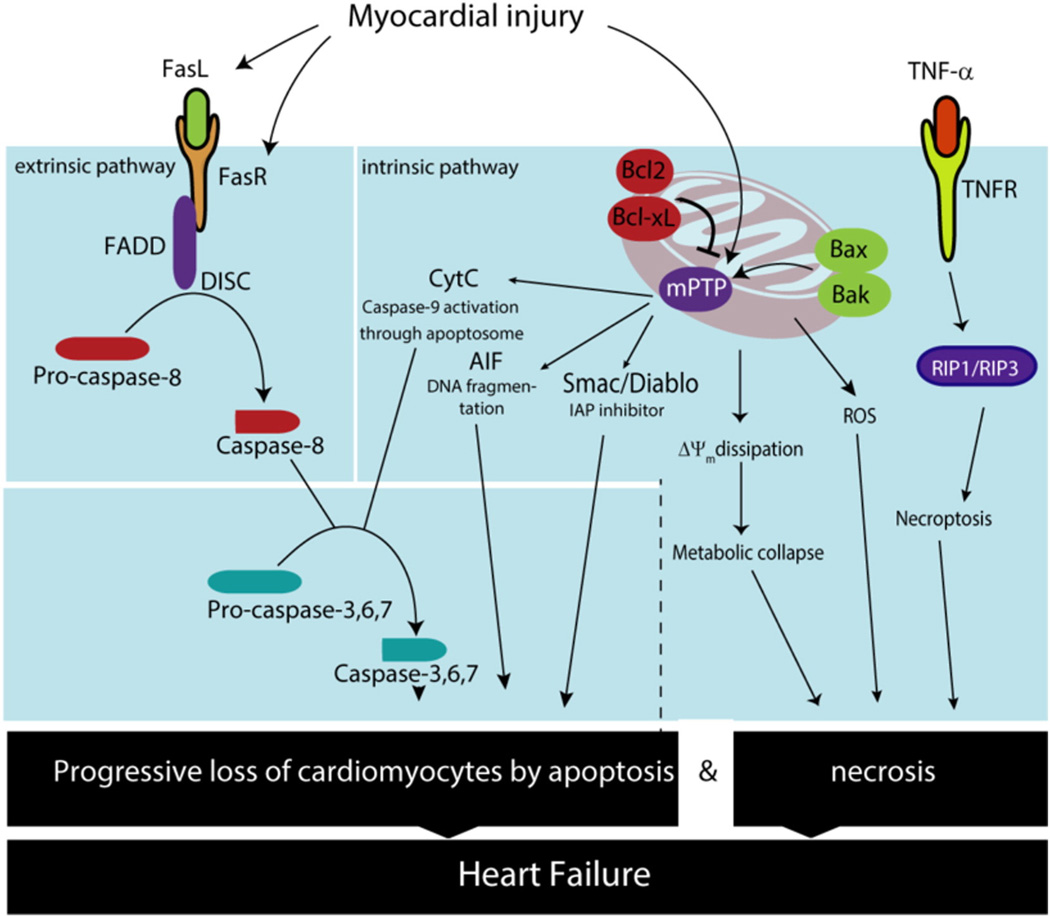
There are two primary pathways of apoptosis, and both the “extrinsic” and “intrinsic” cascades play important roles in cardiomyocyte demise (Fig. 3). These pathways lead to activation of caspase-8 or caspase-9 respectively, which in turn signal downstream to caspase-3 and caspase-7 to trigger cell death. As cardiomyocytes harbor numerous mitochondria, accounting for up to 70% of their volume, it is not surprising that regulation of apoptosis by this organelle plays a significant role in cardiomyocyte homeostasis [83,84]. HF, particularly secondary to ischemic disease, is marked by activation of the intrinsic pathway, a cascade characterized by loss of mitochondrial membrane integrity [85,86]. This, in turn, stimulates mitochondrial outer membrane permeabilization secondary to activation and oligomerization of specific Bcl-2 proteins, such as Bax and Bak. This allows release of multiple proapoptotic factors, including apoptosis-inducing factor (AIF), DIABLO, and cytochrome c from the intermembrane space into the cytoplasm [87,88]. Cytochrome c leads to oligomerization of Apaf-1 (apoptotic protease-activating factor-1) and formation of the apoptosome, leading to subsequent cleavage of caspase-9 and consequent apoptotic cell death [89]. The flavoprotein AIF promotes chromatin condensation and DNA fragmentation, and DIABLO blocks the apoptosis-suppressing actions of IAP, leading to increased inherent caspase activity.
Fig. 3.
Increased levels of apoptosis and necrosis are observed during HF. Activation of both the extrinsic and intrinsic apoptotic pathways have been described in HF. Activation of FasR leads to caspase-8 activation and extrinsic pathway activation. On the other hand, mitochondrial permeabilization leads to cytochrome C, AIF and Smac/Diablo release, which triggers the intrinsic pathway. Similarly, mitochondrial Ca2+ overload leads to mitochondrial membrane potential dissipation (ΔΨm) and ROS production, which ultimately trigger necrosis. In addition, regulated necrosis or necroptosis has emerged as another source for cell death in HF through activation of RIP1/RIP3. Over time, cardiomyocyte death results in a decreasing abundance of functional cardiomyocytes in the failing heart, which further deteriorates cardiac function.
Ca2+ concentrations also play an essential role in apoptosis during HF, as increases in Ca2+ within mitochondria can result in increased ROS and cytochrome c release, as well as activation of ERK and JNK stress pathways [89]. Furthermore, Ca2+ can stimulate calpain, a cysteine protease which can cleave Bax, creating pro-apoptotic fragments [90].
In contrast, the extrinsic apoptotic pathway is launched by activation of so-called death receptors, for example when Fas ligand (FasL) binds its receptor (FasR) [91]. This FasL–FasR interaction triggers Fas-associated death domain protein (FADD) to accumulate caspase-8 molecules, ultimately resulting in their activation [92].
3.1.2. Apoptosis and HF
FasL and FasR are abundant in cardiomyocytes, and their expression increases during pathological cardiac stress or obesity [93–96]. In addition, up-regulation of circulating FasR in murine models of I/R and HF has been long associated with increased cardiomyocyte death [97,98]. In a rat model of autoimmune myocarditis-induced HF, increases in apoptosis and FasL/FasR signaling were reported [99]. Similarly, a study of ischemic HF in sheep reported that increases in LV wall stress were linearly correlated with levels of FasL, and both FasL and caspase-8 were localized by immunohistochemistry to the cardiomyocyte intercalated disk [100]. Some evidence suggests that serum levels of FasL are elevated in patients experiencing an ST segment elevation myocardial infarction, a finding predictive of worse outcomes [101].
Some studies report no correlation between HF and levels of Fas in patients, whereas others have shown that worse NYHA HF class correlates with higher levels of FasR and FasL [102,103]. In a meta-analysis of HF clinical trials testing levosimendan, it was suggested that in patients with decompensated HF, efficacy in reducing hospitalization relates to the drug's ability to reduce soluble apoptosis mediators such as FasL/FasR [104,105]. In a similar fashion, it has been suggested that some of the beneficial effects on reducing pathologic remodeling with olmesartan derive from inhibition of Fas-directed apoptosis [106]. There is also significant evidence that the benefits of beta-blockers and ACE inhibitors in HF derive, at least in part, from decreases in apoptosis and improvements in G-protein signaling [107,108–111].
Also important is the Bcl-2 family of oncogenic proteins, molecules which have either anti-apoptotic (Bcl-2, Bcl-xL) or pro-apoptotic (Bax, Bak) roles. Dysregulation of cardiomyocyte Ca2+ levels during HF can subsequently lead to mitochondrial Ca2+ overload and dissipation of the mitochondrial transmembrane gradient [92]. However, as this loss of transmembrane potential does not appear to require increasing permeability of the outer mitochondrial membrane (or release of cyto-chrome c or caspase), this inner membrane MPTP opening likely has a significant role in necrotic cell death [112]. For instance, in Bax/Bak-deficient cardiomyocytes, thapsigargin, which inhibits SERCA, causes Ca2+ overload and MPTP opening, eventually leading to cellular necrosis [113]. In addition, mice deficient in cyclophilin D- and Bax/Bak, both of which are known regulators of the MPTP, are resistant to necrotic cell death [114].
Apoptosis is driven by Bax/Bak-triggered permeabilization of the mitochondrial outer membrane, which subsequently releases cytochrome c and other apoptotic factors into the cytoplasm [115]. In contrast, Bax and Bak stimulation of sustained MPTP opening on the inner mitochondrial membrane leads to mitochondrial rupture and necrotic cellular demise [112]. Indeed, Bax/Bak-driven opening of the mitochondrial outer membrane and Ca2+ dysregulation may actually enhance MPTP opening at the inner membrane, blurring the lines between necrotic and apoptotic cell death.
Cardiomyocyte-restricted over-expression of Bcl-2 preserves mitochondrial function and structural integrity [116], promotes stabilization of the mPTP, and decreases infarct size after I/R injury [87]. Another study with Bcl-2 transgenic mice also reported partial rescue of HF in a model of inherited cardiomyopathy [117]. Patients with higher levels of myocardial Bcl-2 and BcL-xL also appear to have decreased levels of apoptosis, which can be associated with diminished pathological remodeling in HF [118].
Members of the IAP (inhibitor of apoptosis protein) family, as the name implies, inhibit caspase-driven apoptosis and include XIAP, ts-IAP, survivin, and apollon [119]. In patients with end-stage HF, myocardial levels of IAP are decreased, which may increase the susceptibility of the heart to the pathological effects of excessive apoptosis [120]. Chen and colleagues have also suggested that ARC (apoptosis regulator with caspase recruitment domain), an apoptotic repressor prevalent in cardiac muscle, is degraded by deficiency of SNX13 (sorting nexin-13) with consequent increases in cardiac apoptosis, increases in cardiomyocyte death and worsened cardiac function [121]. Similarly, inhibition of XIAP by XAF1 (XIAP-interacting protein-1) promotes caspase-induced apoptosis in cardiac myocytes. In particular, XAF1 is abundant in the heart and is up-regulated in HF [122,123] suggesting that suppression of XIAP-dependent inhibition may render the heart less susceptible to apoptotic cell death. In a porcine model of cardiac arrest, therapeutic hypothermia appeared to increase levels of survivin and decrease levels of XAF1 and cleaved caspase-3 [123].
IGF-1 is recognized to have many roles in the heart, particularly in physiological cardiac growth, and possibly in protection from I/R and metabolic (e.g. diabetic/high-fat diet) injury [124]. There are also studies suggesting that IGF-1 can promote reverse remodeling in several models of preclinical cardiomyopathy [125]. Clinically, higher levels of circulating IGF-1 predict survival in HF patients [126]. While there appear to be several pathways involved, the ability of IGF-1 to provide salutary effects in HF derives, at least in part, from down-regulation of apoptosis via suppression of Bax and caspase-3 activation [127,128].
Some data link increased guanine nucleotide-binding protein activity and pathological hypertrophy/failure with apoptosis [107]. Several established stimulators of hypertrophy, such as norepinephrine, angiotensin, and endothelin-1, provoke hypertrophy via this G protein pathway. Mice over-expressing Gαq exhibit cardiac hypertrophy and increases in HF markers [129,130]. Furthermore, these Gαq mice manifest worsened cardiac decompensation, pulmonary congestion, and mortality, with increased susceptibility to dilated cardiomyopathy elicited by transverse aortic constriction (TAC) or volume overload. This Gαq-mediated transition to hypertrophy and failure is in part linked to increased rates of cardiomyocyte apoptosis. Further, activation of upstream pathways (e.g. using norepinephrine or angiotensin) or constitutive activation of Gαq increases apoptosis levels in cultured cardiac myocytes, and exacerbated HF has been observed in several preclinical models [107,131].
The primary driver whereby these G proteins activate apoptosis is under debate [132,133]. For instance, in dilated cardiomyopathy, Gαq over-expression is associated with opening of the MPTP and cytochrome c release [130], suggesting a role of the intrinsic apoptotic pathway. Furthermore, utilization of MPTP inhibitors or polycaspase inhibitors partially rescues this phenotype.
One of the proapoptotic Bcl-2 proteins, Nix, is increased in both human and animal models of pressure-overload hypertrophy [134,135]. Transgenic mice expressing cardiomyocyte-restricted Nix die of HF within 2 weeks, whereas mutant mice that over-express Gαq but lack Nix harbor reduced levels of apoptosis and are relatively resistant to pressure overload-induced HF [135].
Scherer and colleagues have engineered transgenic mice expressing a cardiomyocyte-specific cassette encoding a fusion protein consisting of procaspase-8 and amutated FK506-binding protein (FKBP) dimerization domain. In this model, dimerization of the mutant protein can be triggered by injection of AP20187, activating caspase-8-mediated apoptosis [136]. Expression and activation of this transgene causes widespread cardiomyocyte apoptosis with 50% mortality from cardiac catastrophe within 20 h of administration. Similarly, Kitsis and colleagues reported low, but definitively increased, levels of cardiomyocyte apoptosis in a model of transgenic caspase-8-driven apoptosis triggering dilated cardiomyopathy and early mortality [137]. Whereas there is some debate as to whether the presence or absence of GP130, a subunit that is part of the IL-6 family, promotes HF, dysregulation of this protein increases mortality by promoting apoptosis and pathological changes in preclinical models [138]. In addition, the Controlled Rosuvastatin Multinational Trial in HF (CORONA) demonstrated that in elderly patients with ischemic HF, elevations in soluble gp130 correlate with worsening HF as well as increased total and cardiovascular mortality [139].
Caspase-3-specific and polycaspase inhibitors are capable of antagonizing pathological cardiac remodeling, as well as mortality, in several preclinical models of HF [140]. Furthermore, rescue of both I/R injury and HF by these or similar inhibitors suggests that ongoing apoptotic cell death is a significant mechanism contributing to I/R-induced heart disease. In fact, there are multiple lines of evidence that increases in myocardial apoptosis correlate with pathological remodeling and worsened outcomes in ischemic HF. Some evidence suggests that reverse remodeling in the setting of mechanical unloading correlates with decreases in apoptotic index and apoptotic signaling [79,141].
3.1.3. Necrosis and HF
Although necrosis can exist as an unregulated process of cell death involving ATP depletion, cell swelling and ultimately lysis, several forms of regulated necrosis have been described in recent years. Necroptosis is programed cell death by necrosis, triggered by activation of the necrosome, which is formed by receptor interacting protein kinase-1 (RIP1), −3 (RIP3) and substrate mixed lineage kinase like (MLKL) [142]. Diverse stimuli can trigger necroptosis, including TNF-α, Fas-L, and ROS [reviewed in [143]. In cardiomyocytes, necroptosis contributes in part to cell death after I/R injury. In fact, necrostatin-1, an inhibitor of RIP-1, can reduce cardiomyocyte death after I/R injury and mitigate adverse cardiac remodeling [144–146].
More recently, TGFβ-activated kinase 1 (TAK1) has emerged as a factor which plays a pivotal role in regulating cardiomyocyte death by necroptosis in myocardial remodeling and HF [147]. It has been proposed that TAK1 acts as a molecular switch that regulates the formation of two cell death complexes, RIP1-FADD-caspase 8 and RIP1-RIP3 [147]. In addition, TAK1 ablation increases cell death by both apoptosis and necroptosis, a cascade which is blunted by silencing of TNFR1, RIP1, or RIP3 [147].
As outlined above, multiple points in the apoptotic cascade could be envisioned as therapeutic targets. Although the current arsenal of small molecules targeting this death pathway is limited, further elucidation of apoptosis regulation in a cardiomyocyte-specific fashion is warranted. Pan-anti-apoptotic agents may have detrimental effects in conditions such as cancer, in which there is a deficiency in apoptotic cell death. Indeed, it is conceivable that non-selective long-term suppression of apoptosis may not be beneficial. Further, despite an abundance of research effort, it remains unclear whether apoptosis is a primary or secondary event in HF progression. Regardless, there exists clear evidence that manipulation of programmed cardiomyocyte death can have beneficial effects in preclinical models, suggesting promise in the clinical context.
3.2. Autophagy
Autophagy is a phylogenetically conserved process of protein and organelle recycling, essential to cell survival and stress responsiveness. There are 3 main types of autophagy: macroautophagy, microautophagy, and chaperone-mediated autophagy [148]. Our discussion will focus on macroautophagy, as this is the predominant and best characterized form.
The autophagic process involves several steps that lead to formation of double-membrane organelles called autophagosomes within the cytoplasm that sequester intracellular elements (e.g. damaged proteins or organelles). Subsequently, autophagosomes fuse with lysosomes to form autolysosomes with consequent transfer of acidic pH and acid hydrolases. The autolysosome cargo is degraded and ultimately released into the cytoplasm to preserve cellular homeostasis. This pathway involves class III phosphoinositide-3-kinase (PI3K) and Beclin-1 for the initiation and nucleation steps of autophagy, along with a number of so-called ATG (autophagy-related genes) proteins for completion of the autophagosome [149].
In states of cardiac stress, cardiomyocyte autophagy is an indispensable cell survival mechanism [150]. Although there is some variation in the literature, autophagy occurs in failing cardiomyocytes at a prevalence of 0.03–0.08% [151]. In a rodent model of pulmonary hypertension-induced impairment of ventricular relaxation, failing cardiomyocytes were marked by activated autophagy as denoted by increases in LC3-II (a marker of autophagosomes) as well as in the number of autophagic vacuoles [152]. A study of NYHA class II-III HF patients reported autophagic dysregulation, increased LAMP2 levels, and impaired mTOR activity [153]. In explanted cardiac muscle from stage IV HF patients undergoing partial ventriculectomy, electron microscopy demonstrated significantly increased numbers of autophagic vacuoles harboring partially digested intracellular contents [154].
In states of ATP depletion, the heterotrimeric protein AMPK is activated, leading to TSC complex-mediated inhibition of Rheb and subsequently mTOR1 (mechanistic target of rapamycin 1), a negative regulator of autophagy [148,155]. Ca2+/calmodulin-dependent protein kinase 2B (CaMK2B) can also elicit AMPK phosphorylation and activation, increasing the level of autophagic flux within the heart [156]. Alternatively, protein kinase A (PKA), a primary downstream effector of the second messenger cAMP, suppresses cardiomyocyte autophagy by inactivating several ATG proteins (e.g. ATG 8, 13) [157–159].
3.2.1. Selective autophagy
When initially described in the 1960s, autophagy was believed to be a non-specific process utilized by cells for bulk degradation [160]. However, it is clear now that autophagy can be selective in nature, and that there exist important differences between baseline autophagy and targeted autophagy elicited by different stimuli [148]. Although detailed mechanisms are still being elucidated, a number of organelle-specific autophagic pathways have been described. For instance, autophagy of ER, lipids, mitochondria, or protein aggregates are termed reticulophagy, mitophagy, lipophagy, and aggrephagy, respectively. In addition, a variety of cellular stresses, such as energy deficiency, starvation, infection, and protein aggregation likely stimulate autophagic flux in unique ways [155]. It can be surmised then that these distinct forms of autophagy, which recognize different substrates, must have divergent signals and mechanisms driving these differences. However, elucidation of molecular elements governing specific target recognition and autophagic regulation is still in its infancy.
Some evidence suggests that unique forms of autophagy rely on specific autophagy receptors and adaptors that recognize specific substrates, triggering a link between the conserved ATG cascade proteins and unique targets [161]. Thus, although these organelle- and stress-specific forms of autophagy eventually recruit the core ATG-driven autophagy proteins, unique autophagy receptors and adapters may accomplish specific functions to allow for substrate-specific degradation. As one example, receptors defined by a conserved amino-acid sequence termed the LC3-interacting region (LIR) motif bridge autophagic cargo to the LC3 machinery [162]. There exist multiple LIR domains highlighting differences between targeted and baseline autophagy [162].
The established autophagy scaffolding protein p62/SQSTM1 has been implicated in bulk autophagy and in the ubiquitination of peroxisomes and mitochondria during cardiac injury [163,164]. Another scaffolding protein termed autophagy-linked FYVE (ALFY) may help degrade protein aggregates via p62 and ATG5 [165–167]. Histone deacetylase 6 has been suggested to be involved in maturation of ubiquitin-positive autophagosomes and in directing protein aggregates to the aggresome [168–170]. Genetic silencing of ATG5 (a vital autophagy protein involved in the elongation of the autophagosome) elicits dysregulated autophagy with impaired vesicle formation, cardiac dysfunction, ventricular dilatation, and pathological hypertrophy [171,172]. These mutant animals manifest impaired cardiomyocyte autophagy and cardiac dysfunction at 10 months, with elevated mortality by 1 year of age. Alternatively, expression of ATG5 to promote autophagic flux is necessary for proper cardiac remodeling and function following mechanical unloading [173,174].
3.2.2. Basal autophagy
Maintenance of basal levels of autophagy is critical to cardiomyocyte homeostasis, as impairment of any of the essential autophagy factors leads to cellular dysregulation and if prolonged, cardiomyocyte death [150,175–178]. For instance, Danon cardiomyopathy derives from mutations in the gene coding for LAMP2, a protein essential for lysosome-autophagosome fusion. Blunting of autophagic flux by blocking this fusion event leads to pathologic accumulation of undigested material within autophagosomes, triggering cardiomyocyte dysfunction and eventual lethal cardiomyopathy [179–181]. Vps34 is an important mediator of the autophagy initiation complex and is necessary for autophagosome formation. In mice selectively depleted of cardiomyocyte Vps34, impaired autophagy with subsequent contractile dysfunction and pathologic hypertrophy arise at an early age [182,183].
mTOR is a serine/threonine kinase that functions as a master regulator of cardiomyocyte homeostasis, including autophagy. Comprising 2 distinct complexes with differing functions (mTORC1 and mTORC2), mTOR1 is stimulated during states of energy and nutrient plenty, where it serves to inhibit autophagy in part via stimulation of AKT and phosphorylation/deactivation of ULK-1. Dysregulation of mTOR’s control of cardiomyocyte autophagy leads to a variety of pathological outcomes [184]. Members of the Rag family of GTPases sense amino acids, promoting mTOR activation and consequent autophagy inhibition. A transgenic model over-expressing constitutively active RagA in cardiomyocytes is marked by mTORC1 over-activation even during periods of nutrient starvation [185]. This culminates in mice lacking the ability to activate autophagy during the perinatal period, resulting in early mortality. Rheb, a member of the RAS superfamily, is a GTPase that is an established activator of mTORC1. Mice silenced of Rheb selectively in cardiomyocytes manifest overactive autophagy even during feeding and lack normal physiological cardiomyocyte hypertrophy following birth, leading to death by 10 days [186].
Alternatively, selective silencing of mTORC1 in cardiomyocytes triggers cell death and embryonic lethality [187]. Dynamin-related protein 1 (Drp1), a GTPase involved in mitochondrial fission, is also important in the regulation of mitochondrial autophagic flux [188,189]. In particular, conditional cardiomyocyte-deficient Drp1 mice manifest impaired mitochondrial autophagy, resulting in accumulation of dysfunctional mitochondria. These Drp1 knockouts exhibit cardiac dysfunction with demise by 3 months. In cardiomyocyte Drp1 heterozygotes, contractile function was normal at 3 months but deteriorated in the setting of starvation or I/R injury. Together, these findings reinforce the notion that maintenance of basal levels of autophagy is essential for cardiomyocyte survival.
3.2.3. Autophagy in cardiac stress
Recent studies have revealed that autophagy participates in a multitude of physiological and pathophysiological processes. Further, down-or up-regulation of autophagic flux can be beneficial or detrimental, depending on the context and extent of flux change [190–192]. In particular, autophagic flux has been shown to have a particularly important role in the heart [193]. Under resting conditions, cardiomyocytes manifest autophagic turnover of organelles at low basal levels, but this process is altered in stress states such as infarction, metabolic derangements (e.g. diabetes, lipotoxicity), I/R injury, cardiac hypertrophy, and HF [194,195]. Notably, dysregulated autophagy appears to be key in pathological cardiac remodeling [196].
Under normal circumstances or mildly stressed conditions (such as short periods of nutrient depletion), autophagy degrades non-essential proteins and selectively removes damaged organelles to preserve the integrity of the cardiomyocyte. In addition, autophagy has been shown to antagonize apoptotic death and preserve cardiomyocyte mitochondria [116,197].
Mice deficient of lysosome-associated membrane protein 2 (LAMP2), an essential protein for autolysosome formation, manifest impaired autophagy with accumulation of autophagosomes, resulting in cardiomyopathy and HF [198,199]. Cardiomyocytes that lack ATG5 and/ or ATG7 are susceptible to HF triggered by pressure overload or adrenergic stress [174,200]. Ultrastructural analyses of cardiac muscle silenced of ATG5 or ATG7 reveal irregular sarcomeric structures and misshapen mitochondria [178]. Similarly, these isolated cardiomyocytes exhibit increased cell death and worsened hypertrophy in the settings of epinephrine or phenylephrine challenge. These findings suggest that maintenance of a constitutive baseline level of autophagic flux is required for normal cardiomyocyte cellular structure and function, a notion which is consistent with observations in other cell types [192].
There continues to be debate as to whether autophagy is harmful or helpful in the context of HF. With time, however, a consensus is emerging that autophagic flux can be either beneficial or detrimental, depending on the context, proximal trigger, and extent of activation. Work is presently under way to determine the extent to which these changes are quantitative (too much or too little) versus qualitative (autophagy that targets specific cellular elements) [150].
In a model of severe pressure overload-induced HF, Zhu et al. reported that cardiomyocyte autophagy increases in a model of severe TAC and remains chronically elevated, leading to amplified hypertrophic growth and increased rates of functional decline [201]. Cardiomyocyte-specific over-expression of Beclin-1, a protein involved in early and late autophagic flux events, triggered higher levels of stress-induced autophagy and worsened pathological remodeling and survival [201]. Conversely, Beclin-1 haploinsufficient mice manifest decreased levels of autophagy and diminished pathological remodeling [198,201].
At the same time, there is evidence that stimulation of autophagy may rescue pressure overload-induced cardiac hypertrophy and dysfunction via increases in autophagy and mTOR regulation [202]. Nakai et al. reported that autophagy levels are decreased in TAC hearts at 1 week [203]. They also reported that tamoxifen-inducible cre-recombinase-driven silencing of ATG5 in cardiomyocytes leads to an amplified hypertrophic response after TAC. In line with this, several studies have reported that rapamycin, an inhibitor of the autophagy-suppressive mTOR complex 1 and numerous downstream processes, rescues afterload-induced cardiac hypertrophy and can decrease mortality and prolong longevity [184,204]. Together, these data suggest that autophagic dysregulation, whether it is excessive or insufficient, is detrimental in load-induced HF.
4. Linking Ca2+ with autophagy and apoptosis
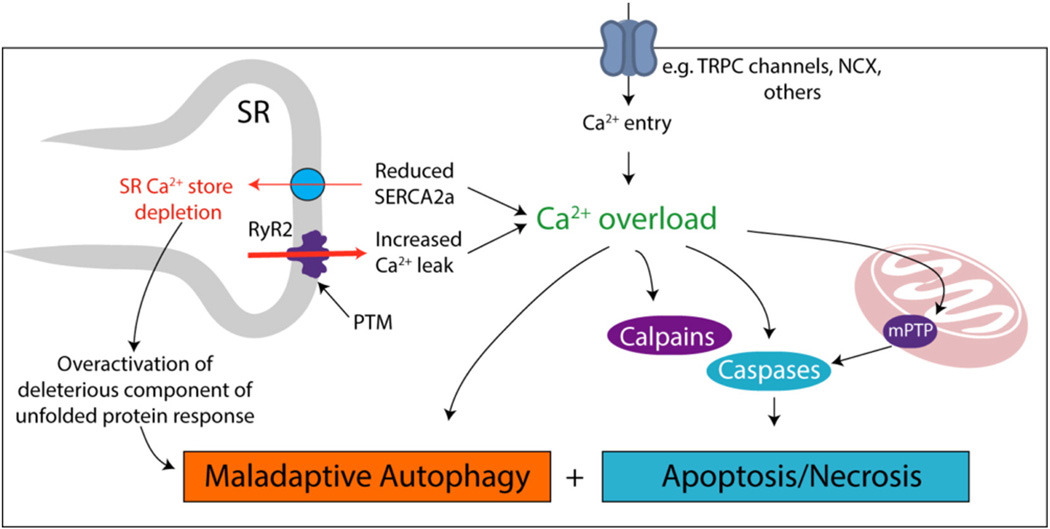
Ca2+ handling, intracellular recycling by autophagy, and programmed cell death by apoptosis are each essential to normal cardiac function, and each is perturbed in disease. Thus, it comes as little surprise that these three fundamental processes are mechanistically linked. That said, these links are only recently being uncovered (Fig. 4).
Fig. 4.
Relationships among disordered Ca2+ homeostasis, autophagy, necrosis and apoptosis in HF. It is generally accepted that depletion of SR Ca2+ stores, coupled with rises in cytosolic Ca2+, perturb EC coupling and elicit changes in cellular signaling events. Post-translational modifications (PTM) of RyR2 and reduced SERCA activity redistribute SR Ca2+ to the cytosol. Increased cytosolic Ca2+ also triggers pathways that contribute to cardiac muscle wasting. Activation of apoptotic pathways (e.g. calpains, caspases, or mPTP opening) has been extensively related to Ca2+ dysregulation, and there is some evidence pointing to altered autophagic signaling as well.
Apart from its role in EC coupling, Ca2+ is an important second messenger than governs a wide range of signaling pathways [205]. Basal Ca2+ levels in cardiomyocytes during diastole are ≈100 nM, and this equilibrium is perturbed in multiple disease states [40,206,207]. For example, mdx mice (a mouse model for Duchenne muscular dystrophy) develop cardiomyopathy at 12–20 months of age reminiscent of that seen in patients [208]. Coincident with this, age-dependent increases in cytosolic Ca2+ are observed in adult cardiomyocytes from mdx mice, reaching ≈400 nM at 1 year [207]. As described above, HF is characterized by increases in diastolic Ca2+ concentration in the cytosol due to alterations in SR (leaky RyR2/reduced SERCA) and sarcolemmal Ca2+ fluxes (increased entry). In addition, altered localization of calsequestrin-2, a major Ca2+ buffer protein in the SR, likely contributes [209]. Persistent elevation of cytosolic Ca2+ or spontaneous Ca2+ release unrelated to systole (e.g. sparks) will activate Ca2+ dependent signaling pathways under basal conditions with a variety of downstream effects.
Depletion of SR Ca2+ stores not only perturbs Ca2+ cycling and contractile performance, it may also trigger additional responses, such as activation of the unfolded protein response (UPR) and cell death pathways, as well as autophagy impairment. For example, pharmacological inhibition of SERCA using thapsigargin activates the UPR, demonstrating that SR Ca2+ levels and the UPR are interconnected [210]. Activation of UPR has been reported in TAC-induced hypertrophy [211] and may be involved in HF progression [212]. The UPR comprises three major cascades: protein kinase RNA-like ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring protein 1α (IRE1α). Together, these pathways modulate gene expression, apopto-sis, autophagy, metabolism, and other events [213]. Ultimately, activation of the UPR serves to restore SR homeostasis by suppressing translation of new proteins, facilitating chaperone-mediated protein processing, and promoting degradation of excess protein. If SR homeostasis cannot be restored, the UPR will ultimately trigger apoptotic cell death.
We reported recently that over-expression of Xbp1s (downstream of IRE1α) blocks I/R injury, diminishing infarct size and preserving contractile function [214]. Conversely, activation of CHOP (downstream of PERK) exacerbates I/R injury [215]. Moreover, we and others have shown that Xbp1s levels are increased in failing human hearts (pre-LVAD) relative to the levels observed after mechanical unloading with a left ventricular assist device (post-LVAD) [214]. This correlates with reported improvements in Ca2+ handling and contraction force/kinetics observed after mechanical unloading [216]. We suggest that, similar to the hyperinsulinemic state in patients with type 2 diabetes or elevated BNP/ANF levels in HF, increased abundance of Xbp1s may function as a compensatory response, serving to mitigate injury [214]. In addition, there is evidence that higher levels of Xbp1s observed in settings of cardiac stress may regulate miRNAs, SERCA, and NCX, promoting beneficial angiogenesis and suppressing pathological remodeling [217–219].
Ca2+-dependent regulation of cell death pathways, both apoptosis and necrosis, occurs at multiple levels. As discussed, calpains are Ca2+-activated proteases linked to both apoptosis and necrosis [220]. Ca2+ overload after myocardial I/R injury increases calpain activation, and inhibition of calpains reduces infarct size [221,222]. Calpain activation is associated with pro-caspase 3, poly-ADP ribose polymerase and AIF cleavage, as well as apoptosis (reviewed in [220]). Similarly, cleavage of pro-caspase-3 and procaspase-9 is governed by Ca2+ [223].
HF is associated with mitochondrial dysfunction, which leads to ROS accumulation, elevations in inflammatory markers, increased apoptosis, and impaired autophagy [197,224–226]. As described earlier, perturbations in Ca2+ homeostasis can trigger MPTP opening and consequent loss of mitochondrial membrane potential, as well as activation of the intrinsic apoptosis cascade via release into the cytoplasm of cytochrome C, AIF and Smac/DIABLO. Furthermore, Bcl-2 and Bax are reported to regulate mitochondrial Ca2+ distribution and permeability, and pathological cardiac hypertrophic stress can promote Bcl-2/Bax-driven Ca2+ dysregulation and apoptosis initiation [227,228]. Recently, it has been proposed that RyR2 Ca2+ leak promotes mitochondrial Ca2+ overload and ROS production in HF, which may contribute to mitochondrial dysfunction and cell death [17].
Links between Ca2+ and autophagy are less well established. Using the Ca2+ phosphate method of DNA transfection in HEK293-T cells, increases in autophagy related to Ca2+, involving Beclin 1 and ATG5, were first described [229]. Elevations in intracellular Ca2+ triggered by other means (e.g. ionomycin, ATP, thapsigargin) in MCF-7 cells are associated with activation of CaMKII-beta and AMPK and rerepression of mTOR inhibition [230].
Recently, a role for Ca2+ in governing autophagy has been highlighted that involves the Ca2+-specific ion channel polycystin-2 (PC2) [231]. By forming a multiprotein ion channel complex with polycystin-1 (PC1), PC2 participates in the function of primary cilia. There, these proteins respond to a variety of extracellular cues, transducing them into changes in intracellular Ca2+ [232]. Changes in conformational shape triggered by shear stress on primary cilia lead to activation of the PC1/PC2 complex and consequent changes in intracellular Ca2+ levels [232]. Now, very recent evidence suggests that PC2-dependent changes in Ca2+ may be important in driving autophagosome formation (unpublished observations). In particular, we have amassed evidence that cardiomyocyte-specific silencing of the PKD2 gene, which codes for PC2, elicits impaired accumulation of autophagosomes and LC3 lipidation, independent of AKT or AMPK activity (unpublished observations). Furthermore, PC2 co-immunoprecipitates with the essential autophagosome initiation protein Beclin 1, and PC2 localizes to the ER. Together, these findings suggest that dysregulated PC2, acting to impair Ca2+ handling and autophagic flux, may participate in the pathogenesis of HF.
It is well established that lysosome acidification depends critically on Ca2+ exchangers interacting with V-ATPases [233]. Release of lysosomal Ca2+ can be induced by Bafilomycin A1 (BafA1), an inhibitor of V-type ATPases, resulting in loss of the pH gradient and suppression of further Ca2+ uptake [234]. Importantly, BafA1 is also an established inhibitor of autophagic flux [235]. Alterations in cytosolic Ca2+ in failing hearts likely alter lysosomal Ca2+ gradients, impairing lysosome function and impacting autophagic flux. Moreover, lysosome Ca2+ signaling regulates the activities of both calcineurin and TFEB, a key regulator of lysosomal biogenesis and autophagy [236].
Interplay between autophagy and ER stress has been described under physiological conditions [237]. In several forms of cardiac pathology, including HF, the UPR is activated [238]. Normally, accumulation of unfolded proteins within the ER triggers dissociation of an inhibitor chaperone, named GRP78, from PERK and IRE1. However, continued accumulation of unfolded protein can lead to dysfunctional UPR signaling with ultimate activation of apoptosis. It has been suggested that GRP78 is essential in mediating the UPR and Ca2+ signaling, as GRP78 can inhibit type 1 cell death induced by ionomycin (a well known apoptosis-inducing Ca2+ ionophore) [239,240]. Alternatively, alterations in the abundance of SERCA protein have been shown to have a role in apoptosis, as thapsigargin, which blocks SERCA, leads to decreased Ca2+ levels and subsequent apoptosis triggered by ER stress [241,242]. Ceramide dysregulation during Ca2+ overload is suggested to induce ROS production, further worsening Ca2+ release and leading to increased levels of apoptosis [243,244].
The ER is a major storage and trafficking site for Ca2+ and chaperone proteins essential for proper Ca2+ handling and protein folding. In particular, the balance between SERCA-dependent Ca2+ entry and Ca2+ leak is essential to maintain ER homeostasis [245]. A significant body of evidence suggests that the ER’s role in cellular stress and cell death is related to Ca2+ and intricate control of autophagy and apoptosis cascades [246–248]. In addition, Ca2+-mediated ER chaperones, such as calreticulin, are involved in protein folding, such that dysregulation of Ca2+ leads to alterations in chaperone capacity, triggering cellular stress [249,250].
Depending on the circumstance, cellular demise can be initiated by Ca2+ release from the ER via multiple mechanisms, leading to Ca2+-driven mitochondrial cell death [247,251]. However, IP3 receptor-mediated Ca2+ delivery to mitochondria can help sustain ATP levels by regulating oxidative phosphorylation, in turn promoting cell survival [249,252]. Clearly, governance of Ca2+ signaling within the cardiomyocyte is central to guiding the cell toward survival or death.
As mentioned previously, Bax and Bak are factors that play a significant role in ER Ca2+-mediated apoptosis. Specifically, rapidly increasing Bax releases Ca2+ from the ER, driving an increase in Ca2+ within mitochondria with subsequent release of cytochrome c [112,113,253]. Likewise, deficiency of Bax and Bak leads to reduction of ER Ca2+ release even in the face of IP3-receptor signaling to increase Ca2+. In part due to their proximity to the ER, Bax and Bak can also negatively affect Bcl-2 and Bcl-XL’s roles in ER Ca2+ regulation. During times of ER stress, Bax/Bak may engage IRE1α leading to UPR activation; subsequent resolution of this can occur via Bax/Bak neutralization by Bcl-2 or Bcl-XL [254,255]. In relation to Ca2+, the protective anti-apoptotic actions of Bcl-2 derive from its lowering of IP3-driven ER Ca2+ levels [256,257].
JNK, an important stress mediator protein, can phosphorylate and inhibit Bcl-2’s protective anti-apoptotic effect by dysregulating Ca2+ in the ER [258,259]. This phosphorylated Bcl-2 in unable to bind and inactivate pro-apoptotic factors (of the BH3 family), and can lead to increased ER Ca2+ release, subsequently causing excess Ca2+ uptake and mitochondrial Ca2+-driven apoptosis [259,260]. Furthermore, during ER stress, CHOP-driven increases in pro-apoptotic Bim can antagonize Bcl-2 and Bcl-XL, limiting its mitochondrial and cytoprotective roles [261,262].
With time, our understanding of the interactions between ER stress, autophagy and apoptosis continues to grow. For instance, Beclin-1, typically thought of simply as an essential autophagy protein involved at the nucleation stage, has been demonstrated to engage the anti-apoptotic factor Bcl-2 in the ER, leading to inhibition of starvation-induced autophagy [263–265]. When there is pathological accumulation of misfolded proteins or dysregulation of Ca2+ levels, ER stress-induced autophagy is activated via IRE1α as part of the UPR. Then, together with TRAF2, JNK activation ensues [266,267]. To support further the UPR’s antagonism of misfolded proteins, particularly when ER capacity is overwhelmed, induction of autophagy takes place, assisting elimination of pathologic proteins. Although ERAD is recognized as the predominant cellular mechanism for removal of unfolded proteins in the context of ER stress, a diversity of studies has shown that autophagy can be potently activated by ER stress [268–270]. The UPR interactions between PERK/elF2α lead not only to ATG12 activation, but PERK also drives an increase in other ATG genes via ATF4 [271,272]. Furthermore, XBP-1 splicing can activate Beclin-1, leading to increased autophagy [273]. Recently, it was reported that JNK-mediated phosphorylation of Bcl-2 facilitates its release of Beclin-1, thereby allowing autophagy to proceed [274,275]. The essential autophagy protein ATG12 can also bind and inhibit Bcl-2 family proteins, leading to increased apoptotic cell death [276,277].
Further evidence for Ca2+’s role in regulating autophagy arises from modulators, such as ionomycin and thapsigargin, that lead to mTOR inhibition and blockage of autophagic flux with autophagosome accumulation [248,278]. Particularly, ER Ca2+ release activates AMPK and CaMKII pathways to stimulate autophagy via mTOR inhibition [279–281]. Alternatively, ER Ca2+ release also may activate protein kinase C (PKC), thereby leading to autophagy activation in an mTOR-independent fashion [282,283]. Clearly, there appear to be multiple mechanisms that drive the interplay between Ca2+, autophagy, apoptosis, and ER stress.
Although much of the discussion focuses on HF stemming from systolic dysfunction, the contributions of apoptosis and autophagy may differ significantly in HFpEF. For instance, there exists evidence that in obesity-induced type 2 diabetes mellitus, increased apoptosis and mitochondrial dysfunction contribute to worsening diastolic dysfunction. Further, eplerenone, a mineralocorticoid antagonist, decreased apoptosis, as evidenced by decreases in caspase-3 activation, TUNEL staining, and nuclear pyknosis [284]. However, the distinction between therapeutically regulating apoptosis in diastolic versus systolic HF is difficult to delineate; there may be beneficial effects on systolic function, as well as diastolic function, such that the salutary effects are multifactorial and difficult to separate definitively. In a study evaluating models of systolic and diastolic HF, it was suggested that adenoviral knockdown of BNIP3 (which harbors the pro-apoptotic BH3 domain) rescued cardiac volume and function and that these benefits derive from decreases in myocardial apoptosis, ER stress, and attenuation of mitochondrial fragmentation [285]. It is important to recognize, however, that BNIP3 has important roles in mediating mitochondrial cell death by regulating ion channels affecting Ca2+ flux between the ER and mitochondria. Therefore, it is possible that BNIP3 knockdown helps balance ER Ca2+ content, mitigating mitochondrial damage and reducing apoptosis, subsequently improving both systolic and diastolic function [286].
In a high salt model of diastolic HF, it was reported that miR-208a decreased apoptosis and increased anti-apoptotic factors, resulting in blunted increases in hypertrophic markers (β-MHC) and cardiac mass, and improvements in diastolic function [287]. In PAH-induced diastolic HF, it was found that autophagy is overactive, contributing to pathological remodeling and cell death. However, treatment with dehydroepian-drosterone, a metabolic intermediate in the biosynthesis of the androgen and estrogen sex steroids, restored autophagy back to baseline levels, resulting in improved diastolic functional parameters (e.g. end-diastolic pressure) [152]. Aging is also clearly associated with diastolic dysfunction, as stiffening of the cardiac ventricles occurs over time; in particular, NAD+-dependent class III histone deacetylases (sirtuins) and ATG protein signaling appear to become impaired with age, contributing to an imbalance and inhibition of basal autophagic flux [288,289]. Caloric restriction and starvation lead to increases in autophagy, and many studies have indicated that caloric restriction blunts the decline in both diastolic and systolic function with age [290,291]. Thus, there exist multiple similarities and differences between autophagy and apoptosis in the regulation of cardiac function, whether it be diastolic or systolic.
5. Conclusions and perspective
HF is a multifaceted syndrome, ever growing in individual and societal impact. It is characterized by profound dysregulation in Ca2+ handling and homeostasis, coupled with alterations in autophagy-driven catabolism and programmed cell death. Recent insights have unveiled novel links among these three biological processes. Indeed, recent studies highlight that these events are tractable by means with potential therapeutic relevance, such as small molecule HDAC (histone deacetylase) inhibitors. Further advances, building on these new discoveries, hold significant promise to afford benefit with clinical impact.
Supplementary Material
Acknowledgments
Sources of funding
This work was supported by grants from the NIH (HL-120732; HL-100401), AHA (14SFRN20510023; 14SFRN20670003), Fondation Leducq (11CVD04) and CPRIT (RP110486P3).
Footnotes
Disclosures
None declared.
Transparency document
The Transparency document associated with this article can be found, in online version.
References
- 1.Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N. Engl. J. Med. 2012;366(1):54–63. doi: 10.1056/NEJMra1112570. [DOI] [PubMed] [Google Scholar]
- 2.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.AlHabib KF, et al. Long-term mortality rates in acute de novo versus acute-on-chronic heart failure: from the Heart Function Assessment Registry Trial in Saudi Arabia. Angiology. 2015;66(9):837–844. doi: 10.1177/0003319714563138. [DOI] [PubMed] [Google Scholar]
- 4.Joseph SM, et al. Acute decompensated heart failure: contemporary medical management. Tex. Heart Inst. J. 2009;36(6):510–520. [PMC free article] [PubMed] [Google Scholar]
- 5.McMurray JJ, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2012;33(14):1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 6.Blecker S, et al. Quality of care for heart failure patients hospitalized for any cause. J. Am. Coll. Cardiol. 2014;63(2):123–130. doi: 10.1016/j.jacc.2013.08.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matza LS, et al. Acute and chronic impact of cardiovascular events on health state utilities. BMC Health Serv. Res. 2015;15:173. doi: 10.1186/s12913-015-0772-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill JA, Olson EN. Cardiac plasticity. N. Engl. J. Med. 2008;358(13):1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 9.Braunwald E. Heart failure. JACC Heart Fail. 2013;1(1):1–20. doi: 10.1016/j.jchf.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Braunwald E. The war against heart failure: the Lancet lecture. Lancet. 2015;385(9970):812–824. doi: 10.1016/S0140-6736(14)61889-4. [DOI] [PubMed] [Google Scholar]
- 11.Sharma K, Kass DA. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circ. Res. 2014;115(1):79–96. doi: 10.1161/CIRCRESAHA.115.302922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrantini C, et al. The transverse-axial tubular system of cardiomyocytes. Cell. Mol. Life Sci. 2013;70(24):4695–4710. doi: 10.1007/s00018-013-1410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bers DM. Cardiac sarcoplasmic reticulum calcium leak: basis and roles in cardiac dysfunction. Annu. Rev. Physiol. 2014;76:107–127. doi: 10.1146/annurev-physiol-020911-153308. [DOI] [PubMed] [Google Scholar]
- 14.Guo A, et al. Emerging mechanisms of T-tubule remodelling in heart failure. Cardiovasc. Res. 2013;98(2):204–215. doi: 10.1093/cvr/cvt020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meissner G. Regulation of ryanodine receptor ion channels through posttransla-tional modifications. Curr. Top. Membr. 2010;66:91–113. doi: 10.1016/S1063-5823(10)66005-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marx SO, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101(4):365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 17.Santulli G, et al. Mitochondrial calcium overload is a key determinant in heart failure. Proc. Natl. Acad. Sci. U. S. A. 2015;112(36):11389–11394. doi: 10.1073/pnas.1513047112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marks AR. Calcium cycling proteins and heart failure: mechanisms and therapeutics. J. Clin. Invest. 2013;123(1):46–52. doi: 10.1172/JCI62834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pleger ST, et al. Cardiac AAV9-S100A1 gene therapy rescues post-ischemic heart failure in a preclinical large animal model. Sci. Transl. Med. 2011;3(92) doi: 10.1126/scitranslmed.3002097. 92ra64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber C, et al. Therapeutic safety of high myocardial expression levels of the molecular inotrope S100A1 in a preclinical heart failure model. Gene Ther. 2014;21(2):131–138. doi: 10.1038/gt.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bers DM. Ryanodine receptor S2808 phosphorylation in heart failure: smoking gun or red herring. Circ. Res. 2012;110(6):796–799. doi: 10.1161/CIRCRESAHA.112.265579. [DOI] [PubMed] [Google Scholar]
- 22.Houser SR. Role of RyR2 phosphorylation in heart failure and arrhythmias: protein kinase A-mediated hyperphosphorylation of the ryanodine receptor at serine 2808 does not alter cardiac contractility or cause heart failure and arrhythmias. Circ. Res. 2014;114(8):1320–1327. doi: 10.1161/CIRCRESAHA.114.300569. (discussion 1327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H, et al. Hyperphosphorylation of the cardiac ryanodine receptor at serine 2808 is not involved in cardiac dysfunction after myocardial infarction. Circ. Res. 2012;110(6):831–840. doi: 10.1161/CIRCRESAHA.111.255158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kranias EG, Hajjar RJ. Modulation of cardiac contractility by the phospholamban/ SERCA2a regulatome. Circ. Res. 2012;110(12):1646–1660. doi: 10.1161/CIRCRESAHA.111.259754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhupathy P, Babu GJ, Periasamy M. Sarcolipin and phospholamban as regulators of cardiac sarcoplasmic reticulum Ca2+ ATPase. J. Mol. Cell. Cardiol. 2007;42(5):903–911. doi: 10.1016/j.yjmcc.2007.03.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haghighi K, Bidwell P, Kranias EG. Phospholamban interactome in cardiac contractility and survival: a new vision of an old friend. J. Mol. Cell. Cardiol. 2014;77:160–167. doi: 10.1016/j.yjmcc.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shareef MA, Anwer LA, Poizat C. Cardiac SERCA2A/B: therapeutic targets for heart failure. Eur. J. Pharmacol. 2014;724:1–8. doi: 10.1016/j.ejphar.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 28.Hasenfuss G, et al. Relation between myocardial function and expression of sarco-plasmic reticulum Ca(2+)-ATPase in failing and nonfailing human myocardium. Circ. Res. 1994;75(3):434–442. doi: 10.1161/01.res.75.3.434. [DOI] [PubMed] [Google Scholar]
- 29.Sande JB, et al. Reduced level of serine(16) phosphorylated phospholamban in the failing rat myocardium: a major contributor to reduced SERCA2 activity. Cardiovasc. Res. 2002;53(2):382–391. doi: 10.1016/s0008-6363(01)00489-8. [DOI] [PubMed] [Google Scholar]
- 30.Li L, et al. Sodium accumulation in SERCA knockout-induced heart failure. Biophys. J. 2012;102(9):2039–2048. doi: 10.1016/j.bpj.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.del Monte F, et al. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation. 1999;100(23):2308–2311. doi: 10.1161/01.cir.100.23.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawase Y, et al. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J. Am. Coll. Cardiol. 2008;51(11):1112–1119. doi: 10.1016/j.jacc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Jessup M, et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 2011;124(3):304–313. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zsebo K, et al. Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure: analysis of recurrent cardiovascular events and mortality. Circ. Res. 2014;114(1):101–108. doi: 10.1161/CIRCRESAHA.113.302421. [DOI] [PubMed] [Google Scholar]
- 35.Greenberg B, et al. Design of a phase 2b trial of intracoronary administration of AAV1/SERCA2a in patients with advanced heart failure: the CUPID 2 trial (calcium up-regulation by percutaneous administration of gene therapy in cardiac disease phase 2b) JACC Heart Fail. 2014;2(1):84–92. doi: 10.1016/j.jchf.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Philipson KD, Nicoll DA. Sodium-calcium exchange: a molecular perspective. Annu. Rev. Physiol. 2000;62:111–133. doi: 10.1146/annurev.physiol.62.1.111. [DOI] [PubMed] [Google Scholar]
- 37.Shattock MJ, et al. Na(+)/Ca(2+) exchange and Na(+)/K(+)-ATPase in the heart. J. Physiol. 2015;593(6):1361–1382. doi: 10.1113/jphysiol.2014.282319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Studer R, et al. Gene expression of the cardiac Na(+)-Ca2+ exchanger in end-stage human heart failure. Circ. Res. 1994;75(3):443–453. doi: 10.1161/01.res.75.3.443. [DOI] [PubMed] [Google Scholar]
- 39.Eder P, Molkentin JD. TRPC channels as effectors of cardiac hypertrophy. Circ. Res. 2011;108(2):265–272. doi: 10.1161/CIRCRESAHA.110.225888. [DOI] [PubMed] [Google Scholar]
- 40.Altamirano F, et al. Ca2+ influx via the Na+/Ca2+ exchanger is enhanced in malignant hyperthermia skeletal muscle. J. Biol. Chem. 2014;289(27):19180–19190. doi: 10.1074/jbc.M114.550764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barrientos G, et al. The Na+/Ca2+ exchange inhibitor 2-(2-(4-(4-nitrobenzyloxy)phenyl)ethyl)isothiourea methanesulfonate (KB-R7943) also blocks ryanodine receptors type 1 (RyR1) and type 2 (RyR2) channels. Mol. Pharmacol. 2009;76(3):560–568. doi: 10.1124/mol.109.057265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Correll RN, et al. Overexpression of the Na+/K+ ATPase alpha2 but not alpha1 isoform attenuates pathological cardiac hypertrophy and remodeling. Circ. Res. 2014;114(2):249–256. doi: 10.1161/CIRCRESAHA.114.302293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maier LS, et al. RAnoLazIne for the treatment of diastolic heart failure in patients with preserved ejection fraction: the RALI-DHF proof-of-concept study. JACC Heart Fail. 2013;1(2):115–122. doi: 10.1016/j.jchf.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Sag CM, et al. Enhanced late INa induces proarrhythmogenic SR Ca leak in a CaMKII-dependent manner. J. Mol. Cell. Cardiol. 2014;76:94–105. doi: 10.1016/j.yjmcc.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 45.Eltit JM, et al. Nonspecific sarcolemmal cation channels are critical for the pathogenesis of malignant hyperthermia. FASEB J. 2013;27(3):991–1000. doi: 10.1096/fj.12-218354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu X, et al. TRPC channels are necessary mediators of pathologic cardiac hypertrophy. Proc. Natl. Acad. Sci. U. S. A. 2010;107(15):7000–7005. doi: 10.1073/pnas.1001825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Makarewich CA, et al. Transient receptor potential channels contribute to pathological structural and functional remodeling after myocardial infarction. Circ. Res. 2014;115(6):567–580. doi: 10.1161/CIRCRESAHA.115.303831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shan D, Marchase RB, Chatham JC. Overexpression of TRPC3 increases apoptosis but not necrosis in response to ischemia-reperfusion in adult mouse cardiomyocytes. Am. J. Physiol. Cell Physiol. 2008;294(3):C833–C841. doi: 10.1152/ajpcell.00313.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, et al. Cardiac-specific knockout of ET(A) receptor mitigates low ambient temperature-induced cardiac hypertrophy and contractile dysfunction. J. Mol. Cell Biol. 2012;4(2):97–107. doi: 10.1093/jmcb/mjs002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dibb KM, et al. A functional role for transverse (t-) tubules in the atria. J. Mol. Cell. Cardiol. 2013;58:84–91. doi: 10.1016/j.yjmcc.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 51.Wei S, et al. T-tubule remodeling during transition from hypertrophy to heart failure. Circ. Res. 2010;107(4):520–531. doi: 10.1161/CIRCRESAHA.109.212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crocini C, et al. Defects in T-tubular electrical activity underlie local alterations of calcium release in heart failure. Proc. Natl. Acad. Sci. U. S. A. 2014;111(42):15196–15201. doi: 10.1073/pnas.1411557111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lyon AR, et al. Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proc. Natl. Acad. Sci. U. S. A. 2009;106(16):6854–6859. doi: 10.1073/pnas.0809777106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferrantini C, et al. Impact of detubulation on force and kinetics of cardiac muscle contraction. J. Gen. Physiol. 2014;143(6):783–797. doi: 10.1085/jgp.201311125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song LS, et al. Orphaned ryanodine receptors in the failing heart. Proc. Natl. Acad. Sci. U. S. A. 2006;103(11):4305–4310. doi: 10.1073/pnas.0509324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nikolaev VO, et al. Beta2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science. 2010;327(5973):1653–1657. doi: 10.1126/science.1185988. [DOI] [PubMed] [Google Scholar]
- 57.Ibarra C, et al. An integrated mechanism of cardiomyocyte nuclear Ca(2+) signaling. J. Mol. Cell. Cardiol. 2014;75:40–48. doi: 10.1016/j.yjmcc.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ibarra C, et al. Local control of nuclear calcium signaling in cardiac myocytes by perinuclear microdomains of sarcolemmal insulin-like growth factor 1 receptors. Circ. Res. 2013;112(2):236–245. doi: 10.1161/CIRCRESAHA.112.273839. [DOI] [PubMed] [Google Scholar]
- 59.Lai NC, et al. Improved function of the failing rat heart by regulated expression of insulin-like growth factor I via intramuscular gene transfer. Hum. Gene Ther. 2012;23(3):255–261. doi: 10.1089/hum.2011.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y, et al. Insulin-like growth factor 1 alleviates high-fat diet-induced myocardial contractile dysfunction: role of insulin signaling and mitochondrial function. Hypertension. 2012;59(3):680–693. doi: 10.1161/HYPERTENSIONAHA.111.181867. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Shah K, et al. Acute non-ST elevation myocardial infarction following paclitaxel administration for ovarian carcinoma: a case report and review of literature. J. Cancer Res. Ther. 2012;8(3):442–444. doi: 10.4103/0973-1482.103530. [DOI] [PubMed] [Google Scholar]
- 62.Rowinsky EK, et al. Cardiac disturbances during the administration of taxol. J. Clin. Oncol. 1991;9(9):1704–1712. doi: 10.1200/JCO.1991.9.9.1704. [DOI] [PubMed] [Google Scholar]
- 63.Crittenden DB, et al. Colchicine use is associated with decreased prevalence of myocardial infarction in patients with gout. J. Rheumatol. 2012;39(7):1458–1464. doi: 10.3899/jrheum.111533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prosser BL, Ward CW, Lederer WJ. X-ROS signaling: rapid mechano-chemo transduction in heart. Science. 2011;333(6048):1440–1445. doi: 10.1126/science.1202768. [DOI] [PubMed] [Google Scholar]
- 65.Zhang C, et al. Microtubule-mediated defects in junctophilin-2 trafficking contribute to myocyte transverse-tubule remodeling and Ca2+ handling dysfunction in heart failure. Circulation. 2014;129(17):1742–1750. doi: 10.1161/CIRCULATIONAHA.113.008452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Casanova P, et al. The cardiovascular effects of colchicine: a comprehensive review. Cardiol. Rev. 2015 doi: 10.1097/CRD.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 67.Chen B, et al. Critical roles of junctophilin-2 in T-tubule and excitation-contraction coupling maturation during postnatal development. Cardiovasc. Res. 2013;100(1):54–62. doi: 10.1093/cvr/cvt180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reynolds JO, et al. Junctophilin-2 is necessary for T-tubule maturation during mouse heart development. Cardiovasc. Res. 2013;100(1):44–53. doi: 10.1093/cvr/cvt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Landstrom AP, et al. Junctophilin-2 expression silencing causes cardiocyte hypertrophy and abnormal intracellular calcium-handling. Circ. Heart Fail. 2011;4(2):214–223. doi: 10.1161/CIRCHEARTFAILURE.110.958694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guo A, et al. Overexpression of junctophilin-2 does not enhance baseline function but attenuates heart failure development after cardiac stress. Proc. Natl. Acad. Sci. U. S. A. 2014;111(33):12240–12245. doi: 10.1073/pnas.1412729111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu CY, et al. Calpain-dependent cleavage of junctophilin-2 and T-tubule remodeling in a mouse model of reversible heart failure. J. Am. Heart Assoc. 2014;3(3):e000527. doi: 10.1161/JAHA.113.000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.L Caldwell J, et al. Dependence of cardiac transverse tubules on the BAR domain protein amphiphysin II (BIN-1) Circ. Res. 2014;115(12):986–996. doi: 10.1161/CIRCRESAHA.116.303448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rizzuto R, Duchen MR, Pozzan T. Flirting in little space: the ER/mitochondria Ca2+ liaison. Sci. STKE. 2004;2004(215):re1. doi: 10.1126/stke.2152004re1. [DOI] [PubMed] [Google Scholar]
- 74.Lopez-Crisosto C, et al. ER-to-mitochondria miscommunication and metabolic diseases. Biochim Biophys. Acta. 2015;1852(10 Pt A):2096–2105. doi: 10.1016/j.bbadis.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 75.Gutierrez T, et al. Alteration in mitochondrial Ca(2+) uptake disrupts insulin signaling in hypertrophic cardiomyocytes. Cell Commun. Signal. 2014;12:68. doi: 10.1186/s12964-014-0068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gomez L, et al. The SR/ER-mitochondria calcium crosstalk is regulated by GSK3beta during reperfusion injury. Cell Death Differ. 2015 doi: 10.1038/cdd.2015.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shalini S, et al. Old, new and emerging functions of caspases. Cell Death Differ. 2015;22(4):526–539. doi: 10.1038/cdd.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Communal C, et al. Functional consequences of caspase activation in cardiac myocytes. Proc. Natl. Acad. Sci. U. S. A. 2002;99(9):6252–6256. doi: 10.1073/pnas.092022999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prescimone T, et al. Cardiac molecular markers of programmed cell death are activated in end-stage heart failure patients supported by left ventricular assist device. Cardiovasc. Pathol. 2014;23(5):272–282. doi: 10.1016/j.carpath.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 80.Niessner A, et al. Prognostic value of apoptosis markers in advanced heart failure patients. Eur. Heart J. 2009;30(7):789–796. doi: 10.1093/eurheartj/ehp004. [DOI] [PubMed] [Google Scholar]
- 81.Mani K. Programmed cell death in cardiac myocytes: strategies to maximize post-ischemic salvage. Heart Fail. Rev. 2008;13(2):193–209. doi: 10.1007/s10741-007-9073-7. [DOI] [PubMed] [Google Scholar]
- 82.van Empel VP, et al. Myocyte apoptosis in heart failure. Cardiovasc. Res. 2005;67(1):21–29. doi: 10.1016/j.cardiores.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 83.Hill JA, Olson EN. Muscle: Fundamental Biology and Mechanisms of Disease. 2012 [Google Scholar]
- 84.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 85.Griffiths EJ. Mitochondria and heart disease. Adv. Exp. Med. Biol. 2012;942:249–267. doi: 10.1007/978-94-007-2869-1_11. [DOI] [PubMed] [Google Scholar]
- 86.Ong SB, et al. The mitochondrial permeability transition pore and its role in myocardial ischemia reperfusion injury. J. Mol. Cell. Cardiol. 2015;78:23–34. doi: 10.1016/j.yjmcc.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 87.Chen Q, et al. Inhibition of Bcl-2 sensitizes mitochondrial permeability transition pore (MPTP) opening in ischemia-damaged mitochondria. PLoS One. 2015;10(3):e0118834. doi: 10.1371/journal.pone.0118834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Halestrap AP, Richardson AP. The mitochondrial permeability transition: a current perspective on its identity and role in ischaemia/reperfusion injury. J. Mol. Cell. Cardiol. 2015;78:129–141. doi: 10.1016/j.yjmcc.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 89.Hongmei Z. Extrinsic and intrinsic apoptosis signal pathway review. Apoptosis Med. 2012 [Google Scholar]
- 90.Potz BA, et al. Calpain inhibition decreases myocardial apoptosis in a swine model of chronic myocardial ischemia. Surgery. 2015;158(2):445–452. doi: 10.1016/j.surg.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Movassagh M, Foo RS. Simplified apoptotic cascades. Heart Fail. Rev. 2008;13(2):111–119. doi: 10.1007/s10741-007-9070-x. [DOI] [PubMed] [Google Scholar]
- 92.Poon IKH, et al. Apoptotic cell clearance: basic biology and therapeutic potential. Nat. Rev. Immunol. 2014;14(3):166–180. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fan Q, et al. Inhibition of Fas-associated death domain-containing protein (FADD) protects against myocardial ischemia/reperfusion injury in a heart failure mouse model. PLoS One. 2013;8(9):e73537. doi: 10.1371/journal.pone.0073537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee SD, et al. Cardiac fas receptor-dependent apoptotic pathway in obese Zucker rats. Obesity (Silver Spring) 2007;15(10):2407–2415. doi: 10.1038/oby.2007.286. [DOI] [PubMed] [Google Scholar]
- 95.Li Y, et al. Critical roles for the Fas/Fas ligand system in postinfarction ventricular remodeling and heart failure. Circ. Res. 2004;95(6):627–636. doi: 10.1161/01.RES.0000141528.54850.bd. [DOI] [PubMed] [Google Scholar]
- 96.R von Harsdorf. “Fas-ten” your seat belt: anti-apoptotic treatment in heart failure takes off. Circ. Res. 2004;95(6):554–556. doi: 10.1161/01.RES.0000143717.70275.8f. [DOI] [PubMed] [Google Scholar]
- 97.Hayakawa K, et al. Sensitivity to apoptosis signal, clearance rate, and ultrastruc-ture of fas ligand-induced apoptosis in in vivo adult cardiac cells. Circulation. 2002;105(25):3039–3045. doi: 10.1161/01.cir.0000018651.89208.69. [DOI] [PubMed] [Google Scholar]
- 98.Lee P, et al. Fas pathway is a critical mediator of cardiac myocyte death and MI during ischemia-reperfusion in vivo. Am. J. Physiol. Heart Circ. Physiol. 2003;284(2):H456–H463. doi: 10.1152/ajpheart.00777.2002. [DOI] [PubMed] [Google Scholar]
- 99.Clarke P, L Tyler K. Apoptosis in animal models of virus-induced disease. Nat. Rev. Microbiol. 2009;7(2):144–155. doi: 10.1038/nrmicro2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jiang L, et al. Cardiomyocyte apoptosis is associated with increased wall stress in chronic failing left ventricle. Eur. Heart J. 2003;24(8):742–751. doi: 10.1016/s0195-668x(02)00655-3. [DOI] [PubMed] [Google Scholar]
- 101.Chang J, et al. High admission glucose levels increase Fas apoptosis and mortality in patients with acute ST-elevation myocardial infarction: a prospective cohort study. Cardiovasc. Diabetol. 2013;12:171. doi: 10.1186/1475-2840-12-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fertin M, et al. Circulating levels of soluble Fas ligand and left ventricular remodeling after acute myocardial infarction (from the REVE-2 study) J. Cardiol. 2012;60(2):93–97. doi: 10.1016/j.jjcc.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 103.Kinugawa T, et al. Proinflammatory cytokine activation is linked to apoptotic mediator, soluble Fas level in patients with chronic heart failure. Int. Heart J. 2012;53 (i3):182–186. doi: 10.1536/ihj.53.182. [DOI] [PubMed] [Google Scholar]
- 104.Koster G, et al. Effects of levosimendan for low cardiac output syndrome in critically ill patients: systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2015;41(2):203–221. doi: 10.1007/s00134-014-3604-1. [DOI] [PubMed] [Google Scholar]
- 105.Silvetti S, et al. Intermittent levosimendan improves mid-term survival in chronic heart failure patients: meta-analysis of randomised trials. Clin. Res. Cardiol. 2014;103(7):505–513. doi: 10.1007/s00392-013-0649-z. [DOI] [PubMed] [Google Scholar]
- 106.Kanamori H, et al. Inhibition of Fas-associated apoptosis in granulation tissue cells accompanies attenuation of postinfarction left ventricular remodeling by olmesartan. Am. J. Physiol. Heart Circ. Physiol. 2007;292(5):H2184–H2194. doi: 10.1152/ajpheart.01235.2006. [DOI] [PubMed] [Google Scholar]
- 107.Loirand G, Sauzeau V, Pacaud P. Small G proteins in the cardiovascular system: physiological and pathological aspects. Physiol. Rev. 2013;93(4):1659–1720. doi: 10.1152/physrev.00021.2012. [DOI] [PubMed] [Google Scholar]
- 108.Faria-Costa G, Leite-Moreira A, Henriques-Coelho T. Cardiovascular effects of the angiotensin type 2 receptor. Rev. Port. Cardiol. 2014;33(7–8):439–449. doi: 10.1016/j.repc.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 109.Qiu XX, et al. The effects of ACEI on calpain-mediated cardiomyocytes apoptosis and cardiac function in diabetic rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2013;29(4):359–362. [PubMed] [Google Scholar]
- 110.Su Q, et al. Effects of pretreatment with metoprolol on cardiomyocyte apoptosis and caspase-8 activation after coronary microembolization in rats. Zhonghua Xin Xue Guan Bing Za Zhi. 2013;41(8):693–697. [PubMed] [Google Scholar]
- 111.Zhang X, et al. Cardiotoxic and cardioprotective features of chronic beta-adrenergic signaling. Circ. Res. 2013;112(3):498–509. doi: 10.1161/CIRCRESAHA.112.273896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Karch J, Molkentin JD. Regulated necrotic cell death: the passive aggressive side of Bax and Bak. Circ. Res. 2015;116(11):1800–1809. doi: 10.1161/CIRCRESAHA.116.305421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Karch J, et al. Necroptosis interfaces with MOMP and the MPTP in mediating cell death. PLoS One. 2015;10(6):e0130520. doi: 10.1371/journal.pone.0130520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Karch J, et al. Bax and Bak function as the outer membrane component of the mitochondrial permeability pore in regulating necrotic cell death in mice. Elife. 2013;2:e00772. doi: 10.7554/eLife.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Poon IK, et al. Apoptotic cell clearance: basic biology and therapeutic potential. Nat. Rev. Immunol. 2014;14(3):166–180. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Biala AK, Kirshenbaum LA. The interplay between cell death signaling pathways in the heart. Trends Cardiovasc. Med. 2014;24(8):325–331. doi: 10.1016/j.tcm.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 117.Chen Z, et al. Overexpression of Bcl-2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. Am. J. Physiol. Heart Circ. Physiol. 2001;280(5):H2313–H2320. doi: 10.1152/ajpheart.2001.280.5.H2313. [DOI] [PubMed] [Google Scholar]
- 118.Latif N, et al. Upregulation of the Bcl-2 family of proteins in end stage heart failure. J. Am. Coll. Cardiol. 2000;35(7):1769–1777. doi: 10.1016/s0735-1097(00)00647-1. [DOI] [PubMed] [Google Scholar]
- 119.Konstantinidis K, Whelan RS, Kitsis RN. Mechanisms of cell death in heart disease. Arterioscler. Thromb. Vasc. Biol. 2012;32(7):1552–1562. doi: 10.1161/ATVBAHA.111.224915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Regula KM, Kirshenbaum LA. Apoptosis of ventricular myocytes: a means to an end. J. Mol. Cell. Cardiol. 2005;38(1):3–13. doi: 10.1016/j.yjmcc.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 121.Li J, et al. SNX13 reduction mediates heart failure through degradative sorting of apoptosis repressor with caspase recruitment domain. Nat. Commun. 2014;5:5177. doi: 10.1038/ncomms6177. [DOI] [PubMed] [Google Scholar]
- 122.Liston P, et al. Identification of XAF1 as an antagonist of XIAP anti-caspase activity. Nat. Cell Biol. 2001;3(2):128–133. doi: 10.1038/35055027. [DOI] [PubMed] [Google Scholar]
- 123.Lee JH, et al. Protective effects of therapeutic hypothermia in post-resuscitation myocardium. Resuscitation. 2012;83(5):633–639. doi: 10.1016/j.resuscitation.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 124.Ren J, Anversa P. The insulin-like growth factor I system: physiological and path-ophysiological implication in cardiovascular diseases associated with metabolic syndrome. Biochem. Pharmacol. 2015;93(4):409–417. doi: 10.1016/j.bcp.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 125.Baines CP, Molkentin JD. STRESS signaling pathways that modulate cardiac myocyte apoptosis. J. Mol. Cell. Cardiol. 2005;38(1):47–62. doi: 10.1016/j.yjmcc.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 126.Arcopinto M, et al. IGF-1 predicts survival in chronic heart failure. Insights from the T.O.S.CA. (Trattamento Ormonale Nello Scompenso CArdiaco) registry. Int. J. Cardiol. 2014;176(3):1006–1008. doi: 10.1016/j.ijcard.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 127.Floratou K, et al. Oxidative stress due to radiation in CD34(+) hematopoietic progenitor cells: protection by IGF-1. J. Radiat. Res. 2012;53(5):672–685. doi: 10.1093/jrr/rrs019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang L, et al. Regulation of cardiomyocyte apoptotic signaling by insulin-like growth factor I. Circ. Res. 1998;83(5):516–522. doi: 10.1161/01.res.83.5.516. [DOI] [PubMed] [Google Scholar]
- 129.O’Connell TD, et al. Cardiac alpha1-adrenergic receptors: novel aspects of expression, signaling mechanisms, physiologic function, and clinical importance. Pharmacol. Rev. 2014;66(1):308–333. doi: 10.1124/pr.112.007203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sakata Y, et al. Decompensation of pressure-overload hypertrophy in G alpha q-overexpressing mice. Circulation. 1998;97(15):1488–1495. doi: 10.1161/01.cir.97.15.1488. [DOI] [PubMed] [Google Scholar]
- 131.D’Angelo DD, et al. Transgenic Galphaq overexpression induces cardiac contractile failure in mice. Proc. Natl. Acad. Sci. U. S. A. 1997;94(15):8121–8126. doi: 10.1073/pnas.94.15.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sato M, et al. Protection of cardiomyocytes from the hypoxia-mediated injury by a peptide targeting the activator of G-protein signaling 8. PLoS One. 2014;9(3):e91980. doi: 10.1371/journal.pone.0091980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Troncoso R, et al. New insights into IGF-1 signaling in the heart. Trends Endocrinol. Metab. 2014;25(3):128–137. doi: 10.1016/j.tem.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 134.Hayakawa Y, et al. Inhibition of cardiac myocyte apoptosis improves cardiac function and abolishes mortality in the peripartum cardiomyopathy of Galpha(q) transgenic mice. Circulation. 2003;108(24):3036–3041. doi: 10.1161/01.CIR.0000101920.72665.58. [DOI] [PubMed] [Google Scholar]
- 135.Yussman MG, et al. Mitochondrial death protein Nix is induced in cardiac hypertrophy and triggers apoptotic cardiomyopathy. Nat. Med. 2002;8(7):725–730. doi: 10.1038/nm719. [DOI] [PubMed] [Google Scholar]
- 136.Holland WL, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 2011;17(1):55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wencker D, et al. A mechanistic role for cardiac myocyte apoptosis in heart failure. J. Clin. Invest. 2003;111(10):1497–1504. doi: 10.1172/JCI17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Askevold ET, et al. Interleukin-6 signaling, soluble glycoprotein 130, and inflammation in heart failure. Curr. Heart Fail. Rep. 2014;11(2):146–155. doi: 10.1007/s11897-014-0185-9. [DOI] [PubMed] [Google Scholar]
- 139.Askevold ET, et al. Soluble glycoprotein 130 predicts fatal outcomes in chronic heart failure: analysis from the controlled rosuvastatin multinational trial in Heart failure (CORONA) Circ. Heart Fail. 2013;6(1):91–98. doi: 10.1161/CIRCHEARTFAILURE.112.972653. [DOI] [PubMed] [Google Scholar]
- 140.Khoynezhad A, Jalali Z, Tortolani AJ. A synopsis of research in cardiac apoptosis and its application to congestive heart failure. Tex. Heart Inst. J. 2007;34(3):352–359. [PMC free article] [PubMed] [Google Scholar]
- 141.Prescimone T, et al. Caspase-1 transcripts in failing human heart after mechanical unloading. Cardiovasc. Pathol. 2015;24(1):11–18. doi: 10.1016/j.carpath.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 142.He S, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137(6):1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 143.Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517(7534):311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 144.Koudstaal S, et al. Necrostatin-1 alleviates reperfusion injury following acute myocardial infarction in pigs. Eur. J. Clin. Investig. 2015;45(2):150–159. doi: 10.1111/eci.12391. [DOI] [PubMed] [Google Scholar]
- 145.Oerlemans MI, et al. Inhibition of RIP1-dependent necrosis prevents adverse cardiac remodeling after myocardial ischemia-reperfusion in vivo. Basic Res. Cardiol. 2012;107(4):270. doi: 10.1007/s00395-012-0270-8. [DOI] [PubMed] [Google Scholar]
- 146.Lim SY, et al. The cardioprotective effect of necrostatin requires the cyclophilin-D component of the mitochondrial permeability transition pore. Cardiovasc. Drugs Ther. 2007;21(6):467–469. doi: 10.1007/s10557-007-6067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li L, et al. Transforming growth factor beta-activated kinase 1 signaling pathway critically regulates myocardial survival and remodeling. Circulation. 2014;130(24):2162–2172. doi: 10.1161/CIRCULATIONAHA.114.011195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat. Cell Biol. 2013;15(7):713–720. doi: 10.1038/ncb2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J. Pathol. 2010;221(1):3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lavandero S, et al. Cardiovascular autophagy: concepts, controversies, and perspectives. Autophagy. 2013;9(10):1455–1466. doi: 10.4161/auto.25969. [DOI] [PubMed] [Google Scholar]
- 151.Hill JA, Lavandero S, Rothermel BA. Chapter 30 — autophagy in cardiac physiology and disease. In: Olson JAHN, editor. Muscle. Boston/Waltham: Academic Press; 2012. pp. 405–422. [Google Scholar]
- 152.Rawat DK, et al. Increased reactive oxygen species, metabolic maladaptation, and autophagy contribute to pulmonary arterial hypertension-induced ventricular hypertrophy and diastolic heart failure. Hypertension. 2014;64(6):1266–1274. doi: 10.1161/HYPERTENSIONAHA.114.03261. [DOI] [PubMed] [Google Scholar]
- 153.Weng TP, et al. Activation of lymphocyte autophagy/apoptosis reflects haemody-namic inefficiency and functional aerobic impairment in patients with heart failure. Clin. Sci. (Lond.) 2014;127(10):589–602. doi: 10.1042/CS20130789. [DOI] [PubMed] [Google Scholar]
- 154.Shimomura H, et al. Autophagic degeneration as a possible mechanism of myocardial cell death in dilated cardiomyopathy. Jpn. Circ. J. 2001;65(11):965–968. doi: 10.1253/jcj.65.965. [DOI] [PubMed] [Google Scholar]
- 155.Hale AN, et al. Autophagy: regulation and role in development. Autophagy. 2013;9(7):951–972. doi: 10.4161/auto.24273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mattiazzi A, et al. Chasing cardiac physiology and pathology down the CaMKII cascade. Am. J. Physiol. Heart Circ. Physiol. 2015;308(10):H1177–H1191. doi: 10.1152/ajpheart.00007.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hardie DG, Ashford ML. AMPK: regulating energy balance at the cellular and whole body levels. Physiology (Bethesda) 2014;29(2):99–107. doi: 10.1152/physiol.00050.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011;13(9):1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Petrovski G, Das DK. Does autophagy take a front seat in lifespan extension? J. Cell. Mol. Med. 2010;14(11):2543–2551. doi: 10.1111/j.1582-4934.2010.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat. Cell Biol. 2010;12(9):814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Johansen T, Lamark T. Selective autophagy mediated by autophagic adapter proteins. Autophagy. 2011;7(3):279–296. doi: 10.4161/auto.7.3.14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Birgisdottir AB, Lamark T, Johansen T. The LIR motif - crucial for selective autophagy. J. Cell Sci. 2013;126(Pt 15):3237–3247. doi: 10.1242/jcs.126128. [DOI] [PubMed] [Google Scholar]
- 163.Zheng Q, et al. Autophagy and p62 in cardiac proteinopathy. Circ. Res. 2011;109(3):296–308. doi: 10.1161/CIRCRESAHA.111.244707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kim PK, et al. Ubiquitin signals autophagic degradation of cytosolic proteins and peroxisomes. Proc. Natl. Acad. Sci. U. S. A. 2008;105(52):20567–20574. doi: 10.1073/pnas.0810611105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Isakson P, Holland P, Simonsen A. The role of ALFY in selective autophagy. Cell Death Differ. 2013;20(1):12–20. doi: 10.1038/cdd.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Clausen TH, et al. p62/SQSTM1 and ALFY interact to facilitate the formation of p62 bodies/ALIS and their degradation by autophagy. Autophagy. 2010;6(3):330–344. doi: 10.4161/auto.6.3.11226. [DOI] [PubMed] [Google Scholar]
- 167.Filimonenko M, et al. The selective macroautophagic degradation of aggregated proteins requires the PI3P–binding protein Alfy. Mol. Cell. 2010;38(2):265–279. doi: 10.1016/j.molcel.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.McLendon PM, et al. Tubulin hyperacetylation is adaptive in cardiac proteotoxicity by promoting autophagy. Proc. Natl. Acad. Sci. U. S. A. 2014;111(48):E5178–E5186. doi: 10.1073/pnas.1415589111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yan J, et al. SQSTM1/p62 interacts with HDAC6 and regulates deacetylase activity. PLoS One. 2013;8(9):e76016. doi: 10.1371/journal.pone.0076016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kirkin V, et al. A role for ubiquitin in selective autophagy. Mol. Cell. 2009;34(3):259–269. doi: 10.1016/j.molcel.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 171.Taneike M, et al. Inhibition of autophagy in the heart induces age-related cardio-myopathy. Autophagy. 2010;6(5):600–606. doi: 10.4161/auto.6.5.11947. [DOI] [PubMed] [Google Scholar]
- 172.Rothermel BA, Hill JA. Autophagy in load-induced heart disease. Circ. Res. 2008;103(12):1363–1369. doi: 10.1161/CIRCRESAHA.108.186551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pyo JO, et al. Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat. Commun. 2013;4:2300. doi: 10.1038/ncomms3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Oyabu J, et al. Autophagy-mediated degradation is necessary for regression of cardiac hypertrophy during ventricular unloading. Biochem. Biophys. Res. Commun. 2013;441(4):787–792. doi: 10.1016/j.bbrc.2013.10.135. [DOI] [PubMed] [Google Scholar]
- 175.Nishida K, Taneike M, Otsu K. The role of autophagic degradation in the heart. J. Mol. Cell. Cardiol. 2015;78:73–79. doi: 10.1016/j.yjmcc.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 176.Jimenez RE, Kubli DA, Gustafsson AB. Autophagy and mitophagy in the myocardium: therapeutic potential and concerns. Br. J. Pharmacol. 2014;171(8):1907–1916. doi: 10.1111/bph.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.De Meyer GR, Martinet W. Autophagy in the cardiovascular system. Biochim. Biophys. Acta. 2009;1793(9):1485–1495. doi: 10.1016/j.bbamcr.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 178.Nakai A, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat. Med. 2007;13(5):619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 179.Hashem SI, et al. Brief report: oxidative stress mediates cardiomyocyte apoptosis in a human model of Danon disease and heart failure. Stem Cells. 2015;33(7):2343–2350. doi: 10.1002/stem.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pedrozo Z, et al. Cardiomyocyte ryanodine receptor degradation by chaperone-mediated autophagy. Cardiovasc. Res. 2013;98(2):277–285. doi: 10.1093/cvr/cvt029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Saftig P, Beertsen W, Eskelinen EL. LAMP-2: a control step for phagosome and autophagosome maturation. Autophagy. 2008;4(4):510–512. doi: 10.4161/auto.5724. [DOI] [PubMed] [Google Scholar]
- 182.Jaber N, Zong WX. Class III PI3K Vps34: essential roles in autophagy, endocytosis, and heart and liver function. Ann. N. Y. Acad. Sci. 2013;1280:48–51. doi: 10.1111/nyas.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jaber N, et al. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc. Natl. Acad. Sci. U. S. A. 2012;109(6):2003–2008. doi: 10.1073/pnas.1112848109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sciarretta S, Volpe M, Sadoshima J. Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ. Res. 2014;114(3):549–564. doi: 10.1161/CIRCRESAHA.114.302022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Efeyan A, et al. Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival. Nature. 2013;493(7434):679–683. doi: 10.1038/nature11745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tamai T, et al. Rheb (Ras homologue enriched in brain)-dependent mammalian target of rapamycin complex 1 (mTORC1) activation becomes indispensable for cardiac hypertrophic growth after early postnatal period. J. Biol. Chem. 2013;288(14):10176–10187. doi: 10.1074/jbc.M112.423640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhu Y, et al. Mechanistic target of rapamycin (Mtor) is essential for murine embryonic heart development and growth. PLoS One. 2013;8(1):e54221. doi: 10.1371/journal.pone.0054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ikeda Y, et al. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ. Res. 2015;116(2):264–278. doi: 10.1161/CIRCRESAHA.116.303356. [DOI] [PubMed] [Google Scholar]
- 189.Ikeda Y, et al. Molecular mechanisms mediating mitochondrial dynamics and mitophagy and their functional roles in the cardiovascular system. J. Mol. Cell. Cardiol. 2015;78:116–122. doi: 10.1016/j.yjmcc.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rifki OF, Hill JA. Cardiac autophagy: good with the bad. J. Cardiovasc. Pharmacol. 2012;60(3):248–252. doi: 10.1097/FJC.0b013e3182646cb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang ZV, Ferdous A, Hill JA. Cardiomyocyte autophagy: metabolic profit and loss. Heart Fail. Rev. 2013;18(5):585–594. doi: 10.1007/s10741-012-9350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lavandero S, et al. Autophagy in cardiovascular biology. J. Clin. Invest. 2015;125(1):55–64. doi: 10.1172/JCI73943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lauer MS, Nakamura R. Reviewing peer review at the NIH. N. Engl. J. Med. 2015;373(20):1893–1895. doi: 10.1056/NEJMp1507427. [DOI] [PubMed] [Google Scholar]
- 194.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev. Cell. 2004;6(4):463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 195.Begley CG, Ioannidis JP. Reproducibility in science: improving the standard for basic and preclinical research. Circ. Res. 2015;116(1):116–126. doi: 10.1161/CIRCRESAHA.114.303819. [DOI] [PubMed] [Google Scholar]
- 196.Xie M, Burchfield JS, Hill JA. Pathological ventricular remodeling: therapies: part 2 of 2. Circulation. 2013;128(9):1021–1030. doi: 10.1161/CIRCULATIONAHA.113.001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hammerling BC, Gustafsson AB. Mitochondrial quality control in the myocardium: cooperation between protein degradation and mitophagy. J. Mol. Cell. Cardiol. 2014;75:122–130. doi: 10.1016/j.yjmcc.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ma X, et al. Autophagy is impaired in cardiac ischemia-reperfusion injury. Autophagy. 2012;8(9):1394–1396. doi: 10.4161/auto.21036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ma X, et al. Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation. 2012;125(25):3170–3181. doi: 10.1161/CIRCULATIONAHA.111.041814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cao DJ, et al. Histone deacetylase (HDAC) inhibitors attenuate cardiac hypertrophy by suppressing autophagy. Proc. Natl. Acad. Sci. U. S. A. 2011;108(10):4123–4128. doi: 10.1073/pnas.1015081108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhu H, et al. Cardiac autophagy is a maladaptive response to hemodynamic stress. J. Clin. Invest. 2007;117(7):1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Li MH, et al. Berberine improves pressure overload-induced cardiac hypertrophy and dysfunction through enhanced autophagy. Eur. J. Pharmacol. 2014;728:67–76. doi: 10.1016/j.ejphar.2014.01.061. [DOI] [PubMed] [Google Scholar]
- 203.Nakai A, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat. Med. 2007;13(5):619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 204.Paul DS, et al. Deficiency of cardiac Acyl-CoA synthetase-1 induces diastolic dysfunction, but pathologic hypertrophy is reversed by rapamycin. Biochim. Biophys. Acta. 2014;1841(6):880–887. doi: 10.1016/j.bbalip.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003;4(7):517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 206.Burr AR, et al. Na+ dysregulation coupled with Ca2+ entry through NCX1 promotes muscular dystrophy in mice. Mol. Cell. Biol. 2014;34(11):1991–2002. doi: 10.1128/MCB.00339-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mijares A, et al. Age-dependent changes in diastolic Ca(2+) and Na(+) concentrations in dystrophic cardiomyopathy: role of Ca(2+) entry and IP3. Biochem. Biophys. Res. Commun. 2014;452(4):1054–1059. doi: 10.1016/j.bbrc.2014.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Duan D. Challenges and opportunities in dystrophin-deficient cardiomyopathy gene therapy. Hum. Mol. Genet. 2006;15:R253–R261. doi: 10.1093/hmg/ddl180. (Spec No 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kho C, Lee A, Hajjar RJ. Altered sarcoplasmic reticulum calcium cycling-targets for heart failure therapy. Nat. Rev. Cardiol. 2012;9(12):717–733. doi: 10.1038/nrcardio.2012.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Timmins JM, et al. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J. Clin. Invest. 2009;119(10):2925–2941. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Park CS, et al. The chemical chaperone 4-phenylbutyric acid attenuates pressure-overload cardiac hypertrophy by alleviating endoplasmic reticulum stress. Biochem. Biophys. Res. Commun. 2012;421(3):578–584. doi: 10.1016/j.bbrc.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 212.Dickhout JG, Carlisle RE, C R. Austin, Interrelationship between cardiac hypertrophy, heart failure, and chronic kidney disease: endoplasmic reticulum stress as a mediator of pathogenesis. Circ. Res. 2011;108(5):629–642. doi: 10.1161/CIRCRESAHA.110.226803. [DOI] [PubMed] [Google Scholar]
- 213.Hetz C, Chevet E, Harding HP. Targeting the unfolded protein response in disease. Nat. Rev. Drug Discov. 2013;12(9):703–719. doi: 10.1038/nrd3976. [DOI] [PubMed] [Google Scholar]
- 214.Wang ZV, et al. Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine biosynthetic pathway. Cell. 2014;156(6):1179–1192. doi: 10.1016/j.cell.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Miyazaki Y, et al. C/EBP homologous protein deficiency attenuates myocardial reperfusion injury by inhibiting myocardial apoptosis and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011;31(5):1124–1132. doi: 10.1161/ATVBAHA.111.224519. [DOI] [PubMed] [Google Scholar]
- 216.Dipla K, et al. Myocyte recovery after mechanical circulatory support in humans with end-stage heart failure. Circulation. 1998;97(23):2316–2322. doi: 10.1161/01.cir.97.23.2316. [DOI] [PubMed] [Google Scholar]
- 217.Duan Q, et al. MicroRNA-214 Is upregulated in heart failure patients and suppresses XBP1-mediated endothelial cells angiogenesis. J. Cell. Physiol. 2015;230(8):1964–1973. doi: 10.1002/jcp.24942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Duan Q, et al. MicroRNA regulation of unfolded protein response transcription factor XBP1 in the progression of cardiac hypertrophy and heart failure in vivo. J. Transl. Med. 2015;13(1):363. doi: 10.1186/s12967-015-0725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Castillero E, et al. Attenuation of the unfolded protein response and endoplasmic reticulum stress after mechanical unloading in dilated cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2015;309(3):H459–H470. doi: 10.1152/ajpheart.00056.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Smith MA, Schnellmann RG. Calpains, mitochondria, and apoptosis. Cardiovasc. Res. 2012;96(1):32–37. doi: 10.1093/cvr/cvs163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Iwamoto H, et al. Calpain inhibitor-1 reduces infarct size and DNA fragmentation of myocardium in ischemic/reperfused rat heart. J. Cardiovasc. Pharmacol. 1999;33(4):580–586. doi: 10.1097/00005344-199904000-00010. [DOI] [PubMed] [Google Scholar]
- 222.Khalil PN, et al. Calpain inhibition reduces infarct size and improves global hemodynamics and left ventricular contractility in a porcine myocardial ischemia/reperfusion model. Eur. J. Pharmacol. 2005;528(1–3):124–131. doi: 10.1016/j.ejphar.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 223.Tantral L, et al. Intracellular calcium release is required for caspase-3 and −9 activation. Cell Biochem. Funct. 2004;22(1):35–40. doi: 10.1002/cbf.1050. [DOI] [PubMed] [Google Scholar]
- 224.Bouras G, et al. Inflammation and chronic heart failure: from biomarkers to novel anti-inflammatory therapeutic strategies. Med. Chem. 2014;10(7):682–699. doi: 10.2174/1573406410666140318113325. [DOI] [PubMed] [Google Scholar]
- 225.Shires SE, Gustafsson AB. Mitophagy and heart failure. J. Mol. Med. (Berl.) 2015;93(3):253–262. doi: 10.1007/s00109-015-1254-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yu P, et al. Class III PI3K–mediated prolonged activation of autophagy plays a critical role in the transition of cardiac hypertrophy to heart failure. J. Cell. Mol. Med. 2015 doi: 10.1111/jcmm.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Viola HM, Hool LC. Targeting calcium and the mitochondria in prevention of pathology in the heart. Curr. Drug Targets. 2011;12(5):748–760. doi: 10.2174/138945011795378603. [DOI] [PubMed] [Google Scholar]
- 228.Kowalczyk JE, Zablocka B. Protein kinases in mitochondria. Postepy Biochem. 2008;54(2):209–216. [PubMed] [Google Scholar]
- 229.Gao W, et al. Induction of macroautophagy by exogenously introduced calcium. Autophagy. 2008;4(6):754–761. doi: 10.4161/auto.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hoyer-Hansen M, et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol. Cell. 2007;25(2):193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 231.Cebotaru V, et al. Polycystin-1 negatively regulates polycystin-2 expression via the aggresome/autophagosome pathway. J. Biol. Chem. 2014;289(10):6404–6414. doi: 10.1074/jbc.M113.501205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Retailleau K, Duprat F. Polycystins and partners: proposed role in mechanosensitivity. J. Physiol. 2014;592(Pt 12):2453–2471. doi: 10.1113/jphysiol.2014.271346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kienzle C, von Blume J. Secretory cargo sorting at the trans-Golgi network. Trends Cell Biol. 2014;24(10):584–593. doi: 10.1016/j.tcb.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 234.Chen X, et al. Autophagy induced by calcium phosphate precipitates targets damaged endosomes. J. Biol. Chem. 2014;289(16):11162–11174. doi: 10.1074/jbc.M113.531855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wong A, et al. Regulation of autophagy in cardiomyocytes by Ins(1,4,5)P(3) and IP(3)-receptors. J. Mol. Cell. Cardiol. 2013;54:19–24. doi: 10.1016/j.yjmcc.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 236.Medina DL, et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Cell Biol. 2015;17(3):288–299. doi: 10.1038/ncb3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Bernales S, McDonald KL, Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 2006;4(12):e423. doi: 10.1371/journal.pbio.0040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Groenendyk J, et al. Biology of endoplasmic reticulum stress in the heart. Circ. Res. 2010;107(10):1185–1197. doi: 10.1161/CIRCRESAHA.110.227033. [DOI] [PubMed] [Google Scholar]
- 239.Luo B, Lee AS. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene. 2013;32(7):805–818. doi: 10.1038/onc.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhu G, Lee AS, Role of the unfolded protein response. GRP78 and GRP94 in organ homeostasis. J. Cell. Physiol. 2015;230(7):1413–1420. doi: 10.1002/jcp.24923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Gong W, et al. Chronic inhibition of cGMP-specific phosphodiesterase 5 suppresses endoplasmic reticulum stress in heart failure. Br. J. Pharmacol. 2013;170(7):1396–1409. doi: 10.1111/bph.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Humeres C, et al. 4-Phenylbutyric acid prevent cytotoxicity induced by thapsigargin in rat cardiac fibroblast. Toxicol. in Vitro. 2014;28(8):1443–1448. doi: 10.1016/j.tiv.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 243.Simon JN, et al. Ceramide-mediated depression in cardiomyocyte contractility through PKC activation and modulation of myofilament protein phosphorylation. Basic Res. Cardiol. 2014;109(6):445. doi: 10.1007/s00395-014-0445-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Holland WL, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 2011;17(1):55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhou X, et al. Trimeric intracellular cation channels and sarcoplasmic/endoplasmic reticulum calcium homeostasis. Circ. Res. 2014;114(4):706–716. doi: 10.1161/CIRCRESAHA.114.301816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Smaili SS, et al. The role of calcium stores in apoptosis and autophagy. Curr. Mol. Med. 2013;13(2):252–265. doi: 10.2174/156652413804810772. [DOI] [PubMed] [Google Scholar]
- 247.Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ. Res. 2010;107(9):1071–1082. doi: 10.1161/CIRCRESAHA.110.227819. [DOI] [PubMed] [Google Scholar]
- 248.Harr MW, Distelhorst CW. Apoptosis and autophagy: decoding calcium signals that mediate life or death. Cold Spring Harb. Perspect. Biol. 2010;2(10):a005579. doi: 10.1101/cshperspect.a005579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Collins HE, et al. Stromal interaction molecule 1 is essential for normal cardiac homeostasis through modulation of ER and mitochondrial function. Am. J. Physiol. Heart Circ. Physiol. 2014;306(8):H1231–H1239. doi: 10.1152/ajpheart.00075.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Mekahli D, et al. Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb. Perspect. Biol. 2011;3(6) doi: 10.1101/cshperspect.a004317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lu FH, et al. Role of the calcium-sensing receptor in cardiomyocyte apoptosis via the sarcoplasmic reticulum and mitochondrial death pathway in cardiac hypertrophy and heart failure. Cell. Physiol. Biochem. 2013;31(4–5):728–743. doi: 10.1159/000350091. [DOI] [PubMed] [Google Scholar]
- 252.Kanekura K, et al. IRE1 prevents endoplasmic reticulum membrane perme-abilization and cell death under pathological conditions. Sci. Signal. 2015;8(382):ra62. doi: 10.1126/scisignal.aaa0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Davis J, et al. Apoptosis repressor with a CARD domain (ARC) restrains Bax-mediated pathogenesis in dystrophic skeletal muscle. PLoS One. 2013;8(12):e82053. doi: 10.1371/journal.pone.0082053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Hetz C, Chevet E, Oakes SA. Proteostasis control by the unfolded protein response. Nat. Cell Biol. 2015;17(7):829–838. doi: 10.1038/ncb3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Hetz C, et al. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312(5773):572–576. doi: 10.1126/science.1123480. [DOI] [PubMed] [Google Scholar]
- 256.Rojas-Rivera D, et al. Alternative functions of the BCL-2 protein family at the endoplasmic reticulum. Adv. Exp. Med. Biol. 2010;687:33–47. doi: 10.1007/978-1-4419-6706-0_2. [DOI] [PubMed] [Google Scholar]
- 257.da Costa Martins PA, et al. MicroRNA-199b targets the nuclear kinase Dyrk1a in an auto-amplification loop promoting calcineurin/NFAT signalling. Nat. Cell Biol. 2010;12(12):1220–1227. doi: 10.1038/ncb2126. [DOI] [PubMed] [Google Scholar]
- 258.Win S, et al. JNK interaction with Sab mediates ER stress induced inhibition of mitochondrial respiration and cell death. Cell Death Dis. 2014;5:e989. doi: 10.1038/cddis.2013.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Merksamer PI, Papa FR. The UPR and cell fate at a glance. J. Cell Sci. 2010;123(Pt 7):1003–1006. doi: 10.1242/jcs.035832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Wang L, Qian L. miR-24 regulates intrinsic apoptosis pathway in mouse cardiomyocytes. PLoS One. 2014;9(1):e85389. doi: 10.1371/journal.pone.0085389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Li Y, et al. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim. Biophys. Sin. (Shanghai) 2015;47(2):146–147. doi: 10.1093/abbs/gmu128. [DOI] [PubMed] [Google Scholar]
- 262.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta. 2013;1833(12):3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Lindqvist LM, et al. Prosurvival Bcl-2 family members affect autophagy only indirectly, by inhibiting Bax and Bak. Proc. Natl. Acad. Sci. U. S. A. 2014;111(23):8512–8517. doi: 10.1073/pnas.1406425111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Luo S, Rubinsztein DC. BCL2L11/BIM: a novel molecular link between autophagy and apoptosis. Autophagy. 2013;9(1):104–105. doi: 10.4161/auto.22399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Luo S, et al. Bim inhibits autophagy by recruiting Beclin 1 to microtubules. Mol. Cell. 2012;47(3):359–370. doi: 10.1016/j.molcel.2012.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zhu X, et al. Ubiquitination of inositol-requiring enzyme 1 (IRE1) by the E3 ligase CHIP mediates the IRE1/TRAF2/JNK pathway. J. Biol. Chem. 2014;289(44):30567–30577. doi: 10.1074/jbc.M114.562868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011;13(3):184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Senft D, Ronai ZA. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem. Sci. 2015;40(3):141–148. doi: 10.1016/j.tibs.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kolattukudy PE, Niu J. Inflammation, endoplasmic reticulum stress, autophagy, and the monocyte chemoattractant protein-1/CCR2 pathway. Circ. Res. 2012;110(1):174–189. doi: 10.1161/CIRCRESAHA.111.243212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Verfaillie T, et al. Linking ER stress to autophagy: potential implications for cancer therapy. Int. J. Cell Biol. 2010;2010:930509. doi: 10.1155/2010/930509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.B’Chir W, et al. The eIF2alpha/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 2013;41(16):7683–7699. doi: 10.1093/nar/gkt563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Teske BF, et al. The eIF2 kinase PERK and the integrated stress response facilitate activation of ATF6 during endoplasmic reticulum stress. Mol. Biol. Cell. 2011;22(22):4390–4405. doi: 10.1091/mbc.E11-06-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Margariti A, et al. XBP1 mRNA splicing triggers an autophagic response in endothelial cells through BECLIN-1 transcriptional activation. J. Biol. Chem. 2013;288(2):859–872. doi: 10.1074/jbc.M112.412783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Tian PG, et al. Spliced XBP1 promotes macrophage survival and autophagy by interacting with Beclin-1. Biochem. Biophys. Res. Commun. 2015;463(4):518–523. doi: 10.1016/j.bbrc.2015.05.061. [DOI] [PubMed] [Google Scholar]
- 275.Luan Q, et al. RIPK1 regulates survival of human melanoma cells upon endoplas-mic reticulum stress through autophagy. Autophagy. 2015;11(7):975–994. doi: 10.1080/15548627.2015.1049800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Haller M, et al. Ubiquitination and proteasomal degradation of ATG12 regulates its proapoptotic activity. Autophagy. 2014;10(12):2269–2278. doi: 10.4161/15548627.2014.981914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Rubinstein AD, et al. The autophagy protein Atg12 associates with antiapoptotic Bcl-2 family members to promote mitochondrial apoptosis. Mol. Cell. 2011;44(5):698–709. doi: 10.1016/j.molcel.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 278.Decuypere JP, et al. mTOR-controlled autophagy requires intracellular Ca(2+) signaling. PLoS One. 2013;8(4):e61020. doi: 10.1371/journal.pone.0061020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.He H, et al. Calcineurin suppresses AMPK-dependent cytoprotective autophagy in cardiomyocytes under oxidative stress. Cell Death Dis. 2014;5:e997. doi: 10.1038/cddis.2013.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Bravo R, et al. Endoplasmic reticulum and the unfolded protein response: dynamics and metabolic integration. Int. Rev. Cell Mol. Biol. 2013;301:215–290. doi: 10.1016/B978-0-12-407704-1.00005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Alers S, et al. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol. Cell. Biol. 2012;32(1):2–11. doi: 10.1128/MCB.06159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Madaro L, et al. Intracellular signaling in ER stress-induced autophagy in skeletal muscle cells. FASEB J. 2013;27(5):1990–2000. doi: 10.1096/fj.12-215475. [DOI] [PubMed] [Google Scholar]
- 283.Sakaki K, Kaufman RJ. Regulation of ER stress-induced macroautophagy by protein kinase C. Autophagy. 2008;4(6):841–843. doi: 10.4161/auto.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Ramirez E, et al. Eplerenone attenuated cardiac steatosis, apoptosis and diastolic dysfunction in experimental type-II diabetes. Cardiovasc. Diabetol. 2013;12:172. doi: 10.1186/1475-2840-12-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Chaanine AH, et al. Potential role of BNIP3 in cardiac remodeling, myocardial stiffness, and endoplasmic reticulum: mitochondrial calcium homeostasis in diastolic and systolic heart failure. Circ. Heart Fail. 2013;6(3):572–583. doi: 10.1161/CIRCHEARTFAILURE.112.000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2014;11(9):507–515. doi: 10.1038/nrcardio.2014.83. [DOI] [PubMed] [Google Scholar]
- 287.Melman YF, Shah R, Das S. MicroRNAs in heart failure: is the picture becoming less miRky? Circ. Heart Fail. 2014;7(1):203–214. doi: 10.1161/CIRCHEARTFAILURE.113.000266. [DOI] [PubMed] [Google Scholar]
- 288.Linton PJ, et al. This old heart: cardiac aging and autophagy. J. Mol. Cell. Cardiol. 2015;83:44–54. doi: 10.1016/j.yjmcc.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Pillai VB, Sundaresan NR, Gupta MP. Regulation of Akt signaling by sirtuins: its implication in cardiac hypertrophy and aging. Circ. Res. 2014;114(2):368–378. doi: 10.1161/CIRCRESAHA.113.300536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ahmet I, et al. Effects of calorie restriction on cardioprotection and cardiovascular health. J. Mol. Cell. Cardiol. 2011;51(2):263–271. doi: 10.1016/j.yjmcc.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Ikeda Y, et al. New insights into the role of mitochondrial dynamics and autoph-agy during oxidative stress and aging in the heart. Oxidative Med. Cell. Longev. 2014;2014:210934. doi: 10.1155/2014/210934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Yin Z, Ren J, Guo W. Sarcomeric protein isoform transitions in cardiac muscle: a journey to heart failure. Biochim. Biophys. Acta. 2015;1852(1):47–52. doi: 10.1016/j.bbadis.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Hamdani N, et al. Sarcomeric dysfunction in heart failure. Cardiovasc. Res. 2008;77(4):649–658. doi: 10.1093/cvr/cvm079. [DOI] [PubMed] [Google Scholar]
- 294.Cotecchia S, et al. The alpha1-adrenergic receptors in cardiac hypertrophy: signaling mechanisms and functional implications. Cell. Signal. 2015;27(10):1984–1993. doi: 10.1016/j.cellsig.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 295.Aronson D, Krum H. Novel therapies in acute and chronic heart failure. Pharmacol. Ther. 2012;135(1):1–17. doi: 10.1016/j.pharmthera.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 296.Vergaro G, et al. Prognostic value of plasma renin activity in heart failure. Am. J. Cardiol. 2011;108(2):246–251. doi: 10.1016/j.amjcard.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 297.Arumugam S, et al. Angiotensin receptor blockers: focus on cardiac and renal injury. Trends Cardiovasc. Med. 2015 doi: 10.1016/j.tcm.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 298.Greco CM, Condorelli G. Epigenetic modifications and noncoding RNAs in cardiac hypertrophy and failure. Nat. Rev. Cardiol. 2015;12(8):488–497. doi: 10.1038/nrcardio.2015.71. [DOI] [PubMed] [Google Scholar]
- 299.Gillette TG, Hill JA. Readers, writers, and erasers: chromatin as the whiteboard of heart disease. Circ. Res. 2015;116(7):1245–1253. doi: 10.1161/CIRCRESAHA.116.303630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ. Res. 2015;116(7):1254–1268. doi: 10.1161/CIRCRESAHA.116.302317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Brown DI, Griendling KK. Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circ. Res. 2015;116(3):531–549. doi: 10.1161/CIRCRESAHA.116.303584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Lim SL, et al. Cardiac endothelium-myocyte interaction: clinical opportunities for new heart failure therapies regardless of ejection fraction. Eur. Heart J. 2015;36(31):2050–2060. doi: 10.1093/eurheartj/ehv132. [DOI] [PubMed] [Google Scholar]
- 303.Orogo AM, Gustafsson AB. Cell death in the myocardium: my heart won’t go on. IUBMB Life. 2013;65(8):651–656. doi: 10.1002/iub.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.