Abstract
Research on how lexical tone is neuroanatomically represented in the human brain is central to our understanding of cortical regions subserving language. Past studies have exclusively focused on tone perception of the spoken language, and little is known as to the lexical tone processing in reading visual words and its associated brain mechanisms. In this study, we performed two experiments to identify neural substrates in Chinese tone reading. First, we used a tone judgment paradigm to investigate tone processing of visually presented Chinese characters. We found that, relative to baseline, tone perception of printed Chinese characters were mediated by strong brain activation in bilateral frontal regions, left inferior parietal lobule, left posterior middle/medial temporal gyrus, left inferior temporal region, bilateral visual systems, and cerebellum. Surprisingly, no activation was found in superior temporal regions, brain sites well known for speech tone processing. In activation likelihood estimation (ALE) meta‐analysis to combine results of relevant published studies, we attempted to elucidate whether the left temporal cortex activities identified in Experiment one is consistent with those found in previous studies of auditory lexical tone perception. ALE results showed that only the left superior temporal gyrus and putamen were critical in auditory lexical tone processing. These findings suggest that activation in the superior temporal cortex associated with lexical tone perception is modality‐dependent. Hum Brain Mapp, 36:304–312, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: functional magnetic resonance imaging, language, pitch, prosody, temporal cortex, activation likelihood estimation
INTRODUCTION
In a tonal language, pitch (i.e., tone) is used to distinguish lexical or grammatical meanings. As tonal languages account for more than 70% of world languages [Yip, 2002], research on how lexical tone is neuroanatomically represented is central to our understanding of cortical regions subserving language. Previous neuroimaging studies have yielded important findings on the brain mechanisms dedicated to the processing of lexical tone in spoken language. For example, activations in bilateral superior temporal gyrus (STG) and left anterior insula cortex have been demonstrated during auditory perception of Mandarin pitch patterns [Klein et al., 2001; Wong et al., 2004; Xi et al., 2010]. When native speakers of Thai and Mandarin performed tone discrimination tasks on Chinese words or resynthesized stimuli by superimposing Thai tones onto Chinese syllables, both groups exhibited stronger activity in the left planum temporale (PT) in response to native than non‐native tones, revealing the role of PT in perceiving lexical tones [Xu et al., 2006]. In a word naming (production) task, Liu et al. [2006] compared the production patterns of Chinese tones and vowels in an adaptation functional magnetic resonance imaging (fMRI) paradigm, and they found that lexical tone production elicited stronger activation in left middle temporal gyrus relative to vowel production. Luo et al. [2006] used a passive oddball paradigm to measure early auditory processing of Chinese lexical tones and consonants in Mandarin natives, demonstrating that preattentive perception of Mandarin tones evoked significant brain activation in the STG bilaterally, with stronger activity in right hemisphere. In a brain structure study, Crinion et al. [2009] identified greater cortical volume in the left insula and right anterior temporal lobe in Chinese speakers when compared to those who do not speak Chinese, implicating the role of temporal lobe in processing linguistic pitch. Using a different approach, neuroimaging studies used an auditory paradigm to train native English speakers to acquire Mandarin tones, revealing that the learning of Mandarin tone classification was associated with increased brain activity in left STG and posterior transverse temporal area (BA 42) [Wang et al., 2003]. When Americans were trained to match pictures with monosyllabic pseudowords that were composed of Mandarin tones superimposed onto English syllables, less successful learners exhibited a smaller gray matter volume in the left Heschl's gyrus compared to successful learners, indicating that the primary auditory regions are involved in linguistic pitch processing [Wong et al., 2008].
Prior research has also indicated the contribution of the frontal cortex in speech tone processing. Activity in the left inferior frontal gyrus is assumed to be associated with tone perception experience in a number of brain mapping experiments by Gandour et al. [1998, 2000, 2002, 2003] and Hsieh et al. [2001]. In a phonological recognition task, direct contrasts of Mandarin tones relative to consonants and rhymes identified significantly stronger brain activation in right fronto‐parietal regions [Li et al., 2010]. Right‐lateralized cortical activations in posterior inferior frontal gyrus were also found in native Mandarin subjects during the production of Chinese lexical tone sequences [Chang et al., 2014].
Although we have gained an in‐depth understanding of the neural basis for auditory tone perception, little is known as to whether these previously identified neural correlates are involved in the visual modality, that is, when lexical tones are read visually. For example, in written Chinese, printed characters map onto monosyllables with tonal information, and thus, how the brain reads tones is fundamental to reading comprehension. Here, we report fMRI results with regard to the neural substrates for lexical tone processing in Chinese reading. We used a simple tone reading task in which Mandarin natives made explicit Mandarin tone discrimination for every visually presented characters. During the scan, subjects were asked to respond by making button presses as quickly and accurately as possible. If the neural basis for lexical tone is modality‐general, we expect to observe brain activations in similar regions in the frontal areas and the temporal cortex that mediate audition of speech tone as reviewed above.
EXPERIMENT 1
Materials and Methods
Participants
We scanned eighteen native Mandarin speaking adults (9 males and 9 females; average age = 21 years, SD = 1.18 years) from Beijing Capital Normal University with normal or correct‐to‐normal vision. They were free from neurological and psychiatric diseases. All participants were strongly right‐handed. They gave informed consents before the study was performed and were paid for participation. One participant was excluded from the analysis due to task incompletion. The study was approved by the Institutional Review Board of Beijing MRI Center for Brain Research.
Materials and Procedures
A total of 96 characters were used in the present experiment. Forty‐eight characters had low number of strokes ranging from 8 to 10 (mean = 9.375, SD = 0.733) (“visually simple characters”) and another 48 characters had high number of strokes ranging from 12 to 17 (mean = 14.313, SD =2.033) (“visually complex characters”). All of the selected characters consisted of three components and were of high frequency of occurrences (Fig. 1). Forty‐eight out of 96 characters carried the falling tone (tone 4), and the other half carried a nonfalling tone (i.e., high level, high rising, or low dipping).
Figure 1.

Examples of the Chinese characters used in the present study. A: Visually simple Chinese single‐character with an up‐down configuration with low stroke numbers. The sample character is pronounced /ma/ with the forth tone; B: Visually complex Chinese single‐character with left‐right configuration with high‐stroke numbers. The sample character is pronounced /zui/ with the third tone.
The experimental design consisted of a tone judgment task (on visually complex and visually simple characters, respectively) and an arrow direction judgment task as baseline. In the tone judgment task, participants were required to judge whether or not a visually exposed character was pronounced as the falling tone (i.e., tone 4). In each trial, a character was exposed in the centre of the screen for 1,000 ms, followed by a 1,000 ms blank screen display. Participants responded by pressing buttons with their right hand to indicate whether the exposed characters carried the forth tone. They were asked to perform the task as accurately and quickly as possible. The arrow direction judgment task involved a 1,000 ms arrow display and a 1,000 ms blank screen in each trial, and the arrows either pointed upward or downward. Participants were required to distinguish the direction of the displayed arrow by pressing the corresponding key with their right index finger and thumb. Task instruction was presented for 2 s before each block. Prior to scanning, all participants were given practice trials to familiarize with task procedures and to make button presses with one hand.
The experiment was conducted with one single run. It began with a 6‐s fixation crosshair, followed by a 2‐s task instruction and a block of six baseline trials. Then the tone judgment tasks (24 s) with task instructions (2 s) were alternated with baseline blocks. The experiment contained four blocks for visually simple characters, four blocks for visually complex characters, and eight blocks of the control task. In each experimental block, 12 characters were visually presented in succession.
Image acquisition
Whole‐brain fMRI data was acquired on a 3T Siemens scanner at Beijing 306 hospital using a T2*‐weighted‐single‐shot gradient echo‐planar images sequence (TE = 30 ms, TR = 2 s, flip angle = 90°, field of view = 21 cm, slice thickness = 4 mm, and image matrix = 64 × 64). Thirty axial slices were collected. Visual stimuli were presented through a projector onto a translucent screen. Participants were immobilized to avoid head movement during the scan.
Image Analysis
The fMRI data analysis was performed with MATLAB software (Version 7.10; Mathworks, Natick, MA) and SPM8 (Wellcome Department of Cognitive Neurology University College London, London). After data conversion and reconstruction, participants' data were preprocessed in batch mode one by one. The first three volumes of each participant's scan were discarded, and the remaining functional images were slice‐time corrected (in ascending order, with reference slice 16), realigned and unwarped. The data were then spatially normalized to Montreal Neurological Institute (MNI) stereotaxic template, resampled into 2 × 2 × 2 mm cubic voxels and spatially smoothed with a full‐width half‐maximum (FWHM) isotropic Gaussian kernel of 8 mm. Participants' data were high‐pass filtered at 128 s. Initial analysis was conducted on an individual subject basis, and the activation t‐map for each subject was generated using the general linear model. Group analysis was done by obtaining contrast images at second‐level random‐effects model. Activation patterns were evaluated by the tone decision on complex characters > baseline and the tone decision on simple characters > baseline contrasts with a one‐sample t‐test (P < 0.05 false discovery rate (FDR‐) corrected; extent threshold = 10), respectively. Then the tone judgment on all characters > baseline contrast images were computed using a one‐sample t‐test (P < 0.05 FDR‐corrected; extent threshold = 10). Another one‐sample t‐test was performed (P < 0.001 uncorrected; extent threshold = 10) to examine the difference in brain activation between high‐stroke and low‐stroke character conditions.
Results and Discussion
Behavioral Performance
Participants were highly accurate in all conditions. It took 477 ms (SD = 52) to perform the arrow direction judgment, with an accuracy of 99.8% (SD = 4.5). Mean accuracy was 90% (SD = 0.07) for tone judgment of visually complex characters and 93% (SD = 0.05) for tone judgment for visually simple characters. Paired t‐tests indicated higher accuracy for simpler characters than more complex characters [t(16)=−2.76, P = 0.014]. After excluding trials where the participants pressed the wrong key, the average reaction time was 827 ms (SD = 129) for the high‐stroke condition and 790 ms (SD = 101) for the low‐stroke condition and the difference was significant [t(16) = 3.11, P = 0.007].
Imaging Results
Our experiment aimed to examine the neural correlates mediating lexical tone perception in reading visual characters. Images of group maps (17 subjects) for character tone reading relative to arrow direction judgment baseline are shown in Figure 2. Significant brain activations are listed in Table 1, encompassing brain regions in frontal lobes, parietal lobes, temporal lobes, and the visual systems bilaterally. Chinese tone judgment yielded greater activity in bilateral insula and visual systems. Activation in the inferior frontal gyrus (BA 45; x = −43, y = 31, z = 15; BA46; x = 27, y = 30, z = 19) and the middle frontal gyrus (BA9; x = −43, y = 8, z = 32; BA10; x = −25, y = −1, z = 56; BA46; x = −45, y = 30, z = 26; x = 34, y = 35, z = 23) occurred bilaterally but was much stronger in the left hemisphere. The left medial frontal gyrus (BA 6; x = −4, y = 8, z = 49; x = −15, y = −11, z = 64), left superior frontal gyrus (BA 6; x = −11, y = 3, z = 63; x = −24, y = −1, z = 58), left inferior parietal lobule (BA 40; x = −43, y = −40, z = 38), left inferior temporal gyrus (BA37; x = −36, y = −58, z = −9; BA19; x = −29, y = −67, z = 0), left middle/medial temporal gyrus (BA19; x = −24, y = −67, z = 21), right fusiform gyrus (BA 18; x = 29, y = −78, z = −13), and cerebellum also contributed to lexical tone discrimination. Subcortical activations were observed in hippocampus, caudate nucleus and putamen.
Figure 2.
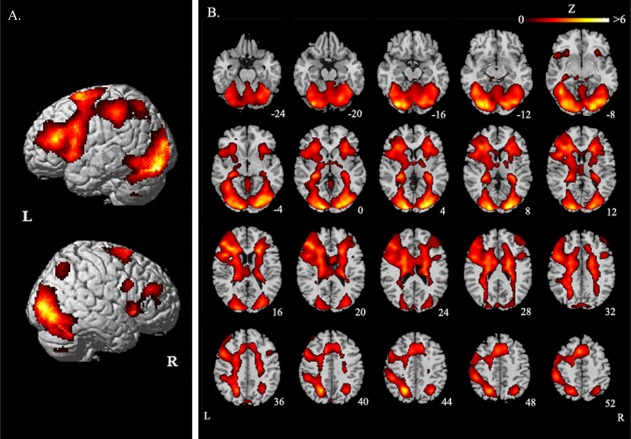
Brain regions with significant activity in tone reading of all characters. A: Lateral view; B: Axial sections. The significant threshold is P < 0.05 FDR‐corrected. The functional maps (in color) are overlaid on the corresponding T1 images (in gray scale). Planes are axial sections, labeled with the height (mm) relative to the bicommisural line. L = the left hemisphere and R = the right hemisphere.
Table 1.
Coordinates of activation peaks
| Regions activated | BA | Z score | x | y | z |
|---|---|---|---|---|---|
| Tone reading of all characters—baseline | |||||
| Occipital | |||||
| L lingual gyrus | 18 | 6.29 | −15 | −95 | −3 |
| R lingual gyrus | 17 | 5.75 | 17 | −91 | −12 |
| R precuneus | 19 | 4.69 | 7 | −65 | 35 |
| R calcarine fissure | 18 | 6.24 | 20 | −93 | 0 |
| R fusiform gyrus | 18 | 5.6 | 29 | −79 | −13 |
| L superior occipital gyrus | 19 | 5.78 | −20 | −63 | 33 |
| Frontal | |||||
| L inferior frontal gyrus | 45 | 5.56 | −43 | 32 | 15 |
| R inferior frontal gyrus | 46 | 3.05 | 27 | 30 | 19 |
| L middle frontal gyrus | 9 | 4.47 | −43 | 8 | 32 |
| 6 | 4.45 | −25 | −1 | 56 | |
| 46 | 4.07 | −45 | 30 | 26 | |
| 6 | 3.04 | −40 | 3 | 49 | |
| R middle frontal gyrus | 46 | 2.85 | 34 | 35 | 23 |
| 6 | 2.56 | 26 | 4 | 51 | |
| 6 | 2.54 | 33 | 10 | 53 | |
| L medial frontal gyrus | 6 | 5.29 | −4 | 8 | 49 |
| 6 | 3.92 | −15 | −11 | 64 | |
| L superior frontal gyrus | 6 | 5.26 | −11 | 3 | 63 |
| 6 | 4.67 | −24 | −1 | 58 | |
| L insula | – | 9.3 | −29 | 20 | 13 |
| – | 5.68 | −32 | 16 | 2 | |
| R insula | – | 3.65 | 29 | 12 | 2 |
| – | 3.53 | 34 | 14 | 0 | |
| Parietal | |||||
| L inferior parietal lobule | 40 | 4.83 | −43 | −40 | 38 |
| Temporal | |||||
| L inferior temporal gyrus | 37 | 8.88 | −36 | −58 | −9 |
| 37 | 4.24 | −29 | −67 | 0 | |
| L middle/medial temporal gyrus | 19 | 4.17 | −24 | −67 | 21 |
| Other areas | |||||
| L hippocampus | – | 8.75 | −31 | −34 | −4 |
| – | 8.07 | −27 | −38 | 1 | |
| R hippocampus | – | 7.63 | 27 | −40 | 3 |
| L cerebellum | – | 6.22 | −29 | −83 | −20 |
| – | 5.68 | −17 | −87 | −19 | |
| R cerebellum | – | 4.84 | −17 | −87 | −19 |
| L putamen | – | 3.53 | −22 | 1 | 5 |
| – | 3.34 | −25 | 2 | 2 | |
| R putamen | – | 4.07 | 26 | 12 | −1 |
| – | 20 | 12 | 0 | ||
| L caudate nucleus | – | 3.91 | −17 | −29 | 14 |
| – | 3.63 | −13 | −5 | 19 | |
| – | 3.36 | −11 | 18 | 9 | |
| R caudate nucleus | – | 5.23 | 15 | −13 | 22 |
| – | 3.51 | 13 | −3 | 19 | |
| – | 3.38 | 12 | 18 | 9 | |
Stereotaxic coordinates (mm) are derived from the human brain atlas of Talairach and Tournoux [1988] and refer to the peak Z scores for each region (P < 0.05 FDR corrected).
The aforementioned patterns of activation are confirmed both in our analysis of the tone judgments of more complex characters contrasted with the baseline, and in our analysis of the tone judgment of simpler characters contrasted with the baseline (Fig. 3A,B).
Figure 3.
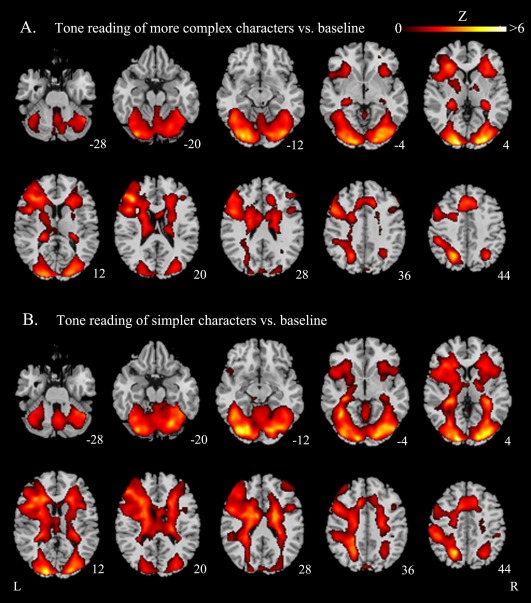
Functional maps: averaged brain activation evoked by tone judgment on Chinese characters. A: Tone reading of visually complex characters relative to baseline; B: Tone reading of visually simple characters relative to baseline. The significant thresholds are P < 0.05 FDR‐corrected for both comparisons. All functional maps (in color) are overlaid on the corresponding T1images (in gray scale). Planes are axial sections, labeled with the height (mm) relative to the bicommisural line. L = left hemisphere and R = right hemisphere.
To determine whether there is a significant difference between the lexical tone processing of visually complex and visually simpler characters, we subtracted brain activation evoked by tone reading of simpler characters from tone reading of more complex characters (P < 0.001 uncorrected). We did not find any significant difference, indicating that brain activations provoked by the orthography‐to‐tone mapping remained stable, though the number of strokes the characters possessed varied.
To further investigate whether or not the activations in the temporal cortex revealed in the above, specifically, the left inferior temporal gyrus (BA37; x = −36, y = −58, z = −9) and the left posterior middle/medial temporal gyrus (BA19; x = −24, y = −67, z = 21), were consistent with those found in previous studies of auditory lexical tone perception, we performed a meta‐analysis to combine results across published experiments using activation likelihood estimation (ALE).
EXPERIMENT 2
Materials and Methods
Literature Selection
There were 14 neuroimaging studies of processing of lexical tones with the spoken language. Among these studies, 11 used an auditory lexical tone perception task [Gandour et al., 1998, 2000, 2002, 2003; Hsieh et al., 2001; Klein et al., 2001; Li et al., 2003, 2010; Wang et al., 2003; Wong et al., 2004; Xu et al., 2006], one used Mandarin tone and vowel production task [Liu et al., 2006], and two measured and contrasted subjects' structural brain volume [Crinion et al., 2009; Wong et al., 2008]. Three selection criteria were included: (1) subjects involved normal and healthy subjects; (2) lexical tone related tasks were used in the studies; (3) significant cortical activation was found in the temporal cortex of the left hemisphere.
According to the mentioned criteria, a set of eight studies with Mandarin and Thai tones was selected for the analysis: four used an explicit tone perception task [Gandour et al., 2003; Klein et al., 2001; Wong et al., 2004; Xu et al., 2006], one used Mandarin tone and vowel production [Liu et al., 2006], two used Mandarin tones training [Wang et al., 2003; Wong et al., 2008], and one measured subjects' structural brain differences [Crinion et al., 2009]. Wong et al. [2008] was excluded from the analysis because three‐dimensional coordinates (x, y, z) were not reported. We entered the data of the rest of the seven mentioned studies (for details, please see Table 2). For these seven studies, four utilized fMRI, two utilized positron emission tomography, and one utilized MRI to acquire brain images. The selected studies for the meta‐analysis had different baseline conditions, however, the experimental tasks or structural images of these studies aided to determine the neural mechanisms subserving auditory lexical tone processing in the temporal cortex of the left hemisphere.
Table 2.
Neuroimaging studies selected for the meta‐analysis
| Literature | Language | n | Scanner | Task | Baseline |
|---|---|---|---|---|---|
| [Klein et al., 2001] | Mandarin | 24 | PET | Tonal discrimination | Silent baseline |
| [Gandour et al., 2003] | Mandarin | 20 | 1.5T | Tonal discrimination | Passive listening |
| [Wang et al., 2003] | Mandarin | 6 | 1.5T | Mandarin tone identification | visual, auditory and motor control tasks |
| [Wong et al., 2004] | Mandarin, English | 14 | PET | Mandarin tone discrimination; English pitch discrimination | Passive Mandarin listening; passive English listening; rest |
| [Xu et al., 2006] | Mandarin, Thai | 20 | 1.5T | Tonal discrimination | Passive listening |
| [Liu et al., 2006] | Mandarin | 10 | 2T MRI | Mandarin pinyin‐naming; Mandarin character‐naming | Fixation |
| [Crinion et al., 2009] | Mandarin, English | 59 | 1.5T MRI | – | – |
n = number of subjects.
Activation Likelihood Estimation
We analyzed all studies which met the inclusion criteria. All MNI coordinates were converted into Talairach space using the icbm2tal transform [Lancaster et al., 2007] implemented in GingerALE software package [Eickhoff et al., 2009, 2011]. ALE maps were generated by the ALE method [Turkeltaub et al., 2002; Laird et al., 2005], using a FWHM of 10 mm. The test was corrected for multiple comparisons using the FDR method with a threshold at P < 0.05 corrected. ALE maps were computed for studies with auditory lexical tone processing. For detailed procedures of using ALE, please refer to Laird et al. [2005].
RESULTS AND DISCUSSION
Table 3 and Figure 4 illustrated the results of our ALE meta‐analysis of the selected literature on auditory lexical tone processing. Two clusters of activation likelihood were identified, one in the left STG (BA42; x = −58, y = −26, z = 12) and the other in the left putamen (x = −32, y = −16, z = 2), with cluster sizes of 288 and 224 mm3, respectively.
Table 3.
ALE Meta‐Analysis of auditory lexical tone processing in left temporal cortex
| Anatomical region | BA | Coordinate (x,y,z) | ALE (10−2) | Vol. (mm3) | ||
|---|---|---|---|---|---|---|
| L superior temporal gyrus | 42 | −58 | −26 | 12 | 1.04 | 288 |
| L putamen | – | −32 | −16 | 2 | 0.98 | 224 |
BA, Brodmann area; L, left.
Figure 4.
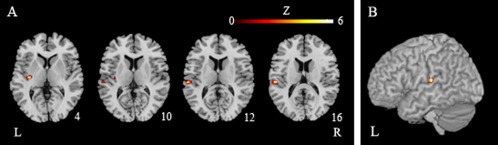
ALE maps of auditory lexical tone processing (P < 0.05, corrected). A: Axial sections; B: Lateral view. The functional maps (in color) are overlaid on the corresponding T1 images (in gray scale). Planes are axial sections, labeled with the height (mm) relative to the bicommisural line. L = the left hemisphere and R = the right hemisphere.
This meta‐analysis indicates that the left STG at BA 42 is responsible for lexical tone processing in the spoken language. This region, however, is spatially different from the two temporal cortex regions we identified in Experiment 1 (i.e., the left inferior temporal gyrus, BA37, and the left posterior middle/medial temporal gyrus, BA19).
GENERAL DISCUSSION
This is the first fMRI study to examine the neural substrates underlying lexical tone perception during silent reading of printed words. Several important findings have been demonstrated. First, a number of brain areas associated with tone perception in spoken language, such as the fronto‐parietal network and the putamen [Gandour et al., 2000, 2003; Hsieh et al., 2001; Klein et al., 2001; Li et al., 2010; Wong et al., 2004; see Zatorre and Gandour, 2008], are demonstrated to be involved in lexical tone reading. Both the left and the right hemispheric regions are engaged, though the left hemispheric activations are much stronger in processing tones of Chinese single characters.
Most importantly, we found that the left STG, a region critical for speech and nonspeech pitch processing widely demonstrated in auditory research [Gandour and Dardarananda, 1983; Gandour, 1992; Kadyamusuma et al., 2011; Liang and van Heuven, 2004; Binder et al., 2000; Price et al., 2005; Zatorre et al., 2002; Gandour et al., 2004; Klein et al., 2001; Luo et al., 2006; Liu et al., 2006; Wang et al., 2003; Wong et al., 2004, 2008; Xu et al., 2006; Zhang et al., 2011], was not involved in lexical tone reading in our experiment. The left posterior middle/medial temporal gyrus at BA 19 was indeed activated in our study, but it is close to the fusiform gyrus and is spatially different from the STG mediating auditory tone perception. The STG is involved in the initial processing of auditory stimuli [Hickok and Poeppel, 2007; Klein et al., 2001] and thus, its activity is assumed to be input‐driven. This reveals that speech tone perception depends on neural mechanisms that are specific to the speech domain. Our results of the tone processing in the visual domain lend support to this input‐dependent hypothesis [Gandour et al., 2000; Liberman and Mattingly, 1989]. Cortical regions critical for lexical tone processing seem to be modality‐specific.
In this study, we did not find activation in the right STG, which has been repeatedly shown to be critical to auditory pitch perception, vocal pitch error detection, and voice control in previous literature (Johnsrude et al., 2000; Zatorre and Berlin, 2001; Robin et al., 1990; Flagmeier et al., 2014; Parkinson et al., 2013). Thus, although both left and right STG are important to pitch modulation and sensory control of vocalization in humans, neither of them is related to lexical tone processing in reading. In fact, even though the posterior middle/medial temporal cortex is relevant to tone reading, its activation is left‐lateralized in our study.
In summary, our findings suggest that the neural circuitry subserving lexical tone processing is differentially engaged depending on the input modality. Further research is needed to directly compare the neural specialization of lexical pitch in auditory and visual domains.
REFERENCES
- Binder J, Frost J, Hammeke T, Bellgowan P, Springer J, Kaufman J, Possing J (2000): Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex 10:512–528. [DOI] [PubMed] [Google Scholar]
- Chang HC, Lee HJ, Tzeng OJL, Kuo WJ (2014): Implicit target substitution and sequencing for lexical tone production in Chinese: An fMRI study. PLoS One 9:e83126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinion JT, Green DW, Chung R, Ali N, Grogan A, Price GR, Mechelli A, Price CJ (2009): Neuroanatomical markers of speaker Chinese. Human Brain Mapp 30:4108–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT (2009): Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: A random‐effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapp 30:2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Roski C, Caspers S, Zilles K, Fox PT (2011): Co‐activation patterns distinguish cortical modules, their connectivity and functional differentiation. Neuroimage 57:938–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagmeier SG, Ray KL, Parkinson AL, Li K, Vargas R, Price LR, Laird AR, Larson CR, Robin DA (2014): The neural changes in connectivity of the voice network during voice pitch perturbation. Brain Lang 132:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandour J (1992): Lexical tones in Thai after unilateral brain damage. Brain Lang 43:275–307. [DOI] [PubMed] [Google Scholar]
- Gandour J, Dardarananda R (1983): Identification of tonal contrasts in Thai aphasic patients. Brain Lang 18:98–114. [DOI] [PubMed] [Google Scholar]
- Gandour J, Wong D, Hutchins G (1998): Pitch processing in the human brain is influenced by language experience. Neuroreport 9:2115–2119. [DOI] [PubMed] [Google Scholar]
- Gandour J, Wong D, Hsieh L, Weinzapfel B, Van Lancker D, Hutchins GD (2000): A crosslinguistic PET study of tone perception. J Cogn Neurosci 12:207–222. [DOI] [PubMed] [Google Scholar]
- Gandour J, Wong D, Talavage T, Dzemidzic M, Satthamnuwong N, Tong Y (2002): A cross linguistic fMRI study of spectral and temporal cues underlying phonological processing. J Cogn Neurosci 14:1076–1087. [DOI] [PubMed] [Google Scholar]
- Gandour J, Xu Y, Wong D, Dzemidzic M, Lowe M, Li X, Tong Y (2003): Neural correlates of segmental and tonal information in speech perception. Human Brain Mapp 20:185–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandour J, Tong Y, Wong D, Talavage T, Dzemidzic M, Xu Y, Li X, Lowe M (2004): Hemispheric roles in the perception of speech prosody. Neuroimage 23:344–357. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D (2007): The cortical organization of speech processing. Nat Rev Neurosci 8:393–402. [DOI] [PubMed] [Google Scholar]
- Hsieh L, Gandour J, Wong D, Hutchins GD (2001): Functional heterogeneity of inferior frontal gyrus is shaped by linguistic experience. Brain Lang 76:227–252. [DOI] [PubMed] [Google Scholar]
- Johnsrude IS, Penhune VB, Zatorre RJ (2000): Funtional specificity in the right human auditory cortex for perceiving pitch direction. Brain: J Neurol 123:155–163. [DOI] [PubMed] [Google Scholar]
- Kadyamusuma, MR , Bleser RD, Mayer J (2011): Lexical tone disruption in Shona after brain damage. Aphasiology 25:1239–1260. [Google Scholar]
- Klein D, Zatorre RJ, Milner B, Zhao V (2001): A cross‐linguistic PET study of tone perception in Mandarin Chinese and English speakers. Neuroimage 13:646–653. [DOI] [PubMed] [Google Scholar]
- Laird AR, McMillan KM, Lancaster JL, Kochunov P, Turkeltaub PE, Pardo JV, Fox PT (2005): A comparison of label‐based meta‐analysis and activation likelihood estimation in the Stroop task. Human Brain Mapp 18:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas‐Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT (2007): Bias between MNI and talairach coordinates analyzed using the ICBM‐152 brain template. Human Brain Mapp 28:1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Heuven V. Jv (2004): Evidence for separate tonal and segmental tiers in the lexical specification of words: A case study of a brain‐damaged Chinese speaker. Brain Lang 91:282–293. [DOI] [PubMed] [Google Scholar]
- Liberman AM, Mattingly IG (1989): A specialization for speech perception. Science 243:489–494. [DOI] [PubMed] [Google Scholar]
- Li X, Gandour J, Talavage T, Wong D, Dzemidzic M, Lowe M, Tong Y (2003): Selective attention to lexical tones recruits left dorsal frontoparietal network. Neuroreport 14:2263–2266. [DOI] [PubMed] [Google Scholar]
- Li X, Gandour JT, Talavage T, Wong D, Hoffa A, Lowe M, Dzemidzic M (2010): Hemispheric asymmetries in phonological processing of tones versus segmental units. Neuroreport 21:690–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Peng D, Ding G, Jin Z, Zhang L, Li K, Chen C (2006): Dissociation in the neural basis underlying Chinese tone and vowel production. Neuroimage 29:515–523. [DOI] [PubMed] [Google Scholar]
- Luo H, Ni J, Li Z, Li X, Zhang D, Zeng G, Chen L (2006): Opposite patterns of hemisphere dominance for early auditory processing of lexical tones and consonants. Proc Natl Acad Sci USA 103:19558–19563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson AL, Korzyukov O, Larson CR, Litvak V, Robin DA (2013): Modulation of effective connectivity during vocalization with perturbed auditory feedback. Neuropsychologia 51:1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C, Thierry G, Griffiths T (2005): Speech‐specific auditory processing: Where is it? Trend Cogn Sci 9:271–276. [DOI] [PubMed] [Google Scholar]
- Robin DA, Tranel D, Damasio H (1990): Auditory perception of temporal and spectral events in patients with focal left and right cerebral lesions. Brain Lang 39:539–555. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar Stereotactic Atlas of the Human Brain. New York: Springer‐Verlag. [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA (2002): Meta‐analysis of the functional neuroanatomy of single‐word reading: method and validation. Neuroimage 16:765–780. [DOI] [PubMed] [Google Scholar]
- Wang Y, Sereno JA, Jongman A, Hirsch J (2003): fMRI evidence for cortical modification during learning of Mandarin lexical tone. J Cogn Neurosci 15:1019–1027. [DOI] [PubMed] [Google Scholar]
- Wong PCM, Parsons LM, Martinez M, Diehl RL (2004): The role of the insular cortex in pitch pattern perception: The effect of linguistic contexts. J Neurosci 24:9153–9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PCM, Warrier CM, Penhune VB, Roy AK, Sadehh A, Parrish TB, Zatorre RJ (2008): Volume of left Heschl's gyrus and linguistic pitch learning. Cereb Cortex 18:828–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi J, Zhang L, Shu H, Zhang Y, Li P (2010): Categorical perception of lexical tones in Chinese revealed by mismatch negativity. Neuroscience 170:223–231. [DOI] [PubMed] [Google Scholar]
- Xu Y, Gandour J, Talavage T, Wong D, Dzemidzic M, Tong Y, Li X, Lowe M (2006): Activation of the left planum temporale in pitch processing is shaped by language experience. Human Brain Mapp 27:173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip M (2002): Tone. Cambridge: CUP. [Google Scholar]
- Zatorre RJ, Belin P (2001): Spectral and temporal processing in human auditory cortex. Cereb Cortex 11:946–953. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Gandour J (2008): Neural specializations for speech and pitch. moving beyond the dichotomies. Philos Trans R Soc Lond B Biol Sci 363:1087–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P, Penhune VB (2002): Structure and function of auditory cortex: Music and speech. Trends Cogn Sci 6:37–46. [DOI] [PubMed] [Google Scholar]
- Zhang L, Xi J, Xu G, Shu H, Wang X, Li P (2011): Cortical dynamics of acoustic and phonological processing in speech perception. PLoS One 6:e20963. [DOI] [PMC free article] [PubMed] [Google Scholar]


