Abstract
A recent fMRI-study revealed neural responses for affective processing of stimuli for which overt attention irrespective of stimulus valence was required in the orbitofrontal cortex (OFC) and bilateral amygdala (AMY): activation decreased with increasing cognitive demand. To further characterize the network putatively related to this attenuation, we here characterized these regions with respect to their functional properties and connectivity patterns in task-dependent and task-independent states. All experiments of the BrainMap database activating the seed regions OFC and bilateral AMY were identified. Their functional characteristics were quantitatively inferred using the behavioral meta-data of the retrieved experiments. Task-dependent functional connectivity was characterized by meta-analytic connectivity modeling (MACM) of significant co-activations with these seed regions. Task-independent resting-state functional connectivity analysis in a sample of 100 healthy subjects complemented these analyses. All three seed regions co-activated with subgenual cingulum (SGC), precuneus (PCu) and nucleus accumbens (NAcc) in the task-dependent MACM analysis. Task-independent resting-state connectivity revealed significant coupling of the seeds only with the SGC, but not the PCu and the NAcc. The former region (SGC) moreover was shown to feature significant resting-state connectivity with all other regions implicated in the network connected to regions where emotional processing may be modulated by a cognitive distractor. Based on its functional profile and connectivity pattern, we suggest that the SGC might serve as a key hub in the identified network, as such linking autobiographic information [PCu], reward [NAcc], (reinforce) values [OFC] and emotional significance [AMY]. Such a role, in turn, may allow the SGC to influence the OFC and AMY to modulate affective processing.
Keywords: Cognition, Emotion, Meta-analytic connectivity-modeling, Resting-state, Subgenual cingulum cortex
Introduction
It has been shown that cognitive distraction can influence affective processing and emotional states by relocation of attentional resources (Blair et al., 2007; Erk et al., 2007; Pessoa et al., 2002; van Dillen and Koole, 2007). In a previous study (Kellermann et al., 2012) examining the modulation of affective processing by concurrent cognitive demand, we found a significant “stimulus valence” (neutral vs. emotional)× “cognitive load” interaction in three regions, namely the bilateral amygdala (laterobasal [LB] and superficial [SF] parts, Amunts et al., 2005), as well as the medial orbitofrontal cortex (OFC). These regions were significantly active during implicit appraisal of emotional stimuli, and this activation was significantly attenuated by the performance of a more challenging working memory task. It is furthermore important to mention that these regions as well as the attenuation effect are not affect-specific, as we found the same interaction effect between “stimulus valence” (emotional vs. neutral) and “cognitive demand” for processing of positive as well as negative emotional stimuli. It was therefore conjectured that these regions are involved in processing affective stimuli independent of their valence and task-relevance, but are at the same time susceptible to attenuation under increased cognitive load. The functional connectivity of these regions and hence their interaction in larger networks, however, remain elusive.
Appraisal of emotional stimuli represents a fundamental feature of affective processing, which is triggered automatically and implicitly by the presentation of emotional stimuli (Grandjean and Scherer, 2008; Grandjean et al., 2008; Scherer, 2001), and has been linked to the assessment of self-relevance (Ellsworth and Scherer, 2003; Sander et al., 2005; Scherer, 2001). This raises the question, whether the regions detailed above interact with the regions involved in self-referential processing. Some indications to this end have in particular been provided for the OFC, which plays a critical role in the representation of reward value (Rolls, 1999) and the individual experiences of pleasantness and unpleasantness (Francis et al., 1999), as well as being discussed to code values or personal (experience based) preferences (Kiebel et al., 2008).
Concepts of amygdala functions have, in turn, evolved from being responsible for indicating threats to a more general perspective with relevance for identification of affective or social salience of stimuli (Adolphs et al., 2002; Phillips et al., 2003; Simmons et al., 2004). The latter concept is supported by amygdala responses to non-aversive stimuli (Ball et al., 2007; Hamann et al., 1999; Taylor et al., 2000) and to tasks requiring social evaluations (Bzdok et al., 2011; Engell et al., 2007; Todorov et al., 2011). Importantly, however, such salience detection may be modulated by distraction or voluntary relocation of attentional resources and hence the, yet elusive, interaction with other cognitive and affective brain regions (Blair et al., 2007; Erk et al., 2007; Kellermann et al., 2012; Pessoa et al., 2002; van Dillen and Koole, 2007).
To better understand the function and connectivity of the three affective regions identified in the previous study as susceptible to cognitive modulation we here further characterized these functionally defined regions using a database driven approach. In particular, our aims were to i) identify regions interacting with the functionally defined seed regions by assessing their task-dependent and task-independent functional connectivity, ii) characterize the functional properties of all implicated regions by quantitative functional inference and iii) probe the potential existence of a central node within this network. To achieve these aims, we used the regions showing the abovementioned interaction-\effect (bilateral AMY and OFC) as seeds for querying the BrainMap database and hereby identifying published neuroimaging studies which feature activation in the respective regions. Reference to this large scale database enables both a quantitative functional inference, i.e., assessment of response characteristics, as well as the delineation of closely interacting (significantly co-activated) regions by meta-analytic connectivity modeling (MACM). Additionally, we performed resting-state functional connectivity analysis in a large sample of healthy subjects to complement the analysis using an independent modality. This seed-based approach thus allows investigation of neuronal networks functionally connected with the seeds independently of the current state (task-dependent – MACM/ task-independent – resting-state). Importantly, our analyses were constrained a priori to regions involved in affective processing. Rather, the behavioral context of our work is set by the experimental contrast used to define the seeds (“stimulus valence”×”cognitive load” interaction) and the analyses then characterized the response-profiles and connectivity of these regions.
We would like to emphasize that the employed approach is aimed at identifying regions showing functional connectivity, i.e., interactions, with the seeds, not regions that may show similar functions. Based on previous fMRI results we thus aimed at mapping regions functionally connected to the three regions in which we found emotional processing to be attenuated by cognitive demand. This is a distinctly different aim from identifying (other) regions in the brain in which affective processing may be modulated through this or other mechanisms, which would require, e.g., a meta-analysis over experiments probing such processes. Analyzing task-dependent and task-independent functional connectivity in turn reveals closely interacting brain regions and hence provides insight into the networks the seed regions are involved in. In this context, it is important to emphasize the aims and limitations of functional connectivity analysis. While these approaches allow the delineation of networks by mapping areas interacting with a seed during the performance of structured tasks (MACM) or in a task-free state of unconstrained cognition (resting-state), they do not allow any causal inference on these interactions. That is, functional connectivity analysis and behavioral profiling as in the present study provide new information on which regions interact with the seeds and what their functions are. But conclusions drawn from this information must be carefully distinguished from direct inference on the causality or context-dependency of the respective interactions. Moreover, it is necessary to point out that functional connections do not necessarily imply a direct, i.e., mono-synaptic, anatomical connection between the involved regions, as functional connectivity may be mediated, through indirect connections. Consequently, the present work is aimed at delineating networks the seeds interact with and characterizing the functions the respective areas are involved in without the notion that these may exert direct, causal influence, e.g., in the context of affect-modulation. In doing so, however, it may serve as a proof-of-principle demonstration how emerging database driven methods may complement and extend neuroimaging results by quantitative behavioral inference and information on their connectivity in order to arrive at a more complete characterization of fMRI results and associated networks.
Materials and methods
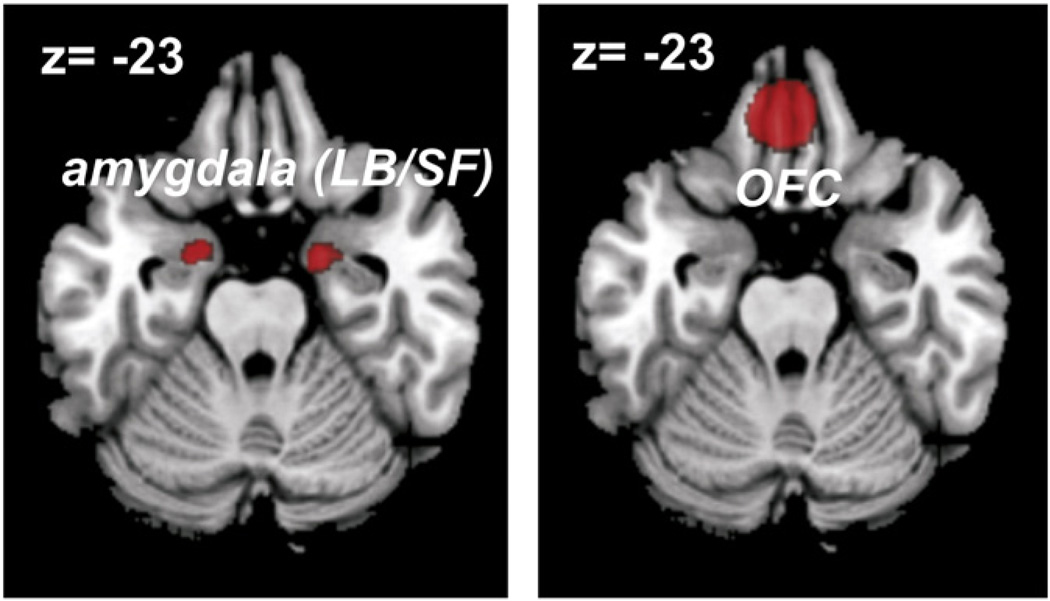
Functional connectivity was assessed for three regions obtained from the previous fMRI-study (Kellermann et al., 2012), which indicated that emotional processing in the (laterobasal [LB] and superficial [SF]) amygdala bilaterally as well as the medial orbitofrontal cortex (OFC) was significantly attenuated by the concurrent performance of a more challenging working memory task. Image VOIs were used for determining the seed regions (Fig. 1), defining them by binarizing the contrast images of the “stimulus valence”×”cognitive load” interaction which represented the significant voxels of this contrast.
Fig. 1.
Seed regions for the functional connectivity analysis. Affective regions activated by the modulation of concurrent cognitive demand (stimulus valence×cognitive load interaction) as revealed in a recent study (Kellermann et al., 2012).
The analysis itself consisted of three aspects: I) Task-based functional connectivity was delineated using meta-analytic connectivity modeling (MACM) on functional neuroimaging experiments of the BrainMap database to identify brain regions that co-activate above chance with the seeds (see Eickhoff et al., 2010; Jakobs et al., 2012; Reetz et al., 2012; Robinson et al., 2012; for example using the same approach and database as in the present paper; cf. Cauda et al., 2011a as well as Koski and Paus, 2000 and Postuma and Dagher, 2006 for alternative implementations of the same concept). II) Functional connectivity in a task-free state was assessed by resting-state correlations, i.e., identification of brain regions showing endogenous activity changes correlated to the time-series of the seeds. III) Functional properties of the seeds and functionally connected areas were delineated by examining the meta-data characteristics of the identified neuroimaging studies of the BrainMap database.
Task-based functional connectivity analysis
The key idea behind meta-analytic connectivity modeling (MACM) is to assess which brain regions are co-activated above chance with a particular seed region in functional neuroimaging experiments (Eickhoff et al., 2010; Laird et al., 2009a). In particular, MACM entails to first identify all experiments in a database that activate a particular brain region and then test for convergence across (all) foci reported in these experiments. Obviously, as experiments were selected by activation in the seeds, highest convergence will be observed in the seed region. Significant convergence of the reported foci in other brain regions, however, indicates consistent co-activation, i.e., functional connectivity with the seed. It should be noted that MACM as a data-driven approach characterizes the co-activations of a given region of interest – independent of how this seed has been defined. That is, while MACM has most commonly been used to investigate the functional connectivity of anatomically defined seed regions (Cauda et al., 2011b; Eickhoff et al., 2010; Robinson et al., 2010), brain regions defined by functional properties as in the current study, may likewise be assessed (e.g. Jakobs et al., 2012).
MACM thus takes advantage of the high standardization in the publication of neuroimaging data, e.g., the ubiquitous adherence to standard coordinate systems and the emergence of large-scale databases which store this information, allowing to establish the convergence of information across studies (Laird et al., 2011a). In particular, anatomical variability due to differences in the size and shape of the brain is removed by spatial normalization algorithms (as implemented, e.g., in FSL, SPM, AFNI or BrainVoyager) in virtually all neuroimaging studies. This ensures that the data for every subject and every cohort is spatially normalized into the same standard brain space. Reporting the results in tables as local maxima of brain activation or structural findings in this standard space, in turn, enables a comparison across studies (Fox, 1995). For good compatibility of the data additional reporting of the reference brain and normalization software used is important as highlighted by a recent guideline paper for reporting neuroimaging data (Poldrack et al., 2008). Given these widely accepted standards, several different neuroimaging databases have been developed to share data, with the most prominent examples being the NeuroSynth (Yarkoni et al., 2011), the Brede database (Nielsen, 2003), the SuMS database (Dickson et al., 2001), fMRIDC (van Horn et al., 2001) and the BrainMap databases (Fox and Lancaster, 2002; Fox et al., 2005; Laird et al., 2005, 2011b).
In this study we employed the latter database (www.brainmap.org), which currently contains the results of over about 2114 functional neuroimaging publications reporting 79,577 activation locations across 9994 experiments. This database contains all significant peak coordinates for all activation foci that are reported in the respective neuroimaging experiments, irrespective of significance threshold. Only fMRI and positron emission tomography studies that reported functional mapping data from healthy participants were considered for the present study. Those investigating age, gender, disease or drug effects were excluded. No further constraints (e.g., on acquisition and analysis details such as fixed- or random-effects inference, experimental design, or stimulation procedures) were enforced. In order to focus on the conceptually unambiguous interpretation of co-activation as shared recruitment by task demands, the present analysis was focused on activation studies and thus excluded deactivation studies. As the first step of the MACM analysis we identified (separately for each seed) all experiments that featured at least one focus of activation within the respective seed (MNI-space). In order to facilitate such filtering, coordinates from all experiments in the BrainMap database reporting their results in Talairach space were converted into MNI coordinates by using Lancaster transformation (Lancaster et al., 2007). Then, all experiments activating the currently considered seed were identified. The retrieval was solely based on the reported activation coordinates, not on any anatomical or functional label. This revealed all experiments which featured at least one activation in the seed regions (amygdala bilaterally and OFC), irrespective of their label in the original publication. This procedure yielded 122 studies of the left amygdala, 189 studies of the right amygdala and 142 studies of the mOFC. Importantly, this number of studies directly reflects the raw data of the location-based search in the BrainMap database, without additional manipulations. For a detailed list of these raw data of the location-based search, indicating the respective publications and contrasts used in the present study, see Supplementary Table S1.
Subsequently, an ALE meta-analysis was performed on the retrieved experiments for each seed using the revised version (Eickhoff et al., 2009; Laird et al., 2009a) of the activation likelihood estimation (ALE) approach implemented in MATLAB (The MathWorks Inc, USA). The concept behind ALE is to treat the foci reported in the associated experiments not as single points, but as centers for 3D Gaussian probability distributions reflecting the associated spatial uncertainty. The width of distribution depends on the number of subjects in the original experiment and should reflect the uncertainty of the reported spatial location (Eickhoff et al., 2009). It is based on empirical data on the between-subject and between-template variance that represents the main components of this uncertainty. The between-subject variance was weighted by the number of investigated subjects per study. Therefore, larger sample sizes should provide more reliable approximations of the “true” activation effect and be modeled by smaller Gaussian distributions (Eickhoff et al., 2009). In this context, it is important to point out, that the modeled activation maps are not intended to reflect the original activation foci of the included imaging experiments but rather provide a probabilistic representation of the reported coordinates by accommodating the associated spatial uncertainty (Eickhoff et al., 2009; Rehme et al., 2012). For each experiment, the probability distributions of all reported foci are then combined into a modeled activation (MA) map (Turkeltaub et al., 2012).
Taking the union across these yielded voxel-wise ALE scores describing the convergence of results at each particular location of the brain. To distinguish ‘true’ convergence between studies from random convergence, i.e., noise, in the proposed revision of the ALE algorithm (Eickhoff et al., 2012), ALE scores are compared to an empirical null-distribution reflecting a random spatial association between experiments, i.e. focusing on inference on the above-chance convergence between studies, not clustering of foci within a particular study. Such null-distribution may be computed by sampling one voxel from each MA-map (representing distinct experiments) at random and then computing the union of them in the same way as for the spatially corresponding voxels in the computation of the ALE map (Eickhoff et al., 2009). The ensuing value thus represents a realization of an ALE value under the null-distribution of spatial independence, i.e., the assumption that spatial location does not matter and any convergence across experiments is purely by chance. This approach, however, has been observed to be computationally inefficient and has thus been superseded by a revised algorithm in which the MA-value found for a particular experiment is represented in a summary histogram (cf, Eickhoff et al., 2012). This histogram summarizes the likelihood of observing any possible MA-value (including zero) when sampling a voxel at random from the respective MA map of this experiment. To derive the null-distribution of ALE values under spatial independence, the histograms are merged across the different experiments considered in the meta-analysis (Eickhoff et al., 2012; Turkeltaub et al., 2012). This histogram-based analysis reflects exactly the same null-hypothesis as the random drawing, but computes the null-distribution much more efficiently. The ensuing null-distribution reflects the chance of observing any particular ALE value under the assumption of spatial independence between the considered experiments, i.e., their MA maps. The p-value of an observed ALE is then given by the proportion of this null-distribution (precisely, its cumulative density function) corresponding equal or higher ALE values. The ALE maps reflecting the convergence of co-activations with any particular seed region were subsequently thresholded at p<0.05 cluster-level corrected (cluster-forming threshold: p<0.001 at voxel-level) and converted into Z-scores for display.
Functional connectivity analysis of resting-state imaging data
Resting-state fMRI images were acquired in 107 volunteers. Among these, 100 subjects (50 female; mean age of male: 43.8 years; SD: 14.85; mean age of female: 44.4 years; SD: 13.5; overall mean age: 44.11 years, overall SD: 14.05) completed the entire scan-session, could clearly indicate that they had not been asleep, and did not show excessive motion or technical problems in their scans. These were then used for further analysis. Right-hand dominance of the participants was established by means of the Edinburgh handedness inventory (Oldfield, 1971). In each age-category (defined by the decades: 21–30, 31–40, 41–50, 51–60, 61–71) 10 female and 10 male subjects were imaged. The youngest subject was 21 years old; the oldest subject was 71 years old. Subjects were recruited via local advertisement and through a database of pre-registered subjects. Subjects with a history of neurological or psychiatric disorders, as assessed by an in-person screening interview, were excluded prior to scanning. Moreover, all subjects were screened using the SKID inventory (Wittchen et al., 1997) and additionally evaluated with respect to cognitive deficits by the mini-mental status-test (MMST) (Folstein et al., 1975). To evaluate depressive/dysthymic symptoms we furthermore employed the Beck’s depression inventory (BDI) (Hautzinger et al., 2006). Only subjects that were confirmed to be psychiatrically and neurologically healthy and did not take any sort of psychiatric or neurological medication were included in the actual imaging study. All subjects gave written informed consent to the study protocol, which had been approved by the local ethics committee of the University of Bonn, as these subjects were scanned as part of a collaborative effort between the Research Centre Jülich and this university.
Before the imaging session, subjects were instructed to keep their eyes closed and just let their mind wander without thinking of anything in particular but not to fall asleep. This was confirmed by post-scan debriefing, where we explicitly asked every subject if they fell asleep, if they kept their eyes closed, if they had felt comfortable in the scanner and if there were any other problems. All of the 100 subjects included in the final study reported that they stayed awake with their eyes closed and did not feel any discomfort or pain.
Images were acquired on a Siemens Tim Trio 3T whole-body scanner (Erlangen, Germany) at the Research Centre Jülich, Germany. For each subject, 300 resting state EPI images were acquired using blood-oxygen-level-dependent (BOLD) contrast [gradient-echo EPI pulse sequence, TR = 2.2 s, TE = 30 ms, flip angle = 90°, in plane resolution = 3.1 × 3.1 mm, 36 axial slices (3.1 mm thickness) covering the entire brain]. The first four images were discarded to allow for magnetic field saturation, the remaining 296 were then processed using SPM8 (www.fil.ion.ucl.ac.uk/spm). Images were first corrected for head movement by affine registration using a two-pass procedure. The mean EPI image for each subject was then spatially normalized to the MNI single subject template (Holmes et al., 1998) using the “unified segmentation” approach (Ashburner and Friston, 2005) and the ensuing deformation was applied to the individual EPI volumes. Finally, images were smoothed by a 5-mm FWHM Gaussian kernel to meet requirements of normal distribution and compensate for residual anatomical variations.
The time-series data of each voxel in the brain was then processed as follows (cf. Eickhoff et al., 2011; Fox et al., 2009; Weissenbacher et al., 2009; zu Eulenburg et al., 2012): In order to reduce spurious correlations, variance that could be explained by the following nuisance variables was removed from each voxel’s time series (Bellec et al., 2006; Fox and Raichle, 2007): i) The six motion parameters derived from the image realignment. ii) The first derivative of the realignment parameters. iii) Mean grey and white matter as well as CSF signal intensity per time-point as obtained from averaging across the voxels attributed to the respective tissue class in the SPM 8 segmentation. iv) Coherent signal changes across the whole brain as reflected by the first five components of a PCA decomposition of the whole-brain time-series (Behzadi et al., 2007; Chai et al., 2012; Thomas et al., 2002). All of these nuisance variables entered the model as first and all but the PCA components also as second order terms. Even though PCA-denoising has been shown to improve specificity of functional connectivity analyses (Behzadi et al., 2007; Chai et al., 2012), we additionally evaluated whether the obtained results were influenced through this approach by performing another set of analyses on the same data without this approach for comparison. Data were then band pass filtered preserving frequencies between 0.01 and 0.08 Hz, since meaningful resting-state correlations will predominantly be found in these frequencies given that the bold-response acts as a low-pass filter itself (Biswal et al., 1995; Fox and Raichle, 2007; Greicius et al., 2003).
As for the MACM analysis described above, the regions engaged in implicit emotional appraisal and modulated by concurrent cognitive demand provided seed regions of interest. Time-courses were extracted for all voxels within the particular cluster that were located in the grey matter of the individual subject as indicated by a segmentation of the individual EPI image (Ashburner and Friston, 2005). The time course of the entire seed region was hereby expressed as the first eigenvariate of the individual voxels. Linear (Pearson) correlation coefficients between the time series of the seed regions and those of all other grey matter voxels in the brain were computed to quantify resting-state functional connectivity. These voxel-wise correlation coefficients were then transformed into Fisher’s Z-scores and tested for consistency across subjects by a second-level analysis of variance (ANOVA, including appropriate non-sphericity correction). The results of this random-effects analysis were then thresholded at a family wise error (FWE)-corrected level of p<0.05.
In order to assess potential age- and gender effects on the functional connectivity analysis, we performed a supplementary second-level analysis of variance (ANOVA) including age and gender as covariates.
Cross-validation of MACM and resting state
To delineate areas showing functional connectivity with the seed regions in a task-dependent (MACM) as well as in the task-free state (resting-state), we performed a conjunction analysis between the MACM and the resting state analyses. That is, we aimed at identifying voxels that showed significant functional connectivity with the seed in the analysis of interactions in both the task-dependent (measured by co-activation across a broad range of experiments using the BrainMap database) and the task-free state (measured by correlations in ongoing activity in 100 subjects). Practically, this was realized by using the regions showing significant co-activations with the three seed regions (MACM analysis on the BrainMap database) as inference masks for the assessment of resting-state connectivity.
Functional characterization
Functional properties were characterized for the three seed regions (amygdalae and OFC) and the areas functionally connected with them. We first identified for each region those experiments in BrainMap that featured at least one focus of activation within this region as detailed above. After that we tested for “Behavioral Domain” (BD), “Paradigm Class” (PC), and “Stimulus Type” (ST) meta-data categories that were significantly overrepresented. Behavioral domains code the mental processes isolated by the statistical contrasts (Laird et al., 2009a, 2009b, 2011a, 2011b) and comprise the main categories cognition, action, perception, emotion, and interoception, as well as their related sub-categories. Paradigm classes categorize the specific task employed (see http://brainmap.org/scribe/ for the complete BrainMap taxonomy). BD, PC, and ST metadata were studied by determining the frequency of category “hits” relative to its distribution across the entire database. In particular, functional roles of the derived clusters were identified by significant over-representation of BDs and PCs in the experiments activating the respective cluster relative to BrainMap using a binominal test (pb0.05, corrected for multiple comparisons using Bonferroni’s method (cf. Nickl-Jockschat et al., 2011)).
Results
Task-based functional connectivity estimated from BrainMap
Meta-analytic connectivity modeling (MACM) of co-activations was performed on the three regions that showed attenuated implicit affective processing during a more challenging working memory task (Kellermann et al., 2012). These ‘affective’ regions that are susceptible to modulation by concurrent cognitive load and represent the seeds for the functional connectivity analysis were the amygdala bilaterally (right amygdala: peak MNI coordinates: −18/−2/−26, cluster-size: 349 voxel; left amygdala: peak MNI coordinate: 26/−3/−17, cluster-size: 89 voxel) and the OFC (peak MNI coordinates: −5/39/−23, cluster-size: 1099 voxel). In reference to cytoarchitectonic maps of the human orbitofrontal cortex, the work of Ongür and Price (2000) as well as that by Petrides and Mackey (2006), we would suggest that our activation and hence region of interest in the OFC is located in area 14 and the medial extreme of area 11 (11 m). It should be noted, however, that probabilistic maps in standard space based on observer-independent cytoarchitectonic examination (cf. Schleicher et al., 2005; Zilles and Amunts, 2010) are not yet available for the OFC. The relevant thresholds use to define all of these three seed regions in the fMRI analysis were: height threshold T=3.11 (cluster forming threshold of voxel-level p<0.001), extent threshold k=85 voxels.
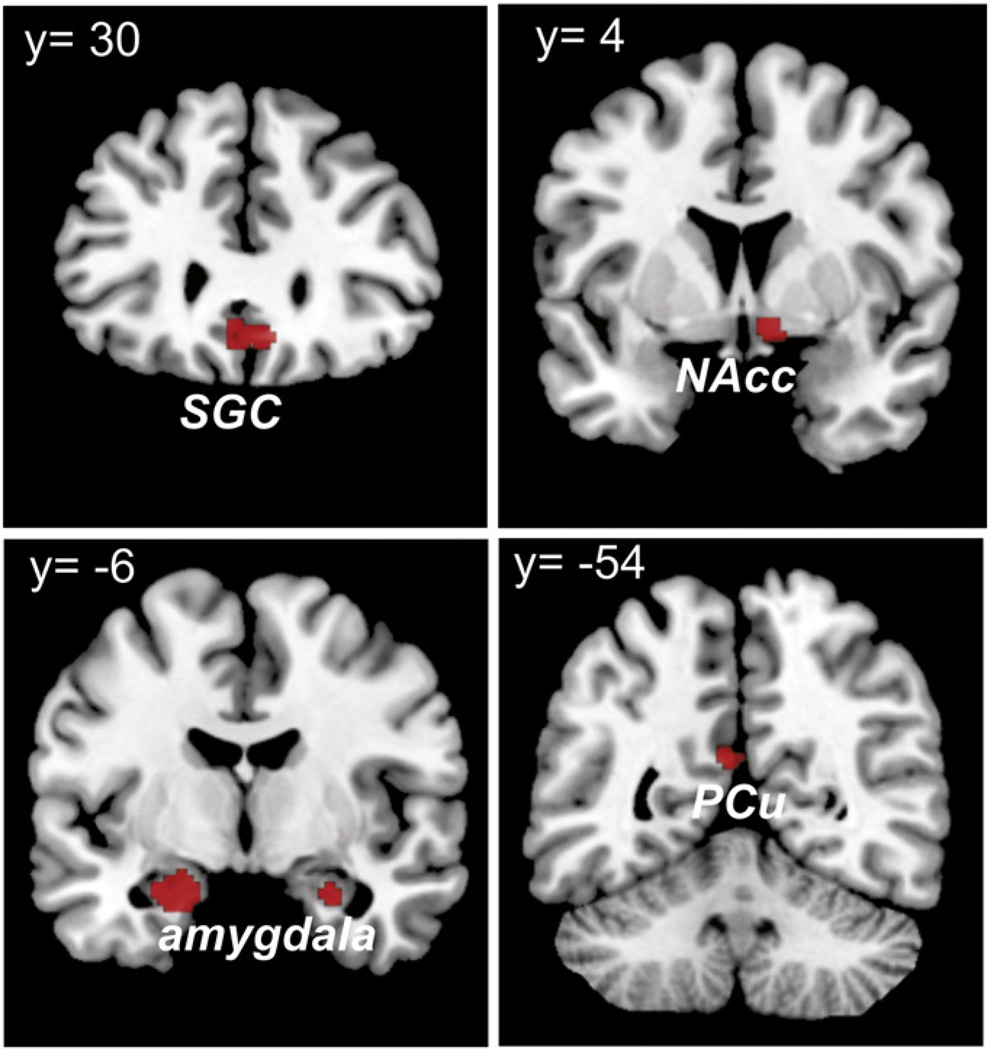
In order to assess brain regions that co-activate and hence interact with all three seeds, we performed a conjunction analysis over the respective thresholded functional connectivity maps (i.e., MACM-maps) using the minimum statistic (Caspers et al., 2010). This conjunction analysis revealed consistent task-based functional connectivity of all three seeds with laterobasal amygdala bilaterally (−18/−14/−17 and 26/−10/−17), subgenual cingulum (SGC) (0/28/−5), right nucleus accumbens (NAcc) (10/−2/−5) and left precuneus (PCu) (−4/−58/25) (Fig. 2).
Fig. 2.
Task-based functional connectivity of the bilateral amygdala and the OFC. The conjunction analysis over the respective functional connectivity maps revealed consistent task-based functional connectivity of all three seeds with the bilateral laterobasal amygdala, the SGC, the right NAcc and the left PCu.
Task-free functional connectivity estimated from resting-state fMRI
This task-based connectivity was then cross-validated against functional connectivity in a task-free resting-state. Testing for the conjunction of the resting-state connectivity maps of the three seeds revealed, apart from the amygdala bilaterally (−24/−12/−21 and 27/−11/−23), significant coupling of all three seeds only with the SGC (maximum at 2/27/−15). Importantly, we could completely confirm these results in the analysis without PCA denoising, where we found local maxima of the significant conjunction-effects in the SGC (−2/33/−9) and in amygdala bilaterally (−25/−12/−24 and 25/−10/−22). It should be noted, that the coordinates previously provided (Task-based functional connectivity estimated from BrainMap) slightly differ from the ones reported here, as the latter pertain to the maximum statistical effect of the resting-state connectivity analysis within the volume defined by the MACM results, whereas the former denote the location of the statistical maximum of the MACM analysis.
Testing for age- and gender effects in the resting-state functional connectivity data (where subject-specific information can be obtained in contrast to the MACM analysis integrating across different neuroimaging studies) showed that neither age nor gender had any statistically detectable effects on the functional connectivity of the assessed seeds. The most significant effect for age was at p<0.003 uncorrected in a cluster of 2 voxels. The analysis of gender effects likewise did not reveal any significant effects (p-value of the most significant voxel was at p<0.008 uncorrected in a cluster of 1 voxel). These effects were also not significant after small volume correction. We thus did not find any evidence for age or gender effects on the functional connectivity of the seed regions (the amygdala and the OFC).
Cross-validation of MACM and resting state
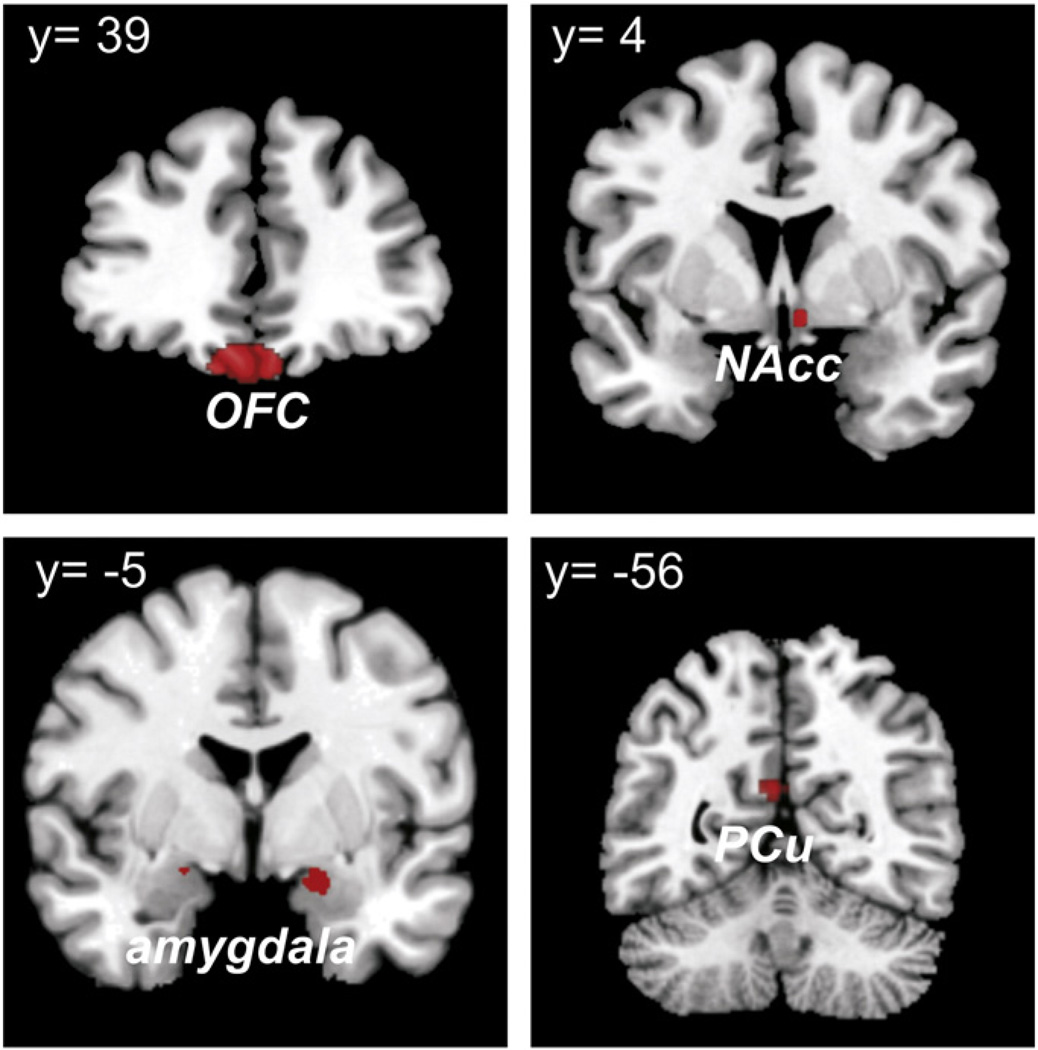
Both task-based and task-free connectivity of the seed regions, in which affective processing was shown to be modulated by cognitive demand, hinted at a role of the SGC as a “hub” in the delineated network. This hypothesis was tested by assessing SGC connectivity in the endogenously controlled resting-state. This analysis revealed that the SGC was significantly coupled with all regions implicated in the network delineated by MACM, i.e., left PCu (−7/−55/21), NAcc (5/3/−10), and amygdala bilaterally (−15/2/−24 and 17/−5/−18) as well as medial OFC (− 3/31/− 21) (Fig. 3). Note again that coordinates for the same region differ between analyses, as these always indicate the respective local maximum.
Fig. 3.
Assessing SGC connectivity in the endogenously controlled resting-state. Connectivity of the SGC during task-free state was revealed with all three seed regions implicated in the network connected to suppressable emotional processing, as delineated by MACM, i.e., the left PCu, NAcc and bilaterally amygdala, as well as the medial OFC.
In line with the almost identical results obtained with and without denoising in the analysis of the seed-connectivity, omitting the PCA-denoising changed the results for the SGC connectivity only slightly. In particular, we found connectivity with all the very same regions as above, although two of these (right amygdala and NAcc) barely failed to reach (corrected) statistical significance but were evident just under the threshold.
For a schematic graph comparing both methods – MACM and resting-state – see Fig. S2 in the supplementary material.
Functional characterization
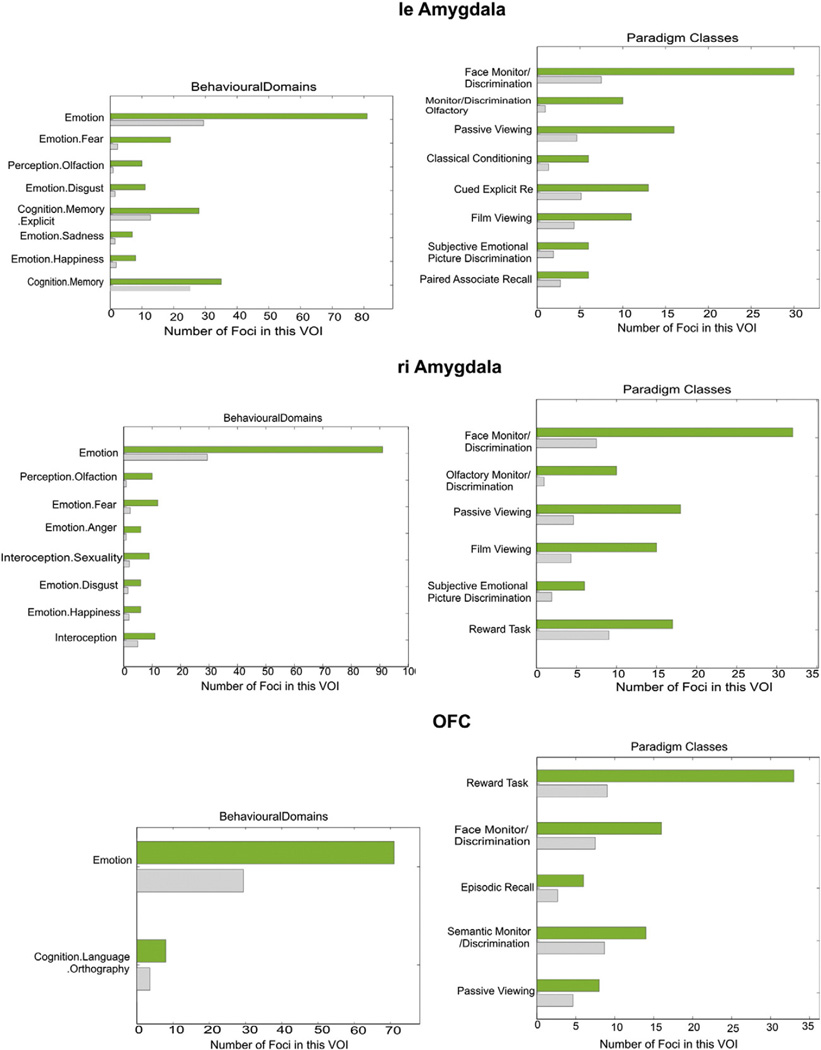
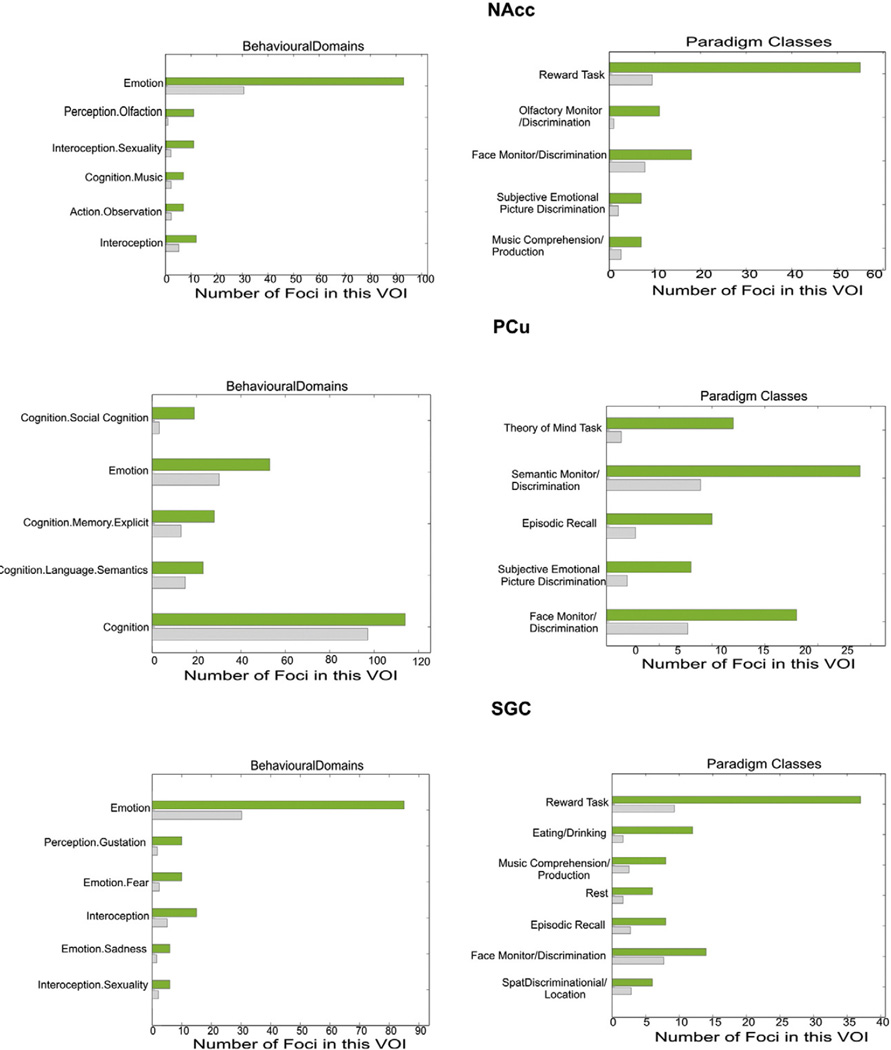
Analysis of behavioral domain profiles and paradigm classes of the three seed regions showed that the seed regions within the left and right amygdala, respectively, were recruited by experiments involving visual stimuli, in particular faces and film clips, as well as by those employing gustatory stimuli and classical conditioning. The right amygdala was additionally associated with reward tasks and interoception, whereas the left amygdala with recognition, associate recall and subjective emotional picture discrimination, as well as (explicit) memory tasks. An association of the amygdala regions on both sides function of the reward tasks was shared with the OFC. Moreover, the latter area (OFC) was also associated with episodic recall and semantic monitoring (Fig. 4).
Fig. 4.
Functional characterization of the three seed regions. The green bar represents found foci in the associated experiments, while the grey bar shows these numbers of foci that were expected over chance to be found. Both amygdala VOIs, though not the OFC were activated significantly above chance by visual and gustatory stimuli, as well as classical conditioning. The right side was additionally associated with reward tasks and interoception, whereas the left side with recognition, associate recall and subjective emotional picture discrimination, as well as (explicit) memory tasks. The first function was shared with OFC, which was also associated with episodic recall and semantic monitoring.
Functional characterization showed association of all co-activated regions, i.e. left PCu, NAcc, bilateral amygdala and SGC, with emotional processing and except PCu also interoception. All regions, except left amygdala and PCu, were activated during reward tasks. In contrast, these two regions (left amygdala and PCu) were activated during memory recall. Both amygdalae, as well as PCu showed activation in episodic memory tasks. However, specifically for PCu, we also found this region activated by tasks of social cognition (theory of mind-tasks). Finally, NAcc and PCu were found to be significantly activated by subjective emotional picture discrimination (Fig. 5).
Fig. 5.
Functional characterization of the co-activated regions. The green bar represents found foci in the associated experiments, while the grey bar shows these numbers of foci that were expected over chance to be found. The co-activated regions (SGC, PCu, NAcc and amygdala) showed associations with emotional processing, episodic memory, interoception and reward, as well as, specific for the precuneus social cognition.
Functional anti-correlations
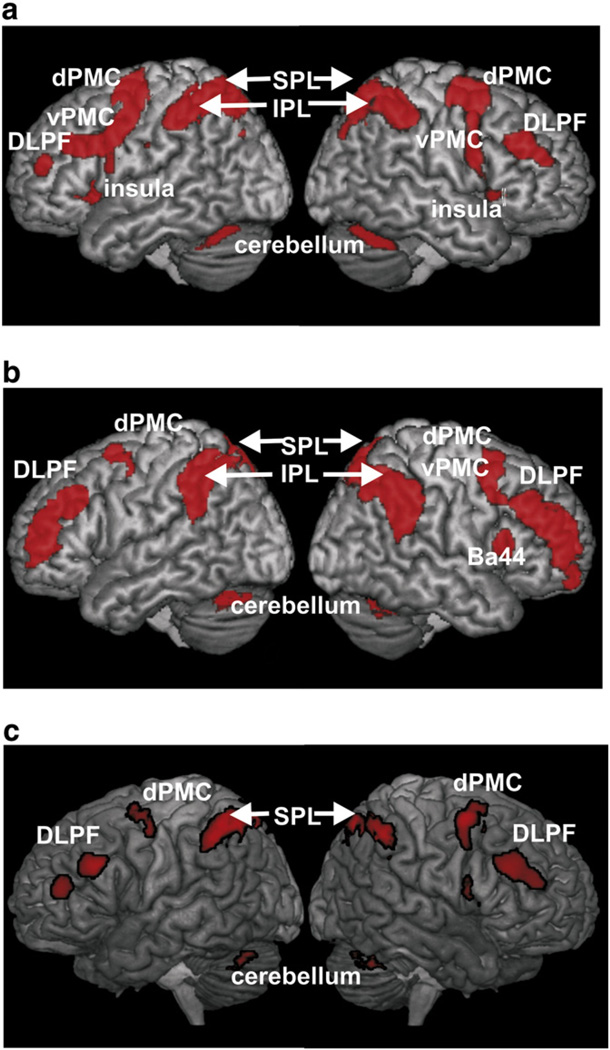
In the previous fMRI study, from which the seeds were derived, we found that increased concurrent cognitive demand was not only associated with attenuation of the seeds for the current experiment but also with increased activation in prefrontal, premotor and parietal cortices (Fig. 6a). In other words, the latter areas showed an anti-correlated pattern of task-based activation in Kellermann et al. (2012) and may thus potentially underlie the attenuation effects in the amygdala and the OFC. To follow up on this observation, we here computed another functional connectivity analysis, this time assessing regions whose resting-state time-courses were significantly anti-correlated with those of the seeds. This analysis revealed negative, i.e., anti-correlated, functional connectivity between the amygdala/OFC and a bilateral fronto-parietal network comprising the dorso-lateral prefrontal cortex, the dorsal premotor cortex, the cerebellum as well as inferior and superior parietal lobules (Fig. 6b). Importantly, this network strongly overlapped with that found to increase activity with higher cognitive load, as demonstrated by a conjunction analysis (Fig. 6c). This significant overlap is particularly important given recent discussions that anti-correlations in resting state analysis may partially be considered methodological artifacts, as it indicates that the ensuing anti-correlated regions are indeed activated by the task under which processing in the seeds is attenuated.
Fig. 6.
Calculating the negative correlation of the three seeds in task-free state demonstrated that the emotional and the cognition network have contrary activation patterns, which might not only be activated in a special fMRI-task, but also in a task-free state. a) Higher concurrent cognitive demand modulated activation in emotional areas (amygdalae and OFC) and evoked stronger activation in the superior parietal lobe and the premotor cortex. b) Functional connectivity analysis, assessing regions whose resting-state time-courses were significantly anti-correlated with those of the seeds. c) Conjunction over regions increasing their activity when going from an easy to a more challenging working-memory task (during which activity in the seeds was attenuated) and anti-correlations with the three seed regions where affective processing is susceptible to attenuation by concurrent cognition.
Discussion
The presented study mapped the network showing functional connectivity with areas that were activated by implicit emotional processing and attenuated by concurrent cognitive demand. In particular, we performed seed-based investigation of three key regions in the affective processing network (bilateral amygdala and OFC), which were based on suppressed processing of emotional stimuli under cognitive demand in a previous fMRI study. Importantly, this is a first demonstration, how a conjunction of several MACM analyses may be employed to identify regions that show significant task-based functional connectivity with more than one seed region. Given that all of the seeds were derived from this same contrast of a functional neuroimaging study, this approach thus combines two innovations to MACM analyses, namely the use of functionally defined seeds and that of conjunctions. Moreover, the methodological purpose was to demonstrate, how functional neuroimaging data may be supplemented by information on task-dependent but also task-independent connectivity as well as quantitative behavioral inference. With this, it exemplifies how these innovative analyses may be used to characterize the functional networks interacting with seed regions derived from fMRI results. Together, these two aspects represent an interesting evolution from previous MACM work on similar, albeit macroanatomically defined, regions (cf. Robinson et al., 2010; Zald et al., 2012). In particular, in contrast to the work of Robinson and colleagues on the amygdala and that of Zald et al. on the orbitofrontal cortex, the current work has an intrinsically close link to functional specialization. Given that the three seeds were obtained from the same fMRI contrast, we can ascribe a particular functionality toward them, in our case the modulation of affective processing by concurrent cognitive demand. The MACM analyses now identify regions that, across many tasks, co-activate with these seeds. Importantly then, the conjunction identifies, which regions are significantly more likely than chance to co-activate with all of these seeds. That is, we can identify common co-activations of functionally defined locations. In our case, this analysis revealed the subgenual cingulate cortex, which itself is known to be strongly associated with mood regulation and dysregulation (depression). It thus appears plausible that the SGC shows significant task-based functional connectivity to all three seeds which were, in turn, defined by showing a modulation of their functional response to affective stimuli by cognitive demand.
Methodological considerations and limitations
Meta-analytic connectivity modeling represents a novel approach to functional connectivity by identifying patterns of co-activation across a large number of subjects (Robinson et al., 2010). In comparison to the widely used ICA-based methods, this approach may enable a deeper understanding of functional properties and cognitive processes that are sustained by the delineated networks as it allows reference to the behavioral meta-data of the tasks recruiting the respective regions. In spite of its emerging success (cf. Cauda et al., 2011a; Eickhoff et al., 2011; Jakobs et al., 2012; Reetz et al., 2012; Wager et al., 2007), a few conceptual peculiarities and potential limitations should be considered.
Technically, it must be noted that the unit of observation in MACM, as a method for testing task-based functional connectivity, is a particular neuroimaging experiment rather than a particular image in a subjects’ time-series, given that MACM assesses correlation of activation (across many different experiments) between brain regions. One potential drawback of MACM is thus that it does not provide information about a particular population of interest or even individual subjects. Hence, it may not be used to assess inter-individual confounds such as age or gender. In the present study, we addressed this limitation by combining MACM with another approach toward functional connectivity analysis, i.e., resting-state correlations. These allowed us to test (and rule out) age and gender related effects.
Another consideration is that sometimes not all regions are equally likely to show activations due to regional differences in effect sizes or spatial variability (Cole and Schneider, 2007). For example, the hippocampus is known to be strongly connected to and its functions modulated by amygdala and prefrontal regions (Depue et al., 2007; Kilpatrick and Cahill, 2003; Peper et al., 2006; Phelps, 2006; Phelps and LeDoux, 2005; Richter-Levin, 2004; Smith et al., 2006), but we did not find this area in the conjunction across the MACM maps of all three seed regions. In the analyses considering only co-activations of the amygdala, in turn, we found effects in the hippocampus as part of a large cluster of significant MACM effects that was spatially contiguous with the seed region. We would hence carefully argue that across many different types of experiments the hippocampus may not be as consistently interacting with the amygdala and OFC as one might have expected. On the other hand, however, this may reflect larger variability and lower signal-to-noise ratios of hippocampal regions in neuroimaging and particularly fMRI experiments as compared to, e.g., the precuneus. Furthermore, several other brain regions involved in affective processing have likewise been discussed as susceptible to modulation by cognitive demand (Bermpohl et al., 2006). For instance, attentional modulation was observed in medial prefrontal cortex, amygdala, dorsal midbrain (Liberzon et al., 2000), anterior and posterior cingulate gyrus (Fichtenholtz et al., 2004), insula, superior temporal gyrus and lateral prefrontal cortex (Keightley et al., 2003; Northoff et al., 2004). The anterior cingulate cortex (ACC), e.g., is known to be implicated in cognitive as well as in affective/emotional processes (Bush et al., 2000). This region, which was not found in the context of our previous experiment and hence not considered in the current follow-up analysis, may therefore represent another critical structure within the affective network susceptible to cognitive demand. In light of the fact that we found and consequently analyzed some but not all previously discussed regions, it must be considered that the methods and paradigms from which conclusions about the abovementioned other areas were derived are quite heterogeneous (see e.g., Lane et al., 1999; Liberzon et al., 2000). Hence, there seems no consensus on which regions involved in affective processing may be susceptible to different cognitive modulations. Moreover, the neuronal distinction between cognition–emotion interactions (Dolcos et al., 2011; Gu et al., 2013), cognitive control/regulation of emotions (Ochsner and Gross, 2005), and cognitive inference with affective processing (Bartholow et al., 2001) remains to be elucidated.
Importantly, task-based functional connectivity analysis is naturally constrained to tasks that can be performed in the scanner. As a consequence networks that may be recruited by more naturalistic behavior may be sparsely represented in the database. Given the broad range of task-results that are stored in the BrainMap database and the data-driven approach, however, we would argue that at least a large amount of possible task-relevant interactions of a particular seed region may be delineated.
A potential drawback of resting state functional connectivity analysis, on the other hand, is its reliance on the raw MRI signal time courses. These are intrinsically noisy due to scanner artefacts, motion-induced effects, and physiological sources such as heart beat and respiratory cycles (Fox et al., 2009; Weissenbacher et al., 2009). Accordingly, reducing the potential for spurious correlations by such perturbations is a key aspect of several factors of data preprocessing by temporal and spatial filtering (Bellec et al., 2006; Fox and Raichle, 2007). It has been noted, however, that such processing steps may themselves introduce (anti-) correlations in the time-series and no final consensus has yet been reached on the optimal preprocessing strategies. Finally, it must be remembered that the physiology underlying resting-state fluctuations and hence also low-frequency correlations remains a matter of conjecture.
In addition to the conceptual differences between resting-state correlations and MACM, these two approaches also differ in the composition of the assessed subject groups. In particular, for the resting-state analysis we recruited a gender-balanced cohort whose age was (in each gender) uniformly distributed between 20 and 70 (with 10 subjects per gender and decade). Additional supplementary analyses moreover showed that there was no statistically detectable effect of age or gender on the functional connectivity of our seeds. For the MACM analysis, in turn, we relied on a large amount of published neuroimaging studies, whose results were synthetized in a purely data-driven manner to identify significant co-activations of the seeds. Evidently, these studies are highly heterogeneous with respect to virtually all socio-demographic variables, e.g., age, gender (–distribution), ethnicity or education level. We would argue that this heterogeneity should enhance generalizability of the obtained results, as it renders these potential confounds non-systematic variations. In other words, whereas resting-state data was obtained in a well-balanced, stratified cohort, the MACM analysis integrates over many different more or less randomized subject characteristics. Nevertheless, it may not be ruled out that the different compositions of the resting-state group and the MACM data may bias these two methods toward different findings even though such biases have not been systematically explored yet. Precisely these concerns, however, were the main reason for using both approaches in conjunction to infer the functional connectivity of the seed regions.
Functional characterization of the seed regions
By examining the functional characteristics of the three seed regions (bilateral amygdala and OFC) via BrainMap, we found all of these regions to be activated during emotion and reward tasks, which may be considered a quantitative confirmation of previous qualitative discussions on the relationship between both processes (Kringelbach and Rolls, 2004; Murray and Wise, 2010; Phan et al., 2002; Sergerie et al., 2008; Wallis, 2007; Zald, 2003). Such association between emotion and reward in turn is not surprising as reward values often reflect the emotional appraisal of information (Schmitz and Johnson, 2007) and the experience of a reward may itself induce a (positive) affect (Keitz et al., 2003). The current data moreover quantitatively confirmed the notion that emotional processing is associated with interoceptive processing (Craig, 2009; Critchley et al., 2005; Pollatos et al., 2007). For example, Pollatos et al. (2007) found that the representation of bodily responses has an essential role for individuals’ emotion, based on the observation that subjects with high interoceptive awareness reported stronger feelings while viewing pleasant and unpleasant pictures.
Anatomical connectivity of the seed regions
Here, we review previous findings on anatomical connections between the seed regions but not without noting that functional connectivity, as a coincidence of spatially remote neurophysiologic events (Eickhoff and Grefkes, 2011; Friston et al., 1996), does not imply any kind of direct connection between the respective regions. Rather, correlation between brain regions might be mediated by monosynaptic connection as well as through relays or may even be spuriously be induced by a third region not involved in the analysis.
Invasive tracing studies in macaque monkeys demonstrated that the medial OFC is anatomically connected with several brain areas both in the prefrontal cortex and the limbic system that are involved in emotional processing (Cavada et al., 2000; Rempel-Clower, 2007). For instance, Carmichael and Price (1995) reported that the OFC can be subdivided into a lateral part which is less limbic related and a posteromedial part, which is strongly limbic related as it is connected with the majority of the examined limbic structures. Projections from the amygdala, hippocampus, perirhinal and entrhinal cortex and the temporal pole could be found in that later region of the OFC (Barbas et al., 2003; Carmichael and Price, 1995; Price, 2007). Moreover, the mOFC was reported to show anatomical connectivity with NAcc (Haber et al., 1995), as the authors reported that the nucleus accumbens in the monkey also receives a dense projection primarily from area 11 (representing the mOFC) and area 13. Connectivity between the mOFC and the SGC has also been demonstrated in non-human primates (Johansen-Berg et al., 2008). Although there are no direct anatomical connections between OFC and precuneus, the absence of direct anatomical connections does not impede functional connectivity between them. Given these diverse and widespread connectivity to limbic and sensory areas, the OFC has been ascribed an important role in emotional behavior as well as the representation of social and affective values (Cavada et al., 2000; Kiebel et al., 2008). This view is well reflected by the functional characterization of the OFC in the present analysis.
While a detailed discussion of the connection patterns of the (primate) amygdala is beyond the scope of this paper (for further reading cf. Sah et al., 2003) we want to point to some findings that are relevant to the observed functional interactions: Several earliest neuroanatomical studies on non-human primates did not reveal inter-hemispheric connections of amygdaloid nuclei (Bailey et al., 1941; Irwin et al., 2003; Lauer, 1945). There is one older investigation that showed direct interhemispheric connectivity of the majority of nuclei in the human brain (Klinger and Gloor, 1960). Moreover, there are several studies that reported intra-amygdalar connections in the rat (Pitkänen et al., 1995; Savander et al., 1995; Petrovich et al., 1996) and in the monkey (Aggleton, 1985; Pitkänen and Amaral, 1998).
The existence of direct, i.e., monosynaptic, structural connection between the amygdalae, in particular in the human brain, remains on open question. In this context, however, it is important to note, that an absence of such connections does by no means rule out a functional connectivity between these areas given that functional connectivity only indicates correlated or coordinated activity which may be mediated by other structures or reflect engagement by the same functional networks.
As mentioned above, there are connections, between mOFC and amygdala (Rempel-Clower, 2007) that may serve complementary functions: the amygdala processed the emotional significance of stimuli (Phelps, 2004), while the OFC is important for appraisal of a stimulus, adapting behavior and the emotional context of a cue (Roberts, 2006; Schultz et al., 2006). In this context, a conspicuous observation from the MACM analysis was that consistent co-activations of the three seed regions were found in the amygdalae but not the OFC. Such asymmetry, however, does not implicate directionality but rather conditionality of functional connectivity. Experiments activating the OFC were more likely than by chance to also activate the amygdala, while this was not the case vice versa. Activation in the amygdala thus does not necessarily entail co-activation of the OFC, whereas activation of the latter is significantly associated with amygdala co-activation. While the functional relevance of this distinction may need further investigation, we would like to note that the missing co-activation of the OFC should most likely not reflect a methodological constraint such as limited coverage of this region, as we found a solid number of 142 studies within BrainMap which featured activation in OFC. However, we cannot entirely rule out the possibility that as differences in magnetic susceptibility at air/tissue boundaries, magnetic field inhomogeneity and the hereby caused geometric distortion and low sensitivity in the OFC may have contributed to this observation. Given that especially the OFC is known as a region often suffering from signal dropout, it remains possible that the lack of OFC coactivation may in part be attributable to methodological issues.
Bidirectional projections between the amygdala to the nucleus accumbens have been observed in all mammalian species (Amaral and Price, 1984; McDonald, 1991) and should (partially) underlie the functional relationship between affect and reward mentioned above (Krettek and Price, 1978; Russchen et al., 1985). Importantly and well in line with the current results, task-dependent functional connectivity of this region with the amygdalae and the OFC have already been shown in a previous MACM investigation (Cauda et al., 2011b). Finally, tracer studies in nonhuman primates have repeatedly demonstrated connections between amygdala and SGC (Freedman et al., 2000). Using fiber tracking based on diffusion weighted imaging, Johansen-Berg et al. (2008) found similar connections between SGC and amygdala in the human brain. While it has been argued that the SGC has connections to different parts of the amygdala in different species (Freedman et al., 2000; Takagishi and Chilba, 1991) there is a growing consensus that the SGC projects in particular to the intercalated nuclei of the amygdala (Freedman et al., 2000) and may exert modulatory effects on different amygdala functions (cf. Paré and Smith, 1993).
In one of the earliest MACM studies, Robinson et al. (2010) examined the connectivity of a macro-anatomically defined amygdala complex as provided by the Harvard-Oxford Structural Probability Atlas. By comparison with the CoCoMac database on structural connectivity in the macaque monkey, they concluded that MACM provides a similar picture of amygdala (functional) connectivity as provided by axonal tracing in non-human primates. Importantly, however, this work pertained to the connectivity of the amygdala complex as a whole. There are, however, several indications, that the amygdala is a heterogeneous structure holding multiple nuclei with differentiated function and connectivity (Adolphs, 2010; Amunts et al., 2005; Bzdok et al., 2012; McGaraughty and Heinricher, 2002). Consequently, specific, e.g., functionally, defined parts of the amygdala do not necessarily need to reflect the overall connectivity pattern for the entire structure. The present paper uses such approach and examines the functional connectivity of a much more confined seed region within the amygdala that was defined by the cluster in which we found a significant modulation of affective processing by cognitive demand that was defined by a particular functional property, i.e., the suppression of affective processing by cognitive demand. That is, whereas the previous work examined the connectivity of the amygdala-complex as a whole, we focused on a specific sub-part of it showing a particular function of interest. We thus address a much more specific question on amygdala connectivity. It may hence not surprise, that in comparison to the previous study by Robinson and colleagues examining the entire amygdala complex, the current work on a specific, functionally defined part of the amygdala yields both congruencies and divergences. An important consistency is the observation of significant functional connectivity of the amygdala with the SGC, the ventral basal ganglia (in our case more specific the NAcc) and the PCu, even though the latter region was just found to be connected with the right AMY in Robinson’s paper. Contrasting our findings, the analysis of the much broader macroanatomically defined amygdala complex also indicated more extensive co-activation with the cingulate cortex (labeled by Robinson and colleagues as BA23, BA32), the frontal gyri (labeled as BA9 and BA47) and the thalamus. Other co-activations not observed in the present study include the bilateral culmen, the anterior insula, the parahippocampal gyri and right middle temporal gyrus labeled as BA37). In summary, we would thus conclude that following what may have been expected from the much more confined, functionally defined seed region, our analysis revealed only a subset of the much broader connectivity network delineated for the entire amygdala by Robinson et al. (2010).
Functional role of the nucleus accumbens
The nucleus accumbens (NAcc) is conceptualized as a crucial node of brain systems regulating motivation and reward (Schultz, 2004) due to its affluent position in the meso-corticolimbic dopaminergic system (Gill et al., 2010; Ikemoto and Panksepp, 1999). Reward function of the NAcc, such as the evaluation of reward expectancy in social, monetary, or drug rewards has consequently been observed in several neuroimaging studies (Kampe et al., 2001; Rademacher et al., 2010). As previously discussed (Functional characterization of the seed regions), there is a strong phenomenological and neurobiological association between emotional processing and reward (Martin-Soelch et al., 2001), which is underlined by the functional connectivity of the NAcc with affective regions as demonstrated in the current and previous study (cf. Ashby et al., 1999; Cauda et al., 2011b). Moreover, the involvement of the brain’s reward circuitry, including NAcc, in appraisal of social stimuli has also been demonstrated (Bzdok et al., 2011). Consequently, reward mechanisms may modulate behavior toward salient social cues (Cardinal et al., 2002; Schilbach et al., 2011) such as facial beauty, which in turn may be appraised in the OFC according to their reward value (Kringelbach and Rolls, 2004). We would hence argue that the NAcc activity reflects the value of someone’s (emotional) appraisal (Aharon et al., 2001) and note that the current data again underline the close relationship between reward and emotional appraisal.
Functional role of the precuneus
The precuneus has been conceptualized as a “higher” association area (Cavanna and Trimble, 2006) involved in visuo-spatial imagery (Le et al., 1998; Nagahama et al., 1999), episodic (Platel et al., 2003; Shallice et al., 1994; Tulving, 1983) and autobiographical memory (McDermott et al., 1999; Svoboda et al., 2006) as well as self-referential processing (Kircher et al., 2000; Lou et al., 2004; Ochsner et al., 2004) and social cognition (Schilbach et al., 2008). The functional characterization by the BrainMap data quantitatively confirmed this view, showing an association between activation within this area and social cognition, as well as subjective emotional picture discrimination and memory. As proposed by Conway (1992) and extended by Schacter et al. (2007) we would thus assume that the precuneus provides retrieval of autobiographical memory and potential projection of prospective accounts and feeling for the self-referential evaluation of (visual) sensory input. Co-activation of precuneus with regions featuring (attenuable) stimulus-driven emotional processing may hence correspond to the integration of one’s own experience into the emotional appraisal of incoming stimuli automatically performed when cognitive load is low.
The SGC as a central node in an (attenuable) emotion-processing network
Convergence of task-dependent and task-free functional connectivity analysis indicated that the subgenual cingulate cortex (SGC) might represent a key node in the delineated network. This hypothesis was corroborated by demonstrating functional connectivity of the SGC with all other regions implicated in this network. In line with this view, dense anatomical connectivity of the SGC within the limbic system has been demonstrated in invasive tracing studies (Carmichael and Price, 1995; Chiba et al., 2001). Neuroimaging studies have associated activity in the SGC with emotionally salient stimuli (Drevets et al., 2008; Elliott et al., 2000), the experience of negative mood states (George et al., 1995; Mayberg et al., 1999) and social cognition (Beauregard et al., 1997; Drevets, 2000; Elliott et al., 2000; Maddock et al., 2003).
Patients with SGC lesions show abnormal responses to emotional stimuli and fail to use reward information in directing social behavior (Damasio et al., 1990). Moreover, abnormal connectivity of the SGC during emotional and cognitive tasks (Chen et al., 2008; Drevets, 1999; Drevets et al., 2008; Johnstone et al., 2007; Matthews et al., 2008) and a reduced volume of this region (Botteron et al., 2002; Coryell et al., 2005) were already demonstrated in patients with depression. Hypofunction or dys-connectivity of the SGC has thus been discussed as possibly indicating susceptibility to affective disorders (Drevets, 2000; Mayberg et al., 1999). Importantly, it has also been ascribed a crucial role for emotion regulation by a recent meta-analysis of various emotion-regulation tasks (Diekhof et al., 2011).
Torta and Cauda (2011) used MACM to investigate the functional connectivity and the functional characteristics of the cingulate cortex. In their study, these authors divided the cingulate cortex into 12 subregions used as seed regions of interest. They reported a ROI subregion of the cingulate cortex which was involved in reward-related and emotional tasks, which closely matches our own findings on this region. Moreover, their findings of functional connectivity with this subregion showed inter alia coactivations with the amygdala, the orbitofrontal cortex and the nucleus accumbens. This is also in good agreement with the present findings. It should be mentioned, however, that we did not perform a new MACM analysis using the SGC as a seed region of interest.
The present data resonates well with this notion and furthermore extends it by demonstrating that the SGC is closely coupled to all regions connected to our seeds. That is, the SGC is coupled to the entire network interacting with three key regions for affective processing and appraisal that are susceptible to cognitive modulation. Albeit somewhat speculative, we would suggest that the SGC might link autobiographic information (PCu), reward (NAcc), reinforcement values (OFC) and emotional significance (AMY). Such a role, in turn, would allow the SGC to exert modulating influences on the latter two structures for the modulation of implicit affective processing.
Conclusion and outlook
We here characterized the function and connectivity of three regions in which we previously found affective processing to be attenuated by concurrent cognitive load. Hereby we could show that the respective network might be formed by regions contributing autobiographic information (PCu), reward (NAcc), reinforcement values (OFC) and emotional significance (AMY). Cross-validation with task-free functional connectivity furthermore demonstrated that in particular the SGC was closely coupled with all other regions implicated in this network. It seems likely that dysregulation of this hub or the connectivity within the delineated network may contribute to the pathophysiology of mood disorders, in particular depression. To evaluate the contribution of regional dysfunction or aberrant connectivity within this network to personality traits and clinical states that entail dysfunctional affective processing remains an important challenge for further research.
Supplementary Material
Acknowledgments
This work was partly funded by the Human Brain Project (R01-MH074457-01A1; S.B.E., P.T.F), the Initiative and Networking Fund of the Helmholtz Association within the Helmholtz Alliance on Systems Biology (Human Brain Model; K.Z., S.B.E.), and the DFG (IRTG 1328, S.B.E., T.S.K.).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.neuroimage.2013.01.046.
References
- Adolphs R. What does the amygdala contribute to social cognition? Ann. N. Y. Acad. Sci. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Damasio H, Tranel D. Neural systems for recognition of emotional prosody: a 3-D lesion study. Emotion. 2002;2:3–51. doi: 10.1037/1528-3542.2.1.23. [DOI] [PubMed] [Google Scholar]
- Aggleton JP. A description of intra-amygdaloid connections in old world monkeys. Exp Brain Res. 1985;57:390–399. doi: 10.1007/BF00236545. [DOI] [PubMed] [Google Scholar]
- Aharon I, Etcoff N, Ariely D, Chabris CF, O’Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32:537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J. Comp. Neurol. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat. Embryol. 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Isen AM, Turken AU. A neuropsychological theory of positive affect and its influence on cognition. Psychol. Rev. 1999;106:529–550. doi: 10.1037/0033-295x.106.3.529. [DOI] [PubMed] [Google Scholar]
- Bailey P, Garol HW, McCullouch WS. Cortical origin and distribution of corpus callosum and anterior commissure in the chimpanzee. Neurophysiology. 1941;4:564–571. [Google Scholar]
- Ball T, Rahm B, Eickhoff SB, Schulze-Bonhage A, Speck O, Mutschler I. Response properties of human amygdala subregions: evidence based on functional MRI combined with probabilistic anatomical maps. PLoS One. 2007;2:e307. doi: 10.1371/journal.pone.0000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Saha S, Rempel-Clower N, Gashghaei T. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci. 2003;10:4–25. doi: 10.1186/1471-2202-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholow BD, Fabiani M, Gratton G, Bettencourt BA. A psychophysiological examination of cognitive processing of and affective responses to social expectancy violations. Psychol. Sci. 2001;12:197–204. doi: 10.1111/1467-9280.00336. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Chertkow H, Gold D, Karama S, Benhamou J, Babins L, Faucher A. Word priming with brief multiple presentation technique: preservation in amnesia. Neuropsychologia. 1997;35:611–621. doi: 10.1016/s0028-3932(96)00108-x. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellec P, Perlbarg V, Jbabdi S, Pélégrini-Issac M, Anton JL, Doyon J, Benali H. Identification of large-scale networks in the brain using fMRI. NeuroImage. 2006;29:1231–1243. doi: 10.1016/j.neuroimage.2005.08.044. [DOI] [PubMed] [Google Scholar]
- Bermpohl F, Pascual-Leone A, Amedi A, Merabet LB, Fregni F, Gaab N, Alsop D, Schlaug G, Northoff G. Attentional modulation of emotional stimulus processing: an fMRI study using emotional expectancy. Hum. Brain Mapp. 2006;27:662–677. doi: 10.1002/hbm.20209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Blair KS, Smith BW, Mitchell DG, Morton J, Vythilingam M, Pessoa L, Fridberg D, Zametkin A, Sturman D, Nelson EE, Drevets WC, Pine DS, Martin A, Blair RJ. Modulation of emotion by cognition and cognition by emotion. NeuroImage. 2007;35:430–440. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botteron KN, Raichle ME, Drevets WC, Heath AC, Todd RD. Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol. Psychiatry. 2002;51:342–344. doi: 10.1016/s0006-3223(01)01280-x. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Caspers S, Kurth F, Habel U, Zilles K, Laird A, Eickhoff SB. ALE meta-analysis on facial judgments of trustworthiness and attractiveness. Brain Struct. Funct. 2011;215:209–223. doi: 10.1007/s00429-010-0287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Laird AR, Zilles K, Fox PT, Eickhoff SB. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Hum. Brain Mapp., in print. 2012 doi: 10.1002/hbm.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J. Comp. Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. NeuroImage. 2010;50:1148–1167. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, Cavanna AE, D’agata F, Sacco K, Duca S, Geminiani GC. Functional connectivity and coactivation of the nucleus accumbens: a combined functional connectivity and structure-based meta-analysis. J. Cogn. Neurosci. 2011a;23:2864–2877. doi: 10.1162/jocn.2011.21624. [DOI] [PubMed] [Google Scholar]
- Cauda F, Giuliano G, Federico D, Sergio D, Katiuscia S. Discovering the somatotopic organization of the motor areas of the medial wall using low-frequency BOLD fluctuations. Hum. Brain Mapp. 2011b;32:1566–1579. doi: 10.1002/hbm.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb. Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Castañón AN, Ongür D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. NeuroImage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Suckling J, Ooi C, Fu CH, Williams SC, Walsh ND, Mitterschiffthaler MT, Pich EM, Bullmore E. Functional coupling of the amygdala in depressed patients treated with antidepressant medication. Neuropsychopharmacology. 2008;33:1909–1918. doi: 10.1038/sj.npp.1301593. [DOI] [PubMed] [Google Scholar]
- Chiba T, Kayahara T, Nakano K. Efferent projections of infralimbic and prelimbic areas of the medial prefrontal cortex in the Japanese monkey, Macaca fuscata. Brain Res. 2001;888:83–101. doi: 10.1016/s0006-8993(00)03013-4. [DOI] [PubMed] [Google Scholar]
- Cole MW, Schneider W. The cognitive control network: integrated cortical regions with dissociable functions. NeuroImage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Conway MA. A structural model of autobiographical memory. In: Conway MA, Spinnler H, Wagenaar WA, editors. Theoretical Perspectives on Autobiological Memory. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. pp. 167–194. [Google Scholar]
- Coryell W, Nopoulos P, Drevets W, Wilson T, Andreasen NC. Subgenual prefrontal cortex volumes in major depressive disorder and schizophrenia: diagnostic specificity and prognostic implications. Am. J. Psychiatry. 2005;162:1706–1712. doi: 10.1176/appi.ajp.162.9.1706. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel-now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Rotshtein P, Nagai Y, O’Doherty J, Mathias CJ, Dolan RJ. Activity in the human brain predicting differential heart rate responses to emotional facial expressions. NeuroImage. 2005;24:751–762. doi: 10.1016/j.neuroimage.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behav. Brain Res. 1990;41:81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Depue BE, Curran T, Banich MT. Prefrontal regions orchestrate suppression of emotional memories via a two-phase process. Science. 2007;317:215–219. doi: 10.1126/science.1139560. [DOI] [PubMed] [Google Scholar]
- Dickson J, Drury H, Van Essen DC. ‘The surface management system’ (SuMS) database: a surface-based database to aid cortical surface reconstruction, visualization and analysis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001;356:1277–1292. doi: 10.1098/rstb.2001.0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. NeuroImage. 2011;58:275–285. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Iordan AD, Dolcos S. Neural correlates of emotion-cognition interactions: a review of evidence from brain imaging investigations. J. Cogn. Psychol. (Hove) 2011;23:669–694. doi: 10.1080/20445911.2011.594433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann. N. Y. Acad. Sci. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog. Brain Res. 2000;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;8:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Grefkes C. Approaches for the integrated analysis of structure, function and connectivity of the human brain. Clin. EEG Neurosci. 2011;42:107–121. doi: 10.1177/155005941104200211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Jbabdi S, Caspers S, Laird AR, Fox PT, Zilles K, Behrens TE. Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. J. Neurosci. 2010;30:6409–6421. doi: 10.1523/JNEUROSCI.5664-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Roski C, Caspers S, Zilles K, Fox PT. Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. NeuroImage. 2011;57:938–949. doi: 10.1016/j.neuroimage.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. NeuroImage. 2012;59:349–361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. Selective attention to emotional stimuli in a verbal go/no-go task: an fMRI study. NeuroReport. 2000;11:1739–1744. doi: 10.1097/00001756-200006050-00028. [DOI] [PubMed] [Google Scholar]
- Ellsworth PC, Scherer KR. Appraisal processes in emotion. In: Davidson R, Scherer KR, Goldsmith HH, editors. Handbook of Affective Sciences. New York: Oxford University Press; 2003. pp. 572–595. [Google Scholar]
- Engell AD, Haxby JV, Todorov A. Implicit trustworthiness decisions: automatic coding of face properties in the human amygdala. J. Cogn. Neurosci. 2007;19:1508–1519. doi: 10.1162/jocn.2007.19.9.1508. [DOI] [PubMed] [Google Scholar]
- Erk S, Kleczar A, Walter H. Valence-specific regulation effects in a working memory task with emotional context NeuroImage. 2007;37:623–632. doi: 10.1016/j.neuroimage.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Fichtenholtz HM, Dean HL, Dillon DG, Yamasaki H, McCarthy G, LaBar KS. Emotion-attention network interactions during a visual oddball task. Brain Res. Cogn. Brain Res. 2004;20:67–80. doi: 10.1016/j.cogbrainres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State (a practical method for grading the state of patients for the clinician) J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fox PT. Spatial normalization origins: objectives, applications, and alternatives. Hum. Brain Mapp. 1995;3:161–164. [Google Scholar]
- Fox PT, Lancaster JL. Opinion: mapping context and content: the BrainMap model. Nat. Rev. Neurosci. 2002;3:319–321. doi: 10.1038/nrn789. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox PT, Laird AR, Fox SP, Fox M, Uecker AM, Crank M, Koenig SF, Lancaster JL. BrainMap taxonomy of experimental design: description and evaluation. Hum. Brain Mapp. 2005;25:185–198. doi: 10.1002/hbm.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J. Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis S, Rolls ET, Bowtell R, McGlone F, O’Doherty J, Browning A, Clare S, Smith E. The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. NeuroReport. 1999;10:453–459. doi: 10.1097/00001756-199902250-00003. [DOI] [PubMed] [Google Scholar]
- Freedman LJ, Insel TR, Smith Y. Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. J. Comp. Neurol. 2000;421:172–188. [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Fletcher P, Liddle PF, Frackowiak RS. Functional topography: multidimensional scaling and functional connectivity in the brain. Cereb. Cortex. 1996;6:156–164. doi: 10.1093/cercor/6.2.156. [DOI] [PubMed] [Google Scholar]
- George MA, Ketter TA, Parekh PI, Horwitz B, Herscovitch P, Post RM. Brain activity during transient sadness and happiness in healthy women. Am. J. Psychiatry. 1995;152:341–351. doi: 10.1176/ajp.152.3.341. [DOI] [PubMed] [Google Scholar]
- Gill TM, Castaneda PJ, Janak PH. Dissociable roles of the medial prefrontal cortex and nucleus accumbens core in goal-directed actions for differential reward magnitude. Cereb. Cortex. 2010;20:2884–2899. doi: 10.1093/cercor/bhq036. [DOI] [PubMed] [Google Scholar]
- Grandjean D, Scherer KR. Unpacking the cognitive architecture of emotion processes. Emotion. 2008;8:341–351. doi: 10.1037/1528-3542.8.3.341. [DOI] [PubMed] [Google Scholar]
- Grandjean D, Sander D, Scherer KR. Conscious emotional experience emerges as a function of multilevel, appraisal-driven response synchronization. Conscious. Cogn. 2008;17:484–495. doi: 10.1016/j.concog.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Liu X, Van Dam NT, Hof PR, Fan J. Cognition-emotion integration in the anterior insular cortex. Cereb. Cortex. 2013;23:20–27. doi: 10.1093/cercor/bhr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. J. Neurosci. 1995;15(7 Pt. 1):4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat. Neurosci. 1999;2:289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Hautzinger M, Keller F, Kühner C. BDI-II; Beck Depressions-Inventar Revision. Frankfurt am Main, Harcourt Test Services. 2006 [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J. Comput. Assist. Tomogr. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res. Brain Res. Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Irwin W, Michael J, Anderle MJ, Abercrombie HC, Schaefer SM, Kalin NH, Davidson RJ. Amygdalar interhemispheric functional connectivity differs between the non-depressed and depressed human brain. NeuroImage. 2003;21:674–686. doi: 10.1016/j.neuroimage.2003.09.057. [DOI] [PubMed] [Google Scholar]
- Jakobs O, Langner R, Caspers S, Roski C, Cieslik EC, Zilles K, Laird AR, Fox PT, Eickhoff SB. Across-study and within-subject functional connectivity of a right temporo-parietal junction subregion involved in stimulus-context integration. NeuroImage. 2012;60:2389–2398. doi: 10.1016/j.neuroimage.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Gutman DA, Behrens TE, Matthews PM, Rushworth MF, Katz E, Lozano AM, Mayberg HS. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb. Cortex. 2008;18:1374–1383. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J. Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampe KK, Frith CD, Dolan RJ, Frith U. Reward value of attractiveness and gaze. Nature. 2001;413:589. doi: 10.1038/35098149. [DOI] [PubMed] [Google Scholar]
- Keightley ML, Winocur G, Graham SJ, Mayberg HS, Hevenor SJ, Grady CL. An fMRI study investigating cognitive modulation of brain regions associated with emotional processing of visual stimuli. Neuropsychologia. 2003;41:585–596. doi: 10.1016/s0028-3932(02)00199-9. [DOI] [PubMed] [Google Scholar]
- Keitz M, Martin-Soelch C, Leenders KL. Reward processing in the brain: a prerequisite for movement preparation? Neural Plast. 2003;10:121–128. doi: 10.1155/NP.2003.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermann TS, Sternkopf MA, Schneider F, Habel U, Turetsky BI, Zilles K, Eickhoff SB. Modulating the processing of emotional stimuli by cognitive demand. Soc. Cogn. Affect. Neurosci. 2012;7(3):263–273. doi: 10.1093/scan/nsq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebel SJ, Daunizeau J, Friston KJ. A hierarchy of time-scales and the brain. PLoS Comput. Biol. 2008;4:e1000209. doi: 10.1371/journal.pcbi.1000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick L, Cahill L. Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. NeuroImage. 2003;20:2091–2099. doi: 10.1016/j.neuroimage.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Kircher TTJ, Senior C, Phillips ML, Benson PJ, Bullmore ET, Brammer M, Simmons A, Williams SCR, Bartels M, David AS. Towards a functional neuroanatomy of self-processing: effects of faces and words. Cogn. Brain Res. 2000;10:133–144. doi: 10.1016/s0926-6410(00)00036-7. [DOI] [PubMed] [Google Scholar]
- Klinger J, Gloor P. The connection of the amygdala and of the anterior temporal cortex in the human brain. J. Comp. Neurol. 1960;115:333–369. doi: 10.1002/cne.901150305. [DOI] [PubMed] [Google Scholar]
- Koski L, Paus T. Functional connectivity of the anterior cingulate cortex within the human frontal lobe: a brain-mapping meta-analysis. Exp. Brain Res. 2000;133:55–65. doi: 10.1007/s002210000400. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J. Comp. Neurol. 1978;15:225–254. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog. Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Laird AR, Lancaster JL, Fox PT. BrainMap: the social evolution of a functional neuroimaging database. Neuroinformatics. 2005;3:65–78. doi: 10.1385/ni:3:1:065. [DOI] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J. Neurosci. 2009a;29:14496–14505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Kurth F, Fox PM, Uecker AM, Turner JA, Robinson JL, Lancaster JL, Fox PT. ALE meta-analysis workflows via the BrainMap database: progress towards a probabilistic functional brain atlas. Front. Neuroinformatics. 2009b;3:23. doi: 10.3389/neuro.11.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Fox PM, Uecker AM, Ray KL, Saenz JJ, Jr, McKay DR, Bzdok D, Laird RW, Robinson JL, Turner JA, Turkeltaub PE, Lancaster JL, Fox PT. The BrainMap strategy for standardization, sharing, and meta-analysis of neuroimaging data. BMC Res. Notes. 2011a;9:349. doi: 10.1186/1756-0500-4-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, Glahn DC, Beckmann CF, Smith SM, Fox PT. Behavioral interpretations of intrinsic connectivity networks. J. Cogn. Neurosci. 2011b;23:4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutiérrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum. Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, Chua PM, Dolan RJ. Common effects of emotional valence, arousal and attention on neural activation during visual processing of pictures. Neuropsychologia. 1999;37:989–997. doi: 10.1016/s0028-3932(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Lauer EW. The nuclear pattern and fiber connections of certain basal telencephalic centers in the macaque. J. Comp. Neurol. 1945;82:215–234. [Google Scholar]
- Le TH, Pardo JV, Hu X. 4 T-fMRI study of nonspatial shifting of selective attention: cerebellar and parietal contributions. J. Neurophysiol. 1998;79:1535–1548. doi: 10.1152/jn.1998.79.3.1535. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Fig LM, Decker LR, Koeppe RA, Minoshimam S. Limbic activation and psychophysiologic responses to aversive visual stimuli. Interaction with cognitive task. Neuropsychopharmacology. 2000;23:508–516. doi: 10.1016/S0893-133X(00)00157-3. [DOI] [PubMed] [Google Scholar]
- Lou HC, Luber B, Crupain M, Keenan JP, Nowak M, Kjaer TW, Sackeim HA, Lisanb SH. Parietal cortex and representation of the mental self. Proc. Natl. Acad. Sci. 2004;101:6827–6832. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum. Brain Mapp. 2003;18:30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Soelch C, Leenders KL, Chevalley AF, Missimer J, Künig G, Magyar S, Mino A, Schultz W. Reward mechanisms in the brain and their role in dependence: evidence from neurophysiological and neuroimaging studies. Brain Res. Brain Res. Rev. 2001;36:139–149. doi: 10.1016/s0165-0173(01)00089-3. [DOI] [PubMed] [Google Scholar]
- Matthews SC, Strigo IA, Simmons AN, Yang TT, Paulus MP. Decreased functional coupling of the amygdala and supragenual cingulate is related to increased depression in unmedicated individuals with current major depressive disorder. J. Affect. Disord. 2008;111:13–20. doi: 10.1016/j.jad.2008.05.022. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am. J. Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Ojemann JG, Petersen SE, Ollinger JM, Snyder AZ, Akbudak E, Conturo TE, Raichle ME. Direct comparison of episodic encoding and retrieval of words: an event-related fMRI study. Memory. 1999;7:661–678. doi: 10.1080/096582199387797. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. NeuroScience. 1991;44:15–33. doi: 10.1016/0306-4522(91)90248-m. [DOI] [PubMed] [Google Scholar]
- McGaraughty S, Heinricher MM. Microinjection of morphine into various amygdaloid nuclei differentially affects nociceptive responsiveness and RVM neuronal activity. Pain. 2002;96:153–162. doi: 10.1016/s0304-3959(01)00440-7. [DOI] [PubMed] [Google Scholar]
- Murray EA, Wise SP. Interactions between orbital prefrontal cortex and amygdala: advanced cognition, learned responses and instinctive behaviors. Curr. Opin. Neurobiol. 2010;20:212–220. doi: 10.1016/j.conb.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Sawamoto N, et al. Transient neural activity in the medial superior frontal gyrus and precuneus time locked with attention shift between object features. NeuroImage. 1999;10:193–199. doi: 10.1006/nimg.1999.0451. [DOI] [PubMed] [Google Scholar]
- Nickl-Jockschat T, Schneider F, Pagel AD, Laird AR, Fox PT, Eickhoff SB. Progressive pathology is functionally linked to the domains of language and emotion: meta-analysis of brain structure changes in schizophrenia patients. Eur. Arch. Psychiatry Clin. Neurosci. 2011;261(Suppl. 2):S166–S171. doi: 10.1007/s00406-011-0249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen FA. The Brede database: a small database for functional neuroimaging. NeuroImage. 2003;19(2) [Google Scholar]
- Northoff G, Heinzel A, Bermpohl F, Niese R, Pfennig A, Pascual-Leone A, Schlaug G. Reciprocal modulation and attenuation in the prefrontal cortex: an fMRI study on emotional-cognitive interaction. Hum. Brain Mapp. 2004;21:202–212. doi: 10.1002/hbm.20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn. Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludloow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC. Reflecting upon feelings: an MRI study of neural systems supporting the attribution of emotion to self and other. J. Cogn. Neurosci. 2004;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Paré D, Smith Y. The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. NeuroScience. 1993;57:1077–1090. doi: 10.1016/0306-4522(93)90050-p. [DOI] [PubMed] [Google Scholar]
- Peper M, Herpers M, Spreer J, Hennig J, Zentner J. Functional neuroimaging of emotional learning and autonomic reactions. J. Physiol. Paris. 2006;99:342–354. doi: 10.1016/j.jphysparis.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Attentional control of the processing of neural and emotional stimuli. Brain Res. Cogn. Brain Res. 2002;15:31–45. doi: 10.1016/s0926-6410(02)00214-8. [DOI] [PubMed] [Google Scholar]
- Petrides M, Mackey S. The orbitofrontal cortex: sulcal and gyral morphology and architecture. In: Zald D, Rauch S, editors. The orbitofrontal Cortex. Oxford: Oxford University Press; 2006. pp. 19–37. [Google Scholar]
- Petrovich GD, Risold PY, Swanson LW. Organization of projections from the basomedial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol. 1996;374:387–420. doi: 10.1002/(SICI)1096-9861(19961021)374:3<387::AID-CNE6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr. Opin. Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu. Rev. Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:1751–1787. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol. Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Stefanacci L, Farb CR, Go GG, LeDoux JE, Amaral DG. Intrinsic connections of the rat amygdaloid complex: projections originating in the lateral nucleus. J Comp Neurol. 1995;356:288–310. doi: 10.1002/cne.903560211. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Amaral DG. Organization of the intrinsic connections of the monkey amygdaloid complex: projections originating in the lateral nucleus. J Comp Neurol. 1998;398:431–458. doi: 10.1002/(sici)1096-9861(19980831)398:3<431::aid-cne9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Platel H, Baron JC, Desgranges B, Bernard F, Eustache F. Semantic and episodic memory of music are subserved by distinct neural networks. NeuroImage. 2003;20:2444–2456. doi: 10.1016/s1053-8119(03)00287-8. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Fletcher PC, Henson RN, Worsley KJ, Brett M, Nichols TE. Guidelines for reporting an fMRI study. NeuroImage. 2008;40:409–414. doi: 10.1016/j.neuroimage.2007.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O, Gramann K, Schandry R. Neural systems connecting interoceptive awareness and feelings. Hum. Brain Mapp. 2007;28:9–18. doi: 10.1002/hbm.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb. Cortex. 2006;16:1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Price JL. Definition of the orbitofrontal cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Ann. N. Y. Acad. Sci. 2007;1121:54–71. doi: 10.1196/annals.1401.008. [DOI] [PubMed] [Google Scholar]
- Rademacher L, Krach S, Kohls G, Irmak A, Gründer G, Spreckelmeyer KN. Dissociation of neural networks for anticipation and consumption of monetary and social rewards. NeuroImage. 2010;49:3276–3285. doi: 10.1016/j.neuroimage.2009.10.089. [DOI] [PubMed] [Google Scholar]
- Reetz K, Dogan I, Rolfs A, Binkofski F, Schultz JB, Laird AR, Fox PT, Eickhoff SB. Investigating function and connectivity of morphometric findings — exemplified on cerebellar atrophy in spinocerebellar ataxia 17 (SCA17) NeuroImage. 2012;62:1354–1366. doi: 10.1016/j.neuroimage.2012.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehme AK, Eickhoff SB, Rottschy C, Fink GR, Grefkes C. Activation likelihood estimation meta-analysis of motor-related neural activity after stroke. NeuroImage. 2012;59:2771–2782. doi: 10.1016/j.neuroimage.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL. Role of orbitofrontal cortex connections in emotion. Ann. N. Y. Acad. Sci. 2007;1121:72–86. doi: 10.1196/annals.1401.026. [DOI] [PubMed] [Google Scholar]
- Richter-Levin G. The amygdala, the hippocampus, and emotional modulation of memory. Neuroscientist. 2004;10:31–39. doi: 10.1177/1073858403259955. [DOI] [PubMed] [Google Scholar]
- Roberts AC. Primate orbitofrontal cortex and adaptive behaviour. Trends Cogn. Sci. 2006;10:83–90. doi: 10.1016/j.tics.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT. Metaanalytic connectivity modeling: delineating the functional connectivity of the human amygdala. Hum. Brain Mapp. 2010;31:173–184. doi: 10.1002/hbm.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Blangero J, Sanghera MK, Pessoa L, Fox PM, Uecker A, Friehs G, Young KA, Griffin JL, Lovallo WR, Fox PT. The functional connectivity of the human caudate: an application of meta-analytic connectivity modeling with behavioral filtering. NeuroImage. 2012;60:117–129. doi: 10.1016/j.neuroimage.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. The Brain and Emotion. Oxford, UK: Oxford University Press; 1999. [Google Scholar]
- Russchen FT, Bakst I, Amaral DG, Price JL. The amygdalostriatal projections in the monkey. An anterograde tracing study. Brain Res. 1985;329:241–257. doi: 10.1016/0006-8993(85)90530-x. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol. Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sander D, Grandjean D, Scherer KR. A systems approach to appraisal mechanisms in emotion. Neural Netw. 2005;18:317–352. doi: 10.1016/j.neunet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Savander V, Go CG, LeDoux JE, Pitkänen A. Intrinsic connections of the rat amygdaloid complex: projections originating in the basal nucleus. J Comp Neurol. 1995;361:345–368. doi: 10.1002/cne.903610211. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Scherer KR. Appraisal considered as a process of multi-level sequential checking. In: Scherer KR, Schorr A, Johnstone T, editors. Appraisal Processes in Emotion: Theory, Methods, Research. New York: Oxford University Press; 2001. pp. 92–120. [Google Scholar]
- Schilbach L, Eickhoff SB, Mojzisch A, Vogeley K. What’s in a smile? Neural correlates of facial embodiment during social interaction. Soc Neurosci. 2008;3:37–50. doi: 10.1080/17470910701563228. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Eickhoff SB, Cieslik E, Shah NJ, Fink GR, Vogeley K. Eyes on me: an fMRI study of the effects of social gaze on action control. Soc. Cogn. Affect. Neurosci. 2011;6:393–403. doi: 10.1093/scan/nsq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleicher A, Palomero-Gallagher N, Morosan P, Eickhoff SB, Kowalski T, de Vos K, Amunts K, Zilles K. Quantitative architectural analysis: a new approach to cortical mapping. Anat. Embryol. (Berl) 2005;210:373–386. doi: 10.1007/s00429-005-0028-2. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC. Relevance to self: a brief review and framework of neural systems underlying appraisal. Neurosci. Biobehav. Rev. 2007;31:585–596. doi: 10.1016/j.neubiorev.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Neural coding of basic reward terms of animal learning theory, game theory, microeconomics, and behavioral ecology. Curr. Opin. Neurobiol. 2004;14:139–147. doi: 10.1016/j.conb.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L. Involvement of primate orbitofrontal neurons in reward, uncertainty, and learning. In: Zald DH, Rauch SL, editors. In The Orbitofrontal Cortex. New York: Oxford University Press; 2006. pp. 173–198. [Google Scholar]
- Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 2008;32:811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Shallice T, Fletcher P, Frith CD, Grasby P, Frackowiak RS, Dolan RJ. Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature. 1994;368:633–635. doi: 10.1038/368633a0. [DOI] [PubMed] [Google Scholar]
- Simmons A, Matthews SC, Stein MB, Paulus MP. Anticipation of emotionally aversive visual stimuli activates right insula. NeuroReport. 2004;15:2261–2265. doi: 10.1097/00001756-200410050-00024. [DOI] [PubMed] [Google Scholar]
- Smith AP, Stephan KE, Rugg MD, Dolan RJ. Task and content modulate amygdala-hippocampal connectivity in emotional retrieval. Neuron. 2006;49:631–638. doi: 10.1016/j.neuron.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;4:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagishi M, Chilba T. Efferent projections of the infralimbic (area 25) region of the medial prefrontal cortex in the rat: an anterograde tracer PHA-L study. Brain Res. 1991;566:26–39. doi: 10.1016/0006-8993(91)91677-s. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Liberzon I, Koeppe RA. The effect of graded aversive stimuli on limbic and visual activation. Neuropsychologia. 2000;38:1415–1425. doi: 10.1016/s0028-3932(00)00032-4. [DOI] [PubMed] [Google Scholar]
- Thomas CG, Harshman RA, Menon RS. Noise reduction in BOLD-based fMRI using component analysis. NeuroImage. 2002;17:1521–1537. doi: 10.1006/nimg.2002.1200. [DOI] [PubMed] [Google Scholar]
- Todorov A, Said CP, Oosterhof NN, Engell AD. Task-invariant brain responses to the social value of faces. J. Cogn. Neurosci. 2011;23:2766–2781. doi: 10.1162/jocn.2011.21616. [DOI] [PubMed] [Google Scholar]
- Torta DM, Cauda F. Different functions in the cingulate cortex, a meta-analytic connectivity modeling study. NeuroImage. 2011;56:2157–2172. doi: 10.1016/j.neuroimage.2011.03.066. [DOI] [PubMed] [Google Scholar]
- Tulving E. Elements of Episodic Memory. New York: Oxford University Press; 1983. [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum. Brain Mapp. 2012;33:1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dillen LF, Koole SL. Clearing the mind: a working memory model of distraction from negative mood. Emotion. 2007;7:715–723. doi: 10.1037/1528-3542.7.4.715. [DOI] [PubMed] [Google Scholar]
- Van Horn JD, Grethe JS, Kostelec P, Woodward JB, Aslam JA, Rus D, Rockmore D, Gazzaniga MS. The Functional Magnetic Resonance Imaging Data Center (fMRIDC): the challenges and rewards of large-scale databasing of neuroimaging studies. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001;356:1323–1339. doi: 10.1098/rstb.2001.0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Lindquist M, Kaplan L. Meta-analysis of functional neuroimaging data: current and future directions. Soc Cogn Affect Neurosci. 2007;2:150–158. doi: 10.1093/scan/nsm015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Annu. Rev. Neurosci. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. NeuroImage. 2009;47:1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Zaudig M, Fydrich T. Strukturiertes Klinisches Interview für DSM-IV. Hogrefe, Göttingen. 1997 [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Text-mining, meta-analysis and machine-learning techniques to generate a large database of mappings between neural and cognitive states: large-scale automated synthesis of human functional neuroimaging data. Nat. Methods. 2011;8:665–870. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res. Brain Res. Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- Zald DH, McHugom M, Ray KL, Glahn DC, Eickhoff SB, Laird AR. Meta-Analytic Connectivity Modeling Reveals Differential Functional Connectivity of the Medial and Lateral Orbitofrontal Cortex. Cereb. Cortex, in print. 2012 doi: 10.1093/cercor/bhs308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Amunts K. Centenary of Brodmann’s map — conception and fate. Nat. Rev. Neurosci. 2010;11:39–45. doi: 10.1038/nrn2776. [DOI] [PubMed] [Google Scholar]
- zu Eulenburg P, Caspers S, Roski C, Eickhoff SB. Meta-analytical definition and functional connectivity of the human vestibular cortex. NeuroImage. 2012;60:162–169. doi: 10.1016/j.neuroimage.2011.12.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.