Abstract
Frankia Sp+ strains maintain their ability to sporulate in symbiosis with actinorhizal plants, producing abundant sporangia inside host plant cells, in contrast to Sp− strains, which are unable to perform in-planta sporulation. We herein examined the role of in-planta sporulation in Frankia infectivity and competitiveness for root infection. Fifteen strains belonging to different Sp+ and Sp− phylogenetic lineages were inoculated on seedlings of Alnus glutinosa (Ag) and A. incana (Ai). Strain competitiveness was investigated by performing Sp−/Sp+ co-inoculations. Plant inoculations were standardized using crushed nodules obtained under laboratory-controlled conditions (same plant species, age, and environmental factors). Specific oligonucleotide primers were developed to identify Frankia Sp+ and/or Sp− strains in the resulting nodules. Single inoculation experiments showed that (i) infectivity by Sp+ strains was significantly greater than that by Sp− strains, (ii) genetically divergent Sp+ strains exhibited different infective abilities, and (iii) Sp+ and Sp− strains showed different host preferences according to the origin (host species) of the inocula. Co-inoculations of Sp+ and Sp− strains revealed the greater competitiveness of Sp+ strains (98.3 to 100% of Sp+ nodules, with up to 15.6% nodules containing both Sp+ and Sp− strains). The results of the present study highlight differences in Sp+/Sp− strain ecological behaviors and provide new insights to strengthen the obligate symbiont hypothesis for Sp+ strains.
Keywords: In-planta sporulation, Frankia, competitiveness, infectivity, host-range, actinorhizal symbiosis
Frankia is a soil filamentous actinobacterium that is capable of forming N2-fixing symbiotic root nodules on a diverse array of actinorhizal plants. Hyphae, diazovesicles (specialized cells for N2 fixation under aerobic conditions), and multilocular sporangia are the typical cell morphological features of Frankia grown in cultures (4, 28). Unlike hyphae and diazovesicles, sporangia are not constantly observed in-planta. Two types of nodules have been identified: Sp+ nodules, hosting abundant sporangia inside plant cells, and sporangia-free Sp− nodules (43). Sp+ nodules have been detected in 9 out of 23 actinorhizal plant genera, and at a high frequency in the genus Alnus (37). Historically, in-planta sporulation is considered to be a genetically stable characteristic (44), suggesting the existence of two types of Frankia strains (Sp+ vs Sp−). However, the inability to isolate strains from Sp+ nodules, despite numerous attempts, has limited their study.
To the best of our knowledge, Sp+ Frankia strains represent a unique model of endophytic symbionts with the ability to sporulate inside host cells. When sporulation occurs during plant infection, for example in the case of pathogenic fungi, spores are only produced on the surface of infected or necrotic tissues (40) and never inside living plant cells. Spores are dormant cells generally described as an optional adaptive strategy permitting spatial and temporal escape from local conditions that are unfavorable to growth (7, 25). In a symbiotic context, the ecological advantage of in-planta sporulation may be linked to the fate of numerous spores produced within Sp+ nodules once released into the soil. After nodule decay, spores provide abundant infective propagules, thereby increasing Sp+ strain infectivity and competitiveness in the soil environment (32). A limited number of studies, using host plant inoculations, have analyzed the behaviors of Sp+ and Sp− strains in the rhizosphere or during root infection, and suggested the higher infectivity of Sp+ (2, 13, 46). Sp+ Frankia strains may also differ in their host specificity (17, 23) and competitiveness for nodulation in co-inoculation assays (18).
However, these findings have been biased by the heterogeneity of the material used as inocula. Due to the non-cultivability of Sp+ strains, Sp− cultured cells have often been used as opposed to Sp+ crushed nodules in inoculation experiments, even though the phenolic compounds and tannins contained in nodule tissues are known to have an impact on symbiotic associations (20, 31). Furthermore, most of these studies were limited to Sp+ nodules from only one host origin (except in the large study by Markham et al. [23]) and, at that time, information was not available on the genetic diversity of Sp+ strains. A recent study conducted on Alnus field nodules from diverse geographical origins revealed at least two divergent lineages among Sp+ strains according to the host plant species: A. incana and A. viridis strains were grouped into clade 1 (1a and 1b, respectively), whereas A. glutinosa Sp+ strains were grouped into clade 5 (32). This genetic diversity suggests diversity in strain life history traits and, thus, is considered to have an impact on the ecological behavior of Sp+ strains. In addition, in the absence of molecular tools available to discriminate between Sp+ and Sp−, strain identification was based on microscopic observations of spores from hand-cut nodule sections. However, it was not possible to rule out false Sp− identification due to delays in the expression of the Sp+ phenotype, and the co-occurrence of both types of strains in the same nodule may have been missed in co-inoculation experiments (3).
Therefore, the aim of the present study was to re-examine the role of Frankia in-planta sporulation in (i) the infectivity and host range of Frankia strains and (ii) their competitiveness for root infection. Fifteen nodule sources of Sp+ and Sp− Frankia strains from three different Alnus species (A. glutinosa, A. incana, and A. viridis) were used, including representative strains from the different Sp+ phylogenetic lineages recently described (32). Sp+ and Sp− inocula were tested on A. glutinosa and A. incana host seedlings, and the resulting nodules were characterized at the molecular level using Sp+ and Sp− strain-specific oligonucleotide primers.
Materials and Methods
Plant culture
A. glutinosa and A. incana seeds from Lyon (Rhône, France) and Fond de France (Isère, France), respectively, were surface-sterilized by agitation for 30 min in absolute alcohol followed by 30 min in Ca(ClO)2 3% (w/v) with 50 μL L−1 of Tween 20, and then rinsed three times in sterile water. Seeds were then incubated at room temperature on Fåhraeus medium (8) agar (10 g L−1) for germination. After 15 d, seedlings were transplanted into hydroponic pouches (8) containing 30 mL of Fåhraeus medium with 5 mM NH4Cl and incubated in a growth chamber (16-h d with 22°C day/18°C night temperatures).
Selection of Sp+ and Sp− Frankia strains from field nodules
Since no pure-cultured Sp+ Frankia have been obtained to date, all Frankia strains were obtained from field nodules, including Sp− nodules, in order to ensure similar inoculation conditions for both types of strains. Nodules containing either Sp+ or Sp− Frankia were collected between September and October 2012 from different sites (previously described by Pozzi et al. [32]) and 3 different Alnus species: A. glutinosa (L.) Gaertn, A. incana (L.) Moench, and A. alnobetula (Ehrh.) K. Koch, hereafter referred to as A. viridis (Chaix) DC (Ag, Ai, and Av, respectively) (Table 1).
Table 1.
Sp+ and Sp− field nodules used as inocula
| Nodule phenotype1 | Alnus species | Site designation2 | Nodule acronym | No. of inoculated seedlings3 | |
|---|---|---|---|---|---|
|
| |||||
| A. glutinosa | A. incana | ||||
| Sp+ | A. glutinosa | Thury | AgTyI.5 | 40 | 25 |
| Le Tremblay, Le-Bourget-du-Lac | AgTrS1 | 40 | 25 | ||
| A. incana | Ornon | AiOR8 | 40 | 24 | |
| Allemont | AiAll | 42 | 27 | ||
| A. viridis | La Bérarde | AvBI.5 | 40 | 25 | |
| Col de la Croix de Fer | AvCf11.1 | 23 | 27 | ||
| La Toussuire | AvToI.2 | 40 | 27 | ||
|
| |||||
| Sp− | A. glutinosa | Arandon | AgARaG1 | 25 | 25 |
| Le Blanchet, Bourget-en-Huile | AgLB4.3 | 26 | 25 | ||
| Le Grand-Lemps | AgGL1 | 22 | NT | ||
| A. incana | Fond-de-France | AiFF2.1 | 25 | 24 | |
| Le Blanchet, Bourget-en-Huile | AiGBh | 17 | NT | ||
| A. viridis | Col de la Croix de Fer | AvCf3 | 22 | NT | |
| Col de la Croix de Fer | AvCf13.1 | 26 | 24 | ||
| Fond-de-France | AvFF1.1 | 28 | 26 | ||
Sp+ and Sp− = in-planta sporulating and non-sporulating phenotypes, respectively.
All sites from Pozzi et al. (32).
NT = non-tested conditions.
The nodule sporulation phenotype was determined (from at least one lobe) by microscopic observations of nodule hand sections stained with Lactophenol Blue (Réactifs RAL, Martillac, France), as previously described (32). Nodules were considered to have the Sp+ phenotype when more than one sporangium was observed in more than 50 infected plant cells. Otherwise, nodules were considered to have the Sp− phenotype. After surface sterilization of the nodules in calcium hypochlorite 3% (w/v) and peeling, nodular DNA was extracted and endophytic Frankia were genetically characterized by pgk and dnaA gene partial amplifications with specific primers (Table 2). Each PCR reaction contained in a final volume of 50 μL: 2 μL of DNA (0.1 μg mL−1), 1× PCR buffer, 0.2 mM of each dNTP, 2 mM MgCl2, 0.5 mM of each primer, 10% DMSO v/v, 16 μg of bovine serum albumin, and 2U Taq DNA polymerase (Invitrogen, Oxon, United Kingdom). The amplification conditions used were: initial denaturation at 94°C for 5 min, and 30 cycles including denaturation at 94°C for 1 min, annealing at 62°C for 1 min, and elongation at 72°C for 1 min. A final 10-min extension step was performed at 72°C. PCR products were purified using a MiniElute PCR purification Kit (Qiagen, Courtaboeuf, France) before sequencing (Biofidal, Villeurbanne, France) with an ABI 3730xl DNA Analyzer (Applied Biosystems, Foster City, California, USA). The resulting sequence alignments and pairwise-distance matrix calculations were performed using BioEdit software (version 7.2.2.). At least 5 lobes were genetically characterized for each nodule. Nodules for which all tested lobes resulted in pgk and dnaA sequences with more than 99.9% similarity were considered to contain a single Frankia strain. All nodules used as inocula in subsequent experiments met this criterion (data not shown). Seven Sp+ strains and 8 Sp− strains were used. Among the Sp+ strains, the two clades previously described (32) were represented with 2 Ag strains belonging to clade 5, and 2 Ai and 3 Av strains belonging to clade 1 (Table 1).
Table 2.
List of PCR primers used for Frankia strain genotyping
| Gene | Primer name1 | Sequence (5′→3′) | Tm (°C) |
|---|---|---|---|
| Field nodule identification (infectivity experiments) | |||
| dnaA | dnAfdT7F | TAATACGACTCACTATAGGGGAGGARTTCACCAACGACTTCAT | 62 |
| dnArvT3R | ATTAACCCTCACTAAAGGGACRGAAGTGCTGGCCGATCTT | ||
| pgk | pgkFwdT3 | ATTAACCCTCACTAAAGGGATGAGGACGATCGACGACCTGC | 62 |
| pgkRevT7 | TAATACGACTCACTATAGGGCGCSAGGAAGGTGAAGCACAT | ||
|
| |||
| Sp+/Sp− discrimination in nodules (co-inoculation experiments) | |||
| dnaA | F2 Sp− | CCATGGAGACGCCGAAGTAC (1341)2 | 65 |
| F3 Sp− | CGTCCGGGATCAGGTCG (1275) | 64–65 | |
| R2 Sp− | CATCGCGATCCTGTCGAAGAAG (1089) | 65 | |
| F3 Sp+ | GCGTCAGGGATCAGGTCA (1275) | 64 | |
| R2 Sp+ | CATCGCGATCCTGTCGAAAAAA (1089) | 64 | |
“fd”, “Fwd”, or “F” in the name indicate forward primers and “rv”, “Rev”, or “R” indicate reverse primers.
Position on the dnaA sequence of the ACN14a strain (GenBank accession NC_008278).
Plant inoculation and growth assessments
Two distinct experiments were carried out in order to compare Sp+ and Sp− strain infectivity (experiment A) and competitiveness (experiment B).
Experiment A (Sp+/Sp− infectivity)
Two-month-old seedlings were transferred to nitrogen-free medium 24 h before inoculation. Each Sp+ and Sp− Frankia strain was inoculated on 17–42 seedlings per condition (Table 1) (total of 760 Ag and Ai seedlings), using a suspension of crushed nodules freshly collected in the field and stored at 4°C for a few days. The inocula density was used as described by Périnet et al. (29), with 2 g of nodules per one thousand seedlings. This relatively low density was selected in order to avoid the saturation of root infection sites, thereby allowing different nodulation rates to be highlighted. In each strain, field nodules were washed in 3% Ca(ClO)2 (w/v) with Tween 20 (50 μL mL−1), rinsed in sterile water, and then crushed with 3% (w/v) polyvinylpyrrolidone (PVP). Nodule suspensions were filtered through a 100-μm mesh filter (17). Plant roots were inoculated with 3 mL of crushed nodule suspensions directly in the hydroponic pouches and the seedlings were then incubated in a plant growth chamber for 5 months. Fåhraeus medium (8) was added weekly. In each plant, the total number of nodules was monitored every 2 weeks, and plant growth was followed by measuring the longest root and stem height.
Experiment B (Sp+/Sp− competitiveness)
Competitiveness experiments focused on Ag infective strains. The two Ag Sp+ strains, AgTrS1 and AgTyI.5, obtained from field nodules (Table 1) were confronted with two in-vitro cultured Sp− strains, ACN14a (28) and Mg60Ag2 (9). These two Sp− strains were selected because of (i) their genetic divergence with Ag Sp+ strains, allowing the design of specific oligonucleotides, and (ii) their different infectivities, with ACN14a being less infective than Mg60Ag2 on Ag. In order to standardize inocula for the competitiveness experiments (same age and plant origin of the crushed nodules), each of the four strains was inoculated on Ag seedlings grown in pouches. After approximately 4 months, the nodules produced were harvested and used to prepare crushed nodule suspensions. Four distinct Sp+/Sp− mixtures (AgTrS1/ACN14a, AgTrS1/Mg60Ag2, AgTyI.5/ACN14a, and AgTyI.5/Mg60Ag2) with 3 different ratios (v/v) were prepared: 15/85, 50/50, and 85/15 (percentage of Sp+ / Sp− inocula in the final volume), leading to 12 distinct conditions. A total of 15–16 plants per condition were inoculated using the previously described protocol. Four additional conditions based on single strain inoculations were included as controls. After 5 months in a growth chamber (the same conditions as those described for Experiment A), nodules were recorded and plant growth assessed as described above.
Genotypic identification of Frankia strains present in nodules of inoculated plants
Experiment A (Sp+/Sp− infectivity)
Five months after inoculation, at least 2 nodules per plant were phenotyped and genotyped using pgk and dnaA gene sequencing, as previously described, in order to ensure that the Frankia strains in the inocula were the same as those found in nodules.
Experiment B (Sp+/Sp− competitiveness)
Under each condition, at least 30 nodules randomly sampled from all plants were surface-sterilized and their DNA extracted as previously described (32). The following Sp+ and Sp− primers specific for dnaA were used: F3 Sp+ and R2 Sp+ primers for Sp+ strains, F3 Sp− and R2 Sp− primers for the ACN14a strain, and F2 and R2 primers for the Mg60Ag2 strain (Table 2). Nodular DNA was PCR amplified with Sp+ and Sp−-specific primers as described above. Amplicons were characterized by 2% w/v agarose gel electrophoresis.
Statistical analyses
Statistical analyses were carried out using R software v. 3.0.1 (41).
Experiment A (Sp+/Sp− infectivity)
Chi-squared statistics with one degree of freedom were used to compare nodulated plant proportions, in order to test the effects of the strain-sporulating phenotype and host plant origin on the ability to infect Ag and Ai seedlings. The effects of possible interactions between these factors (in addition to the factor “species of inoculated seedlings”) on strain infectivity were tested with a Generalized Linear Model (GLM) on nodulated plant proportion data.
An ANOVA (Analysis Of Variance) followed by post hoc analyses (Tukey’s Honestly Significant Difference-HSD-tests) were used to test the effects of the strain phenotype and host plant origin on strain nodulation rates by comparing the log nodule number data (for normalized distribution) of infected plants.
Root length and stem height data were summed to calculate the “growth index” (cm) of nodulated plants. Since “growth index” data were not normally distributed, the effects of the strain-sporulating phenotype on plant development (growth index) was tested using Wilcoxon rank sum statistical tests.
Experiment B (Sp+/Sp− competitiveness)
Plant nodule numbers and growth indices were compared between the different conditions of co-inoculations and Sp+ controls (single Sp+ strain inoculations) in order to test the effects of the mixed strain co-inoculation on nodulation rate and plant development. An ANOVA followed by post hoc analyses (Tukey’s Honestly Significant Difference-HSD-tests) were used to compare the log nodule number data (for normalized distribution) of infected plants. Since growth index data were not normally distributed, Wilcoxon rank sum statistical tests were used.
Results
Infectivity of Sp+ and Sp− Frankia strains and plant growth
Seven Sp+ strains and 8 Sp− strains were inoculated on 456 Ag and 304 Ai seedlings. Three inocula never nodulated under the tested conditions (AvTol.2, AiGBh, and AvFF1.1). In all infective inocula, the first nodules appeared 2 weeks after inoculation and presented at least 3 lobes 60 days after inoculation. Phenotypic and genotypic characterizations always confirmed the presence of the Frankia strain used as the initial inoculum.
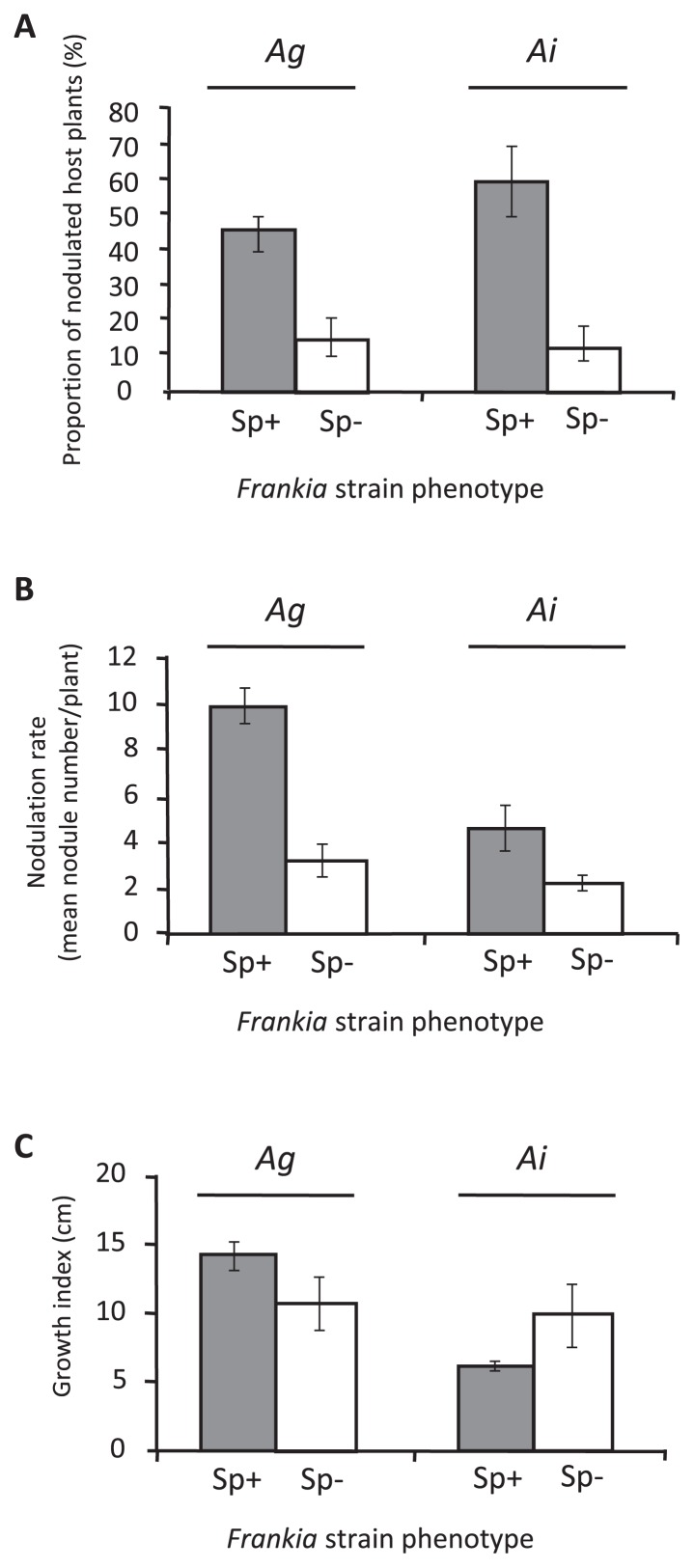
The percentage of nodulated seedlings was significantly higher for Sp+ strains than for Sp− strains, on both Ag and Ai inoculated hosts (Fig. 1A). In total, 46.8% and 13.6% of Ag (Chi2 test, p-value=3.170e−7) and 60.6% and 10.5% of Ai (Chi2 test, p-value=1.350e−13) were infected by Sp+ and Sp− strains, respectively. The nodulation rate (mean nodule number per plant) similarly varied between Sp+ and Sp− strains (Fig. 1B). Sp+ strains produced significantly more nodules than Sp− strains on Ag seedlings (9.9±0.8 vs 3.2±0.9 nodules per plant, respectively, ANOVA, p-value=1.450e−6) and Ai seedlings (4.6±0.8 vs 2.2±0.3 nodules per plant, respectively, ANOVA, p-value=3.360e−3). Av strains presented different behaviors according to the inoculated host plant species (Table 3). While Av strains followed the general scheme on Ai (higher proportion of nodulated seedlings for Sp+ than for Sp− strains), they were weakly infective on Ag, irrespective of their phenotype (less than 6% of nodulated plants).
Fig. 1.
Effects of the Frankia strain-sporulating phenotype (Sp+ or Sp−) on the ability to infect A. glutinosa (Ag) and A. incana (Ai) seedlings (A), their nodulation rate (B), and plant growth (C) with “Growth index” (cm) corresponding to the sum between the root length and stem height data. Fig. B and C only include nodulated plants.
Error bars indicate 95% confidence intervals (CI) computed with the modified Wald method (1) (A) or standard deviations (B and C).
Table 3.
Sp+ and Sp− strain infectivities on Alnus glutinosa and A. incana seedlings.
| Inoculated strains | % of nodulated plants1 | Nodule mean number per plant2 | Plant growth index (cm)2 | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||
| Phenotype | Original Alnus species | A. glutinosa | A. incana | A. glutinosa | A. incana | A. glutinosa | A. incana |
| Sp+ | A. glutinosa | 96.3 (80) | 84.0 (50) | 13.7±0.8 | 5.2±0.5 | 18.7±1.1 | 6.3±0.6 |
| A. incana | 52.4 (82) | 82.4 (51) | 7.0±0.0 | 4.9±0.4 | 6.6±0.4 | 4.5±0.3 | |
| A. viridis | 3.9 (103) | 31.6 (79) | 2.3±0.6 | 3.3±0.7 | 10.8±3.3 | 8.4±0.5 | |
|
| |||||||
| Sp− | A. glutinosa | 27.4 (73) | 16.0 (50) | 3.0±0.9 | 1.9±0.4 | 8.1±1.2 | 6.8±1.2 |
| A. incana | 4.8 (42) | 16.7 (24) | 4.1±0.5 | 3.0±1.2 | 30.7±1.7 | 17.5±5.2 | |
| A. viridis | 5.3 (76) | 2.0 (50) | 1.5±0.5 | 1.0 (1 plant) | 13.7±4.9 | 5.0 (1 plant) | |
(n) number of inoculated seedlings.
only nodulated plants are included.
Multifactorial statistical analyses showed the significant effects of the interactions between strain phenotype, strain origin, and inoculated host species on strain infectivity expressed as the number of nodulated seedlings (GLM, p-value=0.007) and nodule number per plant (ANOVA, p-value=0.007).
The Sp+ strains chosen belonged to two divergent phylogenetic clades correlated to the host plant species: clade 1 (Ai and Av strains) and clade 5 (Ag strains) (Table 1) (32). The infectivity of clade 5 strains was significantly greater than that of clade 1 strains with a higher proportion of nodulated seedlings (91.5% vs 36.2% respectively, Chi2 test, p-value= 2.200e−16) and higher nodulation rate (10.7±0.8 vs 4.1±0.3 nodules per plant, respectively, ANOVA, p-value =1.060e−13).
As expected, Sp+ strains induced a lower plant index on Ai seedlings than Sp− strains (6.1 cm±0.3 and 9.9 cm±2.2, respectively—Wilcoxon rank sum tests, W=981, p-value= 0.024) (Fig. 1C). Conversely, no significant difference was observed on Ag seedlings (14.2 cm±0.9 and 10.7 cm±1.7, respectively—Wilcoxon rank sum tests, W=1309.5, p-value= 0.134). Depending on the strain origin (Ag, Ai, or Av nodules), Sp+ and Sp− inocula had different impacts on plant growth (Table 3): Ag Sp+ strains induced significantly more growth on Ag seedlings (Pairwise Wilcoxon rank sum test, p-value=6.800e−4) than on Ai seedlings (Pairwise Wilcoxon rank sum test, p-value>0.05). Ai Sp+ strains induced significantly less growth on Ai seedlings than Ai Sp− strains (Pairwise Wilcoxon rank sum test, p-value=1.910e−2), and similar results were observed on Ag seedlings (Pairwise Wilcoxon rank sum test, p-value>0.05).
Host compatibility of Sp+ and Sp− Frankia strains
Identical host compatibility was observed for Sp+ and Sp− strains, with both being able to infect Ag and Ai seedlings (Table 3). Among the Sp+ strains tested, host preferences were observed according to their genotype. Clade 5 Sp+ strains infected significantly more Ag than Ai seedlings (96.3% vs 84.0%, respectively—Chi2 test, p-value=0.034), whereas clade 1 Sp+ strains infected significantly more Ai than Ag seedlings (51.5% vs 25.4%, respectively—Chi2 test, p-value=3.61e−6). In contrast, none of the Sp− strains showed significant differences in infected plant proportions between Ag and Ai seedlings (Chi2 test, p-value>0.05), although the same results were observed (different host preferences of Ag, Ai, and Av Sp− strains).
Competitiveness of Sp+ and Sp− co-inoculated Frankia strains
Two Sp+ strains (AgTyI.5 and AgTrS1) and two Sp− strains (ACN14a and Mg60Ag2) were used to assess their competitiveness for Ag infection under co-inoculation conditions. Under single inoculation conditions (control plants), the Sp− strain Mg60Ag2 (9.8±1.4 nodules per plant) and the two Sp+ strains had similar nodulation rates (Tukey’s HSD tests, p-value>0.05). As expected, the ACN14a strain induced significantly fewer nodules than the 3 other strains (3.5±0.9 versus 10.7±0.9 nodules per plant, Tukey’s HSD tests, p-value<0.05).
The Frankia strain(s) present in nodules were identified using dnaA sequences. Irrespective of the strain used and the Sp+/Sp− ratio in the inocula, Sp+ strains were present in 98.3 to 100% of the nodules (Table 4). Under 5 of the tested conditions, Sp+ strains were found to co-exist with the Sp− strain in the same nodule (3.3 to 15.8% of the nodules). Sp− strains never recovered alone, with the exception of the 15/85 AgTyI.5/Mg60Ag2 inoculum, in which 1.7% of the nodules were Sp−. ACN14a, the low infective Sp− strain, was totally absent (100% of Sp+ nodules) in 5 out of the 6 co-inoculation tested conditions.
Table 4.
Competitiveness of co-inoculated Sp+ and Sp− strains on Alnus glutinosa host plants.
| Inoculated strains | Sp+ / Sp− ratio | Nb nodule per plant | Nodule proportions (%)1 | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Sp+ | Sp− | Sp+ | Sp− | Sp+/Sp− | ||
| AgTyI.5 | None (Sp+ control) | 100/0 | 10.0±1.3 | 100.0 | 0.0 | 0.0 |
|
| ||||||
| Mg60Ag2 | 85/15 | 8.2±1.0 | 96.7 | 0.0 | 3.3 | |
| 50/50 | 6.9±1.0 | 84.2 | 0.0 | 15.8 | ||
| 15/85 | 5.9±0.6 | 98.3 | 1.7 | 0.0 | ||
|
| ||||||
| ACN14a | 85/15 | 7.5±1.9 | 100.0 | 0.0 | 0.0 | |
| 50/50 | 7.4±2.2 | 96.6 | 0.0 | 3.4 | ||
| 15/85 | 4.3 *±0.8 | 100.0 | 0.0 | 0.0 | ||
|
| ||||||
| AgTrS1 | None (Sp+ control) | 100/0 | 12.3±2.1 | 100.0 | 0.0 | 0.0 |
|
| ||||||
| Mg60Ag2 | 85/15 | 10.2±1.3 | 96.7 | 0.0 | 3.3 | |
| 50/50 | 8.9±1.9 | 100.0 | 0.0 | 0.0 | ||
| 15/85 | 6.7±1.1 | 96.7 | 0.0 | 3.3 | ||
|
| ||||||
| ACN14a | 85/15 | 12.5±1.9 | 100.0 | 0.0 | 0.0 | |
| 50/50 | 9.0±1.8 | 100.0 | 0.0 | 0.0 | ||
| 15/85 | 6.2±1.2 | 100.0 | 0.0 | 0.0 | ||
Sp+: % of nodules with strain AgTyI.5 or AgTrS1; Sp−: % of nodules with strain Mg60Ag2 or ACN14a; Sp+/Sp−: both Sp+ and Sp− co-existing strains.
Significantly different from the corresponding Sp+ control plants (without Sp− co-inoculation, grey lines), using Tukey’s HSD tests (p-value<0.05).
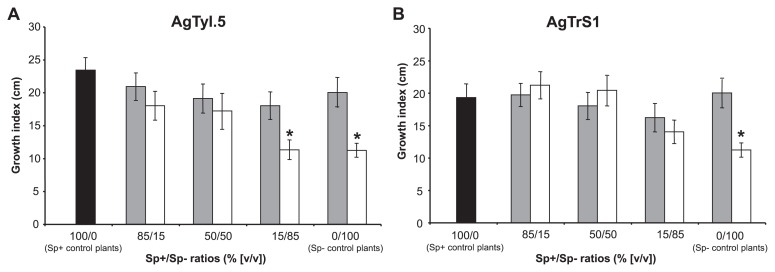
In both Sp+ strains tested, the number of nodules per plant slightly decreased with lower proportions in the inocula (Table 4). However, only the AgTyI.5/ACN14a co-inoculation with the ratio 15/85 was significantly different from the control (the AgTyI.5 single inoculation) (Tukey’s HSD test, p-value=4.464e−3).
No significant effect of the Sp+/Sp− co-inoculation was noted on plant growth, except for the 15/85 AgTyI.5/ACN14a and 100% ACN14a inocula (Sp− control plants), in which growth was significantly less than that in Sp+ control plants (Wilcoxon rank sum tests, p-value<0.05) (Fig. 2).
Fig. 2.
Effects of Sp+/Sp− Frankia strain co-inoculation on plant development. Two Sp+ strains were tested: AgTyI.5 (A) and AgTrS1 (B), in co-inoculations with two Sp− strains (Mg60Ag2 = grey bars and ACN14a = white bars). “Growth index” (cm) was the sum between the root length and stem height data. Error bars indicate 95% confidence intervals (CI). Stars indicate significant differences from the Sp+ control plants tested with Wilcoxon rank sum statistical tests (p-value<0.05).
Discussion
Sp+ Frankia strains represent a unique model of in-planta sporulation. Since bacterial sporulation commonly occurs in order to ensure survival under unfavorable growth conditions, its expression is paradoxical in a symbiotic context in which the nodule supplies a highly favorable ecological niche to the bacteria. Therefore, the role of the numerous spores produced in plant tissues is questionable. They may have an important ecological function in nodule decay, allowing new root infections once released into the rhizosphere. Decaying nodules may provide a source of infective particles depending on the survival and dispersal abilities of the bacteria released in the soil, as well as their competitiveness with other symbiotic strains. We herein attempted to examine Sp+ and Sp− strain behaviors in the rhizosphere by comparing their infectivity and competitiveness under co-inoculation conditions. In order to reflect the fate of decayed nodules in soil, exclusively crushed nodules were used as inocula. Since a large part of the released particles may be carried deep into the soil, with only small amounts reaching the roots, low density inocula were used.
Our results showed that Sp+ strains had (i) greater infectivity on Ag and Ai species (larger proportion of nodulated seedlings and higher nodulation rate—Fig. 1A and B) and (ii) better competitiveness than Sp− strains (Table 4). These two ecological traits are linked because the specific infectivities of two different strains are expected to condition the relative number of nodules they produce when simultaneously inoculated (26). The difference in infectivity and competitiveness between Sp+ and Sp− nodules may have been due to the higher number of infective particles released by Sp+ nodules. While one Sp− nodule releases only hyphae and vesicles in soils, one Sp+ nodule has the ability to also release a large number of spores. Based on microscopic observations of one thousand Sp+ nodule sections, we estimated that between 107 and 108 spores were released from 1 g of nodules (data not shown). The capacity of these spores to become infective propagules is supported by (i) their ability to germinate and form hyphae when transferred to fresh growth medium (16, 42), and (ii) the durability of nodulation capacity of old inocula (likely due to spore survival during culture senescence) (19). Moreover, isolated spores from cultured Frankia were shown to develop higher infectivity than hyphae (5). Spores released by Sp+ nodule tissues may completely invade the vicinity of the roots. Germination may occur in the soil in response to root secondary compounds, producing infective hyphae that may, in turn, induce new infections (16, 22, 42, 49). Alternatively, spores may also directly attach to the roots, as described for many species of sporulating biotrophic fungal pathogens (21, 48). The capacity of spores to rapidly saturate the root system, a limited resource, and, thus, deprive the Sp− strain of available infection sites, may result in competitive exclusion (10, 34) and the elimination of Sp− strains. Our results suggest that this occurs for low infectivity Sp− strains (e.g. ACN14a) (Table 4). Furthermore, the exclusion of Sp− strains by Sp+ strains may be the result of antagonistic interactions between both types of strains. Actinobacteria are known for their capacity to synthesize antibiotics and diverse secondary metabolites involved in antagonistic interactions with other microorganisms. Both competition types (competition for host infection sites or direct antagonism) have previously been described within symbiotic microbial populations (6, 14, 15, 26), and the combination of both cannot be excluded between Sp+ and Sp− strains. However, the non-cultivability of Sp+ strains renders these competition hypotheses hard to test in the case of actinorhizal symbioses.
The infectivity and competitiveness of rhizospheric bacteria may be modulated by the host plant. In the present study, no significant differences were observed in host compatibility between Sp+ and Sp− strains. Despite important variations in strain infectivity according to the inoculated plant species, both types of strains had the ability to infect Ag and Ai species. However, unlike Sp− strains, Sp+ Frankia strains showed differences in their ability to infect both host species. The infectivity of clade 5 (Ag) strains on Ag seedlings was significantly greater than that of clade 1 (Ai and Av) strains, whereas the infectivity of clade 1 strains was significantly greater on Ai seedlings than on Ag seedlings, confirming preliminary results (47) (Table 3). Therefore, Sp+ strains had better compatibility than Sp− strains when inoculated on their original host species, suggesting closer symbiotic relationships. Based on this hypothesis, some Alnus species may preferentially select Sp+ genotypes, as suggested by the higher occurrence of Sp+ strains in Av and Ai alder stands than in Ag alder stands (32). Symbiotic host plants are able to select their microbial partner; defense mechanisms are used during infection to regulate the root infection rate by a given strain (24, 27, 33). In the case of actinorhizal plants, the differential expression of such mechanisms has been reported based on metabolomic or transcriptomic approaches during the early infection steps, depending on the compatibility of the strains (11, 30, 31). Therefore, strain infectivity and competitiveness depend not only on the strain phenotype, but also additional factors, such as host-symbiont specificity, which strongly influence the success of infection.
The significant differences observed in infectivity and competitiveness between Sp+ and Sp− strains may have an impact on plant growth. Previous studies reported that Sp+ strains were less effective (plant dry matter, total N, and/or N-fixed) than Sp− strains (17, 38). By coincidence, both studies mainly based their conclusions on Ai Sp+ nodules and Sp− isolates from various hosts as inocula, tested on Ai host plants. Our results with Ai strains confirmed this conclusion (Fig. 1C). However, by testing different Sp+ and Sp− nodule origins from 3 different Alnus species, we unexpectedly found that Sp+ strains showed higher effectivity than Sp− (e.g. Ag strains on Ag seedlings). Thus, Sp+ and Sp− strains display different host specificities, which impacts not only on their infectivity and competitiveness for root infection, but also their effectivity. Moreover, by including nodules from several geographical origins, we noted a potential site effect on strain behavior (data not shown). Therefore, the hypothesis of Sp+ strains having lower efficiency cannot be fully verified unless numerous host and geographical origins are tested.
Our co-inoculation experiments demonstrated for the first time, using DNA-based genotype characterization, that two distinct Frankia strains inhabit the same nodular lobe (up to 15% of Sp+/Sp− co-inoculations—Table 4). Previous studies reported the presence of different Frankia strains in a single Alnus nodule (3, 39). The co-existence of both Sp+ and Sp− strains within a unique lobe may explain Sp− strain isolation from Sp+ typed nodules. Repeated culturing attempts from Sp+ nodules mostly failed, but led, in rare cases, to the isolation of Frankia strains. However, these cultures never differentiated in-planta sporangia when inoculated on different host species, and were, thus, considered to be Sp− strains (4, 35, 36). Although our protocol aimed at eliminating nodule surface contaminations, we cannot exclude the possibility that some external Frankia cells or their DNA remained and were amplified. Sp+ and Sp− strain localization and their relative proportions inside the nodule warrant further investigations. Additional molecular experiments based on in-situ hybridization from nodule sections and quantitative-PCR will shed light on those recurrent questions.
In conclusion, we herein confirmed the higher infectivity and competitiveness of Sp+ strains than Sp− strains as well as their higher host specificity. Moreover, these traits were differentially expressed depending on the phylogenetic clades that the Sp+ strains belong to. These results, associated with the non-cultivability of Sp+ strains, suggest they are dependent on the host plant for a large part of their life cycle and support the obligate symbiont scenario previously discussed (32, 37). Their higher infectivity and competitiveness may explain why they have been found to be highly invasive in the field (5, 32, 37, 45). The frequency at which Sp+ strains is detected is higher in old alder stands (12, 46), suggesting they may represent the final stage in the succession of Frankia populations (32). To date, Frankia spore persistence in the soil still remains uncharacterized, and further investigations on Sp+ strain detection directly from soil samples are needed in order to shed light on fundamental issues regarding their fitness during their saprophytic life or their fate in soils devoid of host plants.
Acknowledgements
The authors are grateful to the greenhouse platform (Lyon University). We thank the IBIO platform (UMR-CNRS 5557) and Sébastien Devillard for advice on statistical analyses. The authors also thank Elise Simonazzi and Nelly Queruel for their help with experimental assays. Laetitia Cotin-Galvan and Adrien Pozzi were granted a doctoral fellowship by the Ministère de l’Enseignement Supérieur et de la Recherche (MESR; France) through the doctoral school Évolution, Écosystèmes, Microbiologie, Modélisation (ED341 E2M2; Lyon, France).
References
- 1.Agresti A, Coull BA. Approximate is better than exact for interval estimation of binomial proportions. Am J Stat. 1998;52:119–126. [Google Scholar]
- 2.Akkermans A, van Dijk C. The Formation and Nitrogen-fixing Activity of the Root Nodules of Alnus glutinosa Under Field Conditions. In: Nutman EbP., editor. Symbiotic Nitrogen Fixation in Plants. Cambridge University Press; London: 1976. pp. 511–520. [Google Scholar]
- 3.Benson D, Hanna D. Frankia diversity in an alder stand as estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of whole-cell proteins. Can J Bot. 1983;61:2919–2923. [Google Scholar]
- 4.Burgraaf AJP, Quispel A, Tak T, Valstar J. Methods of isolation and cultivation of Frankia species from actinorhizas. Plant Soil. 1981;61:157–168. [Google Scholar]
- 5.Burleigh S, Torrey J. Effectiveness of different Frankia cell types as inocula for the actinorhizal plant Casuarina. Appl Environ Microbiol. 1990;56:2565–2567. doi: 10.1128/aem.56.8.2565-2567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dowling DN, Broughton WJ. Competition for nodulation of legumes. An Rev Microbiol. 1986;40:131–157. doi: 10.1146/annurev.mi.40.100186.001023. [DOI] [PubMed] [Google Scholar]
- 7.Dworkin J, Shah I. Exit from dormancy in microbial organisms. Nat Rev Microbiol. 2010;8:890–896. doi: 10.1038/nrmicro2453. [DOI] [PubMed] [Google Scholar]
- 8.Fåhraeus G. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J Gen Microbiol. 1957;16:374–381. doi: 10.1099/00221287-16-2-374. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez M, Meugnier H, Grimont P, Bardin R. Deoxyribonucleic acid relatedness among members of the genus Frankia. Int J Syst Bacteriol. 1989;39:424–429. [Google Scholar]
- 10.Hibbing M, Fuqua C, Parsek M, Peterson S. Bacterial competition: surviving and thriving in the microbial jungle. Rev Microbiol. 2010;8:15–25. doi: 10.1038/nrmicro2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hocher V, Alloisio N, Auguy F, et al. Transcriptomics of actinorhizal symbioses reveals homologs of the whole common symbiotic signaling cascade. Plant Physiol. 2011;156:700–711. doi: 10.1104/pp.111.174151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holman R, Schwintzer C. Distribution of spore-positive and spore-negative nodules of Alnus incana ssp. rugosa in Maine, USA. Plant Soil. 1987;104:103–111. [Google Scholar]
- 13.Houwers A, Akkermans A. Influence of inoculation on yield of Alnus glutinosa in the Netherlands. Plant Soil. 1981;61:189–202. [Google Scholar]
- 14.Josephson K, Pepper I. Competitiveness and effectiveness of strains of Rhizobium phaseoli isolated from the Sonoran desert. Soil Biol Biochem. 1984;16:651–655. [Google Scholar]
- 15.Koppenhöfer A, Kaya H, Shanmugam S, Wood G. Interspecific Competition between Steinernematid Nematodes within an Insect Host. J Invertebr Pathol. 1995;66:99–103. [Google Scholar]
- 16.Krumholz GD, Chval MS, McBride MJ, Tisa LS. Germination and physiological properties of Frankia spores. Plant Soil. 2003;254:57–67. [Google Scholar]
- 17.Kurdali F, Domenach A, Fernandez M, Capellano A, Moiroud A. Compatibility of Frankia spore positive and spore negative inocula with Alnus glutinosa and Alnus incana. Soil Sci Plant Nutr. 1988;34:451–459. [Google Scholar]
- 18.Kurdali F, Rinaudo G, Moiroud A, Domenach A. Competition for nodulation and 15N2-fixation between a Sp+ and a Sp− Frankia strain from Alnus incana. Soil Biol Biochem. 1990;22:57–64. [Google Scholar]
- 19.Lalonde M, Calvert H. Production of Frankia hyphae and spores as an infective inoculant for Alnus species. In: Gordon JC, Wheeler CT, Perry DA, editors. Symbiotic Nitrogen Fixation in the Management of Temperate Forests. Oregon State University, Forest Research Laboratory; Corvallis, OR: 1979. pp. 95–110. [Google Scholar]
- 20.Laplaze L, Gherbi H, Frutz T, Pawlowski K, Franche C, Macheix JJ, Auguy F, Bogusz D, Duhoux E. Flavan-containing cells delimit Frankia-infected compartments in Casuarina glauca nodules. Plant Physiol. 1999;121:113–122. doi: 10.1104/pp.121.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lazniewska J, Macioszek V, Kononowicz A. Plant-fungus interface: The role of surface structures in plant resistance and susceptibility to pathogenic fungi. Physiol Mol Plant Pathol. 2012;78:24e30. [Google Scholar]
- 22.Mansour S, Dewedar A, Torrey J. Isolation, culture, and behavior of Frankia strain HFPCgI4 from root nodules of Casuarina glauca. Bot Gaz. 1990;151:490–496. [Google Scholar]
- 23.Markham JH. Variability of nitrogen-fixing Frankia on Alnus species. Botany. 2008;86:501–510. [Google Scholar]
- 24.Maunoury N, Redondo-Nieto M, Bourcy M, et al. Differentiation of symbiotic cells and endosymbionts in Medicago truncatula nodulation are coupled to two transcriptome-switches. PLoS One. 2010;5:e9519. doi: 10.1371/journal.pone.0009519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholson WL, Munakata N, Horneck G, Melosh HJ, Setlow P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol Mol Biol Rev. 2000;64:548–572. doi: 10.1128/mmbr.64.3.548-572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicol H, Thornton H. Competition between related strains of nodule bacteria and its influence on infection of the legume host. Proc R Soc. 1941;130:32–59. [Google Scholar]
- 27.Niehaus K, Kapp D, Puhler A. Plant defence and delayed infection of alfalfa pseudonodules induced by an exopolysaccharide (EPS I)-deficient Rhizobium meliloti mutant? Planta. 1993;190:415–425. [Google Scholar]
- 28.Normand P, Lalonde M. Evaluation of Frankia strains isolated from provenances of two Alnus species. Can J Microbiol. 1982;28:1133–1142. [Google Scholar]
- 29.Périnet P, Brouillette J, Fortin J, Lalonde M. Large scale inoculations of actinorhizal plants with Frankia. Plant Soil. 1985;87:175–183. [Google Scholar]
- 30.Popovici J, Comte G, Bagnarol E, Alloisio N, Fournier P, Bellvert F, Bertrand C, Fernandez MP. Differential effects of rare specific flavonoids on compatible and incompatible strains in the Myrica gale-Frankia actinorhizal symbiosis. Appl Env Microbiol. 2010;76:2451–2460. doi: 10.1128/AEM.02667-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Popovici J, Walker V, Bertrand C, Bellvert F, Fernandez MP, Comte G. Strain specificity in the Myricaceae-Frankia symbiosis is correlated to plant root phenolics. Funct Plant Biol. 2011;38:682–689. doi: 10.1071/FP11144. [DOI] [PubMed] [Google Scholar]
- 32.Pozzi AC, Bautista-Guerrero H, Nouioui I, Cotin-Galvan L, Pépin R, Fournier P, Menu F, Fernandez MP, Herrera-Belaroussi A. In-planta sporulation phenotype: a major life-history trait to understand the evolution of Alnus-infective Frankia strains. Env Microbiol. 2014 doi: 10.1111/1462-2920.12644. [DOI] [PubMed] [Google Scholar]
- 33.Pucciariello C, Innocenti G, Van de Velde W, et al. (Homo) glutathione depletion modulates host gene expression during the symbiotic interaction between Medicago truncatula and Sinorhizobium meliloti. Plant Physiol. 2009;151:1186–1196. doi: 10.1104/pp.109.142034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pulliam H. On the relationship between niche and distribution. Ecol Lett. 2000;3:349–361. [Google Scholar]
- 35.Quispel A, Tak T. Studies on the growth of the endophyte of Alnus glutinosa (L.) Vill. in nutrient solutions. New Phytol. 1978;81:587–600. [Google Scholar]
- 36.Racette S, Torrey JG, Berg RH. Sporulation in root nodules of actinorhizal plants inoculated with pure cultured strains of Frankia. Can J Bot. 1991;69:1471–1476. [Google Scholar]
- 37.Schwintzer C. Spore-positive and spore-negative nodules. In: Schwintzer JTCR, editor. The Biology of Frankia and Actinorhizal Plants. Academic Press; New York: 1990. pp. 177–193. [Google Scholar]
- 38.Sellstedt A, Ahlqvist A, Huss-Danell K. Nitrogen fixation and biomass production in symbioses between Alnus incana and Frankia strains with different nitrogen metabolism. Physiol Plant. 1986;66:99–107. [Google Scholar]
- 39.Simonet P, Bosco M, Chapelon C, Moiroud A, Normand P. Molecular characterization of Frankia microsymbionts from spore-positive and spore-negative nodules in a natural alder stand. Appl Environ Microbiol. 1994;60:1335–1341. doi: 10.1128/aem.60.4.1335-1341.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solomon PS, Tan K-C, Oliver RP. Mannitol 1-phosphate metabolism is required for sporulation in-planta of the wheat pathogen Stagonospora nodorum. MPMI. 2005;18:110–115. doi: 10.1094/MPMI-18-0110. [DOI] [PubMed] [Google Scholar]
- 41.Team RDC. R foundation for Statistical Computing. R foundation for Statistical Computing; 2005. R: A language and environment for statistical computing. [Google Scholar]
- 42.Tzean S, Torrey J. Spore germination and the life cycle of Frankia in-vitro. Can J Microbiol. 1989;35:801–806. [Google Scholar]
- 43.van Dijk C, Merkus E. A microscopical study of the development of a spore-like stage in the life cycle of the root nodule endophyte of Alnus glutinosa (L.) Gaertn. New Phytol. 1976;77:73–91. [Google Scholar]
- 44.van Dijk C. Spore formation and endophyte diversity in root nodules of Alnus glutinosa (L.) Vill. New Phytol. 1978;81:601–615. [Google Scholar]
- 45.van Dijk C. Endophyte distribution in the soil. In: Gordon JC, Wheeler CT, Perry DA, editors. Symbiotic nitrogen fixation in the management of temperate forests. Oregon State University, Forest Research Laboratory; Corvallis, OR: 1979. pp. 84–94. [Google Scholar]
- 46.van Dijk C. Ecological aspects of spore formation in Frankia-Alnus symbiosis (Thesis) University of Leiden; The Netherlands: 1984. Ecological aspects of spore formation in Frankia-Alnus symbiosis. [Google Scholar]
- 47.Weber A, Nurmiaho-Lassila E-L, Sundman V. Features of the intrageneric Alnus-Frankia specificity. Physiol Plant. 1987;70:289–296. [Google Scholar]
- 48.Zelinger E, Hawes C, Gurr S, Dewey F. Attachment and adhesion of conidia of Stagonospora nodorum to natural and artificial surfaces. Physiol Mol Plant Pathol. 2006;68:209–215. [Google Scholar]
- 49.Zimpfer JF, McCarty B, Kaelke CM, Mulongwe L, Igual JM, Smyth CA, Dawson JO. Casuarina cunninghamiana cladode extracts increase the Frankia infectious capacity of a tropical soil. Symbiosis. 2002;33:73–90. [Google Scholar]