Abstract
In order to assess the physiological responses of bradyrhizobia and competition for the nodulation of soybean at different temperatures, we investigated the expression of the nodC gene at 20, 25, and 30°C and the abilities of bacteria to nodulate soybean in microcosms at day/night cultivation temperatures of 23/18°C, 28/23°C, and 33/28°C for 16/8 h. We tested five Bradyrhizobium USDA strains: B. diazoefficiens USDA 110T and 122, B. japonicum USDA 123, and B. elkanii USDA 31 and 76T. The expression of nodC was up-regulated by increasing culture temperatures in USDA 110T, 122, 31, and 76T, but was down-regulated in USDA 123. The proportions of USDA 110T and 122 within the community were the greatest at 28/23°C. The population of USDA 31 increased, whereas that of USDA 123 decreased with increasing cultivation temperatures. On the other hand, infection by USDA 76T was not detected, and low numbers of USDA 76T nodules confirmed its poor nodulation ability. These results indicate that the competitiveness of and infection by USDA 110T, 122, 123, and 31 for soybean nodulation depend on cultivation temperatures, and suggest that the temperature dependence of nodC expression affects the bradyrhizobial community structure.
Keywords: bradyrhizobia, nodC gene, temperature, community structure
Soybean (Glycine max [L.] Merr.) is an important crop plant that forms root nodules by infections with rhizobia, which fix atmospheric nitrogen as ammonia through these nodules. Bradyrhizobium diazoefficiens, B. japonicum, and B. elkanii are major soybean-nodulating rhizobia (8, 16, 20). The inoculation of soybean with bradyrhizobia may improve nitrogen fixation, resulting in increased soybean yield. However, the efficiency of the inoculum may be poor if it cannot compete with indigenous bradyrhizobia in the soil or is unable to establish an efficient symbiosis with the host plant due to low compatibility (42). In order to overcome this issue, a clearer understanding of the ecology of indigenous soybean-nodulating rhizobia is needed in terms of their genetic diversity, geographical distribution, compatibility with soybean, and environmental factors associated with the localization and dominance of strains in soil.
Saeki et al. (32) investigated the genetic diversity and geographical distribution of indigenous soybean-nodulating bradyrhizobia collected from five sites in Japan (Hokkaido, Fukushima, Kyoto, Miyazaki, and Okinawa) by analyzing PCR restriction fragment length polymorphisms (RFLP) of the 16S-23S rRNA gene internal transcribed spacer (ITS) region. The distribution of bradyrhizobia strongly correlated with latitude: representative clusters changed from north to south in the order of B. japonicum strains USDA 123, 110 (B. diazoefficiens USDA 110T), 6T, and B. elkanii strain USDA 76T (33, 35, 36). These findings suggested that environmental factors such as temperature influenced the localization of Japanese indigenous bradyrhizobia. Saeki et al. (34) investigated the dominance of three B. japonicum strains and one B. elkanii strain at different temperatures in soil and liquid media, and suggested that temperature affected the occupancy of indigenous bradyrhizobia in soil. Adhikari et al. (1) revealed the genomic diversity of soybean-nodulating bradyrhizobia in relation to climate, as determined by altitude, and to soil properties, such as pH, in Nepal. Suzuki et al. (43) also reported the prominent effects of temperature on competition between B. japonicum and B. elkanii strains that corresponded with the distribution of bradyrhizobial species in Nepal. In USA, the world’s biggest soybean producer, soybean is grown at similar latitudes to those in Japan. Shiro et al. (39) investigated the relationship between the genetic diversity of indigenous soybean-nodulating bradyrhizobia and their geographical distribution in USA using nine soil isolates from eight states: as in Japan, the major clusters changed from B. japonicum USDA 123 in the northern states to B. elkanii in the central and southern states. The indigenous American bradyrhizobial community structure also strongly correlated with latitude. These results suggest a relationship between the geographic distribution of indigenous soybean-nodulating rhizobia and soil temperature (and its variations due to latitude and altitude) as well as soil pH. Shiro et al. (38) investigated the nodulation tendencies and community structures of indigenous bradyrhizobia on soybean cultivars with different Rj (nodulation regulatory gene) genotypes at day/night culture temperatures of 33/28°C, 28/23°C, and 23/18°C for 16/8 h; the findings obtained suggested that the Rj genotype and culture temperature affected the nodulation tendencies and community structures of bradyrhizobia. These findings indicate that changes in bradyrhizobial community structures induced by temperature are caused by differences in the responses of symbiosis-related genes such as nodulation (nod) genes.
In order to test this hypothesis, we investigated the temperature-dependent responses of the nodC gene, which encodes NodC, the first enzyme in the biosynthesis pathway of Nod factor using the substrate UDP-N-acetyl glucosamine (13, 18), and competition for nodulation at different temperatures, with the aim of determining whether the temperature dependency of the expression of the nodC gene contributes to infection by bradyrhizobia for soybean nodulation.
Materials and Methods
Bradyrhizobial strains and culture conditions
B. diazoefficiens strains USDA 110T and 122, B. japonicum strain USDA 123, and B. elkanii strains USDA 31 and 76T were used in the present study. In a quantitative real-time PCR (qPCR) analysis, they were grown in HEPES-MES (HM) broth medium (7) supplemented with 0.1% L-arabinose (37). In order to estimate nodulation abilities, these strains were grown in yeast extract-mannitol broth (YMB) medium (45).
RNA extraction, cDNA synthesis, and qPCR analysis
Regarding RNA extraction, bacteria were pre-cultured at 28°C in 50 mL of HM broth medium for 3 d and then scaled up to 200 mL with the addition of fresh HM broth medium. The bacteria culture was further conducted at 28°C for log-phase growth (OD600 = 0.3–0.5). Cell culture aliquots were diluted to 200 mL with fresh HM broth medium to OD600 = 0.1, and genistein (Nacalai Tesque, Kyoto, Japan) was added to a final concentration of 5 μM in order to induce the expression of nodC (10, 22, 29, 46, 47, 50). These cultures were grown at 20, 25, or 30°C for 24 h, and cells were then immediately harvested by centrifugation and lyophilized. Total RNA was extracted with ISOGEN-LS (Nippon Gene, Tokyo, Japan) according to the manufacturer’s instructions. cDNA was synthesized with the PrimeScript® RT Reagent Kit with gDNA Eraser (Perfect Real Time) (TaKaRa Bio, Shiga, Japan) according to the manufacturer’s instructions. qPCR was performed with SYBR® Premix Ex TaqTM II (Tli RNaseH Plus) (TaKaRa Bio) in the Thermal Cycler Dice® Real Time System TP800 (TaKaRa Bio), and the relative expression quantity of nodC was calculated from TP800 data. The specific primers for nodC and control genes for the qPCR analysis are shown in Table 1. The expression levels of three biological replicates in each treatment were normalized to those of the sigA gene. The sigA gene, which encodes a primary sigma factor, is used as a housekeeping control gene because its expression is independent of temperature (28, 44, 46, 47). Relative gene expression among the treatment groups was quantified using the 2−ΔΔCT method (21, 48). A real-time PCR analysis was conducted at three replications.
Table 1.
Primer information used in an expression analysis of the nodC gene
| Gene | Sequence (5′→3′) | PCR product size (bp) | |
|---|---|---|---|
|
| |||
| Forward primer | Reverse primer | ||
| sigA | ACATGGGCATCAACGTCACC | TCGTTGTCGGTCTCGTCCTC | 84 |
|
nodC for B. diazoefficiens B. japonicum |
CGAGCGATCCGAGATTCAG | ACGTCGGCAGCAAGTATCG | 135 |
| nodC for B. elkanii | TGGACGGTGCTGACGATTG | TGTGAAGCGAGAAGCCGAG | 96 |
Nodulation ability and competition studies using microcosms
In order to estimate nodulation by and competition among bradyrhizobial strains, we performed experiments using soil microcosms and three soybean cultivars (Akishirome, Bragg, and Orihime; non-Rj genotype, 24). Bradyrhizobia were cultured in YMB medium at 28°C for 6 d, and then mixed in combinations of three strains into sterile soil (Andosol, pH [H2O] = 6.46, pH [KCl] = 5.22, EC = 0.03 dS m−1, CEC = 31.2 cmol kg−1) at a bacterial density of 106 cells g−1 dry soil. Four combinations were prepared: A (USDA 31, 110T, and 123), B (USDA 31, 122, and 123), C (USDA 76T, 110T, and 123), and D (USDA 76T, 122, and 123).
In order to isolate the strains from the microcosms, we grew soybeans in 1-L culture pots. The pots were first filled with vermiculite with N-free nutrient solution (30) at 40% (v/v) water content and then autoclaved at 121°C for 20 min. Soybean seeds were surface-sterilized in 70% ethanol for 30 s followed by dilute sodium hypochlorite (0.25% available chlorine) for 3 min, and then washed in sterile distilled water. Microcosm soil (2 to 3 g) was placed in vermiculite at a depth of 2 to 3 cm, and seeds were sown on it. Plants were grown for 4 weeks in a growth chamber at one of the three temperature regimes—low (day/night, 23/18°C for 16/8 h), middle (28/23°C), and high (33/28°C)—with a weekly supply of sterile distilled water. After 4 weeks, 24 nodules were randomly collected and sterilized in 70% ethanol for 3 min followed by dilute sodium hypochlorite for 30 min, and then washed in sterile distilled water. As a negative control, it was confirmed that soybean plants grown without soil, eliminating the possibility of contamination with soybean-nodulating bacteria, formed no nodules.
Soybean-nodulating bradyrhizobia were identified by PCR-RFLP of the 16S-23S rRNA gene ITS region. Total DNA was directly extracted from nodules as described previously (15) with slight modifications (24). Each nodule was homogenized in 50 μL of BL buffer (40 mM Tris-HCl, 1% Tween 20, 0.5% Nonidet P-40, 1 mM EDTA, pH 8.0), 40 μL of sterile distilled water, and 10 μL of proteinase K (1 mg mL−1) and then incubated at 60°C for 20 min and 95°C for 5 min. After centrifugation, the supernatant was collected and used as the PCR template. PCR was performed using TaKaRa Ex Taq® (TaKaRa Bio). Regarding 16S-23S rRNA gene ITS region amplification, we used the primer set BraITS-F (5′-GACTGGGGT GAAGTCGTAAC-3′) and BraITS-R (5′-ACGTCCTTCATCGCC TC-3′) (31). The PCR cycle consisted of an initial 94°C for 5 min; 30 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min; and a final 72°C for 10 min. In the RFLP analysis of the ITS region, the PCR product was digested with MspI (TaKaRa Bio) at 37°C for 16 h (31). Fragments were separated by electrophoresis using 3% agarose gel and visualized with ethidium bromide.
Inoculation test for estimation of nodulation ability
In order to estimate the nodulation ability of each bradyrhizobial strain with soybean, we inoculated each strain into each of the three soybean cultivars (Akishirome, Bragg, and Orihime). These strains were cultured in YMB medium as described above. The cultures were diluted with sterile distilled water to 106 cells mL−1. Soybean seeds were sown into 1-L culture pots as described above without soil and inoculated with 1 mL of diluted bacterial culture per seed. Soybean plants were grown for 3 weeks in a growth chamber (28/23°C for 16/8 h) with a weekly supply of sterile distilled water. After 3 weeks, the nodules were counted. As a negative control, it was confirmed that soybean plants grown without inoculation formed no nodules.
Results
nodC expression levels at different temperatures
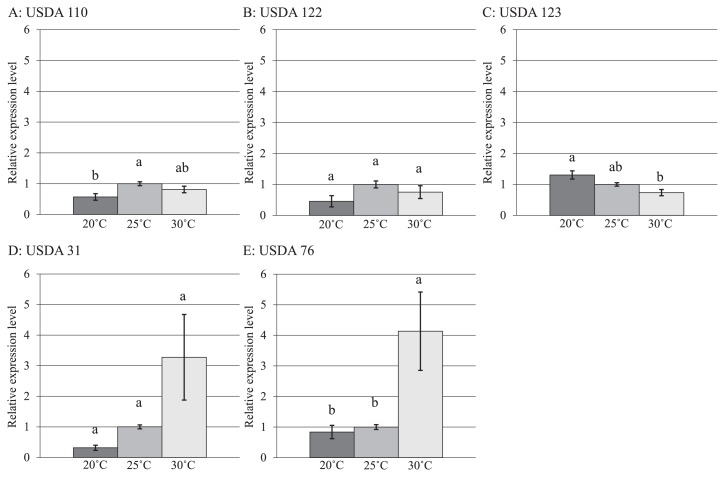
nodC gene expression levels at different temperatures were estimated using the sigA gene as a reference gene because the expression of the 16S rRNA gene exhibits instability with changing temperatures (14). The results obtained revealed different nodC expression levels in each strain at low (20°C), middle (25°C), and high (30°C) temperatures (Fig. 1). nodC expression levels in B. diazoefficiens USDA 110T were significantly higher at the middle temperature than at the low temperature (Fig. 1A). nodC expression levels in B. diazoefficiens USDA 122 were slightly higher at the middle temperature than at the low temperature (Fig. 1B). nodC expression levels in USDA 110T and 122 were higher at the middle temperature than at the high temperature (Fig. 1A, B), indicating that the expression of the nodC gene has an optimum temperature in the vicinity of the middle to high temperatures. nodC expression levels in B. japonicum USDA 123 were significantly lower at the high temperature than at the low temperature (Fig. 1C). nodC expression levels in B. elkanii USDA 31 were slightly higher at the high temperature than at the low and middle temperatures (Fig. 1D). nodC expression levels in B. elkanii USDA 76T were significantly higher at the high temperature than at the low and middle temperatures (Fig. 1E). The increased expression levels observed in USDA 31 and 76T were precipitous (Fig. 1D, E).
Fig. 1.
Relative expression of nodC in five bradyrhizobial strains. Values shown represent mean from three replications ± SE (n=3). Bars with the same superscript letters are not significantly different (Tukey HSD test) at P < 0.05.
Change in nodulation occupancy at different cultivation temperatures
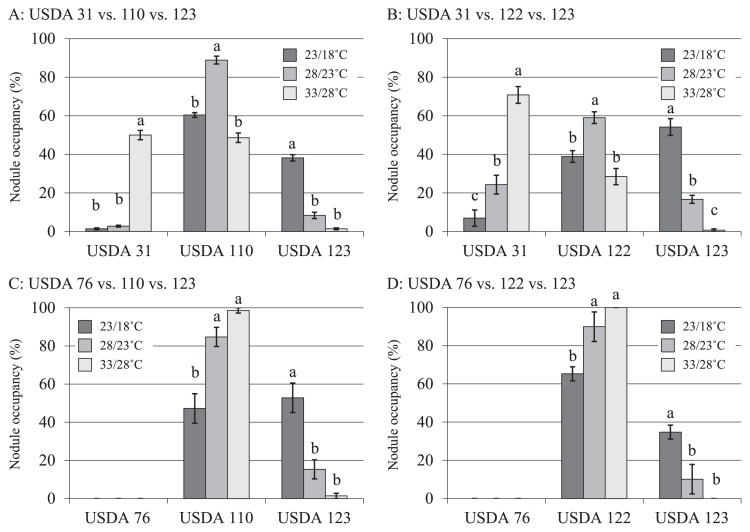
In order to estimate the nodulation rates and competitiveness of strains, we determined the proportion of nodules infected by each strain in three-strain microcosm experiments using a PCR-RFLP analysis of the 16S-23S rRNA gene ITS region. In both combinations in which USDA 31 was present, the nodule occupancy rate of USDA 31 increased significantly at higher cultivation temperatures (Fig. 2A, B). In all four combinations in which USDA 123 was present, the occupancy rate of USDA 123 decreased significantly with increasing cultivation temperatures (Fig. 2A–D). In the presence of USDA 31 and 123, the occupancy rates of USDA 110T and 122 were higher at 28/23°C than at other temperatures (Fig. 2A, B). On the other hand, unlike USDA 31, USDA 76T was not detected in any combination of the nodulation occupancy test (Fig. 2C, D). In association with non-nodulation by USDA 76T, the occupancy rates of USDA 110T and 122 increased at higher cultivation temperatures (Fig. 2C, D).
Fig. 2.
Rate of nodule occupancy by each test strain isolated from soil microcosm experiments using the indicated mixes of three strains. Values shown represent mean from three soybean cultivars, Akishirome, Bragg, and Orihime ± SE (n=3). Bars with the same superscript letters are not significantly different (Tukey HSD test) at P < 0.05.
Nodulation abilities of bradyrhizobial strains
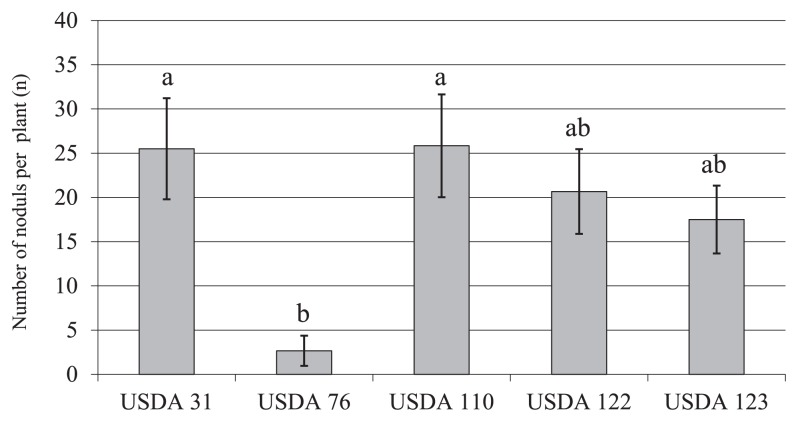
The nodulation ability of each bradyrhizobial strain under non-competitive conditions was shown in Fig. 3. The bradyrhizobial strain that indicated the highest nodulation ability for soybean was B. diazoefficiens USDA 110T. Sub-sequently, the order corresponding to B. elkanii USDA 31, B. diazoefficiens USDA 122, and B. japonicum USDA 123 indicated high nodulation ability for soybean. The nodulation ability of B. elkanii USDA 76T had the lowest value and was significantly different from those of USDA 31 and USDA 110T (Fig. 3).
Fig. 3.
Number of nodules of each test strain on non-Rj genotype soybean. Values shown represent mean from total plant number of six ± SE. Three soybean cultivars, Akishirome, Bragg, and Orihime, were used each two individual. Bars with the same superscript letters are not significantly different (Tukey HSD test) at P < 0.05.
Discussion
The expression level of each nodC gene in the individual strain at each temperature was almost the same when corrected against the sigA gene (Fig. 1). Therefore, nodC genes in the five strains showed strain-specific temperature-dependent changes under our experimental conditions. Although significant differences were not detected among different temperatures in each strain of B. diazoefficiens USDA 122 and B. elkanii USDA 31, the patterns of nodC expression were similar to those of USDA 110T and USDA 76T, for which significant differences were detected, respectively. A significant difference may have been detected in each strain of B. diazoefficiens USDA 122 and B. elkanii USDA 31 by increasing the number of replications. The production of Nod factor (a lipo-chitooligosaccharide), which triggers leguminous plant responses including the initiation of cell division to form nitrogen-fixing root nodules (9, 12), in B. japonicum has been shown to increase at higher rhizosphere temperatures and genistein concentrations (54). The increased production of Nod factor at higher temperatures suggests the up-regulated expression of the nod gene. Begum et al. (5) investigated the effects of incubation temperatures at 15 and 28°C on the expression of nodC in Rhizobium leguminosarum: an incubation temperature of 28°C induced the maximum expression of nodC, while a low incubation temperature reduced its expression. These findings appear to support our results. However, the expression of nodC in USDA 123 decreased from low to high temperatures (Fig. 1C). Soybean-nodulating bradyrhizobia with a similar ITS RFLP type to USDA 123 are tolerant to lower temperatures and, thus, maintain dominance at lower temperatures (32, 34, 39).
In the nodulation ability and competition studies performed using microcosms, USDA 110T and 122 were dominant at 28/23°C (Fig. 2). The occupancy of USDA 31 increased, while that of USDA 123 decreased at higher cultivation temperatures (Fig. 2). USDA 123 may be able to maintain its infectious ability to soybean even under low temperature conditions because the expression of nodC or reverse temperature-dependent expression of nod genes in this strain is less sensitive to temperature (Fig. 1C). These results suggest that USDA 123 nodulates soybean more effectively than other strains under low temperature conditions. However, although USDA 76T indicated the temperature-dependent expression of the nodC gene, nodules infected by USDA 76T were not detected (Fig. 2C, D) possibly because of its low compatibility with or low ability of nodulation on soybean (Fig. 3) despite its nodC expression ability by genistein (Fig. 1E). Therefore, the low competitiveness of USDA 76T for nodulation may have allowed for the increased occupancy of USDA 110T and 122 at higher cultivation temperatures. Since the changes observed in the temperature-dependent expression of nodC in USDA 31, 110T, 122, and 123 were generally consistent with those in nodule occupancy, these results suggest that nodC expression levels affect the nodulation competitiveness of bradyrhizobia. Yokoyama (50) demonstrated that the expression of nod genes in B. japonicum USDA 110 (B. diazoefficiens USDA 110T), B. elkanii USDA 76T, and Bradyrhizobium sp. TARC 64 (isolated from soil in Thailand; 49) depended on incubation temperatures in the range of 20 to 40°C, and suggested that the transcriptional responses of the nod genes of USDA 110 and USDA 76T were distinctly different at 23 to 35°C. Additionally, this study assessed the abilities of various bradyrhizobia (B. japonicum USDA 110, 122 (B. diazoefficiens USDA 110T and 122), 123, 5033; B. elkanii USDA 31, 76T; Bradyrhizobium sp. TARC 64) to nodulate soybean under different temperature conditions (23/18°C, 25/25°C, and 34/28°C), and suggested that B. japonicum strains prefer 23/18°C and 25/25°C, while B. elkanii strains prefer 34/28°C. Banfalvi et al. (4) reported that genistein and soybean seed extract more strongly promoted the expression of nodY and nodC in B. japonicum USDA 110 (B. diazoefficiens USDA 110T) than daidzein. On the other hand, Kosslak et al. (19) investigated the expression of nodABC genes in B. japonicum strains including B. japonicum USDA 110 (B. diazoefficiens USDA 110T) and USDA 123 using isoflavones and soybean root extract, and reported that expression was more strongly induced by daidzein than by genistein. These findings suggest that the induction and expression of nod genes differ with the types of isoflavones secreted from soybean roots and also with strain. Furthermore, a decrease in the rhizosphere temperature was shown to delay the infection of soybean roots by bradyrhizobia, reduce the secretion of genistein from roots, and suppress the expression of nod genes (51, 52, 53). However, it increased the secretion of daidzein (27). In our study, the occupancy of B. japonicum USDA 123 increased as temperature decreased (Fig. 2). In addition to affecting the strength of nod gene expression in USDA 123, lower temperatures might also alter the types and quantities of isoflavones secreted from soybean roots.
Our results suggest that temperature is widely involved in community structure, indigenization, and dominance associated with the expression of nod genes and nodulation abilities of B. diazoefficiens, B. japonicum, and B. elkanii. Furthermore, the nodulation of four out of the five stains tested was temperature dependent, and, thus, the effect of temperature on the expression of nodC is an important factor affecting the nodulation of soybean and the formation of a bradyrhizobial community structure. Recent research indicates that the expression of type III secretion system (T3SS) genes in B. japonicum USDA 110 (B. diazoefficiens USDA 110T) is induced by soybean seed extract and genistein, and suggests that nodulation genes, especially the nolA and nodD2 genes, and T3SS genes play a role in nodulation (47). Therefore, the nodulation of bradyrhizobia may be associated with several factors other than the nodC gene, such as salt and water-deficit stress, the existence of other rhizosphere bacteria, and protein secretion systems (2, 3, 17, 25, 26). Our results also suggest that the expression of nodC in USDA 76T is independent of its compatibility and infection ability. Causatively, the low compatibility of B. elkanii USDA 76T with soybean may be due to a low capacity to produce effective amounts of Nod factor to induce nodulation, which may be due, in turn, to a dysfunction in the nod, noe, and nol genes that function downstream of nodC in the biosynthesis and modification of Nod factor, or of nodIJ genes encoding the ABC family transporters that are involved in the secretion of Nod factor and present in all rhizobia (6, 11, 23, 40, 41). Thus, further studies on infection and compatibility with soybean are needed in order to elucidate bradyrhizobial ecology for nodulation in more detail.
Acknowledgements
This study was supported mainly by JSPS KAKENHI (Grant-in-Aid for Scientific Research (B) no. 16310313), and partly by a grant from the Institute for Fermentation, Osaka, Japan.
References
- 1.Adhikari D, Kaneto M, Itoh K, Suyama K, Pokharel BB, Gaihre YK. Genetic diversity of soybean-nodulating rhizobia in Nepal in relation to climate and soil properties. Plant Soil. 2012;357:131–145. [Google Scholar]
- 2.Atieno M, Herrmann L, Okalebo R, Lesueur D. Efficiency of different formulation of Bradyrhizobium japonicum and effect of co-inoculation of Bacillus subtilis with two different strains of Bradyrhizobium japonicum. World J Microbiol Biotechnol. 2012;28:2541–2550. doi: 10.1007/s11274-012-1062-x. [DOI] [PubMed] [Google Scholar]
- 3.Bai Y, Pan B, Charles TC, Smith DL. Co-inoculation dose and root zone temperature for plant growth promoting rhizobacteria on soybean [Glycine max (L.) Merr] grown in soil-less media. Soil Biol Biochem. 2002;34:1953–1957. [Google Scholar]
- 4.Banfalvi Z, Nieuwkoop A, Schell M, Besl L, Stacey G. Regulation of nod gene expression in Bradyrhizobium japonicum. Mol Gen Genet. 1988;214:420–424. doi: 10.1007/BF00330475. [DOI] [PubMed] [Google Scholar]
- 5.Begum AA, Leibovitch S, Migner P, Zhang F. Specific flavonoids induced nod gene expression and pre-activated nod genes of Rhizobium leguminosarum increased pea (Pisum sativum L.) and lentil (Lens culinaris L.) nodulation in controlled growth chamber environments. J Exp Bot. 2001;52:1537–1543. doi: 10.1093/jexbot/52.360.1537. [DOI] [PubMed] [Google Scholar]
- 6.Cárdenas L, Domínguez J, Santana O, Quinto C. The role of nodI and nodJ genes in the transport of Nod metabolites in Rhizobium etli. Gene. 1996;173:183–187. doi: 10.1016/0378-1119(96)00166-7. [DOI] [PubMed] [Google Scholar]
- 7.Cole MA, Elkan GH. Transmissible resistance to penicillin G, neomycin, and chloramphenicol in Rhizobium japonicum. Antimicrob Agents Chemother. 1973;4:248–253. doi: 10.1128/aac.4.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delamuta JRM, Ribeiro RA, Ormeño-Orrillo E, Melo IS, Martínez-Romero E, Hungria M. Polyphasic evidence supporting the reclassification of Bradyrhizobium japonicum group Ia strains as Bradyrhizobium diazoefficiens sp. nov. Int J Syst Evol Microbiol. 2013;63:3342–3351. doi: 10.1099/ijs.0.049130-0. [DOI] [PubMed] [Google Scholar]
- 9.Dénarié J, Debeellé F, Promé JC. Rhizobium lipochitooligosaccharide nodulation factors: signaling molecules mediating recognition and morphogenesis. Annu Rev Biochem. 1996;65:503–535. doi: 10.1146/annurev.bi.65.070196.002443. [DOI] [PubMed] [Google Scholar]
- 10.Duzan HM, Zhou X, Souleimanov A, Smith DL. Perception of Bradyrhizobium japonicum Nod factor by soybean [Glycine max (L.) Merr.] root hairs under abiotic stress conditions. J Exp Bot. 2004;55:2641–2646. doi: 10.1093/jxb/erh265. [DOI] [PubMed] [Google Scholar]
- 11.Fernández-López M, D’Haeze W, Mergaert P, Verplancke C, Promé JC, Van Montagu M, Holsters M. Role of nodI and nodJ in lipo-chitooligosaccharide secretion in Azorhizobium caulinodans and Escherichia coli. Mol Microbiol. 1996;20:993–1000. doi: 10.1111/j.1365-2958.1996.tb02540.x. [DOI] [PubMed] [Google Scholar]
- 12.Fischer HM. Genetic regulation of nitrogen fixation in rhizobia. Microbiol Rev. 1994;58:352–386. doi: 10.1128/mr.58.3.352-386.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geremia RA, Mergaert P, Geelen D, Van Montagu M, Holsters M. The NodC protein of Azorhizobium caulinodans is an N-acetylglucosaminyltransferase. Proc Natl Acad Sci USA. 1994;91:2669–2673. doi: 10.1073/pnas.91.7.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gustafson AM, O’Connell KP, Thomashow MF. Regulation of Sinorhizobium meliloti 1021 rrnA-reporter gene fusions in response to cold shock. Can J Microbiol. 2002;48:821–830. doi: 10.1139/w02-078. [DOI] [PubMed] [Google Scholar]
- 15.Hiraishi A, Kamagata Y, Nakamura K. Polymerase chain reaction amplification and restriction fragment length polymorphism analysis of 16S rRNA genes from methanogens. J Ferment Bioeng. 1995;79:523–529. [Google Scholar]
- 16.Jordan DC. Transfer of Rhizobium japonicum Buchanan 1980 to Bradyrhizobium gen. nov., a genus of slow-growing, root nodule bacteria from leguminous plants. Int J Syst Bacteriol. 1982;32:136–139. [Google Scholar]
- 17.Jun L, Wen-li X, Ming-chao M, Da-wei G, Xin J, Feng-ming C, De-long S, Hui-jun C, Li L. Proteomic study two Bradyrhizobium japonicum strains with different competitivenesses for nodulation. Agr Sci China. 2011;10:1072–1079. [Google Scholar]
- 18.Kamst E, Pilling J, Raamsdonk LM, Lugtenberg BJ, Spaink HP. Rhizobium nodulation protein NodC is an important determinant of chitin oligosaccharide chain length in Nod factor biosynthesis. J Bacteriol. 1997;179:2103–2108. doi: 10.1128/jb.179.7.2103-2108.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kosslak RM, Joshi RS, Bowen BA, Paaren HE, Appelbaum ER. Strain-specific inhibition of nod gene induction in Bradyrhizobium japonicum by flavonoid compounds. Appl Environ Microbiol. 1990;56:1333–1341. doi: 10.1128/aem.56.5.1333-1341.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuykendall LD, Saxena B, Cevine TE, Udell SE. Genetic diversity in Bradyrhizobium Jordan 1982 and a proposal for Bradyrhizobium elkanii sp. nov. Can J Microbiol. 1992;38:501–505. [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC T method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Mabood F, Souleimanov A, Khan W, Smith DL. Jasmonates induce Nod factor production by Bradyrhizobium japonicum. Plant Physiol Biochem. 2006;44:759–765. doi: 10.1016/j.plaphy.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 23.Mergaert P, Van Montagu M, Holsters M. Molecular mechanisms of Nod factor diversity. Mol Microbiol. 1997;25:811–817. doi: 10.1111/j.1365-2958.1997.mmi526.x. [DOI] [PubMed] [Google Scholar]
- 24.Minami M, Yamakawa T, Yamamoto A, Akao S, Saeki Y. Estimation of nodulation tendency among Rj-genotype soybeans using the bradyrhizobial community isolated from an Andosol. Soil Sci Plant Nutr. 2009;55:65–72. [Google Scholar]
- 25.Miransari M, Smith DL. Alleviating salt stress on soybean (Glycine max (L.) Merr.)—Bradyrhizobium japonicum symbiosis, using signal molecule genistein. Eur J Soil Biol. 2009;45:146–152. [Google Scholar]
- 26.Nápoles MC, Guevara E, Montero F, Rossi A, Ferreira A. Role of Bradyrhizobium japonicum induced by genistein on soybean stressed by water deficit. Spanish J Agr Res. 2009;7:665–671. [Google Scholar]
- 27.Pan B, Smith DL. Genistein and daidzein concentrations and contents in seedling roots of three soybean cultivars grown under three root zone temperatures. J Agronomy Crop Science. 1998;180:77–82. [Google Scholar]
- 28.Pessi G, Ahrens CH, Rehrauer H, Lindemann A, Hauser F, Fischer HM, Hennecke H. Genome-wide transcript analysis of Bradyrhizobium japonicum bacteroids in soybean root nodules. Mol Plant Microbe Interact. 2007;20:1353–1363. doi: 10.1094/MPMI-20-11-1353. [DOI] [PubMed] [Google Scholar]
- 29.Prithiviraj B, Souleimanov A, Zhou X, Smith DL. Differential response of soybean (Glycine max (L.) Merr.) genotype to lipo-chito-oligosaccharide Nod Bj V (C18:1 MeFuc) J Exp Bot. 2000;51:2045–2051. doi: 10.1093/jexbot/51.353.2045. [DOI] [PubMed] [Google Scholar]
- 30.Saeki Y, Akagi I, Takaki H, Nagatomo Y. Diversity of indigenous Bradyrhizobium strains isolated from three different Rj-soybean cultivars in terms of randomly amplified polymorphic DNA and intrinsic antibiotic resistance. Soil Sci Plant Nutr. 2000;46:917–926. [Google Scholar]
- 31.Saeki Y, Aimi N, Hashimoto M, Kaneko A, Yoshida N, Nagatomo Y, Akao S. Grouping of Bradyrhizobium USDA strains by sequence analysis of 16S rDNA and 16S–23S rDNA internal transcribed spacer region. Soil Sci Plant Nutr. 2004;50:517–525. [Google Scholar]
- 32.Saeki Y, Aimi N, Tsukamoto S, Yamakawa T, Nagatomo Y, Akao S. Diversity and geographical distribution of indigenous soybean-nodulating bradyrhizobia in Japan. Soil Sci Plant Nutr. 2006;52:418–426. [Google Scholar]
- 33.Saeki Y, Minami M, Yamamoto A, Akao S. Estimation of the bacterial community diversity of soybean-nodulating rhizobia isolated from Rj-genotype soybean. Soil Sci Plant Nutr. 2008;54:718–724. [Google Scholar]
- 34.Saeki Y, Ozumi S, Yamamoto A, Umehara Y, Hayashi M, Sigua GC. Change in population occupancy of bradyrhizobia under different temperature regimes. Microbes Environ. 2010;25:309–312. doi: 10.1264/jsme2.me10128. [DOI] [PubMed] [Google Scholar]
- 35.Saeki Y. Characterization of soybean-nodulating rhizobial communities and diversity. In: Aleksandra S, editor. Soybean—Molecular Aspects of Breeding. Intech; Rijeka, Croatia: 2011. pp. 163–184. [Google Scholar]
- 36.Saeki Y, Shiro S, Tajima T, Yamamoto A, Sameshima-Saito R, Sato T, Yamakawa T. Mathematical ecology analysis of geographical distribution of soybean-nodulating bradyrhizobia in Japan. Microbes Environ. 2013;28:470–478. doi: 10.1264/jsme2.ME13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sameshima R, Isawa T, Sadowsky MJ, Hamada T, Kasai H, Shutsrirung A, Mitsui H, Minamisawa K. Phylogeny and distribution of extra-slow-growing Bradyrhizobium japonicum harboring high copy numbers of RSα, RSβ and IS1631. FEMS Microbiol Ecol. 2003;44:191–202. doi: 10.1016/S0168-6496(03)00009-6. [DOI] [PubMed] [Google Scholar]
- 38.Shiro S, Yamamoto A, Umehara Y, Hayashi M, Yoshida N, Nishiwaki A, Yamakawa T, Saeki Y. Effect of Rj-genotype and cultivation temperature on the community structure of soybeannodulating bradyrhizobia. Appl Environ Microbiol. 2012;78:1243–1250. doi: 10.1128/AEM.06239-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shiro S, Matsuura S, Saiki R, Sigua GC, Yamamoto A, Umehara Y, Hayashi M, Saeki Y. Genetic diversity and geographical distribution of indigenous soybean-nodulating bradyrhizobia in the United States. Appl Environ Microbiol. 2013;79:3610–3618. doi: 10.1128/AEM.00236-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spaink HP, Wijfjes AH, Lugtenberg BJ. Rhizobium NodI and NodJ play a role in the efficiency of secretion of lipochitin oligosaccharides. J Bacteriol. 1995;177:6276–6281. doi: 10.1128/jb.177.21.6276-6281.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spaink HP, Bloemberg GV, van Brussel AAN, Lugtenberg BJJ, van der Drift KMGM, Haverkamp J, Thomas-Oates JE. Host specificity of Rhizobium leguminosarum is determined by the hydrophobicity of highly unsaturated fatty acyl moieties of the nodulation factors. Mol Plant Microbe Interact. 1995;8:155–164. [Google Scholar]
- 42.Streeter JG. Failure of inoculant rhizobia to overcome the dominance of indigenous strains for nodule formation. Can J Microbiol. 1994;40:513–522. [Google Scholar]
- 43.Suzuki Y, Adhikari D, Itoh K, Suyama K. Effect of temperature on competition and relative dominance of Bradyrhizobium japonicum and Bradyrhizobium elkanii in the process of soybean nodulation. Plant Soil. 2014;374:915–924. [Google Scholar]
- 44.Uchiumi T, Ohwada T, Itakura M, et al. Expression islands clustered on the symbiosis island of the Mesorhizobium loti genome. J Bacteriol. 2004;186:2439–2448. doi: 10.1128/JB.186.8.2439-2448.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vincent JM. International Biological Program. Blackwell Scientific; Oxford, United Kingdom: 1970. A Manual for the Practical Study of the Root-Nodule Bacteria. [Google Scholar]
- 46.Wei M, Yokoyama T, Minamisawa K, et al. Soybean seed extracts preferentially express genomic loci of Bradyrhizobium japonicum in the initial interaction with soybean, Glycine max (L.) Merr. DNA Res. 2008;15:201–214. doi: 10.1093/dnares/dsn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei M, Takeshima K, Yokoyama T, et al. Temperature-dependent expression of type III secretion system genes and its regulation in Bradyrhizobium japonicum. Mol Plant Microbe Interact. 2010;23:628–637. doi: 10.1094/MPMI-23-5-0628. [DOI] [PubMed] [Google Scholar]
- 48.Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase–polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270:41–49. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- 49.Yokoyama T, Ando S, Murakami T, Imai H. Genetic variability of the common nod gene in soybean bradyrhizobia isolated in Thailand and Japan. Can J Microbiol. 1996;42:1209–1218. doi: 10.1139/m96-156. [DOI] [PubMed] [Google Scholar]
- 50.Yokoyama T. Effects of temperature on competition for nodulation in phylogenetically different Bradyrhizobium strains. Jpn J Soil Sci Plant Nutr. 2005;76:599–607. In Japanese. [Google Scholar]
- 51.Zhang F, Smith DL. Effect of low root temperature on the early stages of symbiosis establishment between soybean [Glycine max (L.) Merr.] and Bradyrhizobium japonicum. J Exp Bot. 1994;45:1467–1473. [Google Scholar]
- 52.Zhang F, Smith DL. Genistein accumulation in soybean (Glycine max [L.] Merr.) root systems under suboptimal root zone temperatures. J Exp Bot. 1996;47:785–792. [Google Scholar]
- 53.Zhang F, Charles TC, Pan B, Smith DL. Inhibition of the expression of B. japonicum nod genes at low temperatures. Soil Biol Biochem. 1996;28:1579–1583. [Google Scholar]
- 54.Zhang H, Prithiviraj B, Souleimanov A, D’Aoust F, Charles TC, Driscoll BT, Smith DL. The effect of temperature and genistein concentration on lipo-chitooligosaccharide (LCO) production by wild-type and mutant strains of Bradyrhizobium japonicum. Soil Biol Biochem. 2002;34:1175–1180. [Google Scholar]