Abstract
Background
Neuroblastoma (nbl) is one of the most common solid cancers in children. Prognosis in advanced nbl is still poor despite aggressive multimodality therapy. Furthermore, survivors experience severe long-term multi-organ sequelae. Hence, the identification of new therapeutic strategies is of utmost importance. Cannabinoids and their derivatives have been used for years in folk medicine and later in the field of palliative care. Recently, they were found to show pharmacologic activity in cancer, including cytostatic, apoptotic, and antiangiogenic effects.
Methods
We investigated, in vitro and in vivo, the anti-nbl effect of the most active compounds in Cannabis, Δ9-tetrahydrocannabinol (thc) and cannabidiol (cbd). We set out to experimentally determine the effects of those compounds on viability, invasiveness, cell cycle distribution, and programmed cell death in human nbl SK-N-SH cells.
Results
Both compounds have antitumourigenic activity in vitro and impeded the growth of tumour xenografts in vivo. Of the two cannabinoids tested, cbd was the more active. Treatment with cbd reduced the viability and invasiveness of treated tumour cells in vitro and induced apoptosis (as demonstrated by morphology changes, sub-G1 cell accumulation, and annexin V assay). Moreover, cbd elicited an increase in activated caspase 3 in treated cells and tumour xenografts.
Conclusions
Our results demonstrate the antitumourigenic action of cbd on nbl cells. Because cbd is a nonpsychoactive cannabinoid that appears to be devoid of side effects, our results support its exploitation as an effective anticancer drug in the management of nbl.
Keywords: Neuroblastoma, cannabidiol, Δ9-tetrahydrocannabinol, apoptosis, tumour xenograft models, non-psychoactive cannabinoids
INTRODUCTION
Neuroblastoma (nbl) is the most frequent extracranial solid tumour in childhood. It accounts for approximately 8% of childhood cancers and is characterized by variable clinical behaviour, reflecting molecular differences in the tumour1. Using current risk stratification criteria, approximately 40% of nbl tumours are classified as high-risk. Treatment for children with high-risk nbl involves multimodality therapy, including chemotherapy, autologous stem-cell transplantation, surgery, radiation therapy, and immunotherapy using differentiation therapy. Despite that aggressive approach, children with nbl have very poor outcomes, and the survivors experience serious side effects related to treatment toxicity2. Hence, the need for new and less-toxic therapeutic strategies to treat the disease is urgent.
For millennia, Cannabis sativa has been used in folk medicine to alleviate pain, depression, amenorrhea, inflammation, epilepsy, and numerous other medical conditions3. In cancer patients specifically, cannabinoids are well known to exert palliative effects; their best-established use is the inhibition of chemotherapy-induced nausea and vomiting, but they are also applied for pain alleviation, appetite stimulation, and attenuation of wasting4.
Recently, increasing evidence suggests that Δ9-tetrahydrocannabinol (thc) and cannabidiol (cbd), major components of Cannabis sativa, and synthetic cannabinoids and the endocannabinoid anandamide have antitumour activity5,6. Many adult cancer types (lung cancer, glioma, thyroid cancer, lymphoma, skin cancer, pancreatic cancer, uterine cancer, and breast and prostate carcinoma) have been reported to be sensitive to the antiproliferative action of cannabinoids in a wide variety of experimental models, including cancer cell lines in culture, xenograft mouse models, and genetically engineered mice6.
Cannabinoids act chiefly by activating the specific cannabinoid receptors cb1 and cb26. However, it is now well-established that these molecules also have effects that are cb receptor–independent; other receptors, such as vanilloid receptor 17 and the peroxisome proliferator–activated receptors8, could be responsible for their action.
The mechanisms involved in the antitumour effects of cannabinoids include proliferation inhibition and growth arrest9, induction of apoptosis10,11, stimulation of autophagy12,13, angiogenesis inhibition14, and anti-metastatic effects15–17. However, the antitumourigenic mechanism of action of cbd is as yet unknown18.
At the molecular level, cannabinoids have been shown to trigger changes in various signalling pathways, including Akt/mammalian target of rapamycin complex 119, erk, upregulation of stress-associated transcription factor p819,20, downregulation of matrix metalloproteinase 221, and vascular endothelial growth factor signalling22. Nevertheless, studies exploring the putative antitumourigenic properties of cannabinoids in pediatric tumours are still limited, and the molecular mechanisms underlying the antitumourigenic effect are poorly understood. Recently published data demonstrated the antitumourigenic activity of cannabinoids—mainly thc and synthetic cannabinoids—on alveolar rhabdomyosarcoma and osteosarcoma by inducing apoptosis23 and triggering the endoplasmic reticular stress and autophagy process24.
Our study aimed to characterize both the in vitro and in vivo effects of cannabinoids on another pediatric tumour, nbl, and to unravel the mechanism responsible for those effects. Given our positive results, we suggest that non-thc cannabinoids such as cbd might provide a basis for the development of novel therapeutic strategies in high-risk nbl, without the typical psychotropic effects of thc and without the strong side effects associated with chemotherapeutic agents.
METHODS
Cannabinoids
Δ9-Tetrahydrocannabinol was supplied by Prof. Raphael Mechoulam, Institute for Drug Research, Medical Faculty, The Hebrew University, Ein Kerem Campus, Jerusalem, Israel. Cannabidiol was supplied by THC Pharm GmbH, Frankfurt, Germany.
Cell Cultures
The human nbl cell lines SK-N-SH25 and IMR-3226 were purchased from ATCC (Manassas, VA, U.S.A.) and the European Collection of Authenticated Cell Cultures (Salisbury, U.K.) respectively. The NUB-627 and LAN-1 cell lines were kindly provided by Dr. Shifra Ash, Schneider Children’s Medical Center of Israel28.
SK-N-SH cells were cultured in Eagle minimum essential medium (ATCC), supplemented with 10% fetal bovine serum (fbs) and 100 U/mL penicillin–streptomycin (Gibco, Paisley, U.K.). IMR-32 cells were cultured in Eagle basal medium (Sigma–Aldrich, St. Louis, MO, U.S.A.) supplemented with 2 mmol/L glutamine, 1% non-essential amino acids, 10% fbs, and 100 U/mL penicillin–streptomycin. LAN-1 and NUB-6 cells were cultured in RPMI-1640 (Gibco) supplemented with 10% fbs and 100 U/mL penicillin– streptomycin. All the cell lines were cultured at 37°C in a humidified atmosphere containing 5% CO2.
MTT Test
An mtt assay (Biological Industries, Kibbutz Beit-Haemek, Israel) was used to evaluate the effect of cbd and thc on nbl cell viability. SK-N-SH, LAN-1, IMR-32, and NUB-6 cells (5×103 cells/mL) were plated (200 μL) in triplicate in flat-bottom 96-well plates in the appropriate medium. The cells were allowed to adhere to the plate surface overnight and were then cultured with increasing doses of thc or cbd (0–50 μg/mL) for 24, 48, and 72 hours. Cell viability was then determined by mtt assay, which measures the reduction of mtt to formazan by the mitochondria of viable cells29. Formazan was measured spectrophotometrically by absorption at 560 nm in a PowerWaveX plate reader (BioTek, Winooski, VT, U.S.A.). All experiments were repeated at least 3 times. Cell morphologies were assessed daily by light microscopy.
Microscopy Analysis
One day before treatment, SK-N-SH cells were plated (1×106 cells per 9-cm plate). After 48 hours of incubation with cbd (10 μg/mL), cell morphology changes were assessed by light microscopy (Olympus CKX41: Olympus, Tokyo, Japan).
Cell-Cycle Analysis
One day before thc or cbd treatment, SK-N-SH cells were plated (1×106 cells per 9-cm plate). After 24, 48, and 72 hours of treatment, the cells were washed in phosphate-buffered saline (pbs: Biological Industries), detached using a solution of 0.1% trypsin (Biological Industries), and spun at 1100 rpm. The resulting pellet was resuspended in 250 μL cold pbs, and the cells were fixed overnight with 5 mL cold 75% ethanol (Sigma–Aldrich) and pbs at −20°C. The pellet was then washed twice with cold pbs (followed by centrifugation at 1100 rpm for 7 minutes). Distribution of the cells in G1, S, and G2/M phases of the cell cycle were monitored after nuclei had been stained with 50 μg/mL propidium iodide (Sigma–Aldrich) containing 125 U/mL protease-free rnase (Sigma–Aldrich) in 0.5% Triton (Bio-Lab, Jerusalem, Israel) and had been pbs-buffered for 30 minutes in the dark. The cells were analyzed using an Epics XL-MCL flow cytometer and the FlowJo software application (Beckman Coulter, Brea, CA, U.S.A.).
Apoptotic Cell Death
Annexin V Assay
One day before cbd treatment (7.5 μg/mL and 10 μg/mL for 24 hours and 48 hours), cells were plated (1×106 cells per 9-cm plate). Cells treated with 1–10 μmol/L staurosporine (Sigma–Aldrich) for 24 hours served as a positive control; untreated cells served as a negative control. Treated cells, untreated cells, and positive control cells were harvested, and after the annexin V assay [human recombinant annexin V (apc conjugate, catalogue no. ALX-209–252: Enzo Life Sciences, Ann Arbor, MI, U.S.A.); annexin V binding buffer, no. 556454 (BD Pharmingen, San Diego, CA, U.S.A.); and 7-aminoactinomycin D, no. 559925 (BD Pharmingen)] were analyzed using an Epics XL-MCL flow cytometer and the FlowJo software application.
Caspase Assay
One day before cbd treatment (7.5 μg/mL and 10 μg/mL for 24 hours), cells were plated (1×106 cells per 9-cm plate). Cells were harvested, and proteins were extracted with radioimmunoprecipitation assay buffer (Sigma–Aldrich). Protein concentrations were calibrated using the BCA Protein Assay Reagent Kit (Pierce, Rockford, IL, U.S.A.).
Samples were separated on 12% sds-page (Bio-Rad, Rishon LeZion, Israel) and transferred onto nitro filters (Schleicher and Schuell Bioscience, Dassel, Germany). The blots were reacted using caspase 3 (8G10) rabbit monoclonal antibody (Cell Signalling Technology, Danvers, MA, U.S.A.) as the primary antibody. The secondary antibody, horseradish peroxidase conjugated goat anti-rabbit antibody (Jackson ImmunoResearch Laboratories, Farmington, CT, U.S.A.), was detected by chemiluminescence. Signals were detected using an ECL Kit (Amersham Pharmacia, Little Chalfont, U.K.) and visualized by exposure to radiography film.
Invasion Assay
Tumour cell invasion was assayed in Transwell chambers (Transwell 3422: Corning, Corning, NY, U.S.A.) pre-coated with Cultrex Basement Membrane Extract (Trevigen, Gaithersburg, MD, U.S.A.). Membrane filters were placed in 24-well tissue-culture plates according to manufacturer guidelines. After 24 hours of treatment with cbd (15 μg/mL and 20 μg/mL), cells were harvested, and 2×105 cells suspended in 200 μL serum-free medium were added to the upper surface of each chamber. The bottom of the chamber was filled with 750 μL medium with 10% fbs. After 24 hours in which cells were allowed to migrate to the underside of the membrane, the invaded cells were fixed with paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, U.S.A.) and stained with crystal violet (Sigma–Aldrich).
In Vivo Studies
All experiments involving mice were approved and performed according to the guidelines issued by the Sheba Medical Center Research Committee for the Care and Use of Laboratory Animals (permit no. 803/12).
To study the in vivo antitumour activity of cannabinoids, nbl tumours were induced in nonobese diabetic immunodeficient (nod/scid) mice by subcutaneous injection. Briefly, 1×107 SK-N-SH cells suspended in 100 μL serum-free medium and Cultrex (1:1) were injected subcutaneously into the rear flank of 5- to 8-week-old nod/ scid mice. Mice were maintained in a pathogen-free environment and monitored weekly for tumour growth. Secondary tumours were detected by palpation and were measured with external callipers. Volume was calculated as (width)2 × (length) × 0.52. When tumours had reached an average size of 400 mm3, the mice were randomly assigned to treatment and control groups (each n = 12). They were then injected intraperitoneally for 14 days with thc (20 mg/kg daily), cbd (20 mg/kg daily), or vehicle (ethanol) or were left untreated. At the end of the treatment period, the mice were euthanized, and the tumours were excised and processed for further analyses.
Histology
Formalin-fixed tissues were dehydrated, embedded in paraffin, and sectioned at 4 μm. The slides were warmed to 60°C for 1 hour and then processed by a fully automated protocol. Immunostainings were calibrated on a Benchmark XT staining module (Ventana Medical Systems, Tucson, AZ, U.S.A.). Briefly, after sections had been dewaxed and rehydrated, a CC1 Standard Benchmark XT pre-treatment for antigen retrieval (Ventana Medical Systems) was selected for active caspase 3. Active caspase 3 antibody (Epitomics, Burlingame, CA, U.S.A.) was diluted 1:10 with Antibody Diluent (Ventana Medical Systems) and incubated for 1 hour at 37°C. Detection was performed using an ultraView detection kit (Ventana Medical Systems) and counterstained with hematoxylin (Ventana Medical Systems). After the run on the automated stainer was completed, the slides were dehydrated in 70% ethanol, 95% ethanol, and 100% ethanol (10 s each). Before cover-slipping, the sections were cleared in xylene (10 s) and mounted with Entellan (EMD Millipore, Billerica, MA, U.S.A.). The stained sections were reviewed under light microscopy and analyzed by a pathologist.
Statistical Analysis
Unless otherwise specified, results are shown as means or medians ± standard deviation. A Kruskal–Wallis test, followed by a post hoc Mann–Whitney test, was used to evaluate significant differences in the viability of cell lines, the growth rate of xenografts, and the counts of positive cleaved caspase 3 cells for the various treatment groups. A p value less than 0.05 was considered statistically significant. All analyses were performed using the IBM SPSS Statistics software application (version 21: IBM, Armonk, NY, U.S.A.).
RESULTS
Viability of NBL Cell Lines In Vitro
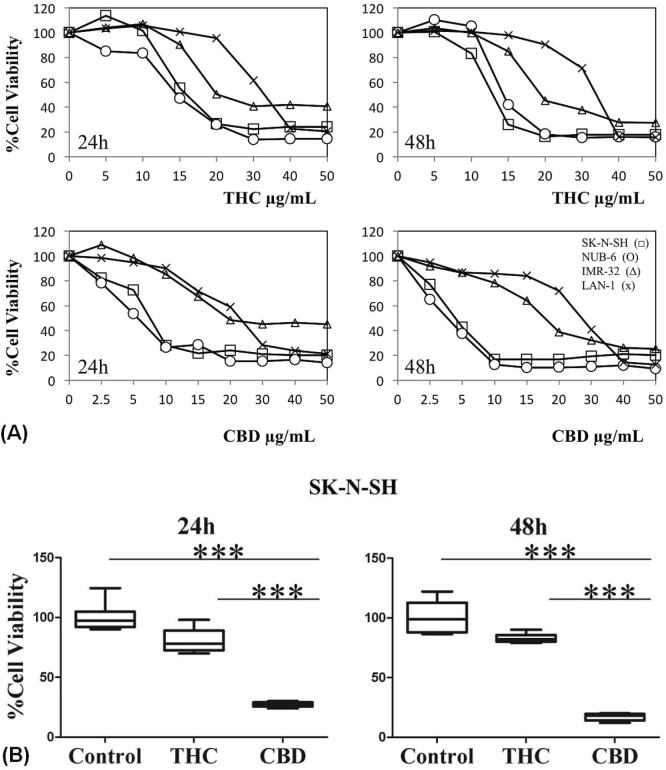
We used an mtt assay to assess the effect of thc and cbd on the viability of the SK-N-SH, NUB-6, IMR-32, and LAN-1 nbl cell lines [Figure 1(A)]. In vitro, after 24 hours of treatment, cbd and thc had already effectively reduced the viability of nbl cell lines in a dose- (0–50 μg/mL) and time-dependent manner, with cbd having the better effect. Better response to treatment was observed in the SK-N-SH and NUB-6 cell lines, as demonstrated by a 50% reduction in cell viability at lower cbd or thc concentrations (5 μg/mL and 15 μg/mL for SK-N-SH and NUB-6 respectively, compared with >20 μg/mL for IMR-32 and LAN-1). The same trend was found, and even enhanced, after treatment with thc and cbd for 48 hours [Figure 1(A)]. More importantly, the response after treatment of SK-N-SH cells with cbd (10 μg/mL) was better than the response after treatment with the same concentration of thc [Figure 1(B), p = 0.0004 for 24 and 48 hours of treatment].
FIGURE 1.
Δ9-Tetrahydrocannabinol (THC) and cannabidiol (CBD) reduce viability of neuroblastoma (NBL) cell lines in vitro, with CBD having a better effect. (A) Cell lines SK-N-SH (open squares), NUB-6 (open circles), IMR-32 (open triangles), and LAN-1 (crosses) were incubated with increasing concentrations (0–50 μg/mL) of THC or CBD for 24 hours and 48 hours. Viability was measured by MTT assay. (B) Mean ± standard deviation of SK-N-SH cell viability after incubation with 10 μg/mL THC or CBD for 24 and 48 hours. *** Denotes a significant change relative to control (p = 0.0004). Data are expressed as a percentage of the vehicle control and are the mean of pooled results from experiments performed in triplicate.
The foregoing data indicate that anti-nbl activity is better with cbd than with thc in all nbl cell lines tested. Because cell lines showed varying sensitivity to cbd, we chose the most sensitive SK-N-SH cell line to confirm the antiproliferative effect of cbd in further in vitro and in vivo experiments.
Cell-Cycle Analysis
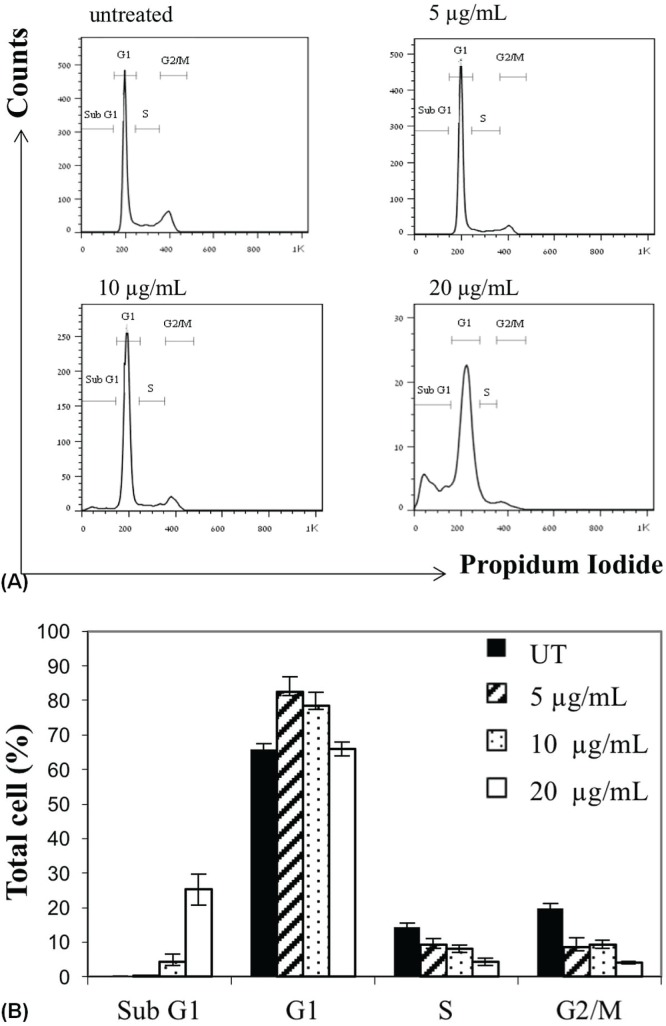
We next studied the effect of 48 hours of treatment with increasing doses of cbd (5–20 μg/mL) on cell-cycle progression [Figure 2(A)]. During treatment with cbd (5 μg/mL), the percentage of SK-N-SH cells sequestered in the G1 compartment rose to 82.4% from 65.8% in untreated control cells [Figure 2(B)]. Accordingly, the percentages of cells in G2 and S phase were found to be decreased, indicating that those cell populations had undergone G1 phase arrest (similar results were obtained when NUB-6 cells were treated with 10 μg/mL cbd; data not shown). Furthermore, an accumulation of SK-N-SH cells in sub-G1 phase [Figure 2(B)] was detected when that line was incubated with 10 μg/mL cbd (4.27%) and 20 μg/mL cbd (25.3%), indicating the possibility that treatment with cbd induced apoptosis in a dose-dependent manner.
FIGURE 2.
Alteration of SK-N-SH cell cycle progression induced by cannabidiol (CBD). (A) Cell cycle analysis in untreated SK-N-SH cells and in cells treated with increasing concentrations of CBD for 48 hours. (B) Change in cell accumulation percentages during cell cycle progression after incubation with CBD for 48 hours. UT = untreated.
Apoptotic Cell Death
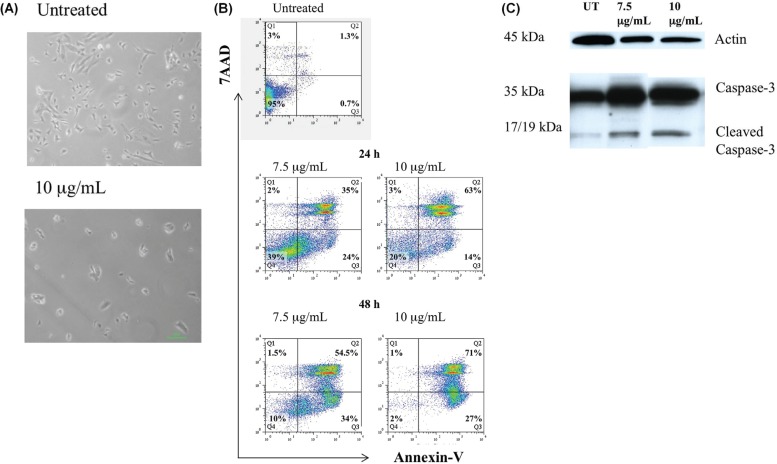
To verify our hypothesis that the reduction in nbl cell viability associated with cbd treatment was indeed attributable to apoptotic cell death, we first examined morphology changes after cbd treatment. Microscopic analysis showed that treatment with 10 μg/mL cbd affected cell morphology; the number of cells that had lost their normal shape, becoming rounded and swollen, and that floated in the medium increased [Figure 3(A)]. Those results confirmed that cbd treatment might induce the appearance of typical features of apoptosis.
FIGURE 3.
Apoptotic effects of cannabidiol (CBD) on SK-N-SH cells. (A) Changes in SK-N-SH cell morphology: untreated cells compared with cells treated with 10 μg/mL CBD for 48 hours. (B) Apoptotic effects of CBD on SK-N-SH cells analyzed by annexin-V assay. Cells were treated with CBD in a dose- and time-dependent manner (7.5 μg/mL, 10 μg/mL; 24 hours, 48 hours) and were stained with annexin-V and 7-amino actinomycin D (7AAD). Q1 = percentage of dead cells; Q2 = percentage of cells in late apoptosis; Q3 = percentage of cells in early apoptosis; Q4 = percentage of live cells. (C) Apoptotic effects of CBD on SK-N-SH cells analyzed by caspase-3 assay. Cells were treated with increasing doses of CBD (7.5 μg/mL, 10 μg/mL) for 24 hours.
Next, we used an annexin V assay to measure the percentage of cells undergoing apoptosis after cbd treatment [Figure 3(B)]. Staurosporine-treated cells were used as a positive control. Treatment of SK-N-SH cells with cbd (7.5 μg/mL and 10 μg/mL) for 24 hours and for 48 hours resulted in an increase in the early apoptotic cell population (annexin V–positive and 7-aminoactinomycin D–negative) in a time-dependent manner. A dose- and time-dependent increase in the late apoptotic cell population was also demonstrated (annexin V–positive and 7-aminoactinomycin D–positive). Early apoptosis was demonstrated in 24% of cells incubated for 24 hours with cbd (7.5 μg/mL), but in only 0.7% of untreated cells. As Figure 3 shows, the proportion of late apoptotic cells increased to 63% from 35% after 24 hours of treatment with increasing concentrations of cbd (10 μg/mL and 7.5 μg/mL respectively)
Finally, to further confirm the apoptotic effects of cbd on SK-N-SH cells, we measured apoptosis by caspase 3 assay [Figure 3(C)]. After 24 hours of treatment with increasing doses of cbd (7.5 μg/mL and 10 μg/mL), a dose-dependent cleavage of caspase 3 was found as evaluated by the appearance of activated p17 and p19 fragments on Western blot analysis.
Altogether, the foregoing results confirm that treatment with cbd induces apoptosis in the SK-N-SH nbl cell line.
Cell Invasiveness
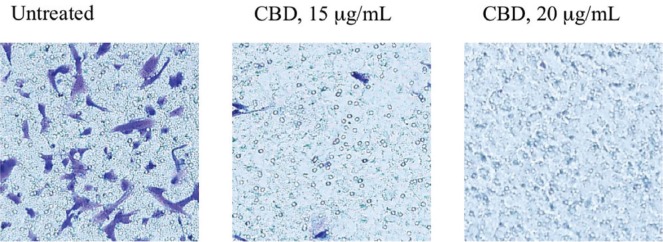
As shown in Figure 4, cell invasion in Transwell chambers was dramatically decreased for SK-N-SH nbl cells treated for 24 hours with cbd (15 μg/mL and 20 μg/mL) than for untreated cells.
FIGURE 4.
Anti-invasiveness effect of cannabidiol (CBD) on SK-N-SH cells. The invasion assays were performed using cell cultures (2×105 cells/well) treated with CBD (15 μg/mL, 20 μg/mL) for 24 hours; results were compared with those for untreated cells (2×105 cells/well). For each well (treated or untreated cells), 10 fields were examined by light microscopy.
Tumour Growth Rate in Mouse Xenograft Model
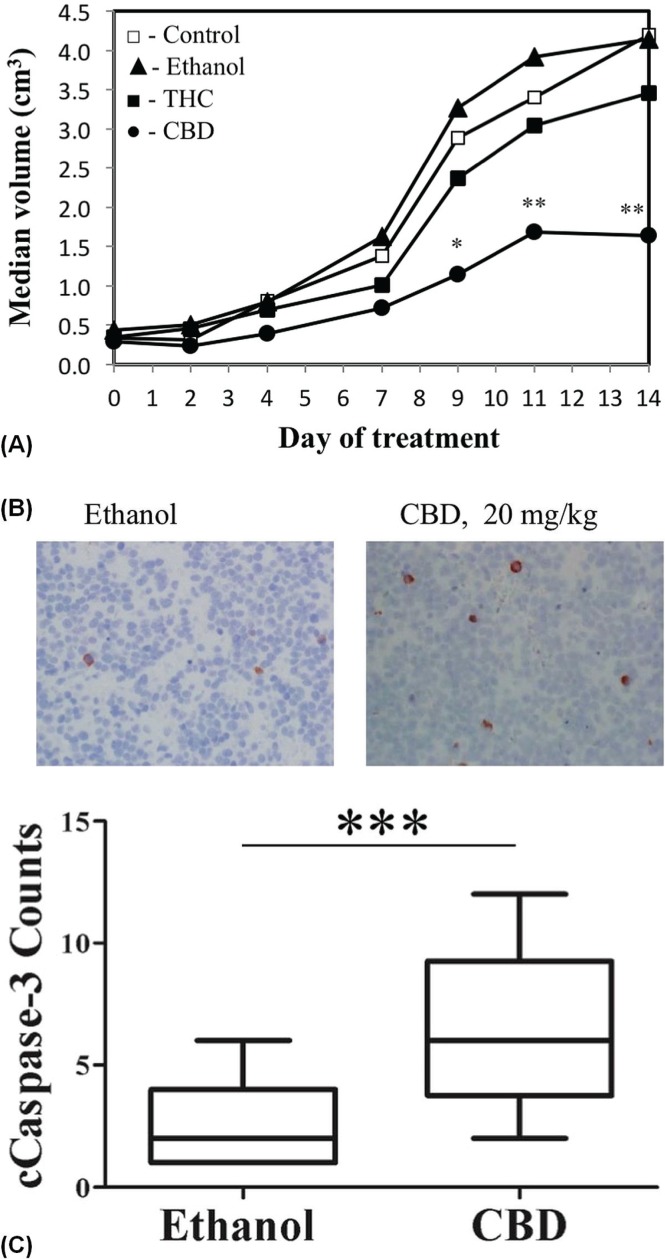
Because tumour regression in an animal xenograft model represents an important endpoint of clinical relevance, we evaluated the ability of cannabinoids to reduce nbl tumour growth in vivo. Tumour xenografts were first generated by subcutaneous injection of SK-N-SH cells into nod/scid mice. The mice were then treated with daily intraperitoneal injections of 20 mg/kg thc, 20 mg/kg cbd, or ethanol vehicle (control), or were left untreated for 14 days.
Tumour growth was significantly reduced in thc- and cbd-treated mice than in the vehicle-treated or untreated mice [Figure 5(A)]. Interestingly, response to treatment was observed to be better in the group treated with cbd than in the group treated with thc: Median xenograft volume at the end of treatment was 2.31 cm3 in the cbd-treated group compared with 4.28 cm3 in the untreated group (p = 0.029) and 4.31 cm3 in the vehicle-treated group (p = 0.036). In the thc-treated group, median volume was 3.46 cm3, which was significant only compared with the untreated group (p = 0.039).
FIGURE 5.
Cannabidiol (CBD) suppresses tumour growth in a mouse xenograft model and increases cleaved caspase-3 staining in treated xenografts. (A) Growth rate of SK-N-SH cell–derived tumour xenografts treated for 14 days with intraperitoneal injections of ethanol-vehicle (n = 12, closed triangles), 20 mg/kg Δ9-tetrahydrocannabinol (n = 12, closed squares), 20 mg/kg CBD (n = 12, closed circles) and untreated controls (n = 12, open squares). Data represents tumour volume during 14 days of treatment. a p < 0.05 and b p < 0.01 for CBD compared with ethanol treatment (Mann–Whitney U-test). (B) Activated caspase-3 immunostaining in SK-NS-H cell–derived tumour xenografts treated with CBD 20 mg/kg or ethanol vehicle for 14 days. (C) Counts of cleaved caspase-3 immunoreactive cells in 18×10 lens fields from xenografts of CBD- and ethanol-treated mice. a p < 0.0001 compared with ethanol.
To further define the in vivo effect of cbd treatment with respect to apoptosis induction, we analyzed tissue obtained from tumour xenografts. Tumours were excised after the last day of treatment, and paraffin-embedded sections were analyzed immunohistochemically with the apoptosis indicator cleaved caspase 3. Cells positive for cleaved caspase 3 were detected with significantly greater frequency in sections of xenografts from cbd-treated mice [Figure 5(B)] than in sections from ethanol-treated mice [p < 0.001, Figure 5(C)].
To summarize, thc and cbd both suppressed the SKN-SH tumour xenograft growth rate, with cbd treatment demonstrating a better effect. Moreover, the better efficacy of cbd and its effect on the induction of activated caspase 3 are consistent with the results obtained in vitro.
DISCUSSION
In recent years, interest in the role of cannabinoids, mainly thc, in cancer therapy has been renewed because of the ability of these molecules to limit tumour cell proliferation and to induce selective cell death5,6,30. The response to treatment with cannabinoids has been investigated and demonstrated in a wide variety of adult tumours30; however, the effect has been studied in only a few pediatric tumours23,24. We therefore investigated the role of cannabinoids in a pediatric tumour, nbl, which is the most frequent extracranial solid tumour of childhood and which still carries a very poor prognosis1.
We focused only on the major compounds in cannabis, thc and cbd. The results obtained in the in vitro studies can be summarized as follows:
■ Both molecules—and cbd in particular—reduced the viability of nbl cells.
■ The effect of cbd seemed to be mediated by apoptotic cell death, as demonstrated by morphology changes, accumulation of sub-G1 cells, annexin V assay, and increased expression of cleaved caspase 3.
■ The invasiveness of nbl cells was also reduced with cbd treatment.
Based on that first set of results, we studied the effect of cbd and thc on xenograft tumours generated in nod/scid mice from SK-N-SH cells that had already demonstrated the greatest sensitivity to the effect of those molecules. In accord with the findings from the in vitro experiments, thc and cbd both reduced the xenograft growth rate, with cbd showing a superior effect.
Our in vitro data suggesting that cbd inhibits the proliferation of, and induces apoptosis in, nbl cells—together with its remarkable effect on nbl xenografts—are, to the best of our knowledge, the first to show an antitumour effect of cbd on nbl cells. Moreover, the results obtained in our study indicate that, of the two cannabinoids tested, cbd was more effective on the SK-N-SH cell line and on xenografts than was the more-studied thc. Those results accord with recently emerging data showing an effect of cbd on other tumours such as glioblastoma and breast, lung, prostate, and colon cancer18,31–34.
Δ9-Tetrahydrocannabinol, the second most abundant cannabinoid in Cannabis sativa, has been shown to induce apoptosis and to inhibit tumour cell viability and invasiveness in various tumours34–38, as our study also demonstrated. Recently, cbd was also reported to enhance the production of reactive oxygen species in cancer cells39, to downregulate the metastatic factor Id1, and to upregulate the pro-differentiation factor Id216,40.
The mechanism by which cbd produces the observed effects has not yet been completely clarified, but seems to be independent of the cb1 and cb2 receptors. Various studies have demonstrated that cbd acts as an agonist for vanilloid receptor 1 and for the trpv2, trpa1, pparg, and 5-ht1a receptors, and as an antagonist for the trpm8 and gpr55 receptors41. However, cbd’s antitumourigenic molecular mechanisms of action have not been studied in nbl. A hint can be found in several reports showing that various nbl cell lines express the foregoing receptors shown to be involved in the action of cbd42–44. Several studies have demonstrated that activation of those receptors with agonists other than cbd mediates cell death in a variety of nbl cell lines, including SK-N-SH45,46, the cbd-responsive cell line in our study.
As a potential therapeutic agent, cbd could have many advantages, especially compared with psychoactive thc. Because most—if not all—of the psychoactive effects of cannabinoids are produced by activation of the central cb1 receptors47, cbd, which has been shown to act independently of cb1, is devoid of psychoactive effects48 and can serve as a more suitable treatment, especially in children. Additionally, it shares the palliative properties and low toxicity profile described for other cannabinoids, has none of the strong side effects associated with chemotherapeutic agents10,18,49, and might have synergistic activity with well-established antineoplastic substances10,50.
The most widely used route of cannabinoid administration is smoking—an unattractive clinical option, particularly in children. Our work indicates that systemic (intraperitoneal) administration of cbd effectively reduces tumour growth, and use in a clinical setting can therefore be based on other routes of administration, such as in oral or oromucosal treatments.
CONCLUSIONS
Our findings about the activity of cbd in nbl support and extend previous findings about the antitumour activities of cbd in other tumours and suggest that cannabis extracts enriched in cbd and not in thc could be suitable for the development of novel non-psychotropic therapeutic strategies in nbl. Use of cbd either as single agent or in combination with existing compounds and chemotherapy agents is a possibility. Combination therapy might improve the antitumourigenic effects of other treatments and allow for a reduction in the chemotherapy dose, minimizing toxicity and long-term sequelae. Future studies are needed to highlight the pathways involved in the antitumourigenic effects of cbd in nbl as demonstrated in the present work.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–20. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 2.London WB, Castel V, Monclair T, et al. Clinical and biologic features predictive of survival after relapse of neuroblastoma: a report from the International Neuroblastoma Risk Group project. J Clin Oncol. 2011;29:3286–92. doi: 10.1200/JCO.2010.34.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mechoulam R, editor. The Pharmacohistory of Cannabis sativa Cannabinoids as Therapeutic Agents. Boca Raton, FL: CRC Press; 1986. pp. 1–19. [Google Scholar]
- 4.Pertwee RG. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br J Pharmacol. 2009;156:397–411. doi: 10.1111/j.1476-5381.2008.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galve-Roperh I, Sanchez C, Cortes ML, Gomez del Pulgar T, Izquierdo M, Guzman M. Anti-tumoral action of cannabinoids: involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat Med. 2000;6:313–19. doi: 10.1038/73171. [DOI] [PubMed] [Google Scholar]
- 6.Velasco G, Sanchez C, Guzman M. Towards the use of cannabinoids as antitumour agents. Nat Rev Cancer. 2012;12:436–44. doi: 10.1038/nrc3247. [DOI] [PubMed] [Google Scholar]
- 7.Zygmunt PM, Petersson J, Andersson DA, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–7. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 8.O’Sullivan SE. Cannabinoids go nuclear: evidence for activation of peroxisome proliferator-activated receptors. Br J Pharmacol. 2007;152:576–82. doi: 10.1038/sj.bjp.0707423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galanti G, Fisher T, Kventsel I, et al. Delta9–tetrahydrocannabinol inhibits cell cycle progression by downregulation of e2f1 in human glioblastoma multiforme cells. Acta Oncol. 2008;47:1062–70. doi: 10.1080/02841860701678787. [DOI] [PubMed] [Google Scholar]
- 10.Carracedo A, Lorente M, Egia A, et al. The stress-regulated protein p8 mediates cannabinoid-induced apoptosis of tumor cells. Cancer Cell. 2006;9:301–12. doi: 10.1016/j.ccr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Calvaruso G, Pellerito O, Notaro A, Giuliano M. Cannabinoid-associated cell death mechanisms in tumor models (review) Int J Oncol. 2012;41:407–13. doi: 10.3892/ijo.2012.1476. [DOI] [PubMed] [Google Scholar]
- 12.Salazar M, Carracedo A, Salanueva IJ, et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of er stress in human glioma cells. J Clin Invest. 2009;119:1359–72. doi: 10.1172/JCI37948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vara D, Salazar M, Olea-Herrero N, Guzman M, Velasco G, Diaz-Laviada I. Anti-tumoral action of cannabinoids on hepatocellular carcinoma: role of ampk-dependent activation of autophagy. Cell Death Differ. 2011;18:1099–111. doi: 10.1038/cdd.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Portella G, Laezza C, Laccetti P, De Petrocellis L, Di Marzo V, Bifulco M. Inhibitory effects of cannabinoid cb1 receptor stimulation on tumor growth and metastatic spreading: actions on signals involved in angiogenesis and metastasis. FASEB J. 2003;17:1771–3. doi: 10.1096/fj.02-1129fje. [DOI] [PubMed] [Google Scholar]
- 15.Qamri Z, Preet A, Nasser MW, et al. Synthetic cannabinoid receptor agonists inhibit tumor growth and metastasis of breast cancer. Mol Cancer Ther. 2009;8:3117–29. doi: 10.1158/1535-7163.MCT-09-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramer R, Hinz B. Inhibition of cancer cell invasion by cannabinoids via increased expression of tissue inhibitor of matrix metalloproteinases-1. J Natl Cancer Inst. 2008;100:59–69. doi: 10.1093/jnci/djm268. [DOI] [PubMed] [Google Scholar]
- 17.McAllister SD, Murase R, Christian RT, et al. Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. Breast Cancer Res Treat. 2011;129:37–47. doi: 10.1007/s10549-010-1177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Massi P, Solinas M, Cinquina V, Parolaro D. Cannabidiol as potential anti-cancer drug. Br J Clin Pharmacol. 2012;75:303–12. doi: 10.1111/j.1365-2125.2012.04298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez del Pulgar T, Velasco G, Sanchez C, Haro A, Guzman M. De novo–synthesized ceramide is involved in cannabinoid-induced apoptosis. Biochem J. 2002;363:183–8. doi: 10.1042/0264-6021:3630183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellert-Miklaszewska A, Kaminska B, Konarska L. Cannabinoids down-regulate pi3k/Akt and erk signalling pathways and activate proapoptotic function of Bad protein. Cell Signal. 2005;17:25–37. doi: 10.1016/j.cellsig.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Blazquez C, Salazar M, Carracedo A, et al. Cannabinoids inhibit glioma cell invasion by down-regulating matrix metalloproteinase-2 expression. Cancer Res. 2008;68:1945–52. doi: 10.1158/0008-5472.CAN-07-5176. [DOI] [PubMed] [Google Scholar]
- 22.Blazquez C, Gonzalez-Feria L, Alvarez L, Haro A, Casanova ML, Guzman M. Cannabinoids inhibit the vascular endothelial growth factor pathway in gliomas. Cancer Res. 2004;64:5617–23. doi: 10.1158/0008-5472.CAN-03-3927. [DOI] [PubMed] [Google Scholar]
- 23.Oesch S, Walter D, Wachtel M, et al. Cannabinoid receptor 1 is a potential drug target for treatment of translocation-positive rhabdomyosarcoma. Mol Cancer Ther. 2009;8:1838–45. doi: 10.1158/1535-7163.MCT-08-1147. [DOI] [PubMed] [Google Scholar]
- 24.Notaro A, Sabella S, Pellerito O, et al. Involvement of par-4 in cannabinoid-dependent sensitization of osteosarcoma cells to trail-induced apoptosis. Int J Biol Sci. 2014;10:466–78. doi: 10.7150/ijbs.8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilbert LC, Wachsman JT. Characterization and partial purification of the plasminogen activator from human neuroblastoma cell line, SK-N-SH. A comparison with human urokinase. Biochim Biophys Acta. 1982;704:450–60. doi: 10.1016/0167-4838(82)90067-X. [DOI] [PubMed] [Google Scholar]
- 26.Tumilowicz JJ, Nichols WW, Cholon JJ, Greene AE. Definition of a continuous human cell line derived from neuroblastoma. Cancer Res. 1970;30:2110–18. [PubMed] [Google Scholar]
- 27.Yeger H, Baumal R, Pawlin G, et al. Phenotypic and molecular characterization of inducible human neuroblastoma cell lines. Differentiation. 1988;39:216–27. doi: 10.1111/j.1432-0436.1988.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 28.Yaari S, Jacob-Hirsch J, Amariglio N, Haklai R, Rechavi G, Kloog Y. Disruption of cooperation between Ras and MycN in human neuroblastoma cells promotes growth arrest. Clin Cancer Res. 2005;11:4321–30. doi: 10.1158/1078-0432.CCR-04-2071. [DOI] [PubMed] [Google Scholar]
- 29.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 30.Sarfaraz S, Adhami VM, Syed DN, Afaq F, Mukhtar H. Cannabinoids for cancer treatment: progress and promise. Cancer Res. 2008;68:339–42. doi: 10.1158/0008-5472.CAN-07-2785. [DOI] [PubMed] [Google Scholar]
- 31.Romano B, Borrelli F, Pagano E, Cascio MG, Pertwee RG, Izzo AA. Inhibition of colon carcinogenesis by a standardized Cannabis sativa extract with high content of cannabidiol. Phytomedicine. 2014;21:631–9. doi: 10.1016/j.phymed.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Nabissi M, Morelli MB, Amantini C, et al. Cannabidiol stimulates Aml-1a–dependent glial differentiation and inhibits glioma stem-like cells proliferation by inducing autophagy in a trpv2-dependent manner. Int J Cancer. 2015;137:1855–69. doi: 10.1002/ijc.29573. [DOI] [PubMed] [Google Scholar]
- 33.Morelli MB, Offidani M, Alesiani F, et al. The effects of cannabidiol and its synergism with bortezomib in multiple myeloma cell lines. A role for transient receptor potential vanilloid type-2. Int J Cancer. 2014;134:2534–46. doi: 10.1002/ijc.28591. [DOI] [PubMed] [Google Scholar]
- 34.Elbaz M, Nasser MW, Ravi J, et al. Modulation of the tumor microenvironment and inhibition of egf/egfr pathway: novel anti-tumor mechanisms of cannabidiol in breast cancer. Mol Oncol. 2015;9:906–19. doi: 10.1016/j.molonc.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ligresti A, Moriello AS, Starowicz K, et al. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J Pharmacol Exp Ther. 2006;318:1375–87. doi: 10.1124/jpet.106.105247. [DOI] [PubMed] [Google Scholar]
- 36.Ramer R, Merkord J, Rohde H, Hinz B. Cannabidiol inhibits cancer cell invasion via upregulation of tissue inhibitor of matrix metalloproteinases-1. Biochem Pharmacol. 2010;79:955–66. doi: 10.1016/j.bcp.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Ramer R, Rohde A, Merkord J, Rohde H, Hinz B. Decrease of plasminogen activator inhibitor-1 may contribute to the anti-invasive action of cannabidiol on human lung cancer cells. Pharm Res. 2010;27:2162–74. doi: 10.1007/s11095-010-0219-2. [DOI] [PubMed] [Google Scholar]
- 38.Aviello G, Romano B, Borrelli F, et al. Chemopreventive effect of the non-psychotropic phytocannabinoid cannabidiol on experimental colon cancer. J Mol Med (Berl) 2012;90:925–34. doi: 10.1007/s00109-011-0856-x. [DOI] [PubMed] [Google Scholar]
- 39.Singer E, Judkins J, Salomonis N, et al. Reactive oxygen species-mediated therapeutic response and resistance in glioblastoma. Cell Death Dis. 2015;6:e1601. doi: 10.1038/cddis.2014.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McAllister SD, Christian RT, Horowitz MP, Garcia A, Desprez PY. Cannabidiol as a novel inhibitor of Id-1 gene expression in aggressive breast cancer cells. Mol Cancer Ther. 2007;6:2921–7. doi: 10.1158/1535-7163.MCT-07-0371. [DOI] [PubMed] [Google Scholar]
- 41.Massi P, Solinas M, Cinquina V, Parolaro D. Cannabidiol as potential anticancer drug. Br J Clin Pharmacol. 2013;75:303–12. doi: 10.1111/j.1365-2125.2012.04298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El Andaloussi-Lilja J, Lundqvist J, Forsby A. trpv1 expression and activity during retinoic acid–induced neuronal differentiation. Neurochem Int. 2009;55:768–74. doi: 10.1016/j.neuint.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 43.Caballero FJ, Soler-Torronteras R, Lara-Chica M, et al. AM404 inhibits nfat and nf-κb signaling pathways and impairs migration and invasiveness of neuroblastoma cells. Eur J Pharmacol. 2015;746:221–32. doi: 10.1016/j.ejphar.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 44.Louhivuori LM, Bart G, Larsson KP, et al. Differentiation dependent expression of trpa1 and trpm8 channels in IMR-32 human neuroblastoma cells. J Cell Physiol. 2009;221:67–74. doi: 10.1002/jcp.21828. [DOI] [PubMed] [Google Scholar]
- 45.Cellai I, Benvenuti S, Luciani P, et al. Antineoplastic effects of rosiglitazone and pparγ transactivation in neuroblastoma cells. Br J Cancer. 2006;95:879–88. doi: 10.1038/sj.bjc.6603344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baek YM, Hwang HJ, Kim SW, et al. A comparative proteomic analysis for capsaicin-induced apoptosis between human hepatocarcinoma (HepG2) and human neuroblastoma (SKN-SH) cells. Proteomics. 2008;8:4748–67. doi: 10.1002/pmic.200800094. [DOI] [PubMed] [Google Scholar]
- 47.Wiskerke J, Pattij T, Schoffelmeer AN, De Vries TJ. The role of cb1 receptors in psychostimulant addiction. Addict Biol. 2008;13:225–38. doi: 10.1111/j.1369-1600.2008.00109.x. [DOI] [PubMed] [Google Scholar]
- 48.Hollister LE, Gillespie H. Interactions in man of delta-9-tetrahydrocannabinol. ii. Cannabinol and cannabidiol. Clin Pharmacol Ther. 1975;18:80–3. doi: 10.1002/cpt197518180. [DOI] [PubMed] [Google Scholar]
- 49.Guzman M, Duarte MJ, Blazquez C, et al. A pilot clinical study of Δ9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br J Cancer. 2006;95:197–203. doi: 10.1038/sj.bjc.6603236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holland ML, Lau DT, Allen JD, Arnold JC. The multidrug transporter abcg2 (bcrp) is inhibited by plant-derived cannabinoids. Br J Pharmacol. 2007;152:815–24. doi: 10.1038/sj.bjp.0707467. [DOI] [PMC free article] [PubMed] [Google Scholar]