Abstract
Purpose
To evaluate the safety, efficacy and biomarkers of short-course proton beam radiation and capecitabine, followed by pancreaticoduodenectomy in a phase 1/2 study in pancreatic ductal adenocarcinoma (PDAC) patients.
Methods and Materials
Patients with radiographically resectable, biopsy-proven PDAC were treated with neoadjuvant short-course (2-week) proton-based radiation with capecitabine, followed by surgery and adjuvant gemcitabine. The primary objective was to demonstrate a rate of toxicity grade ≥3 of <20%. Exploratory biomarker studies were performed using surgical specimen tissues and peripheral blood.
Results
The phase 2 dose was established at 5 daily doses of 5 GyE. Fifty patients were enrolled, of whom 35 patients were treated in the phase 2 portion. There were no grade 4 or 5 toxicities, and only 2 of 35 patients (4.1%) experienced a grade 3 toxicity event (chest wall pain grade 1, colitis grade 1). Of 48 patients eligible for analysis, 37 underwent pancreaticoduodenectomy. Thirty of 37 (81%) had positive nodes. Locoregional failure occurred in 6 of 37 resected patients (16.2%), and distant recurrence occurred in 35 of 48 patients (72.9%). With median follow-up of 38 months, the median progression-free survival for the entire group was 10 months, and overall survival was 17 months. Biomarker studies showed significant associations between worse survival outcomes and the KRAS point mutation change from glycine to aspartic acid at position 12, stromal CXCR7 expression, and circulating biomarkers CEA, CA19-9, and HGF (all, P<.05).
Conclusions
This study met the primary endpoint by showing a rate of 4.1% grade 3 toxicity for neoadjuvant short-course proton-based chemoradiation. Treatment was associated with favorable local control. In exploratory analyses, KRASG12D status and high CXCR7 expression and circulating CEA, CA19-9, and HGF levels were associated with poor survival.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a lethal disease that afflicts ~42,000 patients per year in the United States (1). The available treatments for PDAC have limited efficacy. Even at early stages, only surgical resection affords the potential for cure. However, resected PDAC has high rates of local and distant failure, which remains incurable (2–6). Adjuvant cytotoxic therapies have shown only modest impact on cure rates (7–9), and the role of molecularly targeted agents in perioperative setting remains unknown.
Although the high distant metastatic rate renders a survival benefit with radiation that is difficult to demonstrate, controlling local disease with radiation could alleviate morbidity that adversely affects quality of life. Perioperative radiation therapy can delay systemic therapy or surgery, particularly when delivered preoperatively. Given the high metastatic propensity of even localized PDAC, shorter courses of radiation would be highly desirable. In rectal cancer, short-course (1-week) radiation therapy (5 Gy × 5 fractions) followed by early surgery is an effective way of decreasing pelvic recurrence (10–14). More conformal radiation techniques, such as intensity modulated radiation therapy (IMRT) or proton beam therapy, may allow for delivery of efficacious doses in a shortened schedule. In preclinical evaluations, we demonstrated that proton beam therapy was associated with less radiation dose to adjacent organs than IMRT (15). We have previously reported the feasibility of a proton-based 1-week neoadjuvant chemoradiation schedule followed by early surgery in the phase 1 portion of this trial (16). However, safety and tolerability concerns remain with the use of this approach. In addition, improvements in therapy for this extremely aggressive malignancy will likely require identification and targeting of specific molecular pathways that facilitate metastatic progression. Here, we report safety and efficacy data from the phase 1/2 study. We also report the results of exploratory correlative studies in tissue and blood circulation.
Methods and Materials
Patients
Patients with resectable PDAC were prospectively enrolled in a National Cancer Institute-sponsored clinical trial approved by the institutional review board (NCT00438256). Inclusion criteria included biopsy-proven adenocarcinoma of the pancreatic head or neck amenable to surgical resection with a pancreaticoduodenectomy; Eastern Cooperate Oncology Group 0/1 performance status; a pancreatic protocol computed tomography (CT) scan that, in the judgment of the surgeon and the multidisciplinary team, showed a resectable tumor; and no evidence of metastatic disease based on CT of the chest, abdomen, and pelvis and diagnostic laparoscopy (including cytology). Exclusion criteria included ampullary, biliary, or duodenal cancer, as well as distal tumors of the body or tail of the pancreas; prior therapy for PDAC, any invasive cancer in the last 5 years requiring radiation or chemotherapy, prior radiation therapy to the upper abdomen, and history of dihydropyrimidine dehydrogenase deficiency. Laboratory evaluations included biomarker CA19-9 and CEA levels, electrolytes, complete blood counts, and liver and renal function tests. Patients were required to have absolute neutrophil count (ANC) ≥1500 cells/mm3, a platelet count of >100,000 cells/mm3, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) at ≤2.5 × upper limit of normal (ULN); total bilirubin at ≤2.5 × ULN, if patient had recent biliary stenting, or ≥1.5 × ULN, if no biliary stenting was done; serum creatinine within normal range (0.6–1.5 mg/dL) with a creatinine clearance ≥30 mL/min.
Treatment
Radiation therapy
Gross tumor volume was contoured with the pancreatic protocol CT available. Clinical target volume was defined as gross tumor volume with a 1-cm margin, respecting anatomical boundaries such as stomach and transverse colon, as well as elective nodal coverage including the celiac, portahepatis, superior mesenteric artery and vein, and para-aortic (through the level of the third portion of the duodenum) groups. A planning target expansion was customized using the motion information from the 4-dimensional CT and estimated set-up variation (see Supplementary Material and Table S1) (16).
Treatments were delivered using 240-MeV protons generated from a cyclotron. Proton beam therapy was delivered using 3D passively scattered protons. Most commonly, 3 fields were used, with 2 fields being treated per day.
Chemotherapy
Capecitabine (1650 mg/m2 divided twice daily) was given Monday to Friday for 2 weeks for each dose level.
Supportive treatment
After dose level 1, patients were counseled to use ondansetron, 8 mg orally, 30 to 60 minutes prior to therapy. Additionally, patient therapy was initiated with a proton pump inhibitor if the patient was not already taking one.
Surgery
Patients in dose levels 1 to 3 underwent surgery 3 to 6 weeks following the completion of chemotherapy. In dose level 3 and 4, as well as the phase 2, surgery could be performed as early 1 week after completion of chemotherapy. Repeat CT staging was mandated only if the patient was scheduled for surgery ≥3 weeks after chemotherapy.
Postoperative chemotherapy
We recommended that patients receive gemcitabine chemotherapy for 6 months starting 4 to 10 weeks after surgery.
Follow-up
Patients had follow-up visits every 3 months, with CT scans every 6 months, for a planned follow-up of 5 years. Any other evaluations that were prompted by symptoms, laboratory evaluation, or at the treating physician’s discretion were also used to score events. Oligometastatic disease at first relapse was defined as ≤3 lesions in 1 organ.
Pathological evaluation
Pathology specimens were processed and scored per standard institutional practice, including margin status (pancreatic transection, biliary, uncinate, and retroperitoneal), and nodal status (total assessed, total positive).
Biomarker analyses
Genotyping
Mutational analysis was performed using SNaPshot (Applied Biosystems, Woburn, MA) for resected patients, using a multiplexed DNA sequencing platform (17).
Immunohistochemistry
Surgical specimens were available from the 38 patients who underwent surgical resection or exploration. Five-micrometer-thick sections were cut from formalin-fixed, paraffin-embedded blocks for staining with Masson tri-chrome (to assess fibrosis) or antibodies against SMAD4 (Abcam), HGF (Abcam), cMET (Abcam), SDF1α (Bio-Vision), CXCR4 (R&D Systems, Minneapolis, MN), CXCR7 (Abcam), CD31 (Dako), α-SMA (Sigma), and CD68 (Thermo Scientific). Semiquantitative and quantitative analyses for biomarker expression or tumor-associated macrophage number (estimated by positive staining area ratio) was carried out specifically for the intratumoral and stromal (tumor periphery) compartments, and performed by 2 experienced gastrointestinal pathologists. Because, CXCR4 can be expressed either in the cell cytoplasm or the plasma membrane, analysis was performed separately for cytoplasmic and membranous CXCR4 expression. Quantification of tumor blood vessels was separated for immature (non-α-SMA+ pericyte-covered) versus more mature (α-SMA+ pericyte-covered) vessels.
Cellular and molecular blood biomarkers
Peripheral blood was obtained from 12 consecutive patients prior to neoadjuvant chemoradiation and prior to surgery (after neoadjuvant chemoradiation) in the phase 2 portion, after obtaining institutional review board approval and informed consent. Plasma analysis was carried out for circulating VEGF, PlGF, sVEGFR1, bFGF, IL-1β, IL-6, IL-8, and TNF-α by using multiplex enzyme-linked immunosorbent assay plates (Meso-Scale Discovery, Rockville, MD), and sVEGFR2, HGF, SDF1α, and s-cMET (from R&D Systems). Every sample was run in duplicate. Blood progenitor cells (CD34+CD133+CD45+) and CD14+ monocytes (CD14+CD45+) were enumerated in fresh samples using an LSR-II flow cytometer (BD Biosciences, Franklin Lakes, NJ), as described (18). Biomarker levels measured using quantitative scales were log-transformed, and changes were calculated as on-study-to-baseline value ratios.
Statistical analyses
The phase 1 design, a gradual shortening from 10 fractions in 2 weeks through progressively shorter 5-fraction schedules, has been previously described (16) (Supplementary Table S1). The objective of the phase 2 component was to demonstrate that the highest dose of accelerated chemoradiation tested in phase 1 was associated with an acceptable toxicity profile (<20% overall rate of grade ≥3 adverse events). Overall survival (OS) and progression-free survival (PFS) as well as the time to locoregional failure were secondary endpoints calculated starting from the first day of chemoradiation (see Supplementary Material). Changes in circulating biomarkers were quantified as ratios and tested with the 1-sample exact Wilcoxon test. The association of OS and PFS with tissue and blood biomarkers was assessed using Cox regression to estimate the hazard ratio and to compare groups by test score. Analysis of biomarkers was of an exploratory nature; therefore, we did not adjust P values for multiple comparisons. All P values are based on 2-sided hypothesis tests.
Results
Patient characteristics
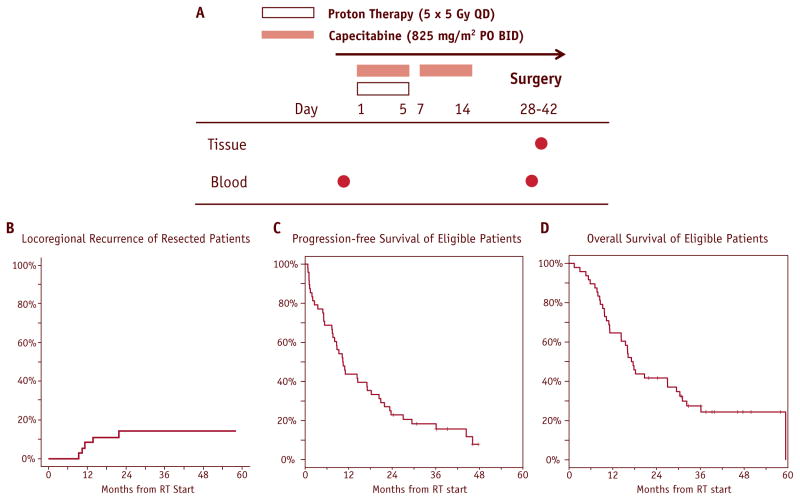
Fifty of 57 patients screened were enrolled in the study (7 were excluded due to metastatic disease found at screening laparoscopy) (Table 1, Fig. 1A). Based on phase 1 data, dose level 4 (5 fractions of 5 Gy-equivalents, or 5 × 5 GyE in 1 week) was selected for the phase 2 component because it showed no DLTs (16).
Table 1.
Patient characteristics (N=50 patients)
| Characteristic | Value | % |
|---|---|---|
| Sex | ||
| Female | n=23 | 46% |
| Male | m = 27 | 54% |
| Age (y) | ||
| Median | 65 | |
| Range | 49–92 | |
| CA19-9 at baseline | ||
| Median | 136.5 | |
| Range | <1–15,151 | |
| Tumor size on CT | ||
| Median | 2.9 cm | |
| Range | 1.1–4.3 | |
Abbreviation: CT = computed tomography.
Fig. 1.
Phase 2 trial of neoadjuvant accelerated short-course radiation therapy with proton beam and capecitabine for resectable pancreatic ductal adenocarcinoma (PDAC). (A) Study design. (B–D) Treatment outcomes of 48 eligible patients: locoregional (resected only) (B), progression-free survival (C), and overall survival (D).
Tolerability
All 35 patients in the phase 2 component completed chemoradiation treatment. Two patients experienced grade 3 toxicity events of colitis and chest wall pain during the preoperative treatment. There were no grade ≥4 toxicities (Table 2).
Table 2.
Preoperative chemoradiation-related toxicity grade 2 or worse (N=35 phase 2 patients)
| Toxicity | No. of patients with grade (%) | No. of patients with grade 3 (%) |
|---|---|---|
| Colitis | 0 | 1 (3%) |
| Nausea and vomiting | 3 (9%) | 0 |
| Constipation | 1 (3%) | 0 |
| Dehydration | 1 (3%) | 0 |
| Diarrhea, no prior colostomy | 1 (3%) | 0 |
| Flatulence | 1 (3%) | 0 |
| Chest wall pain | 0 | 1 (3%) |
| Abdominal pain | 1 (3%) | 0 |
| Limb pain | 1 (3%) | 0 |
| Weight loss | 2 (6%) | 0 |
Surgical outcomes
No patient had surgery delayed due to toxicity. Eleven of 50 patients (22%) did not undergo resection: 1 patient (2%) was ineligible due to a preoperative diagnosis of distal cholangiocarcinoma, 2 patients (4%) due to meta-static progression, and 8 patients (16%) due to unresectable disease at exploration. One of the patients with unresectable disease received intraoperative radiation therapy to a dose of 15 Gy. Another patient went off study after completing chemoradiation but subsequently underwent resection 104 days after the last dose of capecitabine. Median operative time in resected patients was 5:55 hours (range, 3:50–9:28 hours). Median postoperative length of hospital stay was 7 days (range, 5–47 days). Postoperative mortality and morbidity evaluation at 30 days showed no deaths or pancreatic or any other anastomotic leakage. One resected patient was deemed ineligible for outcome analysis due to a final pathologic diagnosis of autoimmune pancreatitis and no evidence of cancer. Thirty-one of 37 eligible resected patients (84%) received postoperative gemcitabine therapy.
Pathological findings
In the 37 eligible resected patients, median pathologic tumor size was 2.9 cm (range, 1.3–4.8 cm). Thirty of 37 patients (81%) had positive nodes, and 6 of 37 patients (16%) had positive margins (Table 3).
Table 3.
Pathologic response
| Primary tumor (N=37 eligible resected patients) | Value |
|---|---|
| RT desmoplasia | |
| No | 26 (70%) |
| Yes | 11 (30%) |
| Tumor size | |
| Median | 2.9 cm |
| Range | 1.3–4.8 |
| Histologic grade | |
| Moderate differentiation | 17 (46%) |
| Poor differentiation | 20 (54%) |
| Perineural invasion | |
| No | 5 (14%) |
| Yes | 32 (86%) |
| Lymphovascular invasion | |
| No | 19 (51%) |
| Yes | 18 (49%) |
| Margin status | |
| Negative | 31 (84%) |
| Positive | 6 (16%) |
| Nodal Involvement | |
| No | 7 (19%) |
| Yes | 30 (81%) |
| Nodes: total assessed | |
| Median | 18 |
| Range | 6–37 |
| Nodes: number positive (among the 30 patients with nodal involvement) | |
| Median | 2 |
| Range | 1–12 |
| Sites of metastatic failure (n=35 patients with distant progression) | |
| Liver* | 20 (57%) |
| Lung* | 14 (40%) |
| Hilar and mediastinal adenopathy* | 1 (3%) |
| Supraclavicular area | 1 (3%) |
| Pleural effusion | 1 (3%) |
Abbreviation: RT = radiation therapy.
Two patients had simultaneous metastatic progression at 2 sites.
Survival outcomes
For all 48 eligible patients (excluding the 2 patients with final diagnosis of cholangiocarcinoma and autoimmune pancreatitis, respectively), the median PFS was 10.4 months (95% confidence interval [CI]: 7.5–17.1 months), and median OS was 17.3 months (95% CI: 11.2–29.5 months) (Fig. 1 C and D). Median follow-up for analysis was 38 months among the 12 patients still alive. The OS rate at 2 years was 42% (95% CI: 28%–55%). For the 37 eligible resected patients, median PFS was 14.5 months (95% CI: 10.2–21.8 months), and median OS was 27.0 months (95% CI: 16.2–32.3 months). Only 6 of 37 eligible resected patients (16%) experienced locoregional recurrence or progression: 1 patient had an isolated local recurrence 16 weeks before progressing to lung metastatic disease, and 5 of 6 patients with locoregional failure had synchronous metastatic disease (Fig. 1B). Thirty-five of 48 patients (73%) developed distant metastases. Initial sites of metastatic failure are listed in Table 3.
Tissue biomarker studies
In the 38 available PDAC surgical specimens, we detected a mutation in the KRAS gene in 31 specimens (82%) and in the TP53 gene in 4 specimens (11%). Both mutations were present in 3 of 38 patients (8%). The most frequent KRAS mutation type was KRASG12D (14 of 38 [37%]), whereas other types were found in lower frequencies (KRASG12V in 10 of 38 [26%]; KRASG12R in 5 of 38 [13%]; KRASG12S in 1 of 38 [3%]; and KRASQ61H in 1 of 38 [3%]). SMAD4 expression was detectable in 12 of 32 patients (38%).
The chemokine SDF1α and its receptors, CXCR4 and CXCR7, were all relatively highly expressed throughout the PDAC tissues but showed differential levels of expression in the tumoral versus the stromal compartments (Table 4). SDF1α and CXCR7 expression predominated intra-tumorally, whereas CXCR4 expression (both cytoplasmic and membranous) was more dominant in stromal cells. In contrast, vascular density was comparable between the 2 compartments, and approximately half of the vessels in these compartments were covered by pericytes. CD68+ macrophage infiltration was more predominant inside the tumor (P=.0029). Tumor-associated fibrosis was pronounced (a median score of 2.3 on a scale of 0–3). Finally, HGF was diffusely detectable in PDAC tissue samples, whereas its receptor, cMET, was expressed in PDAC cells (Supplementary Material Fig. S1).
Table 4.
Tissue biomarker expression and distribution after neoadjuvant proton therapy and chemotherapy in PDACs
| Biomarker | Intratumoral | Stromal | P value |
|---|---|---|---|
| SDF1α (CXCL12) score (n=32) | 2.5 ± 1.0 (32) | 1.8 ± 0.6 (31) | .0001 |
| CXCR4 (cytoplasmic) score | 3.0 ± 1.0 (32) | 3.5 ± 0.8 (31) | .0067 |
| CXCR4 (membranous) score | 1.6 ± 0.7 (32) | 3.5 ± 0.8 (31) | <.0001 |
| CXCR7 score | 3.1 ± 1.0 (32) | 2.3 ± 0.6 (31) | .0004 |
| CD31 + vessel density | 8.7 ± 6.6 (32) | 9.2 ± 6.5 (31) | .45 |
| CD31+/α-SMA + vessel density | 7.5 ± 5.6 (32) | 8.5 ± 4.5 (31) | .21 |
| CD68+ macrophage (area fraction) | 0.056 (0.040–0.081) (32) | 0.037 (0.029–0.051) (32) | .0029 |
| Fibrosis score | 2.3 ± 0.7 (32) | NA | |
Abbreviations: NA = not applicable; PDAC = pancreatic ductal adenocarcinoma.
Data are shown as hazard ratios with 95% confidence intervals.
P values are from a Wald test in a univariable Cox regression.
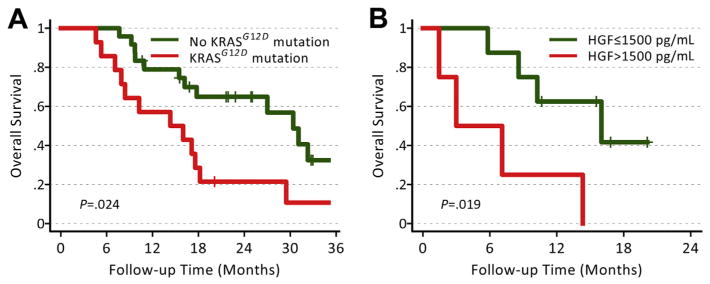
Of the genetic alterations, the presence of KRASG12D, but not any KRAS mutation or SMAD4 status, was associated with poorer PFS (P=.019) and OS (P=.022) (Fig. 2A, Supplementary Material Table S2). Of note, KRASG12D mutation, but not any KRAS mutation, correlated with elevated circulating levels of the cytokine TNF-α (area under the curve receiver operating curve [AUC ROC] = 0.77 [n=8]; P=.036). SMAD4 status did not correlate with PFS or OS (Supplementary Table S2) but showed a tendency to associate with the extent of metastatic disease (Supplementary Material). Finally, we observed a direct association between stromal CXCR7 expression and PFS (P=.0073) and OS (P=.0069) (Supplementary Table S2).
Fig. 2.
Comparison of overall survival according to KRASG12D mutation and elevated baseline hepatocyte growth factor (HGF) in PDAC after neoadjuvant chemoradiation.
Circulating biomarker studies
Of the biomarkers measured in peripheral blood prior to surgery (~2 weeks after neoadjuvant treatment), the levels of VEGF, SDF1α, and bFGF were decreased and those of plasma CAIX and circulating CD14+ monocytes were increased (Supplementary Table S3). High circulating levels of HGF, CEA, and CA19-9 at baseline were associated with poor OS (P<.05) (Fig. 2B, Supplementary Table S4).
Discussion
The role of radiation therapy in resectable PDAC remains controversial (8, 10, 19). Adjuvant chemotherapy alone increases survival compared with surgery alone (8, 9). However, local control remains a challenge: pancreaticoduodenectomy is associated with positive margins rates of approximately 30% (8, 9, 20). When margins of <1 mm are included, most PDAC patients have positive margins (21). Furthermore, approximately 25% of patients have lymph node involvement beyond the peripancreatic nodal field in the hepatic artery or aortocaval regions in addition to the 60% to 80% risk of peripancreatic nodal involvement (9, 22). These factors contribute to high (50%–80%) locoregional failure rates (2–6). Radiation therapy may modify this risk as postoperative chemoradiation studies have shown locoregional failure rates of only 20% to 30% (20, 23, 24), and preoperative chemoradiation trials showed an even lower risk of locoregional recurrence (10%–20%) (25–27). However, the substantial rate of local recurrence after postoperative radiation and the high rate of metastatic disease, which may exceed 75% (20), likely account for the lack of substantial survival benefit with radiation.
Preoperative chemoradiation may improve R0 resection rates and local control (25–27). However, a standard course of preoperative chemoradiation takes approximately 6 weeks, followed by 6 weeks’ delay to surgery. If a patient takes approximately another 6 weeks to recover from surgery, this leads to a delay of more than 4 months in systemic therapy. Short-course radiation followed by early surgery can potentially eliminate this delay to systemic therapy yet maintain efficacious. However, there are a lack of safety data with this approach to the upper abdomen, particularly with elective nodal coverage. Because the intent of preoperative therapy is to replace the postoperative therapy, we felt elective nodal coverage should be added (28). Our study indicates that short-course radiation with proton beam is well tolerated and safe. In addition, despite shortened course radiation, this study showed favorable local control and R0 resection rates. These results are consistent with those of our phase 1 data as well as those of another short-course chemoradiation study that used carbon ion radiation therapy (16,29).
One outstanding question is whether 1 week of proton radiation is required, given that hypofractionated stereo-tactic body radiation therapy (SBRT) is feasible in PDAC. However, one fundamental difference between SBRT and hadron therapies is that the clinical tumor volume (CTV) encompassed elective nodal regions (29). In contrast, with SBRT, only grossly identifiable tumor is targeted, leading to substantially smaller fields of treatment. Our institution attempted the same dose escalation strategy with the same treatment volumes with photons in a separate phase 1 study (30). Ten patients were enrolled with a planned phase 1 enrollment of 12 patients. The study was terminated early due to intraoperative toxicity. Surgeons observed an increased risk of intraoperative fibrosis in patients treated with photons versus those treated with protons (27% vs 63%, respectively), resulting in an increase in median operative time of 69 minutes. Although there were no significant differences in CTV or planning target volumes between patients treated in the proton phase 1 versus those treated in the photon phase 1 study, photon patients had substantially higher stomach and small bowel doses. Accordingly, based on this experience, we have proceeded to develop our proton-based short-course strategy.
We also conducted exploratory correlative studies to evaluate the association between tissue and blood circulating biomarkers with survival outcomes after proton-based chemoradiation. These studies included analyses of frequently mutated genes in PDACs (KRAS and SMAD4) and a panel of biomarkers known to be upregulated after radiation therapy using photons, and also known as mediators of tumor invasion and metastasis (SDF1α/CXCR4 or CXCR7) (31–34). KRAS codon 12 activating mutations are particularly frequent in PDAC (35). Moreover, in transgenic mice, KRASG12D has been shown to drive PDAC formation with high penetrance (36–38). Interestingly, we found that only the KRASG12D mutation was inversely associated with survival. We also detected a correlation between high levels of CXCR7 expression and poor survival. Preclinical studies have linked CXCR7 expression with MAPK activation in PDAC (39), and previous clinical studies indicated that CXCR7 is associated with tumor grade and inversely associated with tumor size (40). Our data support the potential role of this receptor in human PDAC progression though cytotoxic therapies. We found that the degree of fibrosis and macrophage infiltration, as well as SDF1α, CXCR7, and CXCR4 expression levels, were high after neoadjuvant treatment. Of interest, the SDF1α and CXCR7 expression was localized inside the tumor, whereas CXCR4 levels and macrophage infiltration were localized predominantly at the tumor periphery in the stroma-rich compartment. Analysis of the tumor vasculature showed no differences between vessel densities in the 2 compartments and that approximately half of the vessels were covered with pericytes (more mature).
Despite improved local control, treatment did not significantly change serum CEA and CA19-9 levels. However, both CEA and CA19-9 levels were associated with survival outcomes, in line with the modest impact of treatment on systemic disease progression. Thus, inhibiting critical pathways driving PDAC progression in combination with neo-adjuvant chemoradiation may result in more significant survival benefits. We found an association between plasma HGF (a prometastatic protein also referred to as “scatter factor”) (41) and OS. These data should be considered hypothesis generating, and future studies should explore the potential roles of the KRASG12D mutation and HGF/c-MET pathway in PDAC resistance to neoadjuvant therapy.
Conclusions
In conclusion, short-course proton-based chemoradiation is well tolerated and is associated with favorable local control in resectable PDAC. In exploratory analyses, KRASG12D status, CXCR7 expression in tumor periphery, and plasma HGF level were associated with PFS and OS and warrant further exploration in larger studies. We believe that this short course regimen, because of its short duration and excellent tolerability, may be useful after multiagent neo-adjuvant chemotherapy and will be used in a neoadjuvant study comparing gemcitabine/nab-paclitaxel with 5-fluorouracil, irinotecan, and oxaliplatin (FOLFIRINOX) followed by short-course proton-based chemoradiation before surgery.
Supplementary Material
Summary.
We report toxicity, efficacy, and tissue and circulating biomarker data from a phase 1/2 study of preoperative short-course chemoradiation with proton beam therapy and capecitabine, followed by early surgery for resectable pancreatic ductal adenocarcinoma. Treatment was well tolerated and was associated with excellent local control. Exploratory studies showed that KRASG12D status and higher tissue levels of CXCR7 expression and circulating plasma hepatocyte growth factor (HGF) were associated with worse survival after neoadjuvant chemoradiation.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant P01-CA021239; Proton Beam National Cancer Institute/Federal Share Program grants C06-CA059267 (to TFD) and C06-CA059267 (to TSH); a Spiro Award (to DGD); NIH grants P01-CA80124 (to RKJ, YB, and DGD) and R01-CA159258 (to DGD); and American Cancer Society research grant RSG-11-073-01-TBG (to DGD).
Footnotes
Data were presented in abstract form at the 48th and 49th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 2012 and June 2013.
This protocol (NCT00438256) is registered with ClinicalTrials.gov and may be viewed online at http://clinicaltrials.gov/ct2/show/NCT00438256?term=NCT00438256&rank=1.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Griffin JF, Smalley SR, Jewell W, et al. Patterns of failure after curative resection of pancreatic carcinoma. Cancer. 1990;66:56–61. doi: 10.1002/1097-0142(19900701)66:1<56::aid-cncr2820660112>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Nitecki SS, Sarr MG, Colby TV, et al. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg. 1995;221:59–66. doi: 10.1097/00000658-199501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piorkowski RJ, Blievernicht SW, Lawrence W, Jr, et al. Pancreatic and periampullary carcinoma. Experience with 200 patients over a 12 year period. Am J Surg. 1982;143:189–193. doi: 10.1016/0002-9610(82)90064-2. [DOI] [PubMed] [Google Scholar]
- 5.Gudjonsson B. Cancer of the pancreas. 50 years of surgery. Cancer. 1987;60:2284–2303. doi: 10.1002/1097-0142(19871101)60:9<2284::aid-cncr2820600930>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 6.Tepper J, Nardi G, Sutt H. Carcinoma of the pancreas: Review of MGH experience from 1963 to 1973. Analysis of surgical failure and implications for radiation therapy. Cancer. 1976;37:1519–1524. doi: 10.1002/1097-0142(197603)37:3<1519::aid-cncr2820370340>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 7.Conroy T, Desseigne F, Ychou M, et al. Folfirinox versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 8.Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiation therapy and chemotherapy in resectable pancreatic cancer: A randomised controlled trial. Lancet. 2001;358:1576–1585. doi: 10.1016/s0140-6736(01)06651-x. [DOI] [PubMed] [Google Scholar]
- 9.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 10.Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish rectal cancer trial. N Engl J Med. 1997;336:980–987. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- 11.Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215–1223. doi: 10.1002/bjs.5506. [DOI] [PubMed] [Google Scholar]
- 12.Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with t3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01. 04. J Clin Oncol. 2012;30:3827–3833. doi: 10.1200/JCO.2012.42.9597. [DOI] [PubMed] [Google Scholar]
- 13.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 14.Sebag-Montefiore D, Stephens RJ, Steele R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): A multicentre, randomised trial. Lancet. 2009;373:811–820. doi: 10.1016/S0140-6736(09)60484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozak KR, Kachnic LA, Adams J, et al. Dosimetric feasibility of hypofractionated proton radiotherapy for neoadjuvant pancreatic cancer treatment. Int J Radiat Oncol Biol Phys. 2007;68:1557–1566. doi: 10.1016/j.ijrobp.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 16.Hong TS, Ryan DP, Blaszkowsky LS, et al. Phase I study of preoperative short-course chemoradiation with proton beam therapy and capecitabine for resectable pancreatic ductal adenocarcinoma of the head. Int J Radiat Oncol Biol Phys. 2011;79:151–157. doi: 10.1016/j.ijrobp.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 17.Dias-Santagata D, Akhavanfard S, David SS, et al. Rapid targeted mutational analysis of human tumours: A clinical platform to guide personalized cancer medicine. EMBO Mol Med. 2010;2:146–158. doi: 10.1002/emmm.201000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duda DG, Cohen KS, Scadden DT, et al. A protocol for phenotypic detection and enumeration of circulating endothelial cells and circulating progenitor cells in human blood. Nat Protoc. 2007;2:805–810. doi: 10.1038/nprot.2007.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smeenk HG, van Eijck CH, Hop WC, et al. Long-term survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation: Long-term results of EORTC trial 40891. Ann Surg. 2007;246:734–740. doi: 10.1097/SLA.0b013e318156eef3. [DOI] [PubMed] [Google Scholar]
- 20.Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: A randomized controlled trial. JAMA. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 21.Esposito I, Kleeff J, Bergmann F, et al. Most pancreatic cancer resections are r1 resections. Ann Surg Oncol. 2008;15:1651–1660. doi: 10.1245/s10434-008-9839-8. [DOI] [PubMed] [Google Scholar]
- 22.Connor S, Bosonnet L, Ghaneh P, et al. Survival of patients with periampullary carcinoma is predicted by lymph node 8a but not by lymph node 16b1 status. Br J Surg. 2004;91:1592–1599. doi: 10.1002/bjs.4761. [DOI] [PubMed] [Google Scholar]
- 23.Foo ML, Gunderson LL, Nagorney DM, et al. Patterns of failure in grossly resected pancreatic ductal adenocarcinoma treated with adjuvant irradiation +/− 5 fluorouracil. Int J Radiat Oncol Biol Phys. 1993;26:483–489. doi: 10.1016/0360-3016(93)90967-z. [DOI] [PubMed] [Google Scholar]
- 24.Hattangadi JA, Hong TS, Yeap BY, et al. Results and patterns of failure in patients treated with adjuvant combined chemoradiation therapy for resected pancreatic adenocarcinoma. Cancer. 2009;115:3640–3650. doi: 10.1002/cncr.24410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breslin TM, Hess KR, Harbison DB, et al. Neoadjuvant chemo-radiotherapy for adenocarcinoma of the pancreas: Treatment variables and survival duration. Ann Surg Oncol. 2001;8:123–132. doi: 10.1007/s10434-001-0123-4. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman JP, Lipsitz S, Pisansky T, et al. Phase II trial of preoperative radiation therapy and chemotherapy for patients with localized, resectable adenocarcinoma of the pancreas: An Eastern Cooperative Oncology Group study. J Clin Oncol. 1998;16:317–323. doi: 10.1200/JCO.1998.16.1.317. [DOI] [PubMed] [Google Scholar]
- 27.Spitz FR, Abbruzzese JL, Lee JE, et al. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol. 1997;15:928–937. doi: 10.1200/JCO.1997.15.3.928. [DOI] [PubMed] [Google Scholar]
- 28.Abrams RA, Winter KA, Regine WF, et al. Failure to adhere to protocol specified radiation therapy guidelines was associated with decreased survival in RTOG 9704: A phase III trial of adjuvant chemotherapy and chemoradiotherapy for patients with resected adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 2012;82:809–816. doi: 10.1016/j.ijrobp.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shinoto M, Yamada S, Yasuda S, et al. for the Working Group for Pancreas C. Phase 1 trial of preoperative, short-course carbon-ion radiotherapy for patients with resectable pancreatic cancer. Cancer. 2013;119:45–51. doi: 10.1002/cncr.27723. [DOI] [PubMed] [Google Scholar]
- 30.Wo JY, Mamon HJ, Ferrone CR, et al. Phase I study of neoadjuvant accelerated short-course radiation therapy with photons and capecitabine for resectable pancreatic cancer. Radiother Oncol. 2014;110:160–164. doi: 10.1016/j.radonc.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 31.Kozin SV, Kamoun W, Huang Y, et al. Recruitment of myeloid but not endothelial precursor cells facilitates tumor re-growth after local irradiation. Cancer research. 2010;70:5679–5685. doi: 10.1158/0008-5472.CAN-09-4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duda DG, Kozin SV, Kirkpatrick ND, et al. CXCL12 (sdf1alpha)-CXCR4/CXCR7 pathway inhibition: An emerging sensitizer for anticancer therapies? Clin Cancer Res. 2011;17:2074–2080. doi: 10.1158/1078-0432.CCR-10-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kioi M, Vogel H, Schultz G, et al. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 2010;120:694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiratsuka S, Duda DG, Huang Y, et al. C-X-C receptor type 4 promotes metastasis by activating p38 mitogen-activated protein kinase in myeloid differentiation antigen (GR-1)-positive cells. Proc Natl Acad Sci U S A. 2011;108:302–307. doi: 10.1073/pnas.1016917108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iacobuzio-Donahue CA, Velculescu VE, Wolfgang CL, et al. Genetic basis of pancreas cancer development and progression: Insights from whole-exome and whole-genome sequencing. Clin Cancer Res. 2012;18:4257–4265. doi: 10.1158/1078-0432.CCR-12-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aguirre AJ, Bardeesy N, Sinha M, et al. Activated kras and ink4a/arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hingorani SR, Wang L, Multani AS, et al. Trp53r172h and Krasg12d cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 38.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heinrich EL, Lee W, Lu J, et al. Chemokine CXCL12 activates dual CXCR4 and CXCR7-mediated signaling pathways in pancreatic cancer cells. J Transl Med. 2012;10:68. doi: 10.1186/1479-5876-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gebauer F, Tachezy M, Effenberger K, et al. Prognostic impact of CXCR4 and CXCR7 expression in pancreatic adenocarcinoma. J Surg Oncol. 2011;104:140–145. doi: 10.1002/jso.21957. [DOI] [PubMed] [Google Scholar]
- 41.Birchmeier C, Birchmeier W, Gherardi E, et al. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.