Abstract
Alcohol use disorders (AUD) are commonly comorbid with anxiety and mood disorders; however, a strategy for AUD prevention remains unclear in the presence of 3 competing etiological models that each recommends different high-risk groups. Therefore, the investigation of the 3 hypotheses in a characteristically unique cohort is critical to identifying pervasive characteristics of AUD that can inform a universal prevention strategy. The current study evaluated the temporality and onset of comorbid AUD and psychiatric disorders in a representative sample of 528 Ohio Army National Guard soldiers using structured clinical interviews from 2009 to 2012. We examined temporality both statistically and graphically to identify patterns that could inform prevention. General estimating equations with dichotomous predictor variables were used to estimate odds ratios between comorbid psychiatric disorders and AUDs. An annualized rate of 13.5% persons per-year were diagnosed with any AUD between 2010 and 2012. About an equal proportion of participants with comorbid psychiatric disorders and AUD initiated the psychiatric disorder prior to the AUD and half initiated the psychiatric disorder after the AUD. Regardless of onset, however, the majority (80%) AUD initiated during a short interval between the ages of 16 and 23. Focused primary prevention during this narrow age range (16-23 years) may have the greatest potential to reduce population mental health burden of AUD, irrespective of the sequencing of comorbid psychiatric disorder.
Keywords: Prevention, Alcohol-related disorders, Mental disorder, Age of onset, Military personnel
Models of disease etiology that progress from the identification of risk factors to explanation of mechanisms inform interventions to mitigate disease states. In psychiatric epidemiology, decades of research have documented that alcohol use disorders (AUD), including both alcohol abuse and dependence, are commonly comorbid with anxiety and mood disorders in the U.S. population (Hasin, Goodwin, Stinson, & Grant, 2005; Marshall et al., 2012) and there is substantial evidence for a bidirectional relationship between these disorders. Indeed, nearly 1 in 5 individuals diagnosed with a past-year AUD has a comorbid anxiety or mood disorder (Grant et al., 2004; Kenneson, Funderburk, & Maisto, 2013) and those with a primary anxiety or mood disorder are 2-3 times more likely to develop a secondary AUD (Martins & Gorelick, 2011). However, 3 competing models, each suggesting a unique prevention strategy, have all been shown robust in accounting for a range of observed data.
Some studies have found that in those with comorbid AUD and anxiety or mood disorders that the anxiety or mood disorder was primary to the AUD (Bell & Britton, 2014; Crum et al., 2013; Wilk et al., 2010), proposing that AUDs may result as a consequence of self-medicating existing psychiatric disorders. In contrast, other studies have documented that respondents with incident psychiatric disorders also have AUDs, suggesting that problematic alcohol consumption increases psychogenesis through both adverse physiological and contextual changes to the person's life (downward spiral hypothesis)(Falk, Yi, & Hilton, 2008; Marquenie et al., 2007). Finally, a third model posits that an exogenous common risk factor can simultaneously increase the risk of both an AUD and anxiety or mood disorder. For example, several studies have shown that individuals who experience childhood adversities (e.g., family violence, family disruption, parental mental illness) are at an increase risk of developing both major depression and alcohol misuse in adulthood (Green et al., 2010; McLaughlin et al., 2010).
Each of the 3 hypotheses suggests that prevention priorities be focused on different groups at different time periods. First, the self-medicating model would suggest that psychiatric disorders predominantly onset prior to AUD, presupposing that secondary prevention focused on the treatment of psychiatric disorders will offer the greatest reduction in AUD burden to individuals with psychiatric disorders and the population in general. Second, the “downward spiral” model implies that alcohol consumption can initiate chains-of-risk whereby depression is the consequent of a series of negative effects that all began with problematic alcohol consumption. Thus, primary prevention aiming to change social norms about alcohol consumption would have the greatest effect on the population burden of AUD. Third, a shared exogenous factor model calls for a prevention of the primary drivers that increase the risk of subsequent comorbidity—such as, childhood maltreatment that is shown to increase latent liabilities to develop psychopathology (Keyes et al., 2012).
Decades of research in general populations and treatment samples continue to confirm each of these 3 models. Hence, replication studies that continue to focus on risk estimates in a general population are unlikely to provide a more general prevention strategy. Therefore, assessing comorbid disorder etiology in a unique cohort of individuals who are adequately different from extant samples, but have enough in common to expect many shared influences throughout the life course, can help explicate the critical drivers of AUD across the life course that both groups share.
We posit that investigating AUD and comorbid anxiety or mood disorder in a representative military sample will inform similarities and differences between populations that may identify more universal characteristics of AUD. Given the U.S. military force is screened for mental illness vulnerability at enlistment (Jones, Hyams, & Wessely, 2003), experience traumatic events not germane to the general civilian population (e.g., combat), and reside and work in a social environment that treats alcohol use with widespread indifference (G. Ames & Cunradi, 2004; G. M. Ames, Duke, Moore, & Cunradi, 2008), they have different risk factors for comorbid AUD and psychiatric disorders—such that, we would anticipate disorders onset after both the screening for vulnerability at enlistment and subsequent exposure to military specific traumatic events. These population characteristics are likely to both increase the burden of comorbid AUD and psychiatric disorders and provide new insight into the temporal relationship between disorder onsets in both the general U.S. population and military force. However, to our knowledge, no prior studies have examined the temporality of clinically diagnosed comorbid alcohol and psychiatric disorders in a representative military sample.
Alcohol use represents a substantial public health concern in the U.S. military that is exacerbated in the presence of psychiatric disorders. Each year, nearly 35,000 arrests per year result from excessive drinking among service members (Harwood, Zhang, Dall, Olaiya, & Fagan, 2009) and AUDs have been associated with increased soldier misconduct (Hoge et al., 2005) and absenteeism from duty (Armed Forces Health Surveillance Center, 2012). Moreover, alcohol abuse has been documented to increase risk of suicidal behaviors among those with a comorbid psychiatric disorder (Calabrese et al., 2011; Oquendo et al., 2010). Because of the excess risk for morbidity (Blanco et al., 2012) and mortality (Calabrese et al., 2011; Oquendo et al., 2010) that is conferred by a comorbid psychiatric disorder, studies are needed to provide novel insight into the etiology of comorbidity that can inform prevention and treatment priorities in military personnel. Therefore, we used both longitudinal data and retrospectively assessed symptom reports in a representative sample in a U.S. military cohort to respectively investigate (1) the temporal relationship between alcohol use disorders and anxiety or mood disorders and (2) whether alcohol use disorders were more likely to be diagnosed in the presence of a current anxiety or mood disorder.
Methods
Study Sample
Data are based on the in-depth clinical cohort study, nested within the Ohio Army National Guard (OHARNG) Mental Health Initiative (MHI). The OHARNG MHI design has been described in detail elsewhere (Calabrese et al., 2011). The baseline clinical validation subsample (n = 500) was randomly chosen from the OHARNG MHI telephone survey. In addition, we enrolled 105 recently enlisted OHARNG to the clinical validation subsample in 2011 (Year 3) to increase both the analytical power and provide data on the most recent cohort of enlisted soldiers. Of the 605 total participants enrolled in the validation subsample (n = 500 in 2009, plus n = 105 in 2011), we excluded 77 (12.7%) respondents who were assessed only at baseline, retaining a final analytical sample of 528 respondents who contributed a total of 1,210 person-years (2.4± .9 years per person on average). Among these 528 respondents, 374 (70.8%) had complete data, 96 (18.2%) missed one interview, and 58 (11.0%) missed two interviews. The subjects lost to follow up did not differ significantly from those retained in the study on several baseline characteristics that included: current psychiatric diagnoses (e.g., major depression, PTSD), demographic characteristics (e.g., age, sex, education), and military characteristics (i.e., rank, prior lifetime deployment). The Institutional Review Boards at both the University Hospital Case Medical Center (UHCMC) and University of Toledo (UT) approved the study protocol.
Procedures
Across four one-year intervals from 2008 to 2012, study-trained clinicians completed face-to-face diagnostic interviews with each respondent in non-military private locations (e.g., private library room, participant's home). At time 1 (June 2008 to December 2009), study-trained clinicians explained the study, received informed consent, and administered the clinical interview; Time 2 interviews were conducted from January to December 2010; Time 3 interviews from January to December 2011; and Time 4 interviews from January 2012 to February 2013.The interview consisted of questions that assessed soldiers' military experiences, psychiatric health history, treatment history, and socioeconomic circumstances; interviews lasted on average 2.12 hours. The 5 study-trained clinicians performed monthly inter-rater reliability for the Structured Clinical Interview for DSM-IV (SCID) and Clinician-Administered PTSD Scale (CAPS) to assure that the interviewers were standardized in their diagnostic assessment methods and interviewing techniques (Free-marginal Multirater Kappa > .85) (Brennan & Prediger, 1981).
Measures
At the first assessment, the SCID (First, Gibbon, Spitzer, & Williams, 2002) was administered to assess DSM-IV (American Psychiatric Association, 2000) criteria for a lifetime or current AUD, which consist of alcohol dependence or alcohol abuse. Diagnosis of alcohol abuse was determined if s/he experienced one or more problems with functioning in a 12-month period (i.e., failure in obligations, alcohol use in hazardous situations, recurrent legal problems, or continued use despite social or interpersonal problems) that led to “Clinically significant impairment or distress.” Alcohol dependence was diagnosed if s/he exhibited 3 or more dependence symptoms (e.g., tolerance, withdrawal) within a 12-month period. Participants diagnosed with alcohol abuse without dependence (AAWOD) or alcohol dependence (AD) were determined to have any AUD. Retrospective age-of-onset was assessed as part of the interview in a method that has been shown reliable through this form of assessment (Farrer, Florio, Bruce, Leaf, & Weissman, 1989; Prusoff, Merikangas, & Weissman, 1988).
Similar to the methods for assessing lifetime and past-year AUD outlined above, psychiatric disorders were assessed using a diagnostic interview that included the CAPS (Blake et al., 2000) and the SCID (First et al., 2002). At the first assessment, the CAPS was administered twice to assess lifetime and current PTSD symptoms based on the individuals' self-selected “worst” event during their most recent deployment and then again for the “worst” event outside of their most recent deployment (Breslau et al., 1998). We defined a case of PTSD by using the frequency ≥ 1 and intensity ≥ 2 method (Blake et al., 2000). Participant meeting diagnostic criteria on either CAPS were classified as having PTSD. Next, the SCID was administered to assess Axis-I disorders (First et al., 2002). The mental disorders examined included anxiety disorders (panic disorder, agoraphobia without panic disorder, specific phobia, social phobia) and mood disorders (major depressive disorder and bipolar I/II disorders). All diagnoses were based on DSM-IV criteria and a decision tree approach was used to record the presence or absence of each disorder for current and lifetime occurrences. Retrospective age-of-onset was assessed as part of the baseline interview, which has been shown reliable through this form of assessment (Farrer et al., 1989; Prusoff et al., 1988).
Following administration of the CAPS and SCID at the first assessment, study interviewers annually reassessed DSM-IV criteria for psychiatric disorders since the last interview. All psychiatric diagnoses were determined in methods identical to those outlined for the first assessment.
Standard individual characteristics were assessed, including: age at baseline, sex, ethnicity (white, black, or other), education (<HS, HS/GED, some college/AA, college ≤), marital status (single/never married, married, separated/divorced/widowed), rank (enlisted, officer), and lifetime deployment (yes/no).
Statistical Analysis
The temporal relationship between AUD and anxiety or mood disorders was investigated in 2 steps. First, bivariate analyses (two-sided α level ≤ 0.05) were conducted comparing baseline lifetime prevalence of alcohol abuse without dependence, alcohol dependence, and any AUD with relevant sociodemographic and military characteristics. tests or Fisher exact tests were performed for categorical characteristics and student t-test for continuous variables. Second, age-of-onset distributions and temporality were examined graphically by plotting cumulative age-of-onset distributions.
Next, we examined the 1210 person-years of follow-up data and investigated whether an AUD was more likely to be diagnosed in the presence of a current anxiety or mood disorder. As respondents contributed data from multiple time points, we used the generalized estimating equation (GEE) method to estimate adjusted odds-ratios (AOR) and 95% confidence intervals (CI) between past-year comorbid AUD and psychiatric disorders using the proc genmod procedure in SAS v. 9.3 (SAS, Cary NC). All GEE models were adjusted for sociodemographic and military characteristics.
Results
Table 1 displays the baseline demographic and military characteristics of the 528 participants stratified by AAWOD, AD, and any AUD. Participants were predominately male (88%), white (89%), enlisted (89%), had deployed at least once (61%), and had a mean age (± SD) of 31 ± 10 years. At baseline, about a third of participants had a lifetime diagnosis of anxiety (19%) or mood disorder (29%). Of participants who received a lifetime AUD (46%) at baseline, 59% were diagnosed with AAWOD and 41% were diagnosed with AD. In the bivariate analysis, older age, ethnicity, prior deployment, lifetime mood disorder, and lifetime psychotherapy and pharmacotherapy were significantly (p < 0.05) associated with any baseline lifetime AUD diagnosis. Further, lifetime anxiety and mood disorders were significantly associated with AD, but not AAWOD. The period prevalence of any AUD in the sample was 13.5% of persons per year, which included 8.8% and 4.7% per year who were diagnosed with AAWOD and AD, respectively.
Table 1. Baseline characteristics of respondents with alcohol abuse without dependence, alcohol dependence, or alcohol use disorder (N = 528).
| Total (n = 528) | AAWOD | P Value | AD | P Value | Any AUD | P Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Characteristic at Baseline | Absent (n = 384) | Present (n = 144) | Absent (n = 427) | Present (n = 101) | Absent (n = 283) | Present (n = 245) | ||||
| Age, mean (SD), y | 31 (10.0) | 29.9 (9.7) | 31.6 (10.1) | 0.09 | 30.3 (10.1) | 30.9 (8.8) | 0.52 | 29.6 (10.0) | 31.3 (9.6) | 0.04 |
|
| ||||||||||
| Age joining military, mean (SD), y | 21 (4.5) | 21.3 (4.8) | 20.5 (3.6) | 0.03 | 20.9 (4.3) | 21.9 (5.2) | 0.07 | 21.1 (4.6) | 21.1 (4.4) | 0.90 |
|
| ||||||||||
| Sex, No. (%) | 0.33 | 0.01d | 0.27 | |||||||
|
| ||||||||||
| Male | 463 (87.7) | 340 (88.5) | 123 (85.4) | 367 (86.0) | 96 (95.1) | 244 (86.2) | 219 (89.4) | |||
|
| ||||||||||
| Female | 65 (12.3) | 44 (11.5) | 21 (14.6) | 60 (14.1) | 5 (5.0) | 39 (13.8) | 26 (10.6) | |||
|
| ||||||||||
| Ethnicity, No. (%) | 0.74 | 0.03d | 0.02 | |||||||
|
| ||||||||||
| White | 467 (88.6) | 337 (88.0) | 130 (90.3) | 370 (86.9) | 97 (96.0) | 240 (85.1) | 227 (92.7) | |||
|
| ||||||||||
| Black | 36 (6.8) | 28 (7.3) | 8 (5.6) | 34 (8.0) | 2 (2.0) | 26 (9.2) | 10 (4.1) | |||
|
| ||||||||||
| Other | 24 (4.6) | 18 (4.7) | 6 (4.2) | 22 (5.2) | 2 (2.0) | 16 (5.7) | 8 (3.3) | |||
|
| ||||||||||
| Education, (y), No. (%) | 0.24d | 0.09d | 0.11d | |||||||
|
| ||||||||||
| <12 | 5 (1.0) | 5 (1.3) | 0 (0.0) | 5 (1.2) | 0 (0.0) | 5 (1.8) | 0 (0.0) | |||
|
| ||||||||||
| 12 or GED | 93 (17.6) | 72 (18.8) | 21 (14.6) | 68 (15.9) | 25 (24.8) | 47 (16.6) | 46 (18.8) | |||
|
| ||||||||||
| >12 | 430 (81.4) | 307 (80.0) | 123 (85.4) | 354 (82.9) | 76 (75.3) | 231 (81.6) | 199 (81.2) | |||
|
| ||||||||||
| Marital status, No. (%) | 0.51 | 0.08 | 0.18 | |||||||
|
| ||||||||||
| Single, never married | 237 (44.9) | 177 (46.1) | 60 (41.7) | 196 (45.9) | 41 (40.6) | 136 (48.1) | 101 (41.2) | |||
|
| ||||||||||
| Married | 235 (44.5) | 165 (43.0) | 70 (48.6) | 192 (45.0) | 43 (42.6) | 122 (43.1) | 113 (46.1) | |||
|
| ||||||||||
| Separated/Divorced/Widowed | 56 (10.6) | 42 (10.9) | 14 (9.7) | 39 (9.1) | 17 (16.8) | 25 (8.8) | 31 (12.7) | |||
|
| ||||||||||
| Rank, No. (%) | 0.15 | 0.02d | 0.61 | |||||||
|
| ||||||||||
| Enlisted | 468 (88.6) | 345 (89.8) | 123 (85.4) | 372 (88.1) | 96 (95.1) | 249 (88.0) | 219 (89.4) | |||
|
| ||||||||||
| Officer | 60 (11.4) | 43 (10.2) | 21 (14.6) | 55 (12.9) | 5 (5.0) | 34 (12.0) | 26 (10.6) | |||
|
| ||||||||||
| Prior deployment (Yes), No. (%) | 320 (60.6) | 219 (57.0) | 101 (70.1) | 0.006 | 252 (59.0) | 68 (67.3) | 0.12 | 151 (53.4) | 169 (69.0) | 0.0002 |
|
| ||||||||||
| Lifetime psychiatric diagnosesa | ||||||||||
|
| ||||||||||
| Anxiety disorderb | 102 (19.3) | 81 (21.1) | 21 (14.6) | 0.09 | 67 (15.7) | 35 (34.7) | <0.0001 | 46 (16.3) | 56 (22.9) | 0.06 |
|
| ||||||||||
| Mood disorderc | 155 (29.4) | 114 (29.7) | 41 (28.5) | 0.78 | 106 (24.8) | 49 (48.5) | <0.0001 | 65 (23.0) | 90 (36.7) | 0.0005 |
|
| ||||||||||
| Either mood or anxiety disorderbc | 195 (36.9) | 143 (37.2) | 52 (36.1) | 0.81 | 138 (32.3) | 57 (56.4) | <0.0001 | 86 (30.4) | 109 (44.5) | 0.0008 |
|
| ||||||||||
| Treatment | ||||||||||
|
| ||||||||||
| Psychotherapy | 223 (42.2) | 159 (41.4) | 64 (44.4) | 0.53 | 165 (38.6) | 58 (57.4) | 0.0006 | 101 (35.7) | 122 (49.8) | 0.001 |
|
| ||||||||||
| Pharmacotherapy | 157 (29.7) | 101 (26.3) | 56 (38.9) | 0.005 | 117 (27.4) | 40 (39.6) | 0.02 | 61 (21.6) | 96 (39.2) | <0.0001 |
Abbreviations: AAWOD, alcohol abuse without dependence; AD, alcohol abuse; AUD, alcohol use disorder; GED, General Equivalency Diploma
Diagnostic categories are not mutually exclusive
Includes lifetime history of panic disorder, agoraphobia, social phobia, specific phobia, generalized anxiety, obsessive-compulsive, and posttraumatic stress disorders
Includes lifetime history of bipolar I/II, major depression, mania/hypomania, and dysthymia disorders.
Fisher's exact test
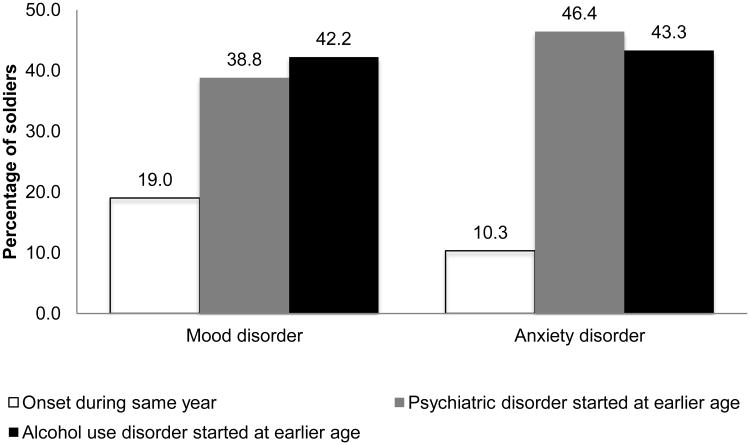
Figure 1 shows temporality between age-of-onset of co-occurrence for participants with lifetime comorbid AUD and mood (n = 116) or anxiety (n = 97) disorders. Three categories were created to examine temporality of comorbid disorders; (i) mood or anxiety disorder onset the same year as the AUD; (ii) anxiety or mood disorder started at an earlier age than the AUD; or (iii) AUD started at an earlier age than the anxiety or mood disorder. Among participants with comorbid mood and AUD, about 42% reported AUD onset first, 39% reported mood disorder onset first, while 19% reported both disorders onset during the same year. Further, among participants with comorbid anxiety disorder, 43% reported AUD onset first, 46% anxiety disorder onset first, and about 10% reported both disorders onset during the same year. Overall, the two distributions were not significantly different (p = 0.18).
Figure 1.
Temporality between age of onset of lifetime co-occurrence for respondents with co-occurrence of alcohol use and mood or anxiety disorders.
-NS difference between mood or alcohol onset first (p = .76)
-Significant difference between either mood (p = .007) or alcohol (p = .002) first versus same time
-NS difference between anxiety or alcohol onset first (p = .83)
-Significant difference between either anxiety (p < .0001) or alcohol (p < .0001) first versus same time.
-Overall, NS difference between two mood and anxiety disorder distributions (p = .18)
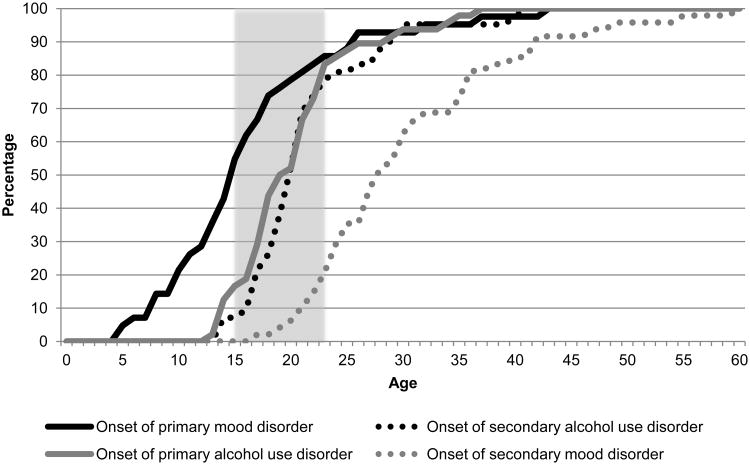
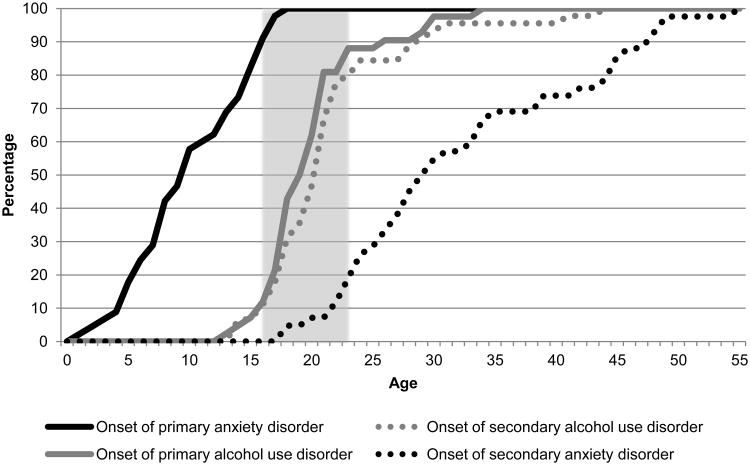
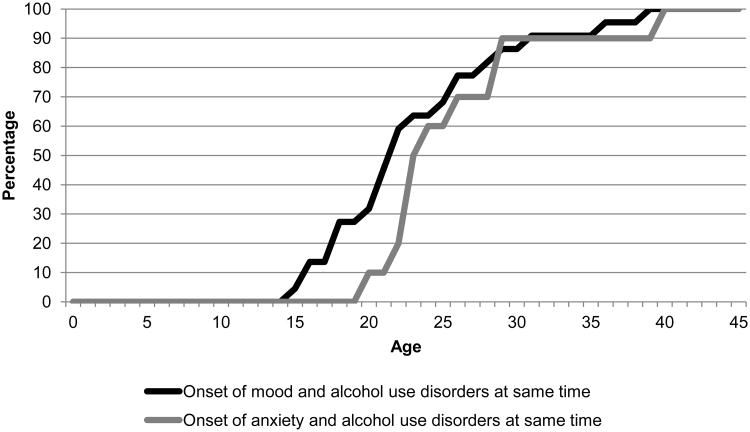
Figure 2, 3, and 4 show the cumulative age-of-onset distribution of the 3 groups described in Figure 1. Figure 2 shows the cumulative age-of-onset distribution of the 39% of participants who reported that mood disorder onset prior to AUD (solid black colored line) and 42% of participants who reported that AUD onset prior to mood disorder (solid grey colored line). Figure 3 shows the same information for anxiety disorder. Both figures show that about 80% of AUD, regardless of whether they onset prior to mood or after mood disorder, initiated during a small interval from about 16 – 23 years. However, the distributions of the anxiety and mood disorders occurred during different age intervals based on their temporality with AUD. Lastly, Figure 4 shows that age-of-onset distributions were similar for participants reporting comorbid AUD and anxiety or mood disorders initiated in the same year.
Figure 2.
Cumulative age of onset distribution of primary and secondary disorders in respondents with co-occurring mood and alcohol use disorders (AUD)
Note: The solid line shows the cumulative age-of-onset distribution of the first onset disorder (i.e., black solid line for mood disorder onset first and solid grey line for AUD onset first), while the dotted line shows the cumulative age-of-onset distribution of the second onset disorder (i.e., grey dotted line for AUD onset second and black dotted line for mood disorder onset second). Shaded area represents ages 16-23.
Figure 3.
Cumulative age of onset distribution of primary and secondary disorders in respondents with co-occurring anxiety and alcohol use disorders (AUD)
Note: The solid line shows the cumulative age-of-onset distribution of the first onset disorder (i.e., black solid line for anxiety disorder onset first and solid grey line for AUD onset first), while the dotted line shows the cumulative age-of-onset distribution of the second onset disorder (i.e., grey dotted line for AUD onset second and black dotted line for anxiety disorder onset second). Shaded area represents ages 16-23.
Figure 4.
Cumulative age of onset distribution of first onset psychiatric and alcohol use disorder in respondents with psychiatric and alcohol use disorders occurring in the same year.
Table 2 shows that after adjusting for sociodemographic and military characteristics that a significantly higher odds of past-year AAWOD, AD, and AUD was observed in respondents with anxiety disorder, mood disorder, or both than those without such disorder, with only comorbid mood disorder and AAWOD not being significant (p = 0.45). In this model, past-year mood disorder increased the odds of comorbid AD by a factor of 4 (AOR = 3.9, 95%CI: 2.4-10.2), while past-year mood or anxiety disorder increased the odds of any comorbid past-year AUD by a factor of 3 (AOR = 3.1, 95%CI: 2.0-4.7).
Table 2. 12-month period prevalence of co-occurring DSM-IV diagnosed alcohol use disorders with anxiety and mood disorders adjusting for sociodemographic and military characteristics*.
| 12-Month co-occurrence | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| AAWOD (n = 106) | Bivariate GEE Regression Analysis, AOR (95%CI)* | AD (n = 57) | Bivariate GEE Regression Analysis, AOR (95%CI)* | Any AUD (n = 163) | Bivariate GEE Regression Analysis, AOR (95%CI)* | |||||
|
|
|
|
||||||||
| Mental disorders | P-Value | P-Value | P-Value | |||||||
| Mood disorder | 0.6 | 0.45 | 1.3 (.7, 2.6) | 19.1 | < .0001 | 5.0 (2.4, 10.2) | 11.7 | <.0001 | 2.4 (1.5, 4.1) | |
|
| ||||||||||
| Anxiety disorder | 14.1 | < .0001 | 2.9 (1.7, 5.0) | 4.9 | 0.03 | 2.2 (1.1, 4.4) | 20.0 | <.0001 | 2.9 (1.8, 4.5) | |
|
| ||||||||||
| Any disorder | 11.6 | < .0001 | 2.4 (1.5, 4.1) | 15.7 | < .0001 | 3.6 (1.9, 6.7) | 27.0 | <.0001 | 3.1 (2.0, 4.7) | |
Abbreviations: AAWOD, alcohol abuse without dependence; AD, alcohol dependence; AUD, alcohol use disorder; GEE, generalized estimating equation; AOR, adjusted odds ration; CI, confidence interval
Adjusted for age, sex, race/ethnicity, marital status, rank, and lifetime deployment
Total person-time = 1210 person years
Discussion
In data from a representative sample assessing reservists' comorbid alcohol and mood or anxiety disorders, we found that AUDs start during a narrow age interval (16-23 years) irrespective of a lifetime comorbid mood or anxiety disorder. Although this finding is unable to resolve the stalemate among the 3 competing etiological models that aim to explain comorbid AUD and psychiatric disorders, we have identified a critical period for AUD onset in persons with comorbidity, robust across diverse samples, which nonetheless can inform prevention priorities. Substance use disorders, unlike all other psychiatric disorders except PTSD, have a necessary exposure (i.e., alcohol consumption) required for a diagnosis. In other words, the presence of an AUD implies alcohol consumption, whereas alcohol consumption does not imply that an AUD will develop. In this context, interventions aimed at persons in this critical window are likely to reach the greatest proportion of high-risk persons.
Our results emphasize the need to focus on methods to intervene with alcohol use among adolescents with and without comorbid mood or anxiety disorder. In particular, since an equal proportion of the participants in our sample reported AUD before the onset of a comorbid psychiatric disorder, and vice versa in the other half of the sample, a high-risk intervention strategy, exclusively targeting adolescents with pre-existing psychiatric disorders, will likely neglect half of the population at risk to develop comorbid disorders. Although our findings are derived from a military sample, these conclusions have similar implications for the college drinking population and general population. Although the military and universities are unable to mitigate existing risk contributed by genetics (Kendler et al., 2012) or adverse childhood events (Keyes et al., 2012), the widespread and permissive use of alcohol in these institutions (G. Ames & Cunradi, 2004; G. M. Ames et al., 2008) may increase exposure to alcohol, exacerbating other early life risk factors for AUD. The Institute of Medicine recommended that the Department of Defense (DoD) should adopt “consistent enforcement of regulations on underage drinking, a reduced number of alcohol outlets, and limited hours of operation of such outlets” to combat the current lenient environment towards alcohol consumption (Institute of Medicine, 2012). Whereas, in the last decade, U.S. universities have effectively confronted problem drinking among students through social advertising and interventions aimed at changing the social norms on university campuses away from excess drinking (Lewis & Neighbors, 2006; Wechsler & Nelson, 2008). The shift on university campuses from a punitive focus to a “social norms approach” could provide insight to the DoD and secondary education on future directions to reduce the burden of alcohol use disorders in the force and general community, respectively.
In the context of the military, our study reported that about half of the participants with lifetime comorbid disorders had developed their AUD by the mean age our sample joined the military (21 years), consistent with previous literature (Kessler et al., 2014), and combat deployment was significantly associated with AAWOD and AUD. This is consistent with extant evidence that about two times as many cases of post-deployment AUD represent a pre-deployment disorder compared to first incident disorder (Kehle et al., 2012). Although deployment may not represent the primary driver of military AUD burden, substantial evidence suggests that combat deployment experiences are associated with screening positive for alcohol misuse (Wilk et al., 2010), psychopathology, and readjustment stress (Hoge et al., 2004). Furthermore, individuals with mental health issues, particularly undiagnosed issues, are likely to exhibit increased alcohol consumption (Bell & Britton, 2014; Cohen, Fink, Sampson, & Galea, 2015). To mitigate chronic psychopathology, these findings emphasize the importance of a continuous systematic monitoring of military personnel serving in high stress positions for early signs of both alcohol use and psychiatric disorders.
We found an equal balance of cases where AUD was primary to the psychiatric disorder and vice versa. Irrespective of the direction of causation, these findings suggest that multiple processes, rather than a single underlying mechanism, are likely to drive this association between AUD and psychiatric disorder. Two processes, in particular, require further investigation. First, temporality is being affected by a third factor not considered in previous studies. This hypothesis suggests a need for a life course framework to explain the complex interplay between genetics and environment in the development of comorbid alcohol use and psychiatric disorders. A recent literature has begun to elucidate the influence of pre-military experiences on military psychopathology, including a study that documented a higher probability of childhood adversity in military personnel than comparable civilians (Blosnich, Dichter, Cerulli, Batten, & Bossarte, 2014) and two papers documenting a majority of military psychiatric diagnoses initiate prior to military service (Kessler et al., 2014; Rosellini et al., 2014). The second hypothesis is that temporality may be disorder specific. Extant literature has documented that an AUD is more likely to onset in the presence of certain primary anxiety (Falk et al., 2008) and mood disorders (Falk et al., 2008; Kenneson et al., 2013) compared to other disorders. Kenneson et al. (2013), for example, showed that compared to those without a current mood disorder, respondents aged 12-17 years with bipolar disorder (aOR=3.7; 95%CI=2.6-5.5) were more likely than those with major depressive disorders (aOR=1.8; 95%CI=1.3-2.4) to develop a subsequent substance use disorder. While our sample size did not provide sufficient power to stratify by psychiatric disorder, future large cohort studies should consider examining disorder specific temporality.
This study is not without limitations. First, age-of-onset were self-reported, which may result some variance with regard to exact age-of-onset. Reassuring in this regard, while longitudinal investigations have documented that persons may fail to remember previously reported disorders (Takayanagi et al., 2014), two longitudinal studies have documented moderate test-retest reliability for age-of-onset questions (Johnson & Mott, 2001; Parra, O'Neill, & Sher, 2003). Further, the interviewers' utilized a method that included probes about symptoms during particular time periods that was previously shown reliable in assessing disorder age-of-onset (Farrer et al., 1989; Prusoff et al., 1988). Second, while military personnel are likely to provide truthful answers if they believe a survey will be used for legitimate purposes and individual answers will remain confidential (Warner et al., 2011), the presence or perception of mental illness stigma (Kim, Thomas, Wilk, Castro, & Hoge, 2010) and a bias against reporting embarrassing behaviors (Tourangeau & Yan, 2007) remain prevalent in the military. Thus, participants were assured that individual answers will remain confidential, both verbally and in writing, prior their volunteering for the study to compensate for this concern. Additionally, confidentiality was improved by having civilian clinicians conduct all assessments in neutral locations without the presence of military personnel.
Our observation that the age-of-onset for most AUD occurred within a narrow period of time (16-23 years), and the knowledge that AUD development can only occur in the presence of an external environmental factor (alcohol), suggests that universal primary prevention strategies (e.g., enforcement of minimum drinking age laws across the military) focused during this age range (i.e., during and after military enlistment) may have the greatest potential to reduce the population mental health burden. In addition, identifying the appropriate treatment is important for those with comorbid disorders. Given the high rates of comorbid alcohol use and psychiatric disorders, clinicians treating either alcohol use disorders or psychiatric disorders should routinely assess potential comorbid conditions to inform prescribed treatment. Recognition of family history for alcohol use and psychiatric disorders is likely the first step to understanding whether a person may be predisposed, and require further education related to early signs, symptoms, and risk factors. In the military setting, this suggests a need for selective prevention towards personnel at elevated risk driven by personal or family history of mental illness.
Acknowledgments
Compliance with Ethical Standards: Funding: This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Joint Warfighter Medical Research Program under Award No. W81XWH-15-1-0080, W81XWH-07-1-0409, and W81XWH-10-1-0579 [DSF, MTB, IL, PC, GHC, LS, ES, TG, ND, TF, PLR, JRC, SG]. The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense. This report was supported by National Institute on Drug Abuse grant T32DA031099 [DSF]. The informatics support for this research was provided by the Michigan State University Clinical and Translational Sciences Institute, through its Biomedical Research Informatics Core.
Footnotes
Disclosures of potential conflicts of interest: The authors declare that they have no conflicts of interest.
Ethical approval: “All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
Informed consent: Informed consent was obtained from all individual participants included in the study.
Contributor Information
David S. Fink, Department of Epidemiology, Mailman School of Public Health, New York, NY
M. Shayne Gallaway, U.S. Army Substance Abuse Program, U.S. Army Garrison Ansbach, Germany.
Marijo B. Tamburrino, Department of Psychiatry, University of Toledo, Toledo, OH
Israel Liberzon, Department of Psychiatry, University of Michigan, Ann Arbor, MI.
Philip Chan, Department of Psychiatry, University Hospitals Case Medical Center, Case Western Reserve University, Cleveland, OH.
Gregory H. Cohen, Department of Epidemiology, Mailman School of Public Health, New York, NY
Laura Sampson, School of Public Health, Boston University, Boston, MA.
Edwin Shirley, Department of Psychiatry, University Hospitals Case Medical Center, Case Western Reserve University, Cleveland, OH.
Toyomi Goto, Department of Psychiatry, University Hospitals Case Medical Center, Case Western Reserve University, Cleveland, OH.
Nicole D'Arcangelo, Department of Psychiatry, University Hospitals Case Medical Center, Case Western Reserve University, Cleveland, OH.
Thomas Fine, Department of Psychiatry, University of Toledo, Toledo, OH.
Philip L. Reed, Biomedical Research and Informatics Center, Michigan State University
Joseph R. Calabrese, Department of Psychiatry, University Hospitals Case Medical Center, Case Western Reserve University, Cleveland, OH
Sandro Galea, School of Public Health, Boston University, Boston, MA.
References
- American Psychiatric Association (2000). DSM-IV-TR: Diagnostic and statistical manual of mental disorders, text revision.
- Ames G, Cunradi C. Alcohol use and preventing alcohol-related problems among young adults in the military. Alcohol Research & Health. 2004;28(4):252–257. [Google Scholar]
- Ames GM, Duke MR, Moore RS, Cunradi CB. The Impact of Occupational Culture on Drinking Behavior of Young Adults in the U.S. Navy. Journal of Mixed Methods Research. 2008;3(2):129–150. doi: 10.1177/1558689808328534. [DOI] [Google Scholar]
- Armed Forces Health Surveillance Center, A. Absolute and relative morbidity burdens attributable to various illnesses and injuries, U.S. Armed Forces, 2011. MSMR. 2012;19(4):4–8. discussion 8-9. [PubMed] [Google Scholar]
- Bell S, Britton A. An exploration of the dynamic longitudinal relationship between mental health and alcohol consumption: a prospective cohort study. BMC Medicine. 2014;12(1):91. doi: 10.1186/1741-7015-12-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D, Weathers FW, Nagy L, Kaloupek D, Klauminzer G, Charney D, et al. Buckley TC. Instruction Manual: Clinician-Administered PTSD Scale. 2000 Retrieved from National Center for Postraumatic Stress Disorder, Behavioral Science Division, Boston, MA. [Google Scholar]
- Blanco C, Alegria AA, Liu SM, Secades-Villa R, Sugaya L, Davies C, Nunes EV. Differences among major depressive disorder with and without co-occurring substance use disorders and substance-induced depressive disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions. The Journal of clinical psychiatry. 2012;73(6):865–873. doi: 10.4088/JCP.10m06673. [DOI] [PubMed] [Google Scholar]
- Blosnich JR, Dichter ME, Cerulli C, Batten SV, Bossarte RM. Disparities in Adverse Childhood Experiences Among Individuals With a History of Military Service. JAMA Psychiatry. 2014;71(9):1041–1048. doi: 10.1001/jamapsychiatry.2014.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan RL, Prediger DJ. Coefficient kappa: Some uses, misuses, and alternatives. Educational and Psychological Measurement. 1981;41(3):687–699. [Google Scholar]
- Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Archives of General Psychiatry. 1998;55(7):626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Prescott M, Tamburrino M, Liberzon I, Slembarski R, Goldmann E, et al. Galea S. PTSD comorbidity and suicidal ideation associated with PTSD within the Ohio Army National Guard. Journal of Clinical Psychiatry. 2011;72(8):1072–1078. doi: 10.4088/JCP.11m06956. [DOI] [PubMed] [Google Scholar]
- Cohen GH, Fink DS, Sampson L, Galea S. Mental Health Among Reserve Component Military Service Members and Veterans. Epidemiologic Reviews. 2015;37(1) doi: 10.1093/epirev/mxu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum RM, Mojtabai R, Lazareck S, Bolton JM, Robinson J, Sareen J, et al. Storr CL. A prospective assessment of reports of drinking to self-medicate mood symptoms with the incidence and persistence of alcohol dependence. JAMA Psychiatry. 2013;70(7):718–726. doi: 10.1001/jamapsychiatry.2013.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk DE, Yi HY, Hilton ME. Age of onset and temporal sequencing of lifetime DSM-IV alcohol use disorders relative to comorbid mood and anxiety disorders. Drug and Alcohol Dependence. 2008;94(1-3):234–245. doi: 10.1016/j.drugalcdep.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer LA, Florio LP, Bruce ML, Leaf PJ, Weissman MM. Reliability of self-reported age at onset of major depression. Journal of Psychiatric Research. 1989;23(1):35–47. doi: 10.1016/0022-3956(89)90015-0. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams DR. User's guide for the structure clinical interview for DSM-IV-TR Axis I disorders. New York, NY: Biometrics Research; 2002. [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, et al. Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2004;61(8):807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Archives of General Psychiatry. 2010;67(2):113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood HJ, Zhang Y, Dall TM, Olaiya ST, Fagan NK. Economic implications of reduced binge drinking among the military health system's TRICARE prime plan beneficiaries. Military Medicine. 2009;174(7):728–736. doi: 10.7205/milmed-d-03-9008. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Archives of General Psychiatry. 2005;62(10):1097–1106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental health problems and barriers to care. New England Journal of Medicine. 2004;351(1):13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- Hoge CW, Toboni HE, Messer SC, Bell N, Amoroso P, Orman DT. The occupational burden of mental disorders in theU.S. military: psychiatric hospitalizations, involuntary separations, and disability. American Journal of Psychiatry. 2005;162(3):585–591. doi: 10.1176/appi.ajp.162.3.585. [DOI] [PubMed] [Google Scholar]
- Johnson TP, Mott JA. The reliability of self-reported age of onset of tobacco, alcohol and illicit drug use. Addiction. 2001;96(8):1187–1198. doi: 10.1080/09652140120060770. [DOI] [PubMed] [Google Scholar]
- Jones E, Hyams K, Wessely S. Review: Screening for vulnerability to psychological disorders in the military: an historical survey. Journal of Medical Screening. 2003;10(1):40–46. doi: 10.1258/096914103321610798. [DOI] [PubMed] [Google Scholar]
- Kehle SM, Ferrier-Auerbach AG, Meis LA, Arbisi PA, Erbes CR, Polusny MA. Predictors of postdeployment alcohol use disorders in National Guard soldiers deployed to Operation Iraqi Freedom. Psychology of Addictive Behaviors. 2012;26(1):42. doi: 10.1037/a0024663. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Chen X, Dick D, Maes H, Gillespie N, Neale MC, Riley B. Recent advances in the genetic epidemiology and molecular genetics of substance use disorders. Nature Neuroscience. 2012;15(2):181–189. doi: 10.1038/nn.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenneson A, Funderburk JS, Maisto SA. Substance use disorders increase the odds of subsequent mood disorders. Drug and Alcohol Dependence. 2013;133(2):338–343. doi: 10.1016/j.drugalcdep.2013.06.011. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Heeringa SG, Stein MB, Colpe LJ, Fullerton CS, Hwang I, et al. for the Army SC. Thirty-Day Prevalence of DSM-IV Mental Disorders Among Nondeployed Soldiers in the US Army: Results from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS) JAMA Psychiatry. 2014 doi: 10.1001/jamapsychiatry.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Eaton NR, Krueger RF, McLaughlin KA, Wall MM, Grant BF, Hasin DS. Childhood maltreatment and the structure of common psychiatric disorders. The British Journal of Psychiatry. 2012;200(2):107–115. doi: 10.1192/bjp.bp.111.093062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim PY, Thomas JL, Wilk JE, Castro CA, Hoge CW. Stigma, barriers to care, and use of mental health services among active duty and National Guard soldiers after combat. Psychiatric Services. 2010;61(6):582–588. doi: 10.1176/appi.ps.61.6.582. [DOI] [PubMed] [Google Scholar]
- Lewis MA, Neighbors C. Social norms approaches using descriptive drinking norms education: A review of the research on personalized normative feedback. Journal of American College Health. 2006;54(4):213–218. doi: 10.3200/JACH.54.4.213-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquenie LA, Schade A, van Balkom AJ, Comijs HC, de Graaf R, Vollebergh W, et al. van den Brink W. Origin of the comorbidity of anxiety disorders and alcohol dependence: findings of a general population study. European Addiction Research. 2007;13(1):39–49. doi: 10.1159/000095814. [DOI] [PubMed] [Google Scholar]
- Marshall BD, Prescott MR, Liberzon I, Tamburrino MB, Calabrese JR, Galea S. Coincident posttraumatic stress disorder and depression predict alcohol abuse during and after deployment among Army National Guard soldiers. Drug and Alcohol Dependence. 2012;124(3):193–199. doi: 10.1016/j.drugalcdep.2011.12.027. [DOI] [PubMed] [Google Scholar]
- Martins SS, Gorelick DA. Conditional substance abuse and dependence by diagnosis of mood or anxiety disorder or schizophrenia in the U.S. population. Drug and Alcohol Dependence. 2011;119(1-2):28–36. doi: 10.1016/j.drugalcdep.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication II: associations with persistence of DSM-IV disorders. Archives of General Psychiatry. 2010;67(2):124–132. doi: 10.1001/archgenpsychiatry.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicine Io. Substance Use Disorders in the U S Armed Forces. 2012 Retrieved from Washington, DC. [Google Scholar]
- Oquendo MA, Currier D, Liu S, Hasin D, Grant B, Blanco C. Increased risk for suicidal behavior in comorbid bipolar disorder and alcohol use disorders. The Journal of clinical psychiatry. 2010;71(7):902. doi: 10.4088/JCP.09m05198gry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra GR, O'Neill SE, Sher KJ. Reliability of self-reported age of substance involvement onset. Psychology of Addictive Behaviors. 2003;17(3):211–218. doi: 10.1037/0893-164x.17.3.211. [DOI] [PubMed] [Google Scholar]
- Prusoff BA, Merikangas KR, Weissman MM. Lifetime prevalence and age of onset of psychiatric disorders: recall 4 years later. Journal of Psychiatric Research. 1988;22(2):107–117. doi: 10.1016/0022-3956(88)90075-1. [DOI] [PubMed] [Google Scholar]
- Rosellini AJ, Heeringa SG, Stein MB, Ursano RJ, Chiu WT, Colpe LJ, et al. Naifeh JA. Lifetime Prevalence of DSM-IV Mental Disorders Among New Soldiers in the US Army: Results from the Army Study to Assess Risk in Servicemembers (ARMY STARRS) Depression and Anxiety. 2014 doi: 10.1002/da.22316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Spira AP, Roth KB, Gallo JJ, Eaton WW, Mojtabai R. JAMA Psychiatry. 2014. Accuracy of Reports of Lifetime Mental and Physical Disorders: Results From the Baltimore Epidemiological Catchment Area Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourangeau R, Yan T. Sensitive questions in surveys. Psychological Bulletin. 2007;133(5):859. doi: 10.1037/0033-2909.133.5.859. [DOI] [PubMed] [Google Scholar]
- Warner CH, Appenzeller GN, Grieger T, Belenkiy S, Breitbach J, Parker J, et al. Hoge C. Importance of anonymity to encourage honest reporting in mental health screening after combat deployment. Archives of General Psychiatry. 2011;68(10):1065–1071. doi: 10.1001/archgenpsychiatry.2011.112. [DOI] [PubMed] [Google Scholar]
- Wechsler H, Nelson TF. What we have learned from the Harvard School of Public Health College Alcohol Study: Focusing attention on college student alcohol consumption and the environmental conditions that promote it. Journal of Studies on alcohol and Drugs. 2008;69(4):481. doi: 10.15288/jsad.2008.69.481. [DOI] [PubMed] [Google Scholar]
- Wilk JE, Bliese PD, Kim PY, Thomas JL, McGurk D, Hoge CW. Relationship of combat experiences to alcohol misuse among U.S. soldiers returning from the Iraq war. Drug and Alcohol Dependence. 2010;108(1-2):115–121. doi: 10.1016/j.drugalcdep.2009.12.003. [DOI] [PubMed] [Google Scholar]