Abstract
Amyotrophic lateral Sclerosis is characterized by progressive loss of motor neurons in the brain and spinal cord. Mutations in several genes, including FUS, TDP43, Matrin 3, hnRNPA2 and other RNA binding proteins, have been linked to ALS pathology. Recently, Pur-alpha a DNA/RNA binding protein was found to bind to C9orf72 repeat expansions and could possibly play a role in the pathogenesis of ALS. When overexpressed, Pur-alpha mitigates toxicities associated with Fragile X tumor ataxia syndrome (FXTAS) and C9orf72 repeat expansion diseases in Drosophila and mammalian cell culture models. However, the function of Pur-alpha in regulating ALS pathogenesis has not been fully understood. We identified Pur-alpha as a novel component of cytoplasmic stress granules (SGs) in ALS patient cells carrying disease-causing mutations in FUS. When cells were challenged with stress, we observed that Pur-alpha co-localized with mutant FUS in ALS patient cells and became trapped in constitutive SGs. We also found that FUS physically interacted with Pur-alpha in mammalian neuronal cells. Interestingly, shRNA mediated knock down of endogenous Pur-alpha significantly reduced formation of cytoplasmic stress granules in mammalian cells suggesting that Pur-alpha is essential for the formation of SGs. Furthermore, ectopic expression of Pur-alpha blocked cytoplasmic mislocalization of mutant FUS and strongly suppressed toxicity associated with mutant FUS expression in primary motor neurons. Our data emphasizes the importance of stress granules in ALS pathogenesis and identifies Pur-alpha as a novel regulator of SG dynamics.
Keywords: ALS, FUS, TDP-43, Pur-alpha, Stress granules, RNA binding proteins, primary motor neurons, Motor neuron diseases, neurodegeneration, C9orf72, amyotrophic lateral sclerosis
Introduction
RNA-binding proteins have been implicated in several neurodegenerative diseases, such as polyglutamine expansion diseases and amyotrophic lateral sclerosis (ALS) [9, 22, 51, 52, 64]. Over the last decade, TDP-43, FUS, Matrin-3, VCP and several other RNA-binding proteins have been found to be linked with causing ALS pathogenesis suggesting that disease-causing mutations perturbing RNA metabolism might be central to causing ALS pathogenesis [26, 51, 52, 64, 68]. This is supported by recent studies showing that ALS-causing mutations in RNA binding proteins cause defects in RNA splicing, stability, transcription, mRNA processing, translation and transport, which may lead to gross functional impairments in several key biological pathways [3, 5–7, 10, 18–23, 25, 30, 31, 33, 34, 60, 61, 63, 65, 66].
Several RNA-binding proteins harboring a prion-like domain have been identified as components of cytoplasmic stress granules (SGs), which are ribonucleoprotein granules (RNP granules) [16, 46, 67, 71]. SGs are highly dynamic cytoplasmic structures known to be involved in regulating RNA homeostasis [59]. SGs form rapidly when cells are exposed to stress-causing agents such as heat, cold, infection or chemicals; these SGs protect cells by sequestering actively translated mRNA and proteins until stressful conditions are relieved. A majority of the RNA-binding proteins involved in ALS pathogenesis are sequestered into SGs, suggesting that these cytoplasmic structures are relevant to ALS [13, 16, 27, 28, 31, 32, 34, 35, 44, 67, 71, 73]. SGs are rapidly formed when cells are challenged with stress-causing agents and they rapidly disassemble when stressful conditions end. ALS-causing mutations in FUS impair SG dynamics by perturbing their assembly and disassembly processes. Similarly, disease-causing mutations in valosin-containing protein (VCP) produce constitutive SGs containing another ALS-causing protein, TDP-43 [16]. Interestingly, knocking down VCP impairs the ability of cells to turnover SGs without affecting their assembly [16]. RNA-binding proteins involved in ALS pathogenesis are important building blocks of SGs under physiological conditions. Pathogenic mutations in proteins such as TDP-43 and FUS perturb SG dynamics, driving misregulation of multiple cellular pathways involved in regulating neuronal function and survival. Despite tremendous progress towards identifying ALS-causing genes and their roles in disease pathogenesis, components involved in regulating SG assembly and disassembly are still largely unknown.
Here, we identified Pur-alpha as a novel component of cytoplasmic stress granules in ALS patient cells carrying disease-causing mutations in FUS. We found that Pur-alpha is sequestered into cytoplasmic stress granules along with mutant FUS and observed that Pur-alpha physically interacts with FUS in mammalian cells. Pur-alpha is a sequence-specific single-stranded DNA- and RNA-binding protein whose sequence element strongly resembles the C9ORF72 hexamer [12]. Pur-alpha deficiency has been linked to disorders in brain development [42]. Surprisingly, we discovered that Pur-alpha is required for the formation of SGs, as knocking down endogenous Pur-alpha prevents formation of SGs in mammalian cells. We were able to restore SG formation by exogenously expressing full length Pur-alpha in cells expressing shRNA against Pur-alpha. Importantly, knocking down Pur-alpha neither perturbed p-body formation nor influenced expression of FUS or G3BP (a component of stress granules). Furthermore, we demonstrate that ectopic expression of Pur-alpha blocks cytoplasmic mislocalization of FUS carrying a disease-causing mutation and suppresses FUS-medicated toxicity in primary motor neurons These observations uncover the role of Pur-alpha in regulating cytoplasmic SG dynamics and cytoplasmic mislocalization and toxicity in FUS-related neurodegeneration.
Materials and Methods
Culturing human lymphoblastoid cells
Human B-lymphoblastoid cells were obtained from Coriell Institute cell repository. ALS patient cells with defined endogenous FUS mutations R521C (ND14790) and R518G (ND14136) were utilized. Age and sex matched population control (ND00066) were used for comparison. All lymphoblastiod cell lines were cultured in suspensions with Advanced RPMI 1640 medium (Life Technologies 12633-012) containing 10% FBS and 1% Glutamax (Life Technologies 35050-061). Cell densities and passage number were constants. Lymphoblastoid cell cultures were not allowed to reach densities below 2×10^6 cells/ml.
Immunofluorescence (IF)
Following fixation with 4% paraformaldehyde, samples were permeabilized with 0.1% Triton X-100 in PBS. Samples were blocked with 0.1% PBST + 5% normal goat serum (NGS). Antibody dilutions were made in blocking solution (5% NGS in 0.1% PBST) and incubated overnight at 4°C on a rocker. After 12 hours of incubation with the primary antibody solution a series of washes was performed with 0.1% PBST (5x 10 minutes each). Secondary antibodies were diluted in blocking solution and incubated with samples at room temperature for 3 hours. Antibodies used were: anti-G3BP mouse (Becton Dickinson catalogue #611126), anti-G3BP rabbit (Proteintech #13057-2-AP) (1:500), anti-Pur-alpha mouse [41] (1:750), anti-GW182, and anti-FUS N-terminal (Bethyl laboratories #A300-302A). As secondary antibodies, we used anti-rabbit AlexaFluor 488 (Invitrogen #A11008), anti-mouse AlexaFluor 488 (Invitrogen # A11029), anti-rabbit AlexaFluor 546 (Invitrogen #A11035), anti-mouse AlexaFluor 546 (Invitrogen #A11030) and anti-rabbit AlexaFluor 647 (Invitrogen #21245), all at a dilution of 1:500. After the staining procedure was complete, DAPI was included in the final wash for 15 minutes. Finally, coverslips were mounted with ProLong Gold (Life Technologies #P36930) and allowed to cure overnight in the dark.
Plasmids
WT FUS, FUS R518K and FUS R521C plasmid constructs are described previously [27, 55, 70]. Since these disease-causing mutations in FUS cause ALS via a similar mechanism i.e. disruption of C-terminal nuclear localization signals (NLS), we used these mutations interchangeably in our manuscript. pBK-CMV HA-Pur-alpha was generated as described [41]. For shRNA knockdown of endogenous human Pur-alpha, shRNA pGFP-C-shLenti (4 unique 29mer target-specific shRNAs) and scrambled shRNA control (Origene TL310036V) were used. For direct visualization of Pur-alpha in unfixed primary motor neuron cultures, we generated an mCherry-tagged Pur-alpha using pmCherry-C1 (Clontech) as a backbone for subcloning Pur-alpha into Xhol and EcoRI sites. To induce expression of FUS using doxycycline, we used the TET-inducible system. The FUS constructs epB-TT-RFP-FLAG-FUS WT and epB-TT-GFP-FLAG-FUS R521C were kindly provided by Dr. Alessandro Rosa.
Generation of deletion constructs
Using the pBK-CMV HA-Pur-alpha WT plasmid as a template, we generated gBlocks Gene Fragments (Integrated DNA Technologies) with flanking unique restriction enzymes sites surrounding the sequence we would use to ligate a new portion of the gene with a sequence missing at either the C-terminal end or N-terminal end. Constructs were analyzed with diagnostic digests, then sequenced in both forward and reverse directions to verify that gBlock fragments containing the deleted portion of the gene were integrated in frame.
Western Blotting
Total lysates were generated from 35,000 cells, denatured using β-mercaptoethanol and Laemmli sample loading buffer. Proteins were separated on Nupage 4–12% PAGE gels (Life Technologies) and were transferred to nitrocellulose membranes using the semidry iBlot transfer system. Membranes were labeled with primary antibodies overnight at room temperature. For visualization, membranes were labeled with LI-COR fluorescent secondary antibodies (Dylight 680 and Dylight 800). The LI-COR Odyssey fluorescence scanner was used to capture images of membranes.
Quantitative PCR
Total RNA was extracted with Trizol (Invitrogen). Total RNA (2 μg) was reverse transcribed using the SuperScript III First-Strand Synthesis System kit (Invitrogen). Gene expression was measured by quantitative real-time PCR using the 7900 HT Fast Real-Time PCR System (Applied Biosystems). The level of each transcript was measured with the threshold cycle (Ct) method. Values were normalized to the mean GAPDH levels.
Assessment of Neuronal Viability
Neuronal viability was examined by MAP-2 immunofluorescence. Degenerated/dead neurons show a striking loss of MAP-2. Motor neuron-enriched cultures were prepared from E13.5 Sprague Dawley rat embryos (Charles River Laboratories) and transfected using Lipofectamine 2000 (Life Technologies) on day 7 of culture. Cell cultures were transfected in duplicate with a) GFP, b) WT FUS-GFP, c) WT FUS-GFP +Pur-alpha-mCherry, d) R521G FUS-GFP, e) R521G FUS-GFP + Pur-alpha-mCherry, and f) Pur-alpha-mCherry. At 72–90 hours post-transfection, cells were fixed with 4% paraformaldehyde in sucrose in PBS for 20 min at room temperature. Cells were labeled with anti-MAP2 antibody (1:500, Millipore) and AlexaFluor 647 goat anti-rabbit secondary antibody (1:500, Life Technologies). GFP and mCherry fluorescence were visualized using FITC and TRITC channels, whereas MAP-2 was visualized in the far red channel. Z stacks of images were captured sequentially to eliminate bleed-through of the fluorophores. Loss of MAP-2 fluorescence was taken as a measure of cell death. Neuronal viability was expressed as a percentage of MAP-2 positive cells. Statistical analysis was performed using Student’s t-test.
Propidium iodide (PI) staining
To examine FUS mediated neuronal death in the presence/absence of Pur-alpha overexpression, motor neurons were transfected as before. 96 hours post transfection cells were processed for immunocytochemistry to label GFP (1:1000, Millipore), HA (1:500, Abcam) and MAP-2 (1:1000, Millipore). Before mounting the coverslips with antifade, cells were exposed to 10 μM propidium iodide (Molecular Probes, Eugene, OR, USA) for 20 min. The PI enters dead cells with loss of membrane integrity and exhibits a red fluorescence (maximum emission at 620 nm). The number of dead cells was measured as percentage of propidium iodide positive cells to total number of transfected neurons identified by MAP-2. Student T test was performed between groups. Error bars represent ± SEM (**P<0.01).
TUNEL assay
Primary neuronal cells were transfected on DIV 9 with mCherry tagged FUS (FUS-WT and FUS-R521C) and either GFP tagged Pur-alpha shRNA1 construct or control shRNA construct using lipofectamine 2000 (Invitrogen). 750 ng of each plasmid constructs were used for all the experiments. 96 hours post transfection, neuronal viability was determined using Click-iT TUNEL Alexa Fluor 647 Imaging assay according to the manufacturer’s instructions (Molecular Probes). Cells were fixed in 4% v/v paraformaldehyde/sucrose for 15 min, permeabilized with 0.25% triton 100 for 10 minutes, and then labeled by TUNEL and counterstained with DAPI. Cells were visualized using Olympus FV1000 microscope (Olympus). The fraction of TUNEL-positive cells as a percentage of total number of transfected neurons was determined for each condition. A minimum of 30 neurons was scored for each condition and data presented as mean and standard error from two independent experiments.
Stress granule induction and quantification
To induce stress granules, cells were treated with 0.5 mM sodium arsenite (Ricca Chem Co #7140-16) in DMEM for 1 hour 30 minutes at 37°C. Immunofluorescence was performed as previously described using a SG marker anti-G3BP antibody. Images were taken on an Olympus FluorView1000 microscope using a 60X objective. Stacks of images were captured from three different fields for each experimental group. Maximum projections were created using ImageJ and were then coded. All images were given to an unbiased analyst who was instructed to count distinct structures using the ImageJ cell counter. Prior to starting the quantification of SGs, the average size of G3BP-positive SGs was determined. G3BP-positive granules within two SD of the mean size of puncta were included in the analysis. For each field of view, the number of DAPI-positive cells was quantitated as a total number of cells. Next, the analyst was told to count the number of cells containing >1 G3BP-positive puncta (n=~300 cells/group). The number of cells containing SGs was divided by the total number of cells to give a percentage of cells containing SGs. For the GFP-shRNA Pur-alpha groups, one of the inclusion criteria was that the cell expressed GFP. Statistical analysis was performed on three independent quantifications per group.
Primary neurons 9 DIV (Days in vitro) were transfected with FUS-R521G GFP alone or with Pur-alpha mCherry using lipofectamine 2000.48 hours post transfection cells were treated with 0.5 mM sodium arsenite for 150 minutes at 37°C. Cells were then processed for immunocytochemistry and labeled with antibodies for GFP (1:1000, Millipore), stress granule marker (SG) G3BP1 (1:500, Proteintech). Cells were counterstained with DAPI and were visualized using Alexa Fluor 647 tagged secondary antibody. All Images were captured using Olympus FV 1000 (60X oil immersion objective and 2.5 optical zoom) confocal microscope. SG number was quantified using Image J (Version 1.50b) after background correction. Data are represented as total number of G3BP1 positive SGs per cell and differences were analyzed by Student’s t test.
Results
Pur-alpha is a component of cytoplasmic stress granules (SGs)
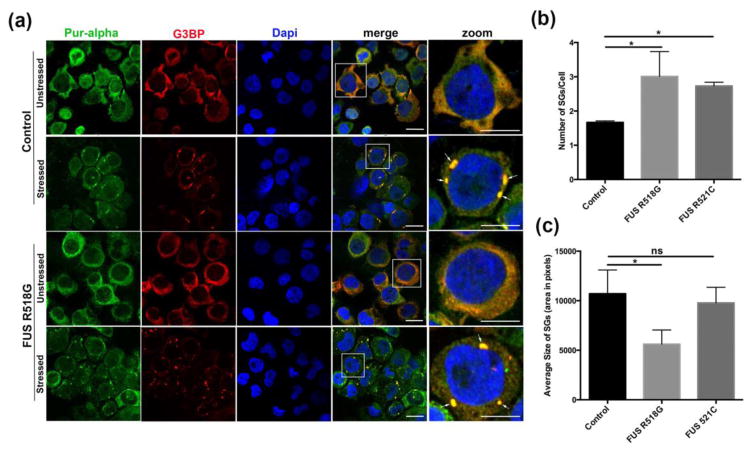
Given the protective role of Pur-alpha in neurodegenerative diseases such as FXTAS and C9orf72 expanded repeat ALS, we examined the role of Pur-alpha in FUS-related neurodegeneration. We determined the subcellular distribution of Pur-alpha in FUS-related neurodegeneration under native and stress conditions. We utilized human lymphoblastoid cell lines from ALS patients carrying disease-causing mutations in FUS and age/sex matched control lines obtained from the Coriell Institute. Under native conditions, Pur-alpha is diffusely distributed in the cytoplasm and the nucleus (Figure 1a). However, when cells were challenged with sodium arsenite, we found that Pur-alpha localized with the cytoplasmic stress granule marker G3BP, suggesting that Pur-alpha is a component of SGs (Figure 1a). We also examined the subcellular distribution of Pur-alpha in ALS patient cells carrying the disease-causing mutation FUS R518G and found that Pur-alpha was localized to SGs under stress conditions. We quantified the number of cells showing SGs in control and ALS patient cells (Figure 1b). We found that ALS patient cells carrying disease-causing mutations (FUS R518G or FUS R521C) contained significantly more SGs than control cells (Figure 1b), indicating that pathological mutations in FUS caused an increase the number of cytoplasmic SGs. Furthermore, we quantified the size of SGs in control cells as well as two ALS patients’ cells but we did not see any significant difference in the size of SGs between the FUS R521C and controls. However, we found a significant difference in the SG-size in the FUS 518G as compared to controls (Figure 1c). We quantified the percentage of cells containing SGs (G3BP-positive) under stress or native conditions. We found that FUS R518G-expressing cells had equal number of Pur-alpha positive SGs as compared to controls suggesting, that Pur-alpha incorporates into SGs equally in FUS R518G expressing cells and controls (Figure S1a). Interestingly, we observed that FUS R518G- or R521C-expressing cells showed significantly higher number of FUS-positive SGs than controls (Figure S1b). Given that FUS carrying ALS-linked mutations (FUS R518G or R521C) become mislocalized to the cytoplasm, it was not surprising to observe significantly more FUS-positive SGs in ALS-patient cells than control cells.
Figure 1. Pur-alpha is a novel component of cytoplasmic stress granules in both control and ALS patient cells.
(a). Control lymphoblastoid cells were stained with anti-Pur-alpha (green) and a stress granule marker anti-G3BP (Red). In unstressed conditions both Pur-alpha and G3BP remain diffuse in the cytoplasm. Under stress conditions (0.5 mM sodium arsenite) both Pur-alpha and G3BP aggregate to form cytoplasmic puncta that colocalize with each other. ALS patient lymphoblastoid cells carrying FUS R518G mutations are found to have diffuse Pur-alpha staining as well as G3BP localizing primarily to the cytoplasm. Upon treatment with sodium arsenite Pur-alpha and G3BP colocalize in cytoplasmic stress granules. Scale bars represent 10 μm. Arrows indicate areas of colocalization. (b). Number of G3BP positive SG were quantified per cell in control, ALS patient Lymphoblastiod cells with FUS R518G, and FUS R521C mutations. (c). Total area of G3BP positive SGs were determined using imageJ by subtracting background to an constant threshold. Total area was divided by the number of SGs to give a relative area in pixels. N=~100 cells/genotype. Averages from 3 fields at 60x were considered in the analysis. ANOVA with Tukey’s Post hoc analysis was applied. (*p< 0.05).
We also validated our findings in a mammalian neuroblastoma cell line (N2a) and in primary motor neurons overexpressing tagged Pur-alpha, and found that Pur-alpha co-localized with endogenous G3BP under stress conditions (Figure S2 and S3). These observations suggest that Pur-alpha is a novel component of SGs in ALS patient cells carrying disease-causing mutations as well as in age/sex matched controls, mammalian neuronal cells (N2a) and mammalian primary motor neurons. Next, we asked if Pur-alpha co-localization with SGs is specific to sodium arsenite treatment or whether Pur-alpha is a component of SGs under other forms of stress. In addition to sodium arsenite treatment, we used two alternate forms of stress-causing agents heat (42°C) and hydrogen peroxide (H2O2). We found that Pur-alpha also co-localized with SGs under these stress conditions (Figure S4 and S5). In general, we observed that heat stress was a more robust inducer of SGs, producing more Pur-alpha positive SGs than H2O2 stress. Altogether, these observations suggest that Pur-alpha is an integral component of SGs under three independent forms of stress.
Pur-alpha is sequestered with mutant FUS in ALS patient cells carrying disease-causing FUS mutations and becomes trapped in constitutive SGs
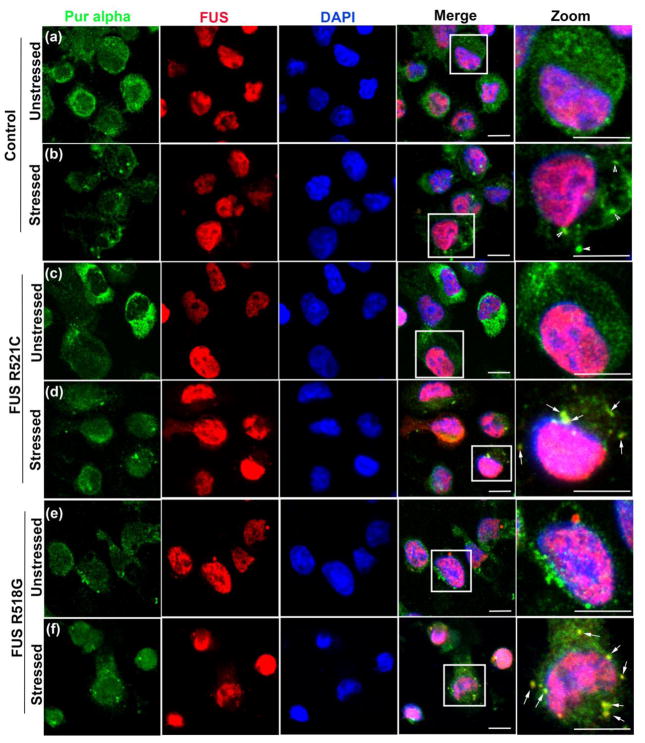
It has been recently shown that ALS-causing mutations in FUS impair SG dynamics by both delaying their assembly and disrupting their turnover [8, 59, 69]. These observations are further supported by the fact that under pathological conditions stalled SGs trap important proteins and mRNAs essential for performing cellular functions, which in turn might lead to cellular dysfunction and degeneration [16]. To determine if Pur-alpha is sequestered with FUS-positive puncta in the cytoplasm under stress conditions, we challenged ALS patient cells expressing FUS R521C and FUS R518G, as well as cells from age/sex matched controls, with sodium arsenite and examined the subcellular distribution of FUS and Pur-alpha proteins. As shown previously, WT FUS is predominantly nuclear whereas FUS carrying ALS-causing mutations is distributed in both the cytoplasm and nucleus [50, 72]. We found that under stress conditions, Pur-alpha co-localizes with FUS in ALS patient cells carrying pathogenic mutations (Figure 2). In control cells, we occasionally observed a small fraction of WT FUS in the cytoplasm; however, we did not find WT FUS localizing with Pur-alpha in the cytoplasm under stressed or unstressed conditions. In contrast to WT FUS, we found that FUS R521C and FUS R518G were frequently incorporated into cytoplasmic SGs in ALS patient cells carrying disease-causing mutations (Figure 2). Furthermore, we found that Pur-alpha and FUS are both components of SGs in mammalian neuronal cells (N2a) expressing FUS R521C under stress conditions (Figure S6). Interestingly, SGs containing Pur-alpha and FUS do not disassemble at the same rate as in control cells when stress conditions are relieved (Figure S15). These observations suggest that Pur-alpha and FUS are trapped in constitutive SGs under pathological conditions and that they are not released after the stress conditions end.
Figure 2. Pur-alpha co-localizes with ALS-linked versions of FUS in cytoplasmic aggregates.
(a). Control lymphoblastoid cell were stained for Pur-alpha and FUS under unstressed conditions. Pur-alpha staining is diffuse in the cytoplasm and FUS localizes primarily in the nucleus. (b). Under stressed (0.5 mM sodium arsenite) conditions Pur-alpha aggregates in the cytoplasm and these puncta are not positive for WT FUS. (c) and (e). FUS ALS patient cells carrying R521C and R518G mutations in unstressed conditions show Pur-alpha staining throughout the cytoplasmic compartment. (d) and (f) Under stress conditions Pur-alpha and FUS aggregate in the cytoplasm. These cytoplasmic puncta colocalize. Arrows indicate areas of colocalization of FUS and Pur-alpha in the cytoplasm. Scale bars represent 10 μm.
Pur-alpha physically interacts with FUS in mammalian neuronal cells
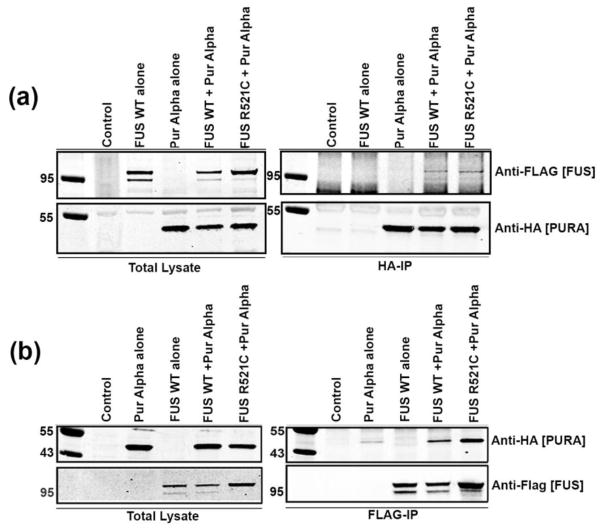
To further examine the relationship between FUS and Pur-alpha, we asked whether these two RNA binding proteins physically interact. We transfected stable mammalian neuroblastoma (N2a) cell lines expressing FLAG-FUS (WT or R521C) with a vector expressing wild type Pur-alpha fused to an HA tag on the amino-terminal portion (HA-Pur-alpha) (Figure 3). FUS and Pur-alpha interaction was analyzed by an immunoprecipitation assay using anti-HA or anti-FLAG antibodies. We found that pulling down Pur-alpha with anti-HA antibody also brings down FUS, as evident from Western blots probed with ant-FLAG (Figure 3a). Similarly, we immunoprecipitated FUS using an anti-FLAG antibody and probed with anti-HA (for Pur-alpha) and found the same pattern of interaction between FUS and Pur-alpha (Figure 3b). We also tested whether ALS-causing mutations in FUS alter the physical interaction between FUS and Pur-alpha. We found that disease-causing mutant FUS retains the ability to interact with Pur-alpha in neuronal cells. These results suggest that Pur-alpha and FUS physically interact, and that disease-causing mutations in FUS do not affect interaction with Pur-alpha in a mammalian neuronal cell line.
Figure 3. Pur-alpha physically interacts with FUS in mammalian cell cultures.
N2A cells stably carrying TET-inducible FLAG-FUS WT and FLAG-FUS R521C constructs were treated with 300 ng/ml of doxycycline to induce FUS expression and were transfected with HA-Pur-alpha. (a). Anti-HA7 was used to immunoprecipitate HA-Pur-alpha. Pur-alpha levels and induced FUS expression levels in total lysate are shown. In the immunoprecipitation (IP), Pur-alpha levels were enriched and FLAG-FUS constructs co-precipitated. (b). FLAG-FUS constructs were again induced with doxycycline and transfected with HA-Pur-alpha. Total lysates indicate Pur-alpha and FUS input levels. IP was performed with anti-FLAG, and FLAG-FUS levels were enriched by IP. HA-Pur-alpha co-precipitated with FLAG-FUS.
Pur-alpha is essential for the formation of cytoplasmic stress granules
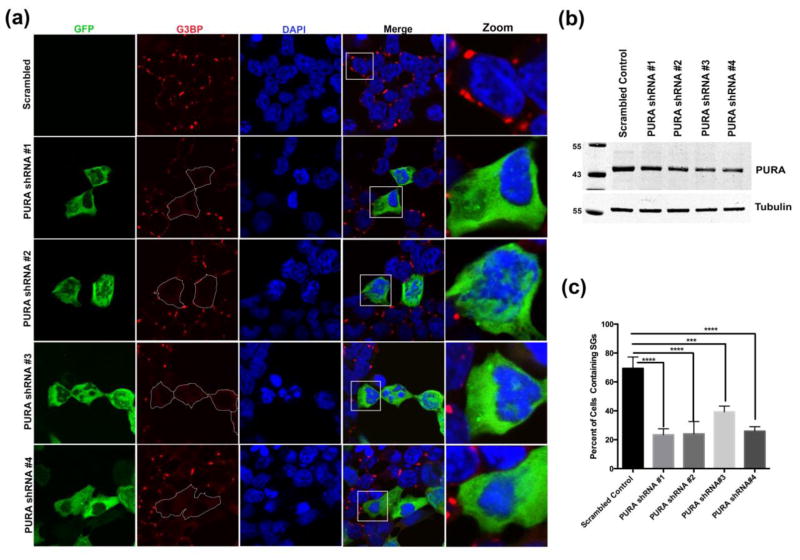
To understand the role of Pur-alpha in regulating cytoplasmic stress granules, we depleted endogenous Pur-alpha using an shRNA approach in the human embryonic kidney 293 T (HEK293T) cell line. We used 4 different shRNAs targeted against multiple functional regions of Pur-alpha to deplete endogenous Pur-alpha. After transfecting HEK293T cells with Pur-alpha shRNA for 24 hours, we challenged the cells with sodium arsenite. We found that knocking down endogenous Pur-alpha significantly impaired the ability of cells to form G3BP-positive cytoplasmic SGs under stress conditions (Figure 4a and c). In addition we used TIAR as a second marker for SGs to validate these findings and found that knocking down Pur-alpha blocks the formation of TIAR-positive SGs (Figure S7). Furthermore, we generated HEK293T cells stably expressing shRNAs and measured the reduction of Pur-alpha by qPCR and Western blot. All 4 shRNAs designed against Pur-alpha depleted endogenous Pur-alpha mRNA and protein at different levels (Figure 4b and S8a). In addition to sodium arsenite treatment, we also challenged the HEK293T cells stably expressing shRNA against Pur-alpha with heat (42°C) and found that knocking down endogenous Pur-alpha significantly impaired the ability of cells to form SGs under these conditions (Figure S9). Intriguingly, we found that knocking down Pur-alpha with shRNA significantly reduced mRNA levels of known SG components such as G3BP1, FMR1, and TIAL1 (Figure S8 b, c, and d). However, knocking down Pur-alpha with shRNA did not reduce G3BP1 protein levels (Figure S10). Interestingly, knocking down endogenous Pur-alpha did not alter FUS protein and RNA levels (Figure S11).
Figure 4. Knockdown of Pur-alpha with shRNA disrupts SG assembly in mammalian cell culture.
HEK293T cells expressing four independent human Pur-alpha shRNA-GFP constructs were stressed with sodium arsenite to determine the effect of knocking down Pur-alpha on SG dynamics. (a). In control HEK293T cells transfected with scrambled shRNA, cytoplasmic SGs (G3BP-Red) formed under sodium arsenite stress (0.5mM for 90 mins). In HEK293T cells transiently expressing four different Pur-alpha shRNA-GFP (Green) constructs (Origene), G3BP-positive SGs did not assemble in the cytoplasm. (b). HEK293T cell stably expressing Pur-alpha shRNA were used to generate total lysates (35,000 cells/well). Proteins in total lysates were resolved by SDS PAGE, Western blots were probed with anti-Pur-alpha and anti-tubulin. Pur-alpha protein levels were substantially reduced in Pur-alpha shRNA samples compared to scrambled shRNA controls. (c) Quantification of the percentage of cells containing SGs (G3BP-positive) under stress conditions. In cells expressing Pur-alpha shRNA, the formation of SGs was significantly inhibited. ANOVA was performed with Tukey’s multiplecomparison. Error bars represent ± SEM. (P=0.001***, 0.0001****).
To determine if depleting endogenous Pur-alpha can disrupt processing bodies (p-body), we stained the HEK293T cells expressing Pur-alpha shRNA with anti-GW182 (a p-body marker) after challenging with stress. We did not observe any change in the p-body number or size in Pur-alpha depleted cells compared to control cells expressing scrambled shRNA, suggesting that depleting endogenous Pur-alpha does not affect p-body formation (Figure S12). These observations indicate that Pur-alpha is essential for the formation of cytoplasmic stress granules, but not for p-bodies, under stress conditions. We also investigated whether knocking down endogenous Pur-alpha has any influence on subcellular distribution of endogenous FUS. We found that knocking down endogenous Pur-alpha does not change nuclear localization of endogenous FUS (Figure S13).
Next, given that knockdown of Pur-alpha inhibited the formation of SGs, we asked if ectopic expression of Pur-alpha has any influence on the formation and disassembly of cytoplasmic SGs in mammalian cells. Using sodium arsenite treatment, we performed a time-course of the induction of SGs to monitor the formation of SG at multiple time points (10, 20, 30, 45 and 60 minutes) post stress. We found that exogenous expression of Pur-alpha led to the formation of SGs after 20 minutes of sodium arsenite treatment, whereas controls at 20 minutes of sodium arsenite treatment did not show the presence of SGs. These results suggest that ectopic expression of Pur- alpha promotes the formation of SGs in mammalian neuronal cells (Figure S14). Since Pur-alpha may accelerate SG dynamics, we tested whether overexpression of Pur-alpha could influence the SG disassembly process. We performed a recovery assay where mammalian neuronal cells were stressed with 0.5 mM sodium arsenite for 1 hour. We then removed the stress causing agent, washed the cells and replaced with medium, allowing them to recover for 2 hours and 30 minutes. Using this recovery assay, we observed that overexpression of Pur-alpha facilitated the disassembly of SGs (Figure S15). These observations indicate that Pur-alpha is important for both assembly and disassembly of cytoplasmic SGs.
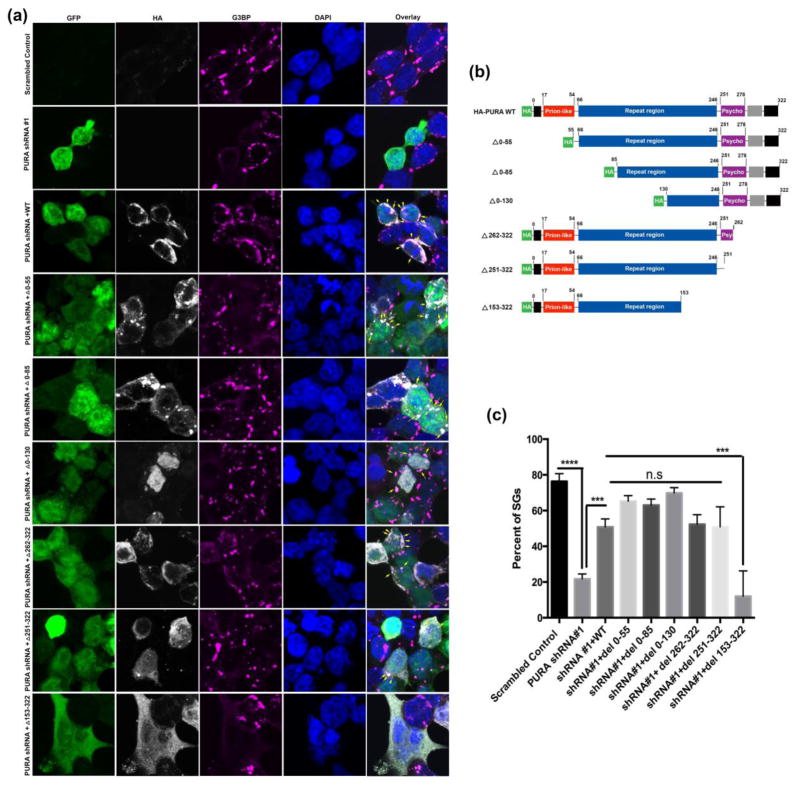
Mapping functional domain (s) of Pur-alpha required for the cytoplasmic SG assembly
The Pur-alpha protein contains several functional domains with distinct biological functions. To determine which domain of Pur-alpha is required for SG assembly, we generated a series of Pur-alpha constructs with deletions in their functional domains (Figure 5b). We found that the Pur-alpha mutant constructs were expressed correctly when transfected into HEK293 cells (Figure 5a). We next asked if ectopic expression of WT Pur-alpha can restore SG assembly in HEK293T cells stably expressing shRNA against Pur-alpha. We found that reintroducing WT Pur-alpha significantly restored SG formation in HEK293T cells expressing shRNA against Pur-alpha (Figure 5a and 5c). These findings indicate that SG assembly is specifically perturbed by knocking down endogenous Pur-alpha levels. Using a bioinformatic predictive algorithm [54] to identify potential functional domains, we found Pur-alpha contains a putative prion-like domain (Figure S16). We reintroduced Pur-alpha with deletions in different functional domains to the HEK293 cells stably expressing shRNA against Pur-alpha. The majority of the mutant Pur-alpha constructs showed a subcellular distribution comparable to that of WT Pur-alpha, except the construct with the deletion in the first N-terminal 85 amino acids. Most of the Pur-alpha mutant constructs were able to restore, with different propensity, SG formation in HEK2932 cells stably expressing Pur-alpha shRNA (Figure 5a). Interestingly, we observed that deleting 160 amino acids from the C-terminus of Pur-alpha spanning the repeat region, psycho domain, C-terminal glutamine-rich and glutamate-rich domains, impaired the ability of Pur-alpha to restore the formation of SGs in HEK293T cells expressing Pur-alpha shRNA, suggesting that this region is critical for the formation of SGs in HEK293T cells.
Figure 5. Pur-alpha functional domains required for SG assembly and incorporation.
HEK293T cell expressing human Pur-alpha shRNA were transfected with deletion constructs to determine the functional domain required to restore SG formation. (a). Immunofluoresence of Pur-alpha shRNA GFP #1 (green), HA-Pur-alpha deletion constructs (white), G3BP (magenta), DAPI (blue). Arrows indicate SGs positive for both Pur-alpha and G3BP. (b) Schematic diagram to demonstrate the functional domains systematically deleted to map the domain required for SG formation and incorporation (c). Quantification of the number of cells containing SGs. Expression of Pur-alpha shRNA cause a significant reduction in the total percentage of cells containing SGs. Co-expression of Pur-alpha WT and other mutations in the presence of Pur-alpha shRNA restored SG formation. Pur-alpha del 153–322 mutant does not restore SG formation. ANOVA was performed with Tukey’s multiple comparison. Error bars represent ± SEM. (P=0.001***, 0.0001****).
Expression of Pur-alpha can block cytoplasmic accumulation of mutant FUS in primary motor neurons
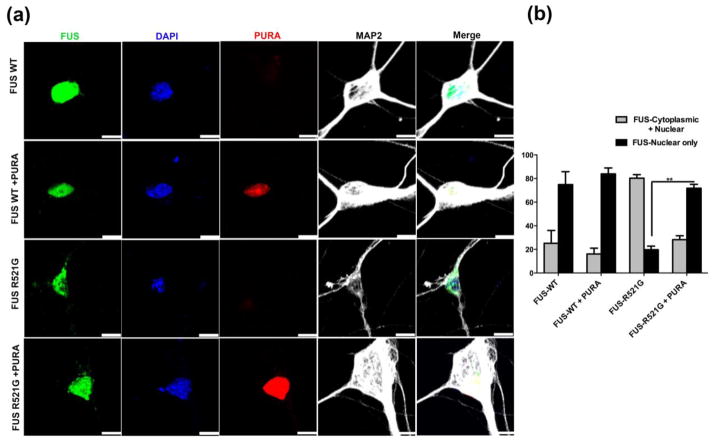
Cytoplasmic mislocalization of FUS carrying disease-causing mutations is a hallmark of disease, as observed in ALS patients and in several experimental models of FUS-related neurodegeneration [50, 56, 72]. We found that blocking cytoplasmic mislocalization of mutant FUS strongly suppressed neurodegenerative phenotypes in a fly model of ALS [55, 56]. We investigated whether Pur-alpha expression can affect cytoplasmic mislocalization of FUS in primary motor neurons expressing FUS R521G. We expressed GFP-tagged FUS WT or GFP-tagged FUS R521G with or without mCherry-tagged Pur-alpha in primary motor neurons. As expected, predominant nuclear localization of WT FUS was seen, whereas FUS R521G was distributed in both the cytoplasm and the nucleus. Interestingly, we found that expression of Pur-alpha significantly blocked cytoplasmic mislocalization of FUS R521G in primary motor neurons (Figure 6). Furthermore, we quantified the number of cells containing FUS localized to the nucleus or the nucleus and cytoplasm. Only about 20% of primary motor neurons expressing FUS R521G had FUS localized to the nucleus. However, Pur-alpha expression in the FUS R521C background restored nuclear localization of FUS in approximately 80% of cells (Figure 6b). Our data suggest that Pur-alpha expression is sufficient to prevent cytoplasmic mislocalization of mutant FUS in primary motor neurons.
Figure 6. Pur-alpha co-expression rescues mutant FUS mislocalization in primary motor neurons.
(a) Representative confocal images of primary motor neurons expressing GFP-FUS-WT and GFP-FUS-R521G alone or coexpressed with mCherry-Pur-alpha. Cytoplasmic mislocalization of FUS-R521G was evident in motor neurons whereas coexpression with Pur-alpha prevented this mislocalization. (b) Bar graph shows the mean percentage of neurons with FUS localized in the nucleus alone or nucleus + cytoplasm under different condition. The percentage of neurons with FUS-R521G in the nucleus alone was significantly increased upon co-expression with Pur-alpha. Student T test were prerformed. Error bars represent ± SEM. (**P=0.01) Scale= 10 μm.
Pur-alpha expression can suppress toxicity associated with mutant FUS in primary motor neurons
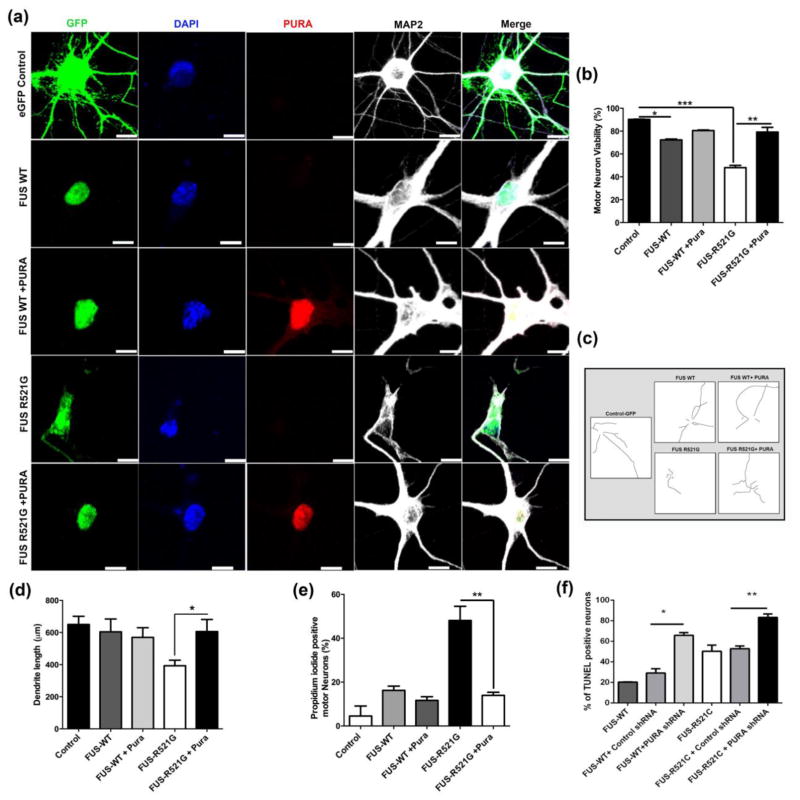
Next, we asked whether expression of Pur-alpha can mitigate toxicity associated with mutant FUS expression in primary motor neurons. We expressed GFP-tagged FUS WT or GFP-tagged FUS R521G with or without mCherry-tagged Pur-alpha in primary motor neurons. We found that expression of FUS R521G, but not FUS WT, disrupted dendritic integrity in primary motor neurons, as evident from anti-MAP2 staining (Figure 7a). Interestingly, expression of Pur-alpha significantly restored viability in primary neuronal cells as compared to FUS WT and control (Figure 7b). To further evaluate the effects of Pur-alpha on primary neurons expressing FUS R521G, we quantified the dendritic morphology and length. Pur-alpha expression mitigated FUS R521C dendritic morphology and ameliorated average dendritic length to control levels (Figure 7c and d). In addition, we measured the soma size and found that primary neurons expressing FUS R521C had reduced soma size, which was increased with Pur-alpha overexpression (Figure S17). We performed Propidium Iodide staining of primary neuronal cells expressing human FUS and found that expression of Pur-alpha significantly suppressed cell death associated with expression of FUS R521G (Figure 7e). In order to further delineate the role of Pur-alpha in regulating FUS-mediated toxicity, we depleted endogenous Pur-alpha in primary neurons expressing WT or mutant version of FUS and performed TUNEL assay. We found that knocking down endogenous FUS significantly enhanced FUS mediated toxicity suggesting that endogenous Pur-alpha plays an important role in regulating FUS toxicity (Figure 7f). Finally, we reasoned if Pur-alpha mediated amelioration of FUS toxicity involve SGs in primary neuronal cells. We expressed FUS R521G in primary neuronal cells and challenged them with sodium arsenite and found that a robust induction of cytoplasmic stress granule formation as expected. We compared the total number of cytoplasmic stress granules (G3BP positive) in cells expressing FUS R521G alone with cells expressing FUS R521G and Pur-alpha together. Two key observations emerged from our abovementioned experimental approach. First, we found that expression of Pur-alpha significantly reduced the total number of cytoplasmic stress granules in primary neuronal cells expressing FUS R521G as compared to cells expressing FUS R521G alone (Figure S18a, b). These observations suggest that cytoplasmic stress granules are cleared much faster when Pur-alpha is overexpressed in mutant FUS expressing cells. Second, we observed that ectopic expression of Pur-alpha significantly reduced the number of FUS- containing SGs (Figure S18c). These observations suggest that Pur-alpha expression is sufficient to suppress toxicity associated with human FUS expression and reduce total number of SGs as well as FUS-positive SGs in neuronal cells.
Figure 7. Pur-alpha co-expression rescues mutant FUS toxicity in primary motor neurons.
(a) Representative confocal images show loss and fragmentation of MAP-2 immunofluorescence in GFP-FUS-R521G transfected motor neurons. In primary motor neurons expressing both mCherry-Pur-alpha and GFP-FUS R521C MAP-2 fragmentation was not seen. (b) Bar graph shows the mean viability of motor neurons. FUS-R521G toxicity was significantly attenuated by Pur-alpha co-expression. (c). Representative dendrite tracings generated using image J. (d). Ectopic expression of Pur-alpha in mutant FUS expressing primary motor neurons rescues dendritic loss. Average dendritic length of motor neurons measured with neurite tracer ImageJ. (*P<0.05, **P=0.01, ***P=0.001). Data are shown as mean ± SEM. Scale= 10 μm. (e) Primary motor neurons expressing FUS R521G show positive Propidium iodide staining indicating cell death that was significantly rescued by overexpression of Pur-alpha. (f) Knocking down endogenous Pur-alpha significantly exacerbated human FUS toxicity in primary neurons. Percentage of TUNEL positive neurons in FUS WT and FUS R521C expressing neurons with and without PURA knockdown were analyzed. Percentage of TUNEL positive neurons were drastically increased in both FUS-WT and FUS-R521C conditions upon PURA shRNA1 expression (P < 0. 05) compared to control shRNA.
Discussion
Recently, RNA granules have been implicated in many human diseases, including neurodegeneration, cancer, autoimmune diseases and infectious diseases [2, 4, 14, 15, 17, 58, 74, 76]. RNA granules (stress granules (SGs), processing bodies (p-bodies), nuclear paraspeckles, neuronal granules etc.) can be classified based on their constituents, subcellular localization (cytoplasmic, nuclear, axonal, etc.), origin (e.g. germ cells or neurons), dynamic behavior and predicted functions (mRNA storage/decay or stress response, etc.) [1, 2]. SGs are physiological structures formed to combat adverse conditions such as infection, oxidative stress, heat and cold) [2, 15]. Recent studies have shown that several RNA-binding proteins implicated in human neurodegenerative diseases are constituents of SGs [48]. Most RNA-binding proteins contain a prion-like domain or low complexity domain, and these proteins are sticky and highly aggregation-prone. Incorporation of RNA-binding proteins and RNAs into cytoplasmic SGs is a highly organized, reversible process. Disease-causing mutations in RNA-bindings proteins (RBPs) might serve to “seed” irreversible aggregation of RBPs and RNAs. These SGs may form or may lead to formation of constitutive SGs. These SGs may form irreversible interactions among SG components that could disrupt SG dynamics, functions of other SG components, and global RNA metabolism, all which would be expected to have deleterious effects on neurons. Our data showing that FUS and Pur-alpha become trapped into cytoplasmic SGs under stress conditions supports this model. These notions have been further supported by evidence that mutations in the RNA-binding protein TIA1 (a component of SGs) can cause defects in RNA splicing and cellular stress which result in Welander distal myopathy [36, 49].
Components of RNA granules have been shown to modify neurodegenerative phenotypes associated with fragile X mental retardation and TDP-43 mediated ALS in Drosophila [24, 47]. It has been recently shown that the expression of ALS-causing protein TDP-43 can upregulate phosphorylated eIF2 alpha levels in Drosophila brains, suggesting a chronic translation arrest stage. In addition, drugs that target the phosphorylated version of eIF2 could suppress neurodegeneration associated with TDP-43 related pathologies [47]. These findings suggest that strategies to ameliorate defects in RNA granule dynamics might serve as potential therapeutic interventions for human neurodegenerative diseases, particularly ALS.
Pur-alpha is a DNA/RNA binding protein involved in regulating multiple aspects of transcription, tumor suppression and RNA transport granules [42, 75]. Pur proteins are highly conserved from bacteria to mammals with only a two amino acid difference between human and murine Pur-alpha [40, 42]. The human Pur proteins family consists of four members; Pur-alpha, Pur-beta, and two isoforms of Pur-gamma [11, 12, 57]. The pur-alpha proteins have been shown to bind purine-rich nucleic acids [11, 12, 38]. There are several functional domains in the Pur-alpha proteins and each domain of the pur-alpha protein is involved in regulating multiple aspects of cellular functions such as transcription, DNA replication, and cell growth [42]. Pur-alpha has an N-terminal glycine-rich domain, a central DNA-binding domain, a prion-like domain and C-terminal glutamine-rich and glutamate-rich domains [42]. Furthermore, heterozygous knockouts of endogenous Pur-alpha in mice have been shown to cause neurological symptoms such as severe tremor and spontaneous seizures indicating the importance of this protein in the mouse nervous system [45].
Recently, mutations have been identified in Pur-alpha that lead to profound neurodevelopmental delay, learning disabilities, neonatal hypotonia, seizures and encephalopathy, suggesting the neuronal functions of Pur-alpha in humans [37, 53]. Pur-alpha plays an important role in the pathogenesis of fragile X tremor ataxia syndrome (FXTAS) and C9orf72 related neurodegeneration in ALS. Pur-alpha colocalizes with FMRP (fragile X mental retardation protein 1 homolog) in the dendrites of rat hippocampal neurons, and the Drosophila homolog of human Pur-alpha interacts with rCGG-containing repeats in the FMR1 gene in a fly model of FXTAS [39, 43]. Interestingly, ectopic expression of Pur-alpha can suppress FXTAS-related neurodegeneration in Drosophila. Similarly, Pur-alpha binds to expanded GGGGCC repeats, and expression of Pur-alpha is sufficient to suppress neurodegenerative phenotypes associated with expanded GGGGCC repeat-mediated neurodegeneration in a Drosophila model of ALS [77]. However, the exact mechanisms of Pur-alpha-mediated suppression of neurodegenerative phenotypes are still not clear.
Our current studies, though highly intriguing, raise many key questions that remain to be answered. It is not yet clear which molecular pathway(s) are regulated by Pur-alpha. Since Pur-alpha is a multi-functional protein, it is possible that it might regulate multiple cellular pathways. A mechanistic understanding of the role of Pur-alpha in relation to other RNA binding proteins is another area of investigation for future studies. Our data show that Pur-alpha expression can cause redistribution of mutant FUS to the nucleus, indicating that Pur-alpha could be involved in nuclear-cytoplasmic shuttling of FUS or other RBPs. The molecular interactions facilitating transport of FUS to the nucleus are still unknown. It is unclear whether the interaction between FUS and Pur-alpha is dependent on RNA. Since both of these proteins are involved in regulating different aspects of RNA metabolism, it is reasonable to believe that FUS and Pur-alpha interaction is RNA-dependent, however it is still not known which functional domains of Pur-alpha or FUS are involved in mediating their physical interaction. Of note, it has been demonstrated that Pur-alpha interacts with FMRP and other RBPs involved in mRNP formation in an RNA-dependent manner [62]. Similarly, RNA-binding ability of FUS is critical for regulating FUS-mediated toxicity, subcellular distribution and incorporation into SGs [27]. Furthermore, it is not clear whether the Pur-alpha and FUS interaction occurs directly or their interaction is mediated by complexing with other RBPs. We observed that Pur-alpha contains a predicted prion-like domain, which is known to function in protein-protein interaction. In addition, Pur-alpha has been shown to bind to proteins and is responsible for Pur Binding Protein (PurBPs) transport into the nucleus during neuronal development [78]. Therefore, Pur-alpha could function directly in transporting RBPs to and from the nucleus. Under pathological conditions, we found that Pur-alpha becomes trapped in cytoplasmic SGs potentially inhibiting its normal function. Pur-alpha is an RNA binding protein; it is still unknown if the RNA-binding ability of Pur-alpha is required for transport of FUS or other RBS to the nucleus. Given that SGs are degraded by autophagy [16, 67], it is possible that Pur-alpha is involved in regulating the autophagy pathway, directly or indirectly. Since autophagy is a druggable pathway, further studies might help in developing therapeutic interventions for neurodegenerative conditions involving perturbed SG dynamics. While our manuscript was under review process, it came to our attention that a study has just been published on line that reported incorporation of Pur-alpha in SGs [29].
Our data suggest that Pur-alpha is a novel component of SGs in ALS patient cells, mammalian neuronal cells and primary motor neurons. We demonstrate that knocking down endogenous Pur-alpha can impair the abilities of cells to form SGs under stress conditions, indicating that Pur-alpha is an essential component of SGs. Interestingly; we observed that Pur-alpha expression can block the cytoplasmic accumulation of mutant FUS protein and suppress toxicity associated with FUS carrying the disease-causing mutation R521G in primary motor neurons. In conclusion, our data demonstrate a role of Pur-alpha in FUS-mediated neurodegeneration and suggest that Pur-alpha expression could help in suppressing toxicity in mammalian neurons through ameliorating the number of SGs.
Supplementary Material
(a). ALS-Patient lymphoblastoid cells carrying FUS R518G and age/sex matched population control were stress with 0.5 mM sodium arsenite for 1 hour 30 minutes and stained for endogenous Pur-alpha (anti-PURA), G3PB, and DAPI. The number of cells containing SGs was counted and the number of cells containing pur-alpha positive SGs was quantified. Percentage of cells colocalized with both pur-alpha and G3BP under stress conditions was determined. Bar graph represents the average from three separate fields. Approximately, 80 % of cells contained G3BP-positive SGs (b). Control, ALS patient cells carrying FUS R518G, and FUS R521C mutations were stained with anti-FUS N-terminal and anti-G3BP. Number of cells containing FUS incorporated into SGs was divided by the total number of cells containing SGs to give a percent of cells with FUS positive SGs. N=~200 cells. ANOVA with Tukey’s posthoc analysis was applied. (**P=0.01).
(a). Neuroblastoma cells (N2A) were stained with Dapi (blue), anti-HA7 (green), and anti-G3BP (red). In unstressed conditions, G3BP was diffuse in the cytoplasm. (b). Under stress (0.5 mM sodium arsenite), G3BP decorated cytoplasmic stress granules. (c). N2A cells transiently transfected with HA-Pur-alpha. Pur-alpha was localized to the cytoplasm. Pur-alpha overexpression did not induce G3BP-positive SGs under unstressed conditions. (d). N2A cells transiently expressing HA-Pur-alpha under stress conditions (0.5 mM sodium arsenite) displayed Pur-alpha in cytoplasmic puncta that colocalized with G3BP in the cytoplasm. Arrowheads indicate cytoplasmic SGs. Arrows indicate SGs containing HA-Pur-alpha.
Primary motor neurons were cultured in normal medium conditions (unstressed) and in medium conditions containing 0.5 mM sodium arsenite for 90 minutes. Under unstressed conditions endogenous Pur-alpha (green) and G3BP (red) appeared diffuse in the cytoplasm. MAP2 chicken antibody (a neuronal marker) was used to label the neuronal processes. Under stress conditions, Pur-alpha was present in large cytoplasmic puncta which colocalized with SG marker G3BP (depicted as large yellow puncta in the merge image). Arrows indicate Pur-alpha incorporation into SGs.
Neuroblastoma cells (N2A) untransfected, transfected with HA-Pur-alpha WT, and TET-inducible FUS-R521C-GFP cells were cultured in DMEM at 37°C on Poly-D Lysine coated coverslips (unstressed). Duplicate groups were cultured in DMEM at 42°C for 3 hours 30 minutes (stressed) then fixed. Immunoflourescense was performed with anti-G3BP (red), anti-HA7 to illuminate HA-Pur-alpha expressing cells (green), and FUS R521C-GFP expressing cells (green). In the Pur-alpha expressing cells colocalization of Pur-alpha (green) and G3BP positive SGs (red)= yellow puncta, in FUS-R521C-GFP (green) and G3BP positive SGs (red)= yellow granules. SGs are indicated with arrows with tails. SGs containing Pur-alpha or FUS are pointed out with arrowheads.
Neuroblastoma cells (N2A) untransfected, transfected with HA-Pur-alpha WT, and TET-inducible FUS-R521C-GFP cells were cultured in DMEM at 37°C (unstressed). Duplicate groups were cultured in 1 mM H2O2 in DMEM for 1 hour 30 minutes then fixed. Immunofluorescence was performed with anti-G3BP (red), anti-HA7 to illuminate HA-Pur-alpha expressing cells (green), and FUS R521C-GFP expressing cells (green). In the Pur-alpha expressing cells colocalization of Pur-alpha (green) and G3BP positive SGs (red) = yellow puncta, in FUS-R521C-GFP (green) and G3BP positive SGs (red)= yellow granules. SGs are indicated with arrows with tails. SGs containing Pur-alpha or FUS are pointed out with arrowheads.
Mammalian neuroblastoma cells (N2a) stably expressing TET inducible GFP-FUS R521C were plated on poly-D lysine coverslips then treated with 300 ng of doxycycline (DOX) for 24 hours to induce expression of FUS. DOX was included in control un-transfected cells and was present in the medium throughout the experiment. Both groups were treated with 0.5 mM sodium arsenite for 90 minutes to induce SGs. After SGs were formed, cells washed with DMEM and allowed to recover in DMEM+DOX for 90 minutes at 37°C. Following the recovery time period Pur-alpha (red) and G3BP (white) in control cells appeared diffuse with few small puncta remaining in the cytoplasm. In cells expressing GFP FUS (green), Pur-alpha (red) remains in G3BP positive SGs that were not resolve when stress was removed (Depicted as Yellow/white puncta in the cytoplasm). Unresolved SGs showing co-localization of FUS, Pur-alpha, and G3BP are indicated with arrows.
HEK293T cells expressing Pur-alpha shRNA-GFP were stressed with 0.5 mM sodium arsenite for 1.5 hour. Cells were stained with anti-TIAR (Red). Cytoplasmic SGs can be seen in adjacent untransfected cells but are absent in cells expressing Pur-alpha shRNA- GFP (green).
(a) qPCR was performed using primers specific to endogenous Pur-alpha as well as GAPDH. (b) qPCR was performed using primers specific to endogenous FMR1 as well as GAPDH. (c) qPCR was performed using primers specific to endogenous G3BP1 as well as GAPDH. (d) qPCR was performed using primers specific to endogenous TIAL1 as well as GAPDH. Relative mRNA levels were quantified and normalized to GAPDH. Graph shows fold changes in mRNA expression. ANOVA was performed with Tukey’s post hoc analysis. Error bars represent ± SEM. n.s = not significant. (*P<0.05, **P=0.01, ***P=0.001).
HEK293T cells expressing Pur-alpha shRNA –GFP were stressed with 42°C for 4 hrs. Cells were fixed then stained with Anti-G3BP (Red) and Dapi (blue). Cytoplasmic SGs in neighboring untransfected cells are indicated with arrows.
(a). HEK293T cells stably expressing pur-alpa shRNA were used to generate total lysates ~35,000 cells per well. Western blot analysis reveals a G3BP positive band at ~70kDa. (b) Band quantification was taken from three separate blots and normalized to tubulin loading control. Average band intensities were analyzed with ANOVA revealing no significant differences across groups.
HEK293T cells stably expressing Pur-alpha shRNA –GFP constructs. (a). Total lysates were made from 35,000 cells and resolved by SDS PAGE. Western blots were probed with anti-FUS (N-term) and anti-tubulin. FUS protein levels were unaltered in Pur-alpha shRNA samples compared to the scrambled shRNA control. (b). qPCR was performed using primers specific to endogenous FUS as well as GAPDH. Relative mRNA levels were quantified and normalized to GAPDH. Graph shows fold changes in FUS mRNA levels. ANOVA was performed with Tukey’s post hoc analysis. Error bars represent ± SEM. n.s = not significant.
HEK293T cells expressing Pur-alpha shRNA constructs were stressed with 0.5mM sodium arsenite then labeled with anti-GW182 [p-body marker]. (a) Immunofluorescence revealed p-bodies were present in control cells expressing scrambled shRNA as well as in cells expressing Pur-alpha shRNA. (b) Quantification indicates that there was no difference in number of p-bodies per cell in Pur-alpha shRNA groups compared to controls. ANOVA was performed with Tukey’s post hoc multiple comparison analysis. The graph shows the average number of p-bodies per cell. n=300 per/group. (n.s= not significant) error bars represent SEM. Arrows indicate p-bodies counted in the quantification.
HEK293T cells expressing Pur-alpha shRNA were labeled for endogenous hFUS [anti-FUS-N-terminal]. FUS (red) was primarily located in the nucleus in control cells expressing scrambled shRNA. In cells expressing Pur-alpha shRNA-GFP (green), the localization did not change.
Neuroblastoma cells, Control, expressing HA-Pur-alpha, and expressing FUS R521C-GFP were treated with 0.5 mM sodium arsenite. Time-lapse induction of SGs was conducted with treatments stopped after 10 mins, 20 mins, 30 mins, 45, mins, and 1 hr. Fixed cells were stained with anti-G3BP (red). In untreated conditions (time 0), G3BP appeared diffuse in the cytoplasm. SG (G3BP positive granules) began to form at 20 minutes time-point in control and FUS R521C conditions. In Pur-alpha over expression SG appear to form as early as 10 mins an earlier time point compared to control.
Neuroblastoma cells (untransfected control, HA-Pur-alpha transient expression, and FUS R521C-GFP inducible expression) were stressed with 0.5 mM sodium arsenite for 1 hour. Stress treatment was removed, washed with DMEM, then replaced with fresh DMEM. Groups were then allowed to recover for 2 hours 30 minutes at 37°C. (a). Schematic depicts the formation of SGs over time. Cells treated with 0.5 mM sodium arsenite for 1 hour showed a robust induction of SGs. Once the stress is removed (replaced with fresh DMEM) SGs begin do dissociate. After 2 hours and 30 minutes SGs have dissolved in control cells. (b) Immunoflourescense shows control cells with disassembled (diffuse red staining in the cytoplasm) SGs and in some cells SG still remaining (red puncta). HA-Pur-alpha expressing cells (green) SGs have dissolved. Cells containing FUS R521C (green) FUS is incorporated into SG (yellow) and these granules do not dissociate after 2 hours 30 minutes post stress. Co-localization is indicated with arrows. (c). At 60x magnification, Z-projections at three separate fields were imaged. Number cells containing >1 SG were quantified and divided by the total number of DAPI positive cells to obtain percentage of cells containing SGs. ANOVA was performed with Tukey’s multiple comparisons post hoc analysis. N= ~250 (Error bars represent ± SEM. (*P<0.05, **P=0.01).
Using a predictive algorithm [54], which has been used to screen other RNA binding proteins for their prion-like properties, we analyzed the Pur-alpha sequence for the presence of prion like domain (s) [48, 54]. We used the full-length amino acid sequence of human Pur-alpha to determine whether the protein contains functional domains with prion-like properties. For comparison, we show that a large portion of the FUS protein is predicted to possess prion-like properties.
Mouse primary motor neurons exogenously expressing FUS R521G, FUS WT, FUS WT + Pur-alpha, and FUS R521G + Pur-alpha were measured using ImageJ. Horizontal and vertical dimensions were measured to quantify the neuron soma size. Student T test was performed between groups. Error bars represent ± SEM (*P<0.05)
(a) Representative confocal images of FUS R521G alone (top panel) and Pur-alpha co-expressing neurons (bottom panel) labeled for G3BP1 after sodium arsenite stress. Co-localization of G3BP1 with mutant FUS is shown by white arrows. Scale= 5 μm (b). SG numbers were significantly reduced in cells co-expressing Pur-alpha (* P= 0.039) (c) FUS positive SGs are significantly less in the cells co-expressing Pur-alpha compared to mutant FUS cells (*P0.034). N=9
References
- 1.Anderson P, Kedersha N. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat Rev Mol Cell Biol. 2009;10:430–436. doi: 10.1038/nrm2694. [DOI] [PubMed] [Google Scholar]
- 2.Anderson P, Kedersha N, Ivanov P. Stress granules, P-bodies and cancer. Biochim Biophys Acta. 2015;1849:861–870. doi: 10.1016/j.bbagrm.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold ES, Ling SC, Huelga SC, Lagier-Tourenne C, Polymenidou M, Ditsworth D, Kordasiewicz HB, McAlonis-Downes M, Platoshyn O, Parone PA, et al. ALS-linked TDP-43 mutations produce aberrant RNA splicing and adult-onset motor neuron disease without aggregation or loss of nuclear TDP-43. Proc Natl Acad Sci U S A. 2013;110:E736–745. doi: 10.1073/pnas.1222809110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ash PE, Vanderweyde TE, Youmans KL, Apicco DJ, Wolozin B. Pathological stress granules in Alzheimer’s disease. Brain Res. 2014;1584:52–58. doi: 10.1016/j.brainres.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayala YM, Pagani F, Baralle FE. TDP43 depletion rescues aberrant CFTR exon 9 skipping. FEBS Lett. 2006;580:1339–1344. doi: 10.1016/j.febslet.2006.01.052. S0014-5793(06)00105-0 [pii]; [DOI] [PubMed] [Google Scholar]
- 6.Barmada SJ, Ju S, Arjun A, Batarse A, Archbold HC, Peisach D, Li X, Zhang Y, Tank EM, Qiu H, et al. Amelioration of toxicity in neuronal models of amyotrophic lateral sclerosis by hUPF1. Proc Natl Acad Sci U S A. 2015;112:7821–7826. doi: 10.1073/pnas.1509744112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barmada SJ, Skibinski G, Korb E, Rao EJ, Wu JY, Finkbeiner S. Cytoplasmic mislocalization of TDP-43 is toxic to neurons and enhanced by a mutation associated with familial amyotrophic lateral sclerosis. J Neurosci. 2010;30:639–649. doi: 10.1523/JNEUROSCI.4988-09.2010. 30/2/639 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baron DM, Kaushansky LJ, Ward CL, Sama RR, Chian RJ, Boggio KJ, Quaresma AJ, Nickerson JA, Bosco DA. Amyotrophic lateral sclerosis-linked FUS/TLS alters stress granule assembly and dynamics. Mol Neurodegener. 2013;8:30. doi: 10.1186/1750-1326-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belzil VV, Gendron TF, Petrucelli L. RNA-mediated toxicity in neurodegenerative disease. Mol Cell Neurosci. 2013;56:406–419. doi: 10.1016/j.mcn.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belzil VV, St-Onge J, Daoud H, Desjarlais A, Bouchard JP, Dupre N, Camu W, Dion PA, Rouleau GA. Identification of a FUS splicing mutation in a large family with amyotrophic lateral sclerosis. J Hum Genet. 2011;56:247–249. doi: 10.1038/jhg.2010.162. jhg2010162 [pii]; [DOI] [PubMed] [Google Scholar]
- 11.Bergemann AD, Johnson EM. The HeLa Pur factor binds single-stranded DNA at a specific element conserved in gene flanking regions and origins of DNA replication. Mol Cell Biol. 1992;12:1257–1265. doi: 10.1128/mcb.12.3.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergemann AD, Ma ZW, Johnson EM. Sequence of cDNA comprising the human pur gene and sequence-specific single-stranded-DNA-binding properties of the encoded protein. Mol Cell Biol. 1992;12:5673–5682. doi: 10.1128/mcb.12.12.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosco DA, Lemay N, Ko HK, Zhou H, Burke C, Kwiatkowski TJ, Jr, Sapp P, McKenna-Yasek D, Brown RH, Jr, Hayward LJ. Mutant FUS proteins that cause amyotrophic lateral sclerosis incorporate into stress granules. Hum Mol Genet. 2010;19:4160–4175. doi: 10.1093/hmg/ddq335. ddq335 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyd JD, Lee-Armandt JP, Feiler MS, Zaarur N, Liu M, Kraemer B, Concannon JB, Ebata A, Wolozin B, Glicksman MA. A high-content screen identifies novel compounds that inhibit stress-induced TDP-43 cellular aggregation and associated cytotoxicity. J Biomol Screen. 2014;19:44–56. doi: 10.1177/1087057113501553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchan JR. mRNP granules. Assembly, function, and connections with disease. RNA Biol. 2014;11:1019–1030. doi: 10.4161/15476286.2014.972208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchan JR, Kolaitis RM, Taylor JP, Parker R. Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function. Cell. 2013;153:1461–1474. doi: 10.1016/j.cell.2013.05.037. S0092-8674(13)00643-0 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchan JR, Kolaitis RM, Taylor JP, Parker R. Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function. Cell. 2013;153:1461–1474. doi: 10.1016/j.cell.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buratti E, Baralle FE. Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. J Biol Chem. 2001;276:36337–36343. doi: 10.1074/jbc.M104236200. M104236200 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Buratti E, Baralle FE. The multiple roles of TDP-43 in pre-mRNA processing and gene expression regulation. RNA Biol. 2010;7:420–429D. doi: 10.4161/rna.7.4.12205. [pii] [DOI] [PubMed] [Google Scholar]
- 20.Buratti E, Brindisi A, Giombi M, Tisminetzky S, Ayala YM, Baralle FE. TDP-43 binds heterogeneous nuclear ribonucleoprotein A/B through its C-terminal tail: an important region for the inhibition of cystic fibrosis transmembrane conductance regulator exon 9 splicing. J Biol Chem. 2005;280:37572–37584. doi: 10.1074/jbc.M505557200. M505557200 [pii]; [DOI] [PubMed] [Google Scholar]
- 21.Buratti E, Dork T, Zuccato E, Pagani F, Romano M, Baralle FE. Nuclear factor TDP-43 and SR proteins promote in vitro and in vivo CFTR exon 9 skipping. EMBO J. 2001;20:1774–1784. doi: 10.1093/emboj/20.7.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colombrita C, Onesto E, Megiorni F, Pizzuti A, Baralle FE, Buratti E, Silani V, Ratti A. TDP-43 and FUS RNA-binding proteins bind distinct sets of cytoplasmic messenger RNAs and differently regulate their post-transcriptional fate in motoneuron-like cells. J Biol Chem. 2012;287:15635–15647. doi: 10.1074/jbc.M111.333450. M111.333450 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper-Knock J, Bury JJ, Heath PR, Wyles M, Higginbottom A, Gelsthorpe C, Highley JR, Hautbergue G, Rattray M, Kirby J, et al. C9ORF72 GGGGCC Expanded Repeats Produce Splicing Dysregulation which Correlates with Disease Severity in Amyotrophic Lateral Sclerosis. PLoS One. 2015;10:e0127376. doi: 10.1371/journal.pone.0127376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cziko AM, McCann CT, Howlett IC, Barbee SA, Duncan RP, Luedemann R, Zarnescu D, Zinsmaier KE, Parker RR, Ramaswami M. Genetic modifiers of dFMR1 encode RNA granule components in Drosophila. Genetics. 2009;182:1051–1060. doi: 10.1534/genetics.109.103234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Alton S, Altshuler M, Lewis J. Studies of alternative isoforms provide insight into TDP-43 autoregulation and pathogenesis. RNA. 2015;21:1419–1432. doi: 10.1261/rna.047647.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Da CS, Cleveland DW. Understanding the role of TDP-43 and FUS/TLS in ALS and beyond. Curr Opin Neurobiol. 2011;21:904–919. doi: 10.1016/j.conb.2011.05.029. S0959-4388(11)00097-3 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daigle JG, Lanson NA, Jr, Smith RB, Casci I, Maltare A, Monaghan J, Nichols CD, Kryndushkin D, Shewmaker F, Pandey UB. RNA-binding ability of FUS regulates neurodegeneration, cytoplasmic mislocalization and incorporation into stress granules associated with FUS carrying ALS-linked mutations. Hum Mol Genet. 2013 doi: 10.1093/hmg/dds526. dds526 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dewey CM, Cenik B, Sephton CF, Johnson BA, Herz J, Yu G. TDP-43 aggregation in neurodegeneration: are stress granules the key? Brain Res. 2012;1462:16–25. doi: 10.1016/j.brainres.2012.02.032. S0006-8993(12)00309-5 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Salvio M, Piccinni V, Gerbino V, Mantoni F, Camerini S, Lenzi J, Rosa A, Chellini L, Loreni F, Carri MT, et al. Pur-alpha functionally interacts with FUS carrying ALS-associated mutations. Cell Death Dis. 2015;6:e1943. doi: 10.1038/cddis.2015.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dini Modigliani S, Morlando M, Errichelli L, Sabatelli M, Bozzoni I. An ALS-associated mutation in the FUS 3′-UTR disrupts a microRNA-FUS regulatory circuitry. Nat Commun. 2014;5:4335. doi: 10.1038/ncomms5335. [DOI] [PubMed] [Google Scholar]
- 31.Dormann D, Rodde R, Edbauer D, Bentmann E, Fischer I, Hruscha A, Than ME, Mackenzie IR, Capell A, Schmid B, et al. ALS-associated fused in sarcoma (FUS) mutations disrupt Transportin-mediated nuclear import. EMBO J. 2010;29:2841–2857. doi: 10.1038/emboj.2010.143. emboj2010143 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Figley MD, Bieri G, Kolaitis RM, Taylor JP, Gitler AD. Profilin 1 associates with stress granules and ALS-linked mutations alter stress granule dynamics. J Neurosci. 2014;34:8083–8097. doi: 10.1523/JNEUROSCI.0543-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finelli MJ, Liu KX, Wu Y, Oliver PL, Davies KE. Oxr1 improves pathogenic cellular features of ALS-associated FUS and TDP-43 mutations. Hum Mol Genet. 2015;24:3529–3544. doi: 10.1093/hmg/ddv104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freibaum BD, Chitta RK, High AA, Taylor JP. Global analysis of TDP-43 interacting proteins reveals strong association with RNA splicing and translation machinery. J Proteome Res. 2010;9:1104–1120. doi: 10.1021/pr901076y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilks N, Kedersha N, Ayodele M, Shen L, Stoecklin G, Dember LM, Anderson P. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol Biol Cell. 2004;15:5383–5398. doi: 10.1091/mbc.E04-08-0715;E04-08-0715. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hackman P, Sarparanta J, Lehtinen S, Vihola A, Evila A, Jonson PH, Luque H, Kere J, Screen M, Chinnery PF, et al. Welander distal myopathy is caused by a mutation in the RNA-binding protein TIA1. Ann Neurol. 2013;73:500–509. doi: 10.1002/ana.23831. [DOI] [PubMed] [Google Scholar]
- 37.Hunt D, Leventer RJ, Simons C, Taft R, Swoboda KJ, Gawne-Cain M, Magee AC, Turnpenny PD, Baralle D study DDD. Whole exome sequencing in family trios reveals de novo mutations in PURA as a cause of severe neurodevelopmental delay and learning disability. J Med Genet. 2014;51:806–813. doi: 10.1136/jmedgenet-2014-102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin P, Duan R, Qurashi A, Qin Y, Tian D, Rosser TC, Liu H, Feng Y, Warren ST. Pur alpha binds to rCGG repeats and modulates repeat-mediated neurodegeneration in a Drosophila model of fragile X tremor/ataxia syndrome. Neuron. 2007;55:556–564. doi: 10.1016/j.neuron.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin P, Zarnescu DC, Zhang F, Pearson CE, Lucchesi JC, Moses K, Warren ST. RNA-mediated neurodegeneration caused by the fragile X premutation rCGG repeats in Drosophila. Neuron. 2003;39:739–747. doi: 10.1016/s0896-6273(03)00533-6. [DOI] [PubMed] [Google Scholar]
- 40.Johnson EM. The Pur protein family: clues to function from recent studies on cancer and AIDS. Anticancer Res. 2003;23:2093–2100. [PubMed] [Google Scholar]
- 41.Johnson EM, Chen PL, Krachmarov CP, Barr SM, Kanovsky M, Ma ZW, Lee WH. Association of human Pur alpha with the retinoblastoma protein, Rb, regulates binding to the single-stranded DNA Pur alpha recognition element. J Biol Chem. 1995;270:24352–24360. doi: 10.1074/jbc.270.41.24352. [DOI] [PubMed] [Google Scholar]
- 42.Johnson EM, Daniel DC, Gordon J. The pur protein family: genetic and structural features in development and disease. J Cell Physiol. 2013;228:930–937. doi: 10.1002/jcp.24237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson EM, Kinoshita Y, Weinreb DB, Wortman MJ, Simon R, Khalili K, Winckler B, Gordon J. Role of Pur alpha in targeting mRNA to sites of translation in hippocampal neuronal dendrites. J Neurosci Res. 2006;83:929–943. doi: 10.1002/jnr.20806. [DOI] [PubMed] [Google Scholar]
- 44.Kedersha NL, Gupta M, Li W, Miller I, Anderson P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J Cell Biol. 1999;147:1431–1442. doi: 10.1083/jcb.147.7.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khalili K, Del Valle L, Muralidharan V, Gault WJ, Darbinian N, Otte J, Meier E, Johnson EM, Daniel DC, Kinoshita Y, et al. Puralpha is essential for postnatal brain development and developmentally coupled cellular proliferation as revealed by genetic inactivation in the mouse. Mol Cell Biol. 2003;23:6857–6875. doi: 10.1128/MCB.23.19.6857-6875.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim HJ, Kim NC, Wang YD, Scarborough EA, Moore J, Diaz Z, MacLea KS, Freibaum B, Li S, Molliex A, et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature. 2013;495:467–473. doi: 10.1038/nature11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim HJ, Raphael AR, LaDow ES, McGurk L, Weber RA, Trojanowski JQ, Lee VM, Finkbeiner S, Gitler AD, Bonini NM. Therapeutic modulation of eIF2alpha phosphorylation rescues TDP-43 toxicity in amyotrophic lateral sclerosis disease models. Nat Genet. 2014;46:152–160. doi: 10.1038/ng.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.King OD, Gitler AD, Shorter J. The tip of the iceberg: RNA-binding proteins with prion-like domains in neurodegenerative disease. Brain Res. 2012;1462:61–80. doi: 10.1016/j.brainres.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klar J, Sobol M, Melberg A, Mabert K, Ameur A, Johansson AC, Feuk L, Entesarian M, Orlen H, Casar-Borota O, et al. Welander distal myopathy caused by an ancient founder mutation in TIA1 associated with perturbed splicing. Hum Mutat. 2013;34:572–577. doi: 10.1002/humu.22282. [DOI] [PubMed] [Google Scholar]
- 50.Kwiatkowski TJ, Jr, Bosco DA, LeClerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–1208. doi: 10.1126/science.1166066. 323/5918/1205 [pii]; [DOI] [PubMed] [Google Scholar]
- 51.Lagier-Tourenne C, Polymenidou M, Cleveland DW. TDP-43 and FUS/TLS: emerging roles in RNA processing and neurodegeneration. Hum Mol Genet. 2010;19:R46–R64. doi: 10.1093/hmg/ddq137. ddq137 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lagier-Tourenne C, Polymenidou M, Hutt KR, Vu AQ, Baughn M, Huelga SC, Clutario KM, Ling SC, Liang TY, Mazur C, et al. Divergent roles of ALS-linked proteins FUS/TLS and TDP-43 intersect in processing long pre-mRNAs. Nat Neurosci. 2012;15:1488–1497. doi: 10.1038/nn.3230. nn.3230 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lalani SR, Zhang J, Schaaf CP, Brown CW, Magoulas P, Tsai AC, El-Gharbawy A, Wierenga KJ, Bartholomew D, Fong CT, et al. Mutations in PURA cause profound neonatal hypotonia, seizures, and encephalopathy in 5q31.3 microdeletion syndrome. Am J Hum Genet. 2014;95:579–583. doi: 10.1016/j.ajhg.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lancaster AK, Nutter-Upham A, Lindquist S, King OD. PLAAC: a web and command-line application to identify proteins with prion-like amino acid composition. Bioinformatics. 2014;30:2501–2502. doi: 10.1093/bioinformatics/btu310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lanson NA, Jr, Maltare A, King H, Smith R, Kim JH, Taylor JP, Lloyd TE, Pandey UB. A Drosophila model of FUS-related neurodegeneration reveals genetic interaction between FUS and TDP-43. Hum Mol Genet. 2011;20:2510–2523. doi: 10.1093/hmg/ddr150. ddr150 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lanson NA, Jr, Pandey UB. FUS-related proteinopathies: Lessons from animal models. Brain Res. 2012 doi: 10.1016/j.brainres.2012.01.039. S0006-8993(12)00100-X [pii]; [DOI] [PubMed] [Google Scholar]
- 57.Liu H, Johnson EM. Distinct proteins encoded by alternative transcripts of the PURG gene, located contrapodal to WRN on chromosome 8, determined by differential termination/polyadenylation. Nucleic Acids Res. 2002;30:2417–2426. doi: 10.1093/nar/30.11.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lloyd RE. Regulation of stress granules and P-bodies during RNA virus infection. Wiley Interdiscip Rev RNA. 2013;4:317–331. doi: 10.1002/wrna.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matus S, Bosco DA, Hetz C. Autophagy meets fused in sarcoma-positive stress granules. Neurobiol Aging. 2014;35:2832–2835. doi: 10.1016/j.neurobiolaging.2014.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morlando M, Dini Modigliani S, Torrelli G, Rosa A, Di Carlo V, Caffarelli E, Bozzoni I. FUS stimulates microRNA biogenesis by facilitating co-transcriptional Drosha recruitment. EMBO J. 2012;31:4502–4510. doi: 10.1038/emboj.2012.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nishimoto Y, Ito D, Yagi T, Nihei Y, Tsunoda Y, Suzuki N. Characterization of alternative isoforms and inclusion body of the TAR DNA-binding protein-43. J Biol Chem. 2010;285:608–619. doi: 10.1074/jbc.M109.022012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ohashi S, Koike K, Omori A, Ichinose S, Ohara S, Kobayashi S, Sato TA, Anzai K. Identification of mRNA/protein (mRNP) complexes containing Puralpha, mStaufen, fragile X protein, and myosin Va and their association with rough endoplasmic reticulum equipped with a kinesin motor. J Biol Chem. 2002;277:37804–37810. doi: 10.1074/jbc.M203608200. [DOI] [PubMed] [Google Scholar]
- 63.Orozco D, Tahirovic S, Rentzsch K, Schwenk BM, Haass C, Edbauer D. Loss of fused in sarcoma (FUS) promotes pathological Tau splicing. EMBO Rep. 2012;13:759–764. doi: 10.1038/embor.2012.90. embor201290 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Polymenidou M, Lagier-Tourenne C, Hutt KR, Bennett CF, Cleveland DW, Yeo GW. Misregulated RNA processing in amyotrophic lateral sclerosis. Brain Res. 2012;1462:3–15. doi: 10.1016/j.brainres.2012.02.059. S0006-8993(12)00391-5 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Polymenidou M, Lagier-Tourenne C, Hutt KR, Huelga SC, Moran J, Liang TY, Ling SC, Sun E, Wancewicz E, Mazur C, et al. Long pre-mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43. Nat Neurosci. 2011;14:459–468. doi: 10.1038/nn.2779. nn.2779 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qiu H, Lee S, Shang Y, Wang WY, Au KF, Kamiya S, Barmada SJ, Finkbeiner S, Lui H, Carlton CE, et al. ALS-associated mutation FUS-R521C causes DNA damage and RNA splicing defects. J Clin Invest. 2014;124:981–999. doi: 10.1172/JCI72723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramaswami M, Taylor JP, Parker R. Altered Ribostasis: RNA-Protein Granules in Degenerative Disorders. Cell. 2013;154:727–736. doi: 10.1016/j.cell.2013.07.038. S0092-8674(13)00946-X [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Renton AE, Chio A, Traynor BJ. State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci. 2014;17:17–23. doi: 10.1038/nn.3584. nn.3584 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ryu HH, Jun MH, Min KJ, Jang DJ, Lee YS, Kim HK, Lee JA. Autophagy regulates amyotrophic lateral sclerosis-linked fused in sarcoma-positive stress granules in neurons. Neurobiol Aging. 2014;35:2822–2831. doi: 10.1016/j.neurobiolaging.2014.07.026. S0197-4580(14)00495-3 [pii]; [DOI] [PubMed] [Google Scholar]
- 70.Scaramuzzino C, Casci I, Parodi S, Lievens PM, Polanco MJ, Milioto C, Chivet M, Monaghan J, Mishra A, Badders N, et al. Protein arginine methyltransferase 6 enhances polyglutamine-expanded androgen receptor function and toxicity in spinal and bulbar muscular atrophy. Neuron. 2015;85:88–100. doi: 10.1016/j.neuron.2014.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shorter J, Taylor JP. Disease mutations in the prion-like domains of hnRNPA1 and hnRNPA2/B1 introduce potent steric zippers that drive excess RNP granule assembly. Rare Dis. 2013;1:e25200. doi: 10.4161/rdis.25200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vance C, Rogelj B, Hortobagyi T, De Vos KJ, Nishimura AL, Sreedharan J, Hu X, Smith B, Ruddy D, Wright P, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–1211. doi: 10.1126/science.1165942. 323/5918/1208 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vance C, Scotter EL, Nishimura AL, Troakes C, Mitchell JC, Kathe C, Urwin H, Manser C, Miller CC, Hortobagyi T, et al. ALS mutant FUS disrupts nuclear localization and sequesters wild-type FUS within cytoplasmic stress granules. Hum Mol Genet. 2013;22:2676–2688. doi: 10.1093/hmg/ddt117. ddt117 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vanderweyde T, Youmans K, Liu-Yesucevitz L, Wolozin B. Role of stress granules and RNA-binding proteins in neurodegeneration: a mini-review. Gerontology. 2013;59:524–533. doi: 10.1159/000354170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.White MK, Johnson EM, Khalili K. Multiple roles for Puralpha in cellular and viral regulation. Cell Cycle. 2009;8:1–7. doi: 10.4161/cc.8.3.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wolozin B. Physiological protein aggregation run amuck: stress granules and the genesis of neurodegenerative disease. Discov Med. 2014;17:47–52. [PMC free article] [PubMed] [Google Scholar]
- 77.Xu Z, Poidevin M, Li X, Li Y, Shu L, Nelson DL, Li H, Hales CM, Gearing M, Wingo TS, et al. Expanded GGGGCC repeat RNA associated with amyotrophic lateral sclerosis and frontotemporal dementia causes neurodegeneration. Proc Natl Acad Sci U S A. 2013;110:7778–7783. doi: 10.1073/pnas.1219643110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zeng LH, Okamura K, Tanaka H, Miki N, Kuo CH. Concomitant translocation of Puralpha with its binding proteins (PurBPs) from nuclei to cytoplasm during neuronal development. Neurosci Res. 2005;51:105–109. doi: 10.1016/j.neures.2004.09.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a). ALS-Patient lymphoblastoid cells carrying FUS R518G and age/sex matched population control were stress with 0.5 mM sodium arsenite for 1 hour 30 minutes and stained for endogenous Pur-alpha (anti-PURA), G3PB, and DAPI. The number of cells containing SGs was counted and the number of cells containing pur-alpha positive SGs was quantified. Percentage of cells colocalized with both pur-alpha and G3BP under stress conditions was determined. Bar graph represents the average from three separate fields. Approximately, 80 % of cells contained G3BP-positive SGs (b). Control, ALS patient cells carrying FUS R518G, and FUS R521C mutations were stained with anti-FUS N-terminal and anti-G3BP. Number of cells containing FUS incorporated into SGs was divided by the total number of cells containing SGs to give a percent of cells with FUS positive SGs. N=~200 cells. ANOVA with Tukey’s posthoc analysis was applied. (**P=0.01).
(a). Neuroblastoma cells (N2A) were stained with Dapi (blue), anti-HA7 (green), and anti-G3BP (red). In unstressed conditions, G3BP was diffuse in the cytoplasm. (b). Under stress (0.5 mM sodium arsenite), G3BP decorated cytoplasmic stress granules. (c). N2A cells transiently transfected with HA-Pur-alpha. Pur-alpha was localized to the cytoplasm. Pur-alpha overexpression did not induce G3BP-positive SGs under unstressed conditions. (d). N2A cells transiently expressing HA-Pur-alpha under stress conditions (0.5 mM sodium arsenite) displayed Pur-alpha in cytoplasmic puncta that colocalized with G3BP in the cytoplasm. Arrowheads indicate cytoplasmic SGs. Arrows indicate SGs containing HA-Pur-alpha.
Primary motor neurons were cultured in normal medium conditions (unstressed) and in medium conditions containing 0.5 mM sodium arsenite for 90 minutes. Under unstressed conditions endogenous Pur-alpha (green) and G3BP (red) appeared diffuse in the cytoplasm. MAP2 chicken antibody (a neuronal marker) was used to label the neuronal processes. Under stress conditions, Pur-alpha was present in large cytoplasmic puncta which colocalized with SG marker G3BP (depicted as large yellow puncta in the merge image). Arrows indicate Pur-alpha incorporation into SGs.
Neuroblastoma cells (N2A) untransfected, transfected with HA-Pur-alpha WT, and TET-inducible FUS-R521C-GFP cells were cultured in DMEM at 37°C on Poly-D Lysine coated coverslips (unstressed). Duplicate groups were cultured in DMEM at 42°C for 3 hours 30 minutes (stressed) then fixed. Immunoflourescense was performed with anti-G3BP (red), anti-HA7 to illuminate HA-Pur-alpha expressing cells (green), and FUS R521C-GFP expressing cells (green). In the Pur-alpha expressing cells colocalization of Pur-alpha (green) and G3BP positive SGs (red)= yellow puncta, in FUS-R521C-GFP (green) and G3BP positive SGs (red)= yellow granules. SGs are indicated with arrows with tails. SGs containing Pur-alpha or FUS are pointed out with arrowheads.
Neuroblastoma cells (N2A) untransfected, transfected with HA-Pur-alpha WT, and TET-inducible FUS-R521C-GFP cells were cultured in DMEM at 37°C (unstressed). Duplicate groups were cultured in 1 mM H2O2 in DMEM for 1 hour 30 minutes then fixed. Immunofluorescence was performed with anti-G3BP (red), anti-HA7 to illuminate HA-Pur-alpha expressing cells (green), and FUS R521C-GFP expressing cells (green). In the Pur-alpha expressing cells colocalization of Pur-alpha (green) and G3BP positive SGs (red) = yellow puncta, in FUS-R521C-GFP (green) and G3BP positive SGs (red)= yellow granules. SGs are indicated with arrows with tails. SGs containing Pur-alpha or FUS are pointed out with arrowheads.
Mammalian neuroblastoma cells (N2a) stably expressing TET inducible GFP-FUS R521C were plated on poly-D lysine coverslips then treated with 300 ng of doxycycline (DOX) for 24 hours to induce expression of FUS. DOX was included in control un-transfected cells and was present in the medium throughout the experiment. Both groups were treated with 0.5 mM sodium arsenite for 90 minutes to induce SGs. After SGs were formed, cells washed with DMEM and allowed to recover in DMEM+DOX for 90 minutes at 37°C. Following the recovery time period Pur-alpha (red) and G3BP (white) in control cells appeared diffuse with few small puncta remaining in the cytoplasm. In cells expressing GFP FUS (green), Pur-alpha (red) remains in G3BP positive SGs that were not resolve when stress was removed (Depicted as Yellow/white puncta in the cytoplasm). Unresolved SGs showing co-localization of FUS, Pur-alpha, and G3BP are indicated with arrows.
HEK293T cells expressing Pur-alpha shRNA-GFP were stressed with 0.5 mM sodium arsenite for 1.5 hour. Cells were stained with anti-TIAR (Red). Cytoplasmic SGs can be seen in adjacent untransfected cells but are absent in cells expressing Pur-alpha shRNA- GFP (green).
(a) qPCR was performed using primers specific to endogenous Pur-alpha as well as GAPDH. (b) qPCR was performed using primers specific to endogenous FMR1 as well as GAPDH. (c) qPCR was performed using primers specific to endogenous G3BP1 as well as GAPDH. (d) qPCR was performed using primers specific to endogenous TIAL1 as well as GAPDH. Relative mRNA levels were quantified and normalized to GAPDH. Graph shows fold changes in mRNA expression. ANOVA was performed with Tukey’s post hoc analysis. Error bars represent ± SEM. n.s = not significant. (*P<0.05, **P=0.01, ***P=0.001).
HEK293T cells expressing Pur-alpha shRNA –GFP were stressed with 42°C for 4 hrs. Cells were fixed then stained with Anti-G3BP (Red) and Dapi (blue). Cytoplasmic SGs in neighboring untransfected cells are indicated with arrows.
(a). HEK293T cells stably expressing pur-alpa shRNA were used to generate total lysates ~35,000 cells per well. Western blot analysis reveals a G3BP positive band at ~70kDa. (b) Band quantification was taken from three separate blots and normalized to tubulin loading control. Average band intensities were analyzed with ANOVA revealing no significant differences across groups.
HEK293T cells stably expressing Pur-alpha shRNA –GFP constructs. (a). Total lysates were made from 35,000 cells and resolved by SDS PAGE. Western blots were probed with anti-FUS (N-term) and anti-tubulin. FUS protein levels were unaltered in Pur-alpha shRNA samples compared to the scrambled shRNA control. (b). qPCR was performed using primers specific to endogenous FUS as well as GAPDH. Relative mRNA levels were quantified and normalized to GAPDH. Graph shows fold changes in FUS mRNA levels. ANOVA was performed with Tukey’s post hoc analysis. Error bars represent ± SEM. n.s = not significant.
HEK293T cells expressing Pur-alpha shRNA constructs were stressed with 0.5mM sodium arsenite then labeled with anti-GW182 [p-body marker]. (a) Immunofluorescence revealed p-bodies were present in control cells expressing scrambled shRNA as well as in cells expressing Pur-alpha shRNA. (b) Quantification indicates that there was no difference in number of p-bodies per cell in Pur-alpha shRNA groups compared to controls. ANOVA was performed with Tukey’s post hoc multiple comparison analysis. The graph shows the average number of p-bodies per cell. n=300 per/group. (n.s= not significant) error bars represent SEM. Arrows indicate p-bodies counted in the quantification.
HEK293T cells expressing Pur-alpha shRNA were labeled for endogenous hFUS [anti-FUS-N-terminal]. FUS (red) was primarily located in the nucleus in control cells expressing scrambled shRNA. In cells expressing Pur-alpha shRNA-GFP (green), the localization did not change.
Neuroblastoma cells, Control, expressing HA-Pur-alpha, and expressing FUS R521C-GFP were treated with 0.5 mM sodium arsenite. Time-lapse induction of SGs was conducted with treatments stopped after 10 mins, 20 mins, 30 mins, 45, mins, and 1 hr. Fixed cells were stained with anti-G3BP (red). In untreated conditions (time 0), G3BP appeared diffuse in the cytoplasm. SG (G3BP positive granules) began to form at 20 minutes time-point in control and FUS R521C conditions. In Pur-alpha over expression SG appear to form as early as 10 mins an earlier time point compared to control.
Neuroblastoma cells (untransfected control, HA-Pur-alpha transient expression, and FUS R521C-GFP inducible expression) were stressed with 0.5 mM sodium arsenite for 1 hour. Stress treatment was removed, washed with DMEM, then replaced with fresh DMEM. Groups were then allowed to recover for 2 hours 30 minutes at 37°C. (a). Schematic depicts the formation of SGs over time. Cells treated with 0.5 mM sodium arsenite for 1 hour showed a robust induction of SGs. Once the stress is removed (replaced with fresh DMEM) SGs begin do dissociate. After 2 hours and 30 minutes SGs have dissolved in control cells. (b) Immunoflourescense shows control cells with disassembled (diffuse red staining in the cytoplasm) SGs and in some cells SG still remaining (red puncta). HA-Pur-alpha expressing cells (green) SGs have dissolved. Cells containing FUS R521C (green) FUS is incorporated into SG (yellow) and these granules do not dissociate after 2 hours 30 minutes post stress. Co-localization is indicated with arrows. (c). At 60x magnification, Z-projections at three separate fields were imaged. Number cells containing >1 SG were quantified and divided by the total number of DAPI positive cells to obtain percentage of cells containing SGs. ANOVA was performed with Tukey’s multiple comparisons post hoc analysis. N= ~250 (Error bars represent ± SEM. (*P<0.05, **P=0.01).
Using a predictive algorithm [54], which has been used to screen other RNA binding proteins for their prion-like properties, we analyzed the Pur-alpha sequence for the presence of prion like domain (s) [48, 54]. We used the full-length amino acid sequence of human Pur-alpha to determine whether the protein contains functional domains with prion-like properties. For comparison, we show that a large portion of the FUS protein is predicted to possess prion-like properties.
Mouse primary motor neurons exogenously expressing FUS R521G, FUS WT, FUS WT + Pur-alpha, and FUS R521G + Pur-alpha were measured using ImageJ. Horizontal and vertical dimensions were measured to quantify the neuron soma size. Student T test was performed between groups. Error bars represent ± SEM (*P<0.05)
(a) Representative confocal images of FUS R521G alone (top panel) and Pur-alpha co-expressing neurons (bottom panel) labeled for G3BP1 after sodium arsenite stress. Co-localization of G3BP1 with mutant FUS is shown by white arrows. Scale= 5 μm (b). SG numbers were significantly reduced in cells co-expressing Pur-alpha (* P= 0.039) (c) FUS positive SGs are significantly less in the cells co-expressing Pur-alpha compared to mutant FUS cells (*P0.034). N=9