Abstract
Background
Fatigue is a common symptom of liver disease but not well-characterized in patients with chronic hepatitis B virus (HBV).
Aims
We assessed the rate of fatigue using a validated instrument in patients with HBV and identified demographic, virologic, and clinical features associated with fatigue in a cross-sectional cohort study from the Hepatitis B Research Network (HBRN).
Methods
Participants were English and Spanish-speaking adults with chronic HBV who were not pregnant nor on treatment. Fatigue was measured using the PROMIS® Fatigue 7-item Short Form.
Results
The sample included 948 adults: median age 42; 51% female; 71% Asian; 74% college educated; 77% employed; 41% inactive HBV carriers; 36% with active chronic disease; and 2% with advanced fibrosis, defined as AST-Platelet Ratio Index (APRI) > 1.50. Patients with chronic HBV had a mean fatigue T-score of 46.8 ±SD=7.9, compared to a mean fatigue T-score of 50.0 ± 10 in the U.S. general population (p<.0001). In univariate analyses, greater fatigue was associated with demographic and clinical features such as female sex, lower income, more comorbidities, higher APRI score, and poorer mental health (p<0.05). In multivariate analysis, female sex (p<.001), poorer mental health (p <.001), APRI score (p=.005) and history of diabetes (p=.039) were the strongest independent predictors.
Conclusions
The frequency of fatigue in this large cohort of North American chronic HBV patients may be equal to or lower than that reported in the U.S. general population. Patients with advanced fibrosis, more comorbidities, and poorer mental health report worse fatigue.
Keywords: Liver, mental health, patient-reported outcomes, PROMIS, symptoms
Introduction
Chronic infection with the hepatitis B virus (HBV) affects approximately 1.25 million individuals in North America, at least 10% of whom will develop cirrhosis, liver failure, or liver cancer in their lifetime(1). In addition to being at increased risk for liver-related mortality and morbidity, patients with chronic HBV may also have disease-related symptoms such as fatigue(2–6). Fatigue is the most commonly reported symptom in patients with chronic viral hepatitis and is associated with reductions in health-related quality of life (HRQOL)(3, 7, 8). In chronic hepatitis C, fatigue scores are higher than in the general population(7, 9) and often associated with demographic factors and poor mental health functioning(9–11). Moreover, patients with advanced liver disease or markers of cirrhosis have more pronounced decrements in HRQOL(8, 9, 11, 12).
Patient-reported outcomes such as fatigue are relatively under-studied in patients with chronic hepatitis B compared to those with hepatitis C. The majority of studies that specifically evaluate fatigue in HBV come from Asian or Middle Eastern countries where the findings may not be applicable to individuals living in North America because of socio-cultural differences including health perceptions about what it means to have HBV(2, 13–15). Studies assessing fatigue in HBV generally have been limited in sample size, and often have been part of larger studies that combined patients with various forms of liver disease(2, 4, 13, 15, 16). Other studies have used single item questions, assessment of serious adverse events, or subscales of HRQOL instruments to measure fatigue(3). Almost all studies have had a primary emphasis on the broader construct of HRQOL which can be affected by numerous patient and environmental characteristics confounding the relationship with disease state(17). Symptoms, such as fatigue, are more directly associated with disease state, but often measured secondarily to HRQOL(2, 4, 14–16, 18). Taken together, standardized measures of fatigue have not been examined as a primary endpoint in any large North American cohort of patients with chronic HBV infection.
The National Institutes of Health (NIH) Patient-Reported Outcomes Measurement Information System® (PROMIS®) initiative has developed a comprehensive set of highly reliable, rigorously validated, precise tools to measure physical, mental, and social functioning and associated symptoms in the general U.S. population and across a wide range of chronic illnesses(19, 20). Fatigue is one of the core symptoms assessed by PROMIS because it is such a pervasive feature of many illnesses and treatments. The PROMIS fatigue surveys are highly reliable, and have been validated in several disease states(19, 21), countries, and ethnic groups(22, 23). Although not previously studied in chronic HBV infection per se, PROMIS measures have been used to measure patient-reported outcomes in other liver disease populations(24–26).
The Hepatitis B Research Network (HBRN), a cooperative network of investigators from clinical centers across the U.S. and Canada, provided a unique opportunity to evaluate fatigue in patients representing all phases of chronic HBV infection. The specific aims of the current HBRN study analysis were to (a) assess fatigue using one of the PROMIS short form surveys and (b) identify clinical, laboratory and demographic factors associated with fatigue in a large multi-ethnic North American population.
Materials and Methods
Study Design
This is a large, multi-center cross-sectional study analyzing data collected from patients enrolled in the HBRN Cohort Study. The HBRN is a cooperative network of investigators from 21 geographically distinct clinical centers across the United States and one in Canada, a data coordinating center, virology testing laboratory and immunology center. The goal of the HBRN is to conduct clinical, scientific, epidemiological and therapeutic research in acute and chronic hepatitis B in both adult and pediatric patients who reside in North America (27). All protocols were approved by the Steering Committee and the Institutional Review Boards or Research Ethics Boards of the participating sites, and all participants provided written informed consent.
Participants
The HBRN observational cohort study enrolls participants with hepatitis B who are above the age of 2 years, are not on antiviral therapy and do not have decompensated chronic hepatitis B nor hepatocellular carcinoma at enrollment. All consenting participants undergo an initial evaluation with collection of demographic information and medical history. In addition, routine laboratory results and virologic testing are done at baseline and at weeks 12, 24, 48, 72, 96, 120, and 144. The NIH PROMIS Fatigue 7-item Short Form (Fatigue SF) was introduced into the observational cohort study after recruitment had begun, and therefore fatigue was assessed at 48 weeks following the baseline visit. In those participants already completing their 48 weeks visit, fatigue was assessed at the week 72 visit. Administration of the Fatigue SF was limited to adults above the age of 18 years who could speak or read English or Spanish. Among 1654 adult patients who were enrolled in the HBRN at the time of this study, 1118 participants completed the Fatigue SF (893 at week 48 and 225 at week 72). The reasons for not completing the Fatigue SF included study discontinuation (n=163), not reaching week 48 or 72 (n=26), lack of IRB approval (n=12 for whom a site did not have the Fatigue SF approved by IRB prior to week 48 or 72 of the study), language barrier (n=175), and incompletion or missed visit (n=160). Of the 1118 who completed the form, 170 were excluded from the current analyses because they were being treated for hepatitis B, were pregnant, or had acute HBV infection. The remaining 948 patients were included in the analytic dataset. The proportion of patients recruited from each center was approximately 50% (range: 22%–73%). Demographic data from the baseline visit and clinical and virological data collected at the same visit as the Fatigue SF assessment were used for these analyses. If covariates were not available at the same time point as the Fatigue SF, values from the previous time point were carried forward.
Measures
Fatigue
The PROMIS Fatigue 7-item Short Form (SF) consists of seven questions that assess the experience of fatigue (e.g., “tired”, “run out of energy”) and interference of fatigue on daily activities (e.g., “work”, “take a bath or shower”) over the past 7 days (see Appendix 1; http://www.nihpromis.org/science/PubsDomain/Fatigue_adult.aspx). The PROMIS Fatigue 7-item SF includes a subset of items from a larger PROMIS item bank of fatigue items for which the items were selected to capture frequency of fatigue experience and interference (e.g., How often did you feel tired?) with five response options ranging from 1 (“never”) to 5 (“always”) (28). Responses are summed so that the final raw score ranges from 7 (lowest possible) to 35 (highest possible). NIH PROMIS provides a scoring table to translate the total raw score into a standardized T-score, which has been calibrated in the US general population to have a mean of 50 and a standard deviation (SD) of 10. The PROMIS T-score metric was based on a sample of 21,133 participants whose internet responses were used to calibrate and derive final T-scores, weighted to the U.S. census norms from 2000(19, 20, 29). A subject with a T-score of 40 is one SD below, and a subject with a T-score of 60 is one SD above the U.S. general population mean. Higher fatigue T-scores indicate higher degrees of fatigue. In previous studies, the Fatigue SF correlated with the full item bank of fatigue items (r = 0.76), correlated with other measures of fatigue (r = .89 –.95) and the reliability of measurement was greater than 0.91 for scores 2 SD below and 4 SD above the mean (19).
Demographic, clinical and virological features
The Fatigue SF scores were analyzed overall in the cohort as well as by multiple demographic features including age, sex, race, marital status, education, employment, income, continent of birth, and years living in the United States and Canada. Clinical features considered in the analyses of fatigue scores included serum alanine aminotransferase (ALT), total bilirubin, albumin, body mass index (BMI), advanced fibrosis as measured by the AST-Platelet Ratio Index (APRI), medication type, and medical comorbidities (i.e., diabetes, hypertension, hyperlipidemia, and other liver diseases such as alcoholic liver disease, non-alcoholic liver disease, autoimmune liver disease, and hepatitis C and D infections). Virological factors analyzed for association with fatigue included clinically-assigned HBV phenotype, presence or absence of HBeAg, HBV DNA level, duration of infection, and presumed source of infection. The HBV phenotype was assigned by clinicians at enrollment based upon common conventions using HBV DNA and ALT levels. The guidelines established by the HBRN are as follows: Inactive HBsAg carriers had HBsAg without HBeAg, normal ALT levels and no or minimal HBV DNA levels in serum (<1000 IU/mL). Immune active chronic hepatitis B was defined by presence of HBsAg and raised ALT levels with moderate or high levels of HBV DNA in serum (>10,000 IU/mL) and were also divided into those with and without HBeAg. Immune tolerant chronic hepatitis B was defined by presence of HBsAg, HBeAg and high levels of HBV DNA in serum, with normal ALT levels. Patients not fitting into any of these 3 patterns were considered “indeterminate.” Since previous studies in chronic hepatitis C demonstrated that fatigue is often associated with mental health functioning, the Medical Outcomes Study 36-item Short Form (MOS SF-36) Mental Health Functioning subscale was used as one of the clinical correlates(30). The Mental Health Functioning subscale includes 5 items which assess feeling “down in the dumps,” “nervous,” “blue/sad,” “happy,” and “peaceful” in the last 7 days; thus the subscale is not contaminated with items that measure fatigue or somatic symptoms. Using an algorithm, the raw score is translated into a norm-based standardized T-score, with a mean of 50 and a standard deviation of 10. Higher scores indicate better mental health functioning.
Data Analytic Plan
The raw scores from the Fatigue SF were translated into standardized T-scores which were calculated using the public software package PROMIScore (http://www.nihpromis.org/resources/resourcehome). All PROMIS measures, including the Fatigue SF, were derived from larger item banks which were evaluated in an initial sample of 21,133 respondents (29). The sample was weighted to have the same distribution of demographic variables as that in the 2000 U.S. Census (29, 31). After calculation of the T-score, a test of whether the mean T-score deviated from 50 was conducted using a two-sided t-test. Subsequently, Fatigue T-scores were described using means, standard deviations, and confidence intervals. Covariates were chosen based upon the previous literature on fatigue in liver disease patients and the clinical judgment and experience of the working group. Across levels of categorical covariates, T-scores were compared using t-tests for two-level variables or analysis of variance F-tests for variables of three or more levels. Linear regression analysis was used to assess the association between fatigue scores and continuous covariates. Multiple linear regression was employed to identify independent predictors of fatigue significant at p<0.05. Age, race, APRI, and viral load were forced into the multivariable model considering their clinical importance. For other covariates, variables significant at p=0.10 in the univariable regression were considered for inclusion in the multivariable regression model and a step-wise variable selection method was used to select a final model. Variables omitted (due to their p-values being greater than 0.10 in the univariable analysis) were checked further by including them individually in the final model to see whether they become statistically significant when adjusted for other variables.
Results
Participant Characteristics
The characteristics of the 948 participants are summarized in Table 1. Over 88% of participants who completed the fatigue survey spoke English as a primary language; the remaining 12% spoke English as a second language and were able to complete the survey. Fifty one percent (n=451) of participants were female, the median age was 42 years, and the median duration of HBV infection 31 years. Participants were predominantly Asian (71%) with only 13% of Caucasian race. The majority (74%) had some college education or above. Participants were primarily employed (77%).
Table 1.
Participant characteristics and unadjusted associations with fatigue, N=948
| Variable | Median(25th:75th) n(%) | Unadjusted Mean/Slope** (95% CI) | p-value |
|---|---|---|---|
| Age | N=945 | ||
| 42.0 (34.0 : 52.0) | 0.03 (−0.01, 0.07) | 0.21 | |
| Sex | N=940 | ||
| Male | 462 (49.1%) | 45.5 (44.79, 46.21) | <0.001 |
| Female | 478 (50.9%) | 47.97 (47.27, 48.67) | |
| Race | N=937 | 0.19* | |
| Asian | 666 (71.1%) | 46.67 (46.07, 47.27) | |
| African American/Other | 154 (16.4%) | 46.17 (44.93, 47.42) | 0.48 |
| Caucasian | 117 (12.5%) | 47.9 (46.47, 49.33) | 0.12 |
| Marital Status | N=925 | 0.001* | |
| Married or living in a marriage-like relationship | 628 (67.9%) | 46.38 (45.76, 46.99) | |
| Never married | 201 (21.7%) | 46.71 (45.62, 47.79) | 0.6 |
| Widowed, divorced, or separated | 96 (10.4%) | 49.51 (47.94, 51.07) | <0.001 |
| Education | N=932 | ||
| Some grade school through HS | 242 (26.0%) | 46.82 (45.83, 47.82) | 0.91 |
| Some college and above | 690 (74.0%) | 46.75 (46.16, 47.34) | |
| Employment | N=936 | ||
| Part-time/full-time | 722 (77.1%) | 46.37 (45.79, 46.94) | |
| Homemaker/retired/unemployed/other | 214 (22.9%) | 48.22 (47.17, 49.27) | 0.002 |
| Income | N=776 | 0.003* | |
| $0 to $49.9K | 338 (43.6%) | 47.8 (46.97, 48.64) | |
| $50K to $99.9K | 212 (27.3%) | 47.09 (46.04, 48.14) | 0.3 |
| 100K to $199.9K | 161 (20.7%) | 45.56 (44.35, 46.76) | 0.003 |
| $200K+ | 65 (8.4%) | 44.91 (43.01, 46.8) | 0.006 |
| Continent at Birth | N=937 | 0.002* | |
| Asia | 606 (64.7%) | 46.78 (46.16, 47.4) | |
| North/South America | 201 (21.5%) | 47.96 (46.88, 49.05) | 0.06 |
| Africa | 92 (9.8%) | 44.1 (42.5, 45.7) | 0.002 |
| Europe/Australia | 38 (4.1%) | 46.49 (44, 48.97) | 0.82 |
| Years in US/Canada | N=856 | 0.07* | |
| <=10 years ago | 192 (22.4%) | 46.03 (44.91, 47.14) | 0.05 |
| >10–20 years ago | 208 (24.3%) | 46.13 (45.06, 47.2) | 0.07 |
| >20 years ago | 456 (53.3%) | 47.34 (46.61, 48.06) | |
| ALT (IU/L) | N=942 | ||
| 30.0 (21.0 : 45.0) | 0 (−0.01, 0.01) | 0.56 | |
| TBili (mg/dL) | N=940 | ||
| 0.6 (0.5 : 0.9) | −1.21 (−2.64, 0.22) | 0.1 | |
| Albumin (g/dL) | N=937 | ||
| 4.3 (4.1 : 4.5) | −2.08 (−3.62, −0.55) | 0.008 | |
| HBV DNA (log10 IU/mL) | N=945 | ||
| 3.4 (2.4 : 4.9) | −0.14 (−0.35, 0.07) | 0.2 | |
| BMI (kg/m2) | N=934 | ||
| 24.1 (21.8 : 27.1) | 0.11 (0.01, 0.21) | 0.029 | |
| Years of HBV Infection | N=641 | ||
| 31.0 (20.0 : 42.0) | 0.05 (0.01, 0.09) | 0.008 | |
| Phenotype | N=938 | 0.61* | |
| Inactive carrier state | 382 (40.7%) | 46.91 (46.12, 47.7) | |
| HBeAg negative chronic hepatitis B | 240 (25.6%) | 47.14 (46.15, 48.14) | 0.72 |
| Indeterminate | 128 (13.6%) | 46.83 (45.46, 48.2) | 0.92 |
| Immune tolerant chronic hepatitis B | 95 (10.1%) | 45.85 (44.26, 47.44) | 0.24 |
| HBeAg positive chronic hepatitis B | 93 (9.9%) | 46.08 (44.48, 47.69) | 0.36 |
| APRI (AST-platelet-ratio index) | N=887 | 0.004* | |
| <=0.50 | 704 (79.4%) | 46.67 (46.09, 47.25) | |
| >0.50–1.50 | 165 (18.6%) | 47.03 (45.82, 48.23) | 0.6 |
| >1.50 | 18 (2.0%) | 52.99 (49.34, 56.64) | <0.001 |
| Presumed Source of Hepatitis B | N=948 | 0.86* | |
| Vertical transmission | 415 (43.8%) | 46.67 (45.91, 47.44) | |
| Horizontal transmission | 175 (18.5%) | 46.49 (45.31, 47.67) | 0.8 |
| Sexually transmitted | 37 (3.9%) | 47.79 (45.23, 50.35) | 0.41 |
| Other | 70 (7.4%) | 47.38 (45.52, 49.25) | 0.49 |
| Unknown/Missing | 251 (26.5%) | 46.77 (45.79, 47.75) | 0.88 |
| Currently Taking Any Prescription Medication | N=945 | ||
| Yes | 224 (23.7%) | 47.89 (46.85, 48.92) | 0.017 |
| No | 46.44 (45.87, 47.02) | ||
| Medical Comorbidities | N=945 | ||
| At least one | 263 (27.8%) | 47.96 (47, 48.91) | 0.005 |
| None | 46.34 (45.74, 46.93) | ||
| Hx Diabetes | N=945 | ||
| Yes | 46 (4.9%) | 50.43 (48.15, 52.71) | 0.001 |
| No | 46.6 (46.09, 47.12) | ||
| Hx Hypertension | N=945 | ||
| Yes | 151 (16.0%) | 47.77 (46.51, 49.03) | 0.1 |
| No | 46.6 (46.05, 47.15) | ||
| Hx Hyperlipidemia | N=944 | ||
| Yes | 128 (13.6%) | 48.77 (47.4, 50.13) | 0.002 |
| No | 46.48 (45.94, 47.02) | ||
| Hx Liver Disease | N=948 | ||
| Yes | 61 (6.4%) | 48.81 (46.82, 50.8) | 0.034 |
| No | 46.60 (46.07, 47.13) | ||
| SF-36 Mental Health Functioning | N=940 | ||
| 54.2 (47.2 : 58.5) | −0.46 (−0.5: −0.41) | <0.001 |
for testing equality of T-scores across all categories
For discrete variables, unadjusted category mean fatigue score. For continuous variables, change in mean fatigue score per unit change in variable.
HBV phenotype was clinician-assigned at the time of enrollment into HBRN: 40.7% were considered inactive carriers, 36% had chronic hepatitis B with disease activity (9.9% with HBeAg and 25.6% without HBeAg), 10.1% had immune tolerant hepatitis B, and 13.6% were designated as “indeterminate”. The median HBV DNA was 3.4 log10 IU/mL, the median ALT was 30 U/L, and 2% of participants had APRI scores (>1.5) suggestive of advanced fibrosis or cirrhosis. Comorbid conditions were not very frequent with only 27.8% of participants having another medical condition besides hepatitis B. The SF-36 Mental Health Functioning subscale norm-based score had a median value of 54.2, with the middle 50% of participants being between 47.2 and 58.5.
Evaluation of fatigue in the cohort
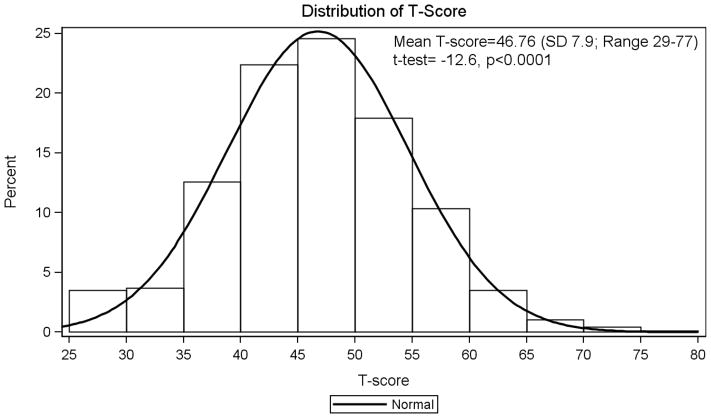
There was excellent internal consistency (over Cronbach’s alpha = 0.84) for the fatigue short form with individual item’s alpha ranging from 0.79 to 0.89. In the HBRN cohort the mean Fatigue raw score was 14.13 (SD=4.48) and the transformed standardized T-score was 46.8 (95% CI 46.3–47.3), which was lower than that of the reference mean for the U.S. general population (T=50). Prior studies suggest that one-half of a SD is equivalent to a moderate effect size and the minimally important difference (MID) in PROMIS scores range from 2–5 points (32). Although the 3.2 difference between the HBRN and PROMIS samples was statistically significant (p<0.0001), it may or may not represent a clinically meaningful difference. The distribution of the fatigue scores in the HBRN cohort is shown in the histogram and density plot of Figure 1, with 33.3% (95% CI: 30.3%–36.4%) of participants having a score greater than the average U.S. fatigue T-score of 50, and 15.4% (95% CI: 13.2% – 17.9%) of participants having fatigue T-scores above 55.
Figure 1.
Distribution of fatigue T-score in HBV patients in the cohort.
Unadjusted and adjusted associations between fatigue and demographic and clinical patient characteristics
In unadjusted analyses, female sex, not being married, not being employed, higher BMI, lower income, higher APRI, being born in North or South America, currently taking medications, having co-morbid conditions (specifically, hyperlipidemia, diabetes, and other liver diseases such as hepatitis C or D), and lower mental health functioning were all significantly (p<0.05) associated with higher fatigue scores (Table 1). In particular, mean fatigue scores were 2.5 points higher in females than males, 1.9 points higher in those not employed compared to those who were employed, and 3.1 points higher in patients widowed, divorced, or separated compared to those who were married.
Among clinical laboratory variables analyzed as continuous variables, serum ALT, AST, bilirubin and alkaline phosphatase levels were not significantly associated with fatigue scores. The calculated APRI score, however, was significantly associated with fatigue scores, being on average 6.3 points higher in participants with APRI >1.5 (T=53) compared to those with levels of <=0.5 (T=46.7) or 0.5–1.5 (T=47.0). Serum albumin levels were also significantly associated with fatigue scores, with a 2.1 point reduction in fatigue for a 1 g/dL increase in albumin. Notably, there were no significant associations between fatigue scores and any virological feature of hepatitis B such as HBeAg status and HBV DNA levels. In addition, fatigue scores were almost identical among the clinically-assigned HBV phenotypes with no differences between inactive HBsAg carriers (46.9) compared to those with HBeAg positive (46.1) or HBeAg negative immune active disease (47.1), or immune tolerant hepatitis B (45.9). In contrast, the presence of other medical comorbidities and worse mental health functioning were both significantly associated with higher levels of fatigue. Participants with one or more comorbidities had 1.6 points higher average fatigue scores compared to those without any comorbidities. For every 10-point decrease in the mental health functioning score, the mean fatigue score increased by 4.6 points.
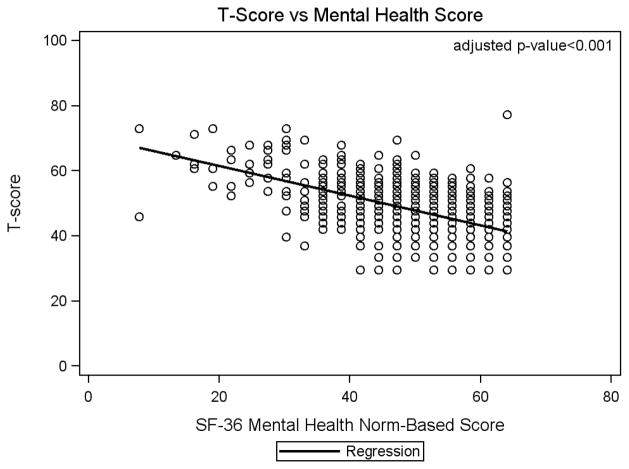
Table 2 shows results for adjusted regression models with race, age, APRI, and viral load forced into the model. Sex, mental health functioning, and having a history of diabetes remained significant independent predictors of fatigue. In the adjusted analysis, the estimated mean fatigue score for females was 2.18 units (95% CI: 1.29 – 3.06) higher than for males (p<0.0001). The estimated mean fatigue score for those with APRI >1.50 (advanced fibrosis) was 4.41 units (95% CI: 1.32 – 7.50) higher than for those with APRI ≤ 0.50 (p=.005). Those with a history of diabetes had a mean fatigue score that was 2.22 (95% CI: 0.11 – 4.32) units higher than those without a history of diabetes (p=0.039). Finally, as mental health functioning deteriorated, mean fatigue scores increased (Figure 2). A 10 unit decrease in mental health functioning was associated with a 4.4 (95% CI: 4.0 – 4.9) unit increase in mean fatigue score (p<0.0001).
Table 2.
Adjusted associations between fatigue and participant characteristics (n=876)
| Variable | Regression Coefficient (95% CI) | p-value |
|---|---|---|
| Race | 0.09 | |
| African-American/Other vs Asian | −1.31 (−2.51, −0.12) | 0.032 |
| Caucasian vs Asian | −0.63 (−2.00, 0.74) | 0.37 |
| Age | 0.01 (−0.02, 0.05) | 0.48 |
| APRI | 0.012* | |
| >1.50 vs <=0.50 | 4.41 (1.32, 7.50) | 0.005 |
| >0.50–1.50 vs <=0.50 | 0.74 (−0.41, 1.89) | 0.21 |
| HBV DNA (log10 IU/mL) | −0.15 (−0.35, 0.05) | 0.15 |
| Female vs Male | 2.18 (1.29, 3.06) | <0.001 |
| History of Diabetes | 2.22 (0.11, 4.32) | 0.039 |
| SF-36 Mental Health Functioning Score | −0.44 (−0.49, −0.40) | <0.001 |
Note:
for testing equality of T-scores across all categories
Race, Age, APRI, and HBV DNA were forced into model
Figure 2. Association between Fatigue T-Score and Mental Health Functioning.
Scatter plot of Fatigue T-Score vs SF-36 Mental Health Functioning subscale norm-based score with regression line. Each circle represents a participant or overlapping of participant points.
Discussion
Fatigue is a common symptom of many medical conditions including diseases of the liver. In this study, fatigue was assessed using a reliable and validated instrument in a large multi-ethnic cohort of patients with chronic HBV infection residing in North America. We utilized the PROMIS Fatigue 7-item short form to measure the frequencyand intensity of fatigue. The PROMIS measure was selected because of its precision in measuring the unidimensional construct of fatigue without being confounded by items that tap other symptoms (e.g., depression) or domains (e.g., HRQOL). Further, it is short, easily utilizable, patient-friendly, and has been applicable to a wide range of disease states, including liver disease(26). The main findings of this study suggest that fatigue is unrelated to most virologic and disease markers of HBV, however it is associated with liver disease severity (i.e., APRI score suggestive of advanced fibrosis), as well as female sex, mental health functioning, and comorbidities such as diabetes.
Several virologic and disease markers of HBV were evaluated in this study, including clinically-assigned HBV phenotype, HBeAg status, HBV DNA levels, serum aminotransferase levels and APRI score. Approximately 50% of the study cohort had immune active HBV disease, however fatigue was not more severe among these patients compared to those with immune inactive or immune tolerant disease phenotypes. The only clinical variable associated with fatigue in the multivariate model, was evidence of advanced liver disease with APRI score of > 1.5. The relationship between severity of liver disease and fatigue thus far in the literature has been equivocal, with one study demonstrating no association(13), but a few suggesting that patients with cirrhosis experience greater fatigue(2, 11, 33) and worse quality of life(5). In a similar population, fatigue levels of patients infected with chronic hepatitis C were not associated with Ishak fibrosis score on liver biopsy, the gold standard for diagnosis of cirrhosis(34). The finding that only an APRI score of >1.5 is associated with fatigue suggests that serious fatigue issues are uncommon in chronic HBV infection unless advanced fibrosis is present. These findings reinforce the need for routine screening for hepatitis B in high risk populations, because patients are unlikely to have symptoms indicative of the presence of liver disease. The absence of symptoms of fatigue also places a burden on the treatment for this disease, the endpoints of which should be prevention of disease progression, not necessarily amelioration of symptoms. The lack of association with most virologic or clinical markers indicates that chronic hepatitis B is generally an asymptomatic disease; however patient-reported fatigue may indicate the presence of advanced fibrosis.
The single, strongest predictor of higher fatigue levels was low mental health functioning, a SF-36 subscale that taps into emotions such as feeling down in the dumps, nervous, blue, sad, happy, and peaceful while not tapping into somatic items, such as fatigue. Other studies have produced similar findings in chronic hepatitis B and C with measures of neuropsychiatric comorbidities, anxiety, and depression predicting higher fatigue levels(4, 5, 10, 11, 13, 14). Collectively, these findings provide important insight into specific patients who may subjectively experience greater fatigue, namely those with higher rates of depressed or anxious mood.
Importantly, higher levels of fatigue were found in women compared to men. This finding is consistently documented in previous studies, including those using the PROMIS fatigue survey in both normal control and in disease populations (35–37) and in women infected with chronic hepatitis C(11). Predisposing vulnerabilities for women (e.g., endocrine and stress-related factors and social-contextual determinants) have been proposed to explain this phenomenon, however, it remains a commonly observed but not well understood association (35).
Several unique aspects of the current study, compared to the existing literature, are noteworthy. Previously, close examination of fatigue severity in patients with chronic hepatitis B has been hampered by relatively small sample sizes or studies that combine hepatitis B with other chronic liver diseases(6, 38, 39). In addition, most studies have not focused on the unique situation in North America with its diverse populations reflecting different sources of infection, varying genotypes and distinct clinical courses of this chronic infection(40). One qualitative study focused on symptoms among U.S. patients with chronic hepatitis B, but the cohort was comprised largely of non-Asian patients who now represent the minority of patients with this disease in the United States(3). Moreover, the primary emphasis has been on understanding the effect of chronic HBV infection on the broader construct of HRQOL which is influenced by many patient and environmental characteristics(17), while specific symptoms such as fatigue have been measured secondarily(2, 4, 14–16, 18). Therefore, the current study broadens our knowledge of patient-reported fatigue in a predominately Asian cohort chronically infected with HBV and residing in North America. It is possible that differences between the HBRN and PROMIS samples, such as race, education, and comorbidities partially explain the statistical differences in fatigue scores, although it remains unclear whether this difference is clinically meaningful. The HBRN and control PROMIS cohorts differed prominently in racial distribution (74% Asian vs 1%), and one might speculate that Asians experience less fatigue than Caucasians. However, we found no significant univariate differences in mean fatigue scores among the Asian (T=46.7), African-American (T=46.2) or Caucasian (T=47.9) patients in the HBRN cohort. Moreover, all three racial groups had median fatigue scores lower than the general US population, suggesting that other factors, not race, likely underlie these differences. On variables available for comparison, the most important difference between the two samples may relate to the fewer comorbidities in the HBRN population (27%) compared to the general population in the U.S. (72%). Nonetheless, no meaningful comparisons can truly be made between the PROMIS sample, which was weighted to have the same distribution of demographics as that in the 2000 U.S. Census, and the HBRN sample of patients who are predominantly Asian, more educated, and have fewer comorbidities.
A few limitations of this study should be noted. Given the specific patient characteristics of this cohort, these findings may not be generalizable to patients with more advanced liver disease or those engaged in active HBV treatment, Non-Asian patients, or individuals residing in other countries where socio-cultural perceptions about having HBV or liver disease may differ. Only 2% of this sample had advanced fibrosis or cirrhosis as estimated by APRI. This may have been because the HBRN network did not enroll patients with decompensated cirrhosis or patients who were already on HBV treatment. Further, patients who were on HBV treatment at the time of fatigue assessment were excluded from the current study in order to focus specifically on fatigue that was not caused by treatment side effects. Of the 130 participants who were excluded from this study due to being on treatment, and who had an APRI score, 12 (9%) had APRI scores > 1.5, substantially higher than the 2% reported in our sample. Therefore, the average fatigue level likely would have been higher had the sample included a greater number of patients with advanced liver disease or on HBV treatment. In this study, we employed APRI as a surrogate for liver histology since liver biopsies were neither necessary nor warranted in many study participants, and when available, were rarely concurrent with administration of the PROMIS fatigue survey. Also, only patients who were English or Spanish speaking were recruited for this study because the PROMIS Fatigue Short form had yet to be translated and tested in other languages. That said, less than a quarter of otherwise eligible patients failed to complete the fatigue instrument due to language barriers. By assessing fatigue at weeks 48 and 72 weeks, we likely missed patients initially diagnosed with more severe disease who required immediate treatment and hence were excluded from these analyses. Our study also did not collect information on anti-depressant or anti-anxiety medication; therefore, the analysis could not be adjusted for the use of these medications. Finally, the external validity of the PROMIS fatigue instrument (or any other fatigue instrument) has not been previously established in individuals with chronic HBV infection or in those from Asian American descent. We chose the PROMIS Fatigue instrument due to its rigorous reliability and validity (e.g., construct, content, and concurrent validity), correlation with items from the larger fatigue bank and other measures of fatigue (e.g., SF-36 Vitality Scale), validity in other medical populations, and to contribute to the overall mission of the NIH PROMIS initiative (20, 41–45). Future studies of fatigue in patients with HBV should include longitudinal analysis of fatigue before, during and after HBV treatment to evaluate trends over time, and assessment of other moderator and mediator variables to better understand which patients, and by which pathways, HBV may be related to fatigue.
In conclusion, in this cohort of North American adults participating in the HBRN study, higher fatigue levels are associated with more advanced liver disease and more comorbid conditions, such as diabetes, but no other disease or virologic markers. Women and patients who experience poorer mental health functioning, such as symptoms of depression or anxiety, also appear to be prone to higher levels of fatigue. In patients who complain of fatigue during the clinical encounter, providers should be suspicious of advanced fibrosis or mental health issues, and refer accordingly for further evaluation.
Acknowledgments
In addition to the authors, the HBRN would like to acknowledge the contributions of the following: Harvard Consortium: Nezam Afdhal, MD, Asad Javaid, MBBS, Jianghe Niu, Johanna Han, Imad Nasser, MD (Beth Israel Deaconess Medical Center, Boston, MA). Minnesota Alliance for Research in Chronic Hepatitis B: Alisha C. Stahler, Linda Stadheim, RN (Mayo Clinic Rochester, Rochester, MN), Mohamed Hassan, MD (University of Minnesota, Minneapolis, MN). Midwest Hepatitis B Consortium: Kathryn Rushing, RN, Rosemary A. Nagy, RDN, LD, MBA, Jacki Cerkoski, RN, MSN (Saint Louis University School of Medicine, St Louis, MO), Debra DeMarco Shaw, RN, BSN, Lisa Kessels, RN, Michael K. Klebert, PhD, RN, ANP-BC (Washington University, St. Louis, MO). University of Toronto Consortium: Seham Noureldin, PhD, Danie La, RN, Lucie Liu, MSc, CCRP, Diana Kaznowski, RN, Jiayun Chen, Doinita Vladutu, Orlando Cerocchi (Toronto Western & General Hospitals, Toronto, Ontario). HBV CRN North Texas Consortium: Stacey Minshall, RN, BSN (Division of Digestive and Liver Diseases, University of Texas Southwestern Medical Center at Dallas, Dallas, Texas), Sheila Bass (University of Texas Southwestern, Dallas, TX), Ethel Sauceda, BS (Baylor University Medical Center, Dallas, TX). Los Angeles Hepatitis B Consortium: Samuel French, MD, Velma Peacock, RN (David Geffen School of Med, UCLA, Los Angeles, CA). San Francisco Hepatitis B Research Group Consortium: Ashley Ungermann, MS, Claudia Ayala, MS, Emma Olson, BS, Ivy Lau, BS (University of California-San Francisco), Veronika Podolskaya, BS, NCPT, Nata DeVole, RN (California Pacific Medical Center, Research Institute). Michigan Hawaii Consortium: Barbara McKenna, MD, Kelly Oberhelman, PAC, Sravanthi Kaza, Bpharm, Cassandra Rodd, BS (University of Michigan, Ann Arbor, MI), Leslie Huddleston, NP, Peter Poerzgen, PhD (The Queen’s Medical Center, University of Hawaii, Honolulu, HI). Chapel Hill, NC Consortium: Jama M. Darling, M.D., A. Sidney Barritt, M.D., Tiffany Marsh, BA, Vikki Metheny, ANP, Danielle Cardona, PA-C (University of North Carolina at Chapel Hill, Chapel Hill, NC). Virginia Commonwealth University Medical Center: Velimir A. Luketic, MD, Paula G Smith, RN, BSN, Charlotte Hofmann, RN (Virginia Commonwealth University Health System, Richmond, VA). PNW/Alaska Clinical Center Consortium: Alycia Wolfstone, RN, MN (University of Washington Medical Center, Seattle WA) Jody Mooney, Lupita Cardona-Gonzalez (Virginia Mason Medical Center, Seattle WA). Liver Diseases Branch, NIDDK, NIH: Nancy Fryzek, RN, BSN, Elenita Rivera, BSN, Nevitt Morris, Vanessa Haynes-Williams. Liver Disease Research Branch, NIDDK, NIH: Jay H. Hoofnagle, MD, Averell H. Sherker, MD, Edward Doo, MD, Rebecca J. Torrance, RN, MS, Sherry R. Hall, MS. Immunology Center: Mary E. Valiga, RN, Keith Torrey, BS, Danielle Levine, BS, James Keith, BS, Michael Betts, PhD (University of Pennsylvania, Philadelphia, PA), Luis J. Montaner, DVM, DPhil (Wistar Institute, Philadelphia, PA). Data Coordinating Center: Michelle Danielson, PhD, Tamara Haller, Geoffrey Johnson, MS, Stephanie Kelley, MS, Sharon Lawlor, MBA, Joan M. MacGregor, MS, Andrew Pelesko, BS, Donna Stoliker, Ella Zadorozny, MS (Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA).
Abbreviations
- HBRN
Hepatitis B Research Network
- PROMIS
Patient Reported Outcomes Measurement Information System
- APRI
AST-Platelet Ratio Index
- HBV
Hepatitis B virus
- NIH
National Institutes of Health
- SD
Standard Deviation
- ALT
Alanine Aminotransferase Test
- BMI
Body Mass Index
- MOS SF-36
Medical Outcomes Study 36-item Short Form
- HrQOL
Health Related Quality of Life
Footnotes
Author Contributions: Donna Evon, Souvik Sarkar and Jay Hoofnagle developed the study concept and design. Geoffrey Johnson and Abdus Wahed conducted the statistical analyses. All authors, including Mandana Khalili, Robert Fontana, and Mauricio Lisker-Melman, were involved in data acquisition and interpretation and drafting and critically revising the manuscript for interpretation and important intellectual content. All authors have made substantial contributions to the research design, data acquisition, analysis and interpretation, and drafting and revising the manuscript and have given approval of the submitted final versions and authorship list.
Declaration of funding interests: The HBRN was funded by a U01 grant from the National Institute of Diabetes and Digestive and Kidney Diseases to the following investigators Lewis R. Roberts, MB, ChB, PhD (DK 082843), Anna Suk-Fong Lok, MD (DK082863), Steven H. Belle, PhD, MScHyg (DK082864), Kyong-Mi Chang, MD (DK082866), Michael W. Fried, MD (DK082867), Adrian M. Di Bisceglie, MD (DK082871), William M. Lee, MD (U01 DK082872), Harry L. A. Janssen, MD, PhD (DK082874), Daryl T-Y Lau, MD, MPH (DK082919), Richard K. Sterling, MD, MSc (DK082923), Steven-Huy B. Han, MD (DK082927), Robert C. Carithers, MD (DK082943), Norah A. Terrault, MD, MPH (U01 DK082944), an interagency agreement with NIDDK: Lilia M. Ganova-Raeva, PhD (A-DK-3002-001) and support from the intramural program, NIDDK, NIH: Marc G. Ghany, MD. Additional funding to support this study was provided to Kyong-Mi Chang, MD, the Immunology Center, (NIH/NIDDK Center of Molecular Studies in Digestive and Liver Diseases P30DK50306, NIH Public Health Service Research Grant M01-RR00040), Richard K. Sterling, MD, MSc (UL1TR000058, NCATS (National Center for Advancing Translational Sciences, NIH), Norah A. Terrault, MD, MPH (CTSA Grant Number UL1TR000004), Michael W. Fried, MD (CTSA Grant Number UL1TR001111), and Anna Suk-Fong Lok (CTSA Grant Number UL1RR024986.) Additional support was provided by Gilead Sciences, Inc. and Roche Molecular Systems via a CRADA through the NIDDK.
Authors’ declaration of personal interests: Mandana Khalili, Geoffrey Johnson, Wahed Abdus, Souvik Sarkar, and Jay Hoofnagle have no conflict of interests to disclose. Donna Evon has served as a consultant for Gilead. Robert Fontana has received research funding from BMS, Vertex, Gilead and Janssen, and has served as a consultant for GSK and Tibotec. Mauricio Lisker-Melman is on the Speaker’s Bureau for Gilead and Simply Speaking.
References
- 1.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661–2. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 2.Bao ZJ, Qiu DK, Ma X, Fan ZP, Zhang GS, Huang YQ, et al. Assessment of health-related quality of life in Chinese patients with minimal hepatic encephalopathy. World J Gastroenterol. 2007;13(21):3003–8. doi: 10.3748/wjg.v13.i21.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hann HW, Han SH, Block TM, Harris M, Maa JF, Fisher RT, et al. Symptomatology and health attitudes of chronic hepatitis B patients in the USA. J Viral Hepat. 2008;15(1):42–51. doi: 10.1111/j.1365-2893.2007.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozkan M, Corapcioglu A, Balcioglu I, Ertekin E, Khan S, Ozdemir S, et al. Psychiatric morbidity and its effect on the quality of life of patients with chronic hepatitis B and hepatitis C. International journal of psychiatry in medicine. 2006;36(3):283–97. doi: 10.2190/D37Y-X0JY-39MJ-PVXQ. [DOI] [PubMed] [Google Scholar]
- 5.Modabbernia A, Ashrafi M, Malekzadeh R, Poustchi H. A review of psychosocial issues in patients with chronic hepatitis B. Archives of Iranian medicine. 2013;16(2):114–22. [PubMed] [Google Scholar]
- 6.Tillmann HL, Wiese M, Braun Y, Wiegand J, Tenckhoff S, Mossner J, et al. Quality of life in patients with various liver diseases: patients with HCV show greater mental impairment, while patients with PBC have greater physical impairment. J Viral Hepat. 2011;18(4):252–61. doi: 10.1111/j.1365-2893.2010.01292.x. [DOI] [PubMed] [Google Scholar]
- 7.Lang CA, Conrad S, Garrett L, Battistutta D, Cooksley WG, Dunne MP, et al. Symptom prevalence and clustering of symptoms in people living with chronic hepatitis C infection. J Pain Symptom Manage. 2006;31(4):335–44. doi: 10.1016/j.jpainsymman.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Foster GR, Goldin RD, Thomas HC. Chronic hepatitis C virus infection causes a significant reduction in quality of life in the absence of cirrhosis. Hepatology. 1998;27(1):209–12. doi: 10.1002/hep.510270132. [DOI] [PubMed] [Google Scholar]
- 9.Kallman J, O’Neil MM, Larive B, Boparai N, Calabrese L, Younossi ZM. Fatigue and health-related quality of life (HRQL) in chronic hepatitis C virus infection. Dig Dis Sci. 2007;52(10):2531–9. doi: 10.1007/s10620-006-9708-x. [DOI] [PubMed] [Google Scholar]
- 10.Dwight MM, Kowdley KV, Russo JE, Ciechanowski PS, Larson AM, Katon WJ. Depression, fatigue, and functional disability in patients with chronic hepatitis C. J Psychosom Res. 2000;49(5):311–7. doi: 10.1016/s0022-3999(00)00155-0. [DOI] [PubMed] [Google Scholar]
- 11.Poynard T, Cacoub P, Ratziu V, Myers RP, Dezailles MH, Mercadier A, et al. Fatigue in patients with chronic hepatitis C. J Viral Hepat. 2002;9(4):295–303. doi: 10.1046/j.1365-2893.2002.00364.x. [DOI] [PubMed] [Google Scholar]
- 12.Younossi Z, Kallman J, Kincaid J. The effects of HCV infection and management on health-related quality of life. Hepatology. 2007;45(3):806–16. doi: 10.1002/hep.21565. [DOI] [PubMed] [Google Scholar]
- 13.Ashrafi M, Modabbernia A, Dalir M, Taslimi S, Karami M, Ostovaneh MR, et al. Predictors of mental and physical health in non-cirrhotic patients with viral hepatitis: a case control study. J Psychosom Res. 2012;73(3):218–24. doi: 10.1016/j.jpsychores.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Poorkaveh A, Modabbernia A, Ashrafi M, Taslimi S, Karami M, Dalir M, et al. Validity, reliability and factor structure of Hepatitis B Quality of Life Questionnaire version 1.0: findings in a large sample of 320 patients. Archives of Iranian medicine. 2012;15(5):290–7. [PubMed] [Google Scholar]
- 15.Pojoga C, Dumitrascu DL, Pascu O, Grigorescu M, Radu C, Damian D. Impaired health-related quality of life in Romanian patients with chronic viral hepatitis before antiviral therapy. Eur J Gastroenterol Hepatol. 2004;16(1):27–31. doi: 10.1097/00042737-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Bondini S, Kallman J, Dan A, Younoszai Z, Ramsey L, Nader F, et al. Health-related quality of life in patients with chronic hepatitis B. Liver Int. 2007;27(8):1119–25. doi: 10.1111/j.1478-3231.2007.01558.x. [DOI] [PubMed] [Google Scholar]
- 17.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. Jama. 1995;273(1):59–65. [PubMed] [Google Scholar]
- 18.Younossi ZM, Boparai N, Price LL, Kiwi ML, McCormick M, Guyatt G. Health-related quality of life in chronic liver disease: the impact of type and severity of disease. Am J Gastroenterol. 2001;96(7):2199–205. doi: 10.1111/j.1572-0241.2001.03956.x. [DOI] [PubMed] [Google Scholar]
- 19.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–94. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reeve BB, Hays RD, Bjorner JB, Cook KF, Crane PK, Teresi JA, et al. Psychometric evaluation and calibration of health-related quality of life item banks: plans for the Patient-Reported Outcomes Measurement Information System (PROMIS) Med Care. 2007;45(5 Suppl 1):S22–S31. doi: 10.1097/01.mlr.0000250483.85507.04. [DOI] [PubMed] [Google Scholar]
- 21.Rothrock NE, Hays RD, Spritzer K, Yount SE, Riley W, Cella D. Relative to the general US population, chronic diseases are associated with poorer health-related quality of life as measured by the Patient-Reported Outcomes Measurement Information System (PROMIS) J Clin Epidemiol. 2010;63(11):1195–204. doi: 10.1016/j.jclinepi.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ow YL, Thumboo J, Cella D, Cheung YB, Yong Fong K, Wee HL. Domains of health-related quality of life important and relevant to multiethnic English-speaking Asian systemic lupus erythematosus patients: a focus group study. Arthritis care & research. 2011;63(6):899–908. doi: 10.1002/acr.20462. [DOI] [PubMed] [Google Scholar]
- 23.Koh O, Lee J, Tan ML, Tai ES, Foo CJ, Chong KJ, et al. Establishing the thematic framework for a diabetes-specific health-related quality of life item bank for use in an english-speaking asian population. PLoS One. 2014;9(12):e115654. doi: 10.1371/journal.pone.0115654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bajaj JS, Thacker LR, Wade JB, Sanyal AJ, Heuman DM, Sterling RK, et al. PROMIS computerised adaptive tests are dynamic instruments to measure health-related quality of life in patients with cirrhosis. Alimentary pharmacology & therapeutics. 2011;34(9):1123–32. doi: 10.1111/j.1365-2036.2011.04842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elliott C, Frith J, Pairman J, Jones DE, Newton JL. Reduction in functional ability is significant postliver transplantation compared with matched liver disease and community dwelling controls. Transplant international: official journal of the European Society for Organ Transplantation. 2011;24(6):588–95. doi: 10.1111/j.1432-2277.2011.01240.x. [DOI] [PubMed] [Google Scholar]
- 26.Newton JL, Elliott C, Frith J, Ghazala C, Pairman J, Jones DE. Functional capacity is significantly impaired in primary biliary cirrhosis and is related to orthostatic symptoms. European journal of gastroenterology & hepatology. 2011;23(7):566–72. doi: 10.1097/MEG.0b013e3283470256. [DOI] [PubMed] [Google Scholar]
- 27.Ghany MG, Perrillo R, Li R, Belle SH, Janssen HL, Terrault NA, et al. Characteristics of Adults in the Hepatitis B Research Network in North America Reflect Their Country of Origin and Hepatitis B Virus Genotype. Clinical Gastroenterology and Hepatology. 2015;13(1):183–92. doi: 10.1016/j.cgh.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia SF, Cella D, Clauser SB, Flynn KE, Lad T, Lai JS, et al. Standardizing patient-reported outcomes assessment in cancer clinical trials: a patient-reported outcomes measurement information system initiative. J Clin Oncol. 2007;25(32):5106–12. doi: 10.1200/JCO.2007.12.2341. [DOI] [PubMed] [Google Scholar]
- 29.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of clinical epidemiology. 2010;63(11):1179–94. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ware JE, Jr, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey Manual and Interpretation Guide. Boston, MA: New England Medical Center, The Health Institute; 1993. [Google Scholar]
- 31.Liu H, Cella D, Gershon R, Shen J, Morales LS, Riley W, et al. Representativeness of the Patient-Reported Outcomes Measurement Information System Internet panel. Journal of clinical epidemiology. 2010;63(11):1169–78. doi: 10.1016/j.jclinepi.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yost KJ, Eton DT, Garcia SF, Cella D. Minimally important differences were estimated for six Patient-Reported Outcomes Measurement Information System-Cancer scales in advanced-stage cancer patients. Journal of clinical epidemiology. 2011;64(5):507–16. doi: 10.1016/j.jclinepi.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buster EH, Hansen BE, Buti M, Delwaide J, Niederau C, Michielsen PP, et al. Peginterferon alpha-2b is safe and effective in HBeAg-positive chronic hepatitis B patients with advanced fibrosis. Hepatology. 2007;46(2):388–94. doi: 10.1002/hep.21723. [DOI] [PubMed] [Google Scholar]
- 34.Sarkar S, Jiang Z, Evon DM, Wahed AS, Hoofnagle JH. Fatigue before, during and after antiviral therapy of chronic hepatitis C: results from the Virahep-C study. J Hepatol. 2012;57(5):946–52. doi: 10.1016/j.jhep.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Junghaenel DU, Christodoulou C, Lai JS, Stone AA. Demographic correlates of fatigue in the US general population: results from the patient-reported outcomes measurement information system (PROMIS) initiative. J Psychosom Res. 2011;71(3):117–23. doi: 10.1016/j.jpsychores.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ricci JA, Chee E, Lorandeau AL, Berger J. Fatigue in the U.S. workforce: prevalence and implications for lost productive work time. Journal of occupational and environmental medicine/American College of Occupational and Environmental Medicine. 2007;49(1):1–10. doi: 10.1097/01.jom.0000249782.60321.2a. [DOI] [PubMed] [Google Scholar]
- 37.Pawlikowska T, Chalder T, Hirsch SR, Wallace P, Wright DJ, Wessely SC. Population based study of fatigue and psychological distress. Bmj. 1994;308(6931):763–6. doi: 10.1136/bmj.308.6931.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lam ET, Lam CL, Lai CL, Yuen MF, Fong DY, So TM. Health-related quality of life of Southern Chinese with chronic hepatitis B infection. Health and quality of life outcomes. 2009;7:52. doi: 10.1186/1477-7525-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karaivazoglou K, Iconomou G, Triantos C, Hyphantis T, Thomopoulos K, Lagadinou M, et al. Fatigue and depressive symptoms associated with chronic viral hepatitis patients. health-related quality of life (HRQOL) Annals of hepatology. 2010;9(4):419–27. [PubMed] [Google Scholar]
- 40.Levy AR, Kowdley KV, Iloeje U, Tafesse E, Mukherjee J, Gish R, et al. The impact of chronic hepatitis B on quality of life: a multinational study of utilities from infected and uninfected persons. Value in health: the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2008;11(3):527–38. doi: 10.1111/j.1524-4733.2007.00297.x. [DOI] [PubMed] [Google Scholar]
- 41.Christodoulou C, Junghaenel DU, DeWalt DA, Rothrock N, Stone AA. Cognitive interviewing in the evaluation of fatigue items: results from the patient-reported outcomes measurement information system (PROMIS) Qual Life Res. 2008;17(10):1239–46. doi: 10.1007/s11136-008-9402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cook KF, Bamer AM, Amtmann D, Molton IR, Jensen MP. Six patient-reported outcome measurement information system short form measures have negligible age- or diagnosis-related differential item functioning in individuals with disabilities. Archives of physical medicine and rehabilitation. 2012;93(7):1289–91. doi: 10.1016/j.apmr.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 43.Junghaenel DU, Schneider S, Stone AA, Christodoulou C, Broderick JE. Ecological validity and clinical utility of Patient-Reported Outcomes Measurement Information System (PROMIS(R)) instruments for detecting premenstrual symptoms of depression, anger, and fatigue. J Psychosom Res. 2014;76(4):300–6. doi: 10.1016/j.jpsychores.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalkanis A, Yucel RM, Judson MA. The internal consistency of PRO fatigue instruments in sarcoidosis: superiority of the PFI over the FAS. Sarcoidosis, vasculitis, and diffuse lung diseases: official journal of WASOG/World Association of Sarcoidosis and Other Granulomatous Disorders. 2013;30(1):60–4. [PubMed] [Google Scholar]
- 45.DeWalt DA, Rothrock N, Yount S, Stone AA. Evaluation of item candidates: the PROMIS qualitative item review. Med Care. 2007;45(5 Suppl 1):S12–21. doi: 10.1097/01.mlr.0000254567.79743.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]