Abstract
Introduction
This study aims to report the outcome and toxicity of combined hyperthermia (HT) and radiation therapy (RT) in treatment of locally advanced or loco-regionally recurrent breast cancer.
Patients and Methods
Patients treated with HT and RT from January 1991 to December 2007 were reviewed. RT doses for previously irradiated patients were >40 Gy and for RT naïve patients >60 Gy, at 1.8–2 Gy/day. HT was planned for 2 sessions/week, immediately after RT, for a minimum of 20 minutes and for >4 sessions. Superficial or interstitial applicators were used with temperature measured by superficial or interstitial thermisters based on target thickness. HT treatment was assessed by thermal equivalent dose (TED), >42.5°C and >43°C. Endpoints included treatment response, lack of local progression (local control), and survival.
Results
127 patients received HT and RT to 167 sites. These included the intact breast (24.4%), chest wall/skin (67.7%), and breast/chest wall and nodes (7.9%). At a median follow-up of 13 months (mean 30±38), improved overall survival was significantly associated with increasing RT dose (p<0.0001), median TED 42.5°C≥200 minutes (p=0.003), and local control (p=0.0002). Local control at last follow-up was seen in 55.1% of patients. Complete response was significantly associated with median TED 42.5°C≥200 minutes (p=0.002) and median TED 43°C≥100 minutes (p=0.03).
Conclusion
HT and RT are effective for locally advanced or recurrent breast cancer in patients that have been historically difficult to treat by RT alone. Over 50% of patients achieved control of locoregional disease. Overall survival was improved with local control.
Keywords: Hyperthermia, Radiation Therapy, Breast Cancer, locoregional recurrence, locally advanced, reirradiation, interstitial applicator
Introduction
Locally advanced or recurrent locoregional breast cancer can be difficult to control with a single treatment modality. Gross disease on the chest wall or breast, or in the regional nodes is often symptomatic and is often psychologically distressful to patients who observe their disease daily. Surgical resection alone is associated with a high risk of failure. Chemotherapy rarely results in complete response. High radiation therapy doses are required for control and can be result in complications. Often, a combination of different modalities is used to increase response and duration of control.(1)
One modality—hyperthermia—can potentiate the effect of radiation and has been shown to improve local control in patients with advanced breast cancer.(2, 3) Hyperthermia refers to the artificial increase in tissue temperature to 40°– 44° C. It is an effective cytotoxic agent in low-pH, hypoxic and nutrient deprived conditions, those that one finds in hypoxic regions of gross tumors. Radiation resistance is often observed in these same tumor regions. In addition, hyperthermia may potentiate RT effect as it may reduce tumor cell repair of sublethal RT-induced DNA damage and may increase tumor oxygenation through improved vasodilatation, blood flow, and a potential change in the tumor cell metabolism. Hyperthermia causes protein denaturation in the cells, which can lead to damage to all intracellular signaling pathways including DNA repair. Duration of HT and timing relative to RT are critical to observe potentiation of RT effect.(4–7)
A number of randomized clinical studies have demonstrated the clinical benefit of combined HT and RT in various tumor sites.(8–11) HT is an underused modality due to the number of available facilities, duration of treatment, difficulty measuring tumor temperature, number of experienced clinicians with the modality, and insurance reimbursement.
We report our clinical experience using hyperthermia and radiation therapy in patients with locally advanced and recurrent breast cancer. Tumor response, treatment outcomes, and adverse effects were evaluated.
Patients and methods
Patients Selection
Hyperthermia records were accessed to identify patients with breast cancer treated to the breast, chest wall, and/or regional lymph nodes with hyperthermia and RT from January 1991 to December 2007. Patients could be treated with palliative or curative intent. After obtaining institutional review board approval, medical records were retrospectively reviewed to determine response, local control, survival, and toxicity.
Radiation Therapy
For RT naïve patients, radiation therapy fields included any gross disease. The entire breast or chest wall were included; regional nodes were generally treated. Typical adjuvant radiation fields for locally advanced breast cancer were used. 50 Gy in 1.8–2 Gy fractions was prescribed to areas without gross disease and a minimum of 60 Gy prescribed to gross disease. Bolus was used when there was skin involvement. Interstitial RT boosts were used to improve dose homogeneity in appropriate patients. For previously treated patients, radiation fields included gross disease plus a margin. A minimum target dose of 40 Gy was planned at 1.8–2 Gy per fractions. Bolus was generally used.
Hyperthermia
HT was delivered twice per week, immediately after RT. A minimum of 4 sessions was planned. More than 1 HT site was treated if tumor extended beyond the diameter of the applicators. Either superficial applicators (MA-120, MA-100, MA-150, MA-201 manufactured by BSD corporation) or interstitial applicators were used and chosen based on estimated thickness and width of the tumor target. Multiple measurement sites were made in each treatment field and intra-tumor measurements made if allowed the ability to place intratumoral thermistors. The goal was to deliver an intra-tumor temperature of >42.5°C for a minimum of 20 minutes and for optimally for >45 minutes. Temperature and HT length could be limited by patient comfort and/or intolerance, including pain. Each HT treatment and the entire HT treatment course were assessed using the thermal equivalent dose (TED), defined as number of minutes at temperatures >42.5°C and 43°C. Since multiple sites of temperature measurement were made, the median TED was determined for each patient, for each treatment, and for the entire treatment course.
Other therapy
Patients received sequential or concurrent systemic therapy or hormonal therapy at the discretion of the treating physicians. Different schedules and types of therapy were used and could not be analyzed for effect. Surgery was not routinely scheduled; 2 patients had mastectomies and patients were treated after resection for positive margins.
Response Assessment
Complete response (CR) was defined by disappearance of tumor in the treatment site. Partial response (PR) was defined as a >50% decrease in tumor thickness by clinical assessment. Response was difficult to assess in patients with large, locally advanced breast cancers. For this reason, freedom from local progression was reported. Local control (LC) was defined as lack of progression in the treated area after maximum response achieved after treatment. Treatment response and tumor status were assessed by the treating physicians (radiation oncology or medical oncology) and were reported regularly in the patients’ charts. Toxicity in the RT and HT fields was categorized by the Common Terminology Criteria (CTC) for AEs, version 3.0. To assess the incidence of severity of complications and their resolution during follow-up, the worst chronic toxicities were reported. Toxicities were registered at each treatment check and at each follow-up visit. Toxicities were coded for telangiectasia, desquamation, ulceration, fibrosis, and abscess formation.
Statistical Analysis
Continuous variables were summarized as means, standard deviations, medians, and ranges and categorical variables were summarized as frequencies and percentages. Survival rates were estimated via the Kaplan-Meier method and median survival times were reported along with 95% confidence limit point estimates. Differences in survival times and local control between groups were assessed by the log-rank test. All analyses were conducted in SAS version 9.4. Overall survival (OS) was measured from the first radiation treatment to death as a result of any cause.
Results
127 female breast cancer patients treated with HT and RT were identified. The median age of patients is 56 years (range 32–106). The median follow-up is 13 months and mean follow-up is 30 months (range 0–182 mo) and includes one patient who died before her first follow-up. Characteristics of tumor in treatment sites are summarized in Table 1. Most patients (79.2%) were treated for recurrent breast cancer on the chest wall. 76% had disease >3 cm in size. 43% had received prior RT to the treatment site. 54.3% of the patients had distant metastases at the time of treatment.
Table (1).
Tumor’s characteristics:
| Characteristics | N | % | |
|---|---|---|---|
| Tumor | |||
| Primary | 26 | 20.5 | |
| Recurrent | 101 | 79.5 | |
| Distant disease at presentation | |||
| Yes | 69 | 54.3 | |
| No | 58 | 45.7 | |
| Time to recurrence | |||
| > 2 years | 49 | 48.5 | |
| ≤ 2 years | 52 | 51.5 | |
| Tumor Stage at first presentation | |||
| I | 14 | 11.0 | |
| II | 51 | 40.2 | |
| III | 34 | 26.7 | |
| IV | 18 | 14.2 | |
| N/A | 10 | 7.9 | |
| Recurrence site | |||
| Breast | 31 | 24.4 | |
| Chest Wall/Skin | 86 | 67.7 | |
| Chest Wall/ Breast and lymph nodes | 10 | 7.9 | |
| Prior RT | |||
| Yes | 54 | 42.5 | |
| No | 73 | 57.5 | |
| Target size | |||
| Extensive (>3 cm) | 96 | 75.6 | |
| Gross (<3 cm) | 13 | 10.2 | |
| Microscopic (positive margins) | 18 | 14.2 |
Radiation therapy (RT)
Radiation therapy delivered is summarized in Table 2. Sixteen percent of patients could not achieve 40 Gy.
Table (2).
Treatment
| Radiation therapy | N | % | |
|---|---|---|---|
| 16 – 39.9 Gy | 20 | 15.7 | |
| 40 – 50 Gy | 53 | 41.7 | |
| > 50 Gy | 54 | 42.5 | |
| Original Surgery | |||
| Biopsy only | 24 | 18.9 | |
| Breast conservative surgery | 29 | 22.8 | |
| Mastectomy | 74 | 58.3 | |
| Chemotherapy | |||
| Yes | 72 | 56.7 | |
| No | 55 | 43.3 | |
| Interstitial hyperthermia | |||
| Yes | 31 | 24.4 | |
| No | 96 | 75.6 | |
| Hyperthermia sites | |||
| 1–2 | 122 | 96 | |
| 3–4 | 2 | 1.6 | |
| 5–6 | 1 | 0.8 | |
| N/A | 2 | 1.6 | |
| Applicators | |||
| MA-100 | 25 | 19.7 | |
| MA-120 | 69 | 54.3 | |
| MA-150 | 5 | 3.9 | |
| Combined applicators / others | 28 | 22 | |
| Number of measurements sites | |||
| < 5 | 11 | 8.7 | |
| 5 – 10 | 110 | 86.6 | |
| > 10 | 2 | 1.6 | |
| N/A | 4 | 3.1 | |
|
In Minutes Median (range) |
|||
| Median TED 42.5°C | |||
| In all patients | 208 (3, 910) | ||
| In controlled patients | 257 (3,788) | ||
| In uncontrolled patients | 193 (10,910) | ||
| Median TED 43°C | |||
| In all patients | 130 (2, 856) | ||
| In controlled patients | 157 (2,494) | ||
| In uncontrolled patients | 120 (7,856) | ||
Chemotherapy
Most patients had been treated with prior chemotherapy. Type and schedule of chemotherapy were not readily available for analysis. Concurrent chemotherapy was used at the discretion of the treating radiation oncologist and medical oncologist. Overall, 56.7% of patients received chemotherapy. Cisplatin-based regimens were often used for potential RT potentiation; however further details about all regimens’ exact dosing, treatment length, and drug combinations are not currently available to be reported.
Treatment Outcome
Treatment outcomes are detailed in Table (3). A CR during treatment was observed in 52.7% and a PR in 26.8%. A minor response was observed in 7.1% and 13.4% of patients showed no response or progressive disease. Two patients were treated by a salvage mastectomy. CR was significantly higher among patients who received a total median TED 42.5°C ≥ 200 minutes (p = 0.002), and median TED 43°C ≥ 100 minutes (p = 0.03). Patients who initially presented with early stage (I and II) disease prior to progression of breast cancer requiring hyperthermia treatment had a higher rate of CR versus those patients who initially presented with advanced stages disease (p= 0.002). Patients who achieved a CR were more likely to exhibit freedom of progression (79.4%) at their last follow up (p < 0.0001). 55.1% of the patients had local control, at a median follow-up of 13 months and a mean follow-up of 30 months (range: 0–182, SD: 38 months). All 18 patients treated for microscopic disease were locally controlled. Notably, only one patient had “0” months of follow; this patient is included per the predetermined study design that incorporated outcomes of all breast cancer patients who received hyperthermia within the study period and per the inclusion criteria. All 18 patients treated for microscopic disease were locally controlled. Using Fisher’s exact test, there was no significant difference in CR among patients who had prior RT (42.5%) versus those who did not (61.1%) (p = 0.49) (Figure 2).
Table (3).
Treatment outcomes
| N | % | ||
|---|---|---|---|
| Treatment response | |||
| CR | 67 | 52.7 | |
| PR | 34 | 26.8 | |
| Minor response | 9 | 7.1 | |
| No Response | 15 | 11.8 | |
| Progressive disease | 2 | 1.6 | |
| Freedom from local progression | |||
| Yes | 70 | 55.1 | |
| No | 55 | 43.3 | |
| N/A (mastectomy) | 2 | 1.6 | |
| Overall Survival (OS) all patients* | In months (95% CI) | ||
| Median OS duration | 16 (12, 21) | ||
| 1-years OS rate (%) | 58.35% (48.94%, 66.63%) |
||
| 2-years OS rate (%) | 40.09% (31.05%, 48.95%) |
||
| 3-years OS rate (%) | 29.59% (21.37%, 38.25%) |
||
| 5-years OS rate (%) | 22.05% (14.65%, 30.43%) |
||
| Median OS stratified by tumor status at the time of hyperthermia treatment | |||
| p = 0.27 | Primary tumor | 14 (8,16) | |
| Recurrent tumor | 21 (12,28) | ||
| Median OS stratified by radiation therapy (RT) dose | |||
| p < 0.0001 | RT <40Gy | 5 (1,20) | |
| RT 40–50Gy | 12 (8,19) | ||
| RT >50Gy | 28 (16,52) | ||
| Median OS stratified by initial stage at first presentation | |||
| p = 0.005 | I | 26 (8,110) | |
| II | 16 (8,33) | ||
| III | 22 (11,47) | ||
| IV | 13 (2,16) | ||
| Median OS stratified by Median TED 42.5°C | |||
| p = 0.003 | Median TED 42.5°C < 200 | 12 (5,21) | |
| Median TED 42.5°C ≥ 200 | 21 (15,41) | ||
| Median OS stratified by Median TED 43°C | |||
| p = 0.09 | Median TED 43°C < 100 | 12 (10,21) | |
| Median TED 43°C ≥ 100 | 27 (15,41) | ||
| Median OS stratified by local control | |||
| p = 0.0002 | Local Control | 22 (16,40) | |
| No Local Control | 9 (5,15) | ||
| Complete response | % | ||
| Complete response (% among patients with various initial stages at first presentation) | |||
| p = 0.002 | I | 64.3% | |
| II | 56.9% | ||
| III | 58.8% | ||
| IV | 11.1% | ||
| Complete response (% among patients with and without median TED 42.5°C ≥ 200 minutes) | |||
| p = 0.002 | Median TED 42.5°C < 200 minutes | 34.7% | |
| Median TED 42.5°C ≥ 200 minutes | 64.1% | ||
| Complete response (% among patients with and without median TED 43°C ≥ 100 minutes) | |||
| p = 0.03 | Median TED 43°C < 100 minutes | 37.5% | |
| Median TED 43°C ≥ 100 minutes | 64.6% | ||
| Complete response (% among patients who did or did not receive chemotherapy) | |||
| p = 0.99 | Chemotherapy | 51.6% | |
| No chemotherapy | 51.5% | ||
| Complete response (% among patients with and without local control) | |||
| P < 0.0001 | Local Control | 79.4% | |
| No Local Control | 18.9% | ||
Survival rates were estimated via the Kaplan-Meier method and median survival times were reported along with 95% confidence limit point estimates. Univariate analysis and comparisons between groups were conducted using the long-rank test, with a p-value of 0.05 indicating significance.
Figure (2).
Percentage of complete response (A) and Grade 3–4 treatment induced adverse events (B) in all patients, those who did not have prior RT, and those who did have prior RT.
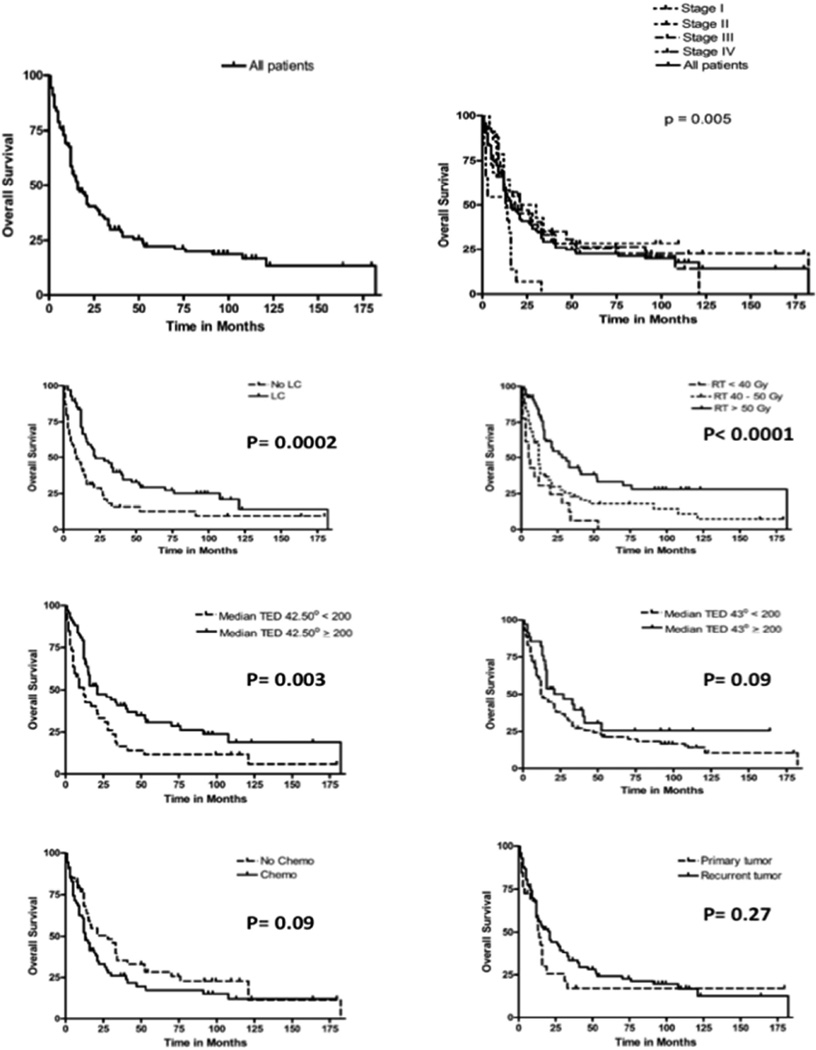
The median OS from treatment was 16 months (95% CI: 12–21 months), and the 1-year, 3-year, and 5-year OS were 58.35%, 29.59%, and 22.05%, respectively. Figure 3 shows OS in all patients, analyzed for radiation dose, hyperthermia dose, chemotherapy, and disease status. Overall survival was impacted significantly by local control, radiation dose, hyperthermia dose, and stage at first presentation. Table (4) lists the hazard ratios associated with risk of death. A microscopic disease, hyperthermia median TED 42.5°C, Radiation > 50Gy, and mastectomy resulted in statistical significant lower risk of death. Via a stepwise selection, another reduced multivariate model revealed that mastectomy (HR 0.622; 95% HR confidence limits 0.396–0.977; p = 0.0392), total radiation > 50 Gy (HR 0.360; 95% HR confidence limits 0.186–0.698; p=0.0025), and a microscopic target (HR 0.306; 95% HR confidence limits 0.138–0.681; p=0.0037) significantly decreased the risk of death. The median TED 42.5°C showed a trend towards decreasing the risk of death (HR 0.667; 95% HR confidence limits 0.431–1.033; p = 0.0697).
Figure (3).
Overall Survival (OS) curves for all patients and stratified by stage at first presentation, local control, Radiation Therapy (RT dose), Median TED 42.5°C, Median TED 43°C, chemotherapy, and primary versus recurrent tumor.
Table (4).
Multivariate analysis for the association between risk of death and various patient’s, tumor’s, and treatment’s characteristics:
| Parameter | Hazard Ratio* |
95% Hazard Ratio Confidence Limits |
p-value | |
|---|---|---|---|---|
| Age | 0.993 | 0.970 | 1.016 | 0.5389 |
| Recurrent disease | 0.456 | 0.115 | 1.800 | 0.2620 |
| Time to recurrence < / >2 years | 0.840 | 0.449 | 1.568 | 0.5833 |
| Chest wall only vs. nodal disease | 1.489 | 0.434 | 5.108 | 0.5267 |
| Breast vs. nodal disease | 0.889 | 0.230 | 3.435 | 0.8644 |
| Mastectomy | 0.399 | 0.194 | 0.820 | 0.0124 |
| Total Radiation: 40–50 Gy | 0.791 | 0.382 | 1.636 | 0.5271 |
| Total Radiation: > 50 Gy | 0.402 | 0.174 | 0.927 | 0.0326 |
| Number of treatment sites = 3,4 | 2.309 | 0.431 | 12.366 | 0.3283 |
| Number of treatment sites = 5,6 | 0.955 | 0.105 | 8.707 | 0.9677 |
| Target size: Gross | 0.448 | 0.180 | 1.113 | 0.0839 |
| Target size: Microscopic (positive margins) | 0.260 | 0.103 | 0.655 | 0.0043 |
| Median TED 42.5°C | 0.426 | 0.229 | 0.793 | 0.0071 |
| Median TED 43°C | 1.459 | 0.722 | 2.947 | 0.2923 |
A hazard ratio less than 1 indicates a decrease in risk and a hazard ratio greater than 1 indicates an increase in risk
Table (3) includes treatment related adverse events. Grade 3–4 desquamation was the most commonly reported treatment-induced adverse event (24.4% of the patients). Ulceration, telangiectasia, and fibrosis were reported in 6.7%, 4.7%, and 6.3% of the patients respectively. One patient had abscess formation. Using Fisher’s exact test, there was no significant difference in Grade 3–4 adverse events among patients who had prior RT (43.8%) versus those who did not (46.3%) (p = 0.86) (Figure 2).
Discussion
Locally advanced and recurrent breast cancer is often a difficult problem facing clinicians, if surgical resection is not possible. Visible or palpable cancer often adds to emotional distress, especially if associated with odor, discharge, or bleeding. Locoregional control in these patients may improve survival, but will enhance quality of life. When good local therapy (including re-irradiation) and optimal systemic treatment are not enough for local control -- either due to extent of local disease or in case of recurrent disease -- more potent strategies are needed for tumor control. In such cases, it becomes impossible to ignore the historic success hyperthermia has with control of refractory lesions.(12)
The mechanism of hyperthermia induced cell killing and tumor regression has been extensively investigated and is likely multifaceted. In addition to direct cell injury from the increased temperature itself (thought to cause damage to critical cytoskeletal and membrane proteins), there is a potentiating effect on radiation therapy.(4–7) This happens through multiple mechanisms. (1) There is better oxygenation of tumor cells due to increased blood flow. Additionally, there may also be a shift in tumor cell metabolism towards an anaerobic state. Together, both factors increase the amount of oxygen available for radiation induced oxygen-mediated damage (via reactive oxygen species) and prevent hypoxia-induced radioresistance.(13–16) (2) There is inhibition of tumor cell DNA repair.(17–19) Disabling the heat-sensitive DNA repair machinery prevents recovery from radiation-induced sublethal and potentially lethal damage, which enhances radiation induced cell kill. (3) Biologically the effects are complementary, yielding synergy when combined with radiotherapy in mechanism of tumor cell injury. Hyperthermia primarily damages proteins, causing cellular injury by denaturing critical cytoskeletal and membrane bound proteins. Also, this is mostly unaffected by oxygenation status or replicative activity. While radiation induced DNA damage is sometimes fully realized with next attempted mitotic activity, hyperthermia has no such cell-cycle limitation. It is as likely to damage both poorly and well differentiated cells and is as likely to injure a tumor cell in the relatively radioresistant S-phase of mitosis. Altogether, the synergy of the different mechanistic actions between the two techniques can cause more balanced tumor cell injury and cell kill, improving overall tumor control.
The clinical ability of improved tumor control with hyperthermia has been demonstrated in multiple sites of disease including breast cancer, head and neck cancer, cervical cancer, melanoma, and soft tissue sarcomas.(12) After an initial randomized controlled trial failed to demonstrate significant benefit (but for which hyperthermia methodology and quality assurance were strongly questioned), a large international cooperative study reported the positive combined results of five randomized control trials (conducted by 4 collaborative groups) that assessed adding hyperthermia to radiation when treating locally advanced or recurrent breast cancer.(20) The combined outcomes of the cohort of 306 patients demonstrated a significant benefit (59% vs 41%, p<0.001) in local control with the addition of hyperthermia, defined as the minimal intratumoral temperature of 43°C even though a significant portion of the study cohort did not meet this criterion.(21) The benefit was most pronounced in patients who had a recurrence in previously irradiated areas (57% vs 31%, OR 4.7, 95% CI 2.4–9.5), which could limit the ability and extent of re-irradiation. Notably, complete response achieved through hyperthermia was more prolonged than if achieved via radiation alone.
A more recent randomized trial of mostly breast cancer patients included rigorous and objective thermal dosimetry to ensure a well-defined hyperthermia treatment arm. 109 patients were randomized to radiation or radiation plus hyperthermia. Patients treated with hyperthermia were planned for at least a dose of 10 cumulative equivalent minutes at 43°C for 90% of measured points. This was in addition to conventionally fractionated external beam radiation to a dose of 60–70Gy or 30–66Gy (median: 41Gy) if previously irradiated. The proportion of patients receiving systemic treatment at the time did not differ between both arms. The complete response rate was significantly higher in patients treated with hyperthermia as opposed to radiation alone – 66.1% vs 42.65. Local control was also more durable with hyperthermia, 48% vs 25% at last follow-up or death. The odds ratio for CR was 2.7 (95%CI, 1.2 to 5.8; p=0.20) in the hyperthermia group. Previously irradiated patients showed the most pronounced benefit in local control (68% vs 24%), thereby suggesting that hyperthermia overcomes the limits of reduced dose in re-irradiation.(2) Use of chemotherapy a sensitizer did not influence response. Notably, 55% of patients maintained freedom from local progression for the duration of their life.
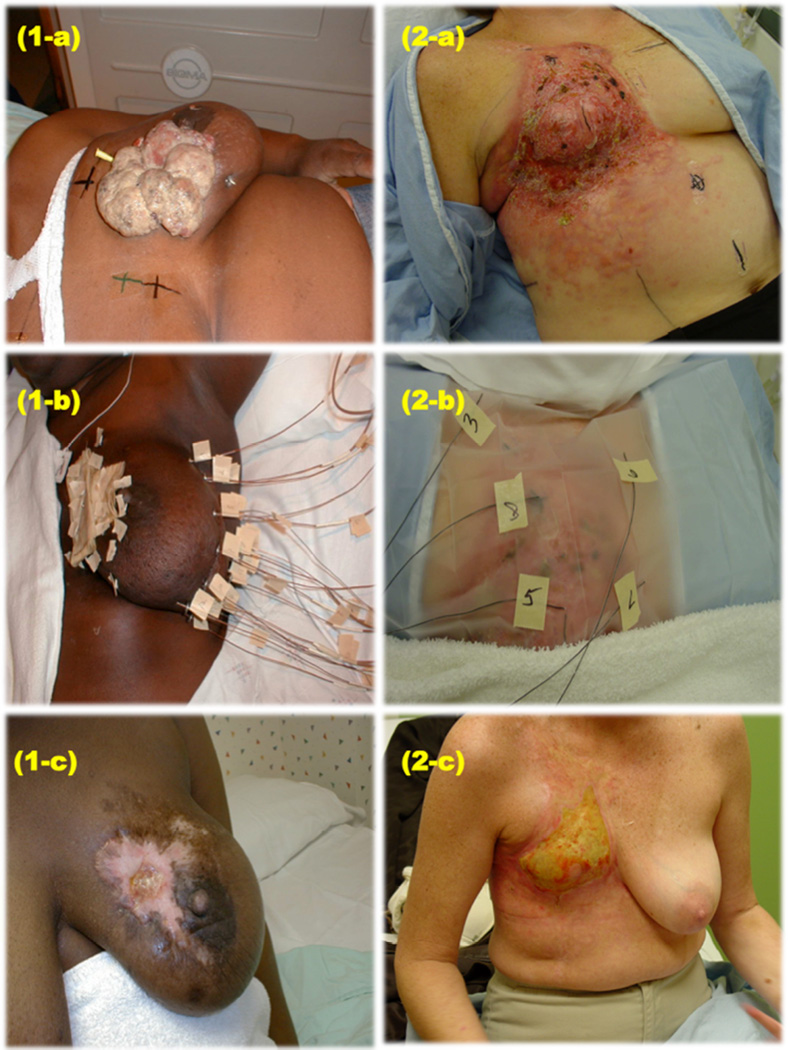
This study confirms the findings of the study by Jones et al.(2) We again demonstrate the utility of combining hyperthermia with radiation for achieving local control. The type of patients referred for hyperthermia in this series generally had difficult local disease to control with radiation and have been heavily treated with systemic therapy (examples, Figure 1). The OS illustrated in figure 3 and Table 3 suggests that survival is impacted by local control and local therapy. This study shows that quality of hyperthermia affects response – both temperature and duration of hyperthermia; the median TED 42.5°C and 43°C was higher in controlled patients and in patients with CR. The control rate in our series is in line with what has been reported by other modern series, despite the fact that patient selection (as opposed to randomization) might have prompted use of this technique for patients with the poorest clinician-predicted chance of local control (i.e. as opposed to radiation alone). Uniquely, our data are specific to women treated for breast cancer (either for a local recurrence or for advanced extent of disease) and in this scenario, add credence to the belief that durable local control might improve overall survival in this disease setting.
Figure (1).
(1-a) Patient A – locally advanced breast cancer, (1-b) Patient A – hyperthermia applicators insertion, (1-c) Patient A at 3 years of follow up, (2-a) Patient B – locally recurrent breast cancer, (2-b) Patient B – hyperthermia applicators insertion, (2-c) Patient B at 6 months of follow up.
To our knowledge, our patient cohort comprises one of the largest modern series to-date, examining the use of hyperthermia in the setting of breast cancer. Most patients were approached aggressively Moreover, with relatively preserved institutional technique and applicator usage among patients, our thermal dosimetric data objectively demonstrate that carefully applied (and measured) hyperthermia can benefit local control. The limitation of this study include it being a single institution retrospective study with a nonstandard approach to systemic treatment (with individualized treatment plans determined per patient suitability and physician preference); the exact details of dose, duration, regimen, etc. of this heterogeneous mix of regimens was not reported. We believe that studying the use of hyperthermia within the context of a controlled prospective clinical trial is required.
Hyperthermia is a well-studied but potentially under-utilized technique that can provide significant clinical benefit through the effects of improved local tumor control. It is known that hyperthermia complements radiation due (in part) to the fact that hyperthermia affects hypoxic and oxic cells equally (unlike radiation and chemotherapy) and because hyperthermia has different patterns of cell cycle toxicity than either radiation or many types of chemotherapy. In general, while the full mechanistic details of its actions are not completely understood, its synergistic addition in enhancing radiation-induced tumor cell kill has been well established. Much work has suggested but not established that hyperthermia may be denaturing DNA repair proteins and therefore acts to prevent DNA repair following treatment with DNA-damaging chemotherapy and radiation. With continued investigation into specific clinical roles and more widespread adoption of its usage, it can continue to establish itself as a powerful oncologic tool. In addition, as new tools become available to deliver hypothermia through the use of heat-labile liposomes and heat-delivering nanomaterials, use of hyperthermia coupled with radiation and chemotherapy may become more important in the anti-cancer therapeutic arsenal.(22–24)
Conclusion
Hyperthermia and radiation therapy is an effective combination in obtaining local control in a group of patients that have been historically difficult to treat by radiation. Overall survival was related to the ability to obtain local control, higher doses of radiation, and higher doses of hyperthermia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: There is no actual or potential conflict of interest with the production and publication of this work. No author has a direct or indirect commercial financial incentive associated with the publication of this article.
References
- 1.Newman EA, Guest AB, Helvie MA, Roubidoux MA, Chang AE, Kleer CG, et al. Changes in surgical management resulting from case review at a breast cancer multidisciplinary tumor board. Cancer. 2006 Nov 15;107(10):2346–2351. doi: 10.1002/cncr.22266. PubMed PMID: 16998942. [DOI] [PubMed] [Google Scholar]
- 2.Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, et al. Randomized trial of hyperthermia and radiation for superficial tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005 May 1;23(13):3079–3085. doi: 10.1200/JCO.2005.05.520. PubMed PMID: 15860867. [DOI] [PubMed] [Google Scholar]
- 3.Varma S, Myerson R, Moros E, Taylor M, Straube W, Zoberi I. Simultaneous radiotherapy and superficial hyperthermia for high-risk breast carcinoma: a randomised comparison of treatment sequelae in heated versus non-heated sectors of the chest wall hyperthermia. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2012;28(7):583–590. doi: 10.3109/02656736.2012.705216. PubMed PMID: 22946861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kampinga HH, Dikomey E. Hyperthermic radiosensitization: mode of action and clinical relevance. International journal of radiation biology. 2001 Apr;77(4):399–408. doi: 10.1080/09553000010024687. PubMed PMID: 11304434. [DOI] [PubMed] [Google Scholar]
- 5.Coss RA, Linnemans WA. The effects of hyperthermia on the cytoskeleton: a review. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 1996 Mar-Apr;12(2):173–196. doi: 10.3109/02656739609022507. PubMed PMID: 8926388. [DOI] [PubMed] [Google Scholar]
- 6.Streffer C. Metabolic changes during and after hyperthermia. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 1985 Oct-Dec;1(4):305–319. doi: 10.3109/02656738509029295. PubMed PMID: 2425020. [DOI] [PubMed] [Google Scholar]
- 7.Vidair CA, Doxsey SJ, Dewey WC. Heat shock alters centrosome organization leading to mitotic dysfunction and cell death. Journal of cellular physiology. 1993 Mar;154(3):443–455. doi: 10.1002/jcp.1041540302. PubMed PMID: 8436595. [DOI] [PubMed] [Google Scholar]
- 8.Franckena M, Stalpers LJ, Koper PC, Wiggenraad RG, Hoogenraad WJ, van Dijk JD, et al. Long-term improvement in treatment outcome after radiotherapy and hyperthermia in locoregionally advanced cervix cancer: an update of the Dutch Deep Hyperthermia Trial. International journal of radiation oncology, biology, physics. 2008 Mar 15;70(4):1176–1182. doi: 10.1016/j.ijrobp.2007.07.2348. PubMed PMID: 17881144. [DOI] [PubMed] [Google Scholar]
- 9.Overgaard J, Gonzalez Gonzalez D, Hulshof MC, Arcangeli G, Dahl O, Mella O, et al. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. European Society for Hyperthermic Oncology. Lancet. 1995 Mar 4;345(8949):540–543. doi: 10.1016/s0140-6736(95)90463-8. PubMed PMID: 7776772. [DOI] [PubMed] [Google Scholar]
- 10.Valdagni R, Amichetti M. Report of long-term follow-up in a randomized trial comparing radiation therapy and radiation therapy plus hyperthermia to metastatic lymph nodes in stage IV head and neck patients. International journal of radiation oncology, biology, physics. 1994 Jan 1;28(1):163–169. doi: 10.1016/0360-3016(94)90154-6. PubMed PMID: 8270437. [DOI] [PubMed] [Google Scholar]
- 11.Vernon CC, Hand JW, Field SB, Machin D, Whaley JB, van der Zee J, et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: results from five randomized controlled trials. International Collaborative Hyperthermia Group. International journal of radiation oncology, biology, physics. 1996 Jul 1;35(4):731–744. doi: 10.1016/0360-3016(96)00154-x. PubMed PMID: 8690639. [DOI] [PubMed] [Google Scholar]
- 12.van der Zee J. Heating the patient: a promising approach? Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2002 Aug;13(8):1173–1184. doi: 10.1093/annonc/mdf280. PubMed PMID: 12181239. [DOI] [PubMed] [Google Scholar]
- 13.Sijens PE, Bovee WM, Seijkens D, Koole P, Los G, van Rijssel RH. Murine mammary tumor response to hyperthermia and radiotherapy evaluated by in vivo 31P-nuclear magnetic resonance spectroscopy. Cancer research. 1987 Dec 15;47(24 Pt 1):6467–6473. PubMed PMID: 3677087. [PubMed] [Google Scholar]
- 14.Song CW, Park H, Griffin RJ. Improvement of tumor oxygenation by mild hyperthermia. Radiation research. 2001 Apr;155(4):515–528. doi: 10.1667/0033-7587(2001)155[0515:iotobm]2.0.co;2. PubMed PMID: 11260653. [DOI] [PubMed] [Google Scholar]
- 15.Oleson JR. Eugene Robertson Special Lecture. Hyperthermia from the clinic to the laboratory: a hypothesis. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 1995 May-Jun;11(3):315–322. doi: 10.3109/02656739509022467. PubMed PMID: 7636318. [DOI] [PubMed] [Google Scholar]
- 16.Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Dodge RK, Charles HC, et al. Radiation therapy and hyperthermia improve the oxygenation of human soft tissue sarcomas. Cancer research. 1996 Dec 1;56(23):5347–5350. PubMed PMID: 8968082. [PubMed] [Google Scholar]
- 17.Raaphorst GP, Yang DP, Ng CE. Effect of protracted mild hyperthermia on polymerase activity in a human melanoma cell line. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 1994 Nov-Dec;10(6):827–834. doi: 10.3109/02656739409012375. PubMed PMID: 7884242. [DOI] [PubMed] [Google Scholar]
- 18.Raaphorst GP, Ng CE, Yang DP. Thermal radiosensitization and repair inhibition in human melanoma cells: a comparison of survival and DNA double strand breaks. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 1999 Jan-Feb;15(1):17–27. doi: 10.1080/026567399285828. PubMed PMID: 10193754. [DOI] [PubMed] [Google Scholar]
- 19.Mivechi NF, Dewey WC. DNA polymerase alpha and beta activities during the cell cycle and their role in heat radiosensitization in Chinese hamster ovary cells. Radiation research. 1985 Sep;103(3):337–350. PubMed PMID: 4041063. [PubMed] [Google Scholar]
- 20.Perez CA, Pajak T, Emami B, Hornback NB, Tupchong L, Rubin P. Randomized phase III study comparing irradiation and hyperthermia with irradiation alone in superficial measurable tumors. Final report by the Radiation Therapy Oncology Group. American journal of clinical oncology. 1991 Apr;14(2):133–141. doi: 10.1097/00000421-199104000-00008. PubMed PMID: 1903023. [DOI] [PubMed] [Google Scholar]
- 21.Sherar M, Liu FF, Pintilie M, Levin W, Hunt J, Hill R, et al. Relationship between thermal dose and outcome in thermoradiotherapy treatments for superficial recurrences of breast cancer: data from a phase III trial. International journal of radiation oncology, biology, physics. 1997 Sep 1;39(2):371–380. doi: 10.1016/s0360-3016(97)00333-7. PubMed PMID: 9308941. [DOI] [PubMed] [Google Scholar]
- 22.Kong G, Anyarambhatla G, Petros WP, Braun RD, Colvin OM, Needham D, et al. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: importance of triggered drug release. Cancer research. 2000 Dec 15;60(24):6950–6957. PubMed PMID: 11156395. [PubMed] [Google Scholar]
- 23.Kumar CS, Mohammad F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Advanced drug delivery reviews. 2011 Aug 14;63(9):789–808. doi: 10.1016/j.addr.2011.03.008. PubMed PMID: 21447363. Pubmed Central PMCID: 3138885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JH, von Maltzahn G, Xu MJ, Fogal V, Kotamraju VR, Ruoslahti E, et al. Cooperative nanomaterial system to sensitize, target, and treat tumors. Proceedings of the National Academy of Sciences of the United States of America. 2010 Jan 19;107(3):981–986. doi: 10.1073/pnas.0909565107. PubMed PMID: 20080556. Pubmed Central PMCID: 2824295. [DOI] [PMC free article] [PubMed] [Google Scholar]