Abstract
In Parkinson’s disease (PD), dopamine neurons in the substantia nigra are degenerated and lost. Cell therapy for PD replaces the lost dopamine neurons by transplanting donor dopamine neural progenitor cells. Cell therapy for PD has been performed in the clinic since the 1980s and uses donor cells from the mesencephalon of aborted embryos. Regenerative medicine for PD using induced pluripotent stem (iPS) cell technology is drawing attention, because it offers a limitless and more advantageous source of donor cells than aborted embryos.
Keywords: Parkinson, dopamine, induced pluripotent stem cell, midbrain
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder that is especially common in the elderly. For this reason, number of patients is expected to surge with aging societies.1,2) PD patients suffer from tremors, rigidity, bradykinesia, and other debilitating symptoms due to the progressive loss of dopaminergic neurons in the substantia nigra. The most standard treatment for PD is medication with L-dopa. L-dopa is used because unlike dopamine it can pass the blood-brain barrier. There, it is converted to dopamine by dopamine neurons. L-dopa is an effective treatment initially, but the progressive loss of dopamine neurons results in the patient’s inability to convert the drug to dopamine regardless of the dosage. Furthermore, patients show side effects, such as on-off phenomena, wearing-off, and dyskinesia. Non-pharmacological therapy, such as deep brain stimulation (DBS),3) gene therapy,4) and cell therapy with stem cells, the main interest of this review, have also been explored.
I. Cell therapy using ventral mesencephalon (VM) from aborted embryos
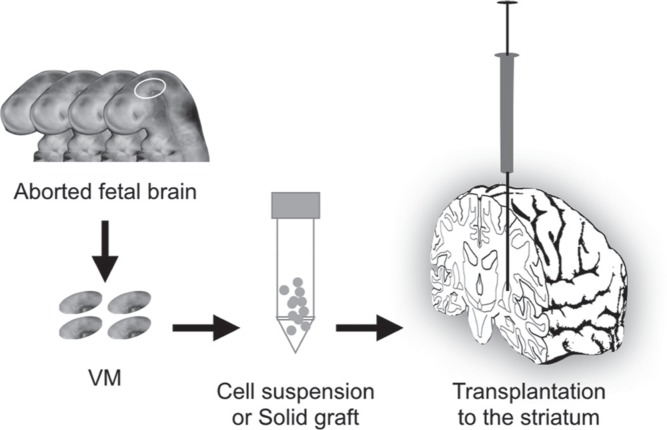
Clinical cell therapy for PD has commonly used fetal VM as the donor source (Fig. 1). Usually, the donor cells are transplanted into the striatum, which is the physiological target of dopamine neurons and is where they are expected to release dopamine locally. The release is dependent on the graft-derived dopamine neurons making local synaptic connections with the host neurons.5) The dopamine is stored in the vesicles of the transplanted cells, and its release is regulated by dopamine transporters in the synaptic terminals. Because the transplanted dopamine neurons convert the administrated L-dopa to dopamine, they function as an effector of the drug therapy. Since the 1980s, animal experiments have shown that transplanted neurons from aborted brain tissue can survive in the striatum after transplantation. Because the brain is considered an immune privileged site,6) it is relatively permissive tissue for immune reactions, which could explain why cell therapy for the brain was successful.7)
Fig. 1.
Fetal ventral mesencephalon (VM) transplantation. VMs from aborted fetuses are dissected, and the tissues are prepared as solid grafts or dissociated cell suspensions. The resulting product is injected through a needle into the patient’s putamen.
Since the late 1980s, clinical trials of cell therapy for PD using fetal VM were begun in the United States and Europe, using around 400 patients. In early open-label trials,8–11) some cases were reported to show markedly improved symptoms and 18F-fluorodopa and 11C-raclopride positron emission tomography (PET) scans have indicated long-lasting survival and functionality of the grafts in these patients.18) The positive results led to two double blind placebo control trials performed in the United States.12,13) However, in the Colorado/Columbia trial, improvement was seen only in younger patients (60 years old or younger) with the standardized test and the motor component of the Unified Parkinson’s disease rating scale (UPDRS), which revealed a 34% improvement in the transplantation group.12) In the Tampa study,13) cells from either one or four donors per side (aged 6–9 weeks) were transplanted and stratification by the median baseline UPDRS score (≦49) indicated that patients with less severe disease who received transplants from four donors improved by a mean of 1.5 [standard deviation (SD) 4.2] points on the UPDRS, compared with a deterioration of 21.4 (SD 43) points in the sham group. However, it was also found that 15%12) to 57%13) of patients in the treated group of these two clinical studies developed graft-induced dyskinesia. Encouragingly, long-term observations and autopsies of grafted patients have revealed that the grafts survived for more than 10 years in patients’ brains,11,14–16) and functional imaging, such as PET, indicated that the grafted cells were functioning as dopamine neurons throughout this period.17,18) Moreover, some patients who received the transplantation had no need for medication after grafting. On the other hand, some reports found Lewy body-like structures in the grafted dopamine neurons. Lewy bodies are abnormal aggregates of proteins and often found in PD.14,15) These aggregates are mainly composed of phosphorylated alpha-synuclein and thought to spread to the grafted dopamine neurons after transplantation. However, the Lewy body-like structures in the grafts were not observed in all patients16,19) and usually found in less than 1–5% of the transplanted neurons.14,15) This finding triggered extensive research on the relationship between alpha-synuclein propagation and PD pathology.20) The effects of Lewy bodies on the function of the grafted neurons, however, remain unclear. The contrasting results using fetal VM transplants indicate that while this approach is effective in some PD cases, the technique still requires improvements with regard to preparation of the donor tissue and immune response.
II. Fundamental problem in fetal VM transplantation
Regardless of the positive effects from cell therapy using aborted embryonic tissue, sufficient donor tissue for the transplantation is difficult to acquire. To get enough cells for one patient, 4 to 10 aborted embryos are needed.13) At the same time, there is an optimal time window (from 9 to 11 gestational weeks) to acquire effective donor cells. Furthermore, there is no established method for the cryopreservation of dopamine neurons in embryonic tissue. Although embryonic tissue can be stored at 4°C for 1 week, the quality of the donor cells reduces over that time. From the ejected brain tissues of the embryo, VM are dissected with scissors and forceps, collected, and often dissociated by enzyme into single cells or cell clumps. These cells are suspended in solution and prepared for the transplantation (Fig. 1). Practically speaking, cells taken from fresh tissues and 1-week stored tissues are mixed for the transplantation. Because the donor cells are a mixture of several embryos, it is also difficult to perform human leukocyte antigen (HLA) typing or examine mixed lymphocyte reaction (MLR). Consequently, it is not easy to standardize the donor quality.
III. Stem cells as the donor source
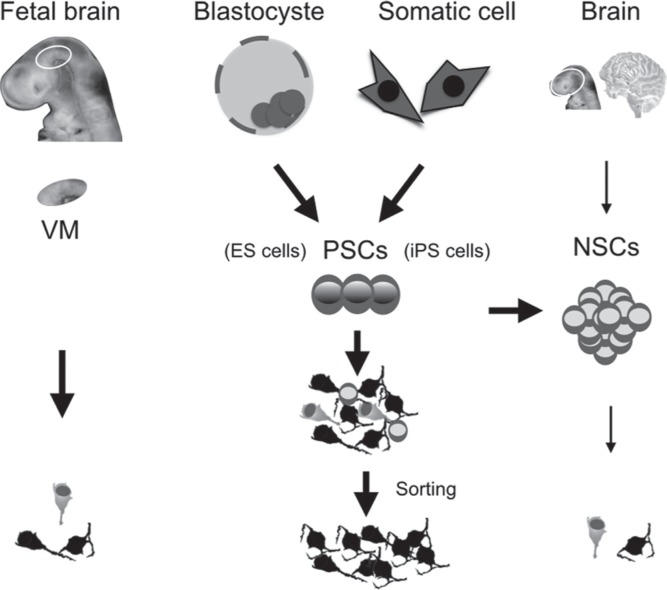
Instead of fetal mesencephalon as the donor source, dopamine neurons can be obtained from neural stem cells (NSCs) or pluripotent stem cells (PSCs; Fig. 2).
Fig. 2.
Donor candidates for cell therapy of Parkinson’s disease. Several sources exist for donor cells. ES cells: embryonic stem cells, iPS cells: induced pluripotent stem cells, NSCs: neural stem cells, PSCs: pluripotent stem cells, VM: ventral mesencephalon.
a. Somatic stem cells and PSCs
Stem cells are defined as cells that have both multipotency and self-renewal. Multipotency describes the ability to differentiate into several different types of cells; self-renewal describes the ability to divide and proliferate into the same cell type. Stem cells can be further divided into somatic stem cells and PSCs.21,22) Somatic stem cells have a limited lineage of differentiation. For example NSCs can differentiate only into neurons, astrocytes, and oligodendrocytes, but never into hepatocytes, cardiac muscles, or other cell types unrelated to the neural system.23) Therefore, somatic stem cells are said to have multipotency. In contrast, the ability to differentiate into any type of cell in the whole body is known as pluripotency, a feature seen in PSCs. Also, although somatic stem cells have the ability of self-renewal, their proliferation activity is lower than that of PSCs.
b. NSCs
In human brain, NSCs exist in the developing nervous system and some parts of the adult brain, such as the hippocampal dentate gyrus and subventricular zone,24,25) and possibly the olfactory bulb. Recently, it was reported that interneurons in the adult human striatum have turnover capacity, which is in contrast with those in rodents.26) For cell therapy purposes, NSCs are typically cultured and expanded in a culture medium supplemented with mitogenic growth factors, such as basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF).23,27) In general, it is difficult to acquire and expand enough NSCs from the brain in vitro to a sufficient number of donor cells. In particular, an efficient induction method from NSCs to mesencephalic dopamine neurons on large scale for clinical application is lacking. It should be noted that in the neural field, regenerative medicine and other medical research primarily aim to reduce neural inflammation and decelerate neural degeneration through the effects of neurotrophic factors secreted by the transplanted stem cells, but not to reconstruct the neural circuit.
c. Induced pluripotent stem (iPS) cells
Like stem cells, PSCs have been broken down into two groups: embryonic stem (ES) cells and iPS cells (Fig. 2). Evans and Kaufman reported the first mouse ES cell in 1981.21) It took another 17 years for human ES cells to be reported by Thomson et al.28) Mouse iPS cells came even later (2006), and human iPS cells soon after (2007), with both reported by the Yamanaka lab.22,29) iPS cells are derived from somatic cells through the transfection of a combination of reprograming factors. The first reported human iPS cells involved the transfection of four genes (Oct3/4, Sox2, KLF4, c-Myc) by retrovirus in adult human fibroblasts.29) Because c-Myc is an oncogene and these original iPS cells needed mouse-derived feeder cells in the culture, they were not practical for clinical application. However, more recent protocols for the establishment and maintenance of iPS cells are both safer and more effective.30–32) Now, researchers can prepare iPS cells from peripheral blood cells, which is less invasive than skin biopsy, by gene transfection without genome insertion or the use of oncogenes like c-Myc, and without the use of feeder cells.32,33) Consequently, iPS cells compatible with clinical application are now available.
IV. Cell therapy using iPS cells
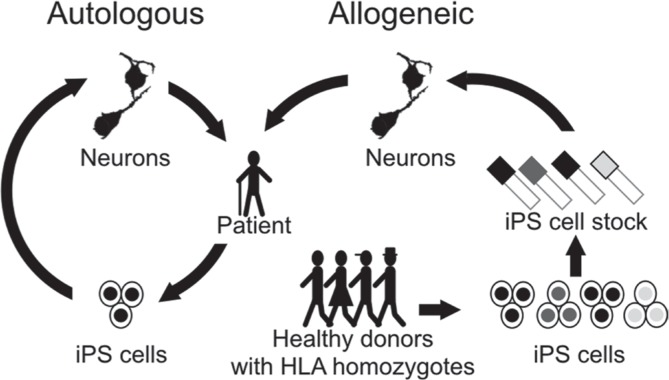
There are several advantages to iPS cells over ES cells. First, iPS cells are established without sacrificing human zygotes, which removes the biggest ethical obstacle against human ES cell studies. The possibility of autologous transplantation is also an advantage of iPS cells.34) In autologous transplantation, the patient’s own somatic cells are used as the original cells. These cells are reprogrammed to iPS cells and then differentiated into the cells required for transplantation. The resulting differentiated cells are expected to have identical HLAs as the patient, meaning that major graft rejection theoretically should not occur. Although the brain is considered an immunologically privileged site, we have shown that there is a difference between autologous cell transplantation and major histocompatibility complex (MHC)-mismatched transplantation.34) Additionally, while autologous cell therapy is ideal theoretically, reprograming the original cells to iPS cells and then preparing them to donor cells from each patient is burdened by high cost and time. As an alternative, Kyoto University has launched the “Stock Project,” which involves the collection of different iPS cell lines from HLA-homozygous donors (Fig. 3). It has been estimated that 50 lines of HLA-homozygous iPS cells will cover 73% of the Japanese population with the matching of three loci (HLA-A, B, and DR).32) However, other minor HLAs, non-HLAs, or the innate immune system, such as macrophage and NK cells, could also contribute to immune reactions. Overall, researchers need to consider the advantages and disadvantages of both autologous and HLA-matched allogeneic transplantation before deciding the cell origin.
Fig. 3.
Two strategies of iPS cell therapy. In autologous transplantation, the patient’s own iPS cells are differentiated into the donor cells. In allogeneic transplantation, HLA-matched transplantation is used. iPS cell lines with a variety of HLA homozygotes are stocked, and the cell line with HLAs that match the patient’s is selected to produce the donor cells. HLA: human leukocyte antigen, iPS: induced pluripotent stem.
V. Induction of dopamine neurons from iPS cells
An important feature of iPS cells is that the same protocol that induces dopamine neurons from ES cells can be used. Generally there are two methods for neural induction from PSCs. One uses a mouse stromal cell line as feeder cells, which have stromal cell derived-inducing activity (SDIA).35) In the other method, PSCs are cultured in cell aggregates, like embryoid bodies (EBs), suspended in the culture medium.36) Making EBs is the standard experimental technique used to show the pluripotency of PSCs in vitro. Recently it was reported that dual inhibition of the BMP and activin signaling cascades induces neural differentiation efficiently.37,38) This dual SMAD inhibition method is now standard for the neural differentiation of PSCs. Dopamine neural progenitors are located in the VM of the developing brain. Using this knowledge, the dual SMAD inhibition method has been optimized with cytokines that function to pattern brain development. These cytokines include sonic hedgehog (SHH), fibroblast growth factor 8 (FGF8), and glycogen synthase kinase 3 beta (GSK3B) inhibitor, which are added to the medium during specific periods of the induction.37,38)
VI. Effect of the graft
As mentioned above, cell therapy with fetal VM for PD has proven effective, especially for patients in the early stage of the disease. Accordingly, if the donor dopamine neural progenitors prepared from stem cells have the same quality as those from fetal VM, one would expect similar gains in PD therapy. However, demonstrating equality between cells taken from fetal VM and those derived from stem cells is not trivial. To begin, the cells must be compared in vitro and in vivo. The function of donor cells in vivo can be tested with PD model animals. Typical PD animal models have their midbrain-striatum dopamine systems selectively destroyed by neurotoxins, such as 6-hydroxydopamine (6-OHDA) for rats and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) for monkeys and mice. The monkey PD model is of special interest because it most resembles human PD patients. For this reason it is preferable to use monkeys in preclinical studies.39,40) Many reports have shown that donor cells derived from iPS cells can improve the motor symptoms of PD animal models.41,42) More importantly, histology of these animals has shown that the grafted dopamine neurons survived and stretched fibers from the graft to the host striatum. Furthermore, iPS cells derived from PD patients could be differentiated to proper dopamine neurons and restore the motor function of a PD model after transplantation.41) This result ensured the effectiveness of autologous cell therapy for PD with iPS cell technology, even though these cells should have higher susceptibility to the disease.
VII. Risk management for clinical application
Any clinical application of an iPS cell-based therapy demands containing the risk of infection and tumorigenicity of the iPS cell-derived donor cells. To that purpose, we need a stable and reproducible differentiation system.
1. Infection
Considering the risk of infection, it is best to avoid materials from non-human animals (xeno-materials) not only for the establishment of iPS cells but also for each step of the neural differentiation. Furthermore, all materials should have sufficient traceability, so that the raw materials can be reevaluated if necessary. To prepare cells with sufficient quality for clinical use demands a cell-processing center (CPC) with good manufacture practice (GMP). An appropriate CPC will have higher costs than the common cell culture rooms typically found in laboratories.
2. Tumorigenicity
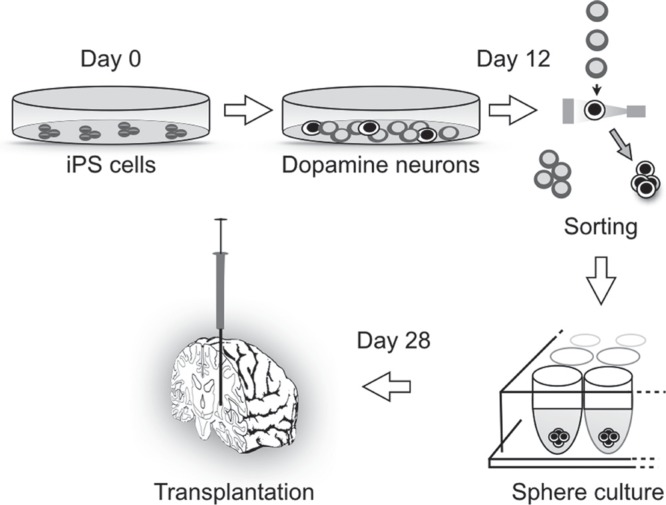
Tumorigenicity is a major problem for any PSC-based therapy. In the past, grafted human cells had sometimes proliferated and formed masses in animal brains that compressed the surrounding brain structure, but that was when differentiation protocols were still relatively unproven.40,43) Even in those cases, malignant features such as metastasis and invasion were never observed. The cause of the tumor formation was attributed to residual immature cells in the graft that had high proliferation activity. Residual PSCs in the donor cells can cause teratoma in the brain, but recent improvements in induction technology have minimized this problem. Still, even if the cells are differentiated into the neural lineage, there is a risk of neural overgrowth of immature proliferative neural cells. Therefore, the preparation of donor cells from PSCs requires control of the direction of the cell phenotype and the extent of maturation.40) If the transplanted neural cells are too mature, however, the rate of survival after grafting decreases. Purification by sorting with antibody has proven effective in preparing donor cells with appropriate maturation and proper phenotype. We are currently using the antibody for Corin to enrich neural progenitors of the midbrain dopaminergic phenotype.42) Corin, a transmembrane serine protease, is expressed in the floor plate of the embryonic brain where dopamine neural progenitors locate. During the differentiation of dopamine neurons from human iPS cells, Corin emerges at day 7 and reaches peak level at 1 month, after which it decreases its expression. In our protocol, we purify Corin positive cells at days 12–14 with fluorescent activated cell sorting (FACS). An immunofluorescence study revealed that the percentage of midbrain dopamine progenitor cells that were LMX1A/FOXA2 positive increased in the Corin positive cell population compared with unsorted cells (75.5% ± 8.2% vs. 47.3% ± 6.6%).42) Thereafter, we continue differentiation of the sorted cells until day 28 (Fig. 4). We have found that transplanting the donor cells after this purification step leads to the survival of more dopamine neurons (tyrosine hydroxylase positive cells: 6,747 ± 2,341 vs. 3,436 ± 2,384 cells/graft) and less proliferation (Ki67 positive cells: 0.06% ± 0.07% vs. 0.86% ± 0.53%), resulting in smaller graft size (3.4 ± 2.9 mm3 vs. 35.0 ± 37.5 mm3).42)
Fig. 4.
iPS cell therapy for Parkinson’s disease. About 12 days after starting the differentiation, the cell population is composed of dopamine neurons and other types of neural cells. The dopamine neurons are sorted by fluorescent activated cell sorting (FACS) with Corin antibody staining. After maturation, the iPS cell-derived dopamine neurons are transplanted at day 28 of differentiation. iPS: induced pluripotent stem.
3. Graft-induced dyskinesia
As mentioned above, L-dopa therapy has the unfortunate side effect of dyskinesia. Dyskinesia has also been reported as a side effect of fetal VM grafts (graft-induced dyskinesia).13,44) The cause of graft-induced dyskinesia has been a matter of debate. These side effects were observed in only some individuals, all of whom had levodopa-induced dyskinesia before grafting. Regarding the mechanisms of graft-induced dyskinesia, two theories have been advanced. The first idea posits that the dyskinesia arises from a non-homogeneous delivery of dopamine cells across the putamen, resulting in striatal dopaminergic hotspots.45–47) The second proposal is that serotonergic neurons in the transplant could be releasing dopamine in an unregulated manner as a false transmitter.48) To avoid this side effect, we have taken care to exclude serotonergic neurons from the donor cell population. Sorting for CORIN+ cells can improve the exclusion, as the percentages of serotonin+ cells per total neurons were 2.5% ± 2.5% and 1.2% ± 0.8% for the unsorted and the day 12 CORIN+ cells, respectively.42) Another hypothesis has the cause of the dyskinesia in the host patients, as patients that show severe fluctuations in symptoms of PD before surgery show a higher risk of graft-induced dyskinesia.
VIII. Plans for clinical study
PD comes in two forms, sporadic and familial, and it is unclear if the two forms share the same mechanisms.1) It is believed that about 10% of PD patients have some mutation in PD-related genes. With no resolution to this argument yet, we have decided to initially limit our clinical study to sporadic patients. The criteria of the patient selection will include the duration of the disease (≧5 years), the phase of the disease (Hoehn & Yahr score at On period ≦III without significant LIDs and that of Off period ≧III), the age at the time of inclusion (50–70 years old), and the response to levodopa (more than 30% improvement in UPDRS-III). The primary endpoint of this study will be the assessment of tumor formation or graft overgrowth by magnetic resonance imaging (MRI) or PET. The secondary endpoint will be the improvement of the neurological status (based on the UPDRS) at the off state and the uptake of F-DOPA as measured by PET. The preparation of donor cells will be performed in a specialized facility at Kyoto University, FiT (Facility for iPS Cell Therapy).
Conclusion
Clinical experience of fetal VM transplantation for PD will make this disease the first neuronal disease to be treated with iPS cell-based therapy. iPS cell-based therapy should provide better quality therapy than fetal VM transplantation and to a much larger number of patients. Following this iPS cell-based PD therapy, it is expected that similar treatments for other CNS disorders, including spinal cord injury, Alzheimer’s disease, Huntington’s disease, epilepsy and more, will soon follow.
Acknowledgments
The authors thank Dr. Peter Karagiannis for reading the manuscript. This study was supported by the following grants: a grant from the Highway Project for Realization of Regenerative Medicine from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and a grant from the Research Center Network for Realization of Regenerative Medicine from the Japan Agency for Medical Research and Development (AMED).
References
- 1). Samii A, Nutt JG, Ransom BR: Parkinson’s disease. Lancet 363: 1783– 1793, 2004. [DOI] [PubMed] [Google Scholar]
- 2). Lees AJ, Hardy J, Revesz T: Parkinson’s disease. Lancet 373: 2055– 2066, 2009. [DOI] [PubMed] [Google Scholar]
- 3). Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, Daniels C, Deutschländer A, Dillmann U, Eisner W, Gruber D, Hamel W, Herzog J, Hilker R, Klebe S, Kloss M, Koy J, Krause M, Kupsch A, Lorenz D, Lorenzl S, Mehdorn HM, Moringlane JR, Oertel W, Pinsker MO, Reichmann H, Reuss A, Schneider GH, Schnitzler A, Steude U, Sturm V, Timmermann L, Tronnier V, Trottenberg T, Wojtecki L, Wolf E, Poewe W, Voges J, German Parkinson Study Group , Neurostimulation Section: A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med 355: 896– 908, 2006. [DOI] [PubMed] [Google Scholar]
- 4). Palfi S, Gurruchaga JM, Ralph GS, Lepetit H, Lavisse S, Buttery PC, Watts C, Miskin J, Kelleher M, Deeley S, Iwamuro H, Lefaucheur JP, Thiriez C, Fenelon G, Lucas C, Brugières P, Gabriel I, Abhay K, Drouot X, Tani N, Kas A, Ghaleh B, Le Corvoisier P, Dolphin P, Breen DP, Mason S, Guzman NV, Mazarakis ND, Radcliffe PA, Harrop R, Kingsman SM, Rascol O, Naylor S, Barker RA, Hantraye P, Remy P, Cesaro P, Mitrophanous KA: Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson’s disease: a dose escalation, open-label, phase 1/2 trial. Lancet 383: 1138– 1146, 2014. [DOI] [PubMed] [Google Scholar]
- 5). Mahalik TJ, Finger TE, Stromberg I, Olson L: Substantia nigra transplants into denervated striatum of the rat: ultrastructure of graft and host interconnections. J Comp Neurol 240: 60– 70, 1985. [DOI] [PubMed] [Google Scholar]
- 6). MEDAWAR PB: Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol 29: 58– 69, 1948. [PMC free article] [PubMed] [Google Scholar]
- 7). Barker RA, Widner H: Immune problems in central nervous system cell therapy. NeuroRx 1: 472– 481, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Brundin P, Pogarell O, Hagell P, Piccini P, Widner H, Schrag A, Kupsch A, Crabb L, Odin P, Gustavii B, Björklund A, Brooks DJ, Marsden CD, Oertel WH, Quinn NP, Rehncrona S, Lindvall O: Bilateral caudate and putamen grafts of embryonic mesencephalic tissue treated with lazaroids in Parkinson’s disease. Brain 123 (Pt 7): 1380– 1390, 2000. [DOI] [PubMed] [Google Scholar]
- 9). Hagell P, Brundin P: Cell survival and clinical outcome following intrastriatal transplantation in Parkinson disease. J Neuropathol Exp Neurol 60: 741– 752, 2001. [DOI] [PubMed] [Google Scholar]
- 10). Hauser RA, Freeman TB, Snow BJ, Nauert M, Gauger L, Kordower JH, Olanow CW: Long-term evaluation of bilateral fetal nigral transplantation in Parkinson disease. Arch Neurol 56: 179– 187, 1999. [DOI] [PubMed] [Google Scholar]
- 11). Mendez I, Dagher A, Hong M, Hebb A, Gaudet P, Law A, Weerasinghe S, King D, Desrosiers J, Darvesh S, Acorn T, Robertson H: Enhancement of survival of stored dopaminergic cells and promotion of graft survival by exposure of human fetal nigral tissue to glial cell line—derived neurotrophic factor in patients with Parkinson’s disease. Report of two cases and technical considerations. J Neurosurg 92: 863– 869, 2000. [DOI] [PubMed] [Google Scholar]
- 12). Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, Eidelberg D, Fahn S: Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med 344: 710– 719, 2001. [DOI] [PubMed] [Google Scholar]
- 13). Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, Shannon KM, Nauert GM, Perl DP, Godbold J, Freeman TB: A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol 54: 403– 414, 2003. [DOI] [PubMed] [Google Scholar]
- 14). Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Björklund A, Widner H, Revesz T, Lindvall O, Brundin P: Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med 14: 501– 503, 2008. [DOI] [PubMed] [Google Scholar]
- 15). Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW: Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med 14: 504– 506, 2008. [DOI] [PubMed] [Google Scholar]
- 16). Hallett PJ, Cooper O, Sadi D, Robertson H, Mendez I, Isacson O: Long-term health of dopaminergic neuron transplants in Parkinson’s disease patients. Cell Rep 7: 1755– 1761, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Ma Y, Tang C, Chaly T, Greene P, Breeze R, Fahn S, Freed C, Dhawan V, Eidelberg D: Dopamine cell implantation in Parkinson’s disease: long-term clinical and (18)F-FDOPA PET outcomes. J Nucl Med 51: 7– 15, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Kefalopoulou Z, Politis M, Piccini P, Mencacci N, Bhatia K, Jahanshahi M, Widner H, Rehncrona S, Brundin P, Björklund A, Lindvall O, Limousin P, Quinn N, Foltynie T: Long-term clinical outcome of fetal cell transplantation for Parkinson disease: two case reports. JAMA Neurol 71: 83– 87, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Mendez I, Viñuela A, Astradsson A, Mukhida K, Hallett P, Robertson H, Tierney T, Holness R, Dagher A, Trojanowski JQ, Isacson O: Dopamine neurons implanted into people with Parkinson’s disease survive without pathology for 14 years. Nat Med 14: 507– 509, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Brundin P, Melki R, Kopito R: Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol 11: 301– 307, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Evans MJ, Kaufman MH: Establishment in culture of pluripotential cells from mouse embryos. Nature 292: 154– 156, 1981. [DOI] [PubMed] [Google Scholar]
- 22). Takahashi K, Yamanaka S: Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663– 676, 2006. [DOI] [PubMed] [Google Scholar]
- 23). Gage FH, Ray J, Fisher LJ: Isolation, characterization, and use of stem cells from the CNS. Annu Rev Neurosci 18: 159– 192, 1995. [DOI] [PubMed] [Google Scholar]
- 24). Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH: Neurogenesis in the adult human hippocampus. Nat Med 4: 1313– 1317, 1998. [DOI] [PubMed] [Google Scholar]
- 25). Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Boström E, Westerlund I, Vial C, Buchholz BA, Possnert G, Mash DC, Druid H, Frisén J: Dynamics of hippocampal neurogenesis in adult humans. Cell 153: 1219– 1227, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, Possnert G, Druid H, Frisén J: Neurogenesis in the striatum of the adult human brain. Cell 156: 1072– 1083, 2014. [DOI] [PubMed] [Google Scholar]
- 27). Ostenfeld T, Svendsen CN: Requirement for neurogenesis to proceed through the division of neuronal progenitors following differentiation of epidermal growth factor and fibroblast growth factor-2-responsive human neural stem cells. Stem Cells 22: 798– 811, 2004. [DOI] [PubMed] [Google Scholar]
- 28). Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM: Embryonic stem cell lines derived from human blastocysts. Science 282: 1145– 1147, 1998. [DOI] [PubMed] [Google Scholar]
- 29). Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S: Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861– 872, 2007. [DOI] [PubMed] [Google Scholar]
- 30). Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S: Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol 26: 101– 106, 2008. [DOI] [PubMed] [Google Scholar]
- 31). Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S: Generation of mouse induced pluripotent stem cells without viral vectors. Science 322: 949– 953, 2008. [DOI] [PubMed] [Google Scholar]
- 32). Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, Hong H, Nakagawa M, Tanabe K, Tezuka K, Shibata T, Kunisada T, Takahashi M, Takahashi J, Saji H, Yamanaka S: A more efficient method to generate integration-free human iPS cells. Nat Methods 8: 409– 412, 2011. [DOI] [PubMed] [Google Scholar]
- 33). Nakagawa M, Taniguchi Y, Senda S, Takizawa N, Ichisaka T, Asano K, Morizane A, Doi D, Takahashi J, Nishizawa M, Yoshida Y, Toyoda T, Osafune K, Sekiguchi K, Yamanaka S: A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci Rep 4: 3594, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Morizane A, Doi D, Kikuchi T, Okita K, Hotta A, Kawasaki T, Hayashi T, Onoe H, Shiina T, Yamanaka S, Takahashi J: Direct comparison of autologous and allogeneic transplantation of iPSC-derived neural cells in the brain of a non-human primate. Stem Cell Reports 1: 283– 292, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Kawasaki H, Mizuseki K, Nishikawa S, Kaneko S, Kuwana Y, Nakanishi S, Nishikawa SI, Sasai Y: Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron 28: 31– 40, 2000. [DOI] [PubMed] [Google Scholar]
- 36). Watanabe K, Kamiya D, Nishiyama A, Katayama T, Nozaki S, Kawasaki H, Watanabe Y, Mizuseki K, Sasai Y: Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci 8: 288– 296, 2005. [DOI] [PubMed] [Google Scholar]
- 37). Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L: Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 27: 275– 280, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38). Morizane A, Doi D, Kikuchi T, Nishimura K, Takahashi J: Small-molecule inhibitors of bone morphogenic protein and activin/nodal signals promote highly efficient neural induction from human pluripotent stem cells. J Neurosci Res 89: 117– 126, 2011. [DOI] [PubMed] [Google Scholar]
- 39). Kikuchi T, Morizane A, Doi D, Onoe H, Hayashi T, Kawasaki T, Saiki H, Miyamoto S, Takahashi J: Survival of human induced pluripotent stem cell-derived midbrain dopaminergic neurons in the brain of a primate model of Parkinson’s disease. J Parkinsons Dis 1: 395– 412, 2011. [DOI] [PubMed] [Google Scholar]
- 40). Doi D, Morizane A, Kikuchi T, Onoe H, Hayashi T, Kawasaki T, Motono M, Sasai Y, Saiki H, Gomi M, Yoshikawa T, Hayashi H, Shinoyama M, Refaat MM, Suemori H, Miyamoto S, Takahashi J: Prolonged maturation culture favors a reduction in the tumorigenicity and the dopaminergic function of human ESC-derived neural cells in a primate model of Parkinson’s disease. Stem Cells 30: 935– 945, 2012. [DOI] [PubMed] [Google Scholar]
- 41). Hargus G, Cooper O, Deleidi M, Levy A, Lee K, Marlow E, Yow A, Soldner F, Hockemeyer D, Hallett PJ, Osborn T, Jaenisch R, Isacson O: Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proc Natl Acad Sci USA 107: 15921– 15926, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Doi D, Samata B, Katsukawa M, Kikuchi T, Morizane A, Ono Y, Sekiguchi K, Nakagawa M, Parmar M, Takahashi J: Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem Cell Reports 2: 337– 350, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43). Morizane A, Takahashi J, Shinoyama M, Ideguchi M, Takagi Y, Fukuda H, Koyanagi M, Sasai Y, Hashimoto N: Generation of graftable dopaminergic neuron progenitors from mouse ES cells by a combination of coculture and neurosphere methods. J Neurosci Res 83: 1015– 1027, 2006. [DOI] [PubMed] [Google Scholar]
- 44). Hagell P, Cenci MA: Dyskinesias and dopamine cell replacement in Parkinson’s disease: a clinical perspective. Brain Res Bull 68: 4– 15, 2005. [DOI] [PubMed] [Google Scholar]
- 45). Ma Y, Feigin A, Dhawan V, Fukuda M, Shi Q, Greene P, Breeze R, Fahn S, Freed C, Eidelberg D: Dyskinesia after fetal cell transplantation for parkinsonism: a PET study. Ann Neurol 52: 628– 634, 2002. [DOI] [PubMed] [Google Scholar]
- 46). Maries E, Kordower JH, Chu Y, Collier TJ, Sortwell CE, Olaru E, Shannon K, Steece-Collier K: Focal not widespread grafts induce novel dyskinetic behavior in parkinsonian rats. Neurobiol Dis 21: 165– 180, 2006. [DOI] [PubMed] [Google Scholar]
- 47). Barker RA, Barrett J, Mason SL, Björklund A: Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson’s disease. Lancet Neurol 12: 84– 91, 2013. [DOI] [PubMed] [Google Scholar]
- 48). Politis M, Wu K, Loane C, Quinn NP, Brooks DJ, Rehncrona S, Bjorklund A, Lindvall O, Piccini P: Serotonergic neurons mediate dyskinesia side effects in Parkinson’s patients with neural transplants. Sci Transl Med 2: 38ra46, 2010. [DOI] [PubMed] [Google Scholar]