Abstract
The sympathetic nervous system (SNS) or neurotransmitters in the bone marrow microenvironment has been known to regulate hematopoietic stem cell (HSC) functions such as self-renewal, proliferation and differentiation. However, the specific role of neuropeptide Y (NPY) in this process remains relatively unexplored. In this study, we demonstrated that NPY deficient mice have significantly reduced HSC numbers and impaired bone marrow regeneration due to apoptotic destruction of SNS fibers and/or endothelial cells. Moreover, NPY treatment prevented bone marrow impairments in a mouse model of chemotherapy-induced SNS injury, while conditional knockout mice lacking the Y1 receptor in macrophages did not restore bone marrow dysfunction in spite of NPY injection. Transforming growth factor-beta (TGF-β) secreted by NPY-mediated Y1 receptor stimulation in macrophages plays a key role in neuroprotection and HSC survival in the bone marrow. Therefore, this study reveals a new role of NPY in bone marrow HSC microenvironment, and provides an insight into the therapeutic application of this neuropeptide. [BMB Reports 2015; 48(12): 645-646]
Keywords: Bone marrow microenvironment, Hematopoietic stem cell, Neuropeptide Y, Regeneration, Sympathetic nervous system
The bone marrow microenvironments are specific sites where stem cells reside and undergo self-renewal and produce a large number of progeny. Hematopoietic stem cells (HSCs) appear to contact osteoblasts in endosteal microenvironments or the sinusoidal endothelium in the perivascular microenvironment. These microenvironments consist of supporting cells that regulate the HSC retention, survival, proliferation and differentiation. Particularly, endothelial cells (ECs) and nestin+ mesenchymal stem cells (MSCs) in the perivascular microenvironment is required for HSCs survival and bone marrow regeneration, and the sympathetic nervous system (SNS) and macrophages are positive regulators of ECs and nestin+ MSCs, which retain the HSCs. Bone marrow innervations have been shown to regulate HSC functions. Sympathetic nerve fibers synapsed on perivascular cells are associated with HSC hibernation and self-renewal in the bone marrow (BM). Moreover, bone marrow nerve injury causes impairment of HSC survival and bone marrow regeneration. Neurotransmitters released from the SNS also play a key role in regeneration, motility and proliferation of bone marrow cells.
Neuropeptide Y (NPY), one of the well-known neurotransmitters, is secreted from the brain or sympathetic nerves in the autonomic system, and has an important role in a variety of physiological processes such as appetite, energy storage, anxiety and pain. Previous studies have demonstrated the role of NPY in BM, which include immune cell homeostasis, bone homeostasis or vascular remodeling through Y receptors expressed in bone marrow cells, especially macrophages, osteoblasts and ECs. Nonetheless, the specific role of NPY in the HSC microenvironment is as yet not clearly determined. In a recent study published in EMBO Journal, we showed an unknown function of NPY in regulating the bone marrow HSC microenvironment.
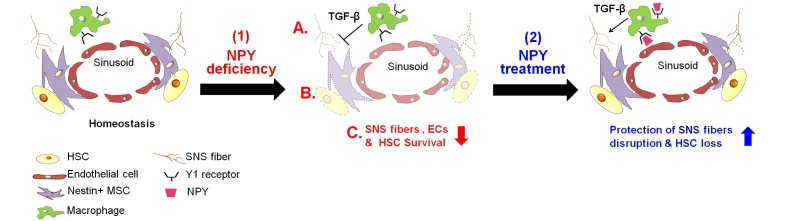
It has been observed that NPY deficiency in the bone marrow environment caused HSC reduction and impaired bone marrow regeneration after bone marrow transplantation. However, this bone marrow dysfunction was not due to cell-autonomous apoptosis, and also the NPY deficient HSCs had normal motility and migration capacity. These results demonstrated that bone marrow microenvironment cells were related to HSC survival in NPY deficient bone marrow. The BM of NPY deficient mice showed increased p53-dependent apoptosis of SNS fibers and CD31+ ECs, resulting in a reduction of these cells. Therefore, these findings indicated that NPY deficiency caused impairment of HSC survival and bone marrow regeneration by reducing the bone marrow microenvironment cells such as SNS fibers and CD31+ ECs. NPY or a Y1 agonist treatment into NPY deficient mice significantly prevented the loss of HSCs in the BM, and the reduction of SNS fiber and CD31+ ECs was also protected. In contrast, the pharmacological Y1 receptor-blockade in mice, by treating with Y1 antagonist, showed reduction in mouse survival, impairment of HSCs and bone marrow microenvironment cell survival after 5FU treatment, which causes ablation of most hematopoietic cells. Overall, these results suggested that an interaction of NPY and the Y1 receptor could mediate the survival of HSCs residing in the perivascular microenvironment by regulation of bone marrow SNS nerves and ECs.
Chemotherapy-induced neuropathy is one of the severe side effects of cancer therapy. Moreover, chronic bone marrow damage by chemotherapy accompanies impaired HSC function or hematopoietic regeneration, and this leads to reduced recovery of bone marrow microenvironment cells. In particular, chemotherapy drugs such as cisplatin induces sympathetic neuropathy by reducing the expression of Th fibers. In our study, cisplatin-induced bone marrow dysfunction and SNS injury were prevented by NPY treatment, which reduced cisplatin-induced apoptosis of BM cells. This protective effect of NPY was not found in conditional Y1 receptor knockout mice in macrophages. To explore the specific mechanism of NPY/Y1 receptor-mediated improvement of cisplatin-induced bone marrow dysfunction, we performed an in vitro experiment evaluating the differentiation of PC12 cells into neurons. PC12 cells exposed to conditioned medium (CM), derived from the NPYtreated control macrophages, showed neural differentiation capacity, but not in Y1 receptor deficient macrophages treated with NPY. Taken together, these results highlight the fact that NPY has a potential therapeutic value for chemotherapy-induced bone marrow abnormalities, and the trophic or bioactive factors secreted from macrophages through NPY/Y1 regulation mediates neural protection. Transforming growth factor-beta (TGF-β) has been known to play a key role in the regulation of neuronal survival, differentiation and repair processes in the nervous system. We confirmed that the PI3K/Akt/mTOR/eIL4E signaling pathway, which produces TGF-β, was activated in macrophages with NPY treatment, resulting in an up-regulation of TGF-β secretion or expression in macrophages. Therefore, these results indicate that TGF-β released by NPY-mediated Y1 receptor stimulation in macrophages is responsible for neuroprotection and HSC survival in bone marrow.
In conclusion, our findings suggest a new role of NPY as a regulator for bone marrow HSC microenvironment (Fig. 1). Moreover, we suggest that NPY or Y1 agonists have a potential clinical utility as neuroprotective agents for patients treated with chemotherapy. With an increase in patients suffering from cancer, the development of drugs is important for reduction of chemotherapy-induced side effects. NPY has a high clinical value to improve bone marrow impairments by chemotherapy, due to a stable peptide that is synthesized naturally. Therefore, the modulation of the endogenous bone marrow HSC microenvironment by NPY could be an effective therapeutic approach. Further studies investigating the functional role of NPY on bone marrow HSC microenvironment under pathological conditions may provide more valuable information on the mechanisms of interaction with the nervous system and HSC microenvironment.
Fig. 1. Model of NPY mediated regulation of HSC microenvironment. Under homeostatic conditions of bone marrow, HSCs reside in the perivascular niche. SNS regulates EC and Nestin+ MSCs associated with HSC retention in perivascular niche. (1) Under condition of NPY deficiency in the bone marrow environment, (A) destruction of SNS fibers and endothelial cell death occurs, and (B) nestin+ MSCs decrease, since macrophages expressing Y1 receptor cannot support these niche cell survival. Thus, (C) survival of HSCs residing in the perivascular niche is reduced. (2) In mouse model of NPY deficiency or chemotherapy-induced SNS nerve injury, NPY treatment promotes neuroprotection from SNS fiber destruction by TGF-β secreted from macrophage through the Y1 receptor, resulting in prevention of HSC loss.
Acknowledgments
This work was supported by Biomedical Research Institute grant, Kyungpook National University Hospital (2012).