Abstract
Background
There has been an increasing role of acellular dermal matrices (ADMs) and synthetic meshes in both single- and two-stage implant/expander breast reconstruction. Numerous alloplastic adjuncts exist, and these vary in material type, processing, storage, surgical preparation, level of sterility, available sizes and cost. However, there is little published data on most, posing a significant challenge to the reconstructive surgeon trying to compare and select the most suitable product. The aims of this systematic review were to identify, summarize and evaluate the outcomes of studies describing the use of alloplastic adjuncts for post-mastectomy breast reconstruction. The secondary aims were to determine their cost-effectiveness and analyze outcomes in patients who also underwent radiotherapy.
Methods
Using the PRSIMA 2009 statement, a systematic review was conducted to find articles reporting on the outcomes on the use of alloplastic adjuncts in post-mastectomy breast reconstruction. Multiple databases were searched independently by three authors (Cabalag MS, Miller GS and Chae MP), including: Ovid MEDLINE (1950 to present), Embase (1980 to 2015), PubMed and Cochrane Database of Systematic Reviews.
Results
Current published literature on available alloplastic adjuncts are predominantly centered on ADMs, both allogeneic and xenogeneic, with few outcome studies available for synthetic meshes. Outcomes on the 89 articles, which met the inclusion criteria, were summarized and analyzed. The reported outcomes on alloplastic adjunct-assisted breast reconstruction were varied, with most data available on the use of ADMs, particularly AlloDerm® (LifeCell, Branchburg, New Jersey, USA). The use of ADMs in single-stage direct-to-implant breast reconstruction resulted in lower complication rates (infection, seroma, implant loss and late revision), and was more cost effective when compared to non-ADM, two-stage reconstruction. The majority of studies demonstrated inferior outcomes in ADM assisted, two-stage expander-to-implant reconstruction compared to non-ADM use. Multiple studies suggest that the use of ADMs results in a reduction of capsular contracture rates. Additionally, the reported beneficial effects of ADM use in irradiated tissue were varied.
Conclusions
ADM assisted two-stage breast reconstruction was associated with inferior outcomes when compared to non-ADM use. However, alloplastic adjuncts may have a role in single stage, direct-to-implant breast reconstruction. Published evidence comparing the long-term outcomes between the different types of adjuncts is lacking, and further level one studies are required to identify the ideal product.
Keywords: Breast reconstruction, breast implants, alloplastic implants, acellular dermal matrices (ADMs)
Introduction
Breast cancer is the most common cancer in women, accounting for 29% of newly diagnosed cancers, and with a lifetime risk of one in eight for females in the United States (1). Numerous options and technical variations exist for post-mastectomy breast reconstruction, and can be categorized into autologous versus alloplastic, immediate versus delayed, as well as single versus two-staged. An estimated one-half to two-thirds of women who undergo a mastectomy will proceed to have an alloplastic reconstruction (2).
Acellular dermal matrices (ADMs) have been used since the 1990s in the areas of burns, head and neck, abdominal wall, hand, nasal as well as lower extremity reconstruction (3-10). These materials are allegedly immunologically inert, and act as biological scaffolds for re-epithelialization, neovascularization and fibroblast infiltration. Duncan first published the use of ADMs in breast surgery in 2001, in which AlloDerm® was utilized in revisional aesthetic surgery to correct implant rippling (11). However, it first used in breast reconstruction in 2005, where Breuing and Warren described the use of AlloDerm® as an inferior sling in single stage (direct to implant) post-mastectomy reconstructions (5). In the same year, Rietjens et al. described the use of a synthetic non-absorbable mesh (Mersilene), to recruit upper abdominal skin for additional soft-tissue coverage of the implant, as well as to recreate the infra-mammary fold (12). Since then, the types and number of alloplastic adjuncts have increased, including ADMs derived from human, bovine and porcine dermis, as well as synthetic meshes. These products vary significantly in their processing, level of sterility, biomechanical properties, thickness, preparation methods and cost (13-15). The use of ADMs in breast reconstruction has gained increasing popularity since its introduction, with an estimated 25% to 75% of tissue expander reconstructions utilizing ADMs (16-19).
Numerous advantages have been proposed with the use of alloplastic adjuncts, including: facilitating immediate implant reconstruction, improved implant positioning via better definition of the infra- and lateral mammary folds, shorter expansion times in tissue-expander reconstructions, improved capsular contracture rates, masking implant rippling, providing an additional layer between the prosthesis and overlying mastectomy skin, reduced rates of implant/expander migration, reduced discomfort during post-operative expansion, and protective effects in patients undergoing radiotherapy (5,20-24). However, there are also concerns regarding potential increased risks of infection, inflammatory reaction, seroma, masking tumour recurrence and significant costs (25-29).
Numerous alloplastic adjuncts exist, and these vary in material type, processing, storage, surgical preparation, level of sterility, available sizes and cost. However, there is little published data on most, posing a significant challenge to the reconstructive surgeon trying to compare and select the most suitable product. The aims of this systematic review were to identify, summarize and evaluate the outcomes of studies describing the use of alloplastic adjuncts for post-mastectomy breast reconstruction. The secondary aims were to determine their cost-effectiveness and to analyze outcomes in patients who also underwent radiotherapy.
Methods
Study identification
Multiple databases were searched independently by three authors (Cabalag MS, Miller GS and Chae MP), including: Ovid MEDLINE (1950 to present), Embase (1980 to 2015), PubMed and Cochrane Database of Systematic Reviews.
The following search terms and Boolean operators were used: (I) (“breast reconstruction” OR “post mastectomy” OR “implant reconstruction” OR “tissue expander” OR “alloplastic”) AND (II) (“acellular dermal matrix” OR “acellular dermal matrices” OR “mesh” OR “synthetic mesh” OR “biological matrix”). Additional searches were conducted using (I) AND (II) AND (“radiotherapy” OR “irradiated”), as well as (I) AND (II) AND (“cost” OR “cost-effectiveness” OR “cost analysis”).
Inclusion criteria
Inclusion criteria for studies reviewed included: (I) meta-analyzes or review articles; (II) adult patients aged 18 years or over undergoing post-mastectomy breast reconstruction; (III) alloplastic breast reconstruction (i.e., tissue-expander and/or implant-based) performed using adjuncts (ADMs and/or synthetic meshes; (IV) studies including outcome measures; (V) case series with more than ten patients; and (VI) English language.
Data extraction
A systematic review was conducted using the PRISMA 2009 statement (30). Data was extracted by three authors (Cabalag MS, Miller GS and Chae MP), and included author, year, journal, study design, level of evidence, outcome details, number of patients (if applicable), and follow-up period. Differences in data extraction were corrected via discussion.
Results
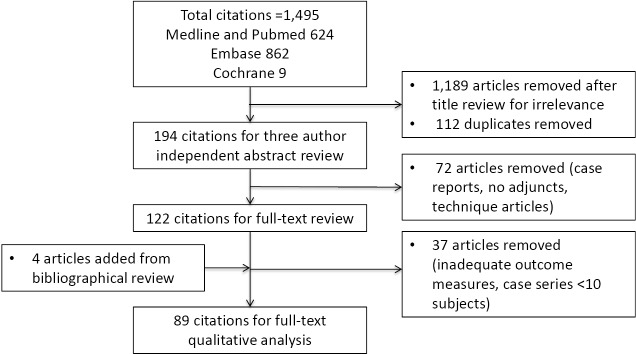
The search was conducted on April 4, 2015, resulting in 1,495 articles, managed using Endnote X7™ (Thomson Reuters, Philadelphia, PA, USA). A summary of the literature review process is shown in Figure 1. After the authors independently assessed the titles for relevance, a total of 1,189 articles were excluded, and 112 duplicates were removed. The abstracts for the remaining articles were then reviewed based on the inclusion criteria, leaving a total of 122 articles for full review. A further four articles were added based on review of bibliographies. Thirty-seven studies were eliminated after full review (inadequate outcome measures, case series <10 subjects). After full text review, analysis and data extraction was conducted for a total of 89 articles. The recommendations of this review are summarized in Table 1. Tables S1,S2,S3,S4 are a summary of the: systematic reviews and meta-analyses; levels III and IV studies; cost-analyzing studies; and studies focusing on synthetic meshes respectively.
Figure 1.
Summary of the literature review process.
Table 1. Summary of recommendations.
| Key points |
|---|
| 1. The overall quality of studies was low, with the majority being of level III to IV evidence (i.e., case series or cohort studies). Additional level I to II evidence are required to validate the use of alloplastic adjuncts |
| 2. Evidence for the use of ADMs in irradiated tissue is varied and inconsistent. However, synthetic mesh should be avoided in patients undergoing radiotherapy |
| 3. Benefits of ADM use include: facilitating single stage, direct-to-implant breast reconstructions; improved cosmesis with better control of the inframammary fold; and shorter expansion times in tissue-expander reconstructions |
| 4. ADM-assisted two stage, expander-to-implant reconstruction led to inferior outcomes when compared to traditional, two-stage submuscular techniques |
| 5. ADM-assisted single stage, direct-to-implant reconstruction resulted in lower overall complication rates (infection, seroma, implant loss and late revision), compared to traditional, two-stage submuscular techniques. However, it was associated with an increased rate of mastectomy skin flap necrosis |
| 6. Cost-analysis studies suggest a cost advantage in ADM-assisted, direct-to-implant reconstruction, compared to non-ADM, two-stage reconstructions |
| 7. The use of ADMs was associated with decreased rates of capsular contracture |
| 8. More studies comparing the long-term outcomes between different alloplastic adjuncts are required to select the best material |
ADMs, acellular dermal matrices.
Types of alloplastic adjuncts available
The types of alloplastic adjuncts in breast reconstruction described in the literature are listed in Table 2 and summarized in Table 3 (14). In summary, they comprise of either ADMs or synthetic meshes. Within ADMs, there are either allografts, derived from cadaveric human skin, or xenografts. There is significantly less published literature on the use of synthetic meshes in post-mastectomy reconstruction.
Table 2. List of alloplastic adjuncts.
| ADMs |
| Allograft |
| AlloDerm® (LifeCell Corp., Branchburg, New Jersey, USA) |
| AlloDerm® Ready to Use (LifeCell Corp., Branchburg, New Jersey, USA) |
| FlexHD® (MTF/Ethicon, Somerville, New Jersey, USA) |
| AlloMax™ (Bard, Warwick, Rhode Island, USA) |
| DermaMatrix® (MTF/Synthes, West Chester, Pennsylvania, USA) |
| DermaCell® (Lifenet, Virginia Beach, Virginia, USA) |
| Xenograft |
| Porcine |
| PermaColl™ (Covidien, Boulder, Colorado, USA) |
| Strattice™ (Lifecell, Branchburg, New Jersey, USA) |
| Protexa (Tecnoss, Mestre, Italy) |
| Fetal bovine |
| SurgiMend® PRS (TEI Biosciences, Boston, Massachusetts, USA) |
| Tutomesh® (RTI Biologics, Alachua, Florida, USA) |
| Synthetic mesh |
| TiLOOP® Bra (PFM Medical, Cologne, Germany) |
| TIGR® Matrix Surgical Mesh (Novus Scientific Pte Ltd, Singapore) |
| Knitted Vicryl Mesh (Vicryl, Ethicon, Somerville, New Jersey, USA) |
ADMs, acellular dermal matrices.
Table 3. Types of alloplastic adjuncts in breast reconstruction.
| Alloplastic adjunct | Product name | Material | Sterility | Use | Contraindications | Other | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acellular dermal matrix | |||||||||||||
| Allograft | AlloDerm® (LifeCell Corp, Branchburg, New Jersey, USA); AlloDerm® Ready to Use (LifeCell Corp., Branchburg, New Jersey, USA) | Cadaveric | Non-sterile, aseptic; | • Freeze dried or pre-hydrated; | • Potential allergen—multiple antibiotics are used in product processing | • Most documented in literature | |||||||
| • Orientation specific; | |||||||||||||
| • Infero-lateral sling; | |||||||||||||
| Terminally sterilized | • Immediate implant or expander based reconstruction; | ||||||||||||
| • Nipple reconstruction; | |||||||||||||
| • Shelf life 2 years | |||||||||||||
| FlexHD® (MTF/Ethicon, Somerville, New Jersey, USA) | Cadaveric | Non-sterile, aseptic | • Pre-hydrated; | • No information available (NIA) | |||||||||
| • Orientation specific; | |||||||||||||
| • Mainly used for abdominal reconstruction; | |||||||||||||
| • Shelf life 3 years | |||||||||||||
| DermaMatrix® (MTF/Synthes, West Chester, Pennsylvania, USA) | Cadaveric | Non-sterile, aseptic | • Freeze dried; | • Not recommended in patients with autoimmune connective tissue disease | |||||||||
| • Orientation specific; | |||||||||||||
| • Immediate implant or expander based reconstruction; | |||||||||||||
| • Shelf life 3 years | |||||||||||||
| AlloMax™ (Bard, Warwick, Rhode Island, USA) | Cadaveric | Terminally sterilized | • Freeze dried; | • NIA | |||||||||
| • Not orientation specific; | |||||||||||||
| • Immediate implant or expander based reconstruction; | |||||||||||||
| • Shelf life 5 years | |||||||||||||
| DermaCell® (Lifenet, Virginia Beach, Virginia, USA) | Cadaveric | Non-sterile, aseptic | • Ready to use; | • Sensitivities to gentamicin and vancomycin | |||||||||
| • Orientation specific; | |||||||||||||
| • Nipple reconstruction; | |||||||||||||
| • Shelf life 2 years | |||||||||||||
| Xenograft | PermaCollTM (Covidien, Boulder, Colorado, USA) | Porcine | Terminally sterilized | • Pre-hydrated; | • Sensitivities to porcine tissue | ||||||||
| • Not orientation specific; | |||||||||||||
| • Mainly used for abdominal reconstruction; | |||||||||||||
| • Not recommended for breast reconstruction due to inadequate laxity | |||||||||||||
| StratticeTM (Lifecell, Branchburg, New Jersey, USA) | Porcine | Terminally sterilized | • Pre-hydrated; | • Sensitivities to porcine tissue | • Highest stiffness and tensile strength of all the acellular dermal matrices (ADMs) | ||||||||
| • Not orientation specific; | |||||||||||||
| • Mainly used for abdominal reconstruction; | |||||||||||||
| • Shelf life 18 months | |||||||||||||
| SurgiMend® PRS (TEI Biosciences, Boston, Massachusetts, USA) | Fetal Bovine | Terminally sterilized | • Pre-hydrated; | • Sensitivities to bovine tissue | |||||||||
| • Not orientation specific; | |||||||||||||
| • Shelf life 3 years | |||||||||||||
| Synthetic mesh | TiLOOP® Bra (PFM Medical, Cologne, Germany) | Titanium coated polypropylene mesh | Terminally sterilized | • Infra-mammary fold like shape; | • Not suitable for revision surgery | ||||||||
| • Comes in three sizes; | |||||||||||||
| • Mainly available in Europe—not yet approved in the United States as of July 2013 | |||||||||||||
| TIGR Matrix Surgical Mesh (Novus Scientific Pte Ltd, Singapore) | Fast-degrading (copolymer of glycolide and trimethylene carbonate) and slow-degrading (copolymer of lactide and trimethylene carbonate) fibers | Terminally sterilized | • Long term, absorbable, macroporous knitted mesh; | • Higher complication rates in irradiated patients | |||||||||
| • Retains mechanics for up to 9 months; | |||||||||||||
| • Totally hydrolysed by 3 years | |||||||||||||
| Knitted Vicryl Mesh (Vicryl, Ethicon, Somerville, New Jersey, USA) | Polyglactin 910 | Terminally sterilized | • Absorbable; | • Higher complication rates in irradiated patients | |||||||||
| • Ready to use; | |||||||||||||
| • Cheap and widely available; | |||||||||||||
| • Minimum inflammatory response and non-allergenic | |||||||||||||
Allogeneic ADMs
AlloDerm® (LifeCell Corp., Branchburg, New Jersey, USA)
First introduced in 1994, AlloDerm was the first human dermis product available, and was initially used for burns reconstruction. It is a cadaveric split-thickness skin graft, in which the epidermis and cells are removed from the skin to reduce its antigenicity. It now comes in two forms: an aseptic, freeze-dried version requiring refrigerated storage and rehydration prior to use; and a newer, sterile, ready to use product. It was first used as an infero-lateral sling in breast reconstruction in 2005, but now also has a role in tissue-expander based as well as nipple reconstructions (5,31,32). Of note, AlloDerm has two distinct surfaces, and thus requires specific orientation during implantation. The dermal side of the product, characterized by the dull, rough texture, is placed against the vascularized wound bed (i.e., the mastectomy skin flaps). AlloDerm is the most extensively studied ADM in breast reconstruction, with 135 references in the PubMed database as of April 2015. Histological studies have demonstrated AlloDerm to be partially integrated into host tissue within 7 days of implantation (33).
FlexHD® (MTF/Ethicon, Somerville, New Jersey, USA)
FlexHD is a pre-hydrated, aseptic, cadaveric dermal matrix, which, similar to AlloDerm is orientation-specific. Rawlani et al. studied the use of FlexHD in 121 breast reconstructions, with complications occurring in 20 breasts (two seromas, eight partial mastectomy flap necroses and nine infections). Furthermore, when compared to the non-irradiated group, the irradiated cohort had a higher rate of complications (13.7% vs. 30.8% respectively) (34).
Allomax™ (Bard, Warwick, Rhode Island, USA)
Previously known as NeoForm®, Allomax™ is a sterile, cadaveric dermal matrix, which is non-orientation specific. Losken et al. published a study involving 22 patients and 31 breast reconstructions, reporting no cases of infection, seroma or foreign body reaction (35).
DermaMatrix® (MTF/Synthes, West Chester, Pennsylvania, USA)
DermaMatrix® is an aseptic, freeze-dried, orientation specific cadaveric allograft. Becker et al. compared DermaMatrix® with AlloDerm® in 30 patients (50 breasts) who underwent immediate expander-based breast reconstruction, in which the only statistically significant difference was a shorter duration in which the drains remained in-situ for AlloDerm® vs. DermaMatrix® (11 vs. 13 days) (36). No significant differences in complication rates (4%) were noted.
DermaCell® (Lifenet, Virginia Beach, Virginia, USA)
DermaCell® is a prehydrated, ready to use cadaveric dermal matrix, which can be stored at room temperature. The literature search revealed three articles on the use of DermaCell® in breast reconstruction, which suggested a relatively low rate of post-operative complications (14,37,38). In a recent case series of ten patients, Bullocks reported two cases of failed tissue-expander reconstructions due to chronic seromas and infection (37). In another recent case series of nine patients, Vashi et al. reported only one patient with bilateral post-mastectomy reconstruction who subsequently developed seromas and infection (38).
Xenogeneic ADMs
Strattice™ (Lifecell, Branchburg, New Jersey, USA)
Strattice™ is a pre-hydrated, terminally sterilized, porcine-derived dermal matrix.
Permacol™ (Covidien, Boulder, Colorado, USA)
Permacol™ is a pre-hydrated, terminally sterilized, porcine-derived dermal matrix. Of note, it is not recommended for breast reconstruction as it lacks adequate laxity to produce natural, ptotic lower pole coverage.
Surgimend® PRS (TEI Biosciences, Boston, Massachusetts, USA)
Surgimend® is the only product comprised of fetal bovine dermal collagen, and is terminally sterilized.
Synthetic mesh
Knitted Vicryl Mesh (Ethicon, Somerville, New Jersey, USA)
Comprised of polyglactin 910, Knitted Vicryl Mesh is cheap, ready to use and widely available. It also exhibits minimal inflammatory reaction, is non-allergenic and resistant to bacteria biofilm formation (39,40).
TiLOOP® Bra (PFM Medical, Cologne, Germany)
TiLOOP Bra is a lightweight, non-absorbable, titanium-coated polypropylene mesh, first approved for use in breast reconstruction in Europe in 2008. It is the most commonly used synthetic mesh in Germany (15). It consists of a monofilament structure and is available in three different bra-like sizes. The mesh comes in an infra-mammary fold like shape, helping to define the lower pole and preventing the implant from bottoming out. Both animal and human studies have demonstrated improved biocompatibility compared to non-titanium coated meshes, with histological evidence of incorporation during the time of expander-implant exchange (41,42). In Europe, the mesh costs €400 (43).
TIGR® Matrix Surgical Mesh (Novus Scientific Pte LTd, Singapore)
TIGR® Matrix Surgical Mesh is a long-term, absorbable synthetic mesh. It is a macroporous mesh knitted from both a fast- (copolymer of glycolide and trimethylene carbonate) and slow-degrading (copolymer of lactide and trimethylene carbonate) fibers. After 2 weeks post implantation, the mesh will become noticeably softer and flexible, with due to the degradation of the fast fibers, which becomes totally resorbed within 4 months. The slow-degrading fibers keep their mechanics for up to 9 months, and are totally hydrolysed after 3 years (13). A 10 cm × 15 cm sheet of TIGR® mesh costs USD $900. A preclinical study has demonstrated that the mesh is rapidly vascularized, demonstrates minimal inflammatory response, and is replaced by well-organized connective tissue over time (44).
Use of ADMs in post-mastectomy breast reconstruction and outcomes compared to non-ADM reconstruction
Currently, ADMs are used in both primary and revisional alloplastic breast reconstructive and aesthetic surgery. Techniques include: (I) expansion of the submuscular pocket to allow for direct-to-implant breast reconstruction (5); (II) expansion of the submuscular pocket to improve two-stage expander-to-implant breast reconstruction (31); (III) providing an interface when performing capsulotomies or capsulectomies for capsular contracture; (IV) correction of symmastia (45); (V) aid in the masking surface irregularities and rippling (23); and (VI) prevention or correction of inframammary fold malposition and ‘bottoming out’ (46).
Use of alloplastic adjuncts in single stage, direct-to-implant reconstruction
Breuing and Warren first described the use of AlloDerm® as an inferior sling in immediate, direct-to-implant post-mastectomy reconstruction (5). The technique re-establishes the lower pole of the pectoralis major muscle, creating a subpectoral-sub-AlloDerm pocket that encloses the implant. The advantages of this method include the ability for a single stage, direct-to-implant reconstruction and its associated cost benefits, the ability to control lower pole fullness by adjusting the width of the sling and providing an additional layer of tissue between skin and implant. In a recent review by Macadam and Lennox, the use of ADMs (AlloDerm®) in direct-to-implant breast reconstruction, when compared to no ADM use in two-stage reconstructions (the Mentor and Allergan core studies) (47-51), resulted in lower rates of capsular contracture (0.3% vs. 8.3-17.1%), seroma (1.2% vs. 4.9%), infection (1.4% vs. 3.2-5.7%), late revisions (8.5% vs. 27-53.3%) and implant loss (1.5% vs. 5.7-7.7%). However, a higher rate of skin flap necrosis was observed (4.7% vs. 2.3%), which may be attributable to increased skin tension due to placement of the implant (52). Of note, the rate of skin flap necrosis is comparable to expander-to-implant reconstructions without the use of ADM published in previous studies (range, 2-6%) (53-56). Similarly, Salzberg et al. demonstrated a low overall complication rate (3.9%) in a retrospective analysis of 260 patients (466 breasts) who underwent single-stage reconstruction with AlloDerm®, with a mean follow up of 29 months (57). Specific complication rates included implant loss (1.3%), flap necrosis (1.1%), hematoma (1.1%), ADM exposure (0.6%), capsular contracture (0.4%) and infection (0.2%). Irradiated breasts had a fourfold higher rate of complications. The low complication rates are also projected long-term, with no complications seen in 354 breasts with more than 1 year of follow-up. A systematic review by Jansen and Macadam further reaffirms the comparable complication rates between AlloDerm®-assisted single stage and non-ADM, two-stage reconstructions (58). Of note, to validate these findings, Zhong et al. are currently conducting a randomized controlled trial comparing direct-to-implant reconstruction with ADM to traditional two-stage non-ADM reconstruction (59).
In contrast, a meta-analysis conducted by Ho et al. revealed higher odds of infection [odds ratio (OR), 2.7; 95 percent confidence interval (95% CI), 1.1-6.4], seroma (OR, 3.9; 95% CI, 2.4-6.2) and reconstructive failure (OR, 3.0; 95% CI, 1.3-6.8) in ADM compared to non-ADM breast reconstructions. However, ADM use was associated with lower rates of capsular contracture. The meta-analysis reviewed a total of 16 studies, most of which did not differentiate between single- or two-stage reconstruction. The most common complication associated with ADM use was skin flap necrosis (10.9%; 95% CI, 8.7-13.5%), followed by seroma (6.9%; 95% CI, 5.3-8.8%), infection (5.7%; 95% CI, 4.3-7.3%), reconstructive failure (5.1%; 95% CI, 3.8-6.7%), cellulitis (2.0%; 95% CI, 1.2-3.1%), hematoma (1.3%; 95% CI, 0.6-2.4%) and capsular contracture (0.6%; 95% CI, 0.1-1.7%).
Vicryl mesh has also been used in immediate single stage reconstructions with favorable results. In a retrospective analysis by Tessler et al., 50 consecutive patients (76 reconstructions) underwent immediate implant-based reconstruction using knitted Vicryl mesh as an inferolateral sling. The overall complication rate was 6.6%, with one case (1.3%) of infection, two cases (2.6%) of mastectomy skin flap necrosis, one case (1.3%) of capsule contracture requiring revision (postradiation), one case (1.3%) of implant failure, and one case of a delayed type IV hypersensitivity reaction. Reported contour and implant positioning were excellent, with a revision rate of 3.9% (three breasts) for size enlargement. Additionally, Garganese et al. have used TiLOOP Bras in immediate implant-based reconstruction in ten patients, reporting no early complications and minimal post-operative pain (60). Klein et al. reported higher complication rates with the use of TiLOOP Bras in immediate reconstruction, with an infection, hematoma and seroma rate of 10.3%, 17.2% and 9.2% respectively (61).
Use of alloplastic adjuncts in two stage, expander-to-implant reconstruction
In 2007, Bindingnavele et al. first described the use of ADMs in a two-stage expander-to-implant reconstructions (31). Alleged advantages include increased intra-operative expansion volumes and thus reduced post-operative expansion time, avoiding the need to raise serratus anterior muscle for lateral prosthesis coverage leading to reduced post-operative pain with expansion, as well as more precise placement of the expander resulting in better lower pole projection and improved aesthetics. However, multiple studies have expressed concern regarding the increased morbidity associated with the use of ADM in two-stage reconstructions. In a series of 283 patients (415 breasts), Chun et al. demonstrated that the use of ADMs increased the odds of seroma by 4.24 times (P=0.018) and infection by 5.37 times (P=0.006), when compared to the non-ADM group (26). This was further confirmed in a meta-analysis performed by Kim et al. comparing the use of ADM (19 studies, n=2,037) and no ADM (35 studies, n=12,847) in two-stage breast reconstruction, reporting inferior outcomes in the ADM group. There were higher rates of seroma (4.8% vs. 3.5%), infection (5.3% vs. 4.7%) and mastectomy flap necrosis (6.9% vs. 4.9%) in the ADM group (62). However, the rate of reconstructive failure was comparable (3.8%). These findings were reinforced by a weighted analysis conducted by Macadam and Lennox for two-stage reconstructions using ADMs, compared to no ADMs, revealing higher rates of seroma (5.8% vs. 4.9%), infection (5.3% vs. 3.2-5.7%), and mastectomy flap necrosis (7.6% vs. 2.3%). However, there were lower rates of capsular contracture (2.6% vs. 8.3-17.1%), and late revisions (10.7% vs. 27-53%). The rate of implant extrusion was comparable (4.9% vs. 5.7-7.7%). Additionally, a meta-analysis by Hoppe et al., consisting of eight studies comparing the use of AlloDerm® in expander-implant reconstruction to traditional submuscular techniques, demonstrated a three-fold increase in the odds seroma formation (OR, 3.00; 95% CI, 1.96-4.61) and a two-fold increase in the odds of infection in the ADM group (OR, 2.33; 95% CI, 1.55-3.49) (63).
In contrast, a systematic review by Sbitany and Serletti comparing the use of ADMs in two-stage reconstruction to standard subpectoral coverage techniques revealed a comparable complication profile, but more rapid reconstruction in the ADM group. There was a significantly higher rate of seroma formation in the ADM group (8.4% vs. 4.3%, P=0.03), but the rate of infection resulting in explantation was similar (3.4% vs. 3.2%, P=0.18, in the ADM and submuscular group respectively). There were also slightly higher rates of hematoma (2.0% vs. 1.2%, P=0.09) and partial mastectomy flap necrosis (9.3% vs. 7.2%, P=0.08) in the ADM compared to the submuscular group, none of which were statistically significant. The ADM group demonstrated higher intra-operative fill volumes (mean of 68.5% of final total volume vs. 24.2%, P=0.01) and a shorter post-operative expansion period (mean of 2.4 fills to achieve final volume vs. 5.1, P=0.03) (64).
Furthermore, a multicenter, blinded randomized, controlled trial comparing the use of ADM in two-stage breast reconstruction showed no significant difference in adverse outcomes (hematoma, seroma and infection) between the ADM and non-ADM group (17% vs. 15% respectively, P=1.00) (65). Furthermore, there were no significant differences in immediate post-operative pain, pain during expansion phase, or the rate of post-operative expansion between the two groups.
A titanium-coated polypropylene mesh, TiLOOP Bra (PFM Medical, Cologne, Germany) is a widely used synthetic adjunct for post-mastectomy reconstruction in Europe. In a retrospective, multicenter analysis by Dieterich et al., 207 patients (231 breasts) underwent either single- or two-stage reconstruction using TiLOOP Bra. The overall complication rate was 29%, with major complications occurring in 13.4% of the cases requiring operative intervention. The rate of mesh removal and implant loss was 7.8% and 8.7% respectively (43). Becker et al. used TIGR® mesh in 11 patients (19 breasts) undergoing two-stage reconstruction, reporting an overall complication rate of 47.3% (one case of flap necrosis, two cases of seroma, three cases of infection/extrusion, one case of rippling, and two cases of asymmetry requiring revision) (13).
Furthermore, Haynes and Kreithen reported on the use of Vicryl mesh in 38 patients (46 breasts) who underwent two-stage reconstructions. The results suggest that Vicryl mesh may be a suitable alternative to ADMs, with an overall complication rate of 15.2% (7 breasts): 3 cases (6.5%) of infections leading to expander removal, 1 case (2.2%) of expander exposure requiring removal in a patient undergoing radiotherapy, 2 cases (4.3%) of mastectomy skin flap necrosis, and 1 case (2.2%) of seroma. However, when analyzing the non-irradiated cohort (38 breasts), the overall complication rate was 10.5% (one case of infection leading to removal of the expander, two cases of mastectomy skin flap necrosis and one case of seroma). The revision rate was 16.2% in the non-irradiated group (two for size change, three for malposition and one for capsular contracture).
Comparison of outcomes between different ADMs
With the great diversity of alloplastic adjuncts available in the market, one of the main challenges faced by reconstructive surgeons is choosing the ideal product. The ideal adjunct would be terminally sterilized, able to be stored without refrigeration, have a long shelf life, not require any preparation (e.g., rehydration or rinsing), result in minimal inflammatory reaction, not require orientation, offer good long-term durability, available in multiple sizes and thickness, as well as be affordable. The majority of published studies focus on AlloDerm®, as it was the first widely available ADM used for breast reconstruction.
Currently, Mendenhall et al. are conducting the largest prospective randomized trial comparing the outcomes after using AlloDerm® versus DermaMatrix® as an inferolateral sling in two stage expander-implant breast reconstruction in 128 patients (199 breasts). Preliminary results demonstrate a significant overall complication rate of 36.2%, with similar rates between the two groups (33.6% in the AlloDerm® and 38.8% in the DermaMatrix® group, P=0.52). In both the AlloDerm® and DermaMatrix® groups, the majority of complications were due to skin necrosis (17.8% vs. 21.4% respectively, P=0.66) and infections (13.9% vs. 16.3% respectively, P=0.29), both of which led to tissue expander losses (5% vs. 11.2% respectively, P=0.11). Of note, the rates of infection and skin necrosis are considerably higher compared to those previously reported (62,64). Complication rates (specifically infection and tissue expander loss) were significantly higher in obese patients, with the authors suggesting that ADM use should be avoided in such patients. Patients reconstructed with AlloDerm® had significantly faster expansion times (42 vs. 70 days, P<0.001).
The use of sterile AlloDerm® Ready to Use, when compared to aseptic AlloDerm®, led to reduced rates of mastectomy skin flap necrosis, seroma and infection (66,67). In contrast, although limited by sample size, a retrospective analysis comparing AlloDerm® (aseptic) with AlloDerm® Ready to Use (sterile) in implant based reconstructions, showed a higher seroma rate with the latter (68). Similarly, in a comparison between AlloDerm® and Strattice for alloplastic breast reconstruction, Glasberg and Light showed a significantly higher seroma rate with the use of AlloDerm® (21.4% vs. 6.3%, P=0.0003). All other complications were similar between the two groups (69). Other studies have shown AlloDerm® has comparable outcomes with DermMatrix, Strattice, SurgiMend, FlexHD, AlloMax and AlloDerm Ready to Use (70-75). Furthermore, Seth et al. showed no significant differences in complication rates between the use of cryopreserved or prehydrated human ADMs (PHADMs) (76).
Furthermore, Mofid et al. conducted a retrospective analysis on the use of Veritas®, a bovine pericardium xenograft, in immediate tissue expander/implant-based breast reconstructions. The overall complication rate was found to be similar, if not lower, compared to the use of AlloDerm® in previous studies (77).
Role of ADM in preventing capsular contracture
Capsular contracture is one of the most common complications in reconstructive breast surgery, with cumulative risks reported to be 12% after 1 year, and increasing to 30% at 5 years post-operatively (78). The aetiology remains unclear, although a common inflammatory pathway has been postulated, leading to increased deposition of collagen around the implant and myofibroblast migration (79-82). The use of ADMs appears to reduce the rate of capsular contracture. A meta-analysis conducted by Ho et al. revealed a pooled capsular contracture rate of 0.6%, significantly lower compared to the 3-18% rate reported in traditional two-stage reconstructions (22,23,83-85). Vardanian et al. studied the use of ADMs in immediate implant based reconstruction, and found a significantly lower rate, and risk of capsular contracture in the ADM group versus the non-ADM group (3.8% vs. 19.4% respectively; OR, 0.18; 95% CI, 0.08-0.43) (24). Basu et al. have also shown the protective effects of ADMs histologically, with intra-operative biopsies of human breast capsules and associated ADM at the time of implant exchange demonstrating decreased capsular fibrosis and fibroblast cellularity relative to controls (86). Multiple other studies have similarly demonstrated a low capsular contracture rate in patients undergoing both single- and two-stage breast reconstruction with ADM, ranging from 0-3.8% (5,24,31,34,57,87,88). Interestingly, in a primate model, Stump et al. have demonstrated the role of AlloDerm® in preventing capsular formation (89). However, further long-term follow up is necessary as the rate of capsular contracture may increase with time.
Role of ADMs in irradiated tissue
There have been mixed reports on the role of ADMs in irradiated tissue. In a study where two AlloDerm implants were placed in the backs of 41 rats that were irradiated, Komorowska-Timek et al. demonstrated that the use of AlloDerm decreased radiation-related inflammation and potentially delayed capsular formation and contraction, with the protective effects still present at 12 weeks (90). Similarly, in a retrospective review of 417 consecutive patients (592 breasts), Seth et al. demonstrated a decreased risk of all complications in irradiated breast tissue reconstructed with ADM, versus the non-ADM group (91). Non-ADM patients who received post-mastectomy radiation therapy were almost three times as likely to have a complication compared to non-irradiated patients (OR, 2.63; P=0.002). Conversely, ADM patients who received radiotherapy did not show a significant increase in the risk of complications compared to the non-irradiated group (OR, 1.90; P=0.10). Additionally, Mitchell suggested a protective effect of ADM in irradiated tissue, in a retrospective series of 103 patients (158 breasts) who underwent ADM assisted reconstruction using Strattice™ (92). Interestingly, no complications occurred in patients who received radiotherapy post reconstruction.
In contrast, Spear et al. investigated the use of AlloDerm in a prospective series of 58 immediate expander-based breast reconstructions, and found that the use of AlloDerm did not protect against the effects of radiotherapy, with an overall complication rate of 71.4% (46). Additionally, Nahabedian found a minor increase in the rates of infection, seroma and wound dehiscence in irradiated versus the non-irradiated groups (21). Twenty-three out of 100 breasts reconstructed with AlloDerm received radiotherapy, and complications included: seroma (13%), infection (8.7%), skin necroses (0%) and dehiscence (13%) versus the non-irradiated AlloDerm group: seroma (2.6%), infection (3.9%), dehiscence (1.3%) and skin necrosis (3.9%). The lack of protective effects in ADM assisted breast reconstruction is further strengthened by a recent meta-analysis conducted by Valdatta et al. (93).
Costs
Conducting cost-benefit analyzes for procedures is complex, as it requires not only the immediate costs of the procedure to be calculated, but also any additional costs that may be incurred post-operatively. Most of the cost analysis studies on the use of ADM have taken into account some, if not all of the significant outcomes associated with breast reconstruction: no complication, seroma, infection, hematoma, capsular contracture, implant exposure with loss, implant exposure with salvage, and skin flap necrosis. The majority of these studies highlight a cost advantage in conducting single stage, direct-to-implant breast reconstructions using ADMs. Using a calculator based on immediate operative costs and expected outcomes, Macadam and Lennox estimated that direct-to-implant reconstruction using ADM was cheaper than two-stage reconstruction without ADM ($11,072 vs. $15,049) (52). Similarly, de Blacam et al. estimated that direct-to-implant reconstruction with ADM was more cost-effective compared to expander-to-implant with ADM, and expander-to-implant with no ADM reconstruction ($5,432.02 vs. $11,255 vs. $10,934 respectively).
Additionally, costs will vary depending on the type and size of alloplastic adjunct used, as well as the country of interest. An inquiry in August 2011 by Cheng et al. revealed that the price of ADMs ranged from approximately USD $21.63-34.76 per centimeter squared (14). However, these prices do not reflect the charges to the patient, and some are still considered experimental and thus are not covered by insurance.
The cost of synthetic meshes is considerably cheaper, with Vicryl mesh costing under USD $200 per breast. With the use of Vicryl mesh in 76 reconstructions, Tessler et al. have reported a saving of USD $172,112 in direct material costs over 10 months (40).
Discussion
First introduced in 1995 for reconstructive burns surgery, ADMs are extracellular matrix grafts which provide a scaffold upon which the patient’s own cells can repopulate and revascularise the implanted tissue (94). Since its introduction for post-mastectomy breast reconstruction in 2005, multiple studies have detailed varied and inconsistent outcomes on the use of alloplastic adjuncts. To date, they can be classified into two main categories, ADMs that are derived from either allogeneic or xenogeneic dermis, as well as synthetic meshes. To date, there are over ten different products available (Table S1). The absence of comparative data between these products makes choosing the ideal material a significant challenge to the reconstructive surgeon. The primary aim of this systematic review was to summarize the published data available for these alloplastic adjuncts, including analyzing outcome data which available, with particular interest in its role in irradiated tissue and cost-effectiveness. Importantly, most of the published data available are on AlloDerm®.
Despite the majority of systematic reviews and meta-analyzes demonstrating inferior outcomes in ADM-assisted breast reconstructions, Macadam and Lennox suggested superior outcomes with the use of ADMs in single stage, direct-to-implant reconstructions, compared to traditional two-stage reconstructions. Reduced rates of seroma, infection, late revisions, implant loss and capsular contracture were observed (52). The direct placement of an implant may lead to a better match in the volumes of the overlying mastectomy skin flap and implant, leading to reduced rates of seroma. However, this needs to be balanced by the higher risk of skin necrosis. The use of the ADM as an inferolateral sling may allow better control of the inframammary fold, leading to improved cosmesis and lower rates of late revision. The reduced frequency of infection may be a consequence of the reduced seroma rate, as well as avoiding the need for repeated expander manipulation for filling and a second surgery for expander-implant exchange.
Based on the available systematic reviews and meta-analyzes, skin flap necrosis was the most common complication post ADM-assisted breast reconstruction, ranging from 1.1-10.9% (52,62-64,84). This is higher when compared to traditional submuscular techniques (range, 2-6%) (53-56). This increased incidence may be attributable to a number of factors, including a higher intra-operative expander fill volume leading to excessive skin tension, and inappropriate preservation of post-mastectomy skin with ADM use. However, a delicate balance needs to be achieved between adequate expander filling to maximize incorporation of the ADM to the mastectomy skin flap, without creating excessive tension. This outcome may potentially improve with increased surgeon experience. More recently, to address this issue, ADMs have been used in staged, immediate (direct-to-implant) breast reconstruction. In patients at high risk of skin flap necrosis, reconstruction using an implant and ADM sling was performed 2 weeks after the initial mastectomy, without the use of interval expanders. Initial results are promising, with no infectious or bleeding complications, and no cases of nipple malposition (95).
One of the main concerns regarding the use of ADMs is the increased risk of infection, as some are ‘aseptic’, and not terminally sterilized (i.e., a sterility assurance level of 10−6). The majority of published evidence confirms this concern, with three meta-analyzes and a systematic review pointing to increased rates of infection in ADM-assisted breast reconstruction compared to standard submuscular techniques (62,63,84,96). A possible explanation for this is that prior to being revascularised, which takes approximately 2 weeks to occur, ADMs may act as a nidus for infection (33). However, there are numerous potential confounding factors that may affect the rate of infection [e.g., patient age, smoking status, diabetes body mass index (BMI), radio- or chemotherapy]. Studies have shown that a higher BMI, higher age, larger breasts (>600 grams), presence of axillary dissection and chemo-radiation are significant risk factors for infection (26,93,97,98). Furthermore, studies may have varying definitions of infection, with a number of studies having both ‘infection’, and ‘cellulitis’, as outcomes of interest, terminally sterilized human ADMs have recently been introduced, including AlloMax and AlloDerm Ready to Use, and xenogeneic ADMs (e.g., Strattice and SurgiMend PRS) are also terminally sterilized, which may theoretically improve the infection rate. Importantly, the red breast syndrome is associated with ADM use, and may be mistaken for infection in some cases. This typically manifests as erythema limited to the region overlying the ADM, and is often self-limiting and not responsive to antibiotics. The underlying aetiology remains unclear, but may represent a delayed hypersensitivity reaction (66,99).
Furthermore, multiple studies have demonstrated a higher rate of seroma in the ADM versus the non-ADM group (62,63,84). This may be a result of a mismatch between the size of the overlying skin envelope and the underlying tissue expander volume. Additionally, seromas are also more likely to form prior to revascularization of the ADM. Further confounding factors, including surgical technique, concomitant axillary node dissection, placement and number of drains may also affect risk of seroma formation.
The use of synthetic mesh, particularly Vicryl mesh, appears to show promising outcomes as a comparable, but cheaper alternative to ADMs. However, one of the major concerns of using absorbable mesh as an inferolateral sling is implant malposition or ‘bottoming out’, in the long-term, as Vicryl mesh is normally resorbed by 3 to 4 weeks (40). The introduction of TIGR® mesh was meant to address this, but published data is scarce and despite a small sample size (19 breasts), demonstrated inferior outcomes (13). Furthermore, the use of TIGR® and Vicryl mesh may be limited to non-irradiated tissue, as the complication rate was significantly higher in irradiated patients (13,100). Further higher powered, long-term studies on the use of these synthetic meshes are needed.
Limitations
Direct comparison between alloplastic adjuncts is challenging, as there are distinct differences between ADMs and synthetic meshes, and also between different types of ADMs themselves. The definition of outcome measures in the included studies may also differ, making direct comparison challenging. For example, seromas may be classified into those that require drainage, or those that are simply observed. Additionally, a limitation inherent in most surgical outcome studies is accounting for the heterogeneity in surgeon skill and technique, which may be an important confounding factor. Related to this is the type of mastectomy performed (simple, skin sparing, nipple sparing, modified radical), and initial fill volumes in tissue expander reconstructions, as these will influence the rate of skin flap necrosis and subsequent complications. Importantly, a significant number of studies did not differentiate between single- and two-stage reconstructions, which may affect the results as these two techniques have different complication profiles. Due to the retrospective nature of the majority of included studies, the number of complications reported may be underestimated. Furthermore, there may be an element of publication bias as researchers are less likely to publish unfavorable results.
Conclusions
The majority of systematic reviews and plural of meta-analysis demonstrate increased complication rates in ADM-assisted expander-implant reconstruction compared to traditional submuscular techniques. However, the potential benefits, including superior outcomes in single-stage direct-to-implant surgery, improved cosmesis, lower costs and reduced incidences of capsular contracture, must also be considered. The reported protective effects of ADMs in irradiated tissue are inconsistent. Additionally, due to the diversity of available products, one of the main challenges is selecting the ideal material. There remains a paucity of literature comparing the long-term outcomes between the different types of alloplastic adjuncts and further studies are required to identify the superior adjunct.
Acknowledgements
None.
Table S1. Summary of systematic reviews and meta-analyses.
| Author, Country | Title | Level of evidence | Year | Type of adjunct | Type of study | Summary |
|---|---|---|---|---|---|---|
| Adetayo, USA | A meta-analysis of outcomes using acellular dermal matrix in breast and abdominal wall reconstructions: event rates and risk factors predictive of complications | III | 2011 | AlloDerm | Meta-analysis | Rates of complications: wound dehiscence 2.1%; seroma rate 4.1%; cellulitis 4.4%, wound infection 5.1%, implant failure 6.1%. Radiation and chemotherapy are significantly associated with the development of cellulitis and seroma, respectively |
| Basu, USA | The role of acellular dermal matrices in capsular contracture: a review of the evidence | III | 2012 | AlloDerm, FlexHD, DermaMatrix, NeoForm, Strattice, Surgimend | Systematic review | Acellular dermal matrice (ADM) use was associated with less capsular contracture [odds ratio (OR), 0.18; 95 percent confidence interval (95% CI), 0.08-0.43], providing the highest level of evidence to date (Level III) |
| Ho, USA | A systematic review and meta-analysis of complications associated with acellular dermal matrix-assisted breast reconstruction | III | 2012 | N/A | Systematic review and meta-analysis | There was an increased likelihood of seroma, infection, and reconstructive failure in ADM-assisted reconstructions. With this information in hand, surgeons who routinely use ADM should consider strategies such as underfilling tissue expanders (TEs) to reduce mastectomy skin flap tension, a defined postoperative course of prophylactic antibiotic therapy, and prolonged use of multiple closed suction drains |
| Hoppe, USA | Complications following expander/implant breast reconstruction utilizing ADM: a systematic review and meta-analysis | III | 2011 | N/A | Meta-analysis | There was more than a 2-fold increase in the number of infections and explanations (OR, 2.33; 95% CI, 1.55-3.49) in the ADM group compared to the control. There was a 3-fold increase in seroma formation (OR, 3.00; 95% CI, 1.96-4.61) in the ADM group compared to the control. There was a significant difference of intraoperative fill volumes between the ADM group compared to the control |
| Jansen, Canada | The use of AlloDerm in postmastectomy alloplastic breast reconstruction: part I. A systematic review | III | 2011 | AlloDerm | Systematic review | Complications using AlloDerm are comparable to those of non-AlloDerm alloplastic reconstructions. AlloDerm appears to confer a low rate of capsular contracture |
| JoAnna, USA | Use of human acellular dermal matrix in implant- based breast reconstruction: evaluating the evidence | IV | 2011 | N/A | Narrative review | There was inconsistent data for commonly perceived advantages, such as: eliminating the need for expanders, increased initial fill volumes, fewer expansions, faster time to reconstruction completion, decreased rate of revision, and improved aesthetic outcome. There was consistent support for a decreased incidence of capsular contracture; however the existing reports have limited long term follow-up. Both long term outcomes and randomized controlled prospective studies are needed in order to definitively evaluate the perceived advantages of ADM in breast reconstruction |
| Kim, USA | A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction | III | 2012 | N/A | Meta-analysis | The meta-analysis suggests that the use of human ADM increases complication rates compared to submuscular expander/implant reconstruction. This must be weighed against its reported advantages in enhancing cosmesis and ameliorating contracture |
| Macadam, Canada | Acellular dermal matrices: Use in reconstructive and aesthetic breast surgery | IV | 2012 | AlloDerm, DermaMatrix, Strattice, Flex HD, SurgiMend | Literature review | The use of ADMs in direct-to-implant reconstruction resulted in lower rates of complications when compared to submusclar techniques. In two-stage expander-to-implant reconstructions, ADM use was associated with higher rates of complications |
| Newman, USA | The true incidence of near-term postoperative complications in prosthetic breast reconstruction utilizing human acellular dermal matrices: a meta-analysis | III | 2011 | Human Acellular Dermal Matrices | Meta-analysis | The true incidence of postoperative complications in the near term utilizing human ADM (HADM) in prosthetic-based breast reconstruction appears to be approximately 12%. The most common complications were flap necrosis (3.3%), seroma (3.3%), and infection (5.6%). The incidence of long-term complications such as capsular contracture remains unknown |
| Phillips, USA | A systematic review of infection rates and associated antibiotic duration in acellular dermal matrix breast reconstruction | III | 2014 | N/A | Systematic review | This article reviewed a pooled 3,189 ADM reconstructions. Mean infection rates varied between 0% and 31.25%, with a combined average of 11.59%. Breast reconstruction is associated with a high infection and overall complication rate. Patients are frequently managed with postoperative antibiotics, although the current literature lacks consensus on the necessary duration following ADM breast reconstruction. The potential increased risk of infection associated with ADM remains controversial, with deficient high-level evidence supporting the necessity for postoperative antibiotics. This study found that ADM reconstruction was associated with a higher infection rate than that reported in patients with non-ADM reconstruction |
| Sbitany, USA | Acellular dermis-assisted prosthetic breast reconstruction: a systematic and critical review of efficacy and associated morbidity | III | 2011 | AlloDerm, Strattice, FlexHD | Systematic review | The use of ADM in two-stage expander/implant reconstruction offers a safety profile similar to that of standard submuscular techniques. Both techniques have shown similar rates of infection ultimately requiring explantation. In addition, ADM offers the advantage of a more rapid reconstruction with less need for manipulation of the prosthetic through filling |
| Valdatta, Italy | Acellular dermal matrices and radiotherapy in breast reconstruction: a systematic review and meta-analysis of the literature | III | 2014 | AlloDerm, Strattice, Allomax, Permacol, Surgimed | Meta-analysis | In ADM assisted reconstructions, radiotherapy resulted in a higher overall complication rate compared to non-irradiated breasts (33% vs. 6%). Specifically, radiotherapy significantly increased the risk of skin necrosis, capsular contracture and implant failure |
| Cleimens, USA | Acellular dermal matrix in irradiated tissue expander/implant-based breast reconstruction: evidence-based review | III | 2012 | N/A | Literature review & case series | The ten clinical studies included 246 irradiated patients. The M. D. Anderson experience included 30 irradiated ADM patients for a total of 276 irradiated patients evaluated in this review. Use of ADM in implant-based breast reconstruction in the setting of radiation therapy did not predispose to higher infection or overall complication rates or prevent bioprosthetic mesh incorporation. Use of ADM for implant-based breast reconstruction does not appear to increase or decrease the risk of complications, but it might provide psychological and aesthetic benefits. Multicenter or single-center randomized controlled trials that provide high-quality, level I evidence are warranted |
| Ibrahim, USA | Acellular dermal matrices in breast surgery: a comprehensive review | IV | 2013 | N/A | Narrative review | The direct comparison of most common ADMs is detailed along with a review of 26 series of breast reconstruction manuscripts involving the usage of ADMs. Specifically, Strattice and Permacol had the highest values of maximum loads sustained, stiffness, and tensile strength. ADMs have a role in breast surgery that continues to be defined. Future long-term follow-up remains crucial to the identification of the optimal biologic mesh |
| Israeli, USA | Complications of acellular dermal matrices in breast surgery | IV | 2012 | N/A | Narrative review | Numerous benefits have been reported with this approach including improved fold control, better support and control of the implant pocket with concomitant reduced risk of malposition, and improved lower pole expansion. Seroma, infection, mastectomy skin necrosis, and expander/implant loss are the most commonly reported complications with this approach, and the incidences vary widely among studies |
N/A, not applicable.
Table S2. Summary of level III and IV studies.
| Author, Country | Title | Level of evidence | Type of adjunct | Type of study | Sample population | Recommendations/conclusions |
|---|---|---|---|---|---|---|
| Appleton, UK | The use of Strattice™ in immediate implant based breast reconstruction in higher risk patients | IV | Strattice | Case series | 22 | This data suggests that Strattice™ may be used in immediate breast reconstruction (IBR) in higher risk patients with acceptable surgical and cosmetic results. We would, however, not utilise it post radiotherapy |
| Bank, USA | Economic analysis and review of the literature on implant-based breast reconstruction with and without the use of the acellular dermal matrix | IV | AlloDerm, Strattice | Retrospective analysis | 61 AlloDerm & 23 Strattice | The use of acellular dermal matrice (ADM) in two-stage reconstruction reduces the number of visits required for reconstructions with 350 mL or more. However, at current pricings, the direct cost of ADM use does not offset the cost savings from the reduced number of visits |
| Barber, UK | Outcome of the use of acellular-dermal matrix to assist implant-based breast reconstruction in a single centre | IV | Strattice, Permacol, AlloDerm | Case series | 156 Strattice, 73 Permacol, 3 AlloDerm; total 232 ADM reconstructions (147 patients) | While offering potential cosmetic and financial benefits, the use of ADM with implant-based reconstructions has a significant rate of implant loss, further surgery and potential delay in adjuvant therapy. These must be considered when planning treatment and consenting patients |
| Brooke, USA | Complications in tissue expander breast reconstruction: a comparison of AlloDerm, DermaMatrix, and FlexHD acellular inferior pole dermal slings | III | AlloDerm, DermaMatrix, FlexHD | Retrospective analysis | 284 breast reconstructions, 49 AlloDerm, 110 DermaMatrix, 62 FlexHD, 64 no ADM at all |
AlloDerm, DermaMatrix, and FlexHD were compared in regards to clinically significant complications (CSCs) with an emphasis on infection and conclude that there is no difference in complication rates or incidence of infection between ADM types. Additionally, no significant increased rates of overall complications and infections are seen with ADM. The use of ADM does provide specific benefits that should not be overlooked with increasing reports of complications |
| Buseman, USA | Comparison of sterile versus nonsterile acellular dermal matrices for breast reconstruction | III | AlloDerm | Retrospective analysis | 58 patients, 9 had the sterile form of ADM placed, 25 non-sterile ADM, and 24 were not reconstructed with ADM | We identified an increased rate of seroma development in our patient population with the use of sterile, ready-to-use ADM for breast reconstruction. Although limited by sample size, no other factors could be identified to cause this increased seroma rate. The only factor that was shown to have an influence on this seroma rate was the use of the sterile, ready-to-use ADM |
| Butterfield, USA | 440 Consecutive immediate, implant-based, single-surgeon breast reconstructions in 281 patients: a comparison of early outcomes and costs between SurgiMend fetal bovine and AlloDerm human cadaveric acellular dermal matrices | III | SurgiMend, AlloDerm | Retrospective analysis | 281 patients had 440 implant-based reconstructions using SurgiMend [222 patients (79.0 percent)] or AlloDerm [59 patients (21.0 percent)]. | No significant differences in complication rates were observed for hematoma, infection, major skin necrosis, or breast implant removal. Seroma was the most prevalent complication; the seroma rate for AlloDerm (15.7 percent) was significantly greater than that for SurgiMend (8.3 percent) |
| Chun, USA | Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications | IV | AlloDerm | Retrospective analysis | 283 patients (415 IBRs): 269 reconstructions with tissue expander/implants with ADMs, and 146 non-ADM tissue expander/implants reconstructions | ADM assisted breast reconstruction is associated with higher rates of postoperative seroma and infection |
| Cayci, USA | Impact and outcome of human acellular dermal matrix size for immediate and two-stage breast reconstruction | III | AlloDerm | Retrospective analysis | 52 patients (88 operated breasts) group A, a small matrix with a surface area of 48 or 96 cm was used. In group B, a larger matrix with either 128 or 160 cm was used | Using larger ADM thereby can reduce the number of subsequent expansions and may even decrease the risk of postoperative complications. Our results also revealed that using a larger human ADM is a safe method that does not increase complications |
| Collis, USA | Acellular dermal matrix slings in tissue expander breast reconstruction: are there substantial benefits? | III | Alloderm, Flex HD | Case control study | 63 patients (106 breasts) in the ADM group and 42 patients (68 breasts) in the control group | Initial intraoperative fill volumes were significantly greater in the ADM group, median 69% full (250 mL) versus 50% full (180 mL; P<0.001). One less office visit was required to complete the fills in the ADM group (P<0.01). Drains were removed 3 days later in the ADM group (P<0.01). Overall complication rate was greater in the ADM group (18.9% vs. 7.4%, P<0.05), with a slightly higher percentage of expanders requiring removal due to infection in the ADM group (5.7% vs. 4.4%, P=NS) |
| Colwell, USA | Retrospective review of 331 consecutive immediate single-stage implant reconstructions with acellular dermal matrix: indications, complications, trends, and costs | III | AlloDerm | Retrospective study | 211 patients had 331 direct-to-implant reconstructions using AlloDerm following nipple-sparing (n=66) or skin-sparing (n=265) mastectomy for cancer (n=216) or prophylaxis (n=115) | Immediate single-stage implant reconstruction using ADM offers a cost-effective reconstruction with a low complication rate. This may be the procedure of choice in select patients |
| Evgeniou, UK | 555 Implant Based Immediate Breast Reconstruction Utilising Strattice(tm) Mesh and Its Impact On Adjuvant Treatment | IV | Strattice | Case series | 21 implant based IBR utilising Strattice™ in 17 patients | 53% (9/17) of patients had complications requiring clinical intervention. We suggest these complications may be addressed at three points; Pre-operatively consideration should be given to the necessity for adjuvant treatment and the type of skin sparing mastectomy procedure. Inter-operatively thorough washing of the mesh, the use of drains and the choice of implant to minimize tension on the skin wound. Post-operatively patience with repeat aspiration of seroma rather than the assumption of mesh infection in patients with a ‘red flare’ reaction. Utilizing these measures complications could be reduced |
| Fung, UK | The matrix: Strattice vs XCM in immediate implant-based breast reconstruction | IV | Strattice™, XCM | Case series | 22 immediate reconstructions, 9 with XCM, 13 with Strattice | Using XCM and Strattice have produced similar short term outcomes in immediate implant-based breast reconstruction, with little difference in handling properties and complications. In the current economic climate of reducing expenditure, the significantly lower price of XCM is a very attractive feature |
| Gamboa-Bobadilla, USA | Implant breast reconstruction using acellular dermal matrix | IV | AlloDerm | Case series | 13 reconstructions in 11 patients | The study demonstrates allogenic dermal grafting provides a satisfactory option for breast reconstruction, with minimal complications |
| Ganske, USA | Minimizing complications with the use of acellular dermal matrix for immediate implant-based breast reconstruction | III | N/A | Retrospective analysis | 179 implant-based reconstructions were compared to results of a series of 150 similar procedures performed by the lead author before institution of the procedural modifications described | Although implant-based breast reconstruction with ADM has previously been associated with increased seroma and infection rates, specific technical measures to reduce seroma formation can significantly reduce the rate of these postoperative complications. We recommend that surgeons performing these procedures consider drainage of both the submastectomy and sub-ADM pocket, a drain removal threshold of less than 20 mL/24 h, as well as the use of soft compression dressings and bras postoperatively. As in most surgical decision-making situations, careful patient selection should be performed to optimize the final reconstructive outcome when using ADM in immediate prosthetic breast reconstruction |
| Glasberg, USA | AlloDerm and Strattice in breast reconstruction: a comparison and techniques for optimizing outcomes | III | AlloDerm, Strattice | Retrospective analysis | 96 patients (126 reconstructions) received AlloDerm, and 90 (144 reconstructions) received Strattice | The use of AlloDerm or Strattice to extend the span of the pectoralis major muscle at the inferior pole allows for easier expansion of the breast lower pole while helping the surgeon to better define the inframammary fold and lateral mammary fold, leading to consistent surgical outcomes. In addition, our data indicate that both ADMs are associated with a low rate of capsular contracture. Meticulous technique is essential for optimizing and achieving consistent results and limiting the risk of complications. Even small technical advancements and changes can yield improved results and outcomes. Overall, the complications in this series were of low severity which, together with the consistent surgical outcomes seen in the authors’ practice, justifies the use of ADMs in breast reconstruction and offsets their initial cost |
| Gubitosi, Italy | Acellular bovine pericardium dermal matrix in immediate breast reconstruction after skin sparing mastectomy | IV | Tutomesh | Case series | A total of 24 patients underwent 28 IBR with Tutomesh ADM implant | The use of Tutomesh® bovine pericardium for immediate breast is safe and technically useful. Complications rate is not high, except for seroma formation that can be reduced by the contemporary use of fibrin sealant |
| Hanna, USA | Comparison study of two types of expander-based breast reconstruction: acellular dermal matrix-assisted versus total submuscular placement | III | AlloDerm | Retrospective analysis | 100 reconstructions on 75 patients | Total complications including seroma, hematoma, infection, skin necrosis, and explantation did not significantly differ between groups (n=13 for ADM vs. n=17 for submuscular, P=0.814). Consistent with prior reports, ADM-based reconstructions were associated with significantly increased intraoperative fill volumes and lower total number of sessions to achieve final volume. Submuscular reconstructions required a significantly higher tissue expander fill volume |
| Hille-Betz, Germany | Breast Reconstruction and Revision Surgery for Implant-associated Breast Deformities Using Porcine Acellular Dermal Matrix: A Multicenter Study of 156 Cases | III | Strattice | Retrospective analysis | 127 patients were identified who underwent breast reconstructions in 156 breasts using an acellular porcine dermal matrix. Split into three groups | Total major complication rate was 7.1%: implant loss (3.2%), skin flap necrosis (2.6%), delayed skin healing (2.6%), hematoma (1.9%), seroma (1.3%), infection (0.6%), and capsular contracture (0.6%). Total minor complication rate was 22.9%, with seroma being the most frequent complication (19.2%). In the group of IBRs, 20.4% of the breasts had received radiotherapy in the past. These patients exhibited a significantly higher rate of seroma than patients without prior radiotherapy (35.0% vs. 14.9%, P=0.031) |
| Jordan, USA | An algorithmic approach for selective acellular dermal matrix use in immediate two-stage breast reconstruction: indications and outcomes |
III | N/A | Retrospective analysis | 193 breasts underwent reconstruction before and 179 underwent reconstruction after implementation of the algorithm | We present an evidence-based, resource-sensitive algorithm for ADM use in breast reconstruction. Our indications and contraindications resulted in a nearly 50 percent reduction in ADM use, with significant cost savings |
| Lanier, USA | The effect of acellular dermal matrix use on complication rates in tissue expander/implant breast reconstruction | III | AlloDerm, Strattice, FlexHD | Retrospective analysis | ADM (n=75) versus standard submuscular placement (n=52) | The ADM group had a statistically significant higher rate of infection (28.9% vs. 12.0%, P=0.022), reoperation (25.0% vs. 8.0%, P=0.011), expander explantation (19.2% vs. 5.3%, P=0.020), and overall complications (46.2% vs. 22.7%, P=0.007). When stratifying by breast size, a higher complication rate was not observed with the use of ADM in breasts less than 600 g, whereas ADM use in breasts larger than 600 g was associated with a statistically significant higher rate of infection when controlling for the occurrence of skin necrosis. The ADM cohort had a significantly higher mean initial tissue expander fill volume (256 vs. 74 mL, P<0.001) and a significantly higher mean initial tissue expander fill ratio (49% vs. 17%, P<0.001) |
| Lardi, UK | Immediate breast reconstruction with acellular dermal matrix: Factors affecting outcome | III | Strattice | Retrospective cohort study | A total of 149 patients underwent 200 reconstructions (110 one-stage and 90 two-stage) following oncologic (134 breasts) or prophylactic (66 breasts) mastectomy | The high rate of early complications in this study was mostly related to patient characteristics and learning curves and highlights the importance of patient selection and technique principles in optimizing the outcome |
| Lee, Korea | A comparative study of CG CryoDerm and AlloDerm in direct-to-implant immediate breast reconstruction | III | Alloderm, Cryoderm | Retrospective analysis | 50 patients who underwent direct-to-implant breast reconstruction using AlloDerm (n=31) or CryoDerm (n=19) | There were no significant differences in the overall incidence of complications (seroma, infection, skin flap necrosis, capsular contracture, and implant loss) between the two groups. Nor was there any significant difference in the duration of drainage |
| Liu A, USA | Postoperative complications in prosthesis-based breast reconstruction using acellular dermal matrix | III | AlloDerm | Retrospective analysis | 470 postmastectomy defects | Patient selection for prosthesis reconstruction involving ADM should be judicious, especially among smokers and patients with elevated body mass index (BMI). Even though the use of ADM allows higher initial volumes and reduced number of expansions, one should be careful about putting in too high of an initial volume |
| Liu D, USA | Comparison of outcomes using AlloDerm versus FlexHD for implant-based breast reconstruction | III | Alloderm, Flex HD | Retrospective analysis | 382 consecutive women (547 total breasts) | There is no significant difference in the complication rates between AlloDerm and FlexHD in IBR. Multivariate analysis suggests that FlexHD may be a risk factor for implant loss |
| Losken, USA | Early Results Using Sterilized Acellular Human Dermis (Neoform) in Post-Mastectomy Tissue Expander Breast Reconstruction | IV | AlloMax | Case series | 22 consecutive patients (31 breasts) | No post-operative complications related to AlloMax |
| Lynch, USA | Dermal autografts as a substitute for acellular dermal matrices (ADM) in tissue expander breast reconstruction: a prospective comparative study | III | AlloDerm | Prospective non-randomised study | 48 patients were enrolled (76 breasts). 27 patients received ADM, and twenty-one patients received dermal autograft | Patients receiving dermal autograft had a lower incidence of major complications and delayed wound healing than patients who received ADM. Despite harvest time, the overall cost of the ADM-assisted expander placement was higher. Dermal autograft-assisted breast reconstruction offers many of the benefits of ADM, but with a lower cost and improved safety profile |
| McCarthy, USA | The use of acellular dermal matrices in two-stage expander/implant reconstruction: a multicenter, blinded, randomized controlled trial | II | AlloDerm | Randomized control trial | 108 consented to participate; 38 were excluded prior to randomization. In total, 70 patients were randomized (36 in ADM and 34 in non-ADM group) | There was no significant difference in adverse outcomes (haematoma, seroma and infection) between the ADM and non-ADM group (17% vs. 15% respectively, P=1.00). The use of ADM in the setting of tissue expander/implant reconstruction neither reduces postoperative pain nor accelerates the rate of postoperative expansion |
| Mendenhall, USA | The BREASTrial: stage I. Outcomes from the time of tissue expander and acellular dermal matrix placement to definitive reconstruction | II | AlloDerm, DermaMatrix | Randomized control trial BREASTrial | 128 patients (199 breasts) were randomized equally over 2.5 years | There was no significant difference between AlloDerm and DermaMatrix groups in overall complication incidence (33.6 percent versus 38.8 percent; P=0.50). Obesity was a predictor of poor ADM biointegration and a longer need for drains, both of which were associated with tissue expander loss. Based on these data, we recommend guarded use of ADM in obese patients |
| Michelotti, USA | Analysis of clinically significant seroma formation in breast reconstruction using acellular dermal grafts | III | AlloDerm, DermaMatrix, FlexHD | Retrospective analysis | 284 consecutive tissue expander-based breast reconstruction (TEBR) | Multivariate analysis identified a strong trend toward FHD as an independent predictor of seroma formation (P=0.061). Although the aesthetic and perioperative benefits of acellular dermal grafts used as an adjunct in tissue expander-based reconstruction cannot be ignored, the safety of this procedure should be constantly reexamined as new biomaterials emerge with differing processing and storage techniques. FHD reconstructions trended toward the formation of seromas with increased frequency as compared to the other available dermal products |
| Myckatyn, USA | The impact of chemotherapy and radiation therapy on the remodeling of acellular dermal matrices in staged, prosthetic breast reconstruction | III | AlloDerm | Prospective observational study | Multiple biopsy specimens were procured from 86 women (n=94 breasts) undergoing exchange of a tissue expander for a breast implant. | Chemotherapy and radiation therapy limit ADM remodeling |
| Nahabedian 1, USA | AlloDerm performance in the setting of prosthetic breast surgery, infection, and irradiation | III | AlloDerm | Retrospective analysis | A total of 361 women and 476 breasts underwent reconstruction or revision with prosthetic devices. Of these, 76 women and 100 breasts underwent reconstruction using AlloDerm assistance | In summary, the incidence of prosthetic infection following breast reconstruction with prosthetic devices remains between 5 and 6 percent in this author’s practice. The use of AlloDerm does not appear to increase or decrease this risk. In the setting of radiation therapy, the incidence of infection, incisional dehiscence, and seroma formation increases slightly in accordance with that associated with the radiation. Delayed healing and skin necrosis were not observed in the women who had prosthetic reconstruction, AlloDerm, and radiation therapy |
| Nahabedian 2, USA | Acellular dermal matrix for secondary procedures following prosthetic breast reconstruction | IV | N/A | Case series | 51 patients | The authors review their cumulative experience between 2004 and 2010 with ADM for the correction of secondary deformities following prosthetic breast reconstruction, focusing on the indications for repair, traditional management, and management with ADM |
| Parks, USA | Human acellular dermis versus no acellular dermis in tissue expansion breast reconstruction | III | AlloDerm | Retrospective analysis | 232 patients and 346 breasts were reconstructed with and 114 patients and 165 breasts without acellular dermis | Seroma occurrence in the acellular dermis group was nearly twice (30.0% versus 15.1%) that of the no acellular dermis breasts, but the tissue expander loss was only slightly higher (11.6% versus 8.5%) and not statistically significant |
| Peled, USA | The effects of acellular dermal matrix in expander-implant breast reconstruction after total skin-sparing mastectomy: results of a prospective practice improvement study | III | AlloDerm | Prospective cohort analysis | 450 cases in 288 patients | ADM use in expander-implant reconstruction after total skin-sparing mastectomy (SSM) reduced major postoperative complications in this study. Maximal benefit is achieved with selected use in patients with thin mastectomy skin flaps and those receiving radiation therapy |
| Pilgrim, UK | Outcomes following implant-based breast reconstruction using Strattice™ acellular dermal matrix | III | Strattice | Case series | 51 implant-based reconstructions 44 patients | While the majority of implant-based breast reconstructions with Strattice™ ADM have a successful outcome, patients should be counselled pre-operatively that complications resulting in revision surgery or implant loss occur relatively frequently despite careful patient selection. The rates in this study are consistent with previously published results for reconstructions using ADMs |
| Potter, UK | Early complications and implant loss in implant-based breast reconstruction with and without acellular dermal matrix (Tecnoss Protexa®): a comparative study | IV | Tecnoss Protexa | Case series | 46 implant-based reconstructions were performed for malignancy (n=31, 67.4%) or prophylaxis (n=15, 32.6%) in 31 women | ADM-assisted implant-based reconstruction with Tecnoss Protexa® is safe and may improve outcomes for women by facilitating a single-stage procedure. Robust prospective evaluation is now needed to definitively evaluate the role of ADM in implant-based breast reconstruction |
| Rawlani, USA | Tissue expander breast reconstruction using prehydrated human acellular dermis | IV | Flex HD | Case series | 121 consecutive tissue expander reconstructions | The outcomes and complication rates of prehydrated HADM (PHADM) tissue expander breast reconstruction are comparable to those reported with freeze-dried human acellular dermis |
| Rundell, USA | Complication prevalence following use of tutoplast-derived human acellular dermal matrix in prosthetic breast reconstruction: A retrospective review of 203 patients | III | AlloMax (Formally neoform, formally totoplast) | Retrospective analysis | 203 patients, 348 breast reconstructions | The complication profile following T-ADM use is this series is comparable to that reported for with other ADM products. T-ADM appears to be a safe and acceptable option for use in ADM-assisted breast reconstruction |
| Salzberg, USA | An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm) | III | AlloDerm | Retrospective analysis | 466 breasts (260 patients) were reconstructed; 68 percent were prophylactic and 32 percent were oncologic case | Human ADM-assisted direct-to-implant breast reconstruction following mastectomy is safe and reliable, with a low overall long-term complication rate. The low incidence of capsular contracture supports the growing body of evidence that human ADM mitigates capsular contracture |
| Sbitany, USA | Acellular dermis-assisted prosthetic breast reconstruction versus complete submuscular coverage: a head-to-head comparison of outcomes | III | AlloDerm | Retrospective analysis | 100 women underwent breast reconstruction with 172 expanders, in 50 using complete submuscular placement and in 50 using partial subpectoral placement with acellular dermis | Acellular dermis–assisted implant breast reconstruction has a safety profile no worse than that of complete submuscular coverage, with the benefit of fewer expansions and the potential for an improved cosmetic outcome |
| Seth, USA | A comparative analysis of cryopreserved versus prehydrated human acellular dermal matrices in tissue expander breast reconstruction | III | Cryopreserved and prehydrated HADM | Retrospective analysis | 255 patients (369 breasts) underwent breast reconstruction utilizing either cryopreserved or prehydrated HADM. | The results of this study suggest that there are no significant differences in complication rates between cryopreserved and prehydrated HADMs |
| Shetty, UK | Learning curve in immediate breast reconstruction with Strattice acellular dermal matrix | IV | Strattice | Case series | 67 patients—40 unilateral & 27 had bilateral reconstruction | Our study has demonstrated a significantly shorter operative time following a learning curve but no difference in the complication rate |
| Spear 1, USA | Two-stage prosthetic breast reconstruction using AlloDerm including outcomes of different timings of radiotherapy | III | AlloDerm | Retrospective analysis | AlloDerm-assisted prosthetic reconstruction was performed in 289 women (428 breasts) | In AlloDerm-assisted prosthetic breast reconstruction, irradiated devices demonstrated higher rates of clinically significant capsular contracture following the first stage. These rates declined considerably on completion of reconstruction, with prostheses irradiated during expansion still having the highest frequency of clinically significant capsular contracture. With the follow-up reported, irradiated devices failed breast reconstruction less frequently and required autologous tissue less often than has been historically reported without ADM |
| Spear 2, USA | Applications of acellular dermal matrix in revision breast reconstruction surgery | III | AlloDerm | Retrospective analysis | 135 revision breast reconstructive procedures using ADM (AlloDerm) in 118 patients (154 breasts) | ADM has proven to be a reliable tool for managing some of the most common and challenging problems in implant-based breast reconstruction. Although there are few published data on the success of more conventional solutions to fold malposition, lower pole support, and capsular contracture, the addition of ADM to buttress these repairs has been shown to provide a high likelihood of success with a low risk of complications |
| Valassiadou, UK | Risk-reducing mastectomy and breast reconstruction for BRCA1, BRCA2 carriers and high-risk women and role of acellular dermal matrices in reducing the risk of revision surgery | IV | Strattice, Surgimend | Case series | 92 risk reducing mastectomy (RRM) procedures in 48 patients (21 patients with previous breast cancer) were analysed | 2-stage implant is superior to 1-stage and use of ADMs is safe with no increased operative complication rate and may play an important role in reducing revision rate |
| Vardanian, USA | Comparison of implant-based immediate breast reconstruction with and without acellular dermal matrix | III | AlloDerm | Retrospective analysis | 203 patients underwent 337 immediate expander-based breast reconstructions [with ADM, n=208 (61.7 percent); without, n=129 (38.3 percent)] | ADM use appears safe and is associated with less capsular contracture and mechanical shift and improvement in the inframammary fold appearance, without increasing postoperative complications |
| Venturi, USA | Evaluating sterile human acellular dermal matrix in immediate expander-based breast reconstruction: a multicenter, prospective, cohort study | III | AlloMax | Prospective cohort analysis | 65 consecutive TEBR in a cohort of patients | The preliminary data suggest that a sterile HADM offers an advantage over aseptic HADM with regard to the rate of infection and seroma. The sterilization process does not negatively impact the incorporation of the graft by the host tissue. Further head-to-head data are necessary to confirm these findings |
| Weichman 1, USA | Sterile “ready-to-use” AlloDerm decreases postoperative infectious complications in patients undergoing immediate implant-based breast reconstruction with acellular dermal matrix | III | AlloDerm | Prospective cohort analysis | A total of 546 reconstructed breasts met inclusion criteria: 64.3 percent (n=351) with no ADM, 16.5 percent (n=90) with aseptic matrix, and 19.2 percent (n=105) with ready-to-use matrix | Although not indicated for all patients, the use of ready-to-use ADM in immediate implant-based breast reconstruction provides an acceptable and useful adjunct. In addition, ready-to-use ADM mitigates the risks of infectious complications in patients undergoing immediate implant-based breast reconstruction when compared with aseptic ADM. However, diabetes mellitus, mastectomy skin flap necrosis, aseptic ADM, and seroma all prove to be independent risk factors for developing perioperative infectious complications |
| Weichman 2, USA | The use of acellular dermal matrix in immediate two-stage tissue expander breast reconstruction | III | AlloDerm | Retrospective analysis | A total of 407 patients underwent 628 immediate two-stage, implant-based breast reconstructions; 442 reconstructions (70.3 percent) used ADM and 186 (29.6 percent) did not | Use of ADM in immediate two-stage, implant-based breast cancer reconstruction is associated with a significant increase in major complications. Therefore, it should only be used in specific patients and in minimal amounts. Indications for its use include single-stage permanent implant reconstruction and inadequate local muscle coverage of the tissue expander |
| Westbroek, USA | Prospective outcomes trial of immediate, implant breast reconstruction using acellular dermal matrix (POBRAD Trial): Pilot series results | IV | SurgiMend | Pilot study | 20 consecutive patients undergoing immediate, implant breast reconstruction were prospectively accrued to the study | This is to our knowledge the only prospectively accrued dataset seeking to critically evaluate the clinical efficacy, cost-benefit value and enhanced aesthetic utility of ADMs (SurgiMend PRS) in the IBR setting. The early results provide new evidence in support of the use ADMs in breast reconstruction and validate our intention to extend the study to a multicentre trial |
| Yuen, USA | Comparison between Freeze-dried and Ready-to-use AlloDerm in Alloplastic Breast Reconstruction | III | AlloDerm | Retrospective analysis | The first 51 patients underwent 96 IBRs with FD AlloDerm. The subsequent 52 patients underwent 100 IBRs with RTU AlloDerm | This study suggests a clinically higher infection rate in IBR with the new version of AlloDerm, RTU, compared with the cryopreserved AlloDerm. Furthermore, this study confirms a significantly higher postoperative complication rate associated with obesity |
N/A, not applicable; NS, not significant.
Table S3. Summary of cost-analyzing studies.
| Author, Country | Title | Year | Type of adjunct | Type of study | Recommendations/conclusions |
|---|---|---|---|---|---|
| Bank, USA | Economic analysis and review of the literature on implant-based breast reconstruction with and without the use of the acellular dermal matrix | 2013 | AlloDerm, Strattice | Retrospective analysis | The use of acellular dermal matrice (ADM) in two-stage reconstruction reduces the number of visits required for reconstructionswith 350 mL or more. However, at current pricings, the direct cost of ADM use does not offset the cost savingsfrom the reduced number of visits |
| Butterfield, USA | 440 Consecutive immediate, implant-based, single-surgeon breast reconstructions in 281 patients: a comparison of early outcomes and costs between SurgiMend fetal bovine and AlloDerm human cadaveric acellular dermal matrices | 2013 | Surgimend, AlloDerm | Retrospective analysis | This study reports the 5-year experience of a single surgeon with use of Surgimend fetal bovine and AlloDerm human cadaveric ADMs in implant-based breast reconstruction. In this retrospective study, one of the larger series published to date, early complications were equivalent between the two ADMs. The cost of Surgimend was lower than the cost of AlloDerm, equating to an approximate $1,000 difference per breast reconstructed for equivalently sized ADM units. Along with other factors, such as product sterility, packaging, preparation time, handling characteristics, and others, these two significant factors should be considered in the choice of an ADM for use in breast reconstruction |
| Colwell, USA | Retrospective review of 331 consecutive immediate single-stage implant reconstructions with acellular dermal matrix: indications, complications, trends, and costs | 2011 | AlloDerm | Retrospective study | Immediate single stage implant (SSI) reconstruction using ADM offers a cost-effective reconstruction with a low complication rate. This may be the procedure of choice in select patients |
| de Blacam, USA | Cost analysis of implant-based breast reconstruction with acellular dermal matrix | 2012 | N/A | Economic analysis | In this study, a decision-analysis model is used to compare costs of 3 implant based techniques of breast reconstruction. Based on Medicare reimbursement, SSI with ADM carries the lowest cost even when the probability of complications is incorporated. When evaluating staged reconstruction, the addition of ADM increases the cost compared with the use of TE/I alone. ADM is becoming commonplace in breast surgery, and it is important that surgeons are aware of the cost implications of its use |
| Jansen, Canada | The use of AlloDerm in postmastectomy alloplastic breast reconstruction: part II. A cost analysis | 2011 | AlloDerm | Systematic review part II—cost-analysis | Although AlloDerm is expensive, it appears to be cost-effective if used for direct-to-implant breast reconstruction |
| Macadam, Canada | Acellular Dermal Matrices: Economic Considerations in Reconstructive and Aesthetic Breast Surgery | 2012 | FlexHD, DermaMatrix, AlloMax, Surgimend. TiMesh, TIGR Matrix | Narrative review—economic considerations of ADMs | Cost considerations of ADM use in breast surgery are described and as an example, a single institution’s experience with implementation of ADM into a pre-existing breast surgery program, is used |
N/A, not applicable.
Table S4. Summary of studies focusing on synthetic meshes.
| Author, Country | Title | Level of evidence | Type of adjunct | Type of study | Sample population | Follow up | Recommendations/conclusions |
|---|---|---|---|---|---|---|---|
| Becker, USA | The use of synthetic mesh in reconstructive, revision, and cosmetic breast surgery | IV | TIGR, Matrix Surgical Mesh | Case series | 11 primary breast reconstructions (19 breasts), 43 secondary reconstructions (77 breasts) | Mean 16.5 months (range, 9.4-26.1 months) | The long-term absorbable synthetic matrix, TIGR® Matrix Surgical Mesh, shows potential when used as temporary reinforcement in patients undergoing breast reconstruction or breast surgery revisions and in primary aesthetic procedures, and it appears to be a viable alter—native to the use of acellular dermal matrices (ADMs) |
| Casella, Italy | TiLoop® Bra mesh used for immediate breast reconstruction: comparison of retropectoral and subcutaneous implant placement in a prospective single-institution series | II | TiLoop® Bra | Prospective Study | 73 breast recons group 1 34 reconstructions in 29 women (standard muscular mesh pocket) group 2 —39 reconstructions in 34 women (prepectoral subcutaneous technique) | 13 & 12 months respectively | A titanium-coated polypropylene mesh used as a tool for immediate definitive implant based breast reconstruction (IBBR) resulted, in the short term, to be safe and effective both for a retropectoral and totally subcutaneous implant placement. Long-term results are forthcoming |
| Dieterich 1, Germany | Implant-based breast reconstruction using a titanium-coated polypropylene mesh (TiLOOP Bra): a multicenter study of 231 cases | III | TiLoop® Bra | Retrospective analysis | 207 patients (231 reconstructions) | Mean 14 months | Major complications occurred in 13.4 percent, minor complications in 15.6 percent, and implant loss in 8.7 percent of patients. Univariate analysis revealed procedure-related risk factors for postoperative complications with a bilateral procedure (P=0.013) or skin expansion before implant surgery (P=0.043). Multivariate analysis confirmed these risk factors and revealed an increased risk for implant loss in patients with skin necrosis (P<0.001) and capsule fibrosis (P<0.001) |
| Dieterich 2, Germany | A short-term follow-up of implant based breast reconstruction using a titanium-coated polypropylene mesh (TiLoop(®) Bra) | III | TiLoop® Bra | Retrospective study | 42 patients | 20±6.9 months (range, 7-33 months) | Although the number of patients is small in this study, evaluated patients and results are comparable in resemblance to previous publications of IBBR with ADM. Titanium coated polypropylene mesh (TCPM) can be a supportive tool to facilitate IBBR by stabilizing the implant pocket. For the right indication TCPM is a less expensive and possible alternative to ADM. Longer follow-up data and additional clinical reports are necessary to evaluate the use of this mesh in daily practice |
| Garganese, Italy | Titanized mesh for immediate prosthetic reconstruction of large/extra-large breasts | IV | TiLoop® Bra | Case series | 10 implant reconstructions with TiLoops | 38 weeks | TiLoop allows one-step prosthetic reconstruction in large and extra-large breasts. Our preliminary data show low rates of early complications and high patient satisfaction. Larger series and longer follow up are needed to assess possible complications after radiotherapy, capsular contracture and recurrence rates |
| Paepke 1, Germany | Mesh supported implant-based reconstructive breast surgery | IV | TiLoop® Bra | Narrative review |
N/A | N/A | Results showed an excellent tissue integration, a less pronounced inflammatory reaction, lower recurrence rate, shorter convalescence time and lower shrinkage rate. Considering the insertion of the titanized polypropylene meshs into breast reconstructive surgery, shape and size were optimized specifically for breast surgery |
| Paepke 2, Germany | Titanized polypropylene mesh Tiloop® Bra in reconstructive breast surgery—Indication and complication rate | III | TiLoop® Bra | Literature review | N/A | N/A | In 231 mesh based procedures complications occurred in 4.8% seroma, in 9.5% superficial hematoma, in 6.1% skin infection, in 3.9% skin necrosis, in 3.5% partial nipple necrosis, in 7.8% re-operations with removal of the mesh and in 2.2% capsular fibrosis. Neither nicotine abuse, hypertension, diabetes mellitus, age >50 years, body mass index (BMI) >27, nor radio- or chemotherapy are significant factors for complications |
| Rezai, Germany | Risk-reducing surgery by skin-sparing mastectomy (SSM) and immediate implant-based reconstruction with and without titanized meshes and quality of life | III | TiLoop® Bra | Case cohort study | This large retrospective case cohort trial analysed 197 skin sparing mastectomies (SSM) in 161 patients (45 bilateral cases) and immediate breast reconstruction (IBR) | N/A | The use of titanized meshes was perceived as favourable with regards to better fitting of the breast in the bra and clothing and yielded low complication rates. The rating of patients in terms of self-perception after the reconstruction was good. Immediate reconstruction has a significant positive impact and gives the patient a considerable physical and psychological support |
| Klein, Germany | Analysis of immediate breast reconstruction with the use of titanized polypropylene mesh (TiLOOP® Bra) | III | TiLoop® Bra | Prospective case series | 87 performed combined SSM and immediate prosthetic breast reconstruction with the usage of TiLoop Bra mesh. | N/A | This analysis showed that the application of titanized polypropylene mesh in immediate reconstructive surgery results in an excellent cosmetic result with greater flexibility in forming the former breast shape. It is a safe procedure with a low rate of complications. Additional follow-up data are now required to assess further data on the cosmetic outcome, patients’ satisfaction and oncologic safety |
| Haynes, USA | Vicryl mesh in expander/implant breast reconstruction: long-term follow-up in 38 patients | IV | Vicryl mesh | Case series | 46 breast reconstructions (38 patients; 35 immediate and 3 delayed). 8 irradiated, 38 breasts non-irradiated | 43 months | In the non-irradiated group (38 breasts), there was one postoperative infection (2.6 percent), which required expander removal. In the irradiated group, there were three complications requiring expander removal (37.5 percent): two infections and one device exposure after irradiation. Significant malposition was not observed in any breast where Vicryl mesh was used, and no visible mesh remained at the time of implant placement. The incidence of symptomatic capsular contracture in non-irradiated breasts was 3.2 percent. At latest follow-up, non-irradiated breasts had an average Baker capsule grade of 1.1, compared with 1.5 in the irradiated group |
| Kim, Korea | The suitability of absorbable mesh insertion for oncoplastic breast surgery in patients with breast cancer scheduled to be irradiated | III | Vicryl mesh | Retrospective analysis | 35 breast cancer patients who received IBR with absorbable mesh insertion at the time of breast conserving surgery followed by radiotherapy were retrospectively studied | N/A | Applying an absorbable mesh for the immediate reconstruction of the breast should be carefully considered in patients with an estimated percentage of breast volume excised of over 30% who are scheduled to be irradiated |
| Lim, Korea | Outcomes of corrective procedure with Vicryl mesh as an oncoplastic surgery of the breast | III | Vicryl mesh | Retrospective analysis | 101 patients, 135 recons—In 79 cases, breast conserving surgery (BCS) with Vicryl mesh implantation was performed; in the other 56 cases, only BCS was performed | N/A | Reconstructive procedures with Vicryl mesh are simple, safe, and less expensive than other plastic reconstruction techniques. This study suggests that the procedure was superior to BCS alone in cosmetic outcomes. We believe that the procedure could become a favorable technique in oncoplastic surgery |
| Tessler, USA | Beyond biologics: absorbable mesh as a low-cost, low-complication sling for implant-based breast reconstruction | IV | Vicryl mesh | Case series | 50 consecutive patients (76 reconstructions) | 1.2 years | Results to date have been encouraging, with a low complication rate (6.6 percent) and excellent aesthetic results. The technique has resulted in $172,112 in direct material cost savings over 10 months. Continued follow-up is planned to evaluate long-term results |
| Ganz, Switzerland | Risks and benefits of using an absorbable mesh in one-stage immediate breast reconstruction: a comparative study | III | Vicryl mesh | Retrospective analysis | 161 IBRs in 139 patients | N/A | Larger implants and perhaps better control of implant position were possible using the Vicryl mesh extension without increasing complications. Because the mesh technique also recreates a slightly ptotic breast, fewer contralateral mastopexies were needed. The Vicryl mesh extension is a low-cost alternative to biological matrices or tissue expanders |
N/A, not applicable.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.American Cancer Society. Breast Cancer Facts & Figures 2013-2014. Atlanta: American Cancer Society, Inc. 2013. [Google Scholar]
- 2.Le GM, O'Malley CD, Glaser SL, et al. reast implants following mastectomy in women with early-stage breast cancer: prevalence and impact on survival. Breast Cancer Res 2005;7:R184-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Achauer BM, VanderKam VM, Celikoz B, et al. Augmentation of facial soft-tissue defects with Alloderm dermal graft. Ann Plast Surg 1998;41:503-7. [DOI] [PubMed] [Google Scholar]
- 4.Bastidas N, Ashjian PJ, Sharma S. Acellular dermal matrix for temporary coverage of exposed critical neurovascular structures in extremity wounds. Ann Plast Surg 2009;62:410-3. [DOI] [PubMed] [Google Scholar]
- 5.Breuing KH, Warren SM. Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg 2005;55:232-9. [DOI] [PubMed] [Google Scholar]
- 6.Buinewicz B, Rosen B. Acellular cadaveric dermis (AlloDerm): a new alternative for abdominal hernia repair. Ann Plast Surg 2004;52:188-94. [DOI] [PubMed] [Google Scholar]
- 7.Chang HS, Lee D, Taban M, et al. “En-glove” lysis of lower eyelid retractors with AlloDerm and dermis-fat grafts in lower eyelid retraction surgery. Ophthal Plast Reconstr Surg 2011;27:137-41. [DOI] [PubMed] [Google Scholar]
- 8.Kokkalis ZT, Zanaros G, Weiser RW, et al. Trapezium resection with suspension and interposition arthroplasty using acellular dermal allograft for thumb carpometacarpal arthritis. J Hand Surg Am 2009;34:1029-36. [DOI] [PubMed] [Google Scholar]
- 9.Sajjadian A, Naghshineh N, Rubinstein R. Current status of grafts and implants in rhinoplasty: Part II. Homologous grafts and allogenic implants. Plast Reconstr Surg 2010;125:99e-109e. [DOI] [PubMed] [Google Scholar]
- 10.Wainwright D, Madden M, Luterman A, et al. Clinical evaluation of an acellular allograft dermal matrix in full-thickness burns. J Burn Care Rehabil 1996;17:124-36. [DOI] [PubMed] [Google Scholar]
- 11.Duncan DI. Correction of implant rippling using allograft dermis. Aesthet Surg J 2001;21:81-4. [DOI] [PubMed] [Google Scholar]
- 12.Rietjens M, De Lorenzi F, Venturino M, et al. The suspension technique to avoid the use of tissue expanders in breast reconstruction. Ann Plast Surg 2005;54:467-70. [DOI] [PubMed] [Google Scholar]
- 13.Becker H, Lind JG, 2nd. The use of synthetic mesh in reconstructive, revision, and cosmetic breast surgery. Aesthetic Plast Surg 2013;37:914-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng A, Saint-Cyr M. Comparison of Different ADM Materials in Breast Surgery. Clin Plast Surg 2012;39:167-75. [DOI] [PubMed] [Google Scholar]
- 15.Dieterich M, Faridi A. Biological Matrices and Synthetic Meshes Used in Implant-based Breast Reconstruction - a Review of Products Available in Germany. Geburtshilfe Frauenheilkd 2013;73:1100-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurunluoglu R, Gurunluoglu A, Williams SA, et al. Current trends in breast reconstruction: survey of American Society of Plastic Surgeons 2010. Ann Plast Surg 2013;70:103-10. [DOI] [PubMed] [Google Scholar]
- 17.JoAnna Nguyen T , Carey JN, Wong AK. Use of human acellular dermal matrix in implant-based breast reconstruction: evaluating the evidence. J Plast Reconstr Aesthet Surg 2011;64:1553-61. [DOI] [PubMed] [Google Scholar]
- 18.Pannucci CJ, Antony AK, Wilkins EG. The impact of acellular dermal matrix on tissue expander/implant loss in breast reconstruction: an analysis of the tracking outcomes and operations in plastic surgery database. Plast Reconstr Surg 2013;132:1-10. [DOI] [PubMed] [Google Scholar]
- 19.Rezak KM, Gillette K, Samson MC, et al. Attitudes toward biological mesh in breast reconstruction: a regional survey of plastic surgeons. Plast Reconstr Surg 2010;126:92e-3e. [DOI] [PubMed] [Google Scholar]
- 20.Gamboa-Bobadilla GM. Implant breast reconstruction using acellular dermal matrix. Ann Plast Surg 2006;56:22-5. [DOI] [PubMed] [Google Scholar]
- 21.Nahabedian MY. AlloDerm performance in the setting of prosthetic breast surgery, infection, and irradiation. Plast Reconstr Surg 2009;124:1743-53. [DOI] [PubMed] [Google Scholar]
- 22.Sbitany H, Sandeen SN, Amalfi AN, et al. Acellular dermis-assisted prosthetic breast reconstruction versus complete submuscular coverage: a head-to-head comparison of outcomes. Plast Reconstr Surg 2009;124:1735-40. [DOI] [PubMed] [Google Scholar]
- 23.Spear SL, Seruya M, Clemens MW, et al. Acellular dermal matrix for the treatment and prevention of implant-associated breast deformities. Plast Reconstr Surg 2011;127:1047-58. [DOI] [PubMed] [Google Scholar]
- 24.Vardanian AJ, Clayton JL, Roostaeian J, et al. Comparison of implant-based immediate breast reconstruction with and without acellular dermal matrix. Plast Reconstr Surg 2011;128:403e-410e. [DOI] [PubMed] [Google Scholar]
- 25.Buck DW, 2nd, Heyer K, Wayne JD, et al. Diagnostic dilemma: acellular dermis mimicking a breast mass after immediate tissue expander breast reconstruction. Plast Reconstr Surg 2009;124:174e-6e. [DOI] [PubMed] [Google Scholar]
- 26.Chun YS, Verma K, Rosen H, et al. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg 2010;125:429-36. [DOI] [PubMed] [Google Scholar]
- 27.de Blacam C, Momoh AO, Colakoglu S, et al. Cost analysis of implant-based breast reconstruction with acellular dermal matrix. Ann Plast Surg 2012;69:516-20. [DOI] [PubMed] [Google Scholar]
- 28.Jansen LA, Macadam SA. The use of AlloDerm in postmastectomy alloplastic breast reconstruction: part II. A cost analysis. Plast Reconstr Surg 2011;127:2245-54. [DOI] [PubMed] [Google Scholar]
- 29.Parikh RP, Pappas-Politis E, Smith PD. Acellular dermal matrix masking detection of recurrent breast carcinoma: a novel complication. Aesthetic Plast Surg 2012;36:149-52. [DOI] [PubMed] [Google Scholar]
- 30.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bindingnavele V, Gaon M, Ota KS, et al. Use of acellular cadaveric dermis and tissue expansion in postmastectomy breast reconstruction. J Plast Reconstr Aesthet Surg 2007;60:1214-8. [DOI] [PubMed] [Google Scholar]
- 32.Nahabedian MY. Secondary nipple reconstruction using local flaps and AlloDerm. Plast Reconstr Surg 2005;115:2056-61. [DOI] [PubMed] [Google Scholar]
- 33.Wong AK, Schonmeyr B, Singh P, et al. Histologic analysis of angiogenesis and lymphangiogenesis in acellular human dermis. Plast Reconstr Surg 2008;121:1144-52. [DOI] [PubMed] [Google Scholar]
- 34.Rawlani V, Buck DW, 2nd, Johnson SA, et al. Tissue expander breast reconstruction using prehydrated human acellular dermis. Ann Plast Surg 2011;66:593-7. [DOI] [PubMed] [Google Scholar]
- 35.Losken A. Early Results Using Sterilized Acellular Human Dermis (Neoform) in Post-Mastectomy Tissue Expander Breast Reconstruction. Plast Reconstr Surg 2009. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 36.Becker S, Saint-Cyr M, Wong C, et al. AlloDerm versus DermaMatrix in immediate expander-based breast reconstruction: a preliminary comparison of complication profiles and material compliance. Plast Reconstr Surg 2009;123:1-6; discussion 107-8. [DOI] [PubMed] [Google Scholar]
- 37.Bullocks JM. DermACELL: a novel and biocompatible acellular dermal matrix in tissue expander and implant-based breast reconstruction. Eur J Plast Surg 2014;37:529-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vashi C. Clinical Outcomes for Breast Cancer Patients Undergoing Mastectomy and Reconstruction with Use of DermACELL, a Sterile, Room Temperature Acellular Dermal Matrix. Plast Surg Int 2014;2014:704323. [DOI] [PMC free article] [PubMed]
- 39.Nyame TT, Lemon KP, Kolter R, et al. High-throughput assay for bacterial adhesion on acellular dermal matrices and synthetic surgical materials. Plast Reconstr Surg 2011;128:1061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tessler O, Reish RG, Maman DY, et al. Beyond biologics: absorbable mesh as a low-cost, low-complication sling for implant-based breast reconstruction. Plast Reconstr Surg 2014;133:90e-9e. [DOI] [PubMed] [Google Scholar]
- 41.Dieterich M, Dieterich H, Timme S, et al. Using a titanium-coated polypropylene mesh (TiLOOP(®) Bra) for implant-based breast reconstruction: case report and histological analysis. Arch Gynecol Obstet 2012;286:273-6. [DOI] [PubMed] [Google Scholar]
- 42.Scheidbach H, Tannapfel A, Schmidt U, et al. Influence of titanium coating on the biocompatibility of a heavyweight polypropylene mesh. An animal experimental model. Eur Surg Res 2004;36:313-7. [DOI] [PubMed] [Google Scholar]
- 43.Dieterich M, Paepke S, Zwiefel K, et al. Implant-based breast reconstruction using a titanium-coated polypropylene mesh (TiLOOP Bra): a multicenter study of 231 cases. Plast Reconstr Surg 2013;132:8e-19e. [DOI] [PubMed] [Google Scholar]
- 44.Hjort H, Mathisen T, Alves A, et al. Three-year results from a preclinical implantation study of a long-term resorbable surgical mesh with time-dependent mechanical characteristics. Hernia 2012;16:191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baxter RA. Intracapsular allogenic dermal grafts for breast implant-related problems. Plast Reconstr Surg 2003;112:1692-6; discussion 1697-8. [DOI] [PubMed]
- 46.Spear SL, Parikh PM, Reisin E, et al. Acellular dermis-assisted breast reconstruction. Aesthetic Plast Surg 2008;32:418-25. [DOI] [PubMed] [Google Scholar]
- 47.Bengtson BP, Van Natta BW, Murphy DK, et al. Style 410 highly cohesive silicone breast implant core study results at 3 years. Plast Reconstr Surg 2007;120:40S-48S. [DOI] [PubMed] [Google Scholar]
- 48.Cunningham B. The Mentor Study on Contour Profile Gel Silicone MemoryGel Breast Implants. Plast Reconstr Surg 2007;120:33S-39S. [DOI] [PubMed] [Google Scholar]
- 49.Cunningham B. The Mentor Core Study on Silicone MemoryGel Breast Implants. Plast Reconstr Surg 2007;120:19S-29S; discussion 30S-32S. [DOI] [PubMed]
- 50.Cunningham B, McCue J. Safety and effectiveness of Mentor's MemoryGel implants at 6 years. Aesthetic Plast Surg 2009;33:440-4. [DOI] [PubMed] [Google Scholar]
- 51.Spear SL, Murphy DK, Slicton A, et al. Inamed silicone breast implant core study results at 6 years. Plast Reconstr Surg 2007;120:8S-16S; discussion 17S-18S. [DOI] [PubMed]
- 52.Macadam SA, Lennox PA. Acellular dermal matrices: Use in reconstructive and aesthetic breast surgery. Can J Plast Surg 2012;20:75-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alderman AK, Wilkins EG, Kim HM, et al. Complications in postmastectomy breast reconstruction: two-year results of the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg 2002;109:2265-74. [DOI] [PubMed] [Google Scholar]
- 54.Cordeiro PG, McCarthy CM. A single surgeon's 12-year experience with tissue expander/implant breast reconstruction: part II. An analysis of long-term complications, aesthetic outcomes, and patient satisfaction. Plast Reconstr Surg 2006;118:832-9. [DOI] [PubMed] [Google Scholar]
- 55.Cordeiro PG, McCarthy CM. A single surgeon's 12-year experience with tissue expander/implant breast reconstruction: part I. A prospective analysis of early complications. Plast Reconstr Surg 2006;118:825-31. [DOI] [PubMed] [Google Scholar]
- 56.Spear SL, Majidian A. Immediate breast reconstruction in two stages using textured, integrated-valve tissue expanders and breast implants: a retrospective review of 171 consecutive breast reconstructions from 1989 to 1996. Plast Reconstr Surg 1998;101:53-63. [DOI] [PubMed] [Google Scholar]
- 57.Salzberg CA, Ashikari AY, Koch RM, et al. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm). Plast Reconstr Surg 2011;127:514-24. [DOI] [PubMed] [Google Scholar]
- 58.Jansen LA, Macadam SA. The use of AlloDerm in postmastectomy alloplastic breast reconstruction: part I. A systematic review. Plast Reconstr Surg 2011;127:2232-44. [DOI] [PubMed] [Google Scholar]
- 59.Zhong T, Temple-Oberle C, Hofer S, et al. The Multi Centre Canadian Acellular Dermal Matrix Trial (MCCAT): study protocol for a randomized controlled trial in implant-based breast reconstruction. Trials 2013;14:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garganese G, Fragomeni S, Cervelli D, et al. Titanized mesh for immediate prosthetic reconstruction of large/extra-large breasts. Int J Gynaecol Obstet 2012;119:S686-S7. [Google Scholar]
- 61.Klein E, Kiechle MB, Paepke S. Analysis of immediate breast reconstruction with the use of titanized polypropylene mesh (TiLOOP® Bra). Eur J Surg Oncol 2013;39:482. [Google Scholar]
- 62.Kim JY, Davila AA, Persing S, et al. A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction. Plast Reconstr Surg 2012;129:28-41. [DOI] [PubMed] [Google Scholar]
- 63.Hoppe IC, Yueh JH, Wei CH, et al. Complications following expander/implant breast reconstruction utilizing acellular dermal matrix: a systematic review and meta-analysis. Eplasty 2011;11:e40. [PMC free article] [PubMed] [Google Scholar]
- 64.Sbitany H, Serletti JM. Acellular dermis-assisted prosthetic breast reconstruction: a systematic and critical review of efficacy and associated morbidity. Plast Reconstr Surg 2011;128:1162-9. [DOI] [PubMed] [Google Scholar]
- 65.McCarthy CM, Lee CN, Halvorson EG, et al. The use of acellular dermal matrices in two-stage expander/implant reconstruction: a multicenter, blinded, randomized controlled trial. Plast Reconstr Surg 2012;130:57S-66S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lewis P, Jewell J, Mattison G, et al. Reducing postoperative infections and red breast syndrome in patients with acellular dermal matrix-based breast reconstruction: the relative roles of product sterility and lower body mass index. Ann Plast Surg 2015;74 Suppl 1:S30-2. [DOI] [PubMed] [Google Scholar]
- 67.Weichman KE, Wilson SC, Saadeh PB, et al. Sterile “ready-to-use” AlloDerm decreases postoperative infectious complications in patients undergoing immediate implant-based breast reconstruction with acellular dermal matrix. Plast Reconstr Surg 2013;132:725-36. [DOI] [PubMed] [Google Scholar]
- 68.Buseman J, Wong L, Kemper P, et al. Comparison of sterile versus nonsterile acellular dermal matrices for breast reconstruction. Ann Plast Surg 2013;70:497-9. [DOI] [PubMed] [Google Scholar]
- 69.Glasberg SB, Light D. AlloDerm and Strattice in breast reconstruction: a comparison and techniques for optimizing outcomes. Plast Reconstr Surg 2012;129:1223-33. [DOI] [PubMed] [Google Scholar]
- 70.Brooke S, Mesa J, Uluer M, et al. Complications in tissue expander breast reconstruction: a comparison of AlloDerm, DermaMatrix, and FlexHD acellular inferior pole dermal slings. Ann Plast Surg 2012;69:347-9. [DOI] [PubMed] [Google Scholar]
- 71.Butterfield JL. 440 Consecutive immediate, implant-based, single-surgeon breast reconstructions in 281 patients: a comparison of early outcomes and costs between SurgiMend fetal bovine and AlloDerm human cadaveric acellular dermal matrices. Plast Reconstr Surg 2013;131:940-51. [DOI] [PubMed] [Google Scholar]
- 72.Endress R, Choi MS, Lee GK. Use of fetal bovine acellular dermal xenograft with tissue expansion for staged breast reconstruction. Ann Plast Surg 2012;68:338-41. [DOI] [PubMed] [Google Scholar]
- 73.Liu DZ, Mathes DW, Neligan PC, et al. Comparison of outcomes using AlloDerm versus FlexHD for implant-based breast reconstruction. Ann Plast Surg 2014;72:503-7. [DOI] [PubMed] [Google Scholar]
- 74.Salzberg CA, Dunavant C, Nocera N. Immediate breast reconstruction using porcine acellular dermal matrix (Strattice™): long-term outcomes and complications. J Plast Reconstr Aesthet Surg 2013;66:323-8. [DOI] [PubMed] [Google Scholar]
- 75.Venturi ML, Mesbahi AN, Boehmler JH, 4th, et al. Evaluating sterile human acellular dermal matrix in immediate expander-based breast reconstruction: a multicenter, prospective, cohort study. Plast Reconstr Surg 2013;131:9e-18e. [DOI] [PubMed] [Google Scholar]
- 76.Seth AK, Persing S, Connor CM, et al. A comparative analysis of cryopreserved versus prehydrated human acellular dermal matrices in tissue expander breast reconstruction. Ann Plast Surg 2013;70:632-5. [DOI] [PubMed] [Google Scholar]
- 77.Mofid MM, Meininger MS, Lacey MS. Veritas® bovine pericardium for immediate breast reconstruction: a xenograft alternative to acellular dermal matrix products. Eur J Plast Surg 2012;35:717-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Handel N, Cordray T, Gutierrez J, et al. A long-term study of outcomes, complications, and patient satisfaction with breast implants. Plast Reconstr Surg 2006;117:757-67; discussion 768-72. [DOI] [PubMed] [Google Scholar]
- 79.Adams WP, Jr. Capsular contracture: what is it? What causes it? How can it be prevented and managed? Clin Plast Surg 2009;36:119-26, vii. [DOI] [PubMed] [Google Scholar]
- 80.Henriksen TF, Fryzek JP, Hölmich LR, et al. Surgical intervention and capsular contracture after breast augmentation: a prospective study of risk factors. Ann Plast Surg 2005;54:343-51. [DOI] [PubMed] [Google Scholar]
- 81.Prantl L, Schreml S, Fichtner-Feigl S, et al. Clinical and morphological conditions in capsular contracture formed around silicone breast implants. Plast Reconstr Surg 2007;120:275-84. [DOI] [PubMed] [Google Scholar]
- 82.Wong CH, Samuel M, Tan BK, et al. Capsular contracture in subglandular breast augmentation with textured versus smooth breast implants: a systematic review. Plast Reconstr Surg 2006;118:1224-36. [DOI] [PubMed] [Google Scholar]
- 83.Clough KB, O'Donoghue JM, Fitoussi AD, et al. Prospective evaluation of late cosmetic results following breast reconstruction: I. Implant reconstruction. Plast Reconstr Surg 2001;107:1702-9. [DOI] [PubMed] [Google Scholar]
- 84.Ho G, Nguyen TJ, Shahabi A, et al. A systematic review and meta-analysis of complications associated with acellular dermal matrix-assisted breast reconstruction. Ann Plast Surg 2012;68:346-56. [DOI] [PubMed] [Google Scholar]
- 85.Macadam SA, Lennox PA. Acellular Dermal Matrices: Economic Considerations in Reconstructive and Aesthetic Breast Surgery. Clin Plast Surg 2012;39:187-216. [DOI] [PubMed] [Google Scholar]
- 86.Basu CB, Leong M, Hicks MJ. Acellular cadaveric dermis decreases the inflammatory response in capsule formation in reconstructive breast surgery. Plast Reconstr Surg 2010;126:1842-7. [DOI] [PubMed] [Google Scholar]
- 87.Mofid MM. Acellular dermal matrix in cosmetic breast procedures and capsular contracture. Aesthet Surg J 2011;31:77S-84S. [DOI] [PubMed] [Google Scholar]
- 88.Zienowicz RJ, Karacaoglu E. Implant-based breast reconstruction with allograft. Plast Reconstr Surg 2007;120:373-81. [DOI] [PubMed] [Google Scholar]
- 89.Stump A, Holton LH, 3rd, Connor J, et al. The use of acellular dermal matrix to prevent capsule formation around implants in a primate model. Plast Reconstr Surg 2009;124:82-91. [DOI] [PubMed] [Google Scholar]
- 90.Komorowska-Timek E, Oberg KC, Timek TA, et al. The effect of AlloDerm envelopes on periprosthetic capsule formation with and without radiation. Plast Reconstr Surg 2009;123:807-16. [DOI] [PubMed] [Google Scholar]
- 91.Seth AK, Hirsch EM, Fine NA, et al. Utility of acellular dermis-assisted breast reconstruction in the setting of radiation: a comparative analysis. Plast Reconstr Surg 2012;130:750-8. [DOI] [PubMed] [Google Scholar]
- 92.Mitchell RE. Porcine acellular dermis-assisted breast reconstruction: influence of adjuvant radiotherapy on complications and outcomes. Plast Reconstr Surg Glob Open 2013;1:e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Valdatta L, Cattaneo AG, Pellegatta I, et al. Acellular dermal matrices and radiotherapy in breast reconstruction: a systematic review and meta-analysis of the literature. Plast Surg Int 2014;2014:472604. [DOI] [PMC free article] [PubMed]
- 94.Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns 1995;21:243-8. [DOI] [PubMed] [Google Scholar]
- 95.Zenn MR. Staged immediate breast reconstruction. Plast Reconstr Surg 2015;135:976-9. [DOI] [PubMed] [Google Scholar]
- 96.Phillips BT, Bishawi M, Dagum AB, et al. A systematic review of infection rates and associated antibiotic duration in acellular dermal matrix breast reconstruction. Eplasty 2014;14:e42. [PMC free article] [PubMed] [Google Scholar]
- 97.Antony AK, McCarthy CM, Cordeiro PG, et al. Acellular human dermis implantation in 153 immediate two-stage tissue expander breast reconstructions: determining the incidence and significant predictors of complications. Plast Reconstr Surg 2010;125:1606-14. [DOI] [PubMed] [Google Scholar]
- 98.Lanier ST, Wang ED, Chen JJ, et al. The effect of acellular dermal matrix use on complication rates in tissue expander/implant breast reconstruction. Ann Plast Surg 2010;64:674-8. [DOI] [PubMed] [Google Scholar]
- 99.Ganske I, Verma K, Rosen H, et al. Minimizing complications with the use of acellular dermal matrix for immediate implant-based breast reconstruction. Ann Plast Surg 2013;71:464-70. [DOI] [PubMed] [Google Scholar]
- 100.Haynes DF, Kreithen JC. Vicryl mesh in expander/implant breast reconstruction: long-term follow-up in 38 patients. Plast Reconstr Surg 2014;134:892-9. [DOI] [PubMed] [Google Scholar]