Abstract
Background
The approach and operative techniques associated with breast reconstruction have steadily been refined since its inception, with abdominal perforator-based flaps becoming the gold standard reconstructive option for women undergoing breast cancer surgery. The current study comprises a cohort of 632 patients, in whom specific operative times are recorded by a blinded observer, and aims to address the potential benefits seen with the use of computer tomography (CT) scanning preoperatively on operative outcomes, complications and surgical times.
Methods
A prospectively recorded, retrospective review was undertaken of patients undergoing autologous breast reconstruction with a DIEP flap at the St Andrews Centre over a 4-year period from 2010 to 2014. Computed tomography angiography (CTA) scanning of patients began in September 2012 and thus 2 time periods were compared: 2 years prior to the use of CTA scans and 2 years afterwards. For all patients, key variables were collected including patient demographics, operative times, flap harvest time, pedicle length, surgeon experience and complications.
Results
In group 1, comprising patients within the period prior to CTA scans, 265 patients underwent 312 flaps; whilst in group 2, the immediately following 2 years, 275 patients had 320 flaps. The use of preoperative CTA scans demonstrated a significant reduction in flap harvest time of 13 minutes (P<0.013). This significant time saving was seen in all flap modifications: unilateral, bilateral and bipedicled DIEP flaps. The greatest time saving was seen in bipedicle flaps, with a 35-minute time saving. The return to theatre rate significantly dropped from 11.2% to 6.9% following the use of CTA scans, but there was no difference in the total failure rate.
Conclusions
The study has demonstrated both a benefit to flap harvest time as well as overall operative times when using preoperative CTA. The use of CTA was associated with a significant reduction in complications requiring a return to theatre in the immediate postoperative period. Modern scanners and techniques can reduce the level of ionising radiation, facilitating patients being able to benefit from the advantages that this preoperative planning can convey.
Keywords: Computer tomography (CT), microsurgery, microvascular, free flap
Introduction
The approach and operative techniques associated with breast reconstruction have steadily been refined since its inception, with abdominal perforator-based flaps becoming the gold standard reconstructive option for women undergoing breast cancer surgery. Surgeons continue to look for ways to improve operative outcomes and minimise complications in this surgery, and preoperative planning has offered a means to achieving these outcomes. The decisions made by the surgeon at every stage of planning and raising of a free flap has the potential to affect the success of the operation, and as such techniques and technologies to help surgeons in this decision making process, has led to a gradual refinement in this decision making.
Perforating vessels arising from the deep inferior epigastric artery are anatomically highly variable in regards to their location, course and calibre. Objective measurements can then provide a road-map of these variables prior to surgery starting has been sought, with computed tomography angiography (CTA) becoming the first such objective imaging modality in this role. CTA of abdominal perforators has been demonstrated to be highly sensitive and specific in the identification of perforator site and calibre (1-3). When compared to the technologies that had been previously used, such as the hand-held Doppler probe and duplex sonography, CTA has been demonstrated to have a far greater level of accuracy and objectivity in its findings (4). The use of CTA imaging in deep inferior epigastric perforator (DIEP) flap planning has since been shown to significantly reduce overall operation time, donor site morbidity and increase flap survival (5,6). However these benefits need to be tempered with the obvious disadvantage of exposure to ionising radiation.
In a resource finite healthcare environment the benefit of a preoperative planning tool which has been shown to reduce complications and operative time, should be assessed as to its net cost benefit or disadvantage. However, studies to date have been limited either in power (number of included patients) or in the level of detail in recording times, focusing on those aspects of surgery most likely to be affected by improved planning. The current study thus comprises a cohort of 632 patients, in whom specific operative times (including flap raise time specifically) are recorded and aims to address the potential benefits seen with the use of CT scanning preoperatively on operative outcomes, complications and surgical times.
Methods
A prospectively recorded, retrospective review was undertaken of patients undergoing autologous breast reconstruction with a DIEP flap at the St Andrews Centre over a 4-year period from 2010 to 2014. Patients operated on by one of the two senior authors were identified during this period. CTA scanning of patients began in September 2012 and thus two time periods were compared: 2 years prior to the use of CTA scans and 2 years afterwards. All patients in both time periods had preoperative hand-held Doppler marking either as primary planning or as an adjunct to CTA, and those patients in the second time period underwent CTA imaging and consultant radiologist-led analysis of suitable perforators. Perforator anatomy was described in respect to vessel location, calibre, and length of intramuscular course, with the operating surgeon able to independently decide which perforator was the primary target for flap perfusion. For all patients, key variables were collected including patient demographics, operative times, flap harvest time, pedicle length, surgeon experience and complications. Statistical analysis was undertaken using SPSS (SPSS Inc., IBM Armok, NY, USA) statistical software. Primary outcomes assessed comprised flap survival, and complications requiring a return to theatre. Data regarding costs were taken directly from the hospital finance department.
Results
In group 1, comprising patients within the period prior to CTA scans, 265 patients underwent 312 flaps; whilst in group 2, the immediately following 2 years, 275 patients had 320 flaps (Table 1). The majority of flaps undertaken in both time periods were unilateral reconstructions, accounting for 63% of all reconstructions prior to CTA scans and 67% of reconstructions after. The use of preoperative CTA scans demonstrated a significant reduction in flap harvest time of 13 minutes (P<0.013). This significant time saving was seen in all flap modifications: unilateral, bilateral and bipedicled DIEP flaps. The greatest time saving was seen in bipedicle flaps, with a 35-minute time saving (Table 2). There was no difference found in the average pedicle lengths raised between the two groups, ruled out as a confounder. The return to theatre rate significantly dropped from 11.2% to 6.9% following the use of CTA scans, but there was no difference in the total failure rate (Table 3). When looking at surgeon grade, there was no difference in flap raise time with the use of CTA between levels of experience, however fellows and registrars demonstrated a significant reduction in complication rates from 4.2% to 0.9%. Two flaps were lost over the entire 4-year period, which was a 0.31% flap loss rate.
Table 1. Total number of flaps and patients.
| Flap type | Before CT |
After CT |
|||
|---|---|---|---|---|---|
| Patients | Flaps | Patients | Flaps | ||
| Unilateral | 197 | 197 | 216 | 216 | |
| Bilateral | 36 | 72 | 29 | 58 | |
| Unilateral bipedicle | 21 | 21 | 14 | 14 | |
| Unilateral stacked | 11 | 22 | 16 | 32 | |
| Total | 265 | 312 | 275 | 320 | |
Table 2. Time for flap harvest.
| Variables assessed | Flap raise time (mins) |
Pedicle length |
|||||
|---|---|---|---|---|---|---|---|
| Without CTA | With CTA | Student t-test | Without CTA | With CTA | Student t-test | ||
| Reconstruction typea | |||||||
| Unilateral | 134 | 122 | 0.05* | 11.6 | 10.3 | 0.067 | |
| Bilateral | 124 | 102 | 0.03* | 11.98 | 9.4 | 0.021* | |
| Unilateral bipedicle | 161 | 126 | 0.046* | 12.8 | 11.3 | 0.068 | |
| Unilateral stacked | 127 | 144 | 0.463 | 10 | 8 | 0.313 | |
| Mean | 136.5 | 123.5 | 0.013* | 11.5 | 9.75 | 0.515 | |
| Grade of surgeonb | |||||||
| Consultant | 109 | 110 | 0.93 | 10.9 | 10.3 | 0.233 | |
| Fellow/registrar | 143 | 139 | 0.71 | 12.1 | 9.8 | 0.001** | |
| Consultant & fellow | 142 | 143 | 0.98 | 11.3 | 10.25 | 0.172 | |
*, P≤0.05; **, P<0.001; a, by flap type; b, by grade of surgeon; CTA, computed tomography angiography.
Table 3. Clinical outcomes for computed tomographic angiography (CTA) versus no CTA for flap planning.
| Variables assessed | Complication rate |
Flap loss |
|||||
|---|---|---|---|---|---|---|---|
| Without CTA | With CTA | Student t-test | Without CTA | With CTA | Student t-test | ||
| Reconstruction typea | |||||||
| Unilateral | 14.0% | 6.2% | 0.032* | 1.0% | 0 | 0.276 | |
| Bilateral | 8.3% | 8.6% | 0.74 | 0.0% | 0 | 0 | |
| Unilateral bipedicle | 10.0% | 14.0% | 0.713 | 0.0% | 0 | 0 | |
| Unilateral stacked | 40.0% | 13.0% | 0.114 | 10.0% | 0 | 0.212 | |
| Mean | 11.2 | 6.9 | 0.023* | 0.64% | 0 | 0.136 | |
| Grade of surgeonb | |||||||
| Consultant | 3.5% | 4.7% | 0.069 | 0.0% | 0 | 0 | |
| Fellow/registrar | 4.2% | 0.9% | 0.004* | 0.3% | 0 | 0.219 | |
| Consultant & fellow | 3.5% | 1.3% | 0.158 | 0.3% | 0 | 0.32 | |
| Total | 11.2% | 6.9% | 0.023* | 0.64% | 0 | 0.136 | |
*, P≤0.05; a, by flap type; b, by grade of surgeon; CTA, computed tomography angiography.
Discussion
Since the advent of preoperative imaging with CTA scanning for DIEP flap planning (7), there have been a number of studies demonstrating both the benefits but also the drawbacks of using this technology in flap planning. Our study is commensurate with the literature with respect to demonstrating a reduction in overall operative time and complication rates (5). We found that with preoperative CTA scans, flap raise was 13 minutes quicker and overall operative time was 44 minutes shorter. The time saved was made even more apparent when looking at the more complex reconstructive cases. Bilateral DIEP flaps were raised 22 minutes faster and bipedicled flaps were raised 35 minutes faster. This decrease in flap raise time has been seen in other published cohorts (6). The ability to plan incisions and plan which perforator to target can be seen to reduce the intraoperative decision making which would otherwise slow down the process (Figures 1,2). Given that there was no difference in pedicle length in both groups, this was able to demonstrate that pedicle length was not being sacrificed as a time saving technique (Figures 3,4). However, the presence of a road map does not suggest that one should follow it at all costs. Clinical judgement must be used to decide a change of course when necessary. One study of 52 DIEP flaps demonstrated 44% involved intraoperative changes due to features not appreciated on the CTA scans (8). Time saving during surgery is associated with decrease morbidity, furthermore using CTA scans have also been shown to reduce a surgeons operative stress which may well have a causal relationship with the decrease morbidity (9).
Figure 1.
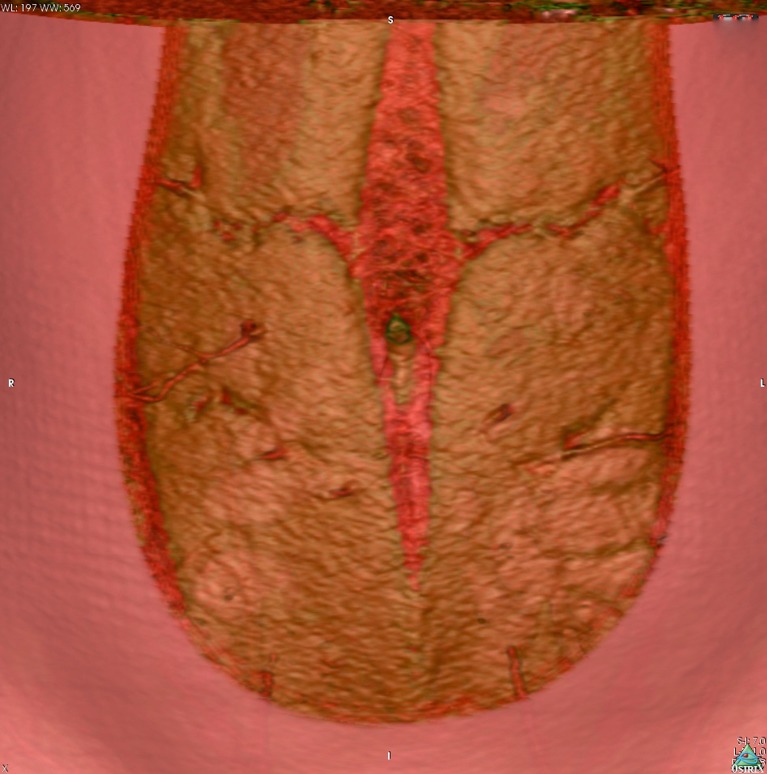
Preoperative computed tomographic angiogram, with volume rendered technique reconstruction, demonstrating an overview of the major abdominal wall perforators for deep inferior epigastric artery (DIEA) perforator flap planning.
Figure 2.
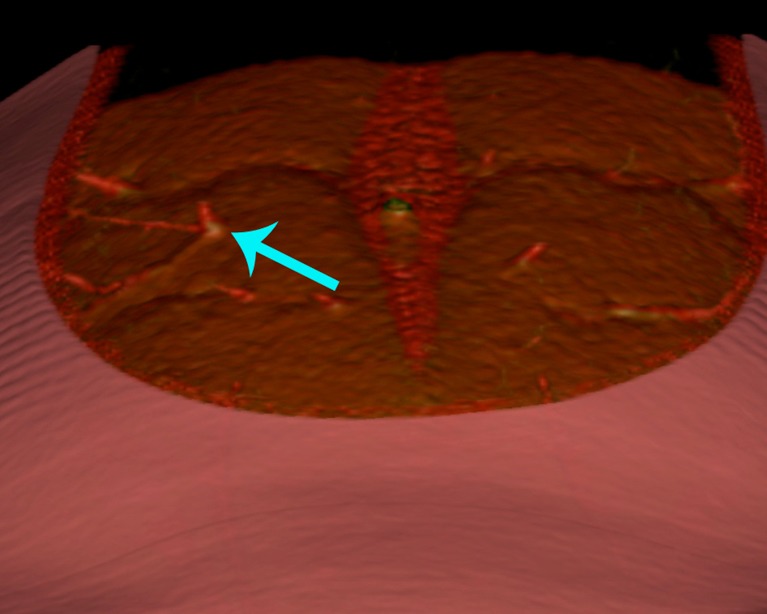
Preoperative computed tomographic angiogram, demonstrating the precise location of emergence of a deep inferior epigastric artery (DIEA) perforator from the anterior rectus sheath.
Figure 3.
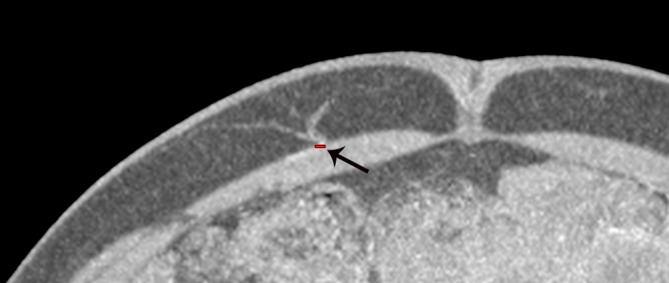
Preoperative computed tomographic angiogram, demonstrating the measurement of luminal calibre of a deep inferior epigastric artery (DIEA) perforator.
Figure 4.

Preoperative computed tomographic angiogram, demonstrating the intramuscular course of a deep inferior epigastric artery (DIEA) perforator.
The greatest disadvantage to preoperative scanning with CTA is the exposure to ionising radiation. A CTA of the abdominal wall is estimated to expose the recipient to between 6 and 10 millisieverts (10). There are adjustments and refinements in the literature which can reduce this exposure to 2 millisieverts and still get accurate information, but such modifications can never remove the potential to induce cancer entirely (11). There is clearly a cost: benefit analysis in assessing this risk, and certainly this risk needs to be assessed in a cumulative fashion rather than assessing only the single dose amount each patient would receive from a single scan. Patients diagnosed with cancer who are offered a breast reconstruction will often have already had a number of exposures to ionising radiation, thus accumulating a conferred additional risk with each further investigation (12). Patient factors such as high body mass index will have an impact on the amount of ionising radiation required to undertake a planning CTA. However, the use of CTA could be similarly seen as a useful surveillance tool at the time of reconstruction for delayed cases, with concurrent scan analysis able to identify other pathologies (13). Often patients complete their entire course of cancer treatment and wait 1 to 2 years for reconstruction, and may even be discharged from oncologic surveillance. The use of CTA in this setting provides a reassuring snap shot into the patients’ preoperative risk of regional or distant recurrence. In our cohort of 275 women, none were found to have tumour recurrence at the time of surgery; however other incidental findings requiring further investigation did occur. Evidence of recurrent of disease has been reported in the literature (13).
There are options for preoperative imaging with scans that do not yield ionising radiation, with these including colour duplex sonography and magnetic resonance imaging (MRI). These options are useful, but have substantial limitations. A systematic review of CTA and contrast enhanced MRA demonstrated no difference in the modalities’ ability to localise perforators preoperatively (14), however resolution of images, availability and cost have made this modality less widely adopted. When comparing colour duplex ultrasonography, many authors have demonstrated this as an operator dependent imaging modality, with highly variable sensitivity (15).
Our data demonstrates that outcomes can be improved, alongside time savings with preoperative CTA scanning. Patients who underwent a pre-operative CTA had 4.3% fewer complications requiring a return to theatre while an inpatient, compared to those who did not undergo a CTA. A significant difference was seen also in the subgroup analysis of flaps raised by fellow or registrar grade surgeons. This would indicate that a road-map provides a greater resource for guidance to a training surgeon than a consultant. In the 275 patients operated on with a CTA scan, there were no flap losses. Although not significant, given that the previous group only had 2 out of 312 flaps lost, it does demonstrate that stepwise technological and process advancements will continue to lead improvements in outcomes.
When assessing the reduction in time and reduction in complications, the use of preoperative CTA can be seen as potential cost saving process. This is essential, given that in a resource finite healthcare environment cost savings need to be considered. Within our operating theatre, the fixed costs of operating were calculated to be £14 GBP/min. This can be calculated to conclude that using a CTA would save £616 per operation. The isolated cost of the CTA is £500, suggesting that the use of CTA is essentially cost neutral. Although this is far from a formal cost analysis, this can be further expanded to included complications and their costs, given that a take-back to theatre places a significant increase on the overall cost of surgery (16). One study demonstrated that staffing costs accounted for 73% of the total cost of DIEP surgery. If the take back rates can be reduced in addition to the overall costs, savings will build.
In conclusion, this is one of the largest series in the literature to compare CTA scanned patients for DIEP flap breast reconstruction with an equivalent non-scanned cohort. The study has demonstrated both a benefit to flap harvest time as well as overall operative times when using preoperative CTA. The use of CTA was associated with a significant reduction in complications requiring a return to theatre in the immediate postoperative period. Modern scanners and techniques can reduce the level of ionising radiation, facilitating patients being able to benefit from the advantages that this preoperative planning can convey.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Rozen WM, Ashton MW, Stella DL, et al. The accuracy of computed tomographic angiography for mapping the perforators of the deep inferior epigastric artery: a blinded, prospective cohort study. Plast Reconstr Surg 2008;122:1003-9. [DOI] [PubMed] [Google Scholar]
- 2.Rozen WM, Ashton MW, Stella DL, et al. The accuracy of computed tomographic angiography for mapping the perforators of the DIEA: a cadaveric study. Plast Reconstr Surg 2008;122:363-9. [DOI] [PubMed] [Google Scholar]
- 3.Alonso-Burgos A, García-Tutor E, Bastarrika G, et al. Preoperative planning of deep inferior epigastric artery perforator flap reconstruction with multislice-CT angiography: imaging findings and initial experience. J Plast Reconstr Aesthet Surg 2006;59:585-93. [DOI] [PubMed] [Google Scholar]
- 4.Rozen WM, Phillips TJ, Ashton MW, et al. Preoperative imaging for DIEA perforator flaps: a comparative study of computed tomographic angiography and doppler ultrasound. Plast Reconstr Surg 2008;121:1-8. [DOI] [PubMed] [Google Scholar]
- 5.Teunis T, Heerma van Voss MR, Kon M, et al. CT-angiography prior to DIEP flap breast reconstruction: a systematic review and meta-analysis. Microsurgery 2013;33:496-502. [DOI] [PubMed] [Google Scholar]
- 6.Ghattaura A, Henton J, Jallali N, et al. One hundred cases of abdominal-based free flaps in breast reconstruction. The impact of preoperative computed tomographic angiography. J Plast Reconstr Aesthet Surg 2010;63:1597-601. [DOI] [PubMed] [Google Scholar]
- 7.Masia J, Clavero JA, Larrañaga JR, et al. Multidetector-row computed tomography in the planning of abdominal perforator flaps. J Plast Reconstr Aesthet Surg 2006;59:594-9. [DOI] [PubMed] [Google Scholar]
- 8.Keys KA, Louie O, Said HK, et al. Clinical utility of CT angiography in DIEP breast reconstruction. J Plast Reconstr Aesthet Surg 2013;66:e61-5. [DOI] [PubMed] [Google Scholar]
- 9.Rozen WM, Anavekar NS, Ashton MW, et al. Does the preoperative imaging of perforators with CT angiography improve operative outcomes in breast reconstruction? Microsurgery 2008;28:516-23. [DOI] [PubMed] [Google Scholar]
- 10.Malhotra A, Chhaya N, Nsiah-Sarbeng P, et al. CT-guided deep inferior epigastric perforator (DIEP) flap localization -- better for the patient, the surgeon, and the hospital. Clin Radiol 2013;68:131-8. [DOI] [PubMed] [Google Scholar]
- 11.Rozen WM, Chubb D, Crossett M, et al. The future in perforator flap imaging: a new technique to substantially reduce radiation dose with computed tomographic angiography. Plast Reconstr Surg 2010;126:98e-100e. [DOI] [PubMed] [Google Scholar]
- 12.Eylert G, Deutinger M, Stemberger A, et al. Evaluation of the perforator CT-angiography with a cancer risk assessment in DIEP flap breast reconstruction. J Plast Reconstr Aesthet Surg 2015;68:e80-2. [DOI] [PubMed] [Google Scholar]
- 13.Rozen WM, Ashton MW, Grinsell D, et al. Establishing the case for CT angiography in the preoperative imaging of abdominal wall perforators. Microsurgery 2008;28:306-13. [DOI] [PubMed] [Google Scholar]
- 14.Cina A, Barone-Adesi L, Rinaldi P, et al. Planning deep inferior epigastric perforator flaps for breast reconstruction: a comparison between multidetector computed tomography and magnetic resonance angiography. Eur Radiol 2013;23:2333-43. [DOI] [PubMed] [Google Scholar]
- 15.Casares Santiago M, García-Tutor E, Rodríguez Caravaca G, et al. Optimising the preoperative planning of deep inferior epigastric perforator flaps for breast reconstruction. Eur Radiol 2014;24:2097-108. [DOI] [PubMed] [Google Scholar]
- 16.Paget JT, Young KC, Wilson SM. Accurately costing unilateral delayed DIEP flap breast reconstruction. J Plast Reconstr Aesthet Surg 2013;66:926-30. [DOI] [PubMed] [Google Scholar]


