Abstract
Mutations in genes encoding tectorial membrane (TM) proteins are a significant cause of human hereditary hearing loss (Hildebrand et al. 2011), and several mouse models have been developed to study the functional significance of this accessory structure in the mammalian cochlea. In this study, we use otoacoustic emissions (OAE), signals obtained from the ear canal that provide a measure of cochlear function, to characterize a mouse in which the TM is detached from the spiral limbus due to an absence of otoancorin (Otoa, Lukashkin et al. 2012). Our results demonstrate that spontaneous emissions (SOAE), sounds produced in the cochlea without stimulation, increase dramatically in mice with detached TMs even though their hearing sensitivity is reduced. This behavior is unusual because wild-type (WT) controls are rarely spontaneous emitters. SOAEs in mice lacking Otoa predominate around 7 kHz, which is much lower than in either WT animals when they generate SOAEs or in mutant mice in which the TM protein Ceacam16 is absent (Cheatham et al. 2014). Although both mutants lack Hensen’s stripe, loss of this TM feature is only observed in regions coding frequencies greater than ~15 kHz in WT mice so its loss cannot explain the low-frequency, de novo SOAEs observed in mice lacking Otoa. The fact that ~80 % of mice lacking Otoa produce SOAEs even when they generate smaller distortion product OAEs suggests that the active process is still functioning in these mutants but the system(s) involved have become less stable due to alterations in TM structure.
Keywords: active process, cochlea, Hensen’s stripe, otoancorin, spontaneous otoacoustic emissions, tectorial membrane
INTRODUCTION
Different sensory organs within the inner ear are associated with unique accessory structures that evolved to optimize hair-cell stimulation for the primary sense, be it auditory or vestibular. In the cochlea, this accessory structure is the tectorial membrane (TM; Fig. 1A), an extracellular matrix that is anchored medially to the surface of the spiral limbus and laterally to the tallest stereocilia of the outer hair cells (OHCs). Even though the stereocilia of the cochlear inner hair cells (IHCs) appear to be free standing (Lim 1971; Dallos et al. 1972), the TM is also thought to facilitate their stimulation (Dallos et al. 1972; Richardson et al. 2008; Lukashkin et al. 2010). The body of the TM contains collagen fibrils embedded in an unusual striated-sheet matrix, the major components of which are alpha and beta-tectorin (Tecta and Tectb, respectively) and Ceacam16 (Legan et al. 1997; Zheng et al. 2011; Kammerer et al. 2012; Cheatham et al. 2014). In the absence of functional Tecta, the TM becomes completely detached from the organ of Corti and the animals suffer a significant hearing loss (Legan et al. 2000; Moreno-Pelayo et al. 2008; Legan et al. 2014).
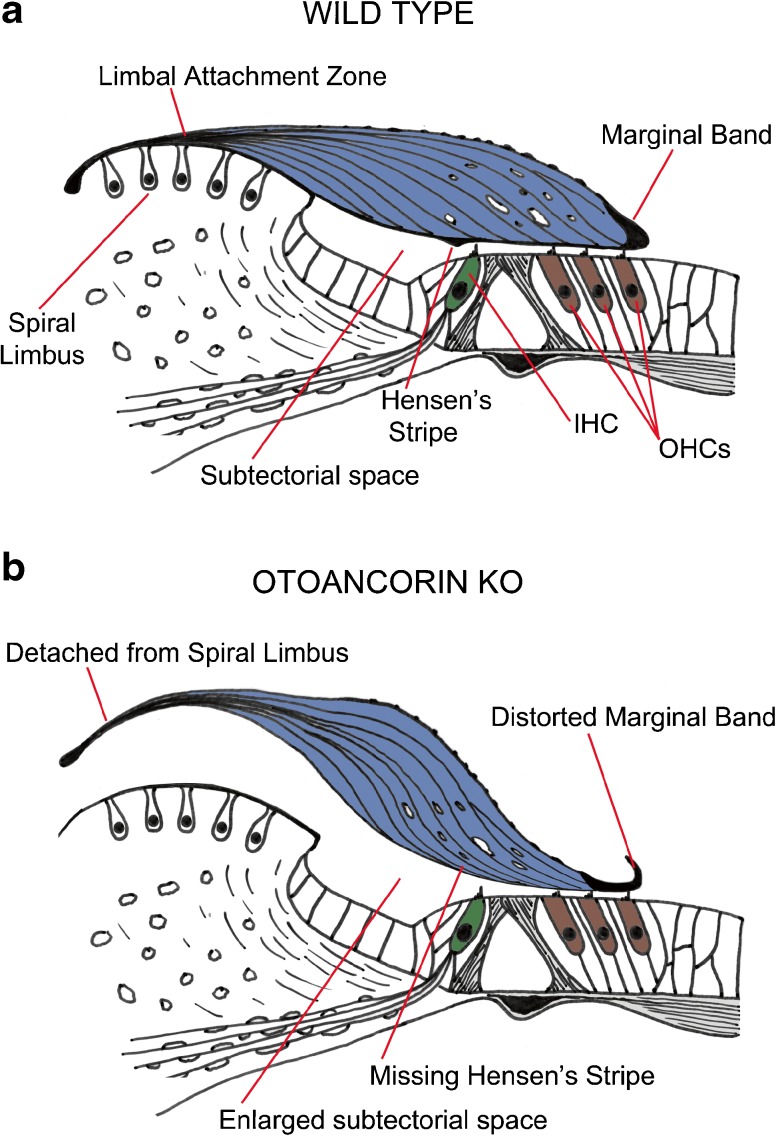
Fig. 1.
Tectorial membrane anatomy. Diagram showing normal (A) and detached (B) tectorial membranes in WT controls and Otoa KO mice. The drawings of the organ of Corti are based on anatomical results published by Lukashkin et al. (2012).
While near-normal auditory sensitivity is observed in young mice lacking Ceacam16, attesting to the fact that the TM remains closely associated with the organ of Corti in these mutants, there is a surprising increase in the number of mice exhibiting spontaneous otoacoustic emissions (SOAEs), sounds that emanate from the cochlea and are thought to reflect the active process that underlies sensitivity and frequency selectivity (Gold 1948; Hudspeth 2014). The SOAEs encountered in Ceacam16 knockout (KO) mice have frequencies that cluster around 23 kHz (Cheatham et al. 2014). Although control wild-type (WT) mice are rarely spontaneous emitters, SOAEs, when present, also originate from this same frequency-encoding region. The increased number of SOAEs encountered in Ceacam16 KO mice correlates with the absence of Hensen’s stripe, a ridge that projects down from the undersurface of the TM. This structure is closely apposed to and lies just medial to the hair bundles of IHCs but is only observed in the basal 60 % of the mouse cochlea, i.e., for regions coding frequencies above 15 kHz (Müller et al. 2005; Cheatham et al. 2014; Legan et al. 2014). Although striated-sheet matrix fails to form in Ceacam16 KO mice and despite the appearance of large holes appearing in the main body of the TM, especially in the apical, low-frequency end of the cochlea, the spatial distribution of these structural defects does not correlate with the observed SOAE frequencies.
In the present study, we characterized otoancorin (Otoa) KO mice (Lukashkin et al. 2012) in which the TM is detached medially from the spiral limbus, Hensen’s stripe is missing, and the extreme lateral region overlying the OHCs is thin and fenestrated (Fig. 1B). Striated-sheet matrix is present within the main body of the TM, but absent from the region where the imprints of the tallest stereocilia of OHCs are observed. Otoa KO mice retain their best sensitivity (as assessed by OAEs) for frequencies encoded at the apex of the cochlea and it is from this region, in the vicinity of 7 kHz, that a profusion of SOAEs is observed. The high incidence of SOAEs in mice with TM defects suggests that anatomical changes in this accessory structure of the cochlea lead to an alteration in the stability of a tightly coupled system that is comprised of the basilar membrane, the outer hair cells and their surrounding supporting cells, and the TM.
MATERIALS AND METHODS
Mice were tested between 3–7 weeks and 6–7 months of age while under anesthesia with ketamine/xylazine (100 mg/kg; 10 mg/kg, IP). The Ceacam16 mice were on a C57BL6J genetic background, while the Otoa mice were on a mixed, variable 129/C57BL6J background, which differs from that in the original publication on this mutant (Lukashkin et al. 2012). Data are also provided for mice (129S7/C57BL6J) lacking prestin. The WT control data were collected over several years from mice on a mixed, variable 129/C57BL6 background. Distortion product OAEs (DPOAE), recorded with a custom probe equipped with a sensitive microphone (Knowles Electronics, FG-3652-CX), were analyzed using Emission Averager (EmAv, Neely and Liu 1994) and additional computer programs written by JH Siegel using Visual Basic. The probe was placed close to the eardrum and sound calibrations in the ear canal of each mouse were performed out to 48 kHz using a chirp stimulus generated in System Response (SysRes, Neely and Stevenson 1992). All signals were generated using a CardDeluxe 24-bit sound card with a sampling rate of 96 kHz. Distortion product otoacoustic emissions were recorded as DPgrams (f2/f1 = 1.2, L1 = L2 = 70 dB) for f2 frequencies between 2 and 47 kHz to cover most of the mouse hearing range. Input-output functions were also collected for selected f2 frequencies with the level of f1 (L1) 10 dB greater than that for f2. Thresholds for 2f1-f2 were then calculated and represent the level of f1 producing a DPOAE of 0 dB. Data are presented as means ± one standard deviation. A one-way analysis of variance (ANOVA) was performed using a Statistical Package for the Social Sciences (SPSS). Post hoc t tests were also acquired. Distortion in the sound was measured using a tubing coupler with a small volume similar to that of the mouse ear canal (Pearce et al. 2001). In order to measure SOAEs, the noise floor in quiet was measured for 3 min 45 s using SysRes by averaging 40 spectra of the canal pressure into a 524,288 point buffer. A fast Fourier transform (FFT) was performed on the time waveform of each sample, and the magnitudes averaged. A moving rectangular window served to smooth the spectrum such that energy was integrated into non-overlapping windows with a width of 93 Hz. Spontaneous emissions were identified as peaks in the noise spectrum. Additional details are provided in our previous publication (Cheatham et al. 2014).
RESULTS
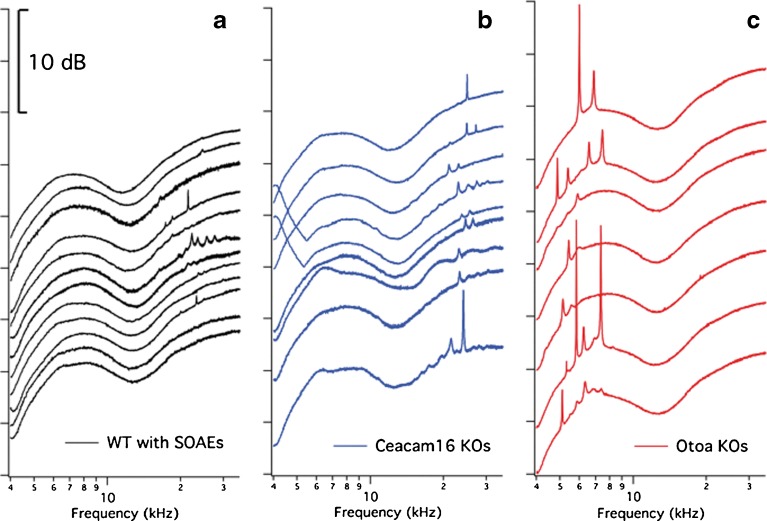
In this report, we focus on otoacoustic emissions (Kemp 1978), which are produced as a by-product of hair-cell function (Jaramillo et al. 1993; Mom et al. 2001; Carvalho et al. 2004; Liberman et al. 2004) and provide a non-invasive tool for studying cochlear processing. Our initial inquiry was to determine the degree to which mice lacking Otoa would display SOAEs since data collected on another TM-mutant mouse revealed that an exceptionally high proportion (70 %) of mice lacking the TM protein Ceacam16 showed this behavior. SOAEs are generated in the absence of stimulation and are thought to signal high gain and instability (Hudspeth 1997) and to reflect those processes required for sharp tuning and sensitivity in the mammalian cochlea. Examples of SOAE traces are provided in Figure 2A for all 12 of the WT mice in our collection that produced SOAEs (8.2 %; 12/147 mice) independent of strain background. These results were obtained over a 9-year period, attesting to the fact that SOAEs in WT mice are uncommon. Of the 77 WT mice examined from the Ceacam16 and Otoa colonies, only six animals showed SOAEs. Although the SOAE frequencies in Ceacam16 KO mice (Fig. 2B) overlap those in controls, SOAEs for Otoa KOs (Fig. 2C) are usually lower in frequency. In our hands, 34 of 43 Otoa KOs (79 %) had measureable SOAEs.
Fig. 2.
SOAE spectra. Representative SOAE spectra are provided for all WT animals (A) in our collection, along with those from a selection of mice lacking Ceacam16 (B) or otoancorin (C). All data are plotted on an ordinate with arbitrary scale, i.e., the 10-dB scale applies to all panels.
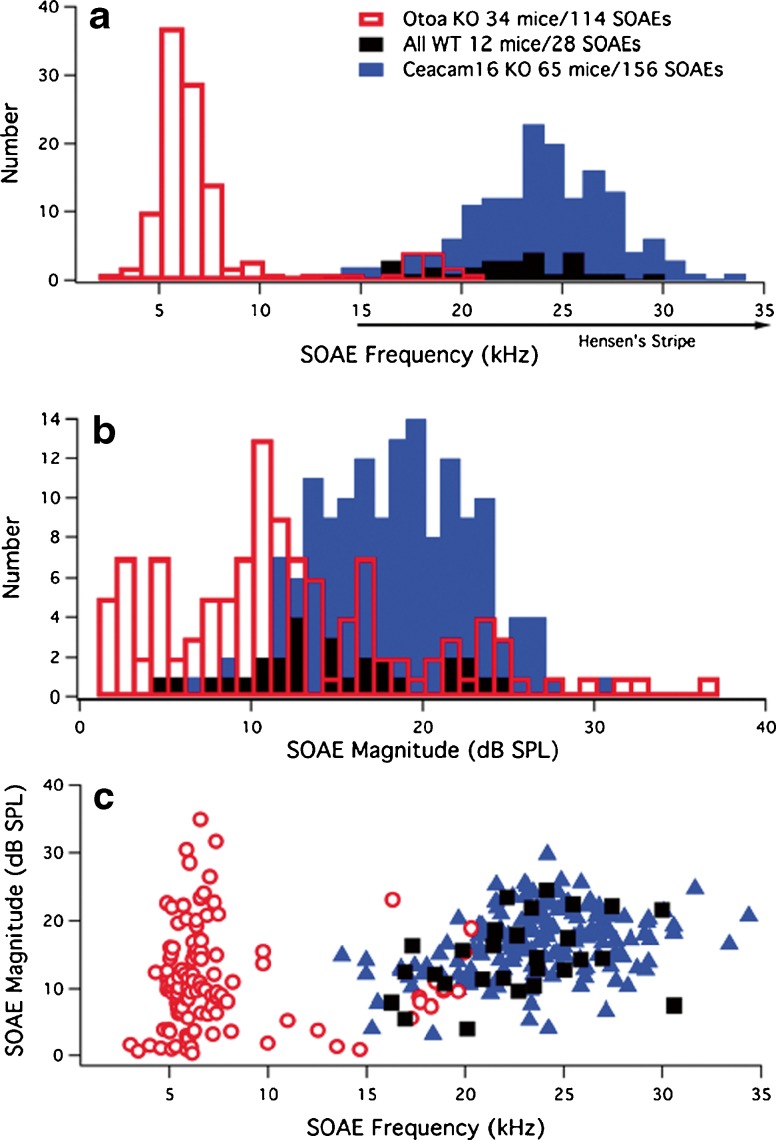
SOAE data are plotted as a bar graph in Figure 3 to show the distribution of their frequencies (Fig. 3A) and magnitudes (Fig. 3B) for the Otoa (red) KO mice, and for reference for the Ceacam16 KOs (blue), as well as for the 12 WT animals that exhibited SOAEs (black). The rare SOAEs in WT mice occur at frequencies corresponding to cochlear locations where Hensen’s stripe is a distinct feature of the TM. The average SOAE frequency in WT mice is 22.6 ± 3.8 kHz, similar to that in Ceacam16 KOs where the average frequency is 23.8 ± 3.7 kHz. Both these frequencies/averages, however, are much higher than the average SOAE frequency in Otoa KOs, which is 7.6 ± 4.1 kHz for the whole population of emissions. Although nine Otoa KO mice produce SOAEs above ~15 kHz, in the same region as those seen in Ceacam16 KOs and WT mice, this behavior is observed only when the animals retain larger high-frequency DPOAEs. Otoa KOs, therefore, generate both de novo low- and higher-frequency SOAEs, with the average frequency for those above 15 kHz being 18.4 ± 1.2 kHz (n = 12) and that for SOAEs below 15 kHz being 6.4 ± 1.8 kHz (n = 102). Also, if a WT mouse is a spontaneous emitter, the average number of SOAEs with different peak frequencies produced per mouse is similar to that encountered in Ceacam16 KOs, i.e., an individual WT mouse that emits generates on average 2.3 SOAEs (28 SOAEs/12 WT mice) and a Ceacam16 KO produces 2.4 (156 SOAEs/65 mice). In contrast, an Otoa KO generates 3.4 (114 SOAEs/34 mice) SOAEs per mouse per recording. Panel B shows that the average magnitude of SOAEs is somewhat smaller for mice lacking otoancorin. The average magnitude for WT controls is 14.7 ± 5.5 dB, that for Ceacam16 KOs, 16.8 ± 4.8 dB and for Otoa KOs, 11.3 ± 7.4 dB. Finally, in Figure 3C, SOAE magnitudes are plotted as a function of SOAE frequency for WT (black squares), Ceacam16 (blue triangles), and Otoa KOs (open red circles). Although some of the SOAEs in the Otoa KO mice are of high magnitude (see Fig. 2C), collectively, they tend to distribute over a larger range than in WT and Ceacam16 KO mice, thereby resulting in a smaller average SOAE magnitude. It is also noted that the Otoa KO mice with SOAEs above ~15 kHz are those animals that produce the largest DPOAEs at high f2 frequencies. In fact, when mice lacking otoancorin retain some sensitivity at high f2 frequencies, they too can exhibit SOAEs in the same region as WT and Ceacam16 KOs.
Fig. 3.
SOAE frequencies and magnitudes. The number of SOAEs is plotted as a function of their peak frequency (A) and magnitude (B). The total number of mice with SOAEs and the total number of SOAEs observed in each mouse group is provided in the legend. The position of Hensen’s stripe in WT mice is also indicated in panel A along the abscissa to show that it is present in controls but only for more basal locations encoding frequencies greater than ~15 kHz (Cheatham et al. 2014; Legan et al. 2014). Panel C provides a plot of SOAE magnitude versus SOAE frequency for WT (black squares), Ceacam16 KOs (blue triangles), and Otoa KOs (red open circles).
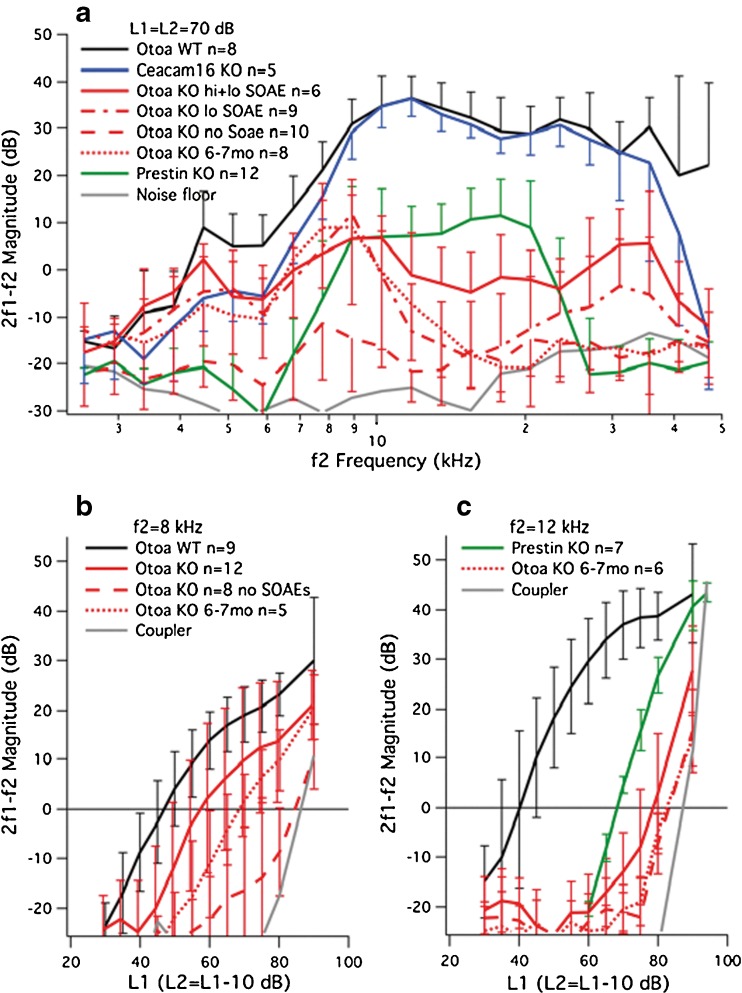
To assess cochlear performance and its relation to the propensity to generate SOAEs, data for DPOAEs at 2f1-f2 are shown in Figure 4A where average magnitudes (± one standard deviation) are plotted as a function of f2 frequency with L1 = L2 = 70 dB SPL. In addition to results from WT controls (black), DPOAEs are included for Ceacam16 (blue) and Otoa KO (red) mice. The latter mutants are separated into four groups: young (3–7 weeks of age) Otoa KOs with both high- (hi) and low- (lo) frequency SOAEs (solid lines) and those with low-frequency SOAEs only (dot-dashed lines) and young Otoa KO mice that did not generate SOAEs (dashed lines) and older Otoa KO mice at 6–7 months of age (dotted lines). As reported previously (Cheatham et al. 2014), mice lacking Ceacam16 retain near-normal DPOAEs when young in spite of the presence of large holes, especially in the apical TM, and the fact that both the striated-sheet matrix and Hensen’s stripe are missing. In contrast, reduced DPOAEs are recorded in Otoa KOs. For young animals generating SOAEs, the largest DPOAE responses are recorded for relatively low f2 frequencies and, in some mice, for higher f2 frequencies as well. Otoa KOs with high-frequency SOAEs produce DPOAEs that are larger than those in mice with only low-frequency SOAEs. This is especially true in the vicinity of f2 = 17.8 kHz, which is near the average SOAE frequency (18.4 ± 1.2 kHz) for mice generating spontaneous emissions above 15 kHz. Mice lacking Otoa that do not exhibit SOAEs have small DPOAEs independent of f2 frequency. In fact, their responses differ from the noise floor only in a narrow frequency range. Data for older Otoa KOs (dotted lines) show DPOAEs at 2f1-f2 but only for low f2 frequencies. Six of these eight older mice lacking otoancorin produced SOAEs, but all were less than 9 kHz. A one-way ANOVA revealed that the WT controls and the various groups of Otoa KOs were statistically different from one another (range of significant effects: F(4,36)=12.08–68.87, p < 0.01). Post hoc t tests also showed that Otoa KOs with high-frequency SOAEs (average SOAE frequency 18.4 kHz) were statistically different (p < 0.01) from KOs with only low-frequency SOAEs at f2 = 17.8 kHz. For comparison, we appended our results from mice lacking prestin to show high-level OAEs measured in a cochlea where the outer hair cells lack somatic electromotility and, hence, amplification (Dallos et al. 2008). The data were obtained from Prestin KOs that produce DPOAEs since emissions in mice lacking prestin are known to be highly variable such that only those with the best sensitivity generate DPOAEs and then only at high stimulus levels (Liberman et al. 2004). Comparisons between mice lacking otoancorin and those lacking prestin indicate that for much of the frequency range of hearing, responses generated in Otoa KO mice are no larger than those for Prestin KOs. In fact, DPOAEs in Otoa KOs exceeded those in mice lacking prestin only at low (<9 kHz) and high (>25 kHz) f2 frequencies, providing evidence for some amplification.
Fig. 4.
DPOAEs. Panel A provides DPOAE magnitudes for 2f1-f2 plotted as a function of f2 frequency for equal-level primaries at 70 dB. Although Ceacam16 KO mice (blue) have near-normal responses when young, the Otoa KOs show reduced responses. The animals lacking Otoa are plotted in red for four different groups: mice producing both high- (hi) and low- (lo) frequency SOAEs with solid lines, those generating low-frequency SOAEs only with dot-dashed lines, those lacking SOAEs with dashed lines, and Otoa KO mice at 6–7 months of age with dotted lines. Results from mice lacking prestin are also appended and plotted in green. Data are presented as means and standard deviations (plotted in one direction only for clarity), and the controls are plotted in black. It is also emphasized that in our hands the Otoa KO mice show smaller DPOAEs than in the initial report on this mouse mutant (Lukashkin et al. 2012). This difference may reflect strain background such that the data reported here are for mice on a mixed, variable 129/B6 background, while those used by Lukashkin et al. were CBA/Ca × B6 F1 hybrids. The backcross to CBA/CaJ appears to minimize the age-related hearing loss known to plague the C57BL6J line. Input-output functions for 2f1-f2 are plotted in panel B for f2 = 8 kHz and in panel C for f2 = 12 kHz. Data for Otoa KO mice in panels B and C are again separated into two groups: those with (red solid lines) and those without (red dashed lines) SOAEs. Input-output functions for older Otoa KO mice are also appended and appear as dotted lines. Panel C also shows results from prestin KO mice (green). Distortion in the sound was measured in a tubing coupler and is indicated in panels B and C by the solid gray line.
Input-output functions were also obtained for DPOAEs at selected f2 frequencies. In Figure 4B, results are provided for f2 = 8 kHz (f1 = 6.667 kHz and 2f1-f2 = 5.332 kHz). Again, the data for mice lacking Otoa are plotted separately for young animals with and without SOAEs and for older KOs. Young Otoa KOs with SOAEs (solid lines) produced larger DPOAEs than mice without SOAEs (dashed lines), and both groups showed greater variability than WT controls. Average DPOAEs for mice at 6–7 months (dashed lines) fall in between the values for the younger mice with and without SOAEs. The average threshold shift for young Otoa KOs with SOAEs was 11.1 dB, while that for young mice lacking SOAEs was 35.4. The average threshold shift for older Otoa KO mice was 21.8 dB. A one-way ANOVA revealed a statistically significant difference between WT and Otoa KO groups (range of significant effects: F(3,30)=4.63–19.47, p = 0.01–0.00). Post hoc t tests also showed that Otoa KO mice with SOAEs were different from mice without SOAEs for L1 = 50 to 80 dB. Between 70 and 80 dB, older mice were also different from Otoa KOs without SOAEs (p < 0.05). Distortion in the sound is also appended as the gray line and indicates that DPOAEs in Otoa KOs without SOAEs may not be biological. Although not shown here, moderate threshold shifts were also obtained for DPOAEs at f2 = 4.5 kHz (12.9 dB) and f2 = 6 kHz (12.6 dB) for Otoa KO mice with SOAEs. Based on these estimates, Otoa KOs with SOAEs were deemed to have suffered a partial loss of gain, especially for f2 frequencies below ~10 kHz. In spite of the reduction in DPOAE magnitudes, most of the Otoa KOs show large and numerous SOAEs in the frequency region between ~4 and 8 kHz where they retain reasonable sensitivity and amplification. Data in Figure 4C show results for f2 = 12 kHz (f1 = 10 kHz, 2f1-f2 = 8 kHz). In this case, the functions are shifted to the right by ~40 dB for all Otoa KO mice independent of age and whether they produced SOAEs or not. A one-way ANOVA revealed that WT and Otoa KO mice were statistically different (range of significant values: F(3,32)=3.06–138.17, p = 0.04–0.0). The decrease in DPOAE magnitude for Otoa KO mice was not unlike that for mice lacking prestin (plotted in green) and signifies high-level production of OAEs at high sound levels in the absence of somatic electromotility.
DISCUSSION
In mice lacking Otoa, where the TM is detached from the limbus, DPOAEs are smaller than in controls. However, in spite of this decrease in DPOAE magnitude, numerous SOAEs are recorded especially at low frequencies. Although the overall increase in expression of SOAEs vis-à-vis WT is similar to that in Ceacam16 KO mice (Cheatham et al. 2014), low-frequency SOAEs are only observed in Otoa KO mice. Therefore, in mice lacking otoancorin, most SOAEs appear to be generated in the apical region of the cochlea where these mice produce their largest DPOAEs and where signal processing appears to be near normal. This observation is consistent with the idea that some degree of amplification is necessary for SOAE generation. In fact, SOAEs can be observed in humans but only when threshold shifts are less than ~20 dB HL (Probst et al. 1991).
It is also emphasized that a small group of good Otoa KO emitters produce signals between ~16 and ~21 kHz, overlapping with those seen in WT and Ceacam16 KO mice. Although some Otoa KO mice at 6–7 months of age retained SOAEs at low frequencies, none of the older mice exhibited high-frequency SOAEs, as their responses to high f2 frequencies were also reduced. Young mice lacking otoancorin can, therefore, generate SOAEs with the same frequencies as Ceacam16 KO and WT mice, but only when they retain some sensitivity at these high frequencies.
Measurements on isolated TMs (Jones et al. 2015) suggest that the striated-sheet matrix can enhance energy dissipation and suppress SOAEs in normal animals. Although this is a possible explanation for the increased incidence of SOAEs in Ceacam16 KO mice, Otoa KO mice retain striated-sheet matrix within the main body of the TM but are, nonetheless, excellent emitters. Hensen’s stripe is also absent from the apical, low-frequency region of the TM in wild-type mice, so the loss of this structural feature from the basal, high-frequency end of the TM is unlikely to account for the increased incidence of low-frequency SOAEs detected in animals where the most prominent anatomical change is detachment of the TM from the spiral limbus.
A recent modeling study (Prodanovic et al. 2015) suggests that viscous damping in the sub-tectorial space influences the oscillatory behavior of the cochlear partition and the dissipation of energy. Loss of limbal attachment and the absence of Hensen’s stripe are both likely to influence sub-tectorial fluid flow and could therefore lead to a change in the production of SOAEs, albeit in different regions of the cochlea. If suppression of spontaneous activity was linked to the complex hydrodynamics of fluid flow within the sub-tectorial space, then detaching the TM from the limbus as in Otoa KO mice and/or removing Hensen’s stripe might be expected to increase SOAE expression. The fact that WT and Ceacam16 KO mice do not emit at low frequencies may relate to the possibility that less than maximal available gain is adequate in normally operating ears. In other words, the system may be more stable in normal ears for regions coding lower-frequency inputs.
Oscillatory hair bundle behavior was first observed in the turtle cochlea (Crawford and Fettiplace 1985) and was predicted in cochlear models (Neely and Kim 1986). Subsequent experiments in non-mammalian vertebrates (Martin et al. 2003) showed that these movements disappeared when the mechanoelectrical transducer channels were blocked and that SOAEs were not observed when an accessory structure was attached to the sense organ (Strimbu et al. 2010). Recent work by Salvi et al. (Salvi et al. 2015) also suggests that hair bundles in mice with TM mutations can oscillate spontaneously due to a change in their mechanical load and that the frequency of these oscillations is expected to decrease with decreasing stiffness. In addition, model predictions indicate that changes in the balance between TM stiffness/mass can also influence SOAE generation (Maoileidigh and Hudspeth 2013). If spontaneous bundle movements change the OHC membrane potential, then a spontaneous change in OHC length could be induced as well. These observations imply that physical changes in the TM can influence SOAE frequency, as well as the degree to which spontaneous activity is generated/suppressed. If the TM-defect in Otoa KOs increases OHC-mediated gain, then the system could move toward a region of instability. A combination of increased gain/instability and reduced suppression may, therefore, account for the SOAEs seen in Otoa mutant mice.
Taken together, these results suggest that the mammalian organ of Corti in normal animals may have evolved to maximize amplification while preserving stability (Salvi et al. 2015). In light of unavoidable viscous losses attendant to the traveling wave in mammals, providing active gain was probably the primary factor that drove evolution of the OHC system, with the additional requirement that stability be maintained. Force-generating outer hair cells, coupled by accessory structures, provide high sensitivity allowing faint sounds to be heard in mammals even at high frequencies. However, the amount of active gain is limited by the added practical requirement that the system remains stable. The high incidence of SOAEs seen in mice with mutations that cause distinct changes in the TM suggests that this accessory structure and the mechanical load that it provides to the sense organ serve to maintain cochlear stability and that a high incidence of SOAEs may be diagnostic of dysfunction (Ruggero et al. 1983).
Acknowledgments
Work was supported by the National Institute on Deafness and Other Communicative Disorders, the National Institutes of Health (Grant DC000089 to M.A.C.), and the Wellcome Trust (Grant 087377 to G.P.R.). We thank Jonathan H. Siegel for wise counsel and technical expertise, as well as Elaine Cheshire Thompson and Trent Nicol for assistance with the statistical analysis.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no competing interests.
Contributor Information
Mary Ann Cheatham, Phone: 847-491-2456, Email: m-cheatham@northwestern.edu.
Aisha Ahmad, Email: Aisha.ahmad@northwestern.edu.
Yingjie Zhou, Email: Yingjie.zhou@northwestern.edu.
Richard J. Goodyear, Email: r.j.goodyear@sussex.ac.uk
Peter Dallos, Email: p-dallos@northwestern.edu.
Guy P. Richardson, Email: g.p.richardson@sussex.ac.uk
References
- Carvalho S, Mom T, Gilain L, Avan P. Frequency specificity of distortion-product otoacoustic emissions produced by high-level tones despite inefficient cochlear electromechanical feedback. J Acous Soc Am. 2004;116:1639. doi: 10.1121/1.1777873. [DOI] [PubMed] [Google Scholar]
- Cheatham MA, Goodyear RJ, Homma K, Legan PK, Korchagina J, Naskar S, Siegel JH, Dallos P, Zheng J, Richardson GP. Loss of the tectorial membrane protein CEACAM16 enhances spontaneous, stimulus-frequency, and transiently evoked otoacoustic emissions. J Neurosci. 2014;34:10325–10338. doi: 10.1523/JNEUROSCI.1256-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AC, Fettiplace R. The mechanical properties of ciliary bundles of turtle cochlear hair cells. J Physiol. 1985;364:359–379. doi: 10.1113/jphysiol.1985.sp015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P, Billone MC, Durrant JD, Wang C, Raynor S. Cochlear inner and outer hair cells: functional differences. Science. 1972;177:356–358. doi: 10.1126/science.177.4046.356. [DOI] [PubMed] [Google Scholar]
- Dallos P, Wu X, Cheatham MA, Gao J, Zheng J, Anderson CT, Jia S, Wang X, Cheng WH, Sengupta S, He DZ, Zuo J. Prestin-based outer hair cell motility is necessary for mammalian cochlear amplification. Neuron. 2008;58:1–7. doi: 10.1016/j.neuron.2008.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold T. Hearing II: the physical basis of the action of the cochlea. Proc R Soc B. 1948;135:492–498. doi: 10.1098/rspb.1948.0025. [DOI] [Google Scholar]
- Hildebrand MS, et al. DFNA8/12 caused by TECTA mutations is the most identified subtype of nonsyndromic autosomal dominant hearing loss. Hum Mutat. 2011;32:825–834. doi: 10.1002/humu.21512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth A. Mechanical amplification of stimuli by hair cells. Curr Opin Neurobiol. 1997;7:480–486. doi: 10.1016/S0959-4388(97)80026-8. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ. Integrating the active process of hair cells with cochlear function. Nat Rev Neurosci. 2014;15:600–614. doi: 10.1038/nrn3786. [DOI] [PubMed] [Google Scholar]
- Jaramillo F, Markin VS, Hudspeth AJ. Auditory illusions and the single hair cell. Nature. 1993;364:527–529. doi: 10.1038/364527a0. [DOI] [PubMed] [Google Scholar]
- Jones GP, Elliott SJ, Russell IJ, Lukashkin AN. Modified protein expression in the tectorial membrane of the cochlea reveals roles for the striated sheet matrix. Biophys J. 2015;108:203–210. doi: 10.1016/j.bpj.2014.11.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammerer R, Ruttiger L, Riesenberg R, Schauble C, Krupar R, Kamp A, Sunami K, Eisenried A, Hennenberg M, Grunert F, Bress A, Battaglia S, Schrewe H, Knipper M, Schneider MR, Zimmermann W. Loss of mammal-specific tectorial membrane component carcinoembryonic antigen cell adhesion molecule 16 (CEACAM16) leads to hearing impairment at low and high frequencies. J Biol Chem. 2012;287:21584–21598. doi: 10.1074/jbc.M111.320481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp DT. Stimulated acoustic emissions from within the human auditory system. J Acoust Soc Am. 1978;64:1386–1391. doi: 10.1121/1.382104. [DOI] [PubMed] [Google Scholar]
- Legan PK, Rau A, Keen JN, Richardson GP. The mouse tectorins. Modular matrix proteins of the inner ear homologous to components of the sperm-egg adhesion system. J Biol Chem. 1997;272:8791–8801. doi: 10.1074/jbc.272.13.8791. [DOI] [PubMed] [Google Scholar]
- Legan PK, Lukashkina VA, Goodyear RJ, Kössl M, Russell IJ, Richardson GP. A targeted deletion in alpha-tectorin reveals that the tectorial membrane is required for the gain and timing of cochlear feedback. Neuron. 2000;28:273–285. doi: 10.1016/S0896-6273(00)00102-1. [DOI] [PubMed] [Google Scholar]
- Legan PK, Goodyear RJ, Morin M, Mencia A, Pollard H, Olavarrieta L, Korchagina J, Modamio-Hoybjor S, Mayo F, Moreno F, Moreno-Pelayo MA, Richardson GP. Three deaf mice: mouse models for TECTA-based human hereditary deafness reveal domain-specific structural phenotypes in the tectorial membrane. Hum Mol Genet. 2014;23:2551–2568. doi: 10.1093/hmg/ddt646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Zuo J, Guinan JJ., Jr Otoacoustic emissions without somatic motility: can stereocilia mechanics drive the mammalian cochlea? J Acoust Soc Am. 2004;116:1649–1655. doi: 10.1121/1.1775275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DJ. Vestibular sensory organs. A scanning electron microscopic investigation. Arch Otolaryngol. 1971;94:69–76. doi: 10.1001/archotol.1971.00770070105013. [DOI] [PubMed] [Google Scholar]
- Lukashkin AN, Richardson GP, Russell IJ. Multiple roles for the tectorial membrane in the active cochlea. Hear Res. 2010;266:26–35. doi: 10.1016/j.heares.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Lukashkin AN, Legan PK, Weddell TD, Lukashkina VA, Goodyear RJ, Welstead LJ, Petit C, Russell IJ, Richardson GP. A mouse model for human deafness DFNB22 reveals that hearing impairment is due to a loss of inner hair cell stimulation. Proc Natl Acad Sci U S A. 2012;109:19351–19356. doi: 10.1073/pnas.1210159109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maoileidigh DO, Hudspeth AJ. Effects of cochlear loading on the motility of active outer hair cells. PNAS. 2013;110:5474–5479. doi: 10.1073/pnas.1302911110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Bozovic D, Choe Y, Hudspeth AJ. Spontaneous oscillation by hair bundles of the bullfrog’s sacculus. J Neurosci. 2003;23:4533–4548. doi: 10.1523/JNEUROSCI.23-11-04533.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mom T, Bonfils P, Gilain L, Avan P. Origin of cubic difference tones generated by high-intensity stimuli: effect of ischemia and auditory fatigue on the gerbil cochlea. J Acoust Soc Am. 2001;110:1477–1488. doi: 10.1121/1.1390337. [DOI] [PubMed] [Google Scholar]
- Moreno-Pelayo MA, Goodyear RJ, Mencia A, Modamio-Hoybjor S, Legan PK, Olavarrieta L, Moreno F, Richardson GP. Characterization of a spontaneous, recessive, missense mutation arising in the Tecta gene. J Assoc Res Otolaryngol. 2008;9:202–214. doi: 10.1007/s10162-008-0116-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, von Hunerbein K, Hoidis S, Smolders JW. A physiological place-frequency map of the cochlea in the CBA/J mouse. Hear Res. 2005;202:63–73. doi: 10.1016/j.heares.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Neely ST, Kim DO. A model for active elements in cochlear biomechanics. J Acoust Soc Am. 1986;79:1472–1480. doi: 10.1121/1.393674. [DOI] [PubMed] [Google Scholar]
- Neely S, Liu Z (1994) EMAV: otoacoustic emission averager. Technical Memo No 17, Boy’s Town National Research Hospital, Omaha NE.
- Neely ST, Stevenson R (1992) SysRes. In: Tech Memo No. 1. Omaha NE: Boystown National Research Hospital.
- Pearce M, Richter CP, Cheatham MA. A reconsideration of sound calibration in the mouse. J Neurosci Methods. 2001;106:57–67. doi: 10.1016/S0165-0270(01)00329-6. [DOI] [PubMed] [Google Scholar]
- Probst R, Lonsbury-Martin BL, Martin GK. A review of otoacoustic emissions. J Acoust Soc Am. 1991;89:2027–2067. doi: 10.1121/1.400897. [DOI] [PubMed] [Google Scholar]
- Prodanovic S, Gracewski S, Nam JH. Power dissipation in the subtectorial space of the mammalian cochlea is modulated by inner hair cell stereocilia. Biophys J. 2015;108:479–488. doi: 10.1016/j.bpj.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GP, Lukashkin AN, Russell IJ. The tectorial membrane: one slice of a complex cochlear sandwich. Curr Opin Otolaryngol Head Neck Surg. 2008;16:458–464. doi: 10.1097/MOO.0b013e32830e20c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero MA, Rich NC, Freyman R. Spontaneous and impulsively evoked otoacoustic emissions: indicators of cochlear pathology? Hear Res. 1983;10:283–300. doi: 10.1016/0378-5955(83)90094-1. [DOI] [PubMed] [Google Scholar]
- Salvi JD, O’Maoileidigh D, Fabella BA, Tobin M, Hudspeth AJ. Control of a hair bundle’s mechanosensory function by its mechanical load. Proc Natl Acad Sci U S A. 2015;112:E1000–E1009. doi: 10.1073/pnas.1501453112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strimbu CE, Kao A, Tokuda J, Ramunno-Johnson D, Bozovic D. Dynamic state and evoked motility in coupled hair bundles of the bullfrog sacculus. Hear Res. 2010;265:38–45. doi: 10.1016/j.heares.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Zheng J, Miller KK, Yang T, Hildebrand MS, Shearer AE, DeLuca AP, Scheetz TE, Drummond J, Scherer SE, Legan PK, Goodyear RJ, Richardson GP, Cheatham MA, Smith RJ, Dallos P. Carcinoembryonic antigen-related cell adhesion molecule 16 interacts with alpha-tectorin and is mutated in autosomal dominant hearing loss (DFNA4) Proc Natl Acad Sci U S A. 2011;108:4218–4223. doi: 10.1073/pnas.1005842108. [DOI] [PMC free article] [PubMed] [Google Scholar]