Abstract
Listening conditions in the real world involve segregating the stimuli of interest from competing auditory stimuli that differ in their sound level and spectral content. It is in these conditions of complex spectro-temporal processing that listeners with age-related hearing loss experience the most difficulties. Envelope following responses (EFRs) provide objective neurophysiological measures of auditory processing. EFRs were obtained to two simultaneous sinusoidally amplitude modulated (sAM) tones from young and aged Fischer-344 rats. One was held at a fixed suprathreshold sound level (sAM1FL) while the second varied in sound level (sAM2VL) and carrier frequency. EFR amplitudes to sAM1FL in the young decreased with signal-to-noise ratio (SNR), and this reduction was more pronounced when the sAM2VL carrier frequency was spectrally separated from sAM1FL. Aged animals showed similar trends, while having decreased overall response amplitudes compared to the young. These results were replicated using an established computational model of the auditory nerve. The trends observed in the EFRs were shown to be due to the contributions of the low-frequency tails of high-frequency neurons, rather than neurons tuned to the sAM1FL carrier frequency. Modeling changes in threshold and neural loss reproduced some of the changes seen with age, but accuracy improved when combined with an additional decrease representing synaptic loss of auditory nerve neurons. Sound segregation in this case derives primarily from peripheral processing, regardless of age. Contributions by more central neural mechanisms are likely to occur only at low SNRs.
Keywords: colliculus, ASSR, auditory nerve, FFR, EFR, cochlear neuropathy, synaptopathy
Introduction
Behaviorally relevant auditory stimuli seldom occur in isolation. They are interspersed with partially or fully overlapping irrelevant sounds. In the classic cocktail party problem, listeners must discriminate their sounds of interest from among competing stimuli of similar or different spectral and temporal content, in conditions where the ambient sound level is often higher than the sounds they are listening to (Bregman 1990). It is in these complex conditions that listeners with age-related hearing loss show the greatest difficulties (Fitzgibbons and Gordon-Salant 1996; Frisina and Frisina 1997).
Age-related hearing deficits occur as a combination of mechanical and neural deficits in the peripheral auditory system and the central auditory pathway. Emerging evidence has shown that the loss of hearing thresholds due to changes in the periphery does not account for all the changes in hearing seen with age (Dubno et al. 1984; Ruggles et al. 2012; Fullgrabe et al. 2015). Neural deficits due to loss of spiral ganglion and ribbon synapses (Sergeyenko et al. 2013) or imbalances in the composition of neurotransmitters (Caspary et al. 2008) in the central auditory pathway could account for the deficits seen in temporal processing and sound segregation at suprathreshold sound levels, which persist in subjects with clinically “normal” hearing thresholds (Hind et al. 2011). These neural deficits can be detected objectively in both human populations and animal models using auditory evoked potentials such as auditory brainstem responses (ABRs) and envelope following responses (EFRs) (Parthasarathy and Bartlett 2011; Ruggles et al. 2012; Sergeyenko et al. 2013; Shaheen et al. 2015). EFRs represent summed, sustained, synchronized activities of populations of neurons from the auditory pathway which provide additional complementary information to metrics obtained from more established responses like ABRs (Parthasarathy et al. 2014), with the major generators being the auditory brainstem and midbrain (Kuwada et al. 2002; Chandrasekaran and Kraus 2010; Parthasarathy and Bartlett 2012).
Perceptual difficulties observed with age in processing competing sound stimuli may depend on a variety of factors, including the fidelity of the auditory information entering the system at the level of the auditory nerve (AN), processing of this information by brainstem, midbrain, and forebrain nuclei in the central auditory pathway, integration of binaural spatial and temporal information, as well as the effects of non-auditory cognitive mechanisms such as attention (Grimault et al. 2001; Alain and McDonald 2007; Bidet-Caulet et al. 2007; Ruggles et al. 2012; Johnsrude et al. 2013; Sergeyenko et al. 2013; Bharadwaj et al. 2014; King et al. 2014). Understanding the changes that occur in processing sound in the auditory periphery will help separate out the deficits inherited from earlier stages from those generated in the later stages of the auditory pathway.
Here, we investigated age-related neurophysiological changes in the processing of two simultaneous sinusoidally amplitude modulated (sAM) tones with varying degrees of spectral overlap. Concurrent sAM stimuli have been used as a model for detection and segregation of auditory objects in which stimulus parameters can be modified in a controlled and predictable manner (Hall and Grose 1991; Bacon and Konrad 1993; Yost and Sheft 1994). These studies suggest varying degrees of interactions in the detection of competing sAM stimuli, depending on sound level and spectral content of each component (Lins and Picton 1995; Herdman and Stapells 2003; McNerney and Burkard 2010; Nakamoto et al. 2010). However, the neurophysiological bases of processing competing sAM tones are poorly understood. Using EFRs, insight into the population neural encoding of these simultaneous sAM stimuli from the early auditory system can be obtained. Here, the effects of a competing second sAM tone on a primary sAM tone of a constant suprathreshold sound level are studied using EFRs in a rodent model of aging. Competing sAM tones were systematically varied in relative sound levels and spectral information. Underlying neural mechanisms were then explored using an established model of the auditory nerve (Zilany et al. 2009; Zilany et al. 2014), which was also used to simulate responses with aging.
Methods
Subjects and Experimental Setup
Ten young (3–5-month old, 275–300 g) and 10 aged (22–24-month old, 400–425 g) male Fischer-344 rats (Taconic and Charles River laboratories) were used in this study. The colony the animals were obtained from had no effect on the fast Fourier transform (FFT) amplitudes of the EFRs obtained (data not shown; for details on FFT amplitudes, see below). The animals were raised in relatively quiet, standard laboratory conditions. All protocols were approved by the Purdue Animal Care and Use Committee (PACUC 1111000167).
The experimental protocols have been described in detail previously (Parthasarathy et al. 2014). The experiments were performed in a 9’ × 9’, double-walled acoustic chamber (Industrial Acoustics Corporation) lined with 2” anechoic foam. The animals were briefly anesthetized using isoflurane (1.5–2 %) for inserting the electrodes and for the intramuscular injection of dexmedetomidine (Dexdomitor, 0.2 mg/kg), an α-adrenergic agonist that acts as a sedative and an analgesic. The anesthesia was then removed and 15 min elapsed before recordings commenced, to allow for the anesthetic effects to wear off. The experiments were terminated if the animal moved its head enough to perturb the recordings or showed any signs of discomfort, as monitored by a video camera in the recording chamber. The evoked potentials were obtained using subdermal needle electrodes (Ambu) and two simultaneous recording channels. One positive electrode (channel 1) was placed on the animal’s forehead along the midline from the Cz to the Fz position, and another positive electrode (channel 2) was placed horizontally, along the inter-aural line, above the location of the inferior colliculus. The negative electrode was placed under the right ear, along the mastoid, and the ground was placed in the nape of the neck. Impedances were ensured to be always less than 1 K as tested using the low-impedance amplifier (RA4LI, Tucker Davis Technologies or TDT). Consistent with the previous findings (Parthasarathy and Bartlett 2012), amplitude modulation (AM) frequencies below 100 Hz were analyzed from channel 2, and AM frequencies above 100 Hz were analyzed from channel 1, to obtain sensitive testing across the entire range of modulation frequencies.
Sounds were generated by SigGenRP (TDT) at a 100-kHz sampling rate, and the acquisition was at a 25-kHz sampling rate. Signal presentation and acquisition were performed by BioSig software (TDT). Waveforms were converted to sounds and delivered via a multichannel processor (RX6, TDT) through a Bowers and Wilkins DM601 speaker. The stimulus was presented free field to the right of the animal, at a distance of 115 cm from speaker to the right ear. The output from the speaker was calibrated free field, using SigCal (TDT) and a Bruel Kjaer microphone with a 0.25-in. condenser, pointed at frontal incidence to the speaker, from the same location as the animal’s right ear, and was found to be within ±6 dB for the frequency range tested (5–15 kHz). Digitized waveforms were recorded with a multichannel recording and stimulation system (RZ-5, TDT) and analyzed with BioSig or custom written programs in MATLAB (Mathworks).
Testing Paradigm, Stimulus Description, Presentation, and Acquisition
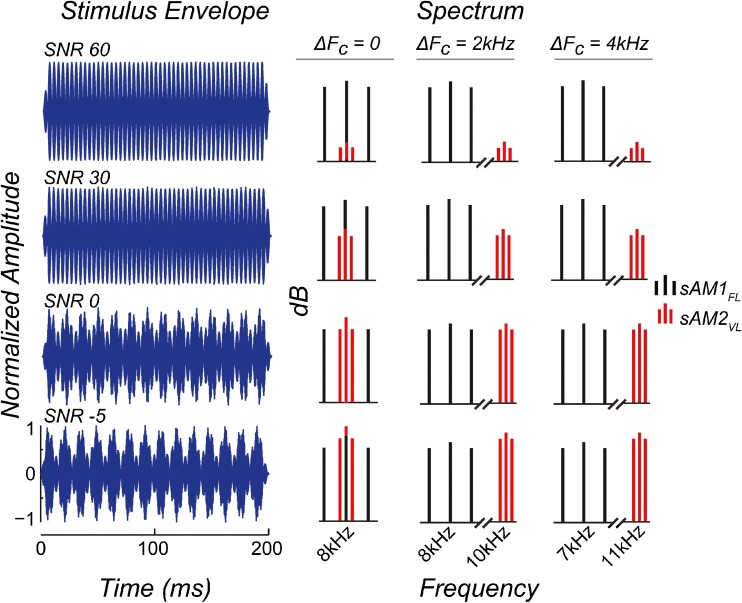
Stimuli for each test condition consisted of two simultaneous sAM tones. The first was a pure tone which was always amplitude modulated at 256 Hz, and presented at a fixed suprathreshold sound level (hereafter referred to as sAM1FL, for fixed level). The optimal sound level of presentation for the sAM1FL was determined as the lowest sound level that produced the maximum response amplitude when presented alone, in quiet, for each animal, consistent with previous studies (Parthasarathy and Bartlett 2012, Parthasarathy et al. 2014). This typically corresponded to 70–75 dB SPL for the young and 80–85 dB SPL for the aged, and represented the maximum ability of that individual animal to represent the target modulation frequency. The second was a pure tone which was always modulated at 71 Hz, and presented simultaneously with the same starting phase. The sound level of the competing sAM tone varied systematically (hereafter referred to as sAM2VL, for varying level) in eight steps to produce different signal-to-noise ratios (SNRs), with “noise” in this case denoting the effect of the interfering sAM tone on the one being measured. The overall sound level of the complex was not adjusted, and hence changed by ∼7 dB between the 60 and −5 dB SNR conditions. The carrier frequencies (FC) of the sAM1FL and sAM2VL tones were varied in three test conditions—a “same” FC condition where carrier frequencies of both sAM tones were at 8 kHz such that the difference ΔFC = 0; a “near” separation condition where the carrier frequency of the sAM1FL tone was 8 kHz, and the carrier frequency of the sAM2VL tone was 10 kHz such that the difference ΔFC = 2 kHz; and a “far” separation condition where the carrier frequency of the sAM1FL was 7 kHz, and the carrier frequency of the sAM2VL tone was 11 kHz such that the ΔFC = 4 kHz. Representative stimuli are presented in Figure 1, both as time-domain waveforms and spectra, for the three test conditions and four representative SNRs. The left column shows the envelope for the four representative SNRs in the ΔFC = 0 condition. However, this can be taken as examples of the envelope for ΔFC = 2 and 4 kHz conditions as well, as the envelope remains largely unchanged. The spectra for the three ΔFC conditions are shown on the right. As noted above, the level of sAM1FL remains fixed across all the conditions (Fig. 1, black vertical lines). The level of sAM2VL (Fig. 1, red vertical lines) varies relative to sAM1FL. At 60 dB SNR, the level of sAM2VL is 60 dB below sAM1FL (Fig. 1, first row); at 30 dB SNR, the level of sAM2VL is 30 dB below sAM1FL (Fig. 1, second row); at 0 dB SNR, the level of sAM1FL and sAM2VL is the same (Fig. 1, third row); or at −5 dB SNR, the level of sAM2VL is five dB above sAM1FL (Fig. 1, bottom row), for the three ΔFC conditions. The carrier frequencies for the three ΔFC conditions were selected to be in the most sensitive region of the rat’s audiogram, which shows minimal changes in thresholds with age (Parthasarathy et al. 2014). The modulation frequencies of the sAM1FL and the sAM2VL were chosen such that the two frequencies are close to two octaves apart but not harmonically related. The modulation depths for the two sAM stimuli were maintained at 100 %.
Fig. 1.
Representative stimuli for the three test conditions, ΔF C = 0, 2, and 4 kHz, in both the time (LEFT) and frequency domains (RIGHT) for the four representative SNR conditions: SNR 60, 30, 0, and −5 dB. For the time-domain waveforms in BLUE ON THE LEFT, X-AXIS represents time in ms, and Y-AXIS represents normalized amplitude. The waveforms shown are for the ΔF C = 0 condition, though the stimulus envelope remains largely unchanged between the three conditions. For the stimuli in the frequency domain (RIGHT), sAM1FL is shown as BLACK LINES, and sAM2VL is shown as RED LINES, with the X-AXIS representing frequency in kHz and Y-AXIS representing relative sound levels in dB.
All sAM tones were 200-ms long, with a 5-ms onset and offset cos2 gate. The sAM1FL-sAM2VL combinations were presented at 3.1 repetitions per second. The acquisition window was 300-ms long, beginning at stimulus onset, and each recorded response was an average of 200 repetitions.
Data Analysis
The data collected were filtered from 30 to 3000 Hz using BioSig (TDT) and exported to MATLAB. Fast Fourier transforms (FFT) were performed on time-domain waveforms obtained from BioSig, starting 10 ms after stimulus onset to exclude transient ABRs, and ending at stimulus offset, using custom programs written in MATLAB. The maximum amplitude of the FFT peak at one of three frequency bins (3 Hz each) around the modulation frequency gave the peak FFT amplitude. This FFT amplitude at the modulation frequency of the target or the masker AM frequency was used as a measure of phase-locking to assess group differences. The noise floor was calculated as the average of five frequency bins (3 Hz each) above and below the central three bins. A response was deemed as significantly above noise if the FFT amplitude was at least 6 dB above the noise floor.
In one case, the FFT amplitudes at sAM1FL obtained for all the other SNR conditions were normalized to the FFT amplitude at 60 dB SNR for each animal, for the three test conditions. Group means and statistical analyses were then performed on these normalized values. Statistically significant group differences were assessed for the FFT amplitudes at sAM1FL or sAM2VL modulation frequencies using repeated measures ANOVA (rmANOVA) performed using custom written scripts in SAS (Proc MIXED, SAS Institute, Cary, NC, USA). A log transformation was applied to the FFT amplitudes to produce a normally distributed data set. No log transformation was applied to the normalized data. Main effects of age, SNR, and carrier frequency combination, as well as their two-way and three-way interactions were tested. Statistically significant differences were calculated using differences in least square means, with a significance criterion of P < 0.05.
Auditory Nerve Model
The activity of the AN to the stimuli was modeled using an established computational model (Zilany et al. 2009; Zilany et al. 2014) which includes estimations of outer hair cell (OHC) and inner hair cell (IHC) function. Model simulations were performed using the sAM1FL-sAM2VL stimuli complexes on neurons with center frequencies (CFs) log-spaced between 5 and 15 kHz according to the cochlear frequency map in rats (Greenwood 1990), at 1000 repetitions per CF. This range comprised all of the driven activity for the frequencies of the stimuli tested as confirmed with a wider range of CFs tested initially. An FFT was performed on the resulting synaptic output, similar to the EFRs, and the FFT amplitude was used as a measure of phase-locking to the sAM1FL and sAM2VL modulation frequencies. The sum of such FFT amplitudes of all the neurons was obtained for low and medium spontaneous rate (SR) (≤18 spikes/s) and high SR (>18 spikes/s) fibers. The FFT amplitudes from these fibers were then combined in a ratio of 60 % high:40 % medium + low, according to known distributions of these AN fibers in other rodent species (Liberman 1978; Schmiedt 1989) to arrive at an estimate of the phase-locking by the AN to the stimulus. For the normalized condition, the amplitudes obtained from the other SNRs in each run were then normalized to FFT amplitude at 60 dB SNR of that run, for each stimulus condition to enable a direct comparison with the normalized EFR data.
For modeling age-related changes in hearing thresholds, the audiograms of the aged animals (Parthasarathy et al. 2014) were used in the “fit audiogram” function of the AN model to arrive at the values for OHC and IHC function. These values were then used in the AN fiber simulations. The FFT amplitudes at AM frequencies were calculated as in the young model. The IHC function in the AN model is assumed to be the loss of spiral ganglions and does not take into account age-related synaptopathy, i.e., the loss of synapses between the IHC and the auditory nerve that precedes the loss of spiral ganglions and is thought to be from the lower SR fibers (Sergeyenko et al. 2013; Kujawa and Liberman 2015). Hence, in further comparisons, the contributions of all the fibers were reduced by 30 or 50 %, or the contributions of just the low and medium SR fibers were reduced by half to mimic the loss of these synapses seen with age.
Sound level of the sAM1FL in the model was maintained at 70 dB SPL for the young and 80 dB SPL for the aged, to reflect the sound levels used in the EFRs, and to compensate for changes in hearing thresholds at the carrier frequency of the stimuli.
Results
Age-Related Deficits in Responses to the Primary sAM1FL and the Competing sAM2VL Tones
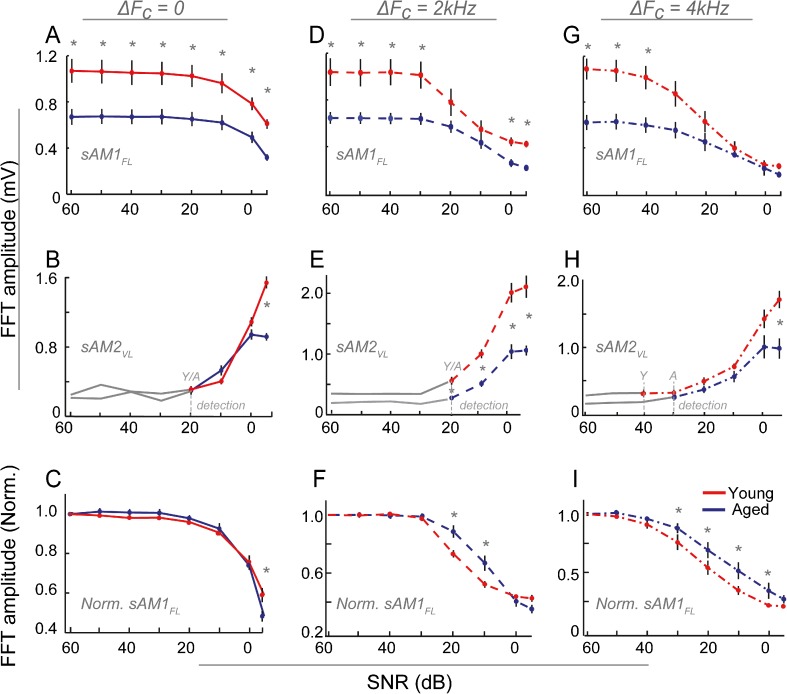
EFRs were obtained in the three test conditions (ΔFC = 0, 2, and 4 kHz) for the young and aged animals. Statistical analysis with rmANOVA using age, SNR, and ΔFC as factors revealed significant main effects of age (F1, 22 = 12.02, P = 0.0022), ΔFC (F2,29 = 107.75, P < 0.0001), and SNR (F7,154 = 185.23, P < 0.0001) for the FFT amplitudes at sAM1FL, as well the interactions between age and SNR (F7,154 = 2.27, P = 0.0318) and between ΔFC and SNR (F14,203 = 15.97, P < 0.0001). When the FFT amplitudes at sAM1FL were normalized to 60 dB SNR for each animal and the means compared between the ages, there were no main effects of age (F1, 18 = 1.79, P = 0.198), while SNR and ΔFC still had significant main effects (F7,126 = 476.7, P < 0.0001 and F2,21 = 256.54, P < 0.0001, respectively). Additionally, for the FFT amplitudes at sAM2VL, similar rmANOVA analysis revealed main effects of age (F1,22 = 27.53, P < 0.0001), ΔFC (F2,29 = 8.56, P = 0.0012), and SNR (F7,154 = 194.11, P < 0.0001) as well as the interaction between age and ΔFC (F2,29 = 8.32, P = 0.0014).
Looking at the trends within and across age, there was no change in the FFT amplitude of the sAM1FL tone as the sAM2VL level varied from 60 to 20 dB SNR in the ΔFC = 0 condition (Fig. 2, A–C). For SNRs lower than 20 dB, there was a decrease in the FFT amplitudes of the sAM1FL with SNR in both the young and aged animals (Fig. 2A). There was a significant, uniform decrease in the amplitude with age across SNRs (Fig. 2A). There was no significant difference in the nature of change with age across SNRs when normalized to 60 dB SNR for each animal, except at −5 dB (Fig. 2C). The physiological detection threshold for sAM2VL was 20 dB SNR for both the young and aged animals (Fig. 2B, gray dashed lines), with responses above the noise floor occurring at SNRs less than 20 dB. No age-related changes were observed, except at −5 dB SNR where the young showed significantly higher amplitudes to the sAM2VL (Fig. 2B).
Fig. 2.
EFRs to sAM1FL tones decrease at low SNRs in young and aged animals. FFT amplitudes at modulation frequency are shown for ΔF C = 0 (A–C), ΔF C = 2 kHz (D–F), and ΔF C = 4 kHz (G–I) conditions, for the sAM1FL (A, D, and G), sAM2VL (B, E, and H), and sAM1FL normalized to 60 dB SNR (C, F, and I), for young (RED) and aged (BLUE) animals. Responses below noise floor are shown in GRAY. The physiological detection thresholds, where the responses first rise above the noise floor, are marked for both the young and aged animals in GRAY DASHED LINES. The X-AXIS represents SNR in dB, and the Y-AXIS represents absolute FFT amplitudes in mV or amplitudes normalized to 60 dB SNR for each animal. ERROR BARS represent standard error. ASTERISKS represent statistically significant differences (P < 0.05, rmANOVA).
In the ΔFC = 2-kHz condition (Fig. 2D–F), the amplitudes to the sAM1FL tone decreased only for SNRs lower than 30 dB (Fig. 2D). The aged animals showed a significant decrease in response to the sAM1FL compared to the young for all SNRs except 20 and 10 dB. This was due to the steeper slope of decrease with SNR in the young compared to the aged, as can be seen when the amplitudes are normalized to 60 dB SNR (Fig. 2F). The physiological detection threshold for sAM2VL was the same as the previous condition, at 20 dB SNR, for both the young and aged animals (Fig. 2E, gray dashed lines), with responses above the noise floor occurring at SNRs ≤20 dB (Fig. 2E). The EFR amplitudes for the sAM2VL in the young were significantly higher than those in the aged for all SNR levels. However, this was due to a larger amplitudes in the young at and below threshold, as witnessed by the gray solid lines in the young being higher than the aged (Fig. 2E), and these differences disappeared when the baselines were normalized to threshold, except at −5 dB SNR (data not shown), similar to the ΔFC = 0 condition.
Trends seen in the ΔFC = 2 kHz condition were amplified in the ΔFC= 4 kHz condition (Fig. 2G–I). Reduction in sAM1FL responses was observed at much higher SNRs in this condition, starting at 40 dB SNR in the young and 30 dB SNR in the aged. This difference with age translated to a decreased normalized amplitude in the young, compared to the aged (Fig. 2I). Young animals showed significantly higher amplitudes than the aged only at 50–60 dB SNR (Fig. 2G). The physiological detection threshold for sAM2VL was lower in this condition compared to the previous two conditions, with responses above the noise floor occurring at 40 dB SNR for the young (Fig. 2H, red line). In the aged, this threshold was higher, with significant responses occurring only at 30 dB SNR (Fig. 2H, blue line). Similar to the other two ΔFC conditions (Fig. 2B, E), significantly higher amplitudes in the young were evident only at the lowest SNRs (Fig. 2H).
Decreased Separation in Responses Between Test Conditions with Age
FFT amplitudes of the sAM1FL normalized to amplitudes at 60 dB SNR for each animal are re-plotted from Figure 2 (C, F, I) in Figure 3 for the three ΔFC test conditions in the young and aged animals. It can be seen that the presence of the sAM2VL tone results in decreases in response amplitudes to the sAM1FL in both the young and aged animals (Fig. 3). Statistical analyses using rmANOVA revealed significant effects of ΔFC and SNR as well as their interaction in both the young and aged animals (young—SNR: F7,63 = 290.30, P < 0.0001; ΔFC: F2,16 = 238, P < 0.0001; SNR X ΔFC: F14,112 = 24, P < 0.0001; aged—SNR: F7,91 = 220.75, P < 0.0001; ΔFC: F2,13 = 78.40, P < 0.0001; SNR X ΔFC: F14,91 = 9.84, P < 0.0001). However, in the young, this decrease is more pronounced, with lower SNRs resulting in a greater separation between the three test conditions (Fig. 3, top). In the aged animals, this amount of separation between the three conditions seen in the young is not present (Fig. 3, bottom). Though the sAM1FL amplitudes significantly decrease with separated carrier frequencies compared to the ΔFC = 0 condition, significant differences between the ΔFC = 2 and 4 kHz conditions are only present between 30 and 10 dB SNR in the older animals.
Fig. 3.
Young animals exhibit a greater separation as carrier frequency difference increases. Comparison of the sAM1FL EFR amplitudes from the three test conditions ΔF C = 0, 2, and 4 kHz normalized to 60 dB SNR for each animal and condition. Responses from the young animals are shown in RED IN THE TOP PANEL, and from the aged animals in BLUE IN THE BOTTOM PANEL. X-AXES represent SNR in dB, and Y-AXES represent normalized FFT amplitudes. ASTERISKS represent statistically significant differences between ΔF C = 0 and the other two conditions; PLUS SIGNS indicate statistically significant differences between ΔF C = 2 kHz and ΔF C = 4 kHz conditions (P < 0.05, rmANOVA).
Exploring the Contributions of the Auditory Nerve to the EFRs Using a Computational Model
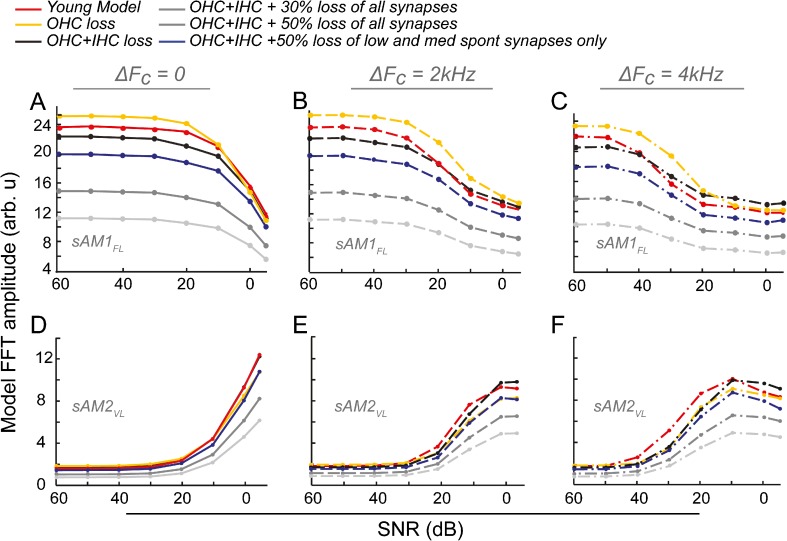
The contributions of the auditory nerve to the EFRs were explored using a computational model, as described in the methods. Log-spaced FCs between 5 and 15 kHz according to the cochlear frequency map in rats (Greenwood 1990) were used to calculate the contributions of all the responsive frequency regions. Responses to the sAM1FL and the sAM2VL, for the three ΔFC conditions, were obtained from the synaptic output of the model. Six modeling conditions were tested. First, a “young” model, where the OHC/IHC functions were normal; second, an “aged OHC loss,” where all changes in hearing thresholds were attributed to OHC loss alone; and third, an “aged OHC/IHC loss,” where 2/3rd of the changes seen in hearing thresholds were attributed to OHC loss and 1/3rd to IHC loss (IHC loss in the model is assumed to be loss of spiral ganglions). In addition, the last three conditions modeled the loss of synapses between the IHC and the auditory nerve seen with age which precedes the loss of spiral ganglions (Sergeyenko et al. 2013). Functionally, this amounts to additional loss of IHC/AN output in the model. This loss of synapses is speculated to be in the low and medium SR fibers (Kujawa and Liberman 2015). Hence, the fourth condition, in addition to OHC and IHC loss, has a 50 % loss of low and medium SR function. The fifth and sixth conditions included a 30 or 50 % loss of function across all SRs, respectively. Figure 4 shows the results of the AN model, for both the sAM1FL and the sAM2VL tones. Trends in the FFT amplitudes to the sAM1FL in the young model are similar to what was observed in the young EFRs for all the three testing conditions (Fig. 4A–C; red lines, compare to Fig. 2A, D, G; red lines). Decreasing the OHC function in the aged model results in increased thresholds, but since the sAM1FL stimuli were presented to the aged at a higher absolute sound level, this results in a higher amplitude of the sAM1FL for the “aged OHC model” (Fig. 4A–C; yellow lines). Adding loss of IHC function in the aged model results in a decrease in the number of responding neurons and hence a decrease in the response amplitude to sAM1FL, with the results approaching those seen in the aged animals (Fig. 4A–C; black lines). Adding an additional 30 or 50 % loss of synapses across SR types results in a decrease in amplitude that appears far greater than those observed in the experimentally measured EFRs (Fig. 4A–C, light and dark gray lines). However, adding a 50 % loss of low and medium SR fibers alone results in outputs that most closely resemble the EFRs from the aged animals (Fig. 4A–C; blue lines, compare to Fig. 2A, D, G; blue lines). This is especially true of the ΔFC = 2 and 4 kHz conditions, where the aged model is much lower than the young for high SNRs, but the difference is smaller for low SNRs, similar to what is seen in the EFRs.
Fig. 4.
A computational model of the auditory nerve recreates EFRs from the young and aged animals. EFR amplitudes from a “young” model (RED) and five “aged” models, with the effects of aging as modeled as of OHC loss alone (YELLOW), OHC and IHC/spiral ganglion loss (BLACK), OHC and IHC loss with an additional 30 % (DARK GRAY) or 50 % (LIGHT GRAY) loss of IHCs across all fiber types, or OHC and IHC loss along with an additional 50 % loss of lower SR fibers only (BLUE) to model different types of synaptic loss for sAM1FL (A–C) and sAM2VL (D–F) in the three test conditions. Comparative FFT amplitudes of the synaptic output from the model are shown on the Y-axes (arbitrary units) and SNR in dB along the X-axes. Compare to EFR amplitudes of the young and aged in Figure 2.
Trends in responses to the sAM2VL as obtained from the “young” model also closely mirror EFRs. Significant responses to sAM2VL begin at 20 dB SNR for the ΔFC = 0- and 2-kHz conditions, but begin at higher SNR of 40 dB for the ΔFC = 4 kHz condition in the young (Fig. 4D–F, red lines, compare to Fig. 2B, E, H). For the aged models, similar to the responses to sAM1FL, responses that most closely mirror the EFRs are seen with modeling loss of OHCs, IHCs, and an additional loss of low and medium SR synapses (Fig. 4D–F, blue lines, compare to Fig. 2A, D, G; blue lines). Loss of synapses across all SR types leads to decreases in amplitudes that are greater than those seen in the EFRs (Fig 4, D–F, dark and light gray lines). The benefit of spectral separation is also not seen in the aged models to the same degree, with higher threshold of responses to sAM2VL in the aged, compared to the young (Fig. 4D–F, blue lines, compare to Fig. 2B, E, H).
Comparing the Three Test Conditions in the Model
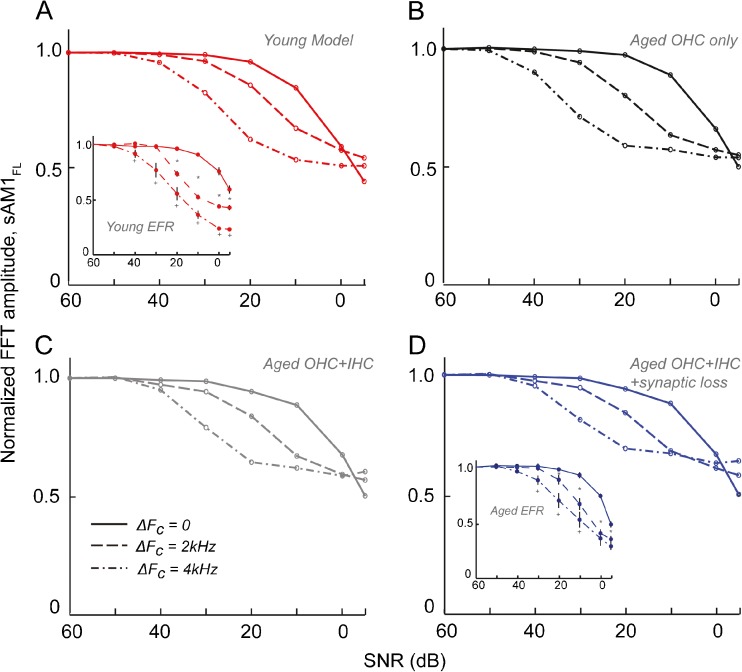
The response amplitudes to sAM1FL for the four modeling conditions—“young,” “aged OHC alone,” “aged OHC + IHC,” and “aged OHC + IHC + synaptic loss in low and medium SR fibers”—were normalized to the 60-dB SNR condition to compare to the trends seen in the EFRs in Figure 3. In the young model, the three conditions were well separated for SNRs between 30 and 0 dB, closely resembling the young EFRs (Fig. 5A, compare to Fig. 3, top; also reproduced in the inset in 5A). Simulating loss of OHC function alone in the aged model does not produce any drastic differences in these trends (Fig. 5B). Adding the loss of IHCs in the model results in the partial overlap of the ΔFC = 2-kHz and ΔFC = 4-kHz conditions (Fig. 5C), while adding the synaptic loss results in a greater overlap of the two separated-FC conditions in the aged model, similar to those seen in the aged EFRs (Fig. 5D, compare to Fig. 3, bottom; also reproduced in the inset in 5D). However, the model did not accurately reproduce either the degree of overlap in the aged for the two FC conditions or the responses in both the young and aged at SNRs of 0 and −5 dB (Fig. 5, compare to Fig. 3). The relative decrease in the response amplitudes with SNRs was also higher in the EFRs compared to the models (Fig. 5, compare to Fig. 3).
Fig. 5.
The model accurately recreates trends seen in EFRs at high SNRs. Comparisons of the FFT amplitudes from the model normalized to 60 dB SNR, for the young model (A) as well as the three aged models (B–D) in the three test conditions. Normalized FFT amplitudes of the synaptic output from the model are shown on the Y-axes and SNR in dB along the X-axes. Compare to EFR amplitudes of the young and aged in Figure 3 (reproduced in the inset in 5A and 5D, same axes as Fig. 5).
Contributions of Various Frequency Regions to the Model Trends
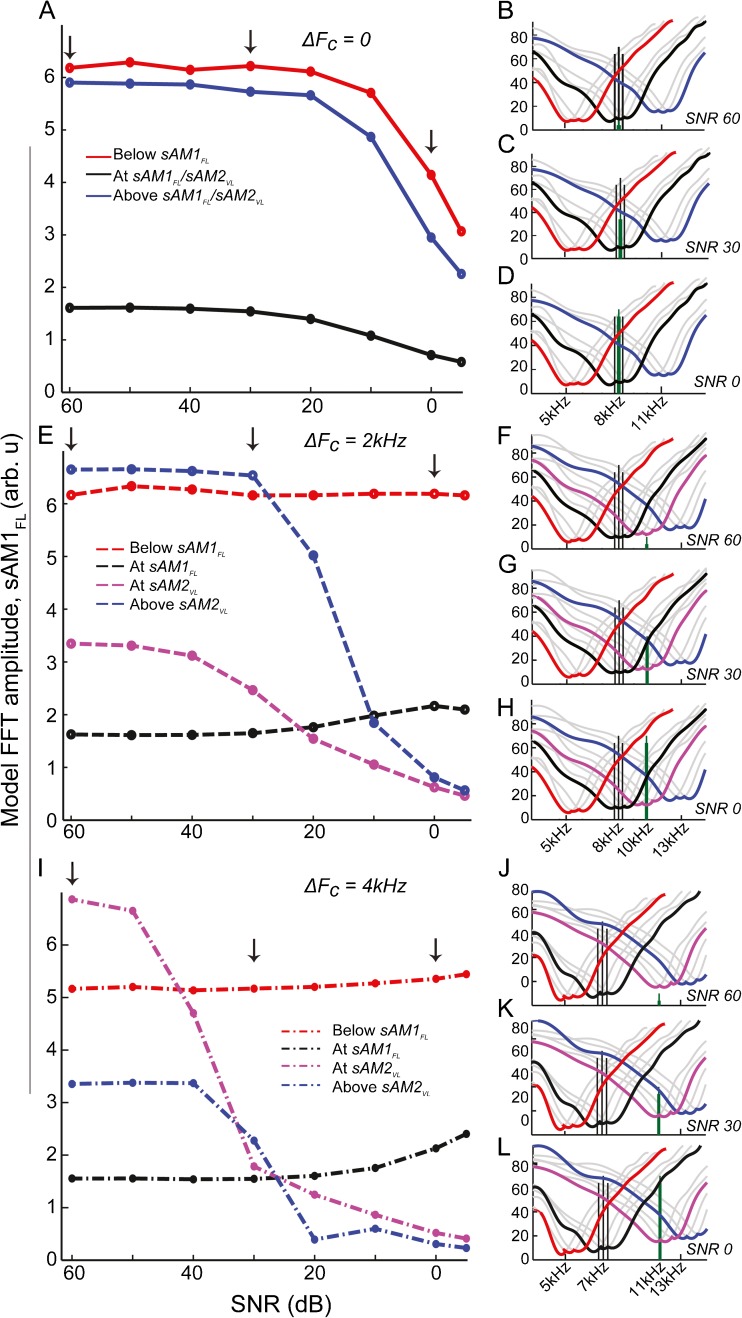
The CFs used in the model spanned the entire responsive range of frequencies for the current stimuli used (5–15 kHz). To understand the trends in the response across the different frequency regions, and how these frequency regions would eventually combine to make up the population EFRs, contributions of neurons from four broad frequency channels in the young model, each consisting of neurons from three adjacent CFs, were plotted for the three test conditions. The frequency regions were: (1) below the FC of sAM1FL, (2) centered at the FC of sAM1FL, (3) centered at the FC of sAM2VL, or (4) above the FC of sAM2VL. The stimuli, as well as the frequency channels, are illustrated for the three ΔFC conditions, for the three representative SNRs, using tuning curves generated using high SR fibers from the model (Fig. 6 right column). The response amplitudes to sAM1FL from these four frequency channels are plotted in Figure 6 (left column) for all the SNR conditions tested in the EFRs, combining data from high, medium, and low SR neurons as described in the “Methods” section.
Fig. 6.
Trends seen in EFRs are due to the contributions of low-frequency tails of high-frequency neurons. The RIGHT COLUMN shows stimuli represented by four frequency regions made up of three neurons of consecutive CFs tested (see “methods” section). Individual frequency response areas of the neurons are in GRAY. Combined frequency regions are outlined for “below sAM1FL” (RED), “at sAM1FL” (BLACK), “at sAM2VL” (MAGENTA), and “above sAM2VL” (BLUE). A–D do not contain magenta, as Fc of sAM1FL = F C of sAM2VL. sAM1FL stimuli are represented as BLACK VERTICAL LINES and sAM2VL as GREEN VERTICAL LINES. Tuning curves were generated using only high SR neurons for illustrative purposes. X-AXES show frequency in kHz, and Y-AXES show sound level in dB SPL. The LEFT COLUMN shows combined model FFT amplitudes for sAM1FL, for the four frequency regions, across all the SNRs, for the three test conditions. X-AXES represent SNR in dB, and Y-AXES represent comparative mean model FFT amplitude (arbitrary units).
For the ΔFC = 0 condition, at SNR60, sAM1FL is being presented at a fixed suprathreshold level, and is being encoded by neurons across all the frequency regions (Fig. 6B, black vertical lines). However, in terms of amplitudes, the neurons above and below the FC of sAM1FL exhibit much greater amplitude than those centered at the FC of sAM1FL (Fig. 6A, left arrow). The sAM2VL is at a low enough level that it does not interfere with the receptive fields of neurons from any of the frequency regions and is below threshold (Fig. 6B, green vertical lines). At SNR30, the level of sAM2VL increases such that it competes with the sAM1FL at the same FC (Fig. 6C), resulting in a slight decrease in amplitude in that region alone, leaving the other regions unaffected (Fig. 6A, middle arrow). At SNR0, the sAM2VL is at a high enough level that it competes with sAM1FL in all the frequency regions (Fig. 6D), resulting in a decrease in amplitude of the sAM1FL (Fig. 6A, right arrow).
In the ΔFC = 2-kHz condition, where the FC of sAM2VL is 2 kHz above that of sAM1FL, neurons below the FC of sAM1FL encode only sAM1FL at all the SNR conditions (Fig. 6F–H, red channel), and are never affected by the presence of sAM2VL, maintaining their amplitudes constantly (Fig. 6E, red line). Neurons centered at the FC of sAM1FL also show similar trends, remaining largely unaffected by the presence of sAM2VL across the SNR conditions (Fig. 6E, black line), since sAM2VL occurs in the high-frequency side of these fibers (Fig. 6F–H, green lines in black channel). For neurons in the frequency region centered at sAM2VL and above it, for high SNRs, the level of sAM2VL is low enough that these fibers predominantly code for sAM1FL (Fig. 6F, green lines in magenta or blue channels; Fig. 6E, left arrow). With increase in the level of sAM2VL (Fig. 6G–H, green lines in magenta and blue channels), these fibers gradually begin to exhibit a decrease in amplitudes to sAM1FL, first in the region centered at sAM2VL (Fig. 6E, middle arrow), and then also in the region above it (Fig. 6E, right arrow).
This trend is further amplified in the ΔFC = 4 kHz condition, where, similar to the previous condition, neurons centered below sAM1FL and at sAM1FLFC remain largely unaffected by the presence of sAM2VL (Fig. 6I, black and red lines). However, neurons centered at sAM2VL and above sAM2VLFCs experience a sharp decrease in amplitudes encoding sAM1FL, with the gradual increase in sound level of sAM2VL (Fig. 6I, magenta and blue lines, middle and right arrow).
Hence, it can be seen that the trends seen in EFRs seem to be primarily driven by higher-frequency neurons above the FC of sAM1FL, rather than those centered at or below the carrier frequency of sAM1FL itself.
Discussion
Detection vs. Fidelity of Neural Coding
In this study, the neural strategies for population encoding of simultaneous AM stimuli and their age-related changes were explored using EFRs. When one sAM tone is presented at a fixed, suprathreshold sound level (sAM1FL), the addition of a second sAM tone varying in level (sAM2VL) produced a decreased response to the first modulation frequency. The decrease in the response to sAM1FL was more pronounced when sAM2VL was spectrally separated from the target (Fig. 3, top). Psychophysical studies using concurrent AM stimuli typically require the listener to detect the presence of a second AM as it decreases in modulation depth (Bacon and Grantham 1989; Yost et al. 1989). The detection of an additional auditory object is facilitated by the degree of spectral separation between the target and the masker (Bacon and Konrad 1993; Micheyl et al. 2006), and the masking is always the greatest when the probe and the masker are spectrally close to one another (Bacon and Konrad 1993; Mendoza et al. 1995).
Though at first glance, this appears to contradict the EFR results found in this study, unlike the psychophysical studies, here, the sAM1FL is always presented at a level which should be well above perceptual detection thresholds. The EFR amplitudes reflect this, being above physiological noise floor for all the SNR and ΔFC conditions in the young and aged animals (Fig. 2A, D, G). Hence, the analogous comparison to the earlier mentioned psychophysical studies would be the “detection” of the sAM2VL, in the presence of sAM1FL. Here, comparable results are seen, with the neural detection threshold of the sAM2VL being 20 dB SNR for the ΔFC = 0 and 2 kHz conditions (∼50 dB SPL in the young), but decreasing to 40 dB SNR for the ΔFC = 4 kHz condition in the young (∼30 dB SPL in the young; Fig. 2B, E, H, gray dashed lines). However, in the aged, this spectral separation does not provide the same benefit, with the detection thresholds decreasing only to 30 dB SNR in the ΔFC = 4 kHz condition (Fig. 2B, E, H; gray dashed lines). This is ∼50 dB SPL, above the auditory thresholds for the aged animals in the frequency regions tested (Parthasarathy et al. 2014).
If the EFR amplitudes are taken as a measure of population temporal fidelity, then, the decrease in sAM1FL amplitudes due to the presence of sAM2VL, while still being significantly above the noise floor, indicates a loss of precision or dispersion in population neural coding rather than a change in detection thresholds. Psychophysical studies have demonstrated similar effects of a higher-frequency masker having a greater effect on the target (Bacon and Moore 1993; Mendoza et al. 1995). The decrease in response amplitudes using multiple AM tones of the same sound level (SNR of 0 dB) has also been previously seen using steady state potentials where significant interactions between the tones take place, especially at moderate to high sound levels of 40–80 dB SPL (Dolphin 1995; Lins and Picton 1995; McNerney and Burkard 2010). In these studies, responses to a given AM decreases in the presence of a separate AM of higher FC (Ross et al. 2003; McNerney and Burkard 2010). Since it is contrary to the typical shapes of tuning curves in AN neurons which show a sharper slope and a higher intensity required for activation on the higher-frequency side (Liberman 1978; Schmiedt 1989), these results were explained as a result of “non-energetic masking” and “central neural mechanisms.” However, in a population response like the EFR, especially for suprathreshold sounds, contributing neurons would not be limited to those near the carrier frequency of the stimulus alone, but rather would encompass a much larger range through activation of the low-frequency tails of their tuning curves, as the results from the AN model show (Fig. 6).
Modeling EFRs Using an AN Model
EFRs provide a way to measure temporal processing from the auditory brainstem and midbrain, and provide distinct information that is complementary to other established metrics such as ABRs (Parthasarathy et al. 2014). However, one of the significant drawbacks of EFRs is the loss of specificity regarding its generators, as opposed to ABRs, which have distinct waveforms whose origins are known (Buchwald and Huang 1975; Hashimoto et al. 1981). The IC and the brainstem are generally regarded as the primary generators of the EFRs (Kiren et al. 1994; Kuwada et al. 2002). Another limitation of any non-invasive auditory evoked potential is distinguishing the contributions of different frequency channels to the response, unlike measurements from single neurons with known CFs.
In this study, the AN model (Zilany et al. 2009; Zilany et al. 2014) was used to further address these issues. By modeling the outputs of the AN model as proxies for the EFRs, the contributions of different peripheral mechanisms were studied, and the contributions of different frequency channels were isolated. The model accurately reproduced the trends seen in the EFRs in the young (Figs. 4 and 5, red lines), suggesting that even by the level of the AN, the neural representations for processing simultaneous AM stimuli by synchronized activity are in place, and further stages of the auditory pathway may only contribute to further refinement of this process. The biggest departures from the EFRs in the “young” model were at low SNRs < 10 dB (Fig. 5A), suggesting that central generators may have a greater effect in these complex listening conditions. Some studies have modeled these central generators as an extension of the AN model (Nelson and Carney 2004) and even used them to model EFRs in cases of auditory neuropathy (Bharadwaj et al. 2014). However, given the differing contributions of the generators to various AM frequencies (Parthasarathy and Bartlett 2012), whether the use of these models would improve the accuracy of the simulated EFRs in this case remains to be explored. The trends seen in the EFRs are primarily driven by the low-frequency tails of high-frequency neurons in these suprathreshold levels (Fig. 6). Most real-world listening conditions, in which elderly listeners have difficulties, involve listening to and discriminating suprathreshold sounds in settings with competing sounds or background noise. Since the high-frequency neurons seem to play a significant role in encoding and discriminating these stimuli, this may explain the benefit older or hearing impaired listeners receive from high-frequency amplification, especially in noisy situations (Turner and Henry 2002; Plyler and Fleck 2006; Moore et al. 2010).
Effects of Aging in Processing Simultaneous sAM Tones
Age-related hearing loss is thought to occur as a combination of peripheral hearing deficits and central processing disorders. In the auditory periphery, OHCs decrease in number and degrade in function with age, resulting in increased thresholds and widened tuning curves (Buckiova et al. 2007; Chen et al. 2009). There is also a decrease in the number of AN fibers, primarily affecting low and medium SR fibers (Schmiedt et al. 1996). In addition, recent evidence has shown that there is an additional loss of synapses between the IHCs and the AN (Sergeyenko et al. 2013) that precedes the loss of the AN fibers with age and is thought to be concentrated in the lower SR fibers (Kujawa and Liberman 2015). In the current study, there was an overall decrease in EFR amplitude to sAM1FL across most SNRs with age (Fig. 2A, D, G, blue). This was not due to changes in hearing thresholds, as the sound levels of the stimuli were adjusted for each animal. Further evidence for this is provided by the model, where changes in thresholds modeled as deficits in the OHCs alone do not significantly change the EFR amplitudes compared to the young, when coupled with an increased stimulus level (Fig. 4, yellow). However, the loss of AN fibers combined with an additional decrease in function of lower SR fibers to model synaptopathy produced an overall decrease in EFR amplitudes and recreated the aged EFRs more accurately (Fig. 4, blue). Loss of synapses equally across all SR fibers resulted in amplitude decreases in the model that were far greater than those observed in the EFRs (Fig. 4, dark and light gray). More information regarding the actual pattern of synaptopathy seen with age will lead to a better refinement of the model to accurately reproduce the EFRs. Central processing changes in aging and other forms of hearing loss manifest as decreases in inhibition (Caspary et al. 2008) as well as changes in excitation due to compensatory mechanisms reacting to the reduced peripheral input (Cui et al. 2007; Miko and Sanes 2009). However, these compensatory mechanisms come at the cost of reduced synchrony of neural representations (Rabang et al. 2012). This could result in the some of the decreased EFR amplitudes with age observed in this study and elsewhere (Clinard et al. 2010; Parthasarathy and Bartlett 2011; Anderson et al. 2012). However, modeling the loss of AN fibers and synapses recreated the majority of the trends observed due to age in this study, suggesting peripheral contributions for the decrease in EFR amplitudes at high SNRs and large AM depths. Departures from the EFRs in the model were observed both in terms of the degree of decrease in amplitudes between the three ΔFC conditions, as well as the nature of the change at low SNR conditions (Fig. 3, compare to Fig. 5). Other studies have also reported age-related changes at lower SNRs, lower modulation depths, differing envelope shapes and frequency modulation (He et al. 2007; Parthasarathy et al. 2010; Parthasarathy and Bartlett 2011; Trujillo and Razak 2013). Whether these differences are due to changes in central processing, or some other peripheral effect that is yet to be accounted for, remains to be explored.
Conclusion
This study presents explanatory physiological evidence for the influence of sound level and spectral separation for processing competing sAM tones in the young and aged animals. The results provide insight into the role of the high-frequency fibers in representing AM stimuli with lower FCs at suprathreshold sound levels. This study also provides evidence for the age-related deficits in processing these competing AM tones due to loss of AN fibers and synapses. These changes can be detected using EFRs, and the use of the AN model can help identify the putative neurophysiological underpinnings of these deficits, providing hypotheses that can be tested in animal models. Our results ascribe nearly all age-related changes in competing AM processing to peripheral changes in hair cells and the AN, especially at high SNRs. However, the responses from low SNRs suggest that more central neurons may be working with degraded peripheral inputs to refine these responses. The roles of the central auditory pathway, as well as the contributions of top down cognitive mechanisms in using these neural signals to obtain perceptual discriminability, remain to be explored.
Acknowledgments
This study was supported by a grant from the National Institutes of Health (NIDCD R01DC011580) to ELB.
Compliance with ethical standards
Conflict of Interest
The authors declare that they have no competing interests.
References
- Alain C, McDonald KL. Age-related differences in neuromagnetic brain activity underlying concurrent sound perception. J Neurosci. 2007;27:1308–1314. doi: 10.1523/JNEUROSCI.5433-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Parbery-Clark A, White-Schwoch T, Kraus N. Aging affects neural precision of speech encoding. J Neurosci. 2012;32(41):14156–64. doi: 10.1523/JNEUROSCI.2176-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon SP, Grantham DW. Modulation masking—effects of modulation frequency, depth, and phase. J Acoust Soc Am. 1989;85:2575–2580. doi: 10.1121/1.397751. [DOI] [PubMed] [Google Scholar]
- Bacon SP, Konrad DL. Modulation detection interference under conditions favoring within- or across-channel processing. J Acoust Soc Am. 1993;93:1012–1022. doi: 10.1121/1.405549. [DOI] [PubMed] [Google Scholar]
- Bacon SP, Moore BCJ. Modulation detection interference—some spectral effects. J Acoust Soc Am. 1993;93:3442–3453. doi: 10.1121/1.405674. [DOI] [PubMed] [Google Scholar]
- Bharadwaj HM, Verhulst S, Shaheen L, Liberman MC, Shinn-Cunningham BG. Cochlear neuropathy and the coding of supra-threshold sound. Front Syst Neurosci. 2014;8:26. doi: 10.3389/fnsys.2014.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidet-Caulet A, Fischer C, Besle J, Aguera PE, Giard MH, Bertrand O. Effects of selective attention on the electrophysiological representation of concurrent sounds in the human auditory cortex. J Neurosci. 2007;27:9252–9261. doi: 10.1523/JNEUROSCI.1402-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregman AS. Auditory scene analysis: the perceptual organization of sound. Cambridge, MA: MIT Press; 1990. [Google Scholar]
- Buchwald JS, Huang CM. Far-field acoustic response—origins in cat. Science. 1975;189:382–384. doi: 10.1126/science.1145206. [DOI] [PubMed] [Google Scholar]
- Buckiova D, Popelar J, Syka J. Aging cochleas in the F344 rat: morphological and functional changes. Exp Gerontol. 2007;42:629–638. doi: 10.1016/j.exger.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol. 2008;211:1781–1791. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran B, Kraus N. The scalp-recorded brainstem response to speech: neural origins and plasticity. Psychophysiology. 2010;47:236–246. doi: 10.1111/j.1469-8986.2009.00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Li MN, Tanaka C, Bielefeld EC, Hu BH, Kermany MH, Salvi R, Henderson D. Aging outer hair cells (OHCs) in the Fischer 344 rat cochlea: function and morphology. Hear Res. 2009;248:39–47. doi: 10.1016/j.heares.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Clinard CG, Tremblay KL, Krishnan AR. Aging alters the perception and physiological representation of frequency: evidence from human frequency-following response recordings. Hear Res. 2010;264:48–55. doi: 10.1016/j.heares.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui YL, Holt AG, Lomax CA, Altschuler RA. Deafness associated changes in two-pore domain potassium channels in the rat inferior colliculus. Neuroscience. 2007;149:421–433. doi: 10.1016/j.neuroscience.2007.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin WF. 16th International-Evoked-Audiometry-Study-Group Meeting. France: Lyon; 1995. The envelope following response to multiple tone pair stimuli; pp. 1–14. [DOI] [PubMed] [Google Scholar]
- Dubno JR, Dirks DD, Morgan DE. Effects of age and mild hearing-loss on speech recognition in noise. J Acoust Soc Am. 1984;76:87–96. doi: 10.1121/1.391011. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons PJ, Gordon-Salant S. Auditory temporal processing in elderly listeners. J Am Acad Audiol. 1996;7:183–189. [PubMed] [Google Scholar]
- Frisina DR, Frisina RD. Speech recognition in noise and presbycusis: relations to possible neural mechanisms. Hear Res. 1997;106:95–104. doi: 10.1016/S0378-5955(97)00006-3. [DOI] [PubMed] [Google Scholar]
- Fullgrabe C, Moore BCJ, Stone MA. Age-group differences in speech identification despite matched audiometrically normal hearing: contributions from auditory temporal processing and cognition. Front Aging Neurosci. 2015;6:347. doi: 10.3389/fnagi.2014.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood DD (1990) A cochlear frequency-position function for several species--29 years later. J Acoust Soc Am 87(6):2592–2605 [DOI] [PubMed]
- Grimault N, Micheyl C, Carlyon RP, Arthaud P, Collet L. Perceptual auditory stream segregation of sequences of complex sounds in subjects with normal and impaired hearing. Br J Audiol. 2001;35:173–182. doi: 10.1080/00305364.2001.11745235. [DOI] [PubMed] [Google Scholar]
- Hall JW, Grose JH. Some effects of auditory grouping factors on modulation detection interference (MDI) J Acoust Soc Am. 1991;90:3028–3035. doi: 10.1121/1.401777. [DOI] [PubMed] [Google Scholar]
- Hashimoto I, Ishiyama Y, Yoshimoto T, Nemoto S. Brain-stem auditory-evoked potentials recorded directly from human brain-stem and thalamus. Brain. 1981;104:841–859. doi: 10.1093/brain/104.4.841. [DOI] [PubMed] [Google Scholar]
- He NJ, Mills JH, Dubno JR. Frequency modulation detection: effects of age, psychophysical method, and modulation waveform. J Acoust Soc Am. 2007;122:467–477. doi: 10.1121/1.2741208. [DOI] [PubMed] [Google Scholar]
- Herdman AT, Stapells DR. Auditory steady-state response thresholds of adults with sensorineural hearing impairments. Int J Audiol. 2003;42:237–248. doi: 10.3109/14992020309078343. [DOI] [PubMed] [Google Scholar]
- Hind SE, Haines-Bazrafshan R, Benton CL, Brassington W, Towle B, Moore DR. Prevalence of clinical referrals having hearing thresholds within normal limits. Int J Audiol. 2011;50:708–716. doi: 10.3109/14992027.2011.582049. [DOI] [PubMed] [Google Scholar]
- Johnsrude IS, Mackey A, Hakyemez H, Alexander E, Trang HP, Carlyon RP. Swinging at a cocktail party: voice familiarity aids speech perception in the presence of a competing voice. Psychol Sci. 2013;24:1995–2004. doi: 10.1177/0956797613482467. [DOI] [PubMed] [Google Scholar]
- King A, Hopkins K, Plack CJ. The effects of age and hearing loss on interaural phase difference discrimination. J Acoust Soc Am. 2014;135:342–351. doi: 10.1121/1.4838995. [DOI] [PubMed] [Google Scholar]
- Kiren T, Aoyagi M, Furuse H, Koike Y (1994) An experimental-study on the generator of amplitude-modulation following response. Acta Oto-Laryngologica 28–33 [PubMed]
- Kujawa SG, Liberman MC (2015) Synaptopathy in the noise-exposed and aging cochlea: primary neural degeneration in acquired sensorineural hearing loss. Hear Res 330:191–199. doi:10.1016/j.heares.2015.02.009 [DOI] [PMC free article] [PubMed]
- Kuwada S, Anderson JS, Batra R, Fitzpatrick DC, Teissier N, D’Angelo WR. Sources of the scalp-recorded amplitude-modulation following response. J Am Acad Audiol. 2002;13:188–204. [PubMed] [Google Scholar]
- Liberman MC. Auditory-nerve response from cats raised in a low-noise chamber. J Acoust Soc Am. 1978;63:442–455. doi: 10.1121/1.381736. [DOI] [PubMed] [Google Scholar]
- Lins OG, Picton TW. Auditory steady-state responses to multiple simultaneous stimuli. Electroencephalogr Clin Neurophysiol. 1995;96:420–432. doi: 10.1016/0168-5597(95)00048-W. [DOI] [PubMed] [Google Scholar]
- McNerney KM, Burkard RF. The effects of a second stimulus on the auditory steady state response (ASSR) from the inferior colliculus of the chinchilla. Int J Audiol. 2010;49:561–573. doi: 10.3109/14992020903473449. [DOI] [PubMed] [Google Scholar]
- Mendoza L, Hall JW, Grose JH. Within-channel and across-channel processes in modulation detection interference. J Acoust Soc Am. 1995;97:3072–3079. doi: 10.1121/1.413105. [DOI] [PubMed] [Google Scholar]
- Micheyl C, Bernstein JGW, Oxenham AJ. Detection and F0 discrimination of harmonic complex tones in the presence of competing tones or noise. J Acoust Soc Am. 2006;120:1493–1505. doi: 10.1121/1.2221396. [DOI] [PubMed] [Google Scholar]
- Miko IJ, Sanes DH. Transient gain adjustment in the inferior colliculus is serotonin- and calcium-dependent. Hear Res. 2009;251:39–50. doi: 10.1016/j.heares.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BCJ, Fullgrabe C, Stone MA. Effect of spatial separation, extended bandwidth, and compression speed on intelligibility in a competing-speech task. J Acoust Soc Am. 2010;128:360–371. doi: 10.1121/1.3436533. [DOI] [PubMed] [Google Scholar]
- Nakamoto KT, Shackleton TM, Palmer AR. Responses in the inferior colliculus of the guinea pig to concurrent harmonic series and the effect of inactivation of descending controls. J Neurophys. 2010;103:2050–2061. doi: 10.1152/jn.00451.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PC, Carney LH. A phenomenological model of peripheral and central neural responses to amplitude-modulated tones. J Acoust Soc Am. 2004;116:2173–2186. doi: 10.1121/1.1784442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A, Bartlett EL. Age-related auditory deficits in temporal processing in F-344 rats. Neuroscience. 2011;192:619–630. doi: 10.1016/j.neuroscience.2011.06.042. [DOI] [PubMed] [Google Scholar]
- Parthasarathy A, Bartlett E. Two-channel recording of auditory-evoked potentials to detect age-related deficits in temporal processing. Hear Res. 2012;289(1-2):52–62. doi: 10.1016/j.heares.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A, Cunningham PA, Bartlett EL. Age-related differences in auditory processing as assessed by amplitude-modulation following responses in quiet and in noise. Front Aging Neurosci. 2010;2:152. doi: 10.3389/fnagi.2010.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A, Datta J, Torres JAL, Hopkins C, Bartlett EL. Age-related changes in the relationship between auditory brainstem responses and envelope-following responses. J Assoc Res Otolaryngol. 2014;15:649–661. doi: 10.1007/s10162-014-0460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plyler PN, Fleck EL. The effects of high-frequency amplification on the objective and subjective performance of hearing instrument users with varying degrees of high-frequency hearing loss. J Speech Lang Hear Res. 2006;49:616–627. doi: 10.1044/1092-4388(2006/044). [DOI] [PubMed] [Google Scholar]
- Rabang CF, Parthasarathy A, Venkataraman Y, Fisher ZL, Gardner SM, Bartlett EL. A computational model of inferior colliculus responses to amplitude modulated sounds in young and aged rats. Front Neural Circuits. 2012;6:77. doi: 10.3389/fncir.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B, Draganova R, Picton TW, Pantev C. Frequency specificity of 40-Hz auditory steady-state responses. Hear Res. 2003;186:57–68. doi: 10.1016/S0378-5955(03)00299-5. [DOI] [PubMed] [Google Scholar]
- Ruggles D, Bharadwaj H, Shinn-Cunningham BG. Why middle-aged listeners have trouble hearing in everyday settings. Curr Biol. 2012;22:1417–1422. doi: 10.1016/j.cub.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt RA. Spontaneous rates, thresholds and tuning of auditory-nerve fibers in the gerbil—comparisons to cat data. Hear Res. 1989;42:23–35. doi: 10.1016/0378-5955(89)90115-9. [DOI] [PubMed] [Google Scholar]
- Schmiedt RA, Mills JH, Boettcher FA. Age-related loss of activity of auditory-nerve fibers. J Neurophys. 1996;76:2799–2803. doi: 10.1152/jn.1996.76.4.2799. [DOI] [PubMed] [Google Scholar]
- Sergeyenko Y, Lall K, Liberman MC, Kujawa SG. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci. 2013;33:13686–13694. doi: 10.1523/JNEUROSCI.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen LA, Valero MD, Liberman MC. Towards a diagnosis of cochlear neuropathy with envelope following responses. J Assoc Res Otolaryngol. 2015;16(6):727–45. doi: 10.1007/s10162-015-0539-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo M, Razak KA. Altered cortical spectrotemporal processing with age-related hearing loss. J Neurophys. 2013;110:2873–2886. doi: 10.1152/jn.00423.2013. [DOI] [PubMed] [Google Scholar]
- Turner CW, Henry BA. Benefits of amplification for speech recognition in background noise. J Acoust Soc Am. 2002;112:1675–1680. doi: 10.1121/1.1506158. [DOI] [PubMed] [Google Scholar]
- Yost WA, Sheft S. Modulation detection interference—across-frequency processing and auditory grouping. Hear Res. 1994;79:48–58. doi: 10.1016/0378-5955(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Yost WA, Sheft S, Opie J. Modulation interference in detection and discrimination of amplitude-modulation. J Acoust Soc Am. 1989;86:2138–2147. doi: 10.1121/1.398474. [DOI] [PubMed] [Google Scholar]
- Zilany MSA, Bruce IC, Nelson PC, Carney LH. A phenomenological model of the synapse between the inner hair cell and auditory nerve: long-term adaptation with power-law dynamics. J Acoust Soc Am. 2009;126:2390–2412. doi: 10.1121/1.3238250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilany MSA, Bruce IC, Carney LH. Updated parameters and expanded simulation options for a model of the auditory periphery. J Acoust Soc Am. 2014;135:283–286. doi: 10.1121/1.4837815. [DOI] [PMC free article] [PubMed] [Google Scholar]