Abstract
Poorer hearing in the presence of background noise is a significant problem for the hearing impaired. Ototoxic drugs, ageing, and noise exposure can damage the sensory hair cells of the inner ear that are essential for normal hearing sensitivity. The relationship between outer hair cell (OHC) loss and progressively poorer hearing sensitivity in quiet or in competing background noise is supported by a number of human and animal studies. In contrast, the effect of moderate inner hair cell (IHC) loss or dysfunction shows almost no impact on behavioral measures of hearing sensitivity in quiet, when OHCs remain intact, but the relationship between selective IHC loss and hearing in noise remains relatively unknown. Here, a moderately high dose of carboplatin (75 mg/kg) that produced IHC loss in chinchillas ranging from 40 to 80 % had little effect on thresholds in quiet. However, when tested in the presence of competing broadband (BBN) or narrowband noise (NBN), thresholds increased significantly. IHC loss >60 % increased signal-to-noise ratios (SNRs) for tones (500–11,300 Hz) in competing BBN by 5–10 dB and broadened the masking function under NBN. These data suggest that IHC loss or dysfunction may play a significant role in listening in noise independent of OHC integrity and that these deficits may be present even when thresholds in quiet are within normal limits.
Keywords: inner hair cell loss, carboplatin, chinchilla, hearing in noise, masking
INTRODUCTION
Normal hearing sensitivity depends on the integrity of two distinct sensory cell types within the cochlea, the mammalian organ of hearing. The electromotile outer hair cells (OHCs) provide active, non-linear amplification in response to basilar membrane displacement (Allen 1980; Johnstone et al. 1986; Preyer and Gummer 1996) whereas inner hair cells (IHCs) passively respond to cochlear mechanics and transduce nearly all of the acoustic energy to afferent type I auditory nerve fibers. Over 90 % of type I afferents innervate IHCs with the remainder (∼10 %) innervating OHCs (Spoendlin 1975). Cochlear damage as a function of noise, ototoxic drugs, or ageing is associated with stereocilia dysfunction in IHCs and OHCs, damage to afferent fibers or synapses, and in many cases partial to complete loss of OHCs (Coleman 1976; McGill and Schuknecht 1976; Prazma et al. 1976; Ryan et al. 1980; Liberman and Dodds 1984; Soucek and Mason 1987). Hearing impairment associated with evidence of OHC damage or loss is generally characterized by increased thresholds and decreased frequency tuning (McGill and Schuknecht 1976; Dallos and Harris 1978; Stebbins et al. 1979; Patuzzi et al. 1989; Ohlms et al. 1991). This reduction in tuning is believed to underlie poorer ability to resolve signals in noise. The effects of selective IHC loss/dysfunction, or afferent degeneration on hearing-in-noise, however, are less understood but have been speculated to play a significant role (Kujawa and Liberman 2009; Lin et al. 2011; Furman et al. 2013).
In chinchillas and other species, OHC dysfunction or loss reduces hearing sensitivity and tuning (Cody and Russell 1985; Borg 1987; Davis et al. 1989; Hamernik et al. 1989; McFadden et al. 2002; Davis et al. 2005). Not all reports, however, attribute poorer tuning to OHC loss (Nienhuys and Clark 1979; Prosen et al. 1989). In one report, psychophysical tuning curves (PTCs) from chinchillas with kanamycin-induced selective OHC loss or mixed OHC/IHC loss showed that when cochlear damage was limited to OHC loss alone, thresholds increased but tuning curves remained normal. In contrast, selective IHC loss resulted in PTCs that were progressively distorted, suggesting that although OHC loss increased threshold, PTCs can remain sharp as long as IHCs are not damaged (Ryan et al. 1979).
In chinchillas with carboplatin-induced evidence of selective IHC loss (Wake et al. 1993; Takeno et al. 1994a, b; Wake et al. 1994; Hofstetter et al. 1997a; Ding et al. 1999b), the compound action potential (CAP) amplitude, a measure of cochlear output, was significantly reduced as a function of IHC loss (Wang et al. 2002; El-Badry and McFadden 2007). However, measurements from individual auditory nerve fibers, presumably synapsed to surviving IHCs, retained sharp tuning and normal thresholds but had shallower rate-level functions and reduced spontaneous and driven rates following carboplatin. Whereas these shallower rate-level functions might not degrade hearing performance in quiet, it is possible that hearing in noise could be substantially degraded prior to the central auditory system (Wang et al. 1997; Wang et al. 2003). In the central auditory system, threshold measures in quiet from the inferior colliculus (IC) and auditory cortex (AC) were also resistant to IHC loss (Salvi et al. 2000a, b; Arnold and Burkard 2002). As long as OHCs were not damaged, thresholds in quiet from areas with sparse IHC populations were only slightly increased in the IC and relatively unchanged at the AC. Collectively, these results suggested that threshold measures in quiet are likely unreliable indicators of IHC pathology. In a previous publication, we demonstrated that behaviorally derived pure-tone thresholds in chinchillas with carboplatin-induced IHC loss showed no significant changes until IHC loss was profound (∼80 %) (Lobarinas et al. 2013). These measures, however, were obtained in the absence of competing background noise. Competing background noise substantially increases the demand on the auditory system, particularly in the presence of missing IHCs. Consequently, we hypothesized that whereas thresholds in quiet showed little sensitivity to extensive IHC loss, hearing in noise would show a significant level of impairment as a function of IHC loss, results that could partially explain performance differences in noise among hearing impaired individuals with similar audiograms.
In the studies presented here, we assessed the relationship among carboplatin-induced IHC loss or dysfunction and (1) audiometric performance in a competing BBN, (2) performance under NBN, and (3) masking functions to determine the effects on off-frequency listening.
MATERIALS AND METHODS
Subjects
Ten, healthy, adult, naïve, male chinchillas were used (400–600 g). Animals were 1–2 years of age at the beginning of the study and were tested over a 9-month period. Five of the ten animals were from a previous publication that focused on thresholds in quiet (Lobarinas et al. 2013).
Subjects were housed in custom individual wire mesh cages in a temperature-controlled room with a 12-h light/dark cycle. Ambient overall sound levels in the chinchilla colony were 60–70-dB sound pressure level (SPL) with most of the energy below 250 Hz. Animals had free access to food and water.
Thresholds in quiet, under broadband noise (BBN), and under narrowband noise (NBN), were assessed before and after treatment with 75 mg/kg of carboplatin (intraperitoneal, i.p.), a dose that produces moderate to severe IHC loss (Hofstetter et al. 1997a, b). All procedures were approved by the University at Buffalo’s and University of Florida’s IACUC committees.
Equipment
During testing, chinchillas were placed in an acoustically transparent (stainless steel bar), operant chamber with dimensions 31.75 cm (W) × 34.29 cm (H) × 25.4 cm (D) (Med Associates, 007-VPX), within a sound-attenuating cubicle (Med Associates, ENV-018V) lined with acoustic foam. The sound-attenuating cubicles were housed in a single-walled sound-attenuating booth (WhisperRoom, MDL 4870S). Within each operant chamber, a photobeam (Med-Associates, ENV-253SD) was placed and centered 19.54 cm above a stainless steel bar grid floor wired for scrambled foot shock (shock cycled serially across rods with a cycle time of 277.2 ms and a stimulation time of 30.8 ms per rod; Med-Associates, ENV-005A-QD and ENV-414S). The output of the photobeam was fed to an adjustable single-channel infrared (IR) controller (Med-Associates, ENV-253B) and then to a real-time processor (Tucker Davis Technologies, RP2.1) connected to a personal computer running custom software. Sound stimuli were presented through a speaker (Fostex, FE127E) placed on the top of the operant chamber (26 cm). Calibration was performed with a sound level meter (Larson Davis 800b) and a 1/2-inch free-field microphone (Larson Davis 2541) placed at the center of the chamber, 13 cm above the stainless steel bar grid floor. Calibration was also performed at each corner of the chamber at the same 13-cm height. Variation among the five measurement locations was less than 2 dB. A piezo buzzer (Radio Shack, 273-059) placed adjacent to the speaker and houselights (Med-Associates, ENV-215 M) were used to provide feedback during testing.
Study Design and Timeline
The overall aim of the study was to evaluate the relationship between carboplatin-induced loss of IHCs or dysfunction and thresholds in the presence of BBN and NBN. Consistent with our previous publications, a within-subjects design was implemented with a pre-carboplatin control condition followed by a post-carboplatin assessment of thresholds in quiet and under both noise conditions. Training and baseline measures occurred over a 2-month period. Following carboplatin treatment, animals were allowed to recover over a 21-day period under veterinary care and observation. Post-carboplatin assessment measures were then obtained over a 4–6-week period. At the end of post-carboplatin testing, animals were killed within 1–2 weeks of the final behavioral test session.
Psychophysical Methods
Hearing thresholds were obtained using a modified descending method of limits with a shock avoidance conditioning procedure described previously (Lobarinas et al. 2013), and similar to shock avoidance procedures from earlier reports (Blakeslee et al. 1978; Salvi et al. 1978; Giraudi et al. 1980; Giraudi-Perry et al. 1982; Salvi and Arehole 1985; Lobarinas et al. 2013). Tonal stimuli were created with a custom Matlab script and RPVDS software running a real-time processor RP2.1 (Tucker Davis Technologies).
Each experimental session contained approximately 120–140 trials. During each trial, six tone bursts (500 ms on/500 ms off, 5-ms rise/fall time) were presented. If the subject reared and broke the plane of the photobeam within the first four of the six tone bursts, the tone burst signal was terminated, the houselight stayed on, and the response was recorded as correct (Hit). However, if the subject failed to respond within the first four tone bursts, both the foot shock (2–5-mA scrambled foot shock) and the buzzer were turned on, the houselights were turned off, and the trial was recorded as an incorrect response (Miss).
During misses, the combination of the shock stimulus and buzzer immediately elicited a response that turned off the shock (Escape). Maximum shock time was limited to 2 s. When tone burst intensity levels fell below 20-dB SPL, only the buzzer and light-off condition (footshock was turned off) signaled a Miss. The use of only the buzzer at low intensities was based on previous methods described by Miller (Miller 1970) and Saunders (Saunders et al. 1977) and similar to those reported by Seaton (Seaton and Trahiotis 1975).
Each session trial was presented using a random intertrial interval (ITI) that ranged from 20 to 60 s. A Modified Method of Limits with a 10-dB down and 5-dB up step size was implemented in order to determine thresholds in quiet. Testing began using a high stimulus level (>60-dB SPL). Within a trial, if a subject produced a correct response, the tone burst intensity was decreased by 10 dB on the subsequent trial. However, failure to respond resulted in a 5-dB intensity increase on the next trial. The false alarm rate (rate of responding when no stimulus was presented) was measured using blank trials (10 % of the trials) to allow for monitoring of false positives and correct rejection. No feedback was provided during blank trials.
Threshold at each frequency was operationally defined as the intensity at which three or more 5-dB reversals occurred. An experimenter that could control stimuli and track animal responses across frequency and intensity monitored each session. The time needed to measure threshold across all stimulus frequencies in a daily session was 50–60 min. The daily thresholds were averaged across 5 days to determine stable baseline pure-tone thresholds at each frequency. Daily threshold measures were considered valid if the false alarm rate was less than 20 % during the session. Following training, false alarm rates remained below 20 %.
Thresholds in Quiet
Thresholds in quiet were assessed using the psychophysical methods described above with tone bursts (500-ms duration, 5-ms rise/fall time) presented at 250–11,300 Hz.
Thresholds in Broadband Noise
Thresholds in broadband noise (BBN) were measured at 250–11,300 Hz by presenting tone bursts in the presence of a continuous BBN with an overall level of 50-dB SPL. Tone bursts were generated using the same equipment as for the quiet condition. The BBN was generated and ran continuously on a separate channel on the RP2.1. The two independent signals were summed in hardware (Tucker Davis Technology, SM3) and delivered to the same speaker.
The BBN was relatively flat from 100 to 16,000 Hz (±2 dB) with a steep roll-off above 20 kHz. The overall level of the noise was 50-dB SPL resulting in a pressure spectrum level (Lps) of ∼7-dB SPL/Hz (Lps = 50 − [10 log (20,000 Hz/1 Hz)]).
Thresholds in Narrowband Noise
The aim of the narrowband noise experiments was to assess the shape of the masking function at three representative frequencies before and after carboplatin as an index of frequency selectivity. To reduce the effects of tone on tone masking such as beats, notches, and combination tones, narrowband noises with a nominal bandwidth of 100 Hz were used (Egan and Hake 1950; Zhang et al. 1990).
Thresholds in narrowband noise (NBN) were measured at 250–11,300 Hz by presenting tone bursts in the presence of NBN centered at 500, 2000, and 4000 Hz. The range of frequencies tested provided assessment of frequencies below, near, and above the center frequency of each NBN masker. The overall level of the NBN was set at 70-dB SPL (BW = 100 Hz). The equipment used to generate the tone bursts and NBN was the same as that used for the quiet and BBN conditions. NBN with a programmed steep high- and low-frequency roll-off (∼36 dB/octave) was generated using software with a digital filter function (TDT system 3 running RPVDS with six biquad filters and four butterworth coefficient functions). Bandwidth was defined by the lower and upper cutoff frequencies (3-dB down point), 50 Hz below and above each center frequency. The shapes of the NBN were confirmed using a 1/2-inch microphone amplified by a Bruel & Kjaer Nexus, fed into a TDT RP2.1 to a computer running a custom FFT Matlab script. The amplitudes were cross-checked with a Larson Davis 824 SLM sound spectrum analyzer function.
Carboplatin
After baseline threshold measures were collected in quiet and under noise, each subject was treated with a single 75 mg/kg intraperitoneal (i.p.) injection of carboplatin (Sigma C2538, cis-Diammine 1,1-cyclobutanedicarboxylate platinum) dissolved in 5 ml of saline. The animals then received daily 10-ml subcutaneous injections of saline for 2 days following carboplatin treatment. Following a 21-day recovery period during which no testing occurred, threshold in quiet, BBN, and NBN were reevaluated.
Cochleograms
At the end of the post-carboplatin hearing assessments, subjects were killed within 1–2 weeks with an overdose of carbon dioxide, decapitated, and the cochleae removed for histological analysis to determine the extent of hair cell loss (Trautwein et al. 1996; Hofstetter et al. 1997a, b; Ding et al. 1999a). Both cochleae were removed carefully, the round window and oval window opened, and a solution of succinate dehydrogenase (SDH; 2.5 ml 0.2 M sodium succinate, 2.5 ml 0.2 M phosphate buffer, pH 7.6, and 5 ml 0.1 % tetranitro blue tetrazolium) was perfused through the round window. The cochleae were then immersed in SDH and incubated at 37 ° C for 45 min. The cochlea were post-fixed with 10 % formalin and stored in this fixative for 24 h. The basilar membrane containing the organ of Corti was dissected from the apex to the base as a flat surface preparation, mounted in glycerin on glass slides, and coverslipped. The organ of Corti was analyzed by light microscopy (Zeiss Standard) at ×400 magnification. Successive segments (0.24 mm) were analyzed for missing IHCs and OHCs. Hair cells were counted as present if the SDH-stained cell bodies were present and visible. Cochleograms were constructed for each ear of each animal, and the percent missing hair cells was plotted as a function of distance from the apex. Percent hair cell loss was determined from laboratory norms established from cochlea obtained from normal chinchillas (n = 9, 1–2 years of age). Percent distance from the apex was also converted to frequency using a cochlear frequency–place map (Greenwood 1990). Cochleograms were constructed for both ears; however, no significant differences in the size or pattern of hair cell loss were observed for the right versus left ears. Therefore, the data presented in RESULTS section show the hair cell lesions from the left ear.
Data Analysis
All subjects were evaluated for thresholds in quiet, thresholds in BBN, and thresholds in NBN. Mean data for the subjects were analyzed using a two-way repeated measures analysis of variance (ANOVA) to ascertain the effect of carboplatin on thresholds as a function of frequency across the three experimental conditions relative to the pre-carboplatin results. All statistical comparisons used an alpha level of 0.05, and post hoc analysis was performed using Tukey tests to avoid type I errors associated with multiple comparisons. Sigma Stat 3.5 was used for all statistical analyses. All results are presented as mean ± standard deviation (SD).
RESULTS
Moderate and Severe Carboplatin-Induced IHC Loss
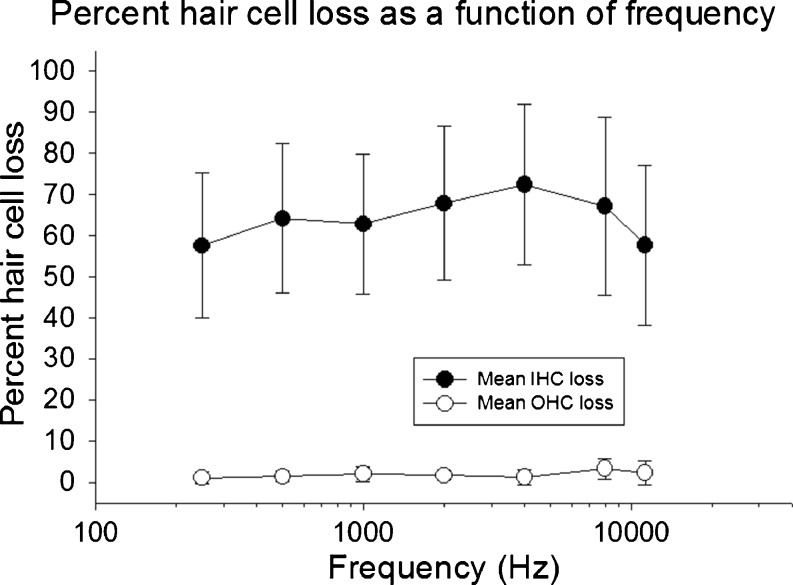
The 75 mg/kg carboplatin treatment produced a mean IHC loss of 64 %. There was no evidence of significant OHC loss, results that were consistent with previous publications (Trautwein et al. 1996; Hofstetter et al. 1997a, b; Wang et al. 1997; Ding et al. 1999b; Lobarinas et al. 2013). The IHC values reported are lower than the 70 % mean loss reported in our recent study (Lobarinas et al. 2013). Figure 1 shows the post-mortem mean IHC and OHC loss across animals. The percent hair cell loss is plotted as a function of the frequency regions tested behaviorally (∼10 % intervals centered on each of the test frequencies). Across animals, the IHC loss was evident across most of the basilar membrane.
FIG. 1.
Mean hair cell loss is shown as a function of test frequency. Carboplatin (75 mg/kg) produced inner hair cell loss of 40–80 %. There was no evidence of outer hair cell loss.
Thresholds in Quiet
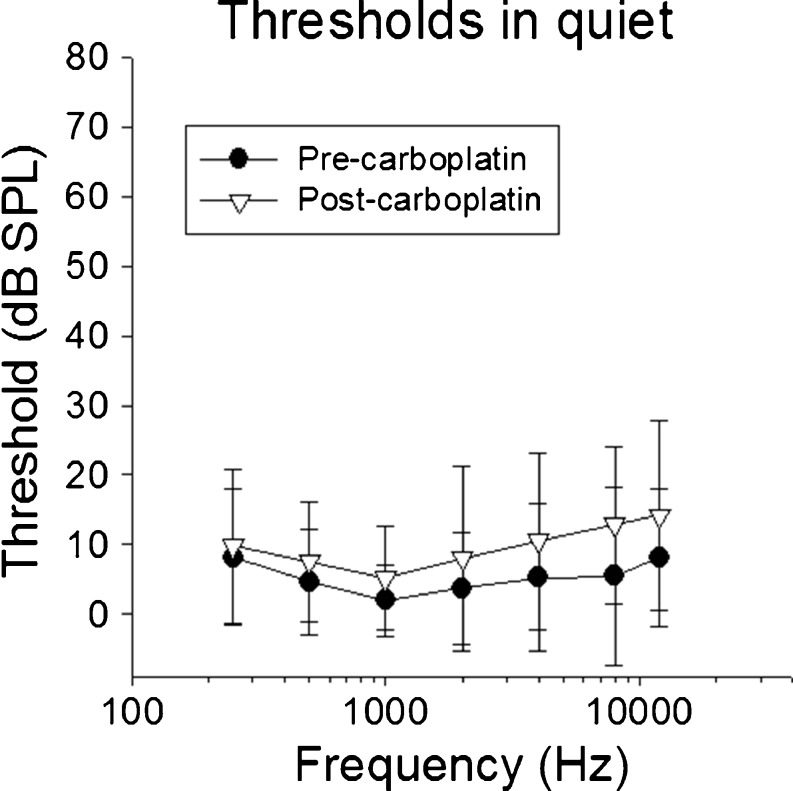
The mean baseline thresholds (Fig. 2) were within 5–8 dB of previously published results in untreated chinchillas using shock avoidance measures (Miller 1970; Blakeslee et al. 1978; Salvi et al. 1978; Santi et al. 1982; Heffner and Heffner 1991; Lobarinas et al. 2013) and 5–10 dB lower at frequencies >4 kHz relative to a food reinforced technique (Clark et al. 1974).
FIG. 2.
Mean thresholds in quiet are shown as a function of test frequency before and after carboplatin. There were no significant changes in thresholds following carboplatin treatment, despite significant inner hair cell loss.
Post-carboplatin (21 day), thresholds increased by 1–5 dB as a function of frequency. Mean thresholds were less than 9-dB SPL below 4000 Hz and 10–14-dB SPL at 4000–11,300 Hz (Fig. 2). A two-way repeated measures ANOVA (Table 1) showed no main effect for carboplatin treatment [F(1,54) = 2.985, p = 0.112] but found a statistically significant effect on frequency (p < 0.001). However, the effect on frequency did not depend significantly on treatment (p = 0.083). These results suggest that carboplatin had no significant impact on thresholds in quiet. There were no significant differences in thresholds across post-carboplatin sessions following the recovery period.
TABLE 1.
Two-way repeated measures ANOVAs performed across the three test conditions
| Condition | Baseline quiet | Baseline BBN | Baseline NBN |
|---|---|---|---|
| Post-carboplatin quiet | [F(1,54) = 2.985, p = 0.112] (n.s.) | ||
| Post-carboplatin BBN | [F(1,54) = 13.117, p = 0.004] | ||
| Post-carboplatin NBN | [F(1324) = 17.885, p = 0.013] |
Statistical results of carboplatin on thresholds in quiet, thresholds in BBN, and thresholds in NBN are shown. Alpha level was set to 0.05. Post hoc analysis was performed using Tukey tests. Carboplatin significantly increased thresholds under both BBN and NBN but had no effect on thresholds in quiet
BBN broadband noise, NBN narrowband noise, n.s. not significant
Thresholds in BBN
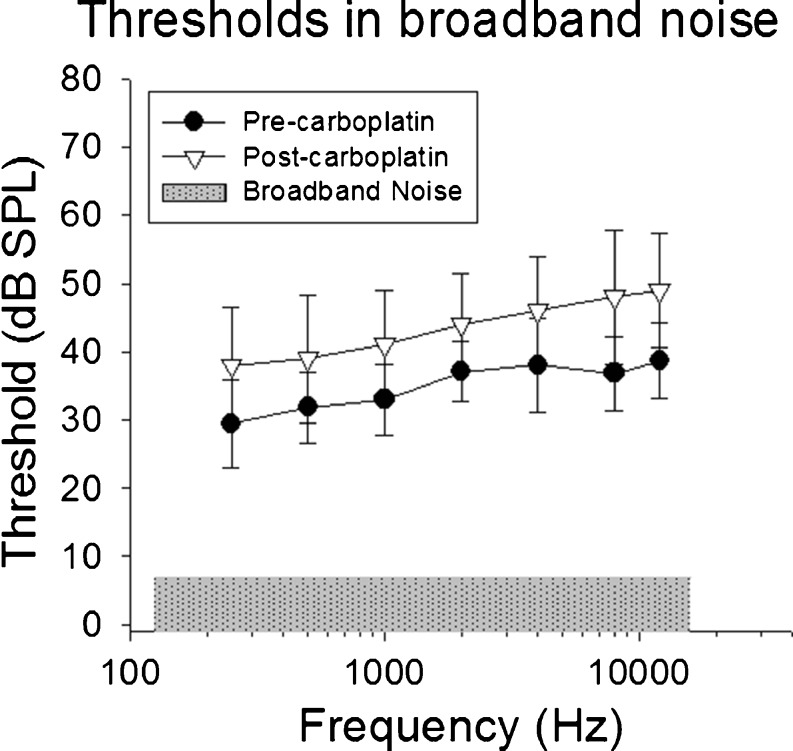
Thresholds across test frequencies 250–11,300 Hz in the presence of the continuous BBN before and after carboplatin treatment are shown in Figure 3. Note that thresholds increased as the target tone frequency increased from 250 to 4000 Hz; these results are consistent with increases in critical bandwidth in chinchillas (Seaton and Trahiotis 1975; Niemiec et al. 1992). Although critical bands were not directly assessed in the present study, a comparison of the critical ratios derived from subtracting Lps from masked threshold shows similar average results as those reported in previous studies in chinchillas using shock avoidance (Seaton and Trahiotis 1975) and food reinforced operant conditioning (Niemiec et al. 1992). A comparison of these results is shown in Table 2. At frequencies above 4000 Hz, however, the slope of the function flattened, suggesting that there were no further increases in the critical ratio from 4000 to 11,300 Hz. These results were consistent with bandwidth broadening results from auditory nerve fibers in chinchillas showing function saturation above 6000 Hz with suprathreshold presentation levels (Temchin et al. 2008).
FIG. 3.
Mean thresholds in competing broadband noise (50-dB SPL overall level, dB/Hz shown as shaded area) are shown as a function of test frequency before and after carboplatin. Unlike thresholds in quiet, treatment with carboplatin significantly increased thresholds in noise by 5–15 dB.
TABLE 2.
Pre-carboplatin treatment critical ratios in decibels, as a function of frequency, for the present study relative to previous reports
| Critical ratio (dB) | 500 Hz | 1000 Hz | 2000 Hz | 4000 Hz |
|---|---|---|---|---|
| Present study (shock avoidance) | 25 | 26 | 30 | 31 |
| Niemiec 1992 (positive reinforcement) | 29 | 26 | 30 | 33 |
| Seaton and Trahiotis 1975 (shock avoidance) | 16 | 19 | 25 | 31 |
| Miller 1970 (shock avoidance) | 22 | 23 | 27 | 30 |
Results were similar to those reported by Miller (1970) and Niemec (1991)
Before carboplatin treatment, thresholds ranged from 30-dB SPL for a tone burst at 500 Hz to 39-dB SPL for a tone burst at 11,300 Hz. Following carboplatin treatment, thresholds in BBN increased at all of the frequencies tested (Fig. 3) with corresponding signal-to-noise ratio (SNR) increases ranging from 6 to 11 dB. A two-way repeated measures ANOVA found a significant effect of frequency [F(6,54) = 20.810, p < 0.001] and treatment with carboplatin [F(1,54) = 13.117, p = 0.004]. The interaction of frequency and carboplatin treatment was not significant. Maximal threshold shift was at 8000 Hz, where an 11-dB increase in SNR was necessary to detect the tone burst relative to baseline measures.
Thresholds in NBN
The mean baseline and post-carboplatin thresholds to tones at 250–11,300 Hz in the presence of 70-dB SPL NBN centered at 500, 2000, and 4000 Hz are shown in Figures 4, 5, and 6. A two-way repeated measures ANOVA found a significant effect of treatment [F(1324) = 17.885, p = 0.013], frequency [F(36,324) = 54.281, p < 0.001], and an interaction effect of frequency and treatment [F(36,324) = 2.296, p = 0.025].
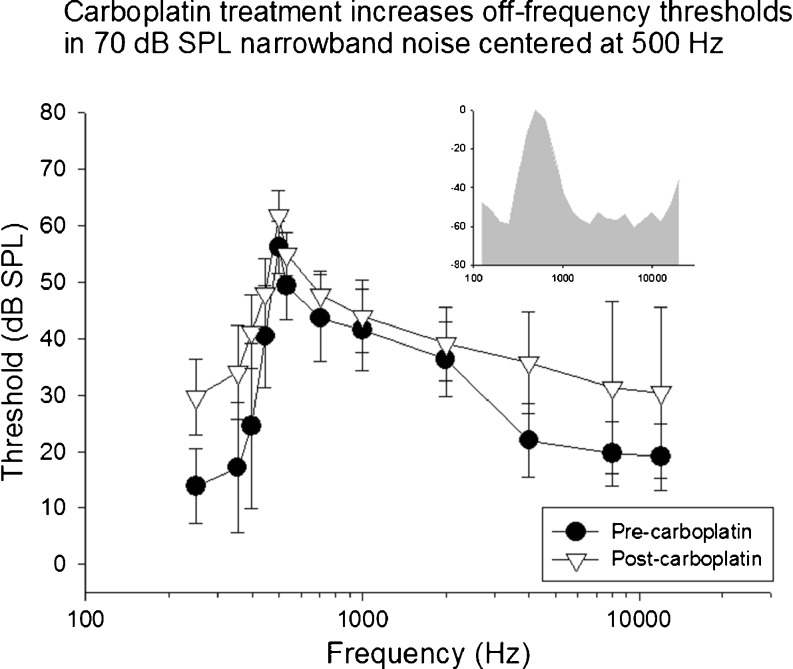
FIG. 4.
Mean thresholds in competing narrowband noise centered at 500 Hz (70-dB SPL overall level) are shown as a function of test frequency before and after carboplatin. Carboplatin treatment significantly increased thresholds across multiple frequencies above and below the frequency of the masker. The spectrum of the masking noise is shown in the insert.
FIG. 5.
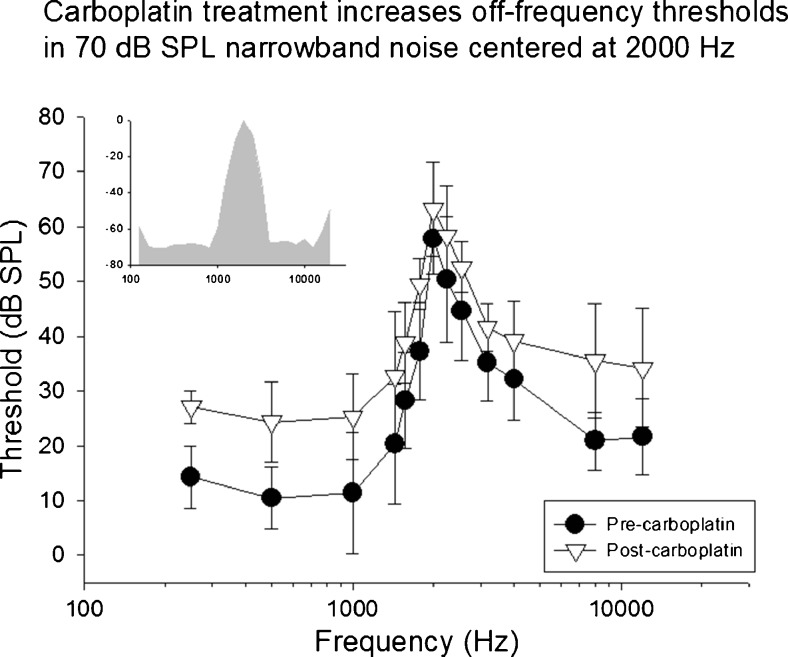
Mean thresholds in competing narrowband noise centered at 2000 Hz (70-dB SPL overall level) are shown as a function of test frequency before and after carboplatin. Carboplatin treatment significantly increased thresholds across multiple frequencies particularly at frequencies distal to the masker. The spectrum of the masking noise is shown in the insert.
FIG. 6.
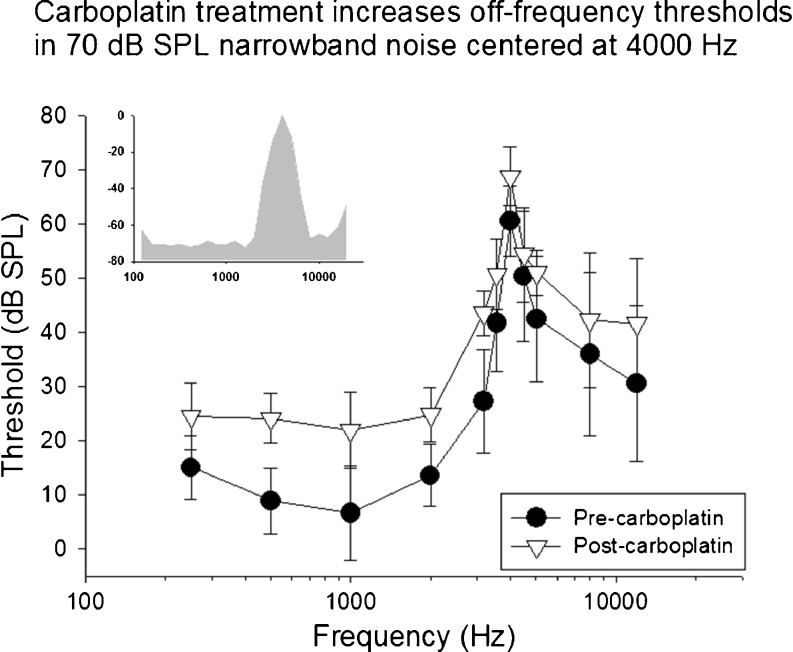
Mean thresholds in competing narrowband noise centered at 4000 Hz (70-dB SPL overall level) are shown as a function of test frequency before and after carboplatin. Following carboplatin treatment thresholds increased across multiple frequencies particularly below the frequency of the masker. The spectrum of the masking noise is shown in the insert.
Prior to carboplatin treatment, low thresholds were observed at frequencies below the frequency of the NBN masker. Thresholds increased as the frequency of the target tones approached the center frequency of the masker. Above the masker frequency, threshold gradually decreased as the frequencies of the target tones moved further away from the maskers’ center frequency. As expected, the highest thresholds were observed at the center frequency of each masker (see insert NBN spectrum). This effect was consistent across all subjects and all three masker conditions. Following carboplatin treatment, thresholds at the center frequency of the masker increased across all conditions relative to baseline. In addition, thresholds to tones distal to the masker center frequency increased significantly. The most distal frequencies below the masker, on average, showed increases of more than 15 dB. The threshold shifts below the center frequencies of the masker were unexpected given the relative amplitude spectrum of the NBN (see inserts Figures 4, 5, and 6).
Summary of Results
The overall results show that there were no significant differences in tone thresholds in quiet between baseline and post-carboplatin treatment that produced IHC dysfunction or loss. Following carboplatin, daily performance did not differ across sessions, suggesting that there were no significant learning effects following the recovery period.
In contrast, thresholds in BBN increased across all frequencies tested following carboplatin. NBN patterns, post-carboplatin, showed increased thresholds at frequencies distal to the masker at both the low and high frequency tails of the function. Table 1 summarizes the statistical results for treatment across test conditions. The results show poorer thresholds in noise across all frequencies under both BBN and NBN following carboplatin treatment even in the presence of a full complement of OHC and thresholds in quiet that were unchanged from baseline levels.
DISCUSSION
The aims of this study were to evaluate the perceptual consequences of IHC loss/dysfunction on hearing in competing BBN or intense NBN. Psychophysical methods were used to evaluate tone thresholds in quiet, in BBN, and in NBN. The measures were obtained before and after carboplatin treatment. The magnitude of the IHC lesion was assessed at the end of the psychophysical studies by anatomical confirmation of IHC and OHC loss as a function of location along the basilar membrane. The within-subjects experimental paradigm employed in this project was powerful in that all of the behavioral measurements were obtained within the same animals followed by anatomical confirmation of the lesion. It is important to note that, as discussed below, carboplatin treatment was systemic. Thus, we cannot discount the possibility that the observed results could be explained by central pathology or a combination of peripheral and central dysfunction. Nonetheless, the presence of significant IHC loss was associated with increased thresholds in noise, but no significant elevation of thresholds in quiet.
Thresholds in Quiet
Baseline audiograms were obtained prior to carboplatin treatment. Thresholds in quiet ranged from 2- to 8-dB SPL from 250 to 11,300 Hz. Half of the study subjects were from a previous study (Lobarinas et al. 2013) and were selected based on quiet thresholds that did not significantly change following carboplatin treatment. The overall average includes these subjects as well as five additional subjects that did not show significant elevations in thresholds in quiet following carboplatin. When compared, our previous publication thresholds were within 5 dB across frequencies 250–4000 Hz and lower above 8000 Hz.
Following carboplatin treatment, there were no significant effects on thresholds in quiet. These results confirm our previous findings that pure-tone thresholds in quiet are relatively insensitive to moderate to severe IHC loss or dysfunction.
Thresholds in BBN
Thresholds for tones at 250–11,300 Hz were obtained in the presence of competing 50-dB SPL BBN before and after carboplatin. Prior to treatment, mean baseline thresholds ranged from 30 to 39 dB SPL with the highest thresholds at 4000–11,300 Hz. The 250–4000-Hz results were consistent with critical ratio data reported previously from 250 to 4000 Hz (Seaton and Trahiotis 1975; Niemiec et al. 1992) with the largest differences observed at 8000 Hz. The signal-to-noise ratio (SNR) changes were statistically significant for all frequencies, suggesting increased difficulty resolving signals in noise despite no evidence of OHC loss and no significant changes in thresholds in quiet. Thus, changes in BBN thresholds after carboplatin appeared to be more sensitive to IHC loss or dysfunction than thresholds obtained in quiet.
There are no behavioral data in the literature showing changes in the critical ratio or the detection of tones in BBN as a function of selective IHC loss/dysfunction. The data presented here show for the first time that there was a significant deterioration in the ability to detect a tone in noise associated with carboplatin-induced inner hair cell loss/dysfunction.
The larger increase in tone-in-noise thresholds at higher frequencies after carboplatin treatment could be interpreted in two ways. The first interpretation is that whereas the width of the critical band may remain unchanged after carboplatin, the input/output function of the neurons contacting residual IHCs becomes shallower. That is, the response to the tone burst embedded in the noise produces a smaller than expected increase in spike rate. This hypothesis is supported by auditory nerve fiber recordings obtained from chinchillas with selective IHC loss/dysfunction (Wang et al. 1997). The decrease in the slope and saturation rate of the input/output functions as well as the reduction in the spontaneous rate could account for the poor response to tones in BBN, that is, a poorer signal (tone response) to noise ratio.
Future studies aimed at measuring the response of single neurons to tones embedded in BBN could be carried out to test this hypothesis. This type of study might need to be carried out at multiple levels of the auditory pathway. Another approach might involve measuring the local field potential amplitude (e.g., AC or IC) in the presence of BBN before and after inducing an IHC lesion (Robertson et al. 1990).
The second, more speculative, interpretation could arise from the critical band hypothesis; the increase of tone-in-noise thresholds could be due to a broadening of internal auditory filters. The critical band widening hypothesis has been evaluated indirectly by assessing physiological tuning curves obtained at multiple stages along the auditory pathway. Recordings made from auditory nerve fibers from chinchillas with selective IHC loss show that sharp neural tuning is retained at the level of the cochlea despite massive IHC loss (Wang et al. 1997). Thus, there is no overt evidence of broader neural tuning at the level of the cochlea for acoustic stimuli in quiet. It is important to note, however, that auditory filter bandwidth slopes at the level of the auditory nerve fibers are input dependent (Temchin et al. 2008) and this level dependency would have to be accounted for in subsequent studies. The effect of competing noise on these functions after carboplatin is unknown.
Single-unit recordings made from the IC in chinchillas with selective IHC loss also show sharp tuning (Wake et al. 1996). Whereas there is no physiological evidence of broader neural tuning at the midbrain to account for the increase in the critical ratio, these experiments would need to be carried out under conditions that would account for the presence of competing background noise. If tuning were retained at lower levels of the auditory system, broadening of neural tuning could occur in the AC. Single-unit AC recordings from chinchillas with carboplatin-induced IHC loss have not yet been carried out so this hypothesis remains untested. At the level of the AC, large changes in neural tuning have been observed as a result of blocking GABA-mediated inhibition with bicuculline (Wang et al. 2000). Many AC neurons lost their finely tuned tips and adopted broadened U-shaped frequency response areas following bicuculline treatment. If selective IHC loss leads to changes in GABA-mediated inhibition, as some studies suggest (Salvi et al. 2000a), then it is possible that broadened tuning at the AC could account for the increase in the hearing-in-noise thresholds reported here. If this were to be true, then the broadened tuning could help explain the low thresholds (increased spectral integration) observed in quiet and the poorer thresholds observed across all frequencies under BBN. Futures studies in the AC of chinchillas with selective IHC loss need to be carried out to test this hypothesis.
Thresholds in NBN
To evaluate tuning characteristics, masking functions were obtained under three NBN masking conditions with center frequencies at 500, 2000, and 4000 Hz before and after carboplatin. As expected, the pre-treatment function was characterized by relatively lower thresholds below the center frequency, maximum thresholds at the center frequency of the masker, and elevated thresholds 1–2 octaves above the masker. However, following carboplatin treatment, we observed unexpected results at thresholds both below and above the center frequencies of the maskers. Although shallow I/O functions and reduced spike rates have been observed in the IC after carboplatin, the high-frequency and low-frequency expansions of the NBN masking are not likely due to frequency selectivity changes along the auditory pathway since single-fiber tuning curves in the auditory nerve and IC remain sharply tuned in animals with large carboplatin-induced IHC lesions (Wake et al. 1996; Wang et al. 1997).
The expansion of the NBN masking patterns could arise if the carboplatin-induced IHC lesions caused frequency response changes at the level of the AC associated with GABA-mediated inhibition, as suggested in the previous section regarding increased thresholds in BBN. A previous study has shown that GABA-mediated inhibition is reduced in the chinchilla AC when IHCs are destroyed by carboplatin (Salvi et al. 2000a). Whereas the application of the GABA-antagonist, bicuculline, to the AC normally leads to a large increase in the amplitude of the AC tone-evoked field potential, in carboplatin-treated chinchillas, bicuculline had little effect on the response, suggesting that GABA-mediated inhibition had already been eliminated. The loss of GABA-mediated inhibition in the AC could facilitate changes in neural frequency response characteristics and lead to increases in threshold above and below the NBN masker. Evidence for this is suggested by the aforementioned studies in which GABA-mediated inhibition is blocked by bicuculline. In these experiments, physiological tuning curves shifted from having low threshold, narrowly tuned tips to low-threshold, broadly tuned tips (Wang et al. 2000). The tuning curve tips were observed to broaden considerably both above and below the characteristic frequency (CF). In the present experiment, chinchillas treated with carboplatin exhibited losses of IHCs across a wide region of the cochlea. Consequently, disinhibition would be expected across a broad range of frequencies. The results from the NBN experiments presented here were consistent with this hypothesis as thresholds for frequencies far below and above the masker center frequency were elevated after carboplatin.
Relationship of IHC Loss to Perceptual Changes
The main findings of this study were that, whereas the degree of IHC loss or dysfunction had little impact on thresholds in quiet (Lobarinas et al. 2013), IHC loss or dysfunction increased thresholds in noise and altered narrowband noise masking patterns in a manner consistent with a reduced ability to resolve signals in competing background noise. Recent human studies have suggested that poorer hearing in noise may be the result of IHC deafferentation (Makary et al. 2011; Bharadwaj et al. 2014). The results presented here support the hypothesis that hearing in noise is impaired as a function of IHC loss or dysfunction, independent of OHC loss. The loss of OHC has been hypothesized to negatively affect hearing in noise due to changes in critical bands. However, abnormal hearing performance in noise varies significantly and does not correlate strongly with audiometry (Vermiglio et al. 2012), a measure that is sensitive to OHC loss. Likewise, some individuals with “normal” audiometric and distortion product otoacoustic emissions (DPOAE) results could exhibit significant difficulties in noise (Bharadwaj et al. 2015).
Thus, functional measures of OHC integrity, such as DPOAE, may have limited utility in predicting functional hearing above threshold. Our own dose response pilot studies as well as previous reports (Trautwein et al. 1996; Hofstetter et al. 1997b) showed no effect of carboplatin at the doses used here on (1) loss of OHC or (2) the presence or amplitude of DPOAEs, results suggesting that the performance deficits in noise were primarily associated with the loss or dysfunction of IHC.
Currently, there are no widely accepted functional tests of IHC loss readily available to clinicians, but increasing test sensitivity to IHC dysfunction may provide significant diagnostic value in relation to functional impairment and differential diagnosis. Such tests could help differentiate central hearing loss from peripheral hearing loss in cases of disproportionally poor hearing in noise. For instance, the use of the ABR wave I amplitude has been suggested as being sensitive to IHC deafferentation (Kujawa and Liberman 2009; Wynne et al. 2013). Electrocochleography (ECOG) can be used to visualize wave I more clearly than in an ABR (Spoendlin and Baumgartner 1977) and reveal potential damage to inner hair cells or afferent dysfunction. The TEN(HL) test can be used to identify cochlear dead regions (Moore et al. 2000) that are presumably devoid of functional inner hair cells. The aforementioned tests can be useful but have limitations in that the relationship between moderate IHC loss and functional deficits require additional studies as shown by the data presented here. In the present study, IHC dysfunction and/or loss due to carboplatin increased thresholds in noise, even though this is typically only associated with OHC dysfunction. It is plausible and likely that hearing loss that results in measurable loss of OHC also damages IHC or afferent fibers. To the degree that hearing-in-noise deficits can be attributed to OHC alone, IHC alone, loss of afferents alone, or some combination has not been fully determined.
The data presented here as well as recent data from Kujawa and Liberman (Kujawa and Liberman 2009) underscore the limitations of relying on thresholds in quiet as an assay of the integrity of the peripheral auditory system and highlight the importance of assessing hearing-in-noise as a routine clinical measure to better evaluate overall hearing ability and identify hidden hearing deficits.
Acknowledgments
The corresponding author gratefully acknowledges Colleen Le Prell for guidance and constructive review on the contents and revisions of the manuscript.
Author Contributions
EL and DD performed data collection and analysis, and all authors contributed to writing the manuscript.
Support
Research reported in this manuscript was supported by the National Institute on Deafness and Other Communication Disorders (NIDCD) of the National Institutes of Health (NIH) under award number R03 DC011612-04. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- ABR
Auditory brainstem response
- AC
Auditory cortex
- CAP
Compound action potential
- CM
Cochlear microphonics
- dB
Decibel
- DPOAE
Distortion product otoacoustic emissions
- IC
Inferior colliculus
- IHC
Inner hair cell
- OHC
Outer hair cell
- PTCs
Psychophysical tuning curves
- SD
Standard deviation
- SEM
Standard error of the mean
- SPL
Sound pressure level
- SDH
Succinate dehydrogenase
References
- Allen JB. Cochlear micromechanics—a physical model of transduction. J Acoust Soc Am. 1980;68:1660–1670. doi: 10.1121/1.385198. [DOI] [PubMed] [Google Scholar]
- Arnold S, Burkard R. Inner hair cell loss and steady-state potentials from the inferior colliculus and auditory cortex of the chinchilla. J Acoust Soc Am. 2002;112:590–599. doi: 10.1121/1.1494991. [DOI] [PubMed] [Google Scholar]
- Bharadwaj HM, Verhulst S, Shaheen L, Liberman MC, Shinn-Cunningham BG. Cochlear neuropathy and the coding of supra-threshold sound. Front Syst Neurosci. 2014;8:26. doi: 10.3389/fnsys.2014.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj HM, Masud S, Mehraei G, Verhulst S, Shinn-Cunningham BG. Individual differences reveal correlates of hidden hearing deficits. J Neurosci. 2015;35:2161–2172. doi: 10.1523/JNEUROSCI.3915-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeslee EA, Hynson K, Hamernik RP, Henderson D. Asymptotic threshold shift in chinchillas exposed to impulse noise. J Acoust Soc Am. 1978;63:876–882. doi: 10.1121/1.381767. [DOI] [PubMed] [Google Scholar]
- Borg E. Loss of hair cells and threshold sensitivity during prolonged noise exposure in normotensive albino rats. Hear Res. 1987;30:119–126. doi: 10.1016/0378-5955(87)90129-8. [DOI] [PubMed] [Google Scholar]
- Clark WW, Clark CS, Moody DB, Stebbins WC. Noise-induced hearing loss in the chinchilla, as determined by a positive-reinforcement technique. J Acoust Soc Am. 1974;56:1202–1209. doi: 10.1121/1.1903409. [DOI] [PubMed] [Google Scholar]
- Cody AR, Russell IJ. Outer hair cells in the mammalian cochlea and noise-induced hearing loss. Nature. 1985;315:662–665. doi: 10.1038/315662a0. [DOI] [PubMed] [Google Scholar]
- Coleman JW. Hair cell loss as a function of age in the normal cochlea of the guinea pig. Acta Otolaryngol. 1976;82:33–40. doi: 10.3109/00016487609120860. [DOI] [PubMed] [Google Scholar]
- Dallos P, Harris D. Properties of auditory nerve responses in absence of outer hair cells. J Neurophysiol. 1978;41:365–383. doi: 10.1152/jn.1978.41.2.365. [DOI] [PubMed] [Google Scholar]
- Davis RI, Ahroon WA, Hamernik RP. The relation among hearing loss, sensory cell loss and tuning characteristics in the chinchilla. Hear Res. 1989;41:1–14. doi: 10.1016/0378-5955(89)90173-1. [DOI] [PubMed] [Google Scholar]
- Davis B, Qiu W, Hamernik RP. Sensitivity of distortion product otoacoustic emissions in noise-exposed chinchillas. J Am Acad Audiol. 2005;16:69–78. doi: 10.3766/jaaa.16.2.2. [DOI] [PubMed] [Google Scholar]
- Ding D, Li M, Zheng X, Wang J, Salvi RJ. Cochleogram for assessing hair cells and efferent fibers in carboplatin-treated ear. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 1999;13:510–512. [PubMed] [Google Scholar]
- Ding DL, Wang J, Salvi R, Henderson D, Hu BH, McFadden SL, Mueller M. Selective loss of inner hair cells and type-I ganglion neurons in carboplatin-treated chinchillas. Mechanisms of damage and protection. Ann NY Acad Sci. 1999;884:152–170. doi: 10.1111/j.1749-6632.1999.tb08640.x. [DOI] [PubMed] [Google Scholar]
- Egan JP, Hake HW. On the masking pattern of a simple auditory stimulus. J Acoust Soc Am. 1950;22:622–630. doi: 10.1121/1.1906661. [DOI] [Google Scholar]
- El-Badry MM, McFadden SL. Electrophysiological correlates of progressive sensorineural pathology in carboplatin-treated chinchillas. Brain Res. 2007;1134:122–130. doi: 10.1016/j.brainres.2006.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman AC, Kujawa SG, Liberman MC. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol. 2013;110:577–586. doi: 10.1152/jn.00164.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudi D, Salvi R, Henderson D, Hamernik R. Gap detection by the chinchilla. J Acoust Soc Am. 1980;68:802–806. doi: 10.1121/1.384818. [DOI] [PubMed] [Google Scholar]
- Giraudi-Perry DM, Salvi RJ, Henderson D. Gap detection in hearing-impaired chinchillas. J Acoust Soc Am. 1982;72:1387–1393. doi: 10.1121/1.388444. [DOI] [PubMed] [Google Scholar]
- Greenwood DD. A cochlear frequency-position function for several species--29 years later. J Acoust Soc Am. 1990;87:2592–2605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- Hamernik RP, Patterson JH, Turrentine GA, Ahroon WA. The quantitative relation between sensory cell loss and hearing thresholds. Hear Res. 1989;38:199–211. doi: 10.1016/0378-5955(89)90065-8. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Heffner HE. Behavioral hearing range of the chinchilla. Hear Res. 1991;52:13–16. doi: 10.1016/0378-5955(91)90183-A. [DOI] [PubMed] [Google Scholar]
- Hofstetter P, Ding D, Salvi R. Magnitude and pattern of inner and outer hair cell loss in chinchilla as a function of carboplatin dose. Audiology. 1997;36:301–311. doi: 10.3109/00206099709071981. [DOI] [PubMed] [Google Scholar]
- Hofstetter P, Ding D, Powers N, Salvi RJ. Quantitative relationship of carboplatin dose to magnitude of inner and outer hair cell loss and the reduction in distortion product otoacoustic emission amplitude in chinchillas. Hear Res. 1997;112:199–215. doi: 10.1016/S0378-5955(97)00123-8. [DOI] [PubMed] [Google Scholar]
- Johnstone BM, Patuzzi R, Yates GK. Basilar membrane measurements and the travelling wave. Hear Res. 1986;22:147–153. doi: 10.1016/0378-5955(86)90090-0. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Dodds LW. Single-neuron labeling and chronic cochlear pathology. III. Stereocilia damage and alterations of threshold tuning curves. Hear Res. 1984;16:55–74. doi: 10.1016/0378-5955(84)90025-X. [DOI] [PubMed] [Google Scholar]
- Lin HW, Furman AC, Kujawa SG, Liberman MC. Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. J Assoc Res Otolaryngol. 2011;12:605–616. doi: 10.1007/s10162-011-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Salvi R, Ding D. Insensitivity of the audiogram to carboplatin induced inner hair cell loss in chinchillas. Hear Res. 2013;302:113–120. doi: 10.1016/j.heares.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makary CA, Shin J, Kujawa SG, Liberman MC, Merchant SN. Age-related primary cochlear neuronal degeneration in human temporal bones. J Assoc Res Otolaryngol. 2011;12:711–717. doi: 10.1007/s10162-011-0283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Jiang H, Woo JM, Salvi RJ. Chinchilla models of selective cochlear hair cell loss. Hear Res. 2002;174:230–238. doi: 10.1016/S0378-5955(02)00697-4. [DOI] [PubMed] [Google Scholar]
- McGill TJ, Schuknecht HF. Human cochlear changes in noise induced hearing loss. Laryngoscope. 1976;86:1293–1302. doi: 10.1288/00005537-197609000-00001. [DOI] [PubMed] [Google Scholar]
- Miller JD. Audibility curve of the chinchilla. J Acoust Soc Am. 1970;48:513–523. doi: 10.1121/1.1912166. [DOI] [PubMed] [Google Scholar]
- Moore BC, Huss M, Vickers DA, Glasberg BR, Alcantara JI. A test for the diagnosis of dead regions in the cochlea. Br J Audiol. 2000;34:205–224. doi: 10.3109/03005364000000131. [DOI] [PubMed] [Google Scholar]
- Niemiec AJ, Yost WA, Shofner WP. Behavioral measures of frequency selectivity in the chinchilla. J Acoust Soc Am. 1992;92:2636–2649. doi: 10.1121/1.404380. [DOI] [PubMed] [Google Scholar]
- Nienhuys TG, Clark GM. Critical bands following the selective destruction of cochlear inner and outer hair cells. Acta Otolaryngol. 1979;88:350–358. doi: 10.3109/00016487909137179. [DOI] [PubMed] [Google Scholar]
- Ohlms LA, Lonsbury-Martin BL, Martin GK. Acoustic-distortion products: separation of sensory from neural dysfunction in sensorineural hearing loss in human beings and rabbits. Otolaryngol Head Neck Surg. 1991;104:159–174. doi: 10.1177/019459989110400203. [DOI] [PubMed] [Google Scholar]
- Patuzzi RB, Yates GK, Johnstone BM. Outer hair cell receptor current and sensorineural hearing loss. Hear Res. 1989;42:47–72. doi: 10.1016/0378-5955(89)90117-2. [DOI] [PubMed] [Google Scholar]
- Prazma J, Postma DS, Pecorak JB, Fischer ND. Ototoxicity of tobramycin sulfate. Laryngoscope. 1976;86:259–268. doi: 10.1288/00005537-197602000-00022. [DOI] [PubMed] [Google Scholar]
- Preyer S, Gummer AW. Nonlinearity of mechanoelectrical transduction of outer hair cells as the source of nonlinear basilar-membrane motion and loudness recruitment. Audiol Neurootol. 1996;1:3–11. doi: 10.1159/000259185. [DOI] [PubMed] [Google Scholar]
- Prosen CA, Halpern DL, Dallos P. Frequency difference limens in normal and sensorineural hearing impaired chinchillas. J Acoust Soc Am. 1989;85:1302–1313. doi: 10.1121/1.397461. [DOI] [PubMed] [Google Scholar]
- Robertson LF, Salvi RJ, Poon M, Powers NL. Evoked potential tone-on-tone masking patterns in the chinchilla. Am J Otol. 1990;11:431–436. [PubMed] [Google Scholar]
- Ryan A, Dallos P, McGee T. Psychophysical tuning curves and auditory thresholds after hair cell damage in the chinchilla. J Acoust Soc Am. 1979;66:370–378. doi: 10.1121/1.383194. [DOI] [PubMed] [Google Scholar]
- Ryan AF, Woolf NK, Bone RC. Ultrastructural correlates of selective outer hair cell destruction following kanamycin intoxication in the chinchilla. Hear Res. 1980;3:335–351. doi: 10.1016/0378-5955(80)90027-1. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Arehole S. Gap detection in chinchillas with temporary high-frequency hearing loss. J Acoust Soc Am. 1985;77:1173–1177. doi: 10.1121/1.392181. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Hamernik RP, Henderson D. Discharge patterns in the cochlear nucleus of the chinchilla following noise induced asymptotic threshold shift. Exp Brain Res. 1978;32:301–320. doi: 10.1007/BF00238704. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear Res. 2000;147:261–274. doi: 10.1016/S0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Ding D, Wang J, Jiang HY. A review of the effects of selective inner hair cell lesions on distortion product otoacoustic emissions, cochlear function and auditory evoked potentials. Noise Health. 2000;2:9–26. [PubMed] [Google Scholar]
- Santi PA, Ruggero MA, Nelson DA, Turner CW. Kanamycin and bumetanide ototoxicity: anatomical, physiological and behavioral correlates. Hear Res. 1982;7:261–279. doi: 10.1016/0378-5955(82)90040-5. [DOI] [PubMed] [Google Scholar]
- Saunders JC, Mills JH, Miller JD. Threshold shift in the chinchilla from daily exposure to noise for six hours. J Acoust Soc Am. 1977;61:558–570. doi: 10.1121/1.381298. [DOI] [PubMed] [Google Scholar]
- Seaton WH, Trahiotis C. Comparison of critical ratios and critical bands in the monaural chinchilla. J Acoust Soc Am. 1975;57:193–199. doi: 10.1121/1.380414. [DOI] [PubMed] [Google Scholar]
- Soucek S, Mason SM. A study of hearing in the elderly using non-invasive electrocochleography and auditory brainstem responses. J Otolaryngol. 1987;16:345–353. [PubMed] [Google Scholar]
- Spoendlin H. Neuroanatomical basis of cochlear coding mechanisms. Audiology. 1975;14:383–407. doi: 10.3109/00206097509071752. [DOI] [PubMed] [Google Scholar]
- Spoendlin H, Baumgartner H. Electrocochleography and cochlear pathology. Acta Otolaryngol. 1977;83:130–135. doi: 10.3109/00016487709128822. [DOI] [PubMed] [Google Scholar]
- Stebbins WC, Hawkins JE, Jr, Johnson LG, Moody DB. Hearing thresholds with outer and inner hair cell loss. Am J Otolaryngol. 1979;1:15–27. doi: 10.1016/S0196-0709(79)80004-6. [DOI] [PubMed] [Google Scholar]
- Takeno S, Harrison RV, Ibrahim D, Wake M, Mount RJ. Cochlear function after selective inner hair cell degeneration induced by carboplatin. Hear Res. 1994;75:93–102. doi: 10.1016/0378-5955(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Takeno S, Harrison RV, Mount RJ, Wake M, Harada Y. Induction of selective inner hair cell damage by carboplatin. Scanning Microsc. 1994;8:97–106. [PubMed] [Google Scholar]
- Temchin AN, Rich NC, Ruggero MA. Threshold tuning curves of chinchilla auditory-nerve fibers. I. Dependence on characteristic frequency and relation to the magnitudes of cochlear vibrations. J Neurophysiol. 2008;100:2889–2898. doi: 10.1152/jn.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautwein P, Hofstetter P, Wang J, Salvi R, Nostrant A. Selective inner hair cell loss does not alter distortion product otoacoustic emissions. Hear Res. 1996;96:71–82. doi: 10.1016/0378-5955(96)00040-8. [DOI] [PubMed] [Google Scholar]
- Vermiglio AJ, Soli SD, Freed DJ, Fisher LM. The relationship between high-frequency pure-tone hearing loss, hearing in noise test (HINT) thresholds, and the articulation index. J Am Acad Audiol. 2012;23:779–788. doi: 10.3766/jaaa.23.10.4. [DOI] [PubMed] [Google Scholar]
- Wake M, Takeno S, Ibrahim D, Harrison R, Mount R. Carboplatin ototoxicity: an animal model. J Laryngol Otol. 1993;107:585–589. doi: 10.1017/S0022215100123771. [DOI] [PubMed] [Google Scholar]
- Wake M, Takeno S, Ibrahim D, Harrison R. Selective inner hair cell ototoxicity induced by carboplatin. Laryngoscope. 1994;104:488–493. doi: 10.1288/00005537-199404000-00016. [DOI] [PubMed] [Google Scholar]
- Wake M, Takeno S, Mount RJ, Harrison RV. Recording from the inferior colliculus following cochlear inner hair cell damage. Acta Otolaryngol. 1996;116:714–720. doi: 10.3109/00016489609137912. [DOI] [PubMed] [Google Scholar]
- Wang J, Powers NL, Hofstetter P, Trautwein P, Ding D, Salvi R. Effects of selective inner hair cell loss on auditory nerve fiber threshold, tuning and spontaneous and driven discharge rate. Hear Res. 1997;107:67–82. doi: 10.1016/S0378-5955(97)00020-8. [DOI] [PubMed] [Google Scholar]
- Wang J, Caspary D, Salvi RJ. GABA-A antagonist causes dramatic expansion of tuning in primary auditory cortex. Neuroreport. 2000;11:1137–1140. doi: 10.1097/00001756-200004070-00045. [DOI] [PubMed] [Google Scholar]
- Wang J, Ding D, Salvi RJ. Functional reorganization in chinchilla inferior colliculus associated with chronic and acute cochlear damage. Hear Res. 2002;168:238–249. doi: 10.1016/S0378-5955(02)00360-X. [DOI] [PubMed] [Google Scholar]
- Wang J, Ding D, Salvi RJ. Carboplatin-induced early cochlear lesion in chinchillas. Hear Res. 2003;181:65–72. doi: 10.1016/S0378-5955(03)00176-X. [DOI] [PubMed] [Google Scholar]
- Wynne DP, Zeng FG, Bhatt S, Michalewski HJ, Dimitrijevic A, Starr A. Loudness adaptation accompanying ribbon synapse and auditory nerve disorders. Brain. 2013;136:1626–1638. doi: 10.1093/brain/awt056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Salvi RJ, Powers N, Wu J. Evoked response narrow-band noise masking patterns in the chinchilla. Hear Res. 1990;47:175–181. doi: 10.1016/0378-5955(90)90175-O. [DOI] [PubMed] [Google Scholar]