Abstract
Background
Inflammatory markers have been shown to play an important role in bone remodeling. The purpose of this study was to investigate the relationship among serum C-reactive protein (CRP), adiponectin, tumor necrosis factor-alpha (TNF-α) and bone health in healthy adults.
Methods
We measured serum levels of CRP, adiponectin, TNF-α as well as lumbar spine and femoral neck bone mineral density (BMD) in 76 adults. Anthropometric measurements and nutrient intake survey of participants were carried out. The participants were divided into two groups (normal BMD group=40; 52.6%, decreased BMD group=36; 47.4%).
Results
The CRP concentration was significantly higher in the decreased BMD group. The adiponectin concentration was lower in the decreased BMD group but the difference was not significant. The TNF-α concentration was higher in the decreased BMD group, the difference was not significant. The participants in the decreased BMD group were found to have lower calcium intakes. The sodium intake of the decreased BMD group was significantly higher. The BMD in the decreased BMD group showed inverse correlations with CRP and dietary sodium intake.
Conclusions
Serum CRP and dietary sodium intake is associated with BMD. Further research is needed to confirm the potential role of inflammatory marker to modulate the effects on bone.
Keywords: Adiponectin, Bone Density, C-reactive protein, Nutritional status
INTRODUCTION
Owing to the recent increases in the average human life expectancy, the public interest in health has been increasing and efforts to lead healthy life and prevent diseases. In particular, with the increase in the elderly population, the prevalence rates and costs of treatment of osteoporosis and fracture have greatly increased. The prevalence of osteoporosis, according to the 2012 Korea National Health and Nutrition Examination Survey, is 34.9% in females and 7.8% in males, and the numbers have been increasing every year. Moreover, considering that the risk of fracture shows a 1.73-fold increase not only in osteoporosis but also in osteopenia, active efforts to prevent osteopenia are required.[1] The highest bone mineral content is formed during the second and third decades of life, and bond loss spontaneously occurs with aging afterward.[2] Various factors, including hormones, nutritional intake of calcium and protein, physical activities, medication use, and smoking, are known to influence the formation and maintenance of bone.[3] Moreover, in order to elucidate the mechanism of correlation of bone mineral density (BMD) with obesity, body fat mass,[4] diabetes,[5] and metabolic diseases,[6] studies are investigating inflammatory markers.[7]
Previous studies suggest that inflammatory response induces the formation of osteoclasts, thereby leading to bone loss. The correlation between bone metabolism and the concentration of C-reactive protein (CRP), which is a typical inflammatory marker, has been observed in immune and inflammatory diseases. High CRP concentration in menopausal women with rheumatoid arthritis was found to be related to high bone turnover,[7] and CRP concentration in premenopausal women was found to significantly correlate with decreases in BMD.[8] Serum adiponectin, which shares structural similarities with tumor necrosis factor-alpha (TNF-α), a potent regulator of osteoclastogenesis, has been reported to inversely correlate with BMD in diabetic patients.[9] However, studies are still ongoing because the exact mechanism has not been elucidated yet. Only a few Korean studies were conducted in adults. Therefore, this study aimed to investigate the influences of BMD status and systemic asymptomatic inflammatory response, and to analyze their relationship in healthy adults.
METHDOS
1. Participants
The participants of this study included healthy adults who visited the health promotion center of a university hospital in Gyeonggi Province. Before the beginning of the study, all the participants were given explanations on the purpose of the study, and the 80 who signed consent forms to participate in the study were selected as our final participants. After all the examinations were completed, four participants whose examinations were omitted were excluded, and data analysis was conducted for the remaining 76 participants. The participants were divided into the following groups according to the lumbar vertebrae and femur neck T-scores obtained during BMD measurement: those with T-scores of ≥-1 were classified as the normal BMD group, and those with T-scores <-1 were classified as the decreased BMD group. This study was approved by the Institutional Review Board of Soonchunhyang University Bucheon Hospital.
2. Anthropometric assessment and biochemical measurement
Height, weight, and body composition were analyzed by using a bio-electrical impedance fatness analyzer (InBody 720; Biospace Co., Seoul, Korea). Waist circumference was measured twice in each participant by using a tapeline while the participant was in a comfortable standing position. The mean of the two values was used. For the blood test, the participants fasted for 12 hr and blood samples were obtained from their brachial veins. Serum that had been centrifuged for 10 min at 2,000 to 4,000 rpm was analyzed by using an automated clinical chemistry analyzer. CRP level was analyzed by using an automated clinical chemistry analyzer (Fuji Dri-Chem 3500i; Fuji Photo Film Ltd., Tokyo, Japan), and adiponectin and TNF-α concentrations were analyzed by using human enzyme-linked immunosorbent assay kits (automatic microplate reader; Molecular Devices, CA, USA). The BMD was measured using the dual-energy X-ray absorptiometry equipment (GE Lunar Prodigy; GE Lunar Corp., Madison, WI, USA) on the participant's lumber spine (LS) and femoral neck (FN).
3. Life habit and nutrient intake survey
The questions on the drinking, smoking and exercise habits were surveyed per individual. Skilled clinical dietitian interviewed participants using photographs of food items and a booklet containing eye measurement data as aids for the nutrient intake survey. Data on dietary intake amount were collected using the 24 hr recall method for three days and intakes of nutrients were analyzed with the computer aided nutritional (CAN) program version 3.0 (Korean Nutrition Society, Seoul, Korea).[10] Nutrient density (ND) was calculated by standardizing the nutrient intake per 1,000 kcal based on the individual's intake amount.
4. Statistical analysis
The mean and standard deviation were calculated using SPSS software program version 18.0 (SPSS Inc., Chicago, IL, USA). All data were reported as mean±standard deviation or as numbers and percentages. Groups comparisons used Chi-square tests for qualitative variables and independent t-test for quantitative variables. The correlation between bone density and its related variables was analyzed using Pearson's correlation coefficient. All results were considered statistically significant when P<0.05 and the significance was presented a)(P<0.05), b)(P<0.01), or c)(P<0.001).
RESULTS
1. Clinical characteristics of the participants
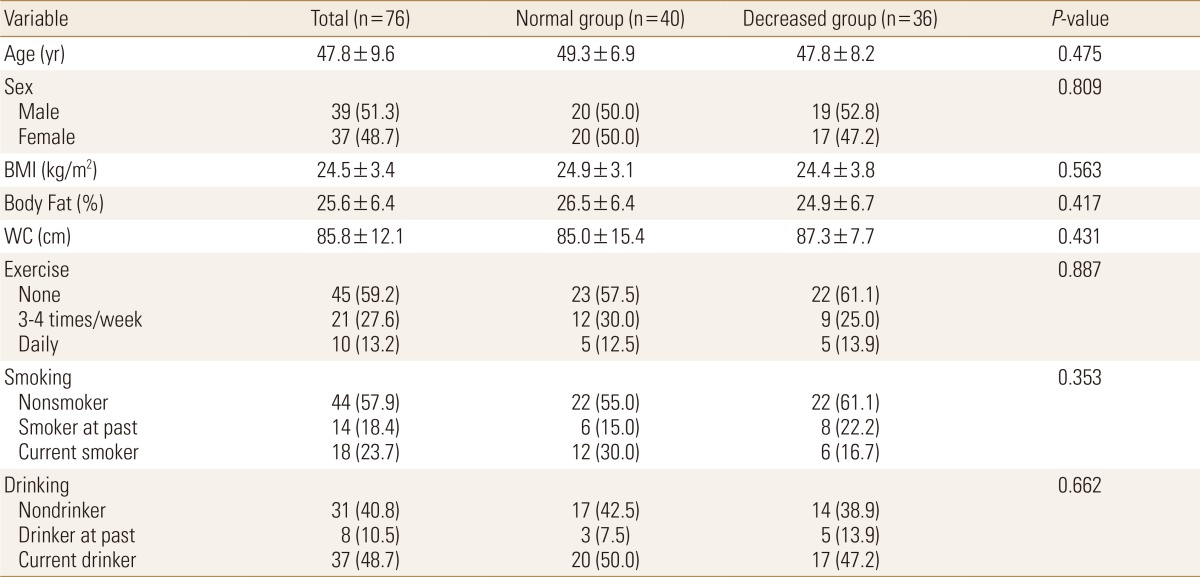
Among the 76 healthy adults, 40 (52.6%) belonged to the normal BMD group and 36 (47.4%) belonged to the decreased BMD group. Among the 36 participants in the decreased BMD group, 31 (86.1%) had osteopenia and five (13.9%) had osteoporosis. The mean age of the participants was 47.8 years. The mean body mass index (BMI), which was 24.5±3.4 kg/m2, was within the overweight range. Similarly, the mean body fat percentage, which was 25.6%±6.4%, showed that the participants were obese. The mean waist circumference of the participants was 85.8 cm, and the groups did not differ significantly in their anthropometric data. With regard to the participants' life habits, 27.6% of them exercised three to four times per week and 13.2% exercised every day. The mean current smoking rate in all the participants was 23.7%, showing a higher smoking rate in the normal BMD group (30.0%) than in the decreased BMD group (16.7%). The mean current drinking rate in all the participants was 48.7%, 50.0% in the normal BMD group, and 47.2% in the decreased BMD group. Although the two groups did not differ significantly in their life habits, the decreased BMD group had lower drinking and smoking rates, as well as exercise rate (Table 1).
Table 1. Anthropometric and life habit data.
Data was presented as mean and standard deviation, frequency and percentage.
BMI, body mass index; WC, waist circumference.
2. Comparison of biochemical data
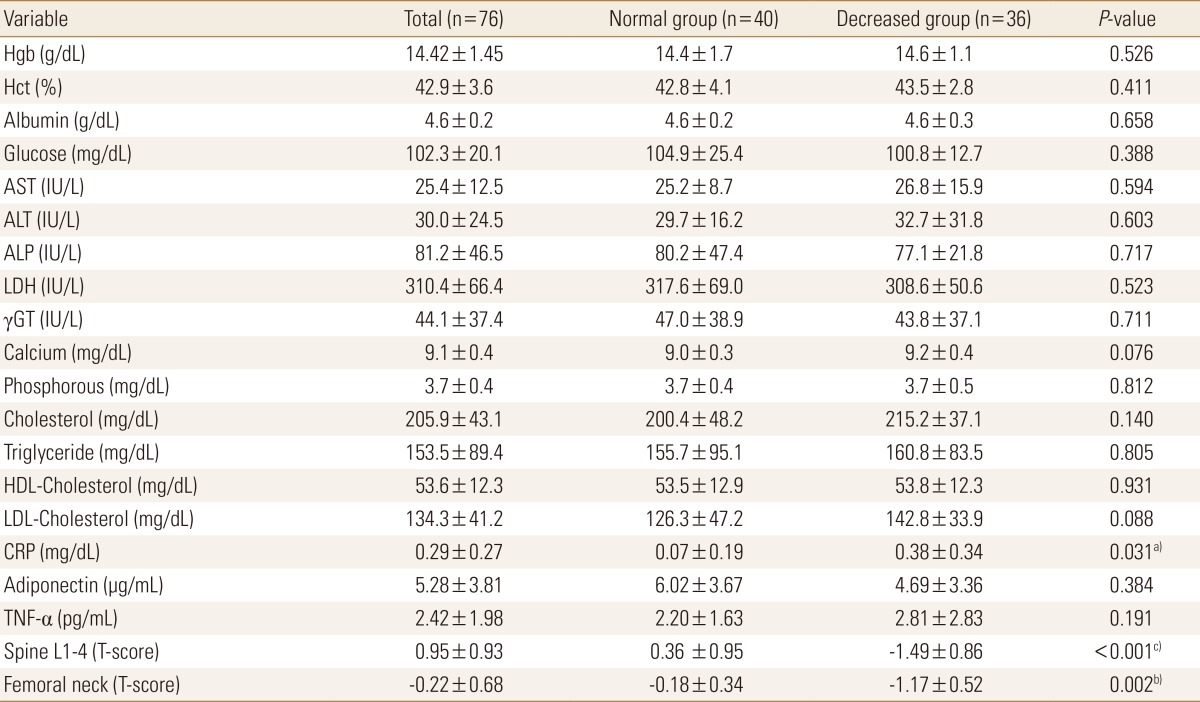
The results of the comparison of the blood test results of the two groups are shown in Table 2. The mean blood test results of all the participants and the two groups were all within the normal ranges. Although all the mean CRP concentrations were within the normal ranges, the CRP concentration in the decreased BMD group was significantly higher. The adiponectin concentration was lower in the decreased BMD group but the difference was not significant. Although the TNF-α concentration was higher in the decreased BMD group, the difference was not significant. Overall, among the three inflammatory markers assessed in this study, only CRP concentration showed a significant difference between the two groups (Table 2).
Table 2. Biochemical data.
Data was presented as mean and standard deviation.
a)P<0.05. b)P<0.01. c)P<0.001.
Hgb, hemoglobin; Hct, hematocrit; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; LDH, lactate dehydronase; γGT, gamma (γ)-glutamyl transferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; CRP, c-reactive protein; TNF-α, tumor necrosis factor-alpha.
3. Nutrient intake status and ND
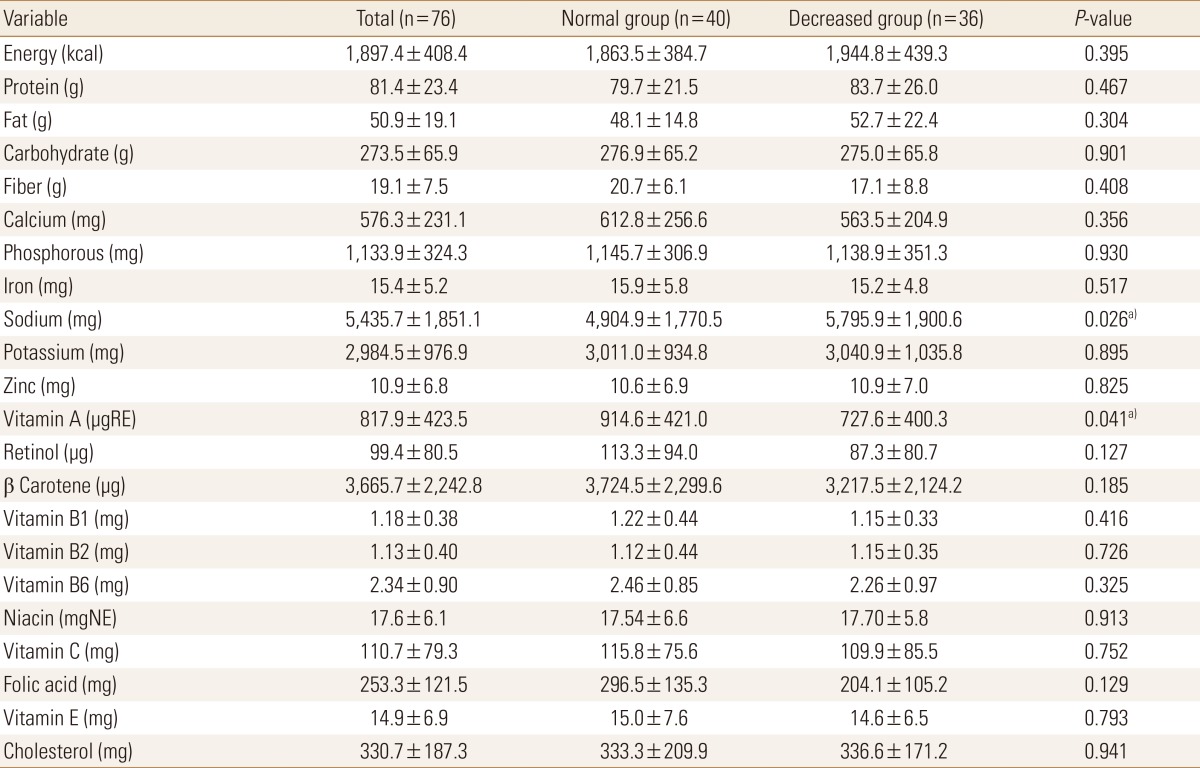
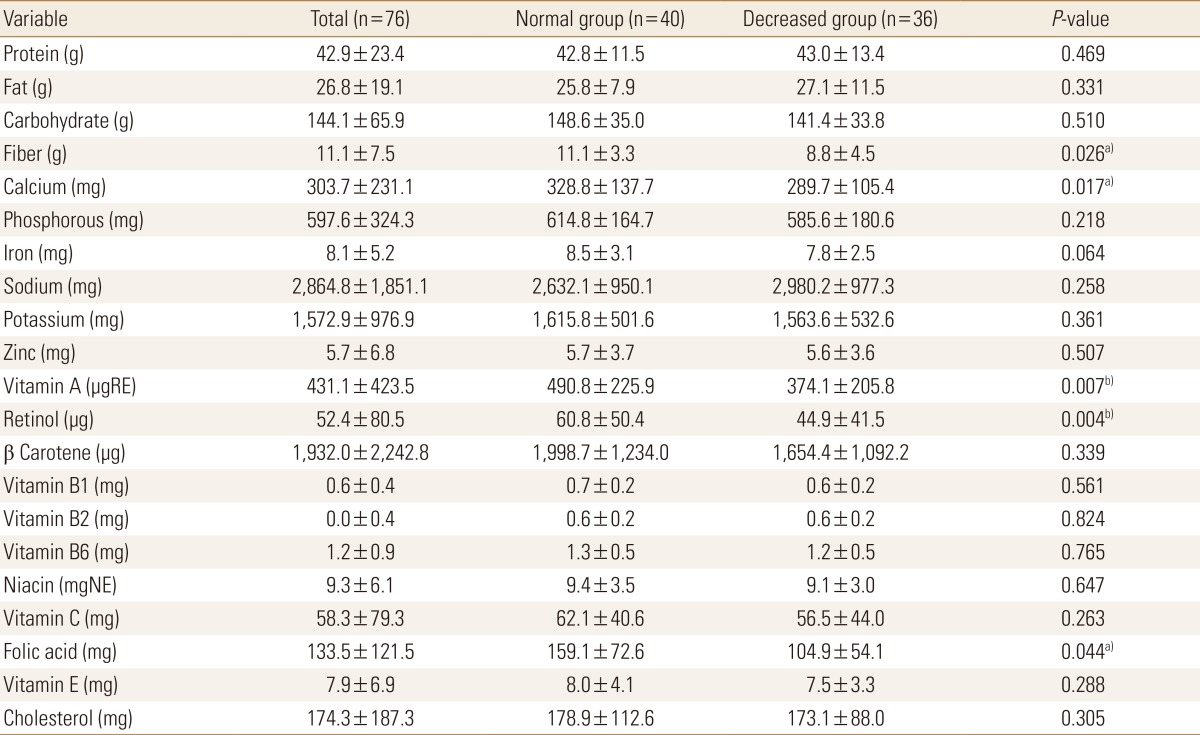
Table 3 shows the comparison of nutrient intake status between the two groups. The decreased BMD group was found to have slightly higher protein and fat intakes. In part of mineral intake, the mean calcium intakes were 576.3±231.1, 612.8±256.6, and 563.5±204.9 mg in all the participants, in the normal BMD group, and in the decreased BMD group, respectively. The participants in the decreased BMD group were found to have lower calcium intakes. The sodium intake of the decreased BMD group was significantly higher. The mean vitamin A intake was significantly lower in the decreased BMD group. Although the intakes of vitamins B1, B6, C, and E, and folic acid were lower in the decreased BMD group, the difference was not significant. In the decreased BMD group, the ND of fiber, calcium, vitamin A, retinol, folic acid were significantly lower (Tables 3, 4).
Table 3. Nutrient intake status.
Data was presented as mean and standard deviation.
a)P<0.05.
Table 4. Comparison of nutrient density.
Data was presented as mean and standard deviation.
a)P<0.05. b)P<0.01.
4. The correlation with inflammatory marker and variables according to BMD
In each of the two groups, the correlation between BMD and the following factors was analyzed: inflammatory markers and nutrients that showed significant differences between the groups (sodium and vitamin A). In the normal BMD group, the correlations between BMD and the other factors were not significant. By contrast, the BMD in the decreased BMD group showed inverse correlations with CRP and dietary sodium intake (Table 5).
Table 5. Correlation coefficients between bone mineral density and inflammatory markers.
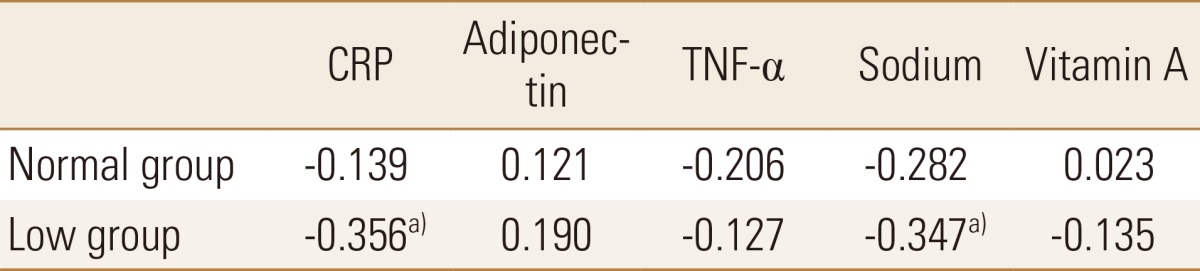
a)P<0.05.
CRP, c-reactive protein; TNF-α, tumor necrosis factor-alpha.
DISCUSSION
Previous studies conducted in South Korea and other countries suggested that inflammatory cytokines are involved in the activation of osteoclasts. Such inflammatory cytokines are known to influence normal bone remodeling, which is not related to inflammatory diseases, and typical cytokines include interleukin 1 (IL-1) and TNF.[11] CRP is a major acute-phase reactant and is considered as a sensitive inflammatory marker. Inflammatory responses strongly stimulate the hepatic CRP production and increase the production of various cytokines such as IL-1, IL-6, and TNF-α, which induce bone resorption by controlling the differentiation and activity of osteoblasts and osteoclasts. Increased bone resorption subsequently leads to increased bone turnover and decreased BMD.[12] To support such hypothesis, Salamone et al.[13] reported that the productions of IL-1, IL-6, and TNF-α in peripheral blood mononuclear cells positively correlated with vertebral bone loss in healthy premenopausal women, and Cohen-Solal et al.[14] reported that the concentrations of IL-1, IL-6, and TNF-α in mononuclear cell culture supernatant positively correlated with bone resorptive activity in menopausal women. Moreover, Scheidt-Nave et al.[15] reported that serum IL-6 concentration can predict femoral bone loss in healthy menopausal women. The aforementioned findings lead us to suspect that inflammatory cytokines are the main mechanisms that link CRP concentration and bone metabolism. Adiponectin, which is a collagenous protein that is specifically expressed in human adipocytes, inversely correlated with obesity.[9] A relatively smaller number of studies have investigated the correlation between serum adiponectin concentration and BMD. An inverse correlation between adiponectin concentration and BMD has been reported in diabetic patients.[16] Adiponectin suppresses the formation of adipocytes by increasing the expression of cyclooxygenase-2 (COX-2) and the secretion of prostaglandin E2 (PG E2) in bone marrow stromal cells. As COX-2 is also known to be involved in the differentiation of cells to osteoblasts, further studies are required to investigate how adiponectin influences bone metabolism through the COX-2 pathway.[17] In our study, the blood test and inflammatory marker results of the healthy adults were all within the normal ranges. However, CRP concentration was significantly higher in the decreased BMD group. In a recent large-scale study, CRP concentration was reported to highly correlate with BMD in both males and females, and to be a useful predictive factor of the occurrence of fractures.[18] In a study conducted with South Korean menopausal women, CRP concentration was found to correlate with BMD.[19] Napoli et al.[18] reported that adiponectin concentration did not correlate with BMD in males but showed significant differences in females. In a cohort study, CRP concentration was found to correlate with femur neck BMD, while TNF-α concentration was found to correlate with spine BMD.[20] When the relationship between BMD and various inflammatory markers was analyzed in this study, BMD significantly correlated only with CRP concentration but did not correlate with other markers.
According to the analysis of the participants' nutrient intake, the participants were taking sufficient amounts of nutrients when compared with the age-matched recommended dietary allowance for South Koreans.[21] However, their intakes of calcium, vitamin B2, and folic acid were insufficient, and their intakes of protein and sodium were excessive. We also analyzed whether nutrient intake differed according to BMD status. In particular, sodium intake was significantly higher and vitamin A intake was significantly lower in the decreased BMD group than in the normal BMD group. Moreover, a negative correlation was observed between BMD and sodium intake was found in the decreased BMD group. As calcium is essential for bone formation and maintenance, and sodium can increase the renal calcium excretion, increased calcium intake and less salty diets should be recommended to prevent and treat osteoporosis. In the future, studies could be conducted to investigate whether secondary factors such as nutritional status influence the correlation between inflammatory markers and BMD. This study has several limitations, including its retrospective cross-sectional design, small number of participants, and inclusion of menopausal women as participants. Nevertheless, this study is significant because no other South Korean study has investigated the relationship of BMD with inflammatory markers and nutrient intake. In the future, large-scale prospective studies are required to investigate the influence of inflammatory markers on BMD.
Footnotes
No potential conflict of interest relevant to this article was reported.
This work was supported by the Soonchunhyang University Research Fund.
The authors wish to thank Eun-Ae Jung librarian and Bora Lee biostatistic consultant who assisted in manuscript editing and statistical advice.
References
- 1.Korean Society for Bone and Mineral Research. Physician's guide for diagnosis & treatment of osteoporosis. 2015. [cited by 2016 Feb 1]. Available from: http://www.ksbmr.org/image/journal/골다공증%20지침서2015_final_1002.pdf.
- 2.Firooznia H, Golimbu C, Rafii M, et al. Quantitative computed tomography assessment of spinal trabecular bone. I. Age-related regression in normal men and women. J Comput Tomogr. 1984;8:91–97. doi: 10.1016/0149-936x(84)90091-2. [DOI] [PubMed] [Google Scholar]
- 3.Matkovic V. Calcium intake and peak bone mass. N Engl J Med. 1992;327:119–120. doi: 10.1056/NEJM199207093270210. [DOI] [PubMed] [Google Scholar]
- 4.Labouesse MA, Gertz ER, Piccolo BD, et al. Associations among endocrine, inflammatory, and bone markers, body composition and weight loss induced bone loss. Bone. 2014;64:138–146. doi: 10.1016/j.bone.2014.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koroglu BK, Kiris F, Ersoy IH, et al. Relation of leptin, adiponectin and insulin resistance to bone mineral density in type 2 diabetic postmenopausal women. Endokrynol Pol. 2011;62:429–435. [PubMed] [Google Scholar]
- 6.Ornstrup MJ, Kjær TN, Harsløf T, et al. Adipose tissue, estradiol levels, and bone health in obese men with metabolic syndrome. Eur J Endocrinol. 2015;172:205–216. doi: 10.1530/EJE-14-0792. [DOI] [PubMed] [Google Scholar]
- 7.Marhoffer W, Stracke H, Masoud I, et al. Evidence of impaired cartilage/bone turnover in patients with active ankylosing spondylitis. Ann Rheum Dis. 1995;54:556–559. doi: 10.1136/ard.54.7.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koh JM, Khang YH, Jung CH, et al. Higher circulating hsCRP levels are associated with lower bone mineral density in healthy pre- and postmenopausal women: evidence for a link between systemic inflammation and osteoporosis. Osteoporos Int. 2005;16:1263–1271. doi: 10.1007/s00198-005-1840-5. [DOI] [PubMed] [Google Scholar]
- 9.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 10.Lim H, Kim HJ, Hong SJ, et al. Nutrient intake and bone mineral density by nutritional status in patients with inflammatory bowel disease. J Bone Metab. 2014;21:195–203. doi: 10.11005/jbm.2014.21.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenzo J. Interactions between immune and bone cells: new insights with many remaining questions. J Clin Invest. 2000;106:749–752. doi: 10.1172/JCI11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinhold B, Rüther U. Interleukin-6-dependent and -independent regulation of the human C-reactive protein gene. Biochem J. 1997;327 pt 2:425–429. doi: 10.1042/bj3270425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salamone LM, Whiteside T, Friberg D, et al. Cytokine production and bone mineral density at the lumbar spine and femoral neck in premenopausal women. Calcif Tissue Int. 1998;63:466–470. doi: 10.1007/s002239900559. [DOI] [PubMed] [Google Scholar]
- 14.Cohen-Solal ME, Graulet AM, Denne MA, et al. Peripheral monocyte culture supernatants of menopausal women can induce bone resorption: involvement of cytokines. J Clin Endocrinol Metab. 1993;77:1648–1653. doi: 10.1210/jcem.77.6.8263153. [DOI] [PubMed] [Google Scholar]
- 15.Scheidt-Nave C, Bismar H, Leidig-Bruckner G, et al. Serum interleukin 6 is a major predictor of bone loss in women specific to the first decade past menopause. J Clin Endocrinol Metab. 2001;86:2032–2042. doi: 10.1210/jcem.86.5.7445. [DOI] [PubMed] [Google Scholar]
- 16.Lenchik L, Register TC, Hsu FC, et al. Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone. 2003;33:646–651. doi: 10.1016/s8756-3282(03)00237-0. [DOI] [PubMed] [Google Scholar]
- 17.Scherer PE, Williams S, Fogliano M, et al. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 18.Napoli N, Pedone C, Pozzilli P, et al. Adiponectin and bone mass density: the InCHIANTI study. Bone. 2010;47:1001–1005. doi: 10.1016/j.bone.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon HG, Bok GH, Lee KW, et al. Relationships of serum leptin and adiponectin concentrations with bone mineral density in Korean postmenopausal women. J Bone Metab. 2006;13:33–39. [Google Scholar]
- 20.Sponholtz TR, Zhang X, Fontes JD, et al. Association between inflammatory biomarkers and bone mineral density in a community-based cohort of men and women. Arthritis Care Res (Hoboken) 2014;66:1233–1240. doi: 10.1002/acr.22270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Korean Nutrition Society. Dietary reference intakes for Koreans. Sejong: Ministry of Health and Welfare; 2015. [Google Scholar]