Abstract
Low circulating levels of total 25-hydroxyvitamin D (25(OH)D) have been associated with an increased risk of adverse effects after cardiac surgery. The metabolites, 25(OH)D2 and 25(OH)D3, provide a good index of vitamin D status. In this study, we examined the association between preoperative plasma levels of total 25(OH)D, 25(OH)D2 and 25(OH)D3 and the risk of postoperative atrial fibrillation (POAF) following open heart surgery. The levels of plasma 25(OH)D2 and 25(OH)D3 in 118 patients, who underwent coronary artery bypass grafting and/or valvular surgery, were measured immediately prior to surgery and on postoperative day 3 by liquid chromatography–tandem mass spectrometry. Patients who developed POAF had higher median plasma levels of 25(OH)D2 than those who remained in sinus rhythm (SR) (P = 0·003), but no significant difference was noted in levels of 25(OH)D3 or total 25(OH)D between the two groups (P > 0·05). By univariate analysis, patients with total 25(OH)D and 25(OH)D2 levels above the median had higher frequency of POAF (P < 0·05) and the incidence of POAF increased significantly with each higher quartile of preoperative plasma levels of 25(OH)D2 (P = 0·001), an association that was independent of confounding factors. In both the SR and POAF groups, the median plasma levels of 25(OH)D2, 25(OH)D3 and total 25(OH)D were lower (P < 0·05) on the third postoperative day compared with preoperatively. Our findings demonstrate that higher plasma levels of 25(OH)D2 are associated with increased risk of POAF, while this is not the case for 25(OH)D3 or total 25(OH)D. The reason for these discrepant results is not clear but warrants further study.
Key words: Atrial fibrillation, Heart surgery, Postoperative state, Vitamin D
Abbreviations: 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D; AF, atrial fibrillation; POAF, postoperative atrial fibrillation; SR, sinus rhythm
Low levels of total 25-hydroxyvitamin D (25(OH)D), the major circulating form of vitamin D, have been associated with various adverse outcomes following open heart surgery, including in-hospital death, myocardial infarction, low cardiac output states and stroke(1–3).
The most important vitamin D compounds are vitamin D2 (ergocalciferol), found in plants and consumed as a supplement or in fortified foods, and vitamin D3 (cholecalciferol), which is synthesised in the human epidermis under the influence of sunlight or consumed in oily fish, fortified foods or as a supplement. 25-Hydroxyergocalciferol (25(OH)D2) and 25-hydroxycholecalciferol (25(OH)D3), which are generally considered to reflect an individual's overall vitamin D status, are produced by hydroxylation in the liver(4). In the kidney, part of total 25(OH)D is converted to the active form of vitamin D, 1,25-dihydroxyvitamin D (1,25(OH)2D), which plays an important role in the regulation of mineral homeostasis and has beneficial effects on cardiovascular function and the immune system(5).
A relationship between vitamin D deficiency and the development of atrial fibrillation (AF) has been suggested, although reported studies have yielded somewhat conflicting results(6–8). However, no studies exist on the possible role of vitamin D metabolites in the development of postoperative AF (POAF), which is surprising in view of the association of vitamin D deficiency with adverse outcomes after cardiac surgery(1–3).
Therefore, this study was carried out to investigate whether higher preoperative plasma levels of 25(OH)D2, 25(OH)D3 and total 25(OH)D are associated with lower incidence of POAF in patients undergoing open heart surgery.
Materials and methods
Subjects
This study was based on prospectively collected data from a randomised, double-blind, placebo-controlled clinical trial designed to examine the effect of n-3 long-chain PUFA on the occurrence of POAF in patients who underwent coronary artery bypass grafting and/or valvular repair surgery. The study was approved by the Bioethics Committee of Landspitali – The National University Hospital of Iceland (62/2004). All patients provided written informed consent. Details of the study design have been published previously(9). In brief, patients scheduled for elective or semi-urgent open heart surgery were evaluated for participation. Patients younger than 40 years of age, those with a history of supraventricular arrhythmia or use of the antiarrhythmic medications amiodarone and/or sotalol, and patients undergoing emergency surgery were excluded. Prior to surgery, all participants answered a questionnaire on lifestyle issues, including consumption of fish and cod liver oil, intake of supplemental n-3 long-chain PUFA capsules, smoking habits, alcohol consumption, height, body weight and medication use. The active treatment consisted of two Omega-3 Forte capsules twice daily, providing a total of 1240 mg of EPA ethyl ester and 1000 mg of DHA ethyl ester but no vitamin D (Omega Forte; Lysi Inc.). The placebo group received 2 g of olive oil in identical capsules (Lysi Inc.). The treatment was initiated 5–7 d before the scheduled date of surgery and continued until the day of discharge from the hospital or for a maximum duration of 2 weeks after the surgery. All patients underwent continuous electrocardiaographic monitoring while hospitalised and the study endpoint, POAF, was defined as an episode of AF lasting more than 5 min.
Measurement of plasma 25-hydroxyvitamin D2 and D3
Plasma samples obtained both immediately before the surgery (preoperatively) and on the third postoperative day (postoperatively), when the risk for POAF is high, were available for 118 of the 168 original randomised controlled trial participants. The plasma samples were stored at −76°C until analysis for 25(OH)D2 and 25(OH)D3 using an MS/MS Vitamin D Kit (Perkin Elmer), and carried out as described previously(10). Briefly, 30 µl of serum were deproteinised in microtitre plates using 120 µl acetonitrile containing [2H3]25(OH)D2 and [2H3]25(OH)D3 as internal standards. The supernatants were transferred to fresh plates and dried under a gentle flow of N2. Subsequently, the samples were derivatised using 4-phenyl-1,2,4-triazoline-3,5-dione (PTAD) dissolved in acetonitrile. The derivatisation reaction was quenched in quenching solution and the samples were subjected to liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis. The LC-MS/MS system consisted of a CTC PAL Autosampler (CTC Analytics), a Thermo Surveyor LC pump and a Thermo TSQ Ultra Triple Quadrupole mass spectrometer (Thermo Scientific). Separation was achieved using a Thermo Gold Hypersil C18 column (50 × 2·1 mm, 3 µm). The analytes were detected as methylamine adducts and the following transitions were used: 619·3/298·1 and 607·3/298·1 for 25(OH)D2 and 25(OH)D3, respectively, 622·3/301·1 and 610·3/298·1 for 25(OH)D2 and 25(OH)D3 internal standards, respectively, and 625·3/298·1 and 613·3/298·1 for 25(OH)D2 and 25(OH)D3 calibration standards, respectively. The assay does not differentiate between epi-25(OH)D3 and 25(OH)D3. Thus the level reported for 25(OH)D3 includes any epi form if present, but the detected level of 25(OH)D2 is unaffected by the epi form. Total plasma 25(OH)D was calculated as the sum of the plasma levels of 25(OH)D2 and 25(OH)D3. The correctness of the method was confirmed through participation in the external control program DEQAS.
Measurement of plasma postoperative C-reactive protein
Routine postoperative care included daily measurements of C-reactive protein using a sandwich enzyme immunoassay (Vitros 5.1 FS Chemistry System; Ortho-Clinical Diagnostic).
Statistical analyses
Mann–Whitney or χ2 tests were used to compare the sinus rhythm (SR) and POAF groups with respect to baseline characteristics. The Wilcoxon signed-rank test was used to compare the differences in plasma levels of 25(OH)D2, 25(OH)D3 and total 25(OH)D between time points in the two study groups. To examine the association between POAF and the preoperative plasma levels of 25(OH)D2, 25(OH)D3 and total 25(OH)D, we compared the rate of POAF between dichotomised levels and quartiles of plasma 25(OH)D2, 25(OH)D3 and total 25(OH)D using the χ2 test and Somers’ d test for ordinal variables. A stepwise multivariable logistic regression was used to examine factors associated with POAF. In the original investigation, there was no difference in POAF incidence between the active treatment and placebo groups, and no association between treatment and outcome. Hence, we have ignored treatment allocation in our analyses. Multivariable linear regression analysis was used to assess predictors of the preoperative plasma levels of 25(OH)D2 and 25(OH)D3 in the whole study group. Data are presented as medians and ranges, percentages or means with their standard errors. A two-sided P value <0·05 was considered statistically significant. All statistical analyses were carried out using SPSS (version 21.0; IBM Corporation).
Results
Baseline characteristics
The baseline characteristics of the patients in the SR (n 52) and POAF (n 66) groups are shown in Table 1. The patients with POAF were older (P = 0·001), they comprised fewer smokers (P = 0·041), and they consumed fish more frequently (P = 0·034) than the SR group. Immediately before the surgery and on the third postoperative day, the median plasma level of 25(OH)D2 was higher in the POAF group than in the SR group (P = 0·003 and P = 0·040, respectively) (Table 2). However, the differences in levels of 25(OH)D3 or total 25(OH)D between the two groups were not significant. In both the SR and POAF groups, the median plasma levels of 25(OH)D2, 25(OH)D3 and total 25(OH)D were lower (P < 0·05) on the third postoperative day compared with the preoperative values (Table 2).
Table 1.
Characteristics of the study subjects
(Medians and ranges, or percentages)
| SR group (n 52) | POAF group (n 66) | ||||
|---|---|---|---|---|---|
| Median | Range | Median | Range | P* | |
| Age (years) | 64·0 | 43–79 | 70·5 | 45–82 | 0·001 |
| BMI (kg/m2) | 28·0 | 20·9–41·3 | 27·1 | 21·0–38·1 | 0·86 |
| Sex (% men) | 78·8 | 81·8 | 0·69 | ||
| Smokers (%) | 26·9 | 12·1 | 0·04 | ||
| Alcohol consumption ≥once per week (%) | 67·3 | 74·2 | 0·42 | ||
| Fish intake ≥once a week (%) | 59·6 | 78·8 | 0·03 | ||
| Use of liquid cod liver oil (%)† | 50·0 | 54·5 | 0·70 | ||
| Use of n-3 long-chain PUFA capsules (%)† | 19·2 | 31·8 | 0·14 | ||
| n-3 Long-chain PUFA treatment (%) | 50·0 | 53·0 | 0·74 | ||
| Diabetes (%) | 17·3 | 12·1 | 0·43 | ||
| Treatment with statins (%) | 84·6 | 72·7 | 0·12 | ||
| CABG only (%) | 78·8 | 66·7 | 0·15 | ||
| Total blood volume in drains (ml) | 710 | 175–3070 | 800 | 96–4980 | 0·58 |
SR, sinus rhythm; POAF, postoperative atrial fibrillation; CABG, coronary artery bypass grafting.
Compared with the SR group (Mann–Whitney test or χ2 test).
Prior to study treatment.
Table 2.
Plasma vitamin D levels of patients in the sinus rhythm (SR) and postoperative atrial fibrillation (POAF) groups, immediately before cardiac surgery (preoperative) and on the third postoperative day (postoperative)
(Medians and ranges)
| Preoperative | Postoperative | ||||
|---|---|---|---|---|---|
| Median | Range | Median | Range | P* | |
| SR group (n 52) | |||||
| 25(OH)D2 (nmol/l) | 0·8 | 0·0–4·4 | 0·75 | 0·0–3·2 | 0·003 |
| 25(OH)D3 (nmol/l) | 37·8 | 7·4–89·1 | 29·4 | 7·6–71·3 | <0·001 |
| Total 25(OH)D (nmol/l) | 39·3 | 8·1–90·5 | 30·5 | 8·6–72·2 | <0·001 |
| POAF group (n 66) | |||||
| 25(OH)D2 (nmol/l) | 1·3† | 0·0–20·8 | 1·0† | 0·0–13·2 | <0·001 |
| 25(OH)D3 (nmol/l) | 51·6 | 8·6–83·5 | 37·7 | 7·3–76·0 | <0·001 |
| Total 25(OH)D (nmol/l) | 52·4 | 8·6–84·9 | 38·3 | 7·3–77·3 | <0·001 |
25(OH)D, 25-hydroxyvitamin D.
Compared with preoperative 25(OH)D2, 25(OH)D3 or total 25(OH)D levels (Wilcoxon signed-rank test).
Median value was significantly different from that of the SR group (P < 0·05; Mann–Whitney test).
Association of plasma 25-hydroxyvitamin D2 and D3 levels with postoperative atrial fibrillation
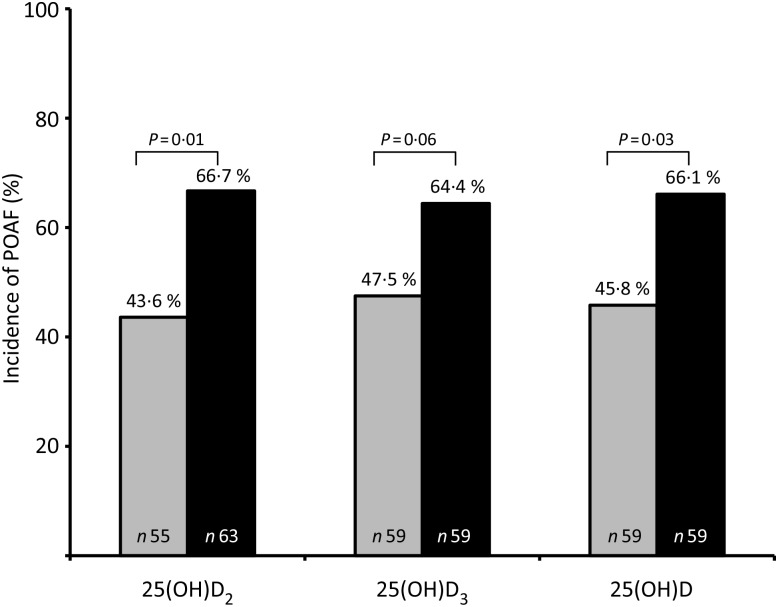
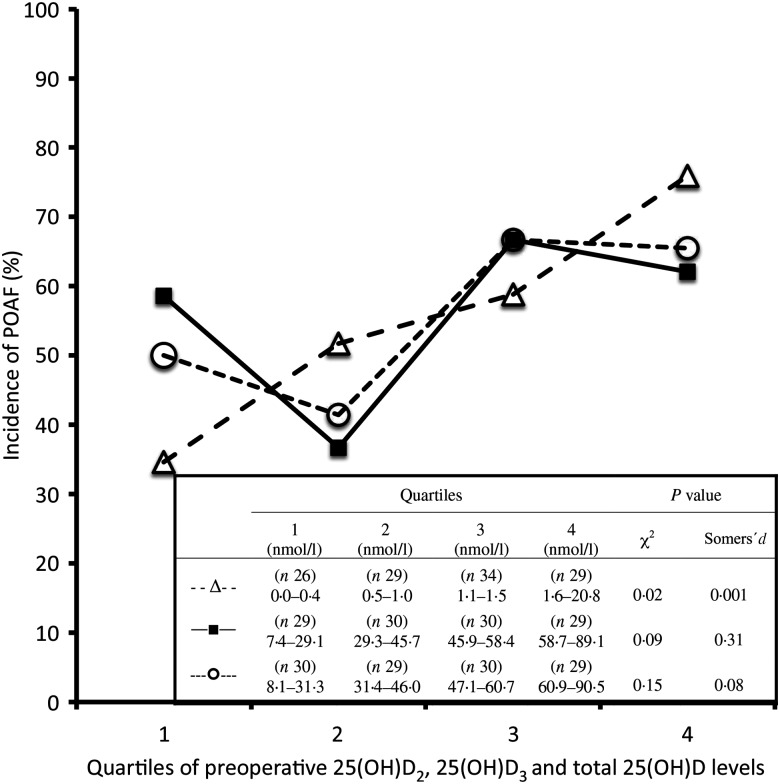
The incidence of POAF was higher (P < 0·05) in those with preoperative plasma levels above the median for 25(OH)D2 (≥1·1 nmol/l) and total 25(OH)D (≥47·1 nmol/l) compared with those below the median, whereas the difference was of borderline significance (P = 0·06) for 25(OH)D3 (≥45·9 nmol/l) (Fig. 1). When the patients were divided into quartiles based on preoperative plasma levels of 25(OH)D2 and 25(OH)D3, the POAF incidence differed for the 25(OH)D2 levels (P = 0·020), with a significant linear trend for an increasing incidence of POAF observed with the higher quartiles (P = 0·001) (Fig. 2). The difference in POAF incidence observed between the quartiles of 25(OH)D3 and total 25(OH)D levels demonstrated a non-significant J-shaped curve (P > 0·05).
Fig. 1.
Incidence of postoperative atrial fibrillation (POAF) according to dichotomised preoperative plasma levels of 25-hydroxyvitamin D2 (25(OH)D2), 25(OH)D3, and total 25(OH)D in patients undergoing open heart surgery. Median levels ≥1·1 nmol/l for 25(OH)D2; ≥45·9 nmol/l for 25(OH)D3; ≥47·1 nmol/l for total 25(OH)D. Data were analysed by Pearson's χ2.  , Below median; ■, above median.
, Below median; ■, above median.
Fig. 2.
Incidence of postoperative atrial fibrillation (POAF) according to quartiles of the preoperative plasma levels (nmol/l) of 25-hydroxyvitamin D2 (25(OH)D2; --Δ--), 25(OH)D3 (–■–) and total 25(OH)D (--○--) in patients undergoing open heart surgery. Data were analysed by Pearson's χ2 and Somers’ d statistics for trend in association between ordinal variables.
Multivariable logistic regression analysis, adjusting for factors known or presumed to affect POAF, showed that higher preoperative plasma levels of 25(OH)D2 were associated with higher risk of developing POAF with OR of 2·747 (95 % CI 1·121, 6·734; P = 0·027), while no significant association between POAF and plasma levels of 25(OH)D3 or total 25(OH)D was observed (Table 3).
Table 3.
Multivariable logistic regression analysis of factors associated with postoperative atrial fibrillation*
| R2 | OR | 95 % CI | P | |
|---|---|---|---|---|
| Age (years) | 0·127 | 1·07 | 1·03, 1·12 | 0·001 |
| Peak postoperative CRP (mg/l) | 0·195 | 1·01 | 1·00, 1·01 | 0·02 |
| Plasma preoperative DHA (%) | 0·241 | 2·63 | 1·10, 6·28 | 0·03 |
| Smoking (reference, non-smoker) | 0·275 | 0·37 | 0·13, 1·05 | 0·06 |
| Type of surgery (reference, valvular or complex procedure) | 0·304 | 0·42 | 0·16, 1·12 | 0·08 |
| BMI (kg/m2) | 0·308 | 0·96 | 0·85, 1·08 | 0·47 |
| 25(OH)D2 (reference, lower half)† | 0·352 | 2·75 | 1·12, 6·73 | 0·03 |
| 25(OH)D3 (reference, lower half)† | 0·309 | 1·10 | 0·45, 2·66 | 0·83 |
| Total 25(OH)D (reference, lower half)† | 0·311 | 1·31 | 0·54, 3·16 | 0·55 |
R2, Nagelkerke's R2; CRP, C-reactive protein; 25(OH)D, 25-hydroxyvitamin D.
Stepwise forward selection of variables, 25(OH)D2, 25(OH)D3 and total 25(OH)D added in the final step in three different models. Cumulative R2 for each step of the regression analysis.
Preoperative plasma 25(OH)D2, 25(OH)D3 and total 25(OH)D levels dichotomised at the median.
Factors predicting the plasma levels of 25-hydroxyvitamin D2 and D3
In the multivariable linear regression model, the preoperative plasma level of 25(OH)D2 was negatively associated with alcohol consumption (β = −0·210; P = 0·024), but no association with the n-3 long-chain PUFA treatment and other factors was detected. To the contrary, 25(OH)D3 was positively associated with cod liver oil supplementation (β = 0·406; P < 0·001) and alcohol consumption (β = 0·193; P = 0·022), but negatively associated with diabetes (β = −0·217; P = 0·012) and smoking (β = −0·206; P = 0·014).
Discussion
In this study, we found higher preoperative plasma 25(OH)D2 levels to be associated with an increased risk of POAF. In contrast, we did not observe an association between plasma levels of 25(OH)D3 or total 25(OH)D and the incidence of POAF. The plasma levels of both 25(OH)D2 and 25(OH)D3 decreased in the early postoperative period.
Previous studies suggest that deficiency of total 25(OH)D is associated with increased prevalence of electrocardiographic abnormalities(11). In a recent prospective cohort study of patients undergoing cardiac surgery, low total 25(OH)D levels were independently associated with the risk of major cardiac and cerebrovascular events (MACCE)(1), although the investigators did not report whether a relationship existed between 25(OH)D and the development of POAF. However, previous work has elucidated a potential role of total 25(OH)D in the development of non-valvular AF(6,7). The results of the present study, following adjustment for age, BMI, smoking, peak postoperative C-reactive protein, preoperative plasma DHA level and valvular surgery or complex surgical procedure(12), showed that higher preoperative plasma levels of 25(OH)D2 were related to the development of POAF. To the contrary, neither 25(OH)D3 nor total 25(OH)D levels were associated with POAF. This finding was somewhat surprising, since median plasma 25(OH)D2 levels in humans are generally low, as exemplified by the patients in the present study. In the POAF group, the highest plasma 25(OH)D2 level was 20·8 nmol/l compared with 4·4 nmol/l in the SR group. A recent study demonstrated the distribution of serum 25(OH)D2 concentrations in samples of Irish adults (n 884), of whom 78·8 % had concentrations above the limit of quantification (1·43 nmol/l), the median concentration being 2·96 nmol/l, and the maximum concentration 27·64 nmol/l(13). According to the authors, the distribution of the serum 25(OH)D2 data suggests that vitamin D2 is ubiquitous in the diet, regardless of food sources. It is therefore likely that dietary differences between study populations explain the observed difference in the levels of 25(OH)D2. While a causal link for the observed association might be sought in the effects of 25(OH)D2 on cardiac function or rhythm, oxidative stress or inflammatory pathways, this remains speculative and it is important that our findings be confirmed in larger studies.
Our study did not have enough power to examine MACCE in relation to vitamin D levels. Prior studies evaluating this association have not examined 25(OH)D2 and 25(OH)D3 separately, only total 25(OH)D(6,7). Hence, it remains unclear whether the effect of the two forms of vitamin D might also be different with respect to MACCE. Nevertheless, it is possible that failure to account for a differential effect of 25(OH)D2 and 25(OH)D3 may explain inconsistency in the results of studies examining the association between vitamin D and AF, an important issue that needs to be addressed in future studies.
Among the conditions that have been linked to deficiency of circulating total 25(OH)D are type 2 diabetes and hypertension, although randomised clinical trials and meta-analyses have yielded inconclusive results(14,15). Recent studies have also shown that exposure to cigarette smoke is associated with lower circulating levels of total 25(OH)D and 25(OH)D3(16,17). Our findings are consistent with data suggesting that low 25(OH)D3 levels are associated with diabetes and cigarette smoking(14,17). It has long been known that excessive alcohol consumption results in perturbations in vitamin D metabolism and that individuals suffering from chronic alcoholism usually have lower levels of circulating total 25(OH)D than non-drinkers(18). However, the results of our study suggest that moderate alcohol consumption may result in lower levels of 25(OH)D2 and higher 25(OH)D3 levels, the latter of which is in line with previous reports(19,20). Thus, our findings indicate that both lifestyle and disease states are related to circulating levels of vitamin D metabolites. Decrease in the plasma 25(OH)D3 level following cardiac surgery may reflect increased conversion into the active form, 1,25(OH)2D(1,20). The effects of anaesthesia and other factors associated with open heart surgery, such as fluid administration, blood loss, fluid shifts and leak of vitamin D-binding proteins into the interstitial space, may also have contributed to the decrease in circulating 25(OH)D levels in the early postoperative period(2,21). Additional supplementation of vitamin D3 in these patients may be required.
While the results of the current study are intriguing, the study is limited by its relatively small sample size, precluding the analysis of more definitive endpoints such as mortality or major complications of the surgical procedure. The sample size is also too limited to reliably examine non-linear relationships between the plasma levels of total 25(OH)D, 25(OH)D2 and 25(OH)D3, and the outcome of interest. However, all patients had plasma levels of total 25(OH)D below 100 nmol/l which is believed to reflect scarcity of 1,25(OH)2D, leading to deficient rather than excessive vitamin D effects(1). Furthermore, the association between PAOF and total 25(OH)D and 25(OH)D3 observed in the univariate analysis may have been rendered insignificant in the multivariate model due to lack of power. Thus, it is important to examine this association in a larger study. The blood samples from the patients were collected throughout the year and circulating total 25(OH)D levels were found to be independent of season (data not shown). This is in accordance with work demonstrating that there is no seasonal variation in circulating total 25(OH)D levels in elderly Icelandic patients, who are living in a country which is geographically located at 64°N and where the use of cod liver oil supplementation is common(22).
The plasma 25(OH)D2 levels were lower in our patients than has been reported previously. This finding is hard to explain by analytical measurement uncertainty, the only plausible exception being batch-to-batch variation. However, the samples were run with matched cases and controls placed adjacent to each other in order to avoid this phenomenon. Therefore, batch-to-batch variation is an unlikely explanation for our findings which can most probably be explained by dietary differences. Finally, this association study cannot establish a cause-and-effect relationship as unknown confounding factors may have influenced the results.
In conclusion, higher plasma levels of 25(OH)D2 were associated with higher incidence of POAF, whereas no association was found for plasma levels of total 25(OH)D and 25(OH)D3. This association of 25(OH)D2 with the risk of POAF needs to be addressed in future studies.
Acknowledgements
The contribution to this work by Ragnhildur Heidarsdottir MS, Lara Bjorgvinsdottir MS and Lilja G. Steinsdottir, Laboratory Assistant at the University of Iceland, is greatly appreciated. We are also grateful to the patients and staff at Landspitali – The National University Hospital of Iceland. This work was supported by grants from the University of Iceland Research Fund, and the Landspitali – The National University Hospital of Iceland Research Fund. O. S. I. supervised the design of the study in collaboration with D. O. A., G. V. S. and R. P.; B. T. recruited the patients and led the surgical team; A. C. analysed the 25(OH)D data together with D. M. H.; G. V. S. collected other data; O. S. I. was responsible for the statistical analyses and interpretation of the data; G. V. S. wrote the initial draft of the manuscript in cooperation with O. S. I.; R. P. critically reviewed the manuscript; all authors contributed to the final version of the manuscript.
There were no conflicts of interest.
References
- 1.Zittermann A, Kuhn J, Ernst JB, et al. (2015) 25-Hydroxyvitamin D, 1,25-dihydroxyvitamin D and postoperative outcome in cardiac surgery. J Clin Endocrinol Metab 100, 72–80. [DOI] [PubMed] [Google Scholar]
- 2.McNally JD & Menon K (2013) Vitamin D deficiency in surgical congenital heart disease: prevalence and relevance. Transl Pediatr 2, 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthews LR, Ahmed Y, Wilson KL, et al. (2012) Worsening severity of vitamin D deficiency is associated with increased length of stay, surgical intensive care unit cost, and mortality rate in surgical intensive care unit patients. Am J Surg 204, 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeLuca HF (2004) Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 80, 6 Suppl., 1689S–1696S. [DOI] [PubMed] [Google Scholar]
- 5.Polly P & Tan TC (2014) The role of vitamin D in skeletal and cardiac muscle function. Front Physiol 5, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demir M, Uyan U & Mehmet M (2014) The effects of vitamin D deficiency on atrial fibrillation. Clin Appl Thromb Hemost 20, 98–103. [DOI] [PubMed] [Google Scholar]
- 7.Chen WR, Liu ZY, Shi Y, et al. (2014) Relation of low vitamin D to nonvalvular persistent atrial fibrillation in Chinese patients. Ann Noninvasive Electrocardiol 19, 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rienstra M, Cheng S, Larson MG, et al. (2011) Vitamin D status is not related to development of atrial fibrillation in the community. Am Heart J 162, 538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heidarsdottir R, Arnar DO, Skuladottir GV, et al. (2010) Does treatment with n-3 polyunsaturated fatty acids prevent atrial fibrillation after open heart surgery? Europace 12, 356–363. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen NO, Strøm M, Boyd HA, et al. (2013) Vitamin D status during pregnancy and the risk of subsequent postpartum depression: a case–control study. PLOS ONE 8, e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuliani TA, Shenoy M, Deshmukh A, et al. (2014) Major electrocardiographic abnormalities and 25-hydroxy vitamin D deficiency: insights from National Health and Nutrition Examination Survey-III. Clin Cardiol 37, 660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skuladottir GV, Heidarsdottir R, Arnar DO, et al. (2011) Plasma n-3 and n-6 fatty acids and the incidence of atrial fibrillation following coronary artery bypass graft surgery. Eur J Clin Invest 41, 995–1003. [DOI] [PubMed] [Google Scholar]
- 13.Cashman KD, Kinsella M, McNulty BA, et al. (2014) Dietary vitamin D2 – a potentially underestimated contributor to vitamin D nutritional status of adults? Br J Nutr 112, 193–202. [DOI] [PubMed] [Google Scholar]
- 14.Khan H, Kunutsor S, Franco OH, et al. (2013) Vitamin D, type 2 diabetes and other metabolic outcomes: a systematic review and meta-analysis of prospective studies. Proc Nutr Soc 72, 89–97. [DOI] [PubMed] [Google Scholar]
- 15.Tamez H, Kalim S & Thadhani RI (2013) Does vitamin D modulate blood pressure? Curr Opin Nephrol Hypertens 22, 204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjørn Jensen C, Thorne-Lyman AL, Vadgård Hansen L, et al. (2013) Development and validation of a vitamin D status prediction model in Danish pregnant women: a study of the Danish National Birth Cohort. PLOS ONE 8, e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulligan JK, Nagel W, O'Connell BP, et al. (2014) Cigarette smoke exposure is associated with vitamin D3 deficiencies in patients with chronic rhinosinusitis. J Allergy Clin Immunol 134, 342–349. [DOI] [PubMed] [Google Scholar]
- 18.Laitinen K, Välimäki M, Lamberg-Allardt C, et al. (1990) Deranged vitamin D metabolism but normal bone mineral density in Finnish noncirrhotic male alcoholics. Alcohol Clin Exp Res 14, 551–556. [DOI] [PubMed] [Google Scholar]
- 19.Lee K (2012) Sex-specific relationships between alcohol consumption and vitamin D levels: The Korea National Health and Nutrition Examination Survey 2009. Nutr Res Pract 6, 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCullough ML, Weinstein SJ, Freedman DM, et al. (2010) Correlates of circulating 25-hydroxyvitamin D: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol 172, 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiemstra TF, Casian A, Boraks P, et al. (2014) Plasma exchange induces vitamin D deficiency. QJM 107, 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steingrimsdottir L, Halldorsson TI, Siggeirsdottir K, et al. (2014) Hip fractures and bone mineral density in the elderly – importance of serum 25-hydroxyvitamin D. PLOS ONE 9, e10. [DOI] [PMC free article] [PubMed] [Google Scholar]