Abstract
Background
Tuberculosis (TB) is the leading cause of death in South Africa. The burden of disease varies by age, with peaks in TB notification rates in the HIV-negative population at ages 0-5, 20-24 and 45-49 years. There is little variation between age groups in the rates in the HIV-positive population. The drivers of this age pattern remain unknown.
Methods
We developed an age-structured simulation model of Mycobacterium tuberculosis (Mtb) transmission in Cape Town, South Africa. We considered five states of TB progression: susceptible, infected (latent TB), active TB, treated TB and treatment default. Latently infected individuals could be re-infected; a previous Mtb infection slowed progression to active disease. We further considered three states of HIV progression: HIV negative, HIV positive, on antiretroviral therapy. To parameterize the model, we analysed treatment outcomes from the Cape Town electronic TB register, social mixing patterns from a Cape Town community and literature estimates for other parameters. To investigate the main drivers behind the age patterns, we conducted sensitivity analyses on all parameters related to the age structure.
Results
The model replicated the age patterns in HIV-negative TB notification rates of Cape Town in 2009. Simulated TB notification rate in HIV-negative patients was 1,000/100,000 person-years (pyrs) in children aged < 5 years and decreased to 51/100,000 in children 5-15 years. The peak in early adulthood occurred at 25-29 years (463/100,000 pyrs). After a subsequent decline, simulated TB notification rates gradually increased from the age of 30 years. Sensitivity analyses showed that the dip after the early adult peak was due to the protective effect of latent TB and that retreatment TB was mainly responsible for the rise in TB notification rates from the age of 30 years.
Conclusion
The protective effect of a first latent infection on subsequent infections and the faster progression in previously treated patients are the key determinants of the age-structure of TB notification rates in Cape Town.
Keywords: Tuberculosis, Age Distribution, Cape Town, Mathematical model
Introduction
The HIV and Tuberculosis (TB) epidemics in South Africa (SA) are among the worst in the world. Tuberculosis (TB) is now the leading cause of natural death in South Africa (Statistics South Africa, 2014a). HIV positive individuals who are infected with Mycrobacterium tuberculosis (Mtb) have a substantially higher risk of developing active TB than their HIV-negative peers (Corbett et al., 2003), whose lifetime risk following a single infection is about 10% (Styblo, 1991). Even though South Africa has implemented the World Health Organization (WHO) “Directly observed therapy, short course” (DOTS, WHO, 1996) strategy, the incidence of TB in South Africa has continued to rise steadily over the past 20 years, and reached a rate of 1,000 cases per 100,000 person-years in 2012 (WHO, 2013). DOTS has been estimated to reduce TB incidence by 11% per year (Dye et al., 1998), but is failing in HIV endemic settings (De Cock and Chaisson, 1999). The HIV-associated TB epidemic partly explains the failure of DOTS to reduce the TB prevalence in SA (Wood et al., 2011b), since about 65% of TB patients in South Africa are co-infected with HIV (WHO, 2014a). Because Mtb infection is preventable and TB disease is curable, effective interventions hold the potential of drastically lowering infection and mortality rates.
In addition to DOTS, HIV-specific interventions have been implemented to control TB in this population, including scaling up the use of antiretroviral therapy (ART) and the “Three I's” strategy: Intensified case finding, Isoniazid preventive therapy (IPT) and infection control (IC) at all clinical encounters (WHO, 2004). Assessing the impact of ART on TB incidence is difficult because ART reduces the risk of TB diseases among HIV-positive individuals and at the same time increases the number of people living with HIV due to reduced HIV-related mortality, thereby increasing the population risk of TB (Bacaer et al., 2008; Bhunu et al., 2009). Mathematical models estimated that universal ART eligibility for all HIV-infected South Africans would reduce the risk of AIDS-related TB by 48% in 2015 (Williams et al., 2010) and reduce new TB cases by 28-37% by 2033 (Pretorius et al., 2014). In contrast, Dodd et al (Dodd et al., 2013) estimated that TB incidence would initially decline, but rebound 20 years after widespread introduction of ART. Results of modeling studies of IPT are also contradictory. Bacaer et al (Bacaer et al., 2008) showed that IPT in HIV-positive individuals could substantially reduce TB notification rates. But Mills et al (Mills et al., 2011) concluded that the predicted effectiveness of IPT would be undermined by repeated re-infections, and Houben et al (Houben et al., 2014) found that IPT did not cure latent Mtb infection in HIV-positive patients.
TB incidence rates are strongly associated with age (Wiker et al., 2010; Wood et al., 2011a). A study of data from the beginning of the 20th century showed that in the United Kingdom age and reinfection after a cured TB episode were important determinants of transmission (Vynnycky and Fine, 1997). The authors concluded that “the sharp peaks in mortality during young adult life were attributable to the combination of a high incidence of (re)infection and a rapid risk of developing disease in late adolescence”. The same age pattern is also seen today in South Africa: a community-based study in Cape Town, showed that the highest burden of TB had shifted from the oldest (>60 years) age group to a younger population (20-39 year olds) (Lawn et al., 2006). In another study, a very distinct age pattern with three notification peaks at ages 0-5 (first peak), 20-24 (second peak), and 45-49 years (third peak) was observed in the HIV-negative population, whereas in the HIV-positive population the burden of TB mirrored age-stratified HIV prevalence (Wood et al., 2011a). The same study also found a high burden of TB in patients who were previously treated, with most patients having no history of treatment failure or default (Wood et al., 2011a).
Despite being of such importance in TB epidemiology, most South African TB models (Aparicio and Castillo-Chavez, 2009; Bacaer et al., 2008; Bhunu et al., 2009; Blower et al., 1995; Castillo-Chavez and Feng, 1997; Hickson et al., 2012; Mills et al., 2011; Ozcaglar et al., 2012; Rodrigues et al., 2007; Roeger et al., 2009; Williams et al., 2010) do not include age, or include age only for HIV incidence but not for rates of progression from latent Mtb infection to active disease or Mtb transmission. We chose to model Cape Town because of the high quality of TB notification data, and the high rates of HIV testing. We aimed to explore the drivers underlying the age-patterns in HIV-negative TB notification rates observed in Cape Town in a mathematical model that includes age-stratification and reinfection.
Methods
Setting
We modeled the TB dynamics in the city of Cape Town, where both TB and HIV are endemic. In 2009, the population of the Cape Town metropolitan area was approximately 3.5 million (“Statistics South Africa,” 2014b). HIV prevalence was estimated at around 5% of the population, and increasing over time (ASSA, 2008). Both HIV care and TB treatment are provided free of charge in government clinics across the city. ART was introduced in 2004 and has been scaled up since, reaching a coverage of 63% in 2013 (Hermans et al., 2015a). According to the 2009 National TB management guidelines, new TB cases are treated for 6 months and TB cases with a history of previous TB for 9 months (Department of Health, 2009). We used these guidelines in our model, because these were the guidelines that influenced the current TB incidence and age structure.
Model structure
We developed an individual-based TB transmission model stratified by five-year age groups. We used an individual-based stochastic simulation model because it allowed us to consider details about individual TB progression depending on age and time since infection and to capture the uncertainty of modeling outputs. Individuals were simulated from birth to death using the disease progression model implemented in the R package gems (Blaser et al., 2015). We considered the following stages of TB progression: susceptible; exposed (latent infection); active disease; treatment; treatment failure/default; and, recovered/susceptible (Figures 1, S1). Individuals latently infected with Mtb could be re-infected by another Mtb strain or progress to active disease. Active TB cases were initiated on treatment at a certain rate. After treatment initiation, patients were cured within six months (nine months in the case of retreatment TB) or they failed treatment. Cured individuals were considered susceptible to new Mtb infections (Marx et al., 2014). In case of reinfection, individuals progressed to active disease at twice the rate as previously uninfected individuals (Wood et al., 2011a).
Figure 1. Model structure.
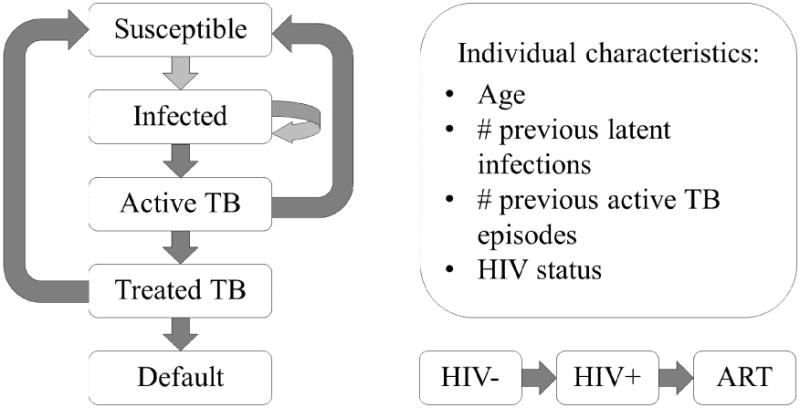
Individuals who start in the susceptible state can be exposed to tuberculosis. Exposed individuals can be exposed to a second strain or progress to active TB. Patients with active TB can recover spontaneously or receive treatment. Treated patients either recover and become susceptible again or they default. Throughout this TB progression, all individuals can also progress in their HIV status, by becoming infected and receiving antiretroviral therapy (ART). Even though it is not represented on the diagram, patients can die at any of the stages.
We modeled HIV status of the patients as HIV-negative, HIV-positive not on ART, and HIV-positive on ART (Figures 1, S2). We assumed HIV incidence was independent of TB progression, but TB progression depended on HIV status. ART initiation was also independent of the natural TB progression, but patients treated for TB were also initiated on ART. We first modeled HIV status over the entire lifespan and then Mtb infection and progression based on HIV status. We considered heterosexual transmission of HIV but ignored vertical transmission. We used separate HIV incidence rates for the pre-ART and ART eras (Table S1). In addition, each simulated individual had characteristics that changed over time: age, number of previous Mtb exposures, and number of previous active TB episodes.
Model parameters and data sources
We used data from the literature and performed dedicated analyses of existing data. HIV incidence rates to parameterize the model, disease progression rates, treatment outcomes (cure, failure) and mortality rates were assumed to depend on age. In HIV-negative children below the age of 5 years, and between 5 and 10 years, we assumed constant TB disease progression rates regardless of the duration of infection. For older HIV-negative individuals, disease progression rates depended on the time since Mtb infection: we fit an exponential decay to the progression rates reported in Ferebee et al (Ferebee, 1970) (Figure S4). Individuals who were latently infected and re-infected with another strain of Mtb had partial protection and progressed from latent to active TB at a rate 0.21 times lower than that of previously uninfected individuals (Andrews et al., 2012). To determine disease progression rates for HIV-positives with and without ART, we applied age-dependent rate ratios to the parameter estimates from the HIV-negative population (Johnson et al., 2013; Sewankambo et al., 2000). Age-specific mortality rates were also determined for HIV-negative people without active TB. We extracted age-specific all-cause mortality rates from the Actuarial Society of South Africa (ASSA) model for the Western Cape (ASSA, 2008). Then we used rate ratios to calculate mortality rates by HIV status and TB status. We extracted age-specific HIV incidence rates for the pre-ART era and for the ART-era from the ASSA model for the Western Cape (ASSA, 2008).
We reanalyzed data from a survey in a semi-urban community in Cape Town (Johnstone-Robertson et al., 2011; Wood et al., 2012) to determine age-specific mixing patterns. During a 24-hour period, 571 randomly selected participants were asked to keep a diary of their indoor contacts and the time spent in each of the following indoor locations: community buildings, crèches/schools, transport, work, household, and other indoor locations. We calculated the average time of close contact of the contacting age group with each other age group. We used this total time spent with other age groups together with the transmission rate per time spent together to calculate the number of new infections.
We analyzed data from the Cape Town metropolitan electronic TB register (ETR), which includes data on all TB cases notified in the city from 2003 onwards to obtain estimates for TB treatment outcomes. Case fatalities were all deaths occurring during treatment; defaults included both treatment failures and stopped treatment. We report the point estimate and the 95% confidence intervals. The University of Cape Town Research Ethics Committee considered these analyses exempt from ethical review as the data used was routinely collected, anonymized and in aggregate.
We incorporated parameter uncertainty into the model by sampling disease progression rates for each patient from a multivariate log-normal distribution. Table 1 shows the parameter values and confidence intervals that we used for the final model. The overall Mtb transmission rate of the model was fitted to TB notification rates in Cape Town from 2009 (Wood et al., 2011a). We initialized the model with 80% susceptible individuals; 20% of the randomly selected individuals were latently infected with Mtb at time zero, and most of them never developed active disease. Median age at initialization was 30.4 years (interquartile range: 14.5-50.1); 17.3% of individuals were HIV-positive. These numbers were the values that the model converged to after starting from different arbitrary values. Assuming that TB does not affect age-structure and HIV incidence, the age-structure and HIV prevalence should stay roughly the same when introducing TB into the model. We modeled temporal trends in the HIV epidemic, by splitting time into a period before the HIV epidemic (1950-1984), a period with HIV and no ART (1950-1984) and a period when ART was available (2005 onwards). Except for the HIV-related parameters we used current parameters instead of trying to estimate all parameters for each of the periods separately.
Table 1.
Parameter description and values for all model parameters.
| Parameter description | Depended on | Stratification | Value | 95% CI | Source |
|---|---|---|---|---|---|
|
| |||||
| Progression from exposed state to active TB (rate per year) | time since infection | Age <= 5 | 0.24 | NA | Smith et al (Smith, 2001) |
| 5 < age <= 10 | 0.02 | NA | |||
| 10 < age | Figure S4 | Ferebee et al (Ferebee, 1970) | |||
|
| |||||
| Rate ratios for progression to active TB | HIV-positive (vs HIV-negative) ART (vs. no ART) Reinfection with additional strain (HIV-negative only) Reinfection after treatment | 6 | 3.5-8 | Corbett et al (Corbett et al., 2003) | |
| 0.50 | 0.37-0.72 | Gupta et al (Gupta et al., 2012) | |||
| 0.21 | 0.14-0.3 | Andrews et al (Andrews et al., 2012) | |||
| 2 | 2-3 | Wood et al (Wood et al., 2011a) and den Boon (den Boon et al., 2007) | |||
|
| |||||
| TB treatment rate per year | HIV-, smear+ | 1.52 | NA | Wood et al (Wood et al., 2007) | |
| HIV-, smear- | 0.15 | NA | |||
| HIV+, smear+ | 0.47 | NA | |||
| HIV+, smear- | 0.23 | NA | |||
|
| |||||
| Spontaneous recovery rate per year | HIV- | 0.25 | NA | Bacaer et al (Bacaer et al., 2008) from Corbett et al (Corbett et al., 2003) and Murray et al (Murray et al., 1990) | |
|
| |||||
| TB treatment outcomes | Age (in 5-year groups), HIV status | First treatment vs. retreatment | Table S2/S3, Figure S6 | Data analysis from Cape Town metropolitan electronic TB register | |
|
| |||||
| Proportion of smear+ | age | HIV-negative HIV-positive |
Table S1 | Wood et al (Wood et al., 2011a) | |
|
| |||||
| HIV incidence | age | Time period (pre-HIV, HIV and ART available) | Table S1 | ASSA model (ASSA, 2008) | |
| ART initiation (for time period where ART was available) | No TB treatment | 0.13 | 0.08-0.29 | Wandel et al (Wandel et al., 2008) | |
| TB treatment | 1.27 | 1.24-1.30 | Kaplan et al (Kaplan et al., 2014) | ||
|
| |||||
| All-cause mortality rate (non-HIV, non-TB) | age | Table S1 | ASSA model (ASSA, 2008) | ||
|
| |||||
| Mortality rate ratio due to untreated active TB | 17.1 | 16.8-17.3 | Drolet et al (Drolet, Godias J and Lowell Anthony M, 1952) | ||
|
| |||||
| Mortality rate ratio for HIV-related mortality | age | Table S1 | Sewankambo et al (Sewankambo et al., 2000) | ||
|
| |||||
| Mortality rate ratio for mortality on ART | age | Table S1 | Johnson et al (Johnson et al., 2013) | ||
|
| |||||
| Transmission rate per year | 10 | 6-15 | Fit to TB notification rates | ||
|
| |||||
| Age-specific mixing | age | Figure S3 | Wood et al (Wood et al., 2012) reanalysis | ||
|
| |||||
| Case detection proportion | age > 16 | 73% | WHO (WHO, 2014b) Dodd et al (Dodd et al., 2014) | ||
| age <= 15 | 35% | ||||
Model update
We modeled a population with a constant size of 10,000 individuals. The population was updated at monthly time-steps as individuals aged and progressed through disease stages. At each time-step, we determined the Mtb transmission potential by 5-year age group. We counted the number of people with active TB and multiplied this by the proportion of smear positive TB cases in an age-group. We calculated age-specific TB incidence rates (in 5-year age-groups) from the age-specific transmission potential, using the age-specific mixing patterns observed in the semi-urban community. All individuals without active TB or TB treatment were considered susceptible to new TB infection. For newly infected individuals, time of death and all other possible event times were simulated again, using the disease progression model gems (Blaser et al., 2015).
Model output
The main model outcomes were age-stratified notifications of active TB disease for HIV-negative and HIV-positive individuals. We assumed that 73% of incident TB cases were notified in adults (WHO, 2014b) and 35% in children (Dodd et al., 2014). At each time-step, we recorded the number of individuals in each disease stage stratified by 5-year age groups. Using these data, we plotted annual TB incidence and prevalence rates to determine convergence from the initial values. After convergence, we plotted the average and 95% confidence interval of active TB notifications by 5-year age group. We also compared demographic outputs such as HIV prevalence, ART coverage and age distribution to the observed demographics.
Sensitivity analyses
We conducted sensitivity analyses for all parameters related to the age-structure to investigate the main drivers of the age-pattern. We varied age-specific mixing patterns, and age-specific HIV incidence rates. We used extreme parameter values (random mixing, only modeling the HIV-negative population) to determine their influence on the model output. We also did a sensitivity analysis of TB progression rates in retreatment TB cases assuming that TB progression was the same in cases reinfected after prior treatment and in primary cases. We conducted an analysis assuming that latent Mtb infection did not have any protective effect on the progression of future infections. We also looked at model outcomes before HIV was introduced into the population and before ART was introduced into the population to determine the impact of HIV and ART on the age structure of TB. We conducted further sensitivity analyses assuming a lower case detection rate in older individuals and assuming that previously treated patients had a higher mortality risk.
Results
Data analyses and model parameters
Of 103,304 new cases of tuberculosis notified between 2003 and 2012, 4.3% died (case fatality rate), 9.4% failed or stopped treatment (default), and 86.3% completed treatment or were cured. Of 41,132 retreatment TB cases, 7.3% died, 19.4% failed treatment and 73.3% completed treatment or were cured. The case fatality rate increased with age for both new and retreatment TB. Treatment outcomes are shown separately for each age group and HIV status in Table S2 for primary disease and in Table S3 for retreatment TB.
Figure S3 shows the age-specific mixing patterns that we observed in a semi urban settlement and used in the model. We observed some age-assortative mixing, and a noticeable decline in overall indoor contact time spent among older individuals. The 35-40 year olds mixed the most, both with each other and with children aged between 0 and 14 years.
Model fit
The modeled notification rates of active TB reproduced the shape of the observed age pattern in TB notification rates in 2009, with a drop in TB notification rates around the age of 5 years, a subsequent peak among 20-25 year old adults and an increase in notification rates among 30 to 50 year old adults. Figure 2 (upper panel) shows simulated TB notification rates by age group and the TB notification rates observed in the HIV-negative population of Cape Town. Some differences were apparent. A second peak occurred at age 45-50 years in the observed TB notification rates. In the simulated TB notification rates, the increase in TB notification rates continued. In the HIV-positive population, neither the notification data nor the simulations indicated that TB incidence varied by age (Figure 2 lower panel).
Figure 2. Age-stratified graph, including uncertainty.
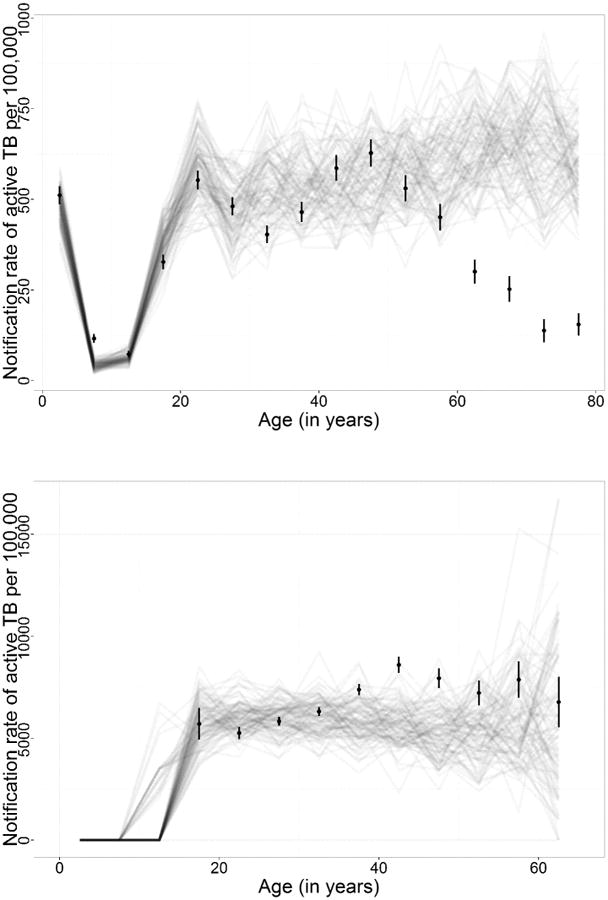
The upper panel shows TB notification rates in the HIV-negative population and the lower panel shows TB notification rates in the HIV-positive population. The dots and confidence intervals represent the data reported in Wood et al (Wood et al., 2011a) and the gray lines represent one model run each.
We estimated the overall Mtb transmission rate to be 10 (6-15) per year. The simulation converged to a stable equilibrium within 10 years after model initialization. The notification rates converge to 1,080 per 100,000 person-years within the first few years. It took about 10 years for the prevalence of active TB to converged to 2,210 per 100,000 persons. Figure S5 shows the convergence of prevalence and incidence after simulation start. HIV prevalence at the end of simulation was 11.6% with an ART coverage of 62%, which compared to estimates in the Western Cape is a higher HIV prevalence than 5.1% (ASSA, 2008) and consistent with ART coverage of 63%. (Hermans et al., 2015a). The age pyramid at the end of simulation was very similar to the 2014 Cape Town age pyramid, with the exception of a higher proportion of children aged 5-20 years (Figure S9).
Sensitivity analyses
Figure 3 shows the results of the sensitivity analyses. The age-specific TB notification rates in HIV-negative individuals for each of the sensitivity analyses are shown. The lines represent the median results and the shaded areas contain 95% of simulations. In Figure 3a we show the base analysis for comparison. In the scenario without protective effect (Figure 3b), we assumed that latent infection with a second strain of Mtb did not provide any protective effect on progression to active disease. In the analysis assuming no faster progression after treatment (Figure 3c), we assumed that TB progression after treatment was exactly the same as the progression for the first TB episode. In Figures 3d-3f we show the sensitivity analyses that made little difference: the analysis assuming no HIV incidence and the analysis assuming random mixing. Figures 3g and 3h show the sensitivity analyses that attempt to explain the decline in TB notification rates in the 50 to 80 year old population.
Figure 3. Sensitivity analyses.
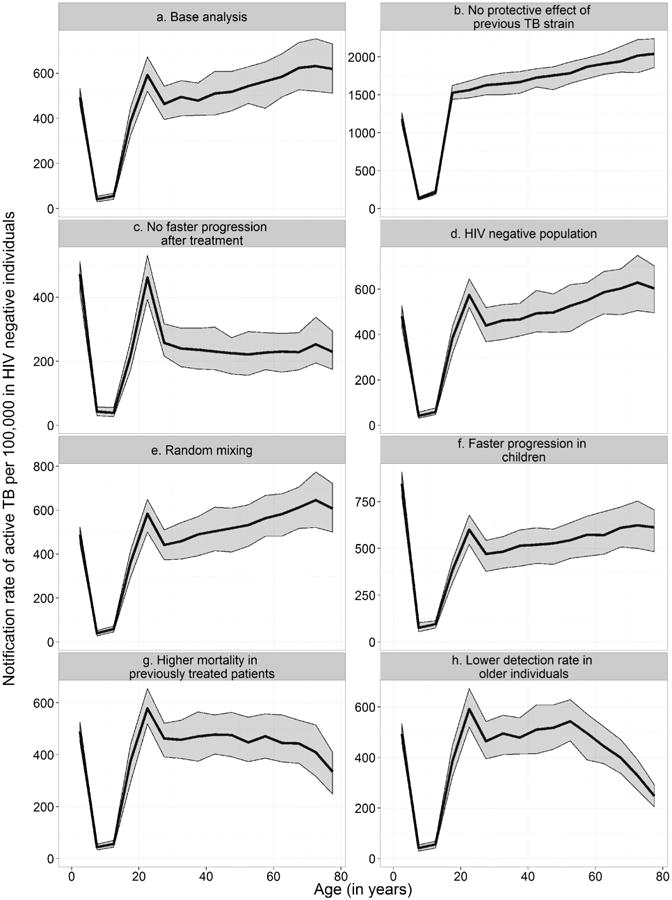
The age-specific TB notification rates in HIV-negative individuals for each of the sensitivity analyses are shown. The lines represent the median results and the shaded areas contain 95% of simulations. Panel a shows the base analysis, Panel b shows the analysis without a protective effect of previous TB strains and Panel c shows the results without faster progression after treatment. Panel d shows the analysis in an entirely HIV-negative population. Panel e shows the analysis where we assumed random mixing. Panel f shows the analysis where we assumed children progressed to active TB twice as fast. Panel g shows the analysis where we assumed a higher mortality in previously treated patients and panel h shows the simulation results assuming lower case detection rate in older individuals. Note that the scales on the y-axis differ.
The model was most sensitive to varying assumptions on the protective effect of previous latent Mtb strains and to retreatment TB progression rates. The assumption of a protective effect had a large influence on the level of the TB notification rates (Figure 3b). When assuming that re-infection with another strain of Mtb while being latently infected did not provide any protective effect on progression to active disease, the overall TB notification rate was much higher (1628 per 100,000 person-years) than in the base analysis (451 per 100,000 person-years). Assuming that retreatment TB progression rates were the same as progression rates for primary TB resulted in the TB notification rates in adults decreasing by 35.3%. This increase was more pronounced (46.6%) among individuals over 50 (Figure 3b). The decline in TB notification rates after early adulthood was not seen in the analysis without the protective effect of a previous latent Mtb infection (Figure 3b). The last peak in adults was absent in the scenario without faster progression to active TB in previously treated patients (Figure 3c). HIV incidence had little effect on the age-structure of TB notification rates in the HIV-negative population (Figures 3d). Overall TB notification rates increased rapidly from less than 500 notifications per 100,000 person years to around 900 notifications per 100,000 person years when HIV was introduced in the model (Figure S7). They decreased slightly to about 700 notifications per 100,000 person years after the introduction of ART. But TB notification rates in HIV negative individuals of all age groups remained stable throughout the three eras (Figure S8). When we assumed random mixing instead of age-assortative mixing, TB notification was essentially unchanged (Figure 3e). If we assumed faster progression rates in children was twice as fast as in our base assumptions, TB notifications in children increased, but the age pattern in adults remained the same.
To explain the lack of fit in 50-80 year old adults we performed additional sensitivity analyses. Having a higher mortality in previously treated patients led to a decline of TB notification rates in older individuals, but it also resulted in a less sharp increase in 30-50 year old adults (Figure 3g). A lower detection rate in older individuals seems to fit the observed age pattern well (Figure 3h).
Discussion
We used a mathematical simulation model to examine the drivers underlying the age-patterns in TB notification rates observed in Cape Town. We replicated the age-structure of TB notifications in Cape Town up to 50 years of age in our model. We found that the first peak in children is likely due to high transmission and rapid progression in children. The second peak is probably explained by relatively rapid progression in early adulthood whereas the increase between 30 and 50 year olds was explained by disease in previously treated patients. Our sensitivity analyses showed that age-specific TB notification rates mostly depended on the protective effect of previous latent infection and the fast progression in previously treated patients. When we assumed no protective effect of previous latent infections, the decline in TB notification rates around the age of 25 years disappeared and TB notification rates remained high in adulthood. This suggests that the decline after the secondary peak in TB notification rates is partially due to a protective effect of latent Mtb infection on progression to active TB upon infection with a second strain. The last increase in TB notification rates disappeared in our sensitivity analysis assuming no faster progression to TB disease in previously treated patients, which suggests that this faster progression determines the increased rates in 45-50 year olds. The sensitivity analyses also suggest that the age-pattern observed in the HIV-negative population is not affected by age-specific social mixing patterns or by HIV-associated TB. Mtb transmission rates also did not affect TB notification rates as much as might have been expected, perhaps because exposure is saturated and most of those exposed to Mtb never develop active TB; additional exposure may not affect TB incidence very much.
In 50-80 year old adults, the model did not reproduce the decline in notification rates observed in the data. This led us to explore additional factors as a possible explanation for the lack of fit. A higher mortality among previously treated patients improved the fit, but a reduced case detection rate in older individuals seemed to fit the observed age pattern the best. However this lower detection incorporated in the model was chosen to fit the age pattern above 50 years. Further research is needed to further elucidate the causes of the declining notification rates at older ages.
Our study has several strengths. We included detailed, time-dependent disease progression rates observed in Cape Town. All parameter values except for the overall transmission rate were extraneously set and parameter uncertainty was included wherever possible. We also conducted detailed sensitivity analyses. There are also several limitations to our model. As outlined above, the model did not fit the age-structured TB notification rates in HIV-negatives perfectly. Since all parameters were set exogenously, we believe that the model fit was good without over-fitting the data. The model did not fit the data well for HIV-positives below the age of 15 and above the age of 40, probably because we did not include mother-to-child transmission of HIV and because of the uncertainty in the denominators in the data. The age distribution of the simulated population differed slightly from the observed data in Cape Town in children and adolescents (Figure S9). We also assumed that HIV incidence was independent of TB status (susceptible, latent infection, disease, recovered), even though van Schalkwyk et al (van Schalkwyk et al., 2014) suggest otherwise. We used average HIV incidence rates for the pre-ART and ART era, which may have affected TB notification rates in the HIV-positive population. We did not use historical parameters to model the pre-HIV era TB epidemic. We feel that that would have added a lot of uncertainty about the model parameters to our study while adding little information. The most important changes in TB incidence during the last 30 years were due to HIV and the introduction of ART. Since our focus was the age structure in an HIV-negative population, which remained similar during the last 10 years and was insensitive to HIV, we do not believe that this is a major limitation. Nevertheless, the rates in the pre-HIV and HIV eras we estimated are very similar to historical rates from this setting (Hermans et al, 2015b). A further limitation is that we did not to include CD4 cell count of HIV-positive patients in the model. This could have impacted the estimates in the HIV-positive population. Since the focus of this study was on HIV-negative individuals and the HIV infected population did not substantially influence the age structure of TB in HIV-negative people, we think adding CD4 would have added unnecessary complexity to the model. We assumed that all patients who cleared active TB naturally or after were treated successfully became susceptible and uninfected. Some accuracy may have been lost when we divided the model into discrete time steps. Our age-mixing matrix may have underestimated the contacts of elderly people, because we only used indoor contacts in schools, work-places, and transport. But this is unlikely to significantly affect our results, since the sensitivity analysis showed that random mixing led to the same age-pattern.
In contrast to the many HIV models in which age-specific prevalence and incidence were used extensively to validate models (Bershteyn et al., 2013; Cambiano et al., 2013), we believe ours is the first mathematical model of Mtb transmission to focus on age-specific TB notifications in sub-Saharan Africa. Although a number of previous TB models were stratified by age (Dye et al., 1998; Schulzer et al., 1994; Stover et al., 2010), they did not compare modeled age-specific TB notifications to available data. For most of them, age-stratification was mainly intended to capture age-specific HIV prevalence. Dodd et al (Dodd et al., 2014) used a static population model and a household model to estimate the incidence of pediatric TB based on adult notification rates. Vynnycky and Fine (Vynnycky and Fine, 1997) explicitly incorporated age-structure in a TB transmission model, and found that age and reinfections were major factors in the spread of TB in England and Wales, where HIV is not a primary driver of TB. Our results extend their findings to settings with endemic HIV. Our estimate of the overall Mtb transmission rate of 10.0 per year was similar to the estimate of Vynnycky and Fine (Vynnycky and Fine, 1999) for 1950 England. It is also consistent with Bacaer et al (Bacaer et al., 2008) who estimated a Mtb transmission rate of 11.4 per year in Cape Town.
Our results have important implications for TB prevention and treatment programs. Our model results suggest that TB interventions, such as DOTS, TB screening, IPT, and ART in HIV-infected TB patients should take age into account. IPT and case finding may be particularly important in young adults considering the rapid progression rates in early adulthood. Similarly, new prevention programs could target case finding at previously treated patients aged 40-50 years. Focusing interventions on the most at-risk age groups may increase their effectiveness (Lawn et al., 2006). More research is needed to determine the implications of the age-dependent progression rates on TB control.
In conclusion, by including age strata, we were able to replicate the age-structure of TB incidence in Cape Town up to 50 years of age. More research is needed to determine the causes for declining TB notification rates with increasing age. The protective effect of a first latent infection on subsequent infections and the faster progression to disease among previously treated patients are the key factors in explaining the age-structure of TB incidence in Cape Town.
Supplementary Material
Highlights.
We modeled the age pattern of the TB transmission dynamics of Cape Town.
We replicated the age pattern in HIV-negative TB notification rates in 2009.
TB declined after age 25 because of a protective effect of previous infection.
The increase in TB in 30-50 year old HIV-negatives was due to retreatment TB.
Acknowledgments
We thank Kali Tal for her editorial work. We also wish to thank the City of Cape Town for providing us with the Electronic TB Register data.
Funding: This work was supported by the National Institute of Allergy and Infectious Diseases [5U01-AI069924–05, R01AI058736 and R01AI093269], the Swiss National Science Foundation [PDFMP3_137106 to NB, PBSKP3_145774 to SH and 32333B_150934 to OK], the European Union [Marie Curie International Outgoing Fellowship for Career Development PIOF-GA-2012-332311 to SH], the Bill & Melinda Gates Foundation [OPP1116641 to RW] and the South African Research Council [MRC-RFA-UFSP-01-2013/CCAMP to CM and RW].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews JR, Noubary F, Walensky RP, Cerda R, Losina E, Horsburgh CR. Risk of progression to active tuberculosis following reinfection with Mycobacterium tuberculosis. Clin Infect Dis. 2012;54:784–91. doi: 10.1093/cid/cir951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio JP, Castillo-Chavez C. Mathematical modelling of tuberculosis epidemics. Math Biosci Eng. 2009;6:209–37. doi: 10.3934/mbe.2009.6.209. [DOI] [PubMed] [Google Scholar]
- ASSA. ASSA AIDS and Demographic model. Actuarial Society of South Africa; 2008. [Google Scholar]
- Bacaer N, Ouifki R, Pretorius C, Wood R, Williams B. Modeling the joint epidemics of TB and HIV in a South African township. J Math Biol. 2008;57:557–93. doi: 10.1007/s00285-008-0177-z. [DOI] [PubMed] [Google Scholar]
- Bershteyn A, Klein DJ, Eckhoff PA. Age-dependent partnering and the HIV transmission chain: a microsimulation analysis. J R Soc Interface R Soc. 2013;10:20130613. doi: 10.1098/rsif.2013.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhunu CP, Garira W, Mukandavire Z. Modeling HIV/AIDS and tuberculosis coinfection. Bull Math Biol. 2009;71:1745–80. doi: 10.1007/s11538-009-9423-9. [DOI] [PubMed] [Google Scholar]
- Blaser N, Vizcaya LS, Estill J, Zahnd C, Kalesan B, Egger M, Keiser O, Gsponer T. gems: An R Package for Simulating from Disease Progression Models. J Stat Softw. 2015;64:1–22. [PMC free article] [PubMed] [Google Scholar]
- Blower SM, McLean AR, Porco TC, Small PM, Hopewell PC, Sanchez MA, Moss AR. The intrinsic transmission dynamics of tuberculosis epidemics. Nat Med. 1995;1:815–21. doi: 10.1038/nm0895-815. [DOI] [PubMed] [Google Scholar]
- Cambiano V, Bertagnolio S, Jordan MR, Lundgren JD, Phillips A. Transmission of Drug Resistant HIV and Its Potential Impact on Mortality and Treatment Outcomes in Resource-Limited Settings. J Infect Dis. 2013;207:S57–S62. doi: 10.1093/infdis/jit111. [DOI] [PubMed] [Google Scholar]
- Castillo-Chavez C, Feng Z. To treat or not to treat: the case of tuberculosis. J Math Biol. 1997;35:629–56. doi: 10.1007/s002850050069. [DOI] [PubMed] [Google Scholar]
- Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–21. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- De Cock KM, Chaisson RE. Will DOTS do it? A reappraisal of tuberculosis control in countries with high rates of HIV infection. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 1999;3:457–465. [PubMed] [Google Scholar]
- Den Boon S, van Lill SWP, Borgdorff MW, Enarson DA, Verver S, Bateman ED, Irusen E, Lombard CJ, White NW, de Villiers C, Beyers N. High Prevalence of Tuberculosis in Previously Treated Patients, Cape Town, South Africa. Emerg Infect Dis. 2007;13:1189–1194. doi: 10.3201/eid1308.051327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health, R. of S.A. South African National Tuberculosis Management Guidelines 2009 2009 [Google Scholar]
- Dodd PJ, Gardiner E, Coghlan R, Seddon JA. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modelling study. Lancet Glob Health. 2014;2:e453–459. doi: 10.1016/S2214-109X(14)70245-1. [DOI] [PubMed] [Google Scholar]
- Dodd PJ, Knight GM, Lawn SD, Corbett EL, White RG. Predicting the Long-Term Impact of Antiretroviral Therapy Scale-Up on Population Incidence of Tuberculosis. PLoS One. 2013;8:e75466. doi: 10.1371/journal.pone.0075466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolet Godias J, Lowell Anthony M. N Y Tuberc Health Assoc. 1952. A half century's progress against Tuberculosis in New York City 1900-1950. [Google Scholar]
- Dye C, Garnett GP, Sleeman K, Williams BG. Prospects for worldwide tuberculosis control under the WHO DOTS strategy. Directly observed short-course therapy. Lancet. 1998;352:1886–91. doi: 10.1016/s0140-6736(98)03199-7. [DOI] [PubMed] [Google Scholar]
- Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis. A general review. Bibl Tuberc. 1970;26:28–106. [PubMed] [Google Scholar]
- Gupta A, Wood R, Kaplan R, Bekker LG, Lawn SD. Tuberculosis incidence rates during 8 years of follow-up of an antiretroviral treatment cohort in South Africa: comparison with rates in the community. PLoS One. 2012;7:e34156. doi: 10.1371/journal.pone.0034156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans SM, Boulle A, Caldwell J, Pienaar D, Wood RW. Temporal trends in TB notification rates during ART scale-up in Cape Town: an ecological analysis. J Int AIDS Soc. 2015a;18(1):20240. doi: 10.7448/IAS.18.1.20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans SM, Horsburgh CR, Wood R. A Century of Tuberculosis Epidemiology in the Northern and Southern Hemisphere: The Differential Impact of Control Interventions. PLoS One. 2015b;10:e0135179. doi: 10.1371/journal.pone.0135179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson RI, Mercer GN, Lokuge KM. A metapopulation model of tuberculosis transmission with a case study from high to low burden areas. PLoS One. 2012;7:e34411. doi: 10.1371/journal.pone.0034411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben RMGJ, Sumner T, Grant AD, White RG. Ability of preventive therapy to cure latent Mycobacterium tuberculosis infection in HIV-infected individuals in high-burden settings. Proc Natl Acad Sci U S A. 2014;111:5325–5330. doi: 10.1073/pnas.1317660111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LF, Mossong J, Dorrington RE, Schomaker M, Hoffmann CJ, Keiser O, Fox MP, Wood R, Prozesky H, Giddy J, Garone DB, Cornell M, Egger M, Boulle A International Epidemiologic Databases to Evaluate AIDS Southern Africa Collaboration. Life expectancies of South African adults starting antiretroviral treatment: collaborative analysis of cohort studies. PLoS Med. 2013;10:e1001418. doi: 10.1371/journal.pmed.1001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone-Robertson SP, Mark D, Morrow C, Middelkoop K, Chiswell M, Aquino LDH, Bekker LG, Wood R. Social mixing patterns within a South African township community: implications for respiratory disease transmission and control. Am J Epidemiol. 2011;174:1246–1255. doi: 10.1093/aje/kwr251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan R, Caldwell J, Middelkoop K, Bekker LG, Wood R. Impact of ART on TB case fatality stratified by CD4 count for HIV-positive TB patients in Cape Town, South Africa (2009-2011) J Acquir Immune Defic Syndr. 2014;66:487–494. doi: 10.1097/QAI.0000000000000201. 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn SD, Bekker LG, Middelkoop K, Myer L, Wood R. Impact of HIV infection on the epidemiology of tuberculosis in a peri-urban community in South Africa: the need for age-specific interventions. Clin Infect Dis. 2006;42:1040–7. doi: 10.1086/501018. [DOI] [PubMed] [Google Scholar]
- Lawn SD, Zumla AI. Tuberculosis. Lancet. 2011;378:57–72. doi: 10.1016/S0140-6736(10)62173-3. [DOI] [PubMed] [Google Scholar]
- Marx FM, Dunbar R, Enarson DA, Williams BG, Warren RM, van der Spuy GD, van Helden PD, Beyers N. The temporal dynamics of relapse and reinfection tuberculosis after successful treatment: a retrospective cohort study. Clin Infect Dis Off Publ Infect Dis Soc Am. 2014;58:1676–1683. doi: 10.1093/cid/ciu186. [DOI] [PubMed] [Google Scholar]
- McBryde ES, Denholm JT. Risk of active tuberculosis in immigrants: effects of age, region of origin and time since arrival in a low-exposure setting. Med J Aust. 2012;197:458–461. doi: 10.5694/mja12.10035. [DOI] [PubMed] [Google Scholar]
- Mills HL, Cohen T, Colijn C. Modelling the performance of isoniazid preventive therapy for reducing tuberculosis in HIV endemic settings: the effects of network structure. J R Soc Interface. 2011;8:1510–20. doi: 10.1098/rsif.2011.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJ, Styblo K, Rouillon A. Tuberculosis in developing countries: burden, intervention and cost. Bull Int Union Tuberc Lung Dis. 1990;65:6–24. [PubMed] [Google Scholar]
- Osler M, Hilderbrand K, Hennessey C, Arendse J, Goemaere E, Ford N, et al. A three-tier framework for monitoring antiretroviral therapy in high HIV burden settings. J Int AIDS Soc. 2014;17:18908. doi: 10.7448/IAS.17.1.18908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcaglar C, Shabbeer A, Vandenberg SL, Yener B, Bennett KP. Epidemiological models of Mycobacterium tuberculosis complex infections. Math Biosci. 2012;236:77–96. doi: 10.1016/j.mbs.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretorius C, Menzies NA, Chindelevitch L, Cohen T, Cori A, Eaton JW, Fraser C, Gopalappa C, Hallett TB, Salomon JA, Stover J, White RG, Dodd PJ. The potential effects of changing HIV treatment policy on tuberculosis outcomes in South Africa: results from three tuberculosis-HIV transmission models. AIDS Lond Engl. 2014;28(Suppl 1):S25–34. doi: 10.1097/QAD.0000000000000085. [DOI] [PubMed] [Google Scholar]
- Rodrigues P, Gomes MG, Rebelo C. Drug resistance in tuberculosis–a reinfection model. Theor Popul Biol. 2007;71:196–212. doi: 10.1016/j.tpb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Roeger LI, Feng Z, Castillo-Chavez C. Modeling TB and HIV co-infections. Math Biosci Eng. 2009;6:815–37. doi: 10.3934/mbe.2009.6.815. [DOI] [PubMed] [Google Scholar]
- Schulzer M, Radhamani MP, Grzybowski S, Mak E, Fitzgerald JM. A mathematical model for the prediction of the impact of HIV infection on tuberculosis. Int J Epidemiol. 1994;23:400–407. doi: 10.1093/ije/23.2.400. [DOI] [PubMed] [Google Scholar]
- Sewankambo NK, Gray RH, Ahmad S, Serwadda D, Wabwire-Mangen F, Nalugoda F, Kiwanuka N, Lutalo T, Kigozi G, Li C, Meehan MP, Brahmbatt H, Wawer MJ. Mortality associated with HIV infection in rural Rakai District, Uganda. AIDS. 2000;14:2391–400. doi: 10.1097/00002030-200010200-00021. [DOI] [PubMed] [Google Scholar]
- Smith KC. Tuberculosis in children. Curr Probl Pediatr. 2001;31:1–30. doi: 10.1016/s1538-5442(01)70040-5. [DOI] [PubMed] [Google Scholar]
- Statistics South Africa. Mortality and causes of death in South Africa, 2012: Findings from death notification 2014a [Google Scholar]
- [accessed 10.23.14];Statistics South Africa [WWW Document] 2014b URL http://beta2.statssa.gov.za/
- Stover J, McKinnon R, Winfrey B. Spectrum: a model platform for linking maternal and child survival interventions with AIDS, family planning and demographic projections. Int J Epidemiol. 2010;39(Suppl 1):i7–10. doi: 10.1093/ije/dyq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styblo K. The impact of HIV infection on the global epidemiology of tuberculosis. Bull Int Union Tuberc Lung Dis. 1991;66:27–32. [PubMed] [Google Scholar]
- Van Schalkwyk C, Variava E, Shapiro AE, Rakgokong M, Masonoke K, Lebina L, Welte A, Martinson N. Incidence of TB and HIV in Prospectively Followed Household Contacts of TB Index Patients in South Africa. PloS One. 2014;9:e95372. doi: 10.1371/journal.pone.0095372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vynnycky E, Fine PE. Interpreting the decline in tuberculosis: the role of secular trends in effective contact. Int J Epidemiol. 1999;28:327–334. doi: 10.1093/ije/28.2.327. [DOI] [PubMed] [Google Scholar]
- Vynnycky E, Fine PE. The natural history of tuberculosis: the implications of age-dependent risks of disease and the role of reinfection. Epidemiol Infect. 1997;119:183–201. doi: 10.1017/s0950268897007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandel S, Egger M, Rangsin R, Nelson KE, Costello C, Lewden C, Lutalo T, Ndyanabo A, Todd J, Van der Paal L, Minga A, Zwahlen M. Duration from seroconversion to eligibility for antiretroviral therapy and from ART eligibility to death in adult HIV-infected patients from low and middle-income countries: collaborative analysis of prospective studies. Sex Transm Infect. 2008;84(Suppl 1):i31–i36. doi: 10.1136/sti.2008.029793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Tuberculosis country profile South Africa [WWW Document] WHO; 2014a. [accessed 10.8.14]. URL http://www.who.int/tb/country/data/profiles/en/ [Google Scholar]
- WHO. Global tuberculosis report 2014. World Health Organization; 2014b. [Google Scholar]
- WHO. Global tuberculosis report 2013. World Health Organisation (WHO); 2013. [Google Scholar]
- WHO. Interim policy on collaborative TB/HIV activities. World Health Organization; Geneva, Switzerland: 2004. [Google Scholar]
- WHO. WHO fact sheet on tuberculosis. 1996 No. No. 104. [Google Scholar]
- WHO. Global tuberculosis report 2013. WHO; [accessed 6.30.14]. [WWW Document], n.d. URL http://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- Wiker HG, Mustafa T, Bjune GA, Harboe M. Evidence for waning of latency in a cohort study of tuberculosis. BMC Infect Dis. 2010;10:37. doi: 10.1186/1471-2334-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BG, Granich R, De Cock KM, Glaziou P, Sharma A, Dye C. Antiretroviral therapy for tuberculosis control in nine African countries. Proc Natl Acad Sci U A. 2010;107:19485–9. doi: 10.1073/pnas.1005660107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R, Lawn SD, Caldwell J, Kaplan R, Middelkoop K, Bekker LG. Burden of new and recurrent tuberculosis in a major South African city stratified by age and HIV-status. PLoS One. 2011a;6:e25098. doi: 10.1371/journal.pone.0025098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R, Lawn SD, Johnstone-Robertson S, Bekker LG. Tuberculosis control has failed in South Africa–time to reappraise strategy. Afr Med J. 2011b;101:111–4. doi: 10.7196/samj.4587. [DOI] [PubMed] [Google Scholar]
- Wood R, Middelkoop K, Myer L, Grant AD, Whitelaw A, Lawn SD, Kaplan G, Huebner R, McIntyre J, Bekker LG. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med. 2007;175:87–93. doi: 10.1164/rccm.200606-759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R, Racow K, Bekker LG, Morrow C, Middelkoop K, Mark D, Lawn SD. Indoor social networks in a South African township: potential contribution of location to tuberculosis transmission. PLoS One. 2012;7:e39246. doi: 10.1371/journal.pone.0039246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


