Abstract
Respiratory motor output after cervical spinal cord injury (cSCI) is profoundly influenced by spinal serotonin. We hypothesized that intraspinal transplantation of embryonic midline brainstem (MB) cells rich in serotonergic raphé neurons would improve respiratory outcomes after cSCI. One week after hemisection of the 2nd cervical segment (C2Hx) a suspension of either embryonic (E14) MB cells, fetal spinal cord cells (FSC), or media only (sham) was delivered to the dorsal C3 spinal cord of adult male rats. Six weeks later, ventilation was evaluated using plethysmography; phrenic nerve activity was evaluated in a subset of rats. Seven of 12 rats receiving MB-derived grafts had clear histological evidence of serotonin-positive neurons in the C3-4 dorsal white matter. The transplantations had no impact on baseline breathing patterns, but during a brief respiratory challenge (7% inspired CO2) rats with successful MB grafts had increased ventilation compared to rats with failed MB grafts, FSC or sham grafts. Recordings from the phrenic nerve ipsilateral to C2Hx also indicated increased output during respiratory challenge in rats with successful MB grafts. We conclude that intraspinal allografting of E14 MB cells can have a positive impact on respiratory motor recovery following high cSCI.
Introduction
Interruption of raphé-spinal tracts by spinal cord injury (SCI) disrupts serotonergic innervation of spinal motor circuits, and this is particularly important in the context of respiratory motor recovery. For example, the spontaneous recovery of phrenic motor output that occurs after cervical SCI (cSCI) correlates with return of spinal serotonin (5-HT) immunoreactivity (Golder and Mitchell, 2005), and is severely blunted by neurotoxic lesions of brainstem raphé nuclei (Golder et al., 2001). In addition, pharmacological treatments which increase 5-HT availability (Zhou and Goshgarian, 2000) or act as 5-HT agonists (Zimmer and Goshgarian, 2006) can substantially increase respiratory motor output following cervical SCI. Transplantation of serotonergic neurons can restore spinal 5-HT after SCI (Eaton et al., 2008; Feraboli-Lohnherr et al., 1997), but respiratory motor recovery has not been targeted with this approach. Slawinska and colleagues showed that intraspinal allografting of embryonic (E14) midline brainstem (MB) tissue containing the B1-3 raphé regions (Konig, 1989) produced robust 5-HT innervation of gray matter caudal to thoracic spinal transection and improved plantar stepping patterns (Slawinska et al., 2013). The profound significance of 5-HT to respiratory recovery after SCI, and past successes with serotonergic transplants targeting thoracic and lumbar motor systems (Feraboli-Lohnherr et al., 1997; Majczynski et al., 2005; Slawinska et al., 2013), led us to hypothesize that intraspinal (mid-cervical) transplantation of embryonic MB cells rich in serotonergic raphé neurons would improve respiratory outcomes after cSCI. A high cervical hemisection SCI model (C2Hx) was used because this lesion induces a persistent reduction in ipsilateral phrenic motor output, rapid-shallow breathing patterns, and reduced 5-HT innervation of the phrenic motor pool.
Materials and Methods
Adult, male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN, USA) were studied; all procedures on live animals were approved by the Institutional Animal Care and Use Committee at the University of Florida. Twenty-five rats received C2Hx injury, and respiratory outcomes were compared between groups receiving mid-cervical injections of cell suspension medium (sham, N=8), cell suspension of embryonic day 14 (E14) fetal spinal cord (FSC, N=5), and E14 MB neurons which included the midline medullary raphé nuclei (N=12). The intraspinal allograft or sham injections were performed one week following C2Hx. Additionally, two spinal intact rats (no prior surgery) were used for histological evaluation of the spinal cord. Embryonic donor tissue was obtained from pregnant female rats of the same strain. Prospective inclusion criteria for all rats included histologically verified anatomically complete hemisection lesions. For MB transplants, prospective criterion for a successful graft was immunohistological detection of 5-HT positive neurons in the immediate region of injection.
SCI and subsequent allografting
Rats were anesthetized with xylazine (10 mg/kg, s.q. Fort Dodge animal Health, IA, USA) and ketamine (140 mg/kg, i.p., Fort Dodge Animal Health, IA, USA), and C2Hx was induced as described previously (Fuller et al., 2008) (see Fig. 1A). For harvest of E14 donor tissue, pregnant rats were anesthetized as described above, embryos removed individually to ice cold Hanks Balanced Salt Solution (HBSS, Invitrogen, Grand Island, NY, USA) followed by dissection of the caudal rhombencephalon, extending from pontine flexure to the rostral cervical spinal cord. The harvest included the B1-3 raphé regions (Konig, 1989) Preliminary immunochemistry experiments confirmed that the midline medullary raphé nuclei were included in the harvest (Fig. 1B). After MB tissue was harvested, it was minced and dissociated by gentle pipetting in HBSS. Tissue was mixed with trypsin-EDTA (0.25% trypsin, 1.0M EDTA, Atlanta Biologicals, Lawrenceville, GA, USA) and incubated for 7-min in a 37°C water bath. Quenching of trypsin activity with fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA, USA) was followed by manual trituration through progressively smaller bore Pasteur pipettes. The suspension was centrifuged (80×g, 10-min), suspended in minimal essential culture medium (Invitrogen Corporation, Grand Island, NY, USA), and adjusted to a concentration of 50,000–75,000 cells/μl. For FSC transplants, fetal cords were isolated in HBSS and processed in the same manner as MB cells. At 1-wk post-C2Hx, anesthesia was induced as described above, the cervical spinal cord was surgically exposed, and 4–5 μl of cell suspension or minimal essential medium (sham) was injected 0.5–0.6 mm below the pial surface with a 31 gauge needle and Hamilton microsyringe (Hamilton Company, Reno, NV). The midline spinal cord was injected 1.0 mm from the caudal edge of the C2Hx lesion. The needle was slowly withdrawn 2-min following completion of the injection to minimize suspension reflux.
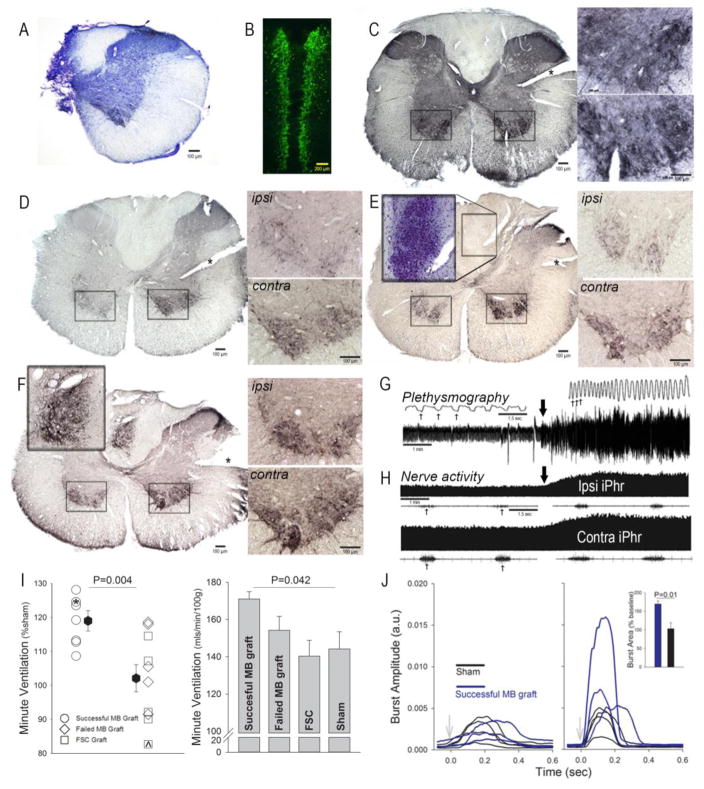
Figure 1.
A: Transverse histological section through C2 spinal cord following chronic C2Hx (cresyl violet stain). B: Coronal section of the E14 midline brainstem (MB) following incubation with 5-HT antibodies (fluorescent secondary antibody). The image shows the area extending from the pontine flexure (rostral) to the rostral cervical spinal cord (caudal). Panels C–F show transverse C3 spinal cord sections incubated with antibodies against 5-HT with a nickel-enhanced DAB reaction. In these panels, an * is placed over the “score” in the tissue denoting the side contralateral (contra) to C2Hx. The ipsilateral (ipsi) and contra ventral horns (boxed areas) are depicted at higher magnification to the right of whole spinal cord sections. C: Section from a spinal-intact rat; note the bilaterally uniform 5-HT immunostaining. D: Section from a sham treated C2Hx rat; note the marked reduction of 5-HT immunostaining in the ipsilateral cord. E: C3 section from a rat which received an E14 FSC cell suspension; the lesion from this animal is depicted in panel A. The inset panel from the midline dorsal spinal cord depicts cresyl violet stained neurons in the adjacent spinal cord serial section (40 μm cuts). The inset clearly shows the location of the FSC transplant. F: Section from C2Hx rat which received an E14 MB cell suspension. The inset panel from the midline dorsal spinal cord depicts the MB graft at a higher magnification. G: Representative airflow traces obtained using whole body plethysmography. Baseline data were recorded during quiet breathing, and this was followed by a brief hypercapnic challenge (7% inspired CO2; onset indicated by the thick arrow). The top traces show an expanded time scale so that single respiratory efforts can be clearly observed (example breaths indicated by the thin arrows). H: Representative recordings of phrenic nerve activity ipsilateral (Ipsi) and contralateral (Contra) to a C2Hx lesion. A compressed record of the integrated phrenic activity (iPhr) during baseline and hypercapnic challenge is shown in the top traces (onset indicated by the thick arrow). The bottom traces show an expanded time scale of the raw burst depicting a few individual inspiratory efforts (thin arrows). I: Minute ventilation during the respiratory challenge. In the first graph, data are expressed relative to breathing in the sham group (%sham). The individual data point marked by ^ is from the animal with histology depicted in Panel E; the individual data point marked by * was obtained from the animal with histology depicted in Panel F. The second graph is a histogram depicting minute ventilation expressed relative to body mass (mls/min100g) in animals with successful or failed MB grafts, and also FSC or sham transplant. One-way ANOVA indicated a difference in breathing across these groups, with successful MB grafts having the highest value. J: Waveform averages of integrated phrenic activity recorded ipsilateral to injury during baseline (left panel) and respiratory challenge (right panel). On average the increase in burst area was greater in rats with successful MB graft compared to sham treatment (inset panel). With the exception of Panel B, all data presented in Figure 1 were obtained at 6 weeks following the transplantation procedures (i.e., 7 weeks post-C2Hx injury).
Plethysmography and phrenic nerve recordings
Plethysmography (Buxco Inc., Wilmington, NC, USA) (Fuller et al., 2008) was used to measure breathing in unanesthetized rats six weeks following cell transplantation. Chamber pressure, temperature and humidity, and rectal temperature were used to calculate tidal volume (ml/breath) and minute ventilation (ml/minute). Baseline recordings were made for 60-min while the chamber was flushed with 21% O2 (balance N2). Subsequently, rats were given a 5-min hypercapnic challenge (7% CO2, 21% O2, balance N2). Baseline data were analyzed over a stable 10-min period immediately prior to the respiratory challenge. Hypercapnic challenge data were averaged over the final 2-min.
The impact of C2Hx on breathing patterns has been well-characterized in the rat model, and here we compared breathing patterns across the experimental groups, all of which had C2Hx. Data were expressed as absolute units (i.e., breaths*min−1), relative to body weight (e.g., ml/100g), or relative to values obtained in sham treated rats (%Sham). Statistical tests included one-way analyses of variance (ANOVA) within each breathing condition, or unpaired t-test. Comparisons of ages and weights were also made using one-way ANOVA.
Neurophysiological studies were conducted 1–2 days after plethysmography. After brief exposure to isoflurane (3–4% in O2) a urethane solution was administered (1.6g/kg in saline, i.p., Sigma, St. Louis, MO, USA). The trachea was cannulated to enable mechanical ventilation and the vagus nerves were sectioned in the mid-cervical region. Pancuronium bromide (2.5 mg/kg i.v.) was given to eliminate spontaneous breathing efforts. Arterial blood pressure (Statham P-10EZ pressure transducer and CP122 AC/DC strain gauge amplifier, Grass Instruments, Warwick, RI, USA) was measured via a femoral catheter. Arterial PO2 (PaO2) and PCO2 (PaCO2), as well as pH, were determined from ~0.2 ml arterial blood samples (i-Stat, Heska, Fort Collins, CO, USA). Rectal temperature was monitored using a thermistor and maintained at 37±1°C using servo-controlled heating pad (model TC-1000, CWE, Ardmore, PA, USA). The end-tidal CO2 partial pressure (PETCO2) was monitored using a mainstream analyzer positioned a few centimeters from the tracheostomy tube (Capnogard, Novametrix Medical Systems, Wallingford, CT, USA).
Extracellular phrenic nerve recordings were made using silver wire electrodes, amplified (1000×) and filtered (band pass 100–10,000 Hz) using a differential A/C amplifier (Model 1700, A-M Systems, Carlsborg, WA, USA). The amplified signal was full-wave rectified and moving averaged (time constant 100 ms; model MA-1000; CWE Inc., Ardmore, PA, USA). Data were digitized using a CED Power 1401 data acquisition interface and recorded using Spike2 software (Cambridge Electronic Design Limited, Cambridge, England). The end-tidal CO2 apneic threshold for inspiratory phrenic activity was determined by gradually increasing the ventilator rate until inspiratory bursting ceased in both phrenic nerves. The ensuing apnea was maintained for 2–3 min, and the ventilator rate was then gradually decreased until inspiratory activity reappeared. The PETCO2 was then maintained 2 mmHg above this value for a 20–30 min baseline period. Rats were then exposed to a 5-min bout of hypercapnia achieved by raising the inspired CO2 until the PETCO2 reached 70 mmHg.
Waveform averages of integrated phrenic signals were created from 2 min segments of data during both the baseline period and the hypercapnic challenge (e.g., an average of 120–150 inspiratory bursts during each period). The waveform averages were scaled identically and plotted on the same axes to enable direct comparison of the appearance of the phrenic burst across MB transplant and sham groups. The burst amplitude was calculated as the raw voltage (labeled as “arbitrary units” or a.u.). The total area of the integrated burst was measured (i.e., a.u.*sec−1), expressed relative to baseline values, and compared using an unpaired t-test.
Spinal cord histology and immunohistochemistry
Rats were anesthetized as described above and systemically perfused with saline followed by 4% paraformaldehyde (Sigma, St. Louis, MO, USA). The cervical spinal cord was removed and 40 μm transverse sections were made using a vibrotome. Prior to incubation with the primary antibody against 5-HT, sections were washed in PBS (0.1 M, pH 7.4, 3×5-min), blocked against endogenous peroxidase activity (30% methanol, 0.6% hydrogen peroxide in 0.1 M PBS, incubated for 20-min), rewashed in PBS, treated with sodium borohydride (1% in dH2O, incubated for 30-min) given a third and final wash with PBS and blocked against nonspecific protein labeling (3% goat serum in 0.1M PBS with 0.03% Triton-X). Sections were then incubated with a rabbit polyclonal antibody against 5-HT (1:20,000; Immunostar, Hudson, WI, USA) with 1% goat serum and 0.03% Triton-X overnight at 4°C. On the following day, tissue was washed in PBS with 0.3% Triton-X (0.1 M, 3×5-min), blocked against nonspecific protein labeling (3% goat serum in 0.1M PBS with 0.03% Triton-X, 30 minutes) and incubated for 30 minutes at room temperature in a biotinylated secondary antibody (goat anti-rabbit; 1:100; Vector Laboratories, Burlingame, CA, USA). This was followed by a second round of washes in PBS with 0.3% Triton-X (3×5-min), a second round of blocking against nonspecific protein labeling (3% goat serum in 0.1M PBS with 0.03% Triton-X, 30 minutes) and a 30-min incubation in rabbit peroxidase anti-peroxidase (1:400; Sigma, St. Louis, MO, USA). Sections were then given a third series of washes in PBS with 0.3% Triton-X, and antigen was visualized with nickel-enhanced diaminobenzidine (DAB with 1% nickel ammonium sulfate; Sigma, St. Louis, MO) in the presence of H2O2. Some tissue sections were counter-stained with Cresyl violet to enable visualization of neuronal cell bodies.
Results
5-HT immunostaining in the uninjured C3-4 spinal cord was bilaterally uniform (Fig. 1C) with dense positive staining in dorsal (laminae I–II), ventral (laminae VII–IX) and intermediate (lamina X) gray matter. Following C2Hx there was a marked laterality to the 5-HT immunostaining pattern with a considerable reduction ipsilateral to the lesion (see Fig. 1D for example from a sham transplant cord). The dissociated FSC grafts could be histologically identified as a cluster of intense neuronal staining in the dorsal spinal cord at the site of injection (Fig. 1E, inset panel depicts cresyl violet stained neurons). The cervical 5-HT immunostaining pattern remained highly lateralized in the FSC transplant group with considerably more positive staining contralateral to C2Hx (Fig. 1E). There was variability in the success of MB transplants, and histological evaluation revealed a distinct cluster of 5-HT immunopositive neurons in the dorsal C4 spinal cord in 7 of 12 rats (Fig. 1F, inset panel shows a MB graft). A similar pattern of positive 5-HT staining in the C3-4 dorsal spinal cord was never observed in uninjured, sham, or FSC transplant animals. In the remaining 5 MB transplant animals, no histological evidence of 5-HT staining could be detected in the proximity of the cell delivery site, and these were classified as failed transplants. After successful MB transplant, 5-HT immunostaining in the C4 spinal cord also showed a lateral pattern with increased staining contralateral to the lesion. Qualitatively, the successful MB group had an apparent increase in the density of 5-HT immunostaining within the intermediate gray matter and ventral horn (lamina X and VII) of the ipsilateral spinal cord (Fig. 1F).
Respiratory output was assessed using plethysmography in unanesthetized rats, and recordings of phrenic nerve activity in anesthetized rats. Fig. 1G depicts an example plethysmography experiment, and Fig. 1H shows an example of phrenic nerve recordings – in both cases note the increase in output during the hypercapnic challenge. The plethysmography recordings revealed no significant differences in breathing frequency, inspiratory tidal volume, or minute ventilation across the four experimental groups regardless of the approach to data quantification (e.g., breaths*min−1, mls*breath−1, %sham response, etc.). Thus, during baseline conditions (21% inspired O2), all rats adopted the rapid-shallow breathing pattern that typifies the C2Hx lesion (Fuller et al., 2008; Lee et al., 2014). However, during the respiratory challenge, rats with histologically verified MB transplant (e.g., Fig. 1F) had a more robust respiratory responses. Average minute ventilation during the respiratory challenge is presented in Fig. 1I, with the data expressed relative to the sham response and also relative to body mass. Both approaches to data analysis indicate that successful MB grafts were associated with an increased ability to ventilate during respiratory stimulation with CO2. This effect was due to an increase in the inspiratory tidal volume in the MB rats (P=0.05 vs. FSC and failed MB groups) versus altered breathing frequency (P=0.33, data not shown). Body weight was similar across experimental groups at the time of the plethysmography data collection (P=0.11; Sham: 384±9 g, FSC: 372±12 g, MB graft: 385±11 g, failed MB graft: 409±15 g).
Phrenic nerve recordings were obtained from a subset of animals, and were made during during a normoxic, normocapnic baseline period, and also during a brief hypercapnic respiratory challenge (Fig. IH). Waveform averaging methods were used to compare the appearance of inspiratory bursts between rats with histologically verified MB transplants (N=3) vs. sham transplant (N=4). The baseline PaO2 (mmHg) tended to be elevated in the MB (214±6) vs. sham group (195±8, P=0.09); PaCO2 was similar between groups (p=0.325; MB: 28±2; sham: 31±3). Baseline mean arterial pressure (mmHg) was not significantly different between groups (MB: 89±6, sham: 105±7; p=0.151). Assessment of the ipsilateral (to C2Hx) phrenic nerve recording data produced a conclusion that was similar to the plethysmography outcome: MB transplant rats could produce greater output during the respiratory challenge. Thus, at baseline (Fig. 1J, left panel), there was no apparent difference in phrenic inspiratory bursting between the two groups. During the hypercapnic challenge, however, 3 of 3 rats with MB transplant had burst amplitudes which exceeded values recorded in the sham group (Fig. 1J, right panel). In addition, quantitative evaluation of the challenge response showed significantly increased area of the inspiratory burst in the MB vs. sham transplant group (%baseline, P=0.01, Fig. 1J, inset).
Discussion
This is the first study to focus on respiratory-related outcomes following transplantation of embryonic MB tissue into the injured cervical spinal cord. The results are consistent with prior literature reports of MB grafting that have focused on serotonergic neurons (Feraboli-Lohnherr et al., 1997; Majczynski et al., 2005; Slawinska et al., 2013), but extend these observations by showing that MB-derived grafts can be successfully applied to the cervical cord following incomplete lesion, and have a functional impact on respiratory motor recovery. We did not determine if the observed motor changes were 5-HT dependent, obvious but multiple prior studies have confirmed that locomotor improvements after MB grafting require 5-HT release from transplanted MB tissue (Feraboli-Lohnherr et al., 1997; Majczynski et al., 2005) and activation of spinal 5HT2 and 5HT7 receptors (Slawinska et al., 2013). Both of these 5-HT receptor subtypes are expressed by spinal respiratory motoneurons, and their activation can trigger increases in phrenic motor activity (Dale et al., 2014). Prior authors have also advanced the hypothesis that embryonic MB raphé cells can innervate developmentally appropriate neuronal populations (e.g., spinal motoneurons and/or interneurons) after spinal transplantation (Privat et al., 1988), and our data are consistent with this concept.
Multiple studies have confirmed that the C2Hx lesion model leads to a persistent low-volume, high frequency ventilation pattern (i.e., rapid-shallow breathing), and a blunted ability to increase tidal volume during a respiratory challenge (reviewed in (Sandhu et al., 2009)). The baseline breathing patterns reported here are consistent with rapid-shallow breathing, and there was no discernable impact of the MB transplant. However, two separate outcome measures point towards the conclusion MB grafts can promote functional recovery during conditions of increased respiratory “drive” or demand. During a brief exposure to elevated inspired CO2 (i.e., hypercapnic respiratory challenge), ventilation was greater in the cohort of rats with histologically verified 5-HT immunopositive grafts. Recordings obtained from the phrenic nerve ipsilateral to the C2Hx lesion, albeit in a subset of rats, were consistent with the plethysmography-derived ventilation data. Indeed, during respiratory challenge, each of the rats with successful MB graft that were neurophysiologically evaluated had ipsilateral phrenic nerve burst output greater than all of the sham treated C2Hx rats, resulting in a highly significant statistical difference even with a relatively small sample size. One logical interpretation of the plethysmography and neurophysiological data is that the MB grafts created conditions in which cervical respiratory motoneurons could be more effectively recruited. In this regard, it is well established that the spinal “crossed-phrenic pathways” which enable post-C2Hx activation of ipsilateral phrenic motoneurons are highly sensitive to 5-HT (Ling et al., 1994; Zhou and Goshgarian, 1999), and indeed require 5-HT innervation to be fully activated (Golder et al., 2001). The mechanisms driving locomotor recovery after MB neuronal transplantation in animals with SCI are also serotonergic (Feraboli-Lohnherr et al., 1997; Majczynski et al., 2005; Slawinska et al., 2013), and thus we hypothesize that the functional respiratory recovery observed following MB transplant occurred via 5-HT related mechanisms.
Previous findings from our group (Lee et al., 2014) merit discussion in light of the current results. The Lee et al. study indicated a functional benefit following mid-cervical en bloc FSC tissue transplantation in rats with acute C2Hx lesions. The primary intent of that study was to determine if spinal neuronal progenitors can have any effect on respiratory outcomes following ipsilateral silencing of the phrenic motoneuron pool. At 5 weeks post- transplantation, we found that rats had similar tidal volumes and minute ventilation as “injury-only” controls. However, after 10 weeks, transplant recipients displayed an increased tidal volume during a respiratory challenge. In the current study, dissociated FSC cells were introduced as a suspension into the C3 dorsal spinal cord at one week post-C2Hx. The fact that we did not see an impact on respiratory outcomes after 6 wks may simply be due to differences in the nature of the graft site (below the lesion and not directly into it), not to mention a very different form of donor tissue preparation (solid versus dissociated tissue). It also is possible that we may have observed results consistent with our previous study had we carried out experiments to 10 weeks. We believe that our FSC suspension grafts still provide a partial control showing that the mere presence of viable donor tissue alone does not guarantee functional impact. Direct comparison of the current data with the Lee et al. study is precluded by a myriad of fundamental transplantation logistics, the resolution of which would require efforts well beyond the scope of this investigation.
The ability to increase breathing during periods of respiratory challenge is of considerable functional importance after SCI. Even modest increases in metabolic rate (e.g., during low-intensity exercise) require substantial increases in ventilation to prevent severe disturbances to arterial blood gas homeostasis. In addition, the impaired ability to perform intense respiratory muscle activation (e.g., during coughing or “sighing”) is the most functionally important motor deficit in those persons with SCI who are able to maintain independent breathing during “eupneic” conditions.
In this study, we observed that 40% of MB-grafted rats showed no 5-HT immunoreactivity at or near the injection site (e.g., C3-5 dorsal midline). Since our purpose was to deliver embryonic 5-HT cells to the injured spinal cord, these rats were classified as having “failed MB transplants” per our prospective inclusions criteria. This conclusion is also supported by the functional respiratory data: when directly compared with the cohort that had successful MB grafts, the failed transplant group had reduced tidal volume and minute ventilation during respiratory challenge (p<0.05). We do know the precise reason for the failed MB grafts, but an immunosuppression regimen may be useful in future studies to increase the overall success rate.
In conclusion, respiratory recovery following high cervical SCI can be influenced by delayed intraspinal delivery of embryonic MB cells. The functional impact of the MB transplant was manifest during periods of increased respiratory “drive”. Overall, the data are consistent with prior reports indicating that transplantation of 5-HT enriched neuronal populations can promote motor recovery after SCI.
Acknowledgments
Support for this work was provided by the National Institutes of Health (NIH): 1R01NS080180-01A1 (DDF). BJD was supported by an NIH NRSA pre-doctoral Fellowship 1F31NS063659-01A2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Dale EA, Ben Mabrouk F, Mitchell GS. Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function. Physiology. 2014;29:39–48. doi: 10.1152/physiol.00012.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton MJ, Pearse DD, McBroom JS, Berrocal YA. The combination of human neuronal serotonergic cell implants and environmental enrichment after contusive SCI improves motor recovery over each individual strategy. Behavioural brain research. 2008;194:236–241. doi: 10.1016/j.bbr.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Feraboli-Lohnherr D, Orsal D, Yakovleff A, Gimenez y Ribotta M, Privat A. Recovery of locomotor activity in the adult chronic spinal rat after sublesional transplantation of embryonic nervous cells: specific role of serotonergic neurons. Exp Brain Res. 1997;113:443–454. doi: 10.1007/pl00005597. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Doperalski NJ, Dougherty BJ, Sandhu MS, Bolser DC, Reier PJ. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Experimental neurology. 2008;211:97–106. doi: 10.1016/j.expneurol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. Journal of Neuroscience. 2005;25:2925–2932. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Reier PJ, Bolser DC. Altered respiratory motor drive after spinal cord injury: Supraspinal and bilateral effects of a unilateral lesion. Journal of Neuroscience. 2001;21:8680–8689. doi: 10.1523/JNEUROSCI.21-21-08680.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig N, Wilkie MB, Lauder J. Dissection of Monoaminergic Neuronal Groups from Embryonic Rat Brain. Alan R. Liss, Inc; 1989. [Google Scholar]
- Lee KZ, Lane MA, Dougherty BJ, Mercier LM, Sandhu MS, Sanchez JC, Reier PJ, Fuller DD. Intraspinal transplantation and modulation of donor neuron electrophysiological activity. Exp Neurol. 2014;251:47–57. doi: 10.1016/j.expneurol.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Bach KB, Mitchell GS. Serotonin reveals ineffective spinal pathways to contralateral phrenic motoneurons in spinally hemisected rats. Experimental brain research. 1994;101:35–43. doi: 10.1007/BF00243214. [DOI] [PubMed] [Google Scholar]
- Majczynski H, Maleszak K, Cabaj A, Slawinska U. Serotonin-related enhancement of recovery of hind limb motor functions in spinal rats after grafting of embryonic raphe nuclei. Journal of neurotrauma. 2005;22:590–604. doi: 10.1089/neu.2005.22.590. [DOI] [PubMed] [Google Scholar]
- Privat A, Mansour H, Geffard M. Transplantation of fetal serotonin neurons into the transected spinal cord of adult rats: morphological development and functional influence. Prog Brain Res. 1988;78:155–166. doi: 10.1016/s0079-6123(08)60278-2. [DOI] [PubMed] [Google Scholar]
- Sandhu MS, Dougherty BJ, Lane MA, Bolser DC, Kirkwood PA, Reier PJ, Fuller DD. Respiratory recovery following high cervical hemisection. Respiratory physiology & neurobiology. 2009;169:94–101. doi: 10.1016/j.resp.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawinska U, Miazga K, Cabaj AM, Leszczynska AN, Majczynski H, Nagy JI, Jordan LM. Grafting of fetal brainstem 5-HT neurons into the sublesional spinal cord of paraplegic rats restores coordinated hindlimb locomotion. Exp Neurol. 2013 doi: 10.1016/j.expneurol.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Goshgarian HG. Effects of serotonin on crossed phrenic nerve activity in cervical spinal cord hemisected rats. Exp Neurol. 1999;160:446–453. doi: 10.1006/exnr.1999.7213. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Goshgarian HG. 5-Hydroxytryptophan-induced respiratory recovery after cervical spinal cord hemisection in rats. J Appl Physiol. 2000;89:1528–1536. doi: 10.1152/jappl.2000.89.4.1528. [DOI] [PubMed] [Google Scholar]
- Zimmer MB, Goshgarian HG. Spinal activation of serotonin 1A receptors enhances latent respiratory activity after spinal cord injury. J Spinal Cord Med. 2006;29:147–155. doi: 10.1080/10790268.2006.11753868. [DOI] [PMC free article] [PubMed] [Google Scholar]