Abstract
Context
The apolipoprotein E ε4 (APOE ε4) allele is a genetic risk factor for Alzheimer disease. Recently, depression has also become recognized as a risk factor for dementia. However, the possible effect of the APOE genotype on the association between depression and dementia is unexamined.
Objective
To examine the independent and combined effects of depression and APOE ε4 on the risk of dementia and its subtypes.
Design
The Honolulu-Asia Aging Study, a population-based prospective cohort study of Japanese American men.
Settings and Participants
Depressive symptoms and presence of the APOE ε4 allele were assessed between March 1991 and October 1993 in 1932 cognitively healthy men aged 71 to 90 years. Incident cases of dementia were diagnosed during approximately 6 years of follow-up based on neurologic assessment at 2 repeated examinations (April 1994–April 1996 and October 1997–February 1999).
Main Outcome Measures
Overall dementia, Alzheimer disease, and vascular dementia.
Results
The interaction of depression and APOE ε4 was statistically significant in the analytical models. Compared with men with neither APOE ε4 nor depression, the risk of dementia in nondepressed men with APOE ε4 was not significant (hazard ratio, 1.1; 95% confidence interval [CI], 0.6–1.8); however, depressed men without APOE ε4 had a 1.6-fold greater risk (95% CI, 0.8–3.0), whereas depressed men with APOE ε4 had a 7.1-fold greater risk (95% CI, 3.0–16.7) of dementia. For subtypes, we found similar increased risks of Alzheimer disease.
Conclusions
The APOE ε4 status modifies the association between depressive symptoms and dementia in elderly men. Because individuals with depressive symptoms and the APOE ε4 allele had a markedly increased risk of dementia, one might be especially watchful for early signs of dementia in the older person with depression who is also positive for the APOE ε4 allele. Because this cohort includes only men, further investigation in women is required.
Depression is often found before or as a coexisting condition with Alzheimer disease (AD), and the presence of depression affects patient outcomes.1 Although depression has been considered to be a response to cognitive decline or an early manifestation of dementia,1 results of prospective studies2–4 and of a recent meta-analysis5 suggest that depression could be a potential risk factor for dementia. The pathologic mechanism linking depression and subsequent dementia is not well understood, but hypotheses include an indirect neurotoxic effect of depression mediated by cortisol-induced hippocampal atrophy or lowered brain-derived neurotrophic factor levels.1 In addition, there may be genetic links between depression and dementia. The apolipoprotein E ε4 (APOE ε4) allele leads to accelerated deposition of amyloid6 and is a major genetic risk factor for AD. The association between APOE ε4 and depression has been examined, but the findings are inconsistent.7–10 This genotype is a possible genetic factor that may affect the depression-dementia association, but the joint effect of depression and APOE genotypes on dementia is unknown. Herein, we hypothesize that the presence of both APOE ε4 and depression leads to a greater risk of dementia than either factor alone. To examine this hypothesis, we investigated the independent and joint effects of depression and APOE ε4 on incident dementia in a well-characterized cohort of men participating in the Honolulu-Asia Aging Study (HAAS).
METHODS
The HAAS was designed to investigate neurodegenerative diseases in old age. This cohort was originally from the Honolulu Heart Program study,11 which commenced in October 1965 as a longitudinal study of risk factors for cardiovascular disease. The cohort consists of Japanese American men born between 1900 and 1919 and living on the island of Oahu, Hawaii, at the initial examination (October 1965–November 1968). The HAAS was initiated at the fourth examination of the Honolulu Heart Program Study, in March 1991 to October 1993, when the men ranged in age from 71 to 93 years.12,13 This study is based on the March 1991 to October 1993 examination and 2 consecutive follow-up examinations (April 1994–April 1996 and October 1997–February 1999), in which final diagnoses for cases of dementia are available and fully validated. The study was approved by the institutional review committee of Kuakini Medical Center. All the participants were informed about the study objectives and provided written informed consent.
ANALYTICAL SAMPLE
Of 3734 participants at the baseline examination, 1932 were included in the analytical sample. We excluded 226 men with prevalent dementia. Because depressive symptoms may be an early manifestation of dementia, 982 men who were possibly cognitively impaired (62 with a Clinical Dementia Rating score of 0.5 and 920 with a baseline Cognitive Abilities Screening Instrument score of <82) were excluded. We also excluded 176 men with incomplete information for depression symptoms and APOE genotypes, 85 men who died before the first follow-up examination, and 333 men who did not attend the follow-up examinations. We compared the baseline characteristics of the 1932 study participants and the 1802 men excluded from the analysis (Table 1). Men who were excluded were older, had less education, had higher midlife blood pressure, had a lower body mass index (BMI) (calculated as weight in kilograms divided by height in meters squared), had lower total cholesterol levels, were more likely to have smoked, and had a higher percentage of abnormal ankle-brachial index (ABI) scores (more atherosclerotic change) compared with men who were included in the analysis. There were no significant differences between the analytical sample and excluded individuals regarding midlife diastolic blood pressure, prevalence of diabetes mellitus, and percentage who were APOE ε4 positive. Because we excluded prevalent dementia and possible dementia cases, we expected that there would be some baseline differences between the analytical and excluded samples on the risk factors for dementia based on studies previously reported.14 The mean follow-up was 6.1 years, with an incidence rate for dementia of 9.2 per 1000 person-years.
Table 1.
Baseline Characteristics of the Study Participants vs Those Excluded From the Analysis
| Included Men (n=1932) | Excluded Men (n=1802) | P Valuea | |
|---|---|---|---|
| Age, mean (SD), y | 76.3 (3.6) | 79.5 (5.1) | <.001 |
| Education >6 y, % | 96.8 | 89.1 | <.001 |
| Midlife BP, mean (SD), mm Hg | |||
| Systolic | 130.7 (17.6) | 133.1 (16.9) | <.001 |
| Diastolic | 82.3 (8.8) | 82.9 (8.5) | .07 |
| Body mass index, mean (SD)b | 23.6 (3.1) | 23.2 (3.0) | <.001 |
| Diabetes mellitus, % | 34.2 | 36.6 | .15 |
| Total cholesterol, mean (SD), mg/dL | 192.2 (30.8) | 186.8 (33.9) | <.001 |
| Never smoked, % | 39.5 | 35.4 | .02 |
| Ankle-brachial index <0.9, % | 7.6 | 13.4 | <.001 |
| Stroke, % | 7.1 | 15.6 | <.001 |
| APOE ε4 allele positive, %c | 17.6 | 19.6 | .14 |
Abbreviations: APOE ε4, apolipoprotein E ε4; BP, blood pressure.
SI conversion factor: To convert cholesterol to millimoles per liter, multiply by 0.0259.
P values are from linear (continuous variables) and logistic (categorical variables) regression models and are adjusted for age at baseline.
Calculated as weight in kilograms divided by height in meters squared.
APOE ε4 allele positive is defined as having at least 1 copy of the ε4 allele.
DIAGNOSIS OF DEMENTIA
Individuals with dementia were identified via screening for cognitive function following a rigorously standardized study protocol. The 3-stage procedure has previously been described in detail.13 Initial screening considered a participant’s age and cognitive performance on the 100-point Cognitive Abilities Screening Instrument, which is a brief, comprehensive measure of intellectual function developed and validated for use in cross-cultural studies.15 The Cognitive Abilities Screening Instrument is a composite of the Hasegawa Dementia Screening Scale (widely used in epidemiologic studies in Japan), the Folstein Mini-Mental State Examination, and the Modified Mini-Mental State Examination. The score range is 0 to 100, with higher scores indicating better cognitive function.15 The Cognitive Abilities Screening Instrument scores were used to identify a subsample for further diagnostic evaluation. The dementia evaluation at all the examinations included a neuropsychological investigation, a proxy interview, a neurologic examination, neuroimaging, and selected blood tests. Final diagnoses were assigned by a consensus panel consisting of a neurologist (G.W.R.) and additional physicians (K.H.M., H.P., and L.R.W.) with expertise in dementia. Dementia was diagnosed according to criteria from the DSM-III-R.16 The diagnosis of AD was adjudicated according to the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer Disease and Related Disorders Association criteria.17 Alzheimer disease was defined to include cases of dementia in which AD was judged to be the sole or primary cause. For the diagnosis of vascular dementia, the State of California Alzheimer’s Disease Diagnostic and Treatment Centers criteria18 were used. Vascular dementia included cases in which a vascular cause was considered the sole or primary cause. The remaining subtypes included subdural hematoma, Parkinson disease, cortical Lewy body disease, Pick disease, and unknown causes.
DEPRESSION SYMPTOMS ASSESSMENT AND APOE GENOTYPING
Depression symptoms at the baseline examination of the HAAS were examined in participants by using an 11-item version of the Center for Epidemiologic Studies Depression Scale.19 Scores range from 0 to 33, and a depression score was valid only if there were fewer than 3 missing answers. Men with scores of 9 or greater were defined as having depressive symptoms, and men with scores of less than 9 were defined as not having depressive symptoms.19 In this article, we refer to the presence of depressive symptoms as “depression” or “depressed”; however, note that these terms are not synonymous with clinically diagnosed depression.
The APOE genotyping was performed by means of polymerase chain reaction amplification followed by restriction enzyme digestion20 at the Bryan Alzheimer’s Disease Research Center at Duke University, Durham, North Carolina. Participants were categorized as APOE ε4 positive if they had at least 1 copy of the ε4 allele and as ε4 negative otherwise.
COVARIATES
Age and years of education were obtained at the baseline examination of the HAAS (1991–1993). Midlife and late-life cardiovascular risk factors that may affect brain and depression symptoms were adjusted for in the statistical models. Midlife systolic and diastolic blood pressures were based on the average values measured during 3 examinations of the Honolulu Heart Program study (1965–1968, 1967–1970, and 1971–1974). The averaged values from the examinations were calculated for the analysis. Midlife cigarette smoking status was based on standard questions administered at these examinations and was coded as never, past, or current smoker.
Late-life variables—the presence of type 2 diabetes mellitus, cholesterol level, BMI, and ABI—were measured in 1991–1993, the baseline examination of the HAAS. The presence of type 2 diabetes mellitus was assessed by participant report of a physician’s diagnosis, the use of diabetes medications, or glucose tolerance testing results. Total cholesterol levels were measured using an Autoanalyzer 1 N24B.11 The ABI, an indicator of subclinical atherosclerosis, was dichotomized at a cutoff point of 0.9, with values less than 0.9 considered to be indicative of peripheral artery disease.21 Previous stroke events were assessed by means of self-report and continuous surveillance of hospital discharge on Oahu.
The level of self-reported memory problems at the baseline of the HAAS was also assessed. Participants were asked whether they had noticed changes during the past 10 to 20 years in their ability to remember the names of people they had just met, the faces of people they had just met, the names of close friends or relatives, or appointments. Each item was scored on a scale from 1 to 5 (1 indicates definitely improved; 2, slightly improved; 3, no change; 4, slightly deteriorated; and 5, definitely deteriorated). The average score based on the 4 items was calculated as a self-reported memory complaints rating of 1 to 5.
STATISTICAL ANALYSIS
Hazard ratios (HRs) for the incidence of dementia and its subtypes were estimated using Cox proportional hazards models adjusted for the covariates described in the previous subsection. In the standard model, we adjusted for age at baseline, years of education, and awareness of memory problems at baseline. In the extended model, we further adjusted for cardiovascular risk factors. We first investigated the estimated risks of dementia and its subtypes in depressed men compared with nondepressed men. Dementia and its subtypes include all types of dementia (all dementia), AD without cerebrovascular disease (pure AD), vascular dementia, and AD including those with cerebrovascular disease (mixed AD). The interaction between depression and APOE ε4 was investigated by including an interaction term (depression × APOE ε4) in the models.
We also estimated the HRs for dementia across 4 groups based on depression status at baseline and APOE ε4 status: men without depression and without APOE ε4 (neither), men without depression and with APOE ε4 (only e4), depressed men without APOE ε4 (only Dep), and depressed men with APOE ε4 (both). The baseline characteristics of the study sample were compared across these 4 strata. Linear regression for continuous variables and logistic regression for categorical variables were performed, with adjustment for age. To calculate the age-adjusted P value for trends across groups, given that the ordering of the 4 groups (neither, only e4, only Dep, and both) is arbitrary in relation to only e4 and only Dep, we combined men with only 1 risk factor, either depression or APOE ε4, into 1 group (single) and created 3 variables with the codes 0, 1, and 2 for neither, single (only e4 and only Dep), and both, respectively. This method enabled us to conduct trend analysis across groups. All analyses were conducted using statistical software (Stata 8; StataCorp, College Station, Texas, and SAS version 8.2; SAS Institute Inc, Cary, North Carolina). P<.05 was considered statistically significant, and all tests were 2-tailed.
RESULTS
Baseline characteristics across the 4 groups (neither, only e4, only Dep, and both) are given in Table 2. The age-adjusted incidences of dementia were 4.2% (neither), 6.3% (only e4), 9.3% (only Dep), and 13.7% (both). No significant difference existed across groups of men with neither risk factor, with a single risk factor, and with both risk factors regarding age at baseline, primary educational level, diabetes mellitus, stroke history, midlife systolic and diastolic blood pressure, cholesterol level, and BMI. However, there was a significant difference across groups regarding abnormal ABI values, indicating that men with both risk factors had the highest frequency of generalized atherosclerosis.
Table 2.
Baseline Characteristics by Groupa
| Neither (n=1439) | Only e4 (n=324) | Only Dep (n=146) | Both (n=23) | P Valueb | |
|---|---|---|---|---|---|
| Age at baseline, examination 4, mean (SD), y | 76.3 (3.6) | 76.1 (3.6) | 76.2 (3.2) | 75.7 (2.2) | .37 |
| Education >6 y, % | 97.8 | 97.5 | 97.1 | 96.7 | .27 |
| Midlife BP, mean (SD), mm Hg | |||||
| Systolic | 129.8 (15.2) | 130.2 (9.0) | 130.7 (10.9) | 131.2 (7.2) | .27 |
| Diastolic | 82.3 (7.6) | 82.7 (5.4) | 83.0 (7.2) | 83.4 (4.3) | .25 |
| Body mass index, mean (SD)c | 23.9 (3.8) | 23.8 (1.8) | 23.6 (2.4) | 23.5 (1.4) | .28 |
| Diabetes mellitus, % | 34.5 | 34.3 | 34.2 | 34.1 | .77 |
| Total cholesterol, mean (SD), mg/dL | 193.3 (30.3) | 193.2 (18.0) | 193.1 (23.0) | 193.0 (14.4) | .44 |
| Never smoked, % | 36.7 | 38.4 | 40.1 | 41.9 | .34 |
| Ankle-brachial index <0.9, % | 6.3 | 8.2 | 10.7 | 13.9 | .03 |
| Stroke, % | 6.4 | 7.8 | 9.3 | 11.1 | .54 |
| Dementia incidence, % | 4.2 | 6.3 | 9.3 | 13.7 | .004 |
Abbreviation: BP, blood pressure.
SI conversion factor: To convert cholesterol to millimoles per liter, multiply by 0.0259.
Neither refers to men without depressive symptoms and apolipoprotein E ε4 (APOE ε4) negative; only e4, men without depressive symptoms and APOE ε4 positive; only Dep, men with depressive symptoms and APOE ε4 negative; and both, men with depressive symptoms and APOE ε4 positive.
P values are from linear (continuous variables) and logistic (categorical variables) regression models for 3 groups (neither, single [combined only e4 and only Dep], and both) and are adjusted for age at baseline.
Calculated as weight in kilograms divided by height in meters squared.
The survival analysis consisted of 2 parts. In the first part, we estimated the risk of dementia in depressed men compared with nondepressed men. The HR in depressed men was approximately 2- to 3-fold greater for all dementia, pure AD, and mixed AD. This association remained after further adjustment of cardiovascular risk factors (Table 3). We also included the interaction term (depression × APOE ε4 allele) in the models and found a significant interaction effect for all dementia, pure AD, and mixed AD (P<.01 for all).
Table 3.
Data for Dementia in Men With Depressive Symptoms
| Standard Modela,b | Extended Modela,b | |
|---|---|---|
| All dementiad | 2.5 (1.5–4.1)e | 2.2 (1.3–3.7)f |
| Pure ADd | 3.0 (1.5–5.9)f | 2.9 (1.4–5.9)f |
| Vascular dementia | 2.2 (0.6–7.4) | 1.3 (0.3–5.8) |
| Mixed ADd | 2.9 (1.6–5.2)e | 2.8 (1.5–5.1)f |
Abbreviations: mixed AD, all Alzheimer disease including those with cerebrovascular disease; pure AD, Alzheimer disease without apparent cerebrovascular disease.
Data are given as hazard ratio (95% confidence interval). The reference group was nondepressed men.
Adjusted for age at examination 4, educational level, and self-reported memory deterioration at baseline.
Adjusted for age at examination 4, educational level, self-reported memory deterioration at baseline, midlife systolic and diastolic blood pressure, diabetes mellitus, smoking status, cholesterol level at examination 4, body mass index at examination 4, and stroke history.
The interaction term (depression × apolipoprotein E ε4) is significant in the standard and extended models (P<.01 for both).
P<.001.
P<.01.
In the second part of the survival analysis, the 4 groups were examined. The cumulative hazard estimate curves and the log-rank test for equality of survivor function ( , P<.001) indicated a different risk of dementia across the groups (Figure). Compared with men with neither APOE ε4 nor depression, depressed men without APOE ε4 had a 1.6-fold greater risk (95% confidence interval [CI], 0.8–3.0), whereas depressed men with APOE ε4 had a 7.1-fold greater risk (95% CI, 3.0–16.7) (Table 4). The risk of dementia in nondepressed men with APOE ε4 was not significant (HR, 1.1; 95% CI, 0.6–1.8). We found similar increased risks of AD and mixed AD.
Figure.
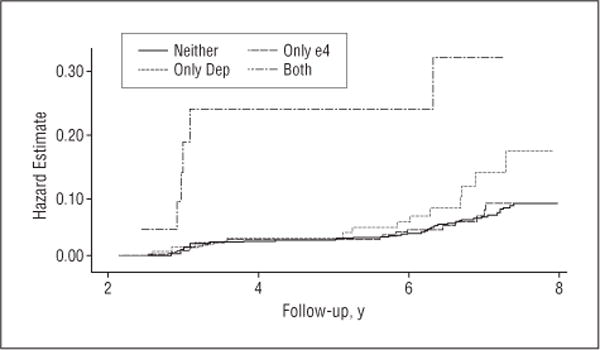
Cumulative hazard estimates for all types of dementia (log-rank test for equality of survivor functions: , P<.001). Neither refers to men without depressive symptoms and apolipoprotein ε4 (APOE ε4) negative; only e4, men without depressive symptoms and APOE ε4 positive; only Dep, men with depressive symptoms and APOE ε4 negative; and both, men with depressive symptoms and APOE ε4 positive.
Table 4.
Data for Incident Dementia and Its Subtypes by the Presence of Depressive Symptoms and APOE ε4a
| Hazard Ratio (95% Confidence Interval)
|
||||
|---|---|---|---|---|
| Neither | Only e4 | Only Dep | Both | |
| All dementia | ||||
| No. of participants | 1439 | 324 | 146 | 23 |
| Standard model | 1 [Reference] | 1.1 (0.6–1.7) | 1.9 (1.1–3.4)b | 7.2 (3.1–16.8)c |
| Extended model | 1 [Reference] | 1.1 (0.6–1.8) | 1.6 (0.8–3.0) | 7.1 (3.0–16.7)c |
| Pure AD | ||||
| No. of participants | 1397 | 318 | 140 | 21 |
| Standard model | 1 [Reference] | 1.5 (0.7–3.0) | 2.3 (1.0–5.3)b | 12.6 (4.4–37.0)c |
| Extended model | 1 [Reference] | 1.6 (0.8–3.1) | 2.2 (0.9–5.2) | 13.0 (4.3–39.5)c |
| Vascular dementia | ||||
| No. of participants | 1382 | 307 | 135 | 18 |
| Standard model | 1 [Reference] | NA | 1.3 (0.3–5.8) | 5.2 (0.7–40.1) |
| Extended model | 1 [Reference] | NA | 0.6 (0.1–4.8) | 4.1 (0.5–33.7) |
| Mixed AD | ||||
| No. of participants | 1408 | 321 | 142 | 22 |
| Standard model | 1 [Reference] | 1.4 (0.8–2.6) | 2.3 (1.1–4.7)b | 10.7 (4.2–27.3)c |
| Extended model | 1 [Reference] | 1.5 (0.8–2.7) | 2.1 (0.9–4.5) | 10.4 (3.9–27.4)c |
Abbreviations: APOE ε4, apolipoprotein E ε4; mixed AD, all Alzheimer disease including those with cerebrovascular disease; NA, not applicable owing to the small number; pure AD, Alzheimer disease without apparent cerebrovascular disease.
Neither refers to men without depressive symptoms and APOE ε4 negative; only e4, men without depressive symptoms and APOE ε4 positive; only Dep, men with depressive symptoms and APOE ε4 negative; and both, men with depressive symptoms and APOE ε4 positive. The standard model was adjusted for age at examination 4, educational level, and self-reported memory deterioration at baseline. The extended model was adjusted for age at examination 4, educational level, self-reported memory deterioration at baseline, midlife systolic and diastolic blood pressure, diabetes mellitus, smoking status, cholesterol level at examination 4, body mass index at examination 4, and stroke history.
P<.05.
P<.001.
COMMENT
We examined the independent and joint effects of depressive symptoms and a genetic risk factor on dementia and its subtypes using dementia-free survival analysis. The main finding in this study is that APOE status modified the association between depressive symptoms and overall dementia, and its subtypes AD and mixed AD, such that elderly men with the APOE ε4 allele and depressive symptoms were at a several-fold greater risk for dementia than were men who were nondepressed and without the ε4 allele and men with only depressive symptoms and men with only the APOE ε4 allele. In the first analysis, we confirmed that the risk of dementia in men with depressive symptoms was approximately 2 to 3 times higher than that in nondepressed men. Because the interaction effects of depression and APOE ε4 genotype were statistically significant, we further examined the independent and combined risks of the presence of depressive symptoms and APOE status on incident dementia. Under the condition in which both factors exist, the risk of dementia was enhanced multiplicatively rather than additively. The enhanced hazard risk in depressed men with the APOE ε4 genotype remained statistically significant even after adjustment for a variety of cardiovascular risk factors.
To our knowledge, the interaction effect between depression and the APOE genotype on dementia has not previously been reported, although a recent study22 from the Mayo Clinic indicated a synergistic interaction between depression and APOE genotype on cognitive decline. The independent risk on dementia in the present cohort is higher in men who have depressive symptoms than in those with the ε4 allele.
Several mechanisms have been proposed to account for the association between depression and dementia. First, late-onset depression has been considered as an early manifestation of AD, and a recent postmortem study23 found increased AD neuropathologic findings in patients with late-onset depression without clinical dementia symptoms. In the present study, we excluded possible cognitively impaired men from the analytical sample and adjusted for the degree of self-reported memory problems and years of education to examine depression’s causal relationship on dementia. Second, some researchers2,24,25 have suggested the possibility of a neurotoxic effect of depression, such as hypothalamus–pituitary–adrenal cortisol–induced hippocampal atrophy or lowered brain-derived neurotrophic factor levels. Third, the association between depression and dementia may be confounded by shared environmental or genetic factors. The apolipoprotein genotype is an important candidate for shared risk factors; however, most studies7,8,26,27 have found no association between APOE and depression. Last, it has been proposed that vascular disease is the underlying condition and may play a mediating role in the depression-dementia causal relationship.22,28 In fact, some studies29,30 indicate that AD and depression share risk factors for vascular disease. Atherosclerotic change starts in the 20s, and by 70 or 80 years of age, most individuals have significant atherosclerosis.30 Cerebrovascular atherosclerosis, arteriosclerosis, and aging-related degenerative changes (such as amyloid deposition and neurofibrillary tangles) are all commonly found in the elderly population without dementia.31,32 Recent studies33 emphasize the important contribution of the vascular component to AD, not only as a primary cause of dementia but also as a cofactor in degenerative dementias. The clinical distinction between AD and vascular dementia may be difficult because the incidence of AD and the severity of atherosclerotic change increase with aging and are often found to coexist in elderly persons.34 On the other hand, the association between depression and deep white matter lesions has been reported.35,36 However, this underlying vascular disease hypothesis remains inconclusive. Recently, a large prospective study37 found no contribution of vascular disease to the depression-dementia association, reporting that depression increased the risk of mild cognitive impairment independent of underlying vascular disease.
Apolipoprotein E plays an important role in lipid transport and metabolism, and previous studies38,39 have found a significant association between the APOE ε4 allele and hypercholesterolemia and increased risk of cardiovascular disease. Haan et al40 reported the modifiable effect of the APOE ε4 allele on the association between atherosclerosis and cognitive decline. They also suggested an important role for vascular diseases in dementia independent of the APOE ε4 allele because they found a much higher risk in individuals with severe atherosclerosis but without the APOE ε4 allele.40 The ABI significantly increased across 3 strata (from neither to single to both) in the baseline characteristic analysis, with the degree of atherosclerotic change being significantly higher in men with both factors. However, adjustment for cardiovascular risk factors did not substantially alter the results of the survival analyses.
This study is unique and has several strengths. It is based on a large, well-characterized cohort, allowing us to study the independent and interactive effects of depression and APOE ε4 status on dementia and its subtypes, and we controlled for a variety of possible confounding factors. In addition, we used a well-structured dementia diagnostic system, and the analytical sample was well-defined to examine the effects of both risk factors. However, the study also has several limitations. First, although the study sample is well-defined by exclusion and adjustment procedures, there may be a type II error if these processes are not sensitive enough to detect all cases with depressive symptoms resulting from the cognitive decline process. Moreover, although the study sample was well-defined, 1802 men were excluded from the analytical sample. Given that this is nearly half of the original HAAS cohort, and that some baseline differences exist between the analytical sample and excluded individuals, there is a possibility that the exclusion process might have affected the analyses. Second, our measurement of depression describes only depression symptoms, which is not synonymous with clinical depression. It is possible that the results may either underestimate or overestimate the association between clinical depression and dementia. However, the presence of depression symptoms has been shown to be associated with subsequent cognitive decline,41–44 and these results support the interpretation that depressive symptoms may predict the development of dementia. Moreover, to identify individuals at increased risk, it seems to be more practical to use a screening measurement of depressive symptoms than to impose a full evaluation for depression. Third, this cohort includes only men, and results may not apply to women. Because it has been recognized that depression is approximately twice as common in women as in men,45,46 further investigation in a cohort of women is required to determine whether a similar relationship exists among APOE, depressive symptoms, and dementia. The underlying cause of a sex difference in depression is not yet fully understood and may include biological differences and the different social roles of men and women and measurement issues.47 However, several studies48–50 have found that the sex difference diminishes or disappears in persons older than 75 or 85 years. Moreover, a recent report from the National Institute of Mental Health51 suggested that there may be more depression cases among men and emphasized the importance of detecting and treating undiagnosed cases. Fourth, owing to the small number of men with vascular dementia, we could not estimate the risk in men who were APOE ε4 allele positive. Last, these results do not provide the long-term effect of early-onset depression on the brain because they pertain to depressive status measured up to approximately 6 years before diagnosis. However, it has been suggested that neurobiologic change during the process of depression may occur even in late-onset depression.52
The present study suggests that the presence of the APOE ε4 allele may create the susceptible condition; however, depressive symptoms further increase the risk of dementia. The multiplicative interaction effect between both factors indicates a pathologic environment that is more predisposing to dementia. Depressed men with the APOE ε4 allele had a 7 times higher risk of dementia, and even depressed men without genetic susceptibility had a 1.5- to 2-fold increased risk compared with nondepressed men. These findings suggest that APOE ε4 status modifies the association between depressive symptoms and dementia in men. Because individuals with depressive symptoms and the APOE ε4 allele had a markedly increased risk of dementia, one might be especially watchful for early signs of dementia in the older person with depression who is also positive for the APOE ε4 allele.
Acknowledgments
Funding/Support: This study was supported by contract N01-AG-4-2149 and the Intramural Research Program, National Institute on Aging, contract N01-HC-05102 from the National Heart, Lung, and Blood Institute, the National Institutes of Health, and the Japan Society for the Promotion of Science.
Footnotes
Author Contributions: Dr Irie had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Disclosure: None reported.
Previous Presentation: This study was presented at the Congress of the Royal Australian and New Zealand College of Psychiatrists; May 3, 2007; Gold Coast, Australia.
References
- 1.Meeks TW, Ropacki SA, Jeste DV. The neurobiology of neuropsychiatric syndromes in dementia. Curr Opin Psychiatry. 2006;19(6):581–586. doi: 10.1097/01.yco.0000245746.45384.0e. [DOI] [PubMed] [Google Scholar]
- 2.Green RC, Cupples LA, Kurz A, Auerbach S, Go R, Sadovnick D, Duara R, Kukull WA, Chui H, Edeki T, Griffith PA, Friedland RP, Bachman D, Farrer L. Depression as a risk factor for Alzheimer disease: the MIRAGE Study. Arch Neurol. 2003;60(5):753–759. doi: 10.1001/archneur.60.5.753. [DOI] [PubMed] [Google Scholar]
- 3.Wilson RS, Barnes LL, Mendes de Leon CF, Aggarwal NT, Schneider JS, Bach J, Pilat J, Beckett LA, Arnold SE, Evans DA, Bennett DA. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology. 2002;59(3):364–370. doi: 10.1212/wnl.59.3.364. [DOI] [PubMed] [Google Scholar]
- 4.Dal Forno G, Palermo MT, Donohue JE, Karagiozis H, Zonderman AB, Kawas CH. Depressive symptoms, sex, and risk for Alzheimer’s disease. Ann Neurol. 2005;57(3):381–387. doi: 10.1002/ana.20405. [DOI] [PubMed] [Google Scholar]
- 5.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63(5):530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cummings JL. Alzheimer’s disease. N Engl J Med. 2004;351(1):56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- 7.Scarmeas N, Brandt J, Albert M, Devanand DP, Marder K, Bell K, Ciappa A, Tycko B, Stern Y. Association between the APOE genotype and psychopathologic symptoms in Alzheimer’s disease. Neurology. 2002;58(8):1182–1188. doi: 10.1212/wnl.58.8.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craig D, Hart DJ, McIlroy SP, Passmore AP. Association analysis of apolipoprotein E genotype and risk of depressive symptoms in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2005;19(2–3):154–157. doi: 10.1159/000082887. [DOI] [PubMed] [Google Scholar]
- 9.Krishnan KR, Tupler LA, Ritchie JC, Jr, McDonald WM, Knight DL, Nemeroff CB, Carroll BJ. Apolipoprotein E-ε4 frequency in geriatric depression. Biol Psychiatry. 1996;40(1):69–71. doi: 10.1016/0006-3223(95)00424-6. [DOI] [PubMed] [Google Scholar]
- 10.Rigaud AS, Traykov L, Caputo L, Coste J, Latour F, Couderc R, Moulin F, Boller F, Forette F. Association of the apolipoprotein E ε4 allele with late-onset depression. Neuroepidemiology. 2001;20(4):268–272. doi: 10.1159/000054801. [DOI] [PubMed] [Google Scholar]
- 11.Syme SL, Marmot MG, Kagan A, Kato H, Rhoads G. Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California: introduction. Am J Epidemiol. 1975;102(6):477–480. doi: 10.1093/oxfordjournals.aje.a112185. [DOI] [PubMed] [Google Scholar]
- 12.Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function: the Honolulu-Asia Aging Study. JAMA. 1995;274(23):1846–1851. [PubMed] [Google Scholar]
- 13.White L, Petrovitch H, Ross GW, Masaki KH, Abbott RD, Teng EL, Rodriguez BL, Blanchette PL, Havlik RJ, Wergowske G, Chiu D, Foley DJ, Murdaugh C, Curb JD. Prevalence of dementia in older Japanese-American men in Hawaii: the Honolulu-Asia Aging Study. JAMA. 1996;276(12):955–960. [PubMed] [Google Scholar]
- 14.White L, Launer L. Relevance of cardiovascular risk factors and ischemic cerebrovascular disease to the pathogenesis of Alzheimer disease: a review of accrued findings from the Honolulu-Asia Aging Study. Alzheimer Dis Assoc Disord. 2006;20(3 suppl 2):S79–S83. doi: 10.1097/00002093-200607001-00012. [DOI] [PubMed] [Google Scholar]
- 15.Teng EL, Hasegawa K, Homma A, Imai Y, Larson E, Graves A, Sugimoto K, Yamaguchi T, Sasaki H, Chiu D. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6(1):45–58. doi: 10.1017/s1041610294001602. [DOI] [PubMed] [Google Scholar]
- 16.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Third. Washington, DC: American Psychiatric Association; 1987. Revised. [Google Scholar]
- 17.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 18.Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s Disease Diagnostic and Treatment Centers. Neurology. 1992;42(3, pt 1):473–480. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- 19.Takeshita J, Masaki K, Ahmed I, Foley DJ, Li YQ, Chen R, Fujii D, Ross GW, Petrovitch H, White L. Are depressive symptoms a risk factor for mortality in elderly Japanese American men? the Honolulu-Asia Aging Study. Am J Psychiatry. 2002;159(7):1127–1132. doi: 10.1176/appi.ajp.159.7.1127. [DOI] [PubMed] [Google Scholar]
- 20.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31(3):545–548. [PubMed] [Google Scholar]
- 21.Murabito JM, Evans JC, Larson MG, Nieto K, Levy D, Wilson PW. The ankle-brachial index in the elderly and risk of stroke, coronary disease, and death: the Framingham Study. Arch Intern Med. 2003;163(16):1939–1942. doi: 10.1001/archinte.163.16.1939. [DOI] [PubMed] [Google Scholar]
- 22.Geda YE, Knopman DS, Mrazek DA, Jicha GA, Smith GE, Negash S, Boeve BF, Ivnik RJ, Petersen RC, Pankratz VS, Rocca WA. Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: a prospective cohort study. Arch Neurol. 2006;63(3):435–440. doi: 10.1001/archneur.63.3.435. [DOI] [PubMed] [Google Scholar]
- 23.Sweet RA, Hamilton RL, Butters MA, Mulsant BH, Pollock BG, Lewis DA, Lopez OL, DeKosky ST, Reynolds CF., III Neuropathologic correlates of late-onset major depression. Neuropsychopharmacology. 2004;29(12):2242–2250. doi: 10.1038/sj.npp.1300554. [DOI] [PubMed] [Google Scholar]
- 24.Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60(8):804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- 25.Rapp MA, Schnaider-Beeri M, Grossman HT, Sano M, Perl DP, Purohit DP, Gorman JM, Haroutunian V. Increased hippocampal plaques and tangles in patients with Alzheimer disease with a lifetime history of major depression. Arch Gen Psychiatry. 2006;63(2):161–167. doi: 10.1001/archpsyc.63.2.161. [DOI] [PubMed] [Google Scholar]
- 26.Lyketsos CG, Baker L, Warren A, Steele C, Brandt J, Steinberg M, Kopunek S, Baker A. Depression, delusions, and hallucinations in Alzheimer’s disease: no relationship to apolipoprotein E genotype. J Neuropsychiatry Clin Neurosci. 1997;9(1):64–67. doi: 10.1176/jnp.9.1.64. [DOI] [PubMed] [Google Scholar]
- 27.Harwood DG, Barker WW, Ownby RL, St George-Hyslop P, Duara R. Apolipoprotein-E (APO-E) genotype and symptoms of psychosis in Alzheimer’s disease. Am J Geriatr Psychiatry. 1999;7(2):119–123. [PubMed] [Google Scholar]
- 28.Fuhrer R, Dufouil C, Dartigues JF. Exploring sex differences in the relationship between depressive symptoms and dementia incidence: prospective results from the PAQUID Study. J Am Geriatr Soc. 2003;51(8):1055–1063. doi: 10.1046/j.1532-5415.2003.51352.x. [DOI] [PubMed] [Google Scholar]
- 29.Iadecola C, Gorelick PB. Converging pathogenic mechanisms in vascular and neurodegenerative dementia. Stroke. 2003;34(2):335–337. doi: 10.1161/01.str.0000054050.51530.76. [DOI] [PubMed] [Google Scholar]
- 30.Rasgon N, Jarvik L. Insulin resistance, affective disorders, and Alzheimer’s disease: review and hypothesis. J Gerontol A Biol Sci Med Sci. 2004;59(2):178–183. doi: 10.1093/gerona/59.2.m178. [DOI] [PubMed] [Google Scholar]
- 31.Korczyn AD. Alzheimer’s disease and vascular brain lesions. J Neurol Sci. 2005;231(1–2):1–2. doi: 10.1016/j.jns.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 32.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease: the Nun Study. JAMA. 1997;277(10):813–817. [PubMed] [Google Scholar]
- 33.Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62(7):1148–1155. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 34.Korczyn AD. The underdiagnosis of the vascular contribution to dementia. J Neurol Sci. 2005:229–230. 3–6. doi: 10.1016/j.jns.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 35.de Groot JC, de Leeuw FE, Oudkerk M, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and depressive symptoms in elderly adults. Arch Gen Psychiatry. 2000;57(11):1071–1076. doi: 10.1001/archpsyc.57.11.1071. [DOI] [PubMed] [Google Scholar]
- 36.Minett TS, Dean JL, Firbank M, English P, O’Brien JT. Subjective memory complaints, white-matter lesions, depressive symptoms, and cognition in elderly patients. Am J Geriatr Psychiatry. 2005;13(8):665–671. doi: 10.1176/appi.ajgp.13.8.665. [DOI] [PubMed] [Google Scholar]
- 37.Barnes DE, Alexopoulos GS, Lopez OL, Williamson JD, Yaffe K. Depressive symptoms, vascular disease, and mild cognitive impairment: findings from the Cardiovascular Health Study. Arch Gen Psychiatry. 2006;63(3):273–279. doi: 10.1001/archpsyc.63.3.273. [DOI] [PubMed] [Google Scholar]
- 38.Lahoz C, Schaefer EJ, Cupples LA, Wilson PW, Levy D, Osgood D, Parpos S, Pedro-Botet J, Daly JA, Ordovas JM. Apolipoprotein E genotype and cardiovascular disease in the Framingham Heart Study. Atherosclerosis. 2001;154(3):529–537. doi: 10.1016/s0021-9150(00)00570-0. [DOI] [PubMed] [Google Scholar]
- 39.McCarron MO, Delong D, Alberts MJ. APOE genotype as a risk factor for ischemic cerebrovascular disease: a meta-analysis. Neurology. 1999;53(6):1308–1311. doi: 10.1212/wnl.53.6.1308. [DOI] [PubMed] [Google Scholar]
- 40.Haan MN, Shemanski L, Jagust WJ, Manolio TA, Kuller L. The role of APOE ε4 in modulating effects of other risk factors for cognitive decline in elderly persons. JAMA. 1999;282(1):40–46. doi: 10.1001/jama.282.1.40. [DOI] [PubMed] [Google Scholar]
- 41.Raji MA, Reyes-Ortiz CA, Kuo YF, Markides KS, Ottenbacher KJ. Depressive symptoms and cognitive change in older Mexican Americans. J Geriatr Psychiatry Neurol. 2007;20(3):145–152. doi: 10.1177/0891988707303604. [DOI] [PubMed] [Google Scholar]
- 42.Chodosh J, Kado DM, Seeman TE, Karlamangla AS. Depressive symptoms as a predictor of cognitive decline: MacArthur Studies of Successful Aging. Am J Geriatr Psychiatry. 2007;15(5):406–415. doi: 10.1097/01.JGP.0b013e31802c0c63. [DOI] [PubMed] [Google Scholar]
- 43.Paterniti S, Verdier-Taillefer MH, Dufouil C, Alperovitch A. Depressive symptoms and cognitive decline in elderly people: longitudinal study. Br J Psychiatry. 2002;181:406–410. doi: 10.1192/bjp.181.5.406. [DOI] [PubMed] [Google Scholar]
- 44.Yaffe K, Blackwell T, Gore R, Sands L, Reus V, Browner WS. Depressive symptoms and cognitive decline in nondemented elderly women: a prospective study. Arch Gen Psychiatry. 1999;56(5):425–430. doi: 10.1001/archpsyc.56.5.425. [DOI] [PubMed] [Google Scholar]
- 45.Blehar MC, Oren DA. Gender differences in depression. Medscape Womens Health. 1997;2(2):3. [PubMed] [Google Scholar]
- 46.Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, Joyce PR, Karam EG, Lee CK, Lellouch J, Lépine JP, Newman SC, Rubio-Stipec M, Wells JE, Wickramaratne PJ, Wittchen H, Yeh EK. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276(4):293–299. [PubMed] [Google Scholar]
- 47.Romans SE, Tyas J, Cohen MM, Silverstone T. Gender differences in the symptoms of major depressive disorder. J Nerv Ment Dis. 2007;195(11):905–911. doi: 10.1097/NMD.0b013e3181594cb7. [DOI] [PubMed] [Google Scholar]
- 48.Bergdahl E, Gustavsson JM, Kallin K, von Heideken Wågert P, Lundman B, Bucht G, Gustafson Y. Depression among the oldest old: the Umeå 85+ study. Int Psychogeriatr. 2005;17(4):557–575. doi: 10.1017/S1041610205002267. [DOI] [PubMed] [Google Scholar]
- 49.Van’t Veer-Tazelaar PJ, van Marwijk HW, Jansen AP, Rijmen F, Kostense PJ, van Oppen P, van Hout HP, Stalman WA, Beekman AT. Depression in old age (75+): the PIKO study. J Affect Disord. 2008;106(3):295–299. doi: 10.1016/j.jad.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Stek ML, Gussekloo J, Beekman AT, van Tilburg W, Westendorp RG. Prevalence, correlates and recognition of depression in the oldest old: the Leiden 85-plus study. J Affect Disord. 2004;78(3):193–200. doi: 10.1016/S0165-0327(02)00310-5. [DOI] [PubMed] [Google Scholar]
- 51.National Institute of Mental Health. Men and depression. 2005 http://www.nimh.nih.gov/health/publications/men-and-depression/men-and-depression.shtml. Accessed December 10, 2007.
- 52.Alexopoulos GS. Vascular disease, depression, and dementia. J Am Geriatr Soc. 2003;51(8):1178–1180. doi: 10.1046/j.1532-5415.2003.51373.x. [DOI] [PubMed] [Google Scholar]


