Abstract
Ethanol (EtOH) has effects on numerous cellular molecular targets, and alterations in synaptic function are prominent among these effects. Acute exposure to EtOH activates or inhibits the function of proteins involved in synaptic transmission, while chronic exposure often produces opposing and/or compensatory/homeostatic effects on the expression, localization, and function of these proteins. Interactions between different neurotransmitters (e.g., neuropeptide effects on release of small molecule transmitters) can also influence both acute and chronic EtOH actions. Studies in intact animals indicate that the proteins affected by EtOH also play roles in the neural actions of the drug, including acute intoxication, tolerance, dependence, and the seeking and drinking of EtOH. This chapter reviews the literature describing these acute and chronic synaptic effects of EtOH and their relevance for synaptic transmission, plasticity, and behavior.
Keywords: GABA, Glutamate, Monoamine, Neuropeptide, Neurotransmitter receptor, Presynaptic, Postsynaptic, Protein phosphorylation, Synaptic plasticity, Intoxication, Tolerance, Dependence
1 Acute Ethanol Actions
Ethanol (EtOH) produces intoxication through actions on the central nervous system (CNS) at concentrations ranging from low to ~100 mM (at least in non-tolerant humans and experimental animals). A number of proteins involved in synaptic transmission are altered by EtOH effects within this concentration range. The target proteins include, but are not limited to, ion channels, neurotransmitter receptors, and intracellular signaling proteins. The first section of this article will review the literature describing the most prominent acute EtOH effects on synaptic transmission in the CNS. This review is not meant to be comprehensive, but rather to cover those effects that have been observed most consistently and are thought to contribute to intoxication.
1.1 Ligand-Gated Ion Channels and Postsynaptic Ethanol Effects
Ion channels are among the best characterized targets for acute EtOH actions (Lovinger 1997; Vengeliene et al. 2008). Ligand-gated ion channels (LGICs) are heteromeric proteins that bind extracellular neurotransmitters or intracellular messengers and transduce that binding energy into opening of an intrinsic ion pore (Collingridge et al. 2009). Among those channels activated by extracellular neurotransmitters there are three classes.
1.1.1 Cys-loop Ligand-Gated Ion Channels
The “cys-loop” LGICs are pentameric proteins characterized by an obligatory cysteine double bond in the N-terminal binding domain. Each subunit protein contains an extracellular ligand-binding domain, four membrane spanning domains, and one large intracellular loop domain that also serves as a “portal” for ion permeability. This receptor class includes proteins with cation-permeable pores, the nicotinic acetylcholine (nAChR) and serotonin3 (5-HT3) receptors, as well as those with anion-permeable pores, the γ-aminobutyric acidA (GABAA), and strychnine-sensitive glycine (GlyR) receptors. This class of receptors is distributed throughout the peripheral and central nervous systems.
Generally, acute EtOH exposure enhances the function of cys-loop LGICs (Aguayo et al. 2002; Harris 1999; Lovinger 1997; Perkins et al. 2010), but instances of inhibition of the nAChRs and GABAARs have been reported (Aguayo et al. 2002; Cardoso et al. 1999; Davis and De Fiebre 2006; Marszalec et al. 1994; Roberto et al. 2003). The most common EtOH action is to potentiate channel opening in the presence of a low concentration of agonist by increasing the probability of channel opening (Zhou et al. 1998), and/or increasing agonist affinity (Tonner and Miller 1995; Welsh et al. 2009). This potentiating effect can influence both synaptic and extrasynaptic receptors (Sebe et al. 2003; Ye et al. 2001; Eggers and Berger 2004; Ziskind-Conhaim et al. 2003) (Fig. 1). For example, EtOH has been shown to increase the amplitude and/or duration of GABAA and GlyR-mediated inhibitory postsynaptic currents (IPSCs) (Sebe et al. 2003; Ziskind-Conhaim et al. 2003).
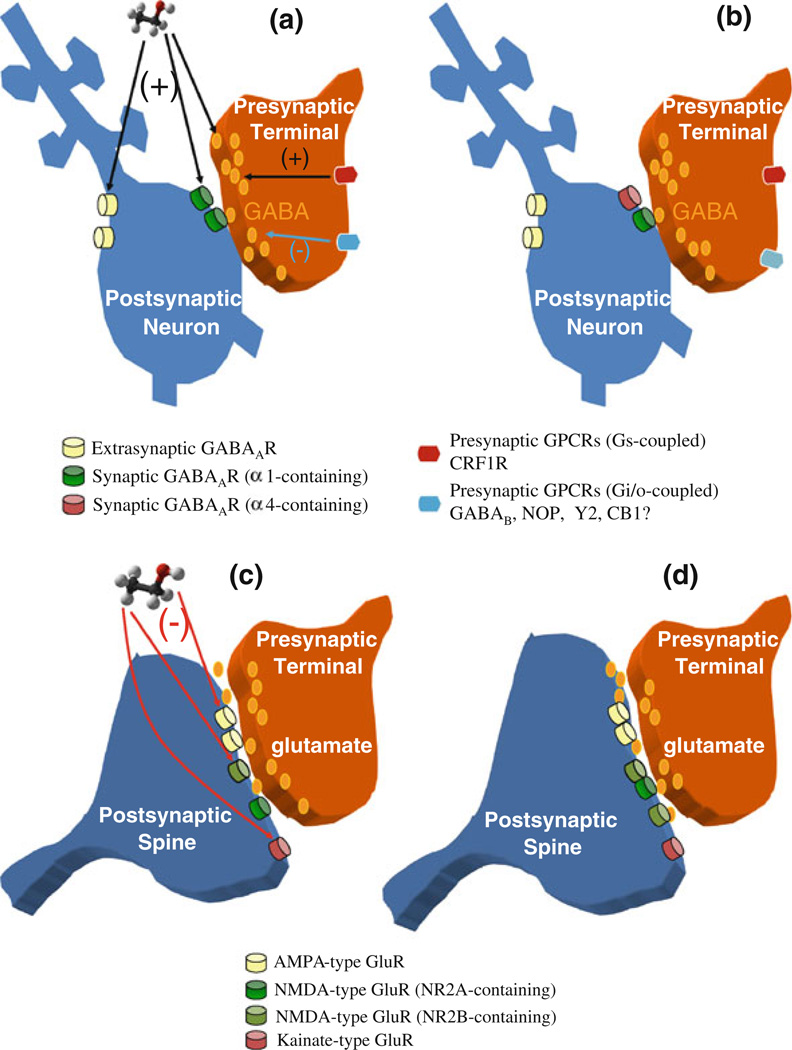
Fig. 1.
Acute and chronic EtOH effects on GABAergic and glutamatergic synaptic transmission. a Schematic diagram of a GABAergic synapse, including presynaptic GPCRs that modulate neurotransmitter release, and postsynaptic ionotropic receptors (located both at synapses and extrasynaptically) that mediate fast synaptic transmission. The predominant presynaptic effect of acute EtOH is potentiation of GABA release (most likely by increasing the probability of vesicle fusion). This presynaptic potentiation may involve neuromodulators such as CRF, and activation of presynaptic GPCRs and downstream signaling pathways. Postsynaptically, EtOH potentiates ionotropic GABAA receptor function. Increases in synaptic GABAAR function prolong synaptic responses, while potentiation of extrasynaptic receptors increases tonic current that affects neuronal excitability, b Changes in GABAergic synapses following chronic EtOH exposure. Presynaptically, the release of GABA is decreased. Alterations in levels of neuromodulators that act on GPCRs, as well as altered function of presynaptic GPCRs may contribute to these changes. Postsynaptically, the subunit composition of GABAARs is altered, often including increased synaptic α4-containing receptors, and fewer α1-containing synaptic receptors. Synaptic α4-containing receptors may be less sensitive to acute EtOH, promoting tolerance to synaptic effect of the drug, c Schematic diagram of a glutamatergic synapse on a dendritic spine, including postsynaptic ionotropic receptors that mediate fast synaptic transmission. The predominant effect of acute EtOH is to inhibit ionotropic glutamate receptor function, and all subclasses of these receptors are sensitive to EtOH inhibition. The most potent effects have been observed at kainate and NMDA receptor subtypes, d Changes in glutamatergic synapses following chronic EtOH exposure. Presynaptically, the release of glutamate is enhanced. Postsynaptically, NMDAR function is increased, most likely due to increased receptor density at the synapse. There is also evidence for increased numbers of NR2B-containing NMDARs. There is also evidence of increased volume of the dendritic spine.
EtOH potentiation of GABAA receptor function has been extensively studied. There are 19 subunit proteins that contribute to the formation of GABAA receptors (International Union of Basic and Clinical Pharmacology, IUPHAR, database http://www.iuphar-db.org/index.jsp). Many of these subunit combinations have been examined for function and pharmacology in heterologous expression systems. To briefly summarize a large body of data, there is evidence that EtOH potentiates the function of α/β/γ-subunit-containing receptors, as well as those containing α4 or α6 along with β and δ subunits (Olsen et al. 2007; Lobo and Harris 2008; Mihic and Harris 1995; McCool et al. 2003). However, none of these findings has been uniformly replicated in all laboratories that have examined EtOH effects in heterologous systems (reviewed in Lovinger and Homanics 2007; Aguayo et al. 2002). Using cultured and isolated neurons, several investigators have observed potentiation of GABAAR function (Celentano et al. 1988; Reynolds and Prasad 1991; Aguayo 1990; Nishio and Narahashi 1990; Sapp and Yeh 1998), but this sort of effect has not been observed in every neuronal type examined (e.g. McCool et al. 2003; White et al. 1990; Yamashita et al. 2006). A tonic GABAA-mediated current is observed in many CNS neurons, and is thought to reflect the function of extrasynaptic, high affinity GABA receptors containing the δ receptor subunit (Hanchar et al. 2005). The Potentiation of this tonic current has been observed in recordings from cerebellum, hippocampus, and thalamus using the brain slice preparation (Hanchar et al. 2005; Wei et al. 2004; Glykys et al. 2007; Jia et al. 2008, although see Botta et al. 2007).
Recent studies suggest that EtOH potentiation of GABAA receptor function depends on protein phosphorylation. Messing and co-workers have shown that activity of the epsilon subunit of protein kinase C (PKC) is necessary for EtOH potentiation of γ2-subunit-containing GABAA receptors expressed heterologously in a mammalian cell line (Qi et al. 2007). This PKC action appears to involve phosphorylation of a specific serine residue on the γ2 subunit. This finding may explain data from previous studies indicating the involvement of PKC in EtOH potentiation of GABAergic transmission (Weiner et al. 1994). However, in this earlier study it was not clear if the EtOH effects on transmission involved pre or postsynaptic mechanisms. A parallel line of investigation indicates that PKCδ is necessary for EtOH potentiation of tonic current involving δ-subunit-containing GABAARs (Choi et al. 2008). It is not yet clear whether acute EtOH exposure activates PKC phosphorylation of the GABAAR or whether phosphorylation on key amino acid residues is permissive for EtOH potentiation of receptor function, and this will be an interesting topic for future research.
EtOH potentiation of glycine-activated chloride channels appears to be dependent on receptor subunit composition. Potentiation is consistently greater at receptors containing the α1 subunit (Davies et al. 2003; Mascia et al. 1996; Mihic et al. 1997), at least when expressed in xenopus laevis oocytes (Valenzuela et al. 1998b, although see McCool et al. 2003; Yevenes et al. 2008). Receptors containing the α2 subunit also exhibit EtOH potentiation (McCool et al. 2003), but may be less sensitive than those containing the α1 subunit (Mascia et al. 1996). Inclusion of the β subunit along with α2 eliminates potentiation (McCool et al. 2003). Potentiation has also been observed in neurons from the brain and spinal cord, particularly in regions where the α1 subunit is expressed (Aguayo et al. 1996; Ye et al. 2001). Potentiation of the function of GABAA and glycine receptors is thought to increase inhibition of neurons. The relative influence of effects on synaptic versus extrasynaptic channels in producing this inhibition remains to be determined.
Acute EtOH exposure potentiates the function of 5-HT3 receptors that contain an intrinsic cation channel (Lovinger 1991; Machu and Harris 1994). It is yet to be determined whether this action alters pre or postsynaptic mechanisms activated by this receptor.
1.1.2 Ionotropic Glutamate Receptors
The ionotropic glutamate receptors (iGluRs) constitute the second class of neurotransmitter- activated LGICs. Three major classes of iGluRs exist, the AMPA receptors (AMPARs, gene name GRIA, made by GluRs1-4), the NMDA receptors (NMDARs1-3, gene name GRIN), and the kainate receptors (KARs, made by GluRs5-7 and KAs1-2, gene name GRIK). These receptors are now thought to be tetrameric and each subunit contains a large N-terminal domain and an extracellular loop domain that together participate in ligand binding via a “venus fly-trap” motif (Gouaux 2004). The subunits have three membrane-spanning domains and a re-entrant pore-loop that forms the ion conduction pathway, as well as intracellular loops and a large intracellular C-terminal domain. The iGluRs are all cationpermeable, with varying ratios of Na+, K+ and Ca2+ selectivity. These receptors are present on all CNS neurons, where they mediate fast synaptic transmission and activation of intracellular signaling.
EtOH has consistent inhibitory actions on iGluRs (although see Lu and Yeh 1999) (Fig. 1c, d). Inhibition of NMDARs at EtOH concentrations associated with intoxication is the best characterized of these effects (Criswell et al. 2003; Dildy and Leslie 1989; Hoffman et al. 1989; Lima-Landman and Albuquerque 1989; Lovinger et al. 1989). The synaptic responses mediated by NMDARs are also reduced by EtOH (Lovinger et al. 1990; Morrisett and Swartzwelder 1993; Roberto et al. 2004b; Wang et al. 2007).
Functional NMDARs always contain an obligatory NR1 subunit in combination with at least one NR2 or NR3 subunit. While EtOH inhibits all NMDAR subtypes, differences in the sensitivity to inhibition have been observed for recombinant with receptors containing different subunit compositions. The most common observation is that EtOH is less potent at receptors containing the NR1/2C composition in comparison to those containing NR1/2A or NR1/2B (Masood et al. 1994; Chu et al. 1995, but see Kuner et al. 1993; Lovinger 1995). There are several splice variants of the NR1-subunit, and a recent comprehensive study by Woodward and co-workers showed that the NR1 splicing status, in combination with the identity of the co-assembled NR2 subunit, has small but reliable effects on EtOH sensitivity (Jin and Woodward 2006). This NR1 splice variant effect could account for the previous difference in reports of low EtOH sensitivity of NR2C-containing receptors. Receptors containing the NR3 subunit are relatively insensitive to inhibition by EtOH, but inclusion of the NR2B-subunit enhances the EtOH inhibitory action on NR3-containing receptors (Jin et al. 2008). In addition, Mg2+ enhances EtOH inhibition of several NR1/2 and N1/2/3 receptor combinations, especially when NR2B is present (Jin et al. 2008). This finding may account for the larger effect of EtOH on NR2B containing NMDARs seen in some neuronal preparations (e.g. Fink and Göthert 1996; Lovinger 1995).
EtOH also inhibits the function of AMPARs, and effects can be seen at concentrations as low as 10 mM (Akinshola 2001; Akinshola et al. 2003; Dildy-Mayfield and Harris 1992; Möykkynen et al. 2003; Nieber et al. 1998; Wirkner et al. 2000). In neurons from the brain, EtOH generally shows lower potency for inhibition of AMPARs in comparison to NMDARs (Frye and Fincher, 2000; Lovinger et al. 1989; Lovinger 1995). The EtOH sensitivity of recombinant AMPAR receptors is not greatly altered by changing the receptor subunit composition (Lovinger 1993), although the potency of EtOH is slightly higher for inhibition of GluR1-containing in contrast to GluR3-containing GluRs in X. laevis oocytes (Akinshola 2001). In addition, recombinant AMPA receptors containing GluRs 2 and 3 exhibit slightly decreased EtOH sensitivity in comparison to those containing GluRs1, 2, and 3 or 3 alone (Akinshola et al. 2003). Recent studies suggest that this EtOH action involves increased receptor desensitization (Möykkynen et al. 2003, 2009), and thus the drug has little impact on AMPAR-mediated synaptic responses at most synapses given that desensitization does not contribute to the amplitude or time course of excitatory postsynaptic currents (EPSCs) (Lovinger et al. 1990; Ariwodola et al. 2003, but see Nie et al. 1993, 1994; Roberto et al. 2004b; Mameli et al. 2005; Zhu et al. 2007).
Inhibition of KAR-mediated responses has been observed at quite low EtOH concentrations (Costa et al. 2000; Lack et al. 2008; Valenzuela et al. 1998a; Weiner et al. 1999). However, direct examination of KAR-mediated ion current has yielded mixed results, at least for the receptor constructs examined to date (Dildy-Mayfield and Harris 1992; Valenzuela et al. 1998a). Thus, it is not yet clear whether EtOH inhibition of KAR function involves a direct effect on protein function or a more indirect action. EtOH inhibition of iGluRs is generally thought to dampen neuronal excitability in many brain regions by reducing excitatory synaptic drive and inhibiting synaptic plasticity that requires iGluR activation.
1.1.3 Purinergic Ligand-Gated Ion Channels
The third major subtype of LGIC is the P2X purinergic receptor subclass. P2X receptors are trimeric (Mio et al. 2005) with each subunit containing an N-terminal ligand binding domain, two membrane-spanning domains linked by an extracellular ligand binding domain, and a C-terminal intracellular domain of moderate length. The second membrane-spanning domain appears to serve as the lining for the ion conduction pathway. EtOH inhibits the function of most P2X receptor subtypes, with some effects reported at concentrations associated with intoxication (Davies et al. 2002; Li et al. 1993). The P2X4 receptor appears to be the most sensitive to inhibition by EtOH, while P2X3 receptors exhibit EtOH-induced potentiation (Davies et al. 2002, 2005). At present, the physiologic consequences of P2X inhibition are unclear.
1.2 G protein-Coupled Receptors and Roles in Ethanol Effects
The majority of neurotransmitter receptors are members of the G protein-coupled receptor (GPCR) superfamily. These receptors are specialized for binding a neurotransmitter, and this binding stimulates rearrangement of the protein to favor activation of intracellular signaling proteins known to bind GTP and GDP. In the GTP-bound state, the G protein is activated. Several forms of intracellular signaling proteins are affected by activated G proteins, including proteins that generate small molecule second messengers, as well as protein kinases and ion channels. Thus, G protein activation can affect neurophysiology fairly directly by altering ion channel function, and can have a long-lasting influence on neuronal function by altering intracellular signaling and even gene expression.
Receptor-activated G proteins are heterotrimeric, consisting of α, β, and γ subunits. The β and γ subunits form a tight complex, but when the G protein is activated the α subunit affinity for the β/γ complex is reduced. The result is that two signaling elements arise from the G protein activation and can act on different intracellular targets. The GPCRs act predominantly on three G protein subclasses: Gi/o, Gq-like, and Gs-like (Wickman and Clapham 1995). The Gi/o G protein class has net inhibitory effects on neuronal function, through actions of both the α and β/γ protein subunits. For example, the α subunit inhibits the enzyme adenylyl cylase (AC) that normally generates the second messenger cAMP. The β/γ subunits activate potassium channels that inhibit neuronal activity (the so-called G protein-activated inward rectifier, GIRK, potassium channels). The β/γ subunits also inhibit the function of voltage-gated calcium channels, leading to inhibition of neurotransmitter release, and also appear to have more direct effects on vesicle fusion (Dolphin 2003; Elmslie 2003; Miller 1998; Wu and Saggau 1994). The Gq-like α subunits activate protein and lipid signaling pathways that activate ion channels that excite neurons, inhibit potassium channels, and increase neurotransmitter release. Thus, activation of the Gq-subclass generally has a net excitatory effect on neuronal activity and synaptic transmission. The proximal effects of Gs-like G protein activation are not always clear. The α subunit of these G proteins stimulates AC/cAMP formation which can enhance synaptic transmission and inhibits some potassium channels. The effects on ion channel function of the different G proteins are outlined in detail in previous review articles (Dolphin 2003; Elmslie 2003; Wickman and Clapham 1995).
Direct effects of acute EtOH on the function of GPCRs and G proteins are generally weak. Furthermore, the physiologic impact of these actions is not always clear. However, there are mechanisms involving these molecules that are influenced by EtOH. Studies beginning in the 1980s showed that EtOH can stimulate cAMP formation (Luthin and Tabakoff 1984; Rabin and Molinoff 1981). This may be due to direct EtOH actions on AC, but other proteins that influence GPCRs and their signaling might play roles in the neural actions of EtOH (Bjork et al. 2008). The physiologic consequences of this AC activation have long been unclear. However, recent studies indicate that acute EtOH exposure can increase neurotransmitter release (described in greater detail later in this review, Fig. 1), and activation of AC is a strong candidate to mediate these effects (Kelm et al. 2008).
In heterologous expression systems, EtOH has been shown to inhibit responses to activation of GPCRs that couple to Gq-like G proteins. These findings mostly involve demonstrations that pharmacologically relevant concentrations of EtOH reduce the ability of the GPCRs to activate a calcium-dependent chloride current in the Xenopus laevis oocyte preparation (Minami et al. 1997a, b, 1998). Among the GPCRs that have been examined in this context are metabotropic glutamate receptors (mGluRs), muscarinic ACh receptors and serotonin type 2 receptors. The observation that these three receptor effects are all inhibited despite differences in the structures of the receptor molecules themselves, indicates that the EtOH target site is likely downstream of the receptor itself. Indeed there is some evidence for involvement of protein kinase C, at least in the inhibition of muscarinic AChR-induced responses (Minami et al. 1997b).
EtOH can also potentiate the function of GIRK-type potassium channels (Aryal et al. 2009; Kobayashi et al. 1999; Lewohl et al. 1999). This effect occurs at concentrations associated with intoxication. The net effect of GIRK activation is to inhibit neuronal activity. This action of EtOH was originally observed in cerebellar granule neurons (Lewohl et al. 1999), and subsequent studies have indicated similar actions in midbrain dopaminergic neurons (Federici et al. 2009). EtOH effects on this G protein target may contribute to intoxication. Studies by Blednov et al. (2001) indicate that loss of the GIRK2 channel subunit alters acute EtOH actions. There is certainly a need for additional studies of how GIRK activation might contribute to intoxication.
1.3 Presynaptic Effects of Ethanol
EtOH potentiation of GABAergic synaptic inhibition is now known to result from both pre and postsynaptic actions. As discussed in the section on LGICs, the postsynaptic effects result from potentiation of GABAA/anion channels. Recent studies indicate that EtOH also acts to enhance GABA release from presynaptic terminals, and this action contributes to enhanced synaptic inhibition (reviewed in Siggins et al. 2005) (Fig. 1). Increases in fast GABAergic synaptic transmission during EtOH treatment have been observed in cerebellum, hippocampus, ventral tegmental area (VTA), hypoglossal nucleus, and amygdala, both basolateral and central nuclei (Ariwodola and Weiner 2004; Ming et al. 2006; Kelm et al. 2007; Theile et al. 2008; Zhu and Lovinger 2006; Roberto et al. 2003; Sebe et al. 2003; Ziskind-Conhaim et al. 2003). These studies have been carried out mostly in brain slices and isolated brain neurons. Examination of spontaneous and miniature GABAergic IPSCs allows investigators to determine whether the frequency of synaptic events is altered (a likely presynaptic change), or whether the amplitude is affected (likely a postsynaptic change). Such analyses have consistently shown that sISPC and mIPSC frequencies are increased at EtOH concentrations associated with intoxication, at least in the amygdala, cerebellum, hippocampus and VTA (Ariwodola and Weiner 2004; Zhu and Lovinger 2006; Theile et al. 2008; Roberto et al. 2003; Kelm et al. 2007). These effects are rapid at onset and rapidly reversible following EtOH removal from tissue.
At present, little is known about the mechanisms underlying EtOH potentiation of GABA release. The increase in mIPSC frequency suggests that the site of EtOH action is downstream of action potential generation and calcium entry into the presynaptic terminal. Experiments in the cerebellum and VTA suggest that EtOH interacts with mechanisms involved in intracellular calcium release, perhaps increasing calcium concentrations in the presynaptic terminal (Kelm et al. 2007; Theile et al. 2009). It would be helpful to know whether EtOH increases calcium concentrations in the relevant population of GABAergic presynaptic terminals. However, this is difficult to determine given the small size (<1 µM diameter) of terminals, and the diversity of subtypes of terminals found on any given neuron.
The role of intracellular signaling pathways in this potentiating EtOH effect has also been examined. It is well established that activation of AC or PKC potentiates transmission at synapses throughout the nervous system (see Leenders and Sheng 2005; Nguyen and Woo 2003 for review). Thus, it is logical to speculate that these signaling molecules might play a role in the acute alcohol action. Potentiation of GABA release onto cerebellar Purkinje neurons is eliminated in the presence of AC and protein kinase A (PKA) inhibitors (Kelm et al. 2008), and is also affected by compounds targeting phospholipase C and PKC (Kelm et al. 2010). The potentiating effect of EtOH is impaired in central amygdala (CeA) in mice that lack PKCε (Bajo et al. 2008). Thus, PKC is implicated in both the pre and postsynaptic effects of EtOH at GABAergic synapses. It is notable that GABA release appears to be increased in the PKCε knockout mice prior to EtOH exposure, and thus the effect in this case may be more akin to occlusion rather than blockade of the drug action. It remains to be determined whether the effects of EtOH on these signaling molecules are direct or indirect.
In contrast to the effects on GABA release, the vast majority of studies indicate that acute EtOH either has no effect or inhibits release of glutamate (reviewed in Siggins et al. 2005, although see Xiao et al. 2009; Eggers and Berger 2004). These findings suggest a fundamental difference between GABAergic and glutamatergic terminals in most brain regions that may be useful in determining what factors contribute to EtOH sensitivity of release.
1.4 Monoamines and Neurotransmitter Transport
Acute EtOH effects on neurotransmitter transport have been investigated using brain tissue and heterologous expression systems. In vivo studies indicate that EtOH increases monoamine levels in brain (reviewed in Gonzales et al. 2004, LeMarquand et al. 1994; Thielen et al. 2001). However, most studies of neurotransmitter transporters show them to be relatively insensitive to EtOH. However, increased cell surface expression of the dopamine transporter (DAT) was observed when this protein was heterologously expressed (Mayfield et al. 2001; Maiya et al. 2002). This effect would most likely decrease striatal dopamine during acute in vivo EtOH exposure in rodents, and thus does not help to explain the findings from in vivo studies. However, there is some controversy as to whether EtOH has potent effects on dopamine uptake measured in brain tissue using voltammetric techniques (Jones et al. 2006; Mathews et al. 2006; Robinson et al. 2005; Yavich and Tiihonen 2000). The EtOH-induced increase in striatal DA levels is unperturbed in DAT knockout mice, suggesting that the drug action responsible for this effect does not involve the transporter (Mathews et al. 2006). Furthermore, studies using in vitro voltammetry and in vivo microdialysis to measure dopamine levels indicate that direct infusion of EtOH into striatum does not alter DA levels (Mathews et al. 2006; Yan 2003; Yim et al. 1998). Thus, the physiologic impact of alterations in DAT function is not yet clear.
Examination of EtOH effects on the brain serotonergic system has yielded interesting findings. In addition to potentiating 5-HT3 receptor function, as mentioned in the previous section on ligand-gated ion channels, inhibition of 5-HT1c by EtOH has also been reported (Sanna et al. 1994) although it is not clear whether this inhibition results from a direct effect on the receptor or on downstream signaling mechanisms. Exposure to acute EtOH also increases extracellular 5-HT levels in brain (LeMarquand et al. 1994; Thielen et al. 2001), and a recent report indicates that reduced 5-HT uptake may contribute to this effect as well as to the acute intoxicating effects of EtOH (Daws et al. 2006). However, EtOH effects on serotonin and other monoamines require further examination.
1.5 Ethanol and Synaptic Plasticity
Long-lasting changes in the efficacy of synaptic transmission are thought to contribute to brain development, learning and memory, and addiction (Hyman et al. 2006; Kauer and Malenka 2007). The most commonly studied forms of long-lasting synaptic plasticity are long-term potentiation (LTP), a persistent increase in synaptic transmission, and long-term depression (LTD), a persistent decrease in transmission. These types of plasticity are usually brought about by repetitive patterned activation of afferent inputs to a given postsynaptic neuron.
Effects of EtOH on LTP have been studied in different brain regions, but the majority of information comes from studies of the Schaffer collateral inputs to the CA1 pyramidal neurons of the hippocampal formation (Blitzer et al. 1990; Morrisett and Swartzwelder 1993; Mulkeen et al. 1987; Sinclair and Lo 1986). Acute EtOH exposure generally suppresses the induction of LTP at this and other synapses (Yin et al. 2007; Blitzer et al. 1990; Givens and McMahon 1995; Morrisett and Swartzwelder 1993; Mulkeen et al. 1987; Sinclair and Lo 1986; Wayner et al. 1993; Weitlauf et al. 2004). Effects occur at EtOH concentrations associated with intoxication, and in some studies at surprisingly low concentrations (Blitzer et al. 1990; Fujii et al. 2008). EtOH also inhibits LTP induced by kainate receptor activation in the basolateral amygdala (Lack et al. 2008).
There is not as much information regarding EtOH effects on LTD. Two prominent subtypes of LTD can be elicited in the hippocampal CA1 region. The most widely studied form of LTD is induced by repetitive low-frequency synaptic activation, and requires activation of NMDA receptors (Dudek and Bear 1992; Mulkey and Malenka 1992). In the hippocampal CA1 region LTD is enhanced by exposure to EtOH at a concentration associated with strong intoxication (Hendricson et al. 2002), although this observation has not been consistent (Izumi et al. 2005).
Other forms of LTD observed in hippocampus and elsewhere involve activation of mGluRs (reviewed in Lüscher and Huber 2010). One report indicates that EtOH, at concentrations associated with severe intoxication, prevents mGluR-LTD at hippocampal synapses (Overstreet et al. 1997). At glutamatergic synapses onto cerebellar Purkinje neurons mGluR-LTD involves decreased surface expression and function of AMPARs (Ito 2001). Acute EtOH exposure inhibits this cerebellar LTD (Belmeguenai et al. 2008; Su et al. 2010), most likely due to inhibition of voltage-gated calcium channels and mGluR function. This finding is intriguing given that acute EtOH is known to impair motor coordination, and cerebellar function has been implicated in these effects. In the dorsal striatum, LTD involving these receptors also requires endocannabinoid (EC) signaling from the post to the presynaptic neuron (retrograde EC signaling) and subsequent activation of CB1 cannabinoid receptors (Gerdeman et al. 2002). The expression of this form of LTD appears to be on the presynaptic side of the synapse. Acute EtOH increases the expression of this EC-dependent mGluR-LTD in dorsal striatum (Yin et al. 2007). It is presently not clear what mechanisms contribute to this effect of EtOH.
2 Chronic Ethanol Actions
2.1 Chronic Ethanol Effects on Glutamatergic Transmission and Glutamate Roles in Synaptic Plasticity
Chronic EtOH treatment in animals provides critical information relevant to central changes that take place during long-term alcohol abuse in humans. Persistent EtOH exposure produces both tolerance and dependence. Tolerance is manifested as a decreased behavioral response to EtOH that implies a decrease in the intoxicating effects and other responses to the drug. Therefore, higher amounts of EtOH are required to achieve the same intoxicating effects seen with acute drug administration. EtOH dependence is generally described by symptomology elicited during and following withdrawal from EtOH (Heilig et al. 2010). These effects include anxiety, dysphoria and increased seizure susceptibility, hyperalgesia and disruption of sleep states (Enoch 2008; Grobin et al. 1998; Kumar et al. 2009). Chronic EtOH treatment is known to induce many neuroadaptative changes in the CNS involving both glutamatergic and GABAergic synaptic transmission.
The majority of work on chronic EtOH effects on glutamatergic transmission has focused on changes in glutamate receptors, particularly in light of the sensitivity of these receptors to acute EtOH actions (see previous discussion). Chronic EtOH exposure generally produces an increase in the function of NMDARs and in NMDAR-mediated glutamatergic synaptic transmission (Cebere et al. 1999; Grover et al. 1998; Gulya et al. 1991; Lack et al. 2007; Smothers et al. 1997) (Fig. 1d). Initial studies examined effects of receptor activation on neuronal calcium and nitric oxide signals either in preparations made from EtOH-exposed animals or in cultured neurons treated with EtOH in the medium (Grover et al. 1998; Gulya et al. 1991; Chandler et al. 1997; Iorio et al. 1992; Smothers et al. 1997). Exposure to EtOH for days to weeks increased NMDAR agonist-induced increases in intracellular calcium. These effects could be observed at EtOH concentrations that did not alter neuronal viability and did not affect baseline intracellular calcium levels. Furthermore, changes in responses to NMDAR activation were consistently larger than changes in the effects of activation of other ionotropic glutamate receptors (Chandler et al. 1997; Gulya et al. 1991; Smothers et al. 1997). Direct examination of ion current through the NMDAR pore has revealed effects consistent with a chronic EtOH-induced upregulation of NMDAR function (Floyd et al. 2003; Grover et al. 1998). An increase in the component of current mediated by NR2B-containing receptors has also been observed (Floyd et al. 2003; Kash et al. 2009; Roberto et al. 2004b, 2006). Interestingly, acute EtOH inhibition of NMDARs in most brain regions is still intact or even increased after chronic in vivo exposure (Floyd et al. 2003; Roberto et al. 2006; Roberto et al. 2004b), although a small decrease in inhibition was observed in medial septum/diagonal band neurons (Grover et al. 1998). Evidence of tolerance to EtOH inhibition during acute exposure has also been observed in hippocampal slices (Grover et al. 1994; Miyakawa et al. 1997). Overall, it appears that NMDAR function is still suppressed during intoxication even after prolonged EtOH exposure, and thus the increase in NMDAR function is likely to be dramatic after EtOH withdrawal following chronic exposure. One consequence of the increase in NMDAR-mediated calcium influx appears to be an increase in susceptibility to excitotoxic effects of NMDA (Chandler et al. 1993; Iorio et al. 1993), although enhanced NMDAR-mediated neuroprotection can also be observed in young cerebellar granule neurons (Pantazis et al. 1998). It has thus been postulated that excitotoxicity during EtOH withdrawal contributes to alcohol-related neuronal loss in the brain.
The mechanisms underlying the increase in NMDAR function are still under investigation, but several interesting facets of the story have already emerged. Analysis of receptor function and pharmacology, as well as examination of receptor subunit expression and location, indicate that receptors containing the NR2B subunit are the subtypes most strongly affected by chronic EtOH exposure (Carpenter-Hyland et al. 2004; Floyd et al. 2003; Kash et al. 2009; Roberto et al. 2004b) (Fig. 1d). The molecular basis of increased NR2B function is less clear. While some investigators have reported increases in NR2B mRNA expression following chronic alcohol exposure in vitro (Hu et al. 1996; Snell et al. 1996), and in vivo (Follesa and Ticku 1995; Kash et al. 2009; Roberto et al. 2006) such increases have not been observed in every brain region (Cebere et al. 1999; Floyd et al. 2003; Läck et al. 2005). Increases in NR2B, and to a lesser extent NR2A, protein expression have also been observed using immunologic techniques after both in vitro and in vivo EtOH exposure (Kash et al. 2005; Obara et al. 2009; Snell et al. 1996). However, other investigators did not observe increased expression of this protein. Increased expression of mRNA and protein for other NR subunits and particular NR1 splice variants has been observed in some brain regions following chronic EtOH exposure (Raeder et al. 2008; Trevisan et al. 1994; Roberto et al. 2006; Winkler et al. 1999, but see Morrow et al. 1994), but there is less evidence for increased receptor function as a result of these increases. Thus, it is not clear whether increased subunit expression is the driving force behind increased receptor function, and if so, what mechanisms underlie the increase in expression or trafficking.
Changes in subcellular distribution of receptors may also contribute to altered NMDAR function following chronic EtOH exposure. In cultured hippocampal neurons, exposure to EtOH leads to increased NMDAR expression in dendritic spines, the location of glutamatergic synapses (Carpenter-Hyland et al. 2004). This increased trafficking to spines is accompanied by an increase in the contribution of NMDARs to glutamatergic transmission, but does not appear to involve increased NMDAR protein expression. The synaptic NMDARs observed following chronic EtOH exposure appear to contain the NR2B subunit. Increases in the contribution of NMDARs to glutamatergic synaptic transmission have also been observed following subacute (10 s of seconds or minutes) EtOH exposure, and NR2Bcontaining receptors also appear to contribute to these increases (Wang et al. 2007; Yaka et al. 2003). Tyrosine phosphorylation by a Fyn-like kinase has been implicated in these rapid increases in the function of NR2B-containing receptors (Wang et al. 2007), but it is yet to be determined whether this mechanism plays a role in chronic EtOH effects on the receptor.
Chronic EtOH effects on AMPA and kainate receptors have been examined, with variable results. Increases in AMPA receptor subunit mRNA have been observed in hippocampus following chronic EtOH exposure (Bruckner et al. 1997). Expression of AMPAR subunit proteins was also induced by chronic exposure in primary cortical cultures (Chandler et al. 1999), while increased AMPAR binding was observed in cortical membranes from EtOH exposed animals (Haugbol et al. 2005). Evidence of increased AMPAR function has also been reported following chronic EtOH exposure, as measured with intracellular calcium signals in cerebellar Purkinje neurons (Netzeband et al. 1999), and AMPA receptor-mediated synaptic responses are increased in basolateral amygdala (Lack et al. 2007). This latter effect was observed following during withdrawal but not just after the end of chronic EtOH exposure. However, other studies have reported that AMPAR expression and function are not altered following chronic EtOH exposure (e.g. Smothers et al. 1997). The factors that underlie this variability in findings may include the type of preparation examined, the duration and pattern of EtOH exposure, and whether assays were performed just after the end of drug exposure or after withdrawal had been allowed to proceed. With respect to kainate receptors, Chandler and collaborations (Chandler et al. 1999) observed no change in receptor expression in cultured cortical neurons following chronic EtOH exposure. In contrast, enhancement of both subunit protein and kainate receptor function was found in cultured hippocampal neurons (Carta et al. 2002), and chronic intermittent EtOH increased KAR-mediated synaptic transmission in basolateral amygdala (Lack et al. 2009).
Chronic EtOH intake has also been shown to enhance intracellular signaling associated with mGluRs, particularly mGluR5, in the nucleus accumbens (NAc) (Cozzoli et al. 2009). While chronic EtOH drinking can induce increases in mGluR1 and mGluR5 protein expression in NAc and amygdala (Szumlinski et al. 2008; Obara et al. 2009), changes in mGluR5 signaling in NAc are not always associated with an increase in the protein itself (Szumlinski et al. 2008). In cultured cerebellar Purkinje neurons, exposure to EtOH for 11 days produced a decrease in mGluR-induced dendritic calcium signals (Netzeband et al. 2002). Clearly, more work is needed to determine how signaling by the many mGluR subtypes changes with long-term EtOH exposure and drinking.
Measurements of extracellular glutamate levels in brain have generally shown increases produced by chronic EtOH exposure, especially after withdrawal or repeated cycles of withdrawal (Dahchour and De Witte 1999, 2003; Rossetti and Carboni 1995; Roberto et al. 2004b). These findings have generally been derived from measurements using in vivo microdialysis in brain. However, microdialysis measures of this type must be interpreted carefully, as both synaptic and nonsynaptic sources of glutamate contribute to the extracellular pool of this amino acid. Indeed, there is mounting evidence that changes in the cystine/glutamate exchanger generate increases in extracellular glutamate produced by some drugs of abuse (Kalivas 2009). Evidence of increased synaptic glutamate release has been observed in amygdala following chronic EtOH treatment (Lack et al. 2007; Zhu et al. 2007; Roberto et al. 2004b). Decreases in glutamate uptake have also been noted following chronic EtOH exposure (Melendez et al. 2005). There may be multiple factors that contribute to increased extracellular glutamate levels and increased glutamatergic transmission following chronic EtOH exposure and withdrawal.
Despite the evidence that NMDAR function and extracellular glutamate levels are increased following chronic EtOH exposure, studies of hippocampal LTP indicate that this form of synaptic plasticity is decreased under the same conditions (Durand and Carlen 1984; Roberto et al. 2002, although see Fujii et al. 2008). Similar results have been obtained in the amygdala (Stephens et al. 2005). It is not yet clear what factors underlie the decrease in LTP, but it is most likely that the loss of plasticity involves mechanisms occurring downstream of NMDAR activation in the LTP induction process.
2.2 Chronic Ethanol and GABAergic Transmission: Postsynaptic Effects
Chronic EtOH treatment is known to induce many neuroadaptative changes in the CNS. Over the past 20 years, it has been widely demonstrated that GABAergic transmission is sensitive to EtOH in distinct brain regions and is clearly involved in EtOH tolerance and dependence (Eckardt et al. 1998; Grobin et al. 1998). Chronic EtOH exposure often results in the development of tolerance to many GABAergic effects of the drug including the anxiolytic, sedative, ataxic, and positive reinforcing effects (Kumar et al. 2004, 2009). Substantial evidence suggests that these behavioral and neural adaptations involve marked changes in the expression profile of specific GABAA receptor subunits (Grobin et al. 1998) and in the pharmacological properties of GABAA receptors (Kang et al. 1998b) (Fig. 1).
Chronic EtOH administration differentially altered the expression of distinct GABAA receptor subunit mRNAs and peptide levels in various brain regions. In the cerebral cortex, both mRNA and peptide levels for GABAA receptor α1, α2, and α3 subunits were decreased (Devaud et al. 1995, 1997). In contrast, both α4, β1, β2, β3, γ1 and γ2 subunit mRNA and peptide levels were increased (Devaud et al. 1995, 1997). These alterations in the subunit expression affect the GABAA receptor assemblage and consequently, also affect receptor function and binding. It has been reported that recombinant GABAA receptors with α4β2γ2 subunits are less sensitive to GABA and benzodiazepines compared to α1β2γ2 receptors (Whittemore et al. 1996). Therefore, these alterations may account for the decreased sensitivity to GABA in cerebral cortical synaptoneurosomes (Morrow et al. 1988) and benzodiazepines in cortical membrane vesicles (microsacs) (Buck and Harris 1990). Following chronic EtOH exposure, acute EtOH did not facilitate the GABA or muscimol-stimulated Cl- uptake in cortex (Morrow et al. 1988) and in cerebellum (Allan and Harris 1987). In the cerebellum, chronic EtOH exposure decreased GABAA receptor α1 subunit mRNA and increased α6 subunit mRNA (Mhatre and Ticku 1992; Morrow et al. 1992). Chronic EtOH administration also decreased the polypeptide levels of the δ subunit of GABAA receptors in the rat cerebellum and hippocampus, whereas there were no changes in the δ subunit polypeptide levels in the rat cerebral cortex (Marutha Ravindran et al. 2007). Furthermore, chronic EtOH administration caused a down-regulation of native δ subunit-containing GABAA receptor assemblies in the rat cerebellum as determined by [(3)H]muscimol binding to the immunoprecipitated receptor assemblies (Marutha Ravindran et al. 2007).
The alterations in GABAA receptor gene expression are regionally and temporally dependent. For example, chronic EtOH consumption produced a significant increase in the level of GABAA receptor α4 subunit peptide in the hippocampus following 40 days but not 14 days exposure (Matthews et al. 1998). The relative expression of hippocampal GABAA receptor α1, α2, α3, β(2/3), or γ2 subunits was not altered by either period of chronic EtOH exposure (Charlton et al. 1997; Matthews et al. 1998). Hippocampal α1 subunit immunoreactivity and mRNA content were also significantly reduced after 12 weeks of treatment, but not after 4 weeks of exposure. In contrast, α5 mRNA content was increased in this brain region. In marked contrast, chronic EtOH consumption for both 14 (Devaud et al. 1997) and 40 (Devaud et al. 1997; Matthews et al. 1998) days significantly increased the relative expression of cerebral cortical GABAA receptor α4 subunits and significantly decreased the relative expression of α1 subunits (Devaud et al. 1997; Matthews et al. 1998). These findings indicate that chronic EtOH consumption alters GABAA receptor gene expression in the hippocampus but in a different manner from that in either the cerebral cortex or the cerebellum. In addition, these alterations are dependent on the duration of EtOH exposure (Grobin et al. 1998).
The Olsen and Spigelman groups have developed a chronic intermittent EtOH treatment paradigm in which rats are given a 5–6 g/kg dose of EtOH on alternate days for 60 treatments (120 days). This chronic administration of EtOH to rats on an intermittent regimen, for 60 repeated intoxicating doses and repeated withdrawal episodes, increases levels of α4 subunit mRNA in hippocampus with no significant change in the mRNAs for the α5 subunit (Mahmoudi et al. 1997). Similarly, rats that were exposed to intermittent episodes of intoxicating EtOH and withdrawal showed increased hippocampal α4 subunit peptide expression (Cagetti et al. 2003) and alteration in the pharmacological responses of GABAA receptors to benzodiazepine agonists and inverse agonists (Cagetti et al. 2003). The mRNA levels for the γ2S and γ1 subunits were also elevated. In CA1 pyramidal slices from chronic intermittent EtOH exposed rats, the baseline decay time of GABAAR-mediated mIPSCs was decreased, and the positive GABA receptor modulation of mIPSCs was also reduced compared with control rats. However, mIPSC potentiation by the α-preferring benzodiazepine ligand bretazenil was maintained, and mIPSC potentiation by Ro15-4513 was increased (Cagetti et al. 2003; Liang et al. 2009).
In the VTA, levels of α1 subunit immunoreactivity were significantly decreased after 12 weeks but not 1–4 weeks of treatment (Charlton et al. 1997). Papadeas et al. (2001) found that in the amygdala, α1 and α4 subunit expression was significantly decreased after two weeks of chronic EtOH consumption. In the nucleus accumbens (NAC), α4 subunit expression was decreased, but α1 subunit expression was not altered. In the VTA, there were no changes in α1 and α4 subunit expressions. Muscimol-stimulated Cl− uptake was enhanced in the extended amygdala, but not the NAC of EtOH-dependent rats. These results suggest that chronic EtOH exposure alters GABAA receptor expression in the amygdala and NAC and that decreased expression of α4 subunits is associated with increases in GABAA receptor function in the amygdala but not the NAC (Papadeas et al. 2001).
Alterations in subunit assembly could induce alterations in the functional properties of GABAA receptors without alterations in the total number of receptors (Devaud et al. 1995; Kumar et al. 2009; Morrow et al. 1992). The expression of GABAA receptors involves a highly regulated process of synthesis, assembly, endocytosis, and recycling or degradation. Changes in the expression and composition of various GABAA receptors could result from selective endocytosis, recycling, and/or trafficking of newly synthesized receptors to the cell surface. GABAA receptor trafficking on the cell surface following EtOH consumption is thought to contribute to the development of EtOH-dependence (Kumar et al. 2004). It has been reported by Kumar et al. (2003) that chronic EtOH exposure selectively increases the internalization of α1 GABAA receptors with no change in the internalization of α4 GABAA receptors into clathrin coated vesicles of the cerebral cortex. There is also a decrease in α1 GABAA receptors and a significant increase in α4 subunit peptide in the synaptic fraction following chronic EtOH exposure. These results suggest that the regulation of intracellular trafficking following chronic EtOH administration may alter the subtypes of GABAA receptors on the cell surface and may account for changes in the pharmacological properties of GABAA receptors (Kumar et al. 2004) (Fig. 1).
Clathrin and the adaptor complex (AP) play a crucial role in the internalization of GABAA receptors following chronic EtOH administration. Notably, in the intracellular fraction, the clathrin-α1-GABAA receptor complex is increased following chronic EtOH administration (Kumar et al. 2004). Specific GABAA receptor subunits (β2 and/or γ2) are required for recognition of the receptor by the AP-2 that precedes clathrin-dependent endocytosis (Herring et al. 2003; Kittler et al. 2008). Chronic EtOH exposure induces an increase in the expression of α4-, β2-, and β3- GABAA receptor subunits in the cerebral cortex and all of these subunits contain consensus phosphorylation sites for PKC. In contrast, α1, α2, and α3 GABAA receptor subunits are decreased in the cortex and these subunits do not contain consensus phosphorylation sites for PKC. Hence, it has been hypothesized that PKC may phosphorylate the GABAA receptor subunits and/or AP-2 following chronic EtOH administration, altering the recognition and endocytosis of GABAA receptors by blocking AP-2 binding (Macdonald 1995; Mohler et al. 1996). A single dose of EtOH also increases the internalization of GABAA receptor α4 and δ subunits (Liang et al. 2007). In rat hippocampus, chronic EtOH exposure induces a decrease in the tyrosine kinase phosphorylation of α1 subunits, an increase of β2 subunits and no alteration in γ2 subunits (Marutha Ravindran et al. 2007).
GABAA receptor trafficking is regulated by many protein kinases, including PKC, PKA, and fyn. However, to date, the role of these protein kinases has not yet been studied in the trafficking of GABAA receptors, especially following EtOH exposure. Chronic EtOH consumption decreases association of PKCγ with α1 GABAA receptors and increases association of PKCγ with α4 GABAA receptors, accompanied by a decreased expression of the α1 subunit and an increased expression of α4 at the cell surface in cerebral cortex (Kumar et al. 2002). However, there were no alterations in the association of PKCγ with GABAA receptors in the α1 subunit expression following chronic EtOH administration in the hippocampus (Kumar et al. 2004). The increased association of PKCγ with α4 GABAA receptors may phosphorylate GABAA receptor subunits and prevent recognition of the receptor by AP-2, thus preventing its internalization. Indeed, phosphorylation of GABAA receptor subunits reduced the binding of receptors with AP-2 and subsequent internalization (Kittler et al. 2008). Moreover, reduced PKC-dependent GABAA receptor phosphorylation increases receptor binding to the AP-2 and promotes receptor endocytosis (Terunuma et al. 2008). Chronic activation of PKA in cerebellar granule cells increases cell surface expression of GABAA receptor α1 subunit (Ives et al. 2002). EtOH exposure alters expression and translocation of PKA (Diamond and Gordon 1994; Newton and Messing 2006) suggesting that PKA is likely also involved in the trafficking of GABAA receptors following EtOH exposure. Future studies will determine the specific role of various protein kinases in GABAA receptor trafficking following chronic EtOH administration.
Post-translational modifications such as phosphorylation and glycosylation of GABAA receptors may play a role in the development of EtOH-dependence. In particular, phosphorylation of GABAA receptors has been demonstrated to modulate receptor function. In Xenopus oocytes and isolated mouse brain membrane vesicles (microsacs), PKC and PKA phosphorylation of GABAA receptors decreases receptor activation (Kellenberger et al. 1992; Krishek et al. 1994; Leidenheimer et al. 1992). Phosphorylation by CAM kinase II or tyrosine kinase enhances GABAA receptor function (Churn et al. 2002; Valenzuela et al. 1995). As discussed previously, acute EtOH induces changes in GABAA receptor function that may be dependent on phosphorylation of particular proteins. Chronic EtOH exposure might be expected to result in long-term changes in second messenger systems, including kinase activity. However, the heterogeneity of GABAA receptors expressed in vivo has precluded definitively answering this question and none of these studies have directly demonstrated that phosphorylation is involved in EtOH modulation of GABAA receptor function. The exact mechanisms involved in the alteration of GABAA receptor function following chronic EtOH exposure still remain to be determined.
From the preceding review, it is clear that the majority of the early studies characterizing chronic effects of EtOH on GABAergic transmission focused mainly on postsynaptic properties and the subunit composition of the GABAA receptors themselves. Some of the disparity in the findings across laboratories on postsynaptic sites of EtOH action may reflect the differences in the chronic EtOH treatment duration and protocol, brain region examined, and methods of assessing receptor function. Most of these studies were generally in agreement that chronic EtOH exposure and withdrawal did not result in dramatic decreases in the number of GABAA receptors in most brain regions. However, many of these studies reported marked alterations in the expression of specific GABAA receptor subunits and hypothesized that those changes in the subunit composition of the GABAA receptors may account for the physiologic and pharmacologic alterations in GABAergic signaling associated with chronic EtOH administration (Grobin et al. 1998).
Of particular clinical importance is the development of tolerance and dependence to EtOH, and it is likely that adaptive changes in synaptic function in response to ethanol’s actions on GABAA receptors play a role in this process. Indeed, it is well known that chronic EtOH treatment can lead to tolerance and physical dependence (Chandler et al. 1998) and withdrawal following long-term EtOH consumption is associated with increased neuronal excitability (Kliethermes 2005; Weiner and Valenzuela 2006). These alterations have been hypothesized to represent, in part, a compensatory adaptation to the in vitro acute facilitatory effects of EtOH on GABAergic synapses (Siggins et al. 2005; Weiner and Valenzuela 2006). Few studies have reported the effects of long-term EtOH exposure on GABAergic synaptic transmission looking at both postsynaptic and presynaptic mechanisms using in vitro brain slice methods.
As described above, the adaptive changes in GABAA receptor expression are thought to lead to a pronounced hypofunction of GABAergic neurotransmission and possibly the development of tolerance to the in vitro acute effects of EtOH on these synapses. In the hippocampus, there is a decrease in the threshold for seizure induction by the GABAA receptor antagonist pentylenetetrazole (Kokka et al. 1993) and a decrease in GABAA receptor activity in hippocampal slices that also lasts for at least 40 days after the last EtOH dose (Cagetti et al. 2003; Kang et al. 1996; Liang et al. 2004, 2009). Using analysis of tetrodotoxin (TTX)-resistant mIPSCs recorded from CA1 pyramidal neurons of chronic EtOH exposed and control rats, this group demonstrated a significant decrease in the amplitude and decay of these responses (Cagetti et al. 2003) possibly reflecting the observed alteration in the expression of α1 and α4 subunits. The mIPSC frequency is also slightly decreased, suggesting that chronic EtOH exposure may also be associated with a presynaptic decrease in GABA release at these synapses (see later section). Importantly, the pharmacological alterations in the properties of GABAergic synapses were consistent with the observed changes in subunit expression. For example, diazepam and the neurosteroid alphaxalone did not have any effect on mIPSCs in slices from chronic EtOH exposed rats (Cagetti et al. 2003), possibly reflecting the loss of α1 and γ-subunits, respectively.
On the other hand, drugs with some selectivity for α4-subunits (e.g., RO 15-4513 and DMCM) showed an increased modulation of mIPSCs possibly reflecting the increase in α4 subunit expression (Kang et al. 1996, 1998a, b). Interestingly, the evoked IPSCs were still sensitive to alphaxalone (Kang et al. 1998b) suggesting differences in the populations of GABAA receptors that underlie evoked and mIPSCs. In addition, the acute effect of EtOH on evoked IPSCs was significantly increased in slices from chronic EtOH-exposed rats (Kang et al. 1998a, b). Liang et al. (2004) have also compared the effects of chronic EtOH exposure on synaptic and extra-synaptic receptor functions in CA1 neurons. These investigators found similar alterations in the synaptic mIPSCs and the tonic extrasynaptic GABAA receptor-mediated conductance associated with chronic EtOH exposure. Both mIPSCs and the tonic current show profound tolerance to α1-containing GABAA receptor selective doses of diazepam and zolpidem (Cagetti et al. 2003). As previously demonstrated (Grobin et al. 2000), chronic EtOH exposure results in a decrease in BZP-sensitive α1-subunits and an increase in BZP-insensitive α4-subunits at synaptic receptors. Thus, THIP (a high affinity and efficacy agonist of the α4-containing GABAA receptors and a partial agonist at most other GABAA receptor assemblies) activated the tonic GABA current in slices from control-untreated rats and had little effect in slices from chronic EtOH exposed rats (Liang et al. 2004). However, THIP depressed mIPSCs in control-untreated rats but strongly increased mIPSCs in chronic EtOH-treated rats. In addition, the chronic EtOH-treated rats show a modest tolerance to the soporific effects of THIP and no change in its anxiolytic effects (Liang et al. 2004).
In the previous decade, non-human primates (Cynomolgus macaques) have been a powerful model to study the effects of long-term EtOH consumption (Vivian et al. 2001). Ongoing research in the Weiner lab has provided the first evidence of neuroadaptations in the GABAergic synapses in monkey hippocampus (Weiner et al. 2005). In this paradigm of EtOH self-administration, cynomolgus macaques are trained to self administer a 4% EtOH solution on an operant panel and then given 22 h daily access to the EtOH solution. Control subjects were age-and sex-matched animals that had free access to food and water but were not exposed to the operant panels. The preliminary in vitro electrophysiologic findings revealed a significant increase in paired-pulse facilitation (PPF) of GABAA IPSCs in dentate granule cells in slices prepared immediately following the last day of 18 months of daily EtOH drinking. Their finding is consistent with a decrease in release probability (see later section) and is in agreement with the decrease in mIPSC frequency observed in rats following chronic intermittent EtOH exposure (Cagetti et al. 2003). Interestingly, there was lack of tolerance for both the acute facilitatory effect of EtOH and flunitrazepam on evoked GABAA IPSCs (Weiner et al. 2005). Using the same paradigm of EtOH self-administration, whole-cell patch clamp recordings on acutely dissociated amygdala neurons from EtOH-exposed cynomolgus macaques showed a decrease in the effect of flunitrazepam on the currents gated by exogenous GABA application compared with amygdala neurons from control animals (Anderson et al. 2007; Floyd et al. 2004). However, the modest inhibition of GABA-gated currents induced by acute EtOH was not affected by the chronic EtOH consumption. In addition, mRNA expression levels for the β, γ, and δ subunits in total amygdala RNA isolated from control and EtOH-drinking animals were measured. Chronic EtOH significantly reduced amygdala β1 and γ2 subunit expression. Overall, these findings demonstrate that chronic EtOH self-administration reduces the benzodiazepine sensitivity of amygdala GABAA receptors and this reduced sensitivity may reflect decreased expression of the γ subunit.
Roberto et al. (2004a) recently assessed whether GABAergic synaptic changes occur with EtOH-dependence in rat central amygdala (CeA) slices. To obtain dependent rats, these investigators used an EtOH vapor inhalation method (Rogers et al. 1979). In this study, male Sprague–Dawley rats were exposed to a continuous EtOH vapor for 2–3 weeks with a targeted blood alcohol level of 150–200 mg/dL. Control rats were maintained in similar chambers without EtOH vapor. On experiment days, the chronic EtOH-treated rats were maintained in the EtOH vapor chamber until preparation of the CeA slices, and recordings of GABAergic transmission were made in EtOH-free solution 2–8 h after cutting the slices (Roberto et al. 2004a). The evoked IPSCs in CeA neurons from EtOH-dependent rats were significantly larger than in naïve rats. In EtOH-dependent rats, the mean baseline amplitude of mIPSCs was also significantly increased compared to naïve rats, suggesting a post synaptic effect of chronic EtOH (Roberto et al. 2004a). However, possible changes in the expression of GABAA receptor subunits were not characterized. It was also found that the baseline PPF ratio of IPSCs was significantly decreased and the mIPSC frequency was higher in neurons of EtOH-dependent rats compared to naïve rats, suggesting that GABA release was augmented in chronic EtOH treated rats (Roberto et al. 2004a) (see later section on presynaptic change).
In addition, acute EtOH (44 mM) increased IPSCs, decreased the PPF ratio of IPSCs and increased the mIPSCs frequency to the same extent in EtOH-dependent rats and naïve rats, suggesting a lack of tolerance for the acute EtOH effects (Roberto et al. 2004a). One of the most consistent findings from these recent studies is the lack of tolerance for the acute potentiating effect of EtOH on GABAergic synapses. These studies suggest that GABAergic mechanisms may not be associated with the tolerance that is known to develop with some of the behavioral effects of EtOH (e.g. ataxia, sedation). Additional studies will be needed to more carefully determine the molecular mechanisms responsible for these adaptive changes in different brain regions and length/duration of EtOH exposure required to induce such neuroadaptations in GABAergic synapse. Moreover, these data also suggest that, as with the acute effects of EtOH, long-term exposure to EtOH results in both pre and postsynaptic alterations and these changes may differ between brain regions (Siggins et al. 2005; Weiner and Valenzuela 2006).
2.3 Chronic Ethanol and GABAergic Transmission: Presynaptic Effects
There are only a few studies reporting that chronic EtOH exposure can alter GABAergic transmission by effects on GABA release. Short in vitro chronic EtOH exposure (one day) induced a transient decrease in mIPSC duration in cultured cortical neurons. Chronic EtOH exposure did not change mIPSC frequency nor did it produce a substantial cross-tolerance to a benzodiazepine in cortical neurons (Fleming et al. 2009). The results suggest that EtOH exposure in vitro has limited effects on synaptic GABAAR function and action potential-independent GABA release in cultured neurons. This group also investigated the effect of chronic EtOH exposure on GABA release in cultured hippocampal neurons (Fleming et al. 2009). These investigators found that chronic EtOH exposure did not alter mIPSC kinetics and frequencies in hippocampal neurons (Fleming et al. 2009). These results suggest that EtOH exposure in cultured cortical and hippocampal neurons may not reproduce all the effects that occur in vivo and in acute brain slices.
In fact, more results generated using in vitro brain slices show a stronger effect of EtOH on GABA release, as discussed earlier in this review (Fig. 1). In vitro brain slice preparations provide a number of highly sensitive experimental strategies that can be employed to detect presynaptic changes in transmitter release (for reviews of these approaches, see Siggins et al. 2005; Weiner and Valenzuela 2006).
Studies in the hippocampus show that chronic EtOH exposure decreased long-term potentiation (LTP) by increasing the electrically stimulated (but not basal) release of tritiated GABA pre-loaded in CA1 hippocampal slices (Tremwel et al. 1994). The GABA uptake or GABAAR function was not altered, and this effect may be due to alterations in the muscarinic receptor regulation of GABA release at presynaptic terminals (Hu et al. 1999). In addition, studies using the GABAB receptor agonist baclofen to reduce release of tritiated GABA suggest that a change in GABAB auto-receptors on GABAergic terminals may also contribute to this effect of chronic EtOH exposure on LTP (Peris et al. 1997) (see later GABAB paragraph). For a general review of brain region specific EtOH actions on the GABA system see (Criswell and Breese 2005; Siggins et al. 2005; Weiner and Valenzuela 2006). More recent studies also reported that chronic EtOH consumption induces tolerance to the impairing effects of acute EtOH treatment on induction of LTP in rat CA1 slices (Fujii et al. 2008). In CA1 slices from control rats, stable LTP was induced by tetanic stimulation, and LTP induction was blocked if the tetanus was delivered in the presence of 8.6 mM EtOH or muscimol. A decrease in the stimulation threshold for inducing LTP was found in hippocampal slices from chronic EtOH-treated rats. In addition, application of EtOH or muscimol did not affect LTP induction in these cells, suggesting that the effects of chronic EtOH exposure on LTP induction are mediated by a reduction in GABAergic inhibition in hippocampal CA1 neurons (Fujii et al. 2008).
Weiner et al. (2004) found that voluntary EtOH drinking is associated with a significant increase in paired-pulse plasticity at GABAergic synapses in dentate gyrus neurons from the hippocampal formation of monkeys (cynomolgus macaques), consistent with a reduction in GABA release probability. In addition, a lack of tolerance to the facilitating effects of both acute EtOH and flunitrazepam on the GABAA IPSCs was reported.
In contrast, Melis et al. (2002) reported that a single EtOH exposure in vivo induces a long-lasting facilitation of GABA transmission in the VTA of EtOH-preferring C57BL/6 mice. These investigators observed that evoked GABAA IPSCs in dopaminergic neurons of EtOH-treated animals exhibited paired-pulse depression (PPD) compared with saline-treated animals, which exhibited PPF (Melis et al. 2002). An increase in frequency of mIPSCs was also observed in the EtOH-treated animals. Moreover, the GABAB receptor antagonist, CGP35348, shifted PPD to PPF, indicating that presynaptic GABAB receptor activation, likely attributable to GABA spillover, might play a role in mediating PPD in the EtOH-treated mice (see later GABAB paragraph). In a more recent study, the same group (Wanat et al. 2009) demonstrated that EtOH exposure also increased GABA release onto VTA dopamine neurons in EtOH non-preferring DBA/2 mice. However, a single EtOH exposure reduced glutamatergic transmission and LTP in VTA dopamine neurons from the EtOH non-preferring DBA strain but not EtOH-preferring C57BL/6 mice (Wanat et al. 2009).
Additional data from Roberto et al. (2004a, 2010) further suggest that chronic EtOH exposure can affect CeA GABA release, perhaps via an action on GABAergic terminals. Baseline GABAA IPSCs were significantly higher, and baseline PPF of GABAA IPSCs was significantly smaller in CeA neurons from EtOH-dependent rats compared to non-dependent rats, suggesting that evoked GABA release was augmented after chronic EtOH exposure. These investigators also reported an increase in the baseline frequency of mIPSCs in CeA neurons from EtOH-dependent rats compared to that of naïve controls. Acute superfusion of EtOH significantly enhanced GABAA IPSCs, decreased the PPF ratio of IPSCs, and increased the mIPSC frequency to the same extent in CeA slices from EtOH-dependent rats and naïve rats, suggesting a lack of tolerance to the presynaptic acute EtOH effects (Roberto et al. 2004a). In addition, these investigators estimated the interstitial GABA levels in CeA using microdialysis in freely moving rats. In agreement with the in vitro electrophysiologic results, the in vivo data showed a fourfold increase of baseline dialysate GABA concentrations in CeA of EtOH-dependent rats compared to naïve rats. Moreover, local administration of EtOH by dialysis increased the dialysate GABA levels in CET rats. These findings again indicate a lack of tolerance to presynaptic acute EtOH effects on GABA release in CeA of CET rats (Roberto et al. 2004a). These studies strengthen the possibility that chronic as well as acute EtOH may alter the function of the GABAergic synapses acting at both the postsynaptic site and presynaptic terminals. Altogether, these data suggest that long-term exposure to EtOH causes changes at GABAergic synapses that may differ between brain regions and with the duration of chronic exposure. Further studies will be needed to more carefully determine the specific exposure durations required to elicit these changes in GABAergic synapses, the molecular mechanisms responsible for these adaptive changes, as well as their behavioral consequences with respect to withdrawal and dependence.
Another area in which action of EtOH on GABA function has been implicated is withdrawal from chronic EtOH. Withdrawal results in an increased sensitivity to induction of seizures (Allan and Harris 1987; Frye et al. 1983). Several functional and behavioral studies on benzodiazepines and other drugs with GABA mimetic action reduced such withdrawal-related hyper-excitability (Breese et al. 2006; McCown et al. 1985; Roberto et al. 2008; Ticku and Burch 1980). Collectively, these results offer strong support for the hypothesis that at least a part of the action of EtOH was mediated by effects on neural functions associated with GABA transmission and that these effects play an important role in the maintenance of addictive drinking behavior.
2.4 γ-Aminobutyric AcidB Receptors and Chronic Ethanol Actions
Several studies demonstrated GABAB receptor involvement in the effects of EtOH. For instance, GABAB receptor antagonists enhance the ability of acute EtOH to facilitate GABA transmission in the hippocampus (Ariwodola and Weiner 2004; Wan et al. 1996; Wu and Saggau 1994) and NAc (Nie et al. 2000). Ariwodola and Weiner (2004) suggested that the effect of EtOH to facilitate GABA transmission is limited because of GABA feedback on presynaptic GABAB receptors (Fig. 1). The presence of GABAB receptors accounted for the difference in sensitivity to EtOH influences on GABA transmission in specific subfields of the hippocampus (Weiner et al. 1997). On the other hand, GABAB receptors did not influence GABA release from neurons in the CeA (Roberto et al. 2003). Thus, the involvement of GABAB receptors on GABA release in various brain regions may not be universal, suggesting that the presence or absence of presynaptic GABAB receptors may be an important determinant for the regional specificity of EtOH to affect GABA transmission (Ariwodola and Weiner 2004).
As mentioned above, Peris et al. (1997) showed that chronic EtOH treatment, sufficient for decreasing LTP in rats, also increased 3H-GABA release from hippocampal slices in these same animals. These investigators characterized presynaptic auto-receptor modulation of 3H-GABA release in hippocampal slices from control and EtOH-dependent rats. Effects of a GABAB receptor agonist (baclofen) and antagonist [2-hydroxy (OH)-saclofen] on electrically stimulated 3H-GABA release from superfused hippocampal slices were examined. Baclofen decreased stimulated release in a dose-dependent manner and the antagonist 2-OH-saclofen increased release consistent with the presence of presynaptic GABAB auto-receptors in hippocampus. The GABAA antagonist bicuculline did not significantly modulate basal or stimulated release. Presynaptic modulation of release by baclofen and 2-OH-saclofen was decreased in animals 48 h after withdrawal from EtOH. Using quantitative autoradiographic techniques, the density of 3H-baclofen binding sites in the hippocampus was not affected by chronic EtOH exposure, whereas the density of 3H-bicuculline binding sites was increased by 28% in EtOH-treated rats. These data may explain how chronic EtOH treatment increases presynaptic regulation of GABA release from hippocampus that may contribute to the decrease in LTP seen in rats after chronic EtOH exposure (Peris et al. 1997).
Another study assessed the impact of EtOH on postsynaptic GABAB receptors via baclofen-induced hyperpolarization of hippocampal CA1 and CA3 pyramidal neurons. These receptors activate outward K+ currents via a pertussis toxin-sensitive G protein cascade to reduce membrane potential during the slow inhibitory postsynaptic potential and may play a role in EtOH intoxication and withdrawal excitability. In both types of pyramidal neurons, baclofen applied consecutively in increasing concentrations caused concentration-dependent hyperpolarization. There were no significant differences in resting membrane potential, input resistance, maximum baclofen-induced hyperpolarization, or EC50 between CA1 and CA3 neurons, although slope values were significantly smaller in the former neurons. These parameters were not significantly changed in the presence of EtOH 10–100 mM. Chronic EtOH treatment (12 days) did not shift sensitivity or maximum response to baclofen in CA1 neurons. These results suggest that GABAB receptors in this model were essentially insensitive to EtOH (Frye and Fincher 1996).
Melis et al. (2002) linked the long-lasting potentiation of GABAergic synapses on dopaminergic neurons in the VTA by systemic EtOH to an effect on presynaptic GABAB receptors. Moreover, the frequency (but not the amplitude) of mIPSCs was also significantly higher in VTA neurons of EtOH-treated animals compared to controls, further supporting an increased probability of presynaptic GABA release independent of neuronal discharge in VTA neurons treated with EtOH. Interestingly, the GABAB receptor antagonist, CGP 35348, shifted PPD to PPF in EtOH-treated animals by increasing the amplitude of the second evoked GABAA IPSC and without affecting GABAA IPSC in the saline-treated animals. In addition, both the frequency and the amplitude of mIPSCs were unaffected by CGP 35348 in both groups of mice. Thus, the PPD observed in the EtOH-treated mice could result from an increased probability of GABA release, which might in turn lead to activation of presynaptic GABAB receptors and decrease the second IPSC. These results further support the hypothesis that GABA levels are increased after EtOH exposure, leading to spillover onto presynaptic GABAB receptors, whose activation leads to inhibition of release (Hausser and Yung 1994; Melis et al. 2002).
In a recent study, Roberto et al. (2008) reported neuroadaptations in GABAB receptors in CeA after chronic EtOH exposure. The sensitivity of GABA IPSCs to the GABAB receptor antagonist CGP 55845A and agonist baclofen was decreased after chronic EtOH, suggesting downregulation of this system. Specifically, the GABAB receptor antagonist, CGP 55845A significantly increased the mean amplitude of evoked IPSCs (by 12 ± 5%) in CeA from naïve rats. This increase in the IPSC amplitude was associated with a significant decrease in PPF, suggesting a tonic activation of presynaptic GABAB receptors in naïve rats. In contrast, in CeA from EtOH-dependent rats, CGP 55845A did not alter the mean evoked IPSCs (98 ± 4%) and did not affect mean PPF. Baclofen (10 µM) markedly depressed evoked GABA-IPSC amplitudes in neurons of naïve rats (to 38% of control), with recovery during washout. The baclofen-induced inhibition of GABA IPSCs was significantly reduced (to 86% of control) in neurons of EtOH-dependent rats. In addition, in CeA neurons from EtOH-dependent rats, baclofen-induced depression was associated with a smaller increase of the PPF ratio of GABA IPSCs compared to that in neurons of naïve rats. These data suggest that the downregulation of the GABAB system associated with EtOH-dependence may explain in part the increased GABAergic tone reported in dependent rats (Roberto et al. 2008).
3 Neuropeptide Roles in Acute and Chronic Alcohol Actions
Neuropeptides are potent neuromodulators in the CNS whose actions are mediated via GPCRs. In contrast to classical neurotransmitters, neuropeptides are released in a frequency-dependent fashion and often have a longer half-life of activity after release. These factors, among others, enable neuropeptides to produce long-lasting effects on cellular functions such as excitatory and inhibitory synaptic transmission, neuronal excitability, and gene transcription (Gallagher et al. 2008). Thus, a long-lasting dysregulation of neuropeptides could have significant effects on the activity of neurons and consequently, behavior.
3.1 Corticotropin-Releasing Factor
Corticotropin-releasing factor (CRF) is a 41-amino acid polypeptide that has a major role in coordinating the stress response of the body by mediating hormonal, autonomic, and behavioral responses to stressors. CRF (originally called corticotropin- releasing hormone, although the International Union of Pharmacology designation is CRF) was identified through classic techniques of peptide sequencing (Vale et al. 1981). Subsequently, genes encoding three paralogs of CRF—urocortins 1, 2, and 3 (Ucn 1, Ucn 2, Ucn 3), were identified by modern molecular biologic approaches. Ucn 2 and Ucn 3 are also referred to as stresscopin-related peptide and stresscopin, respectively. CRF and the urocortins have been implicated in the modulation of multiple neurobiologic systems, including those that regulate feeding, anxiety and depression, hypothalamic-pituitary-adrenal (HPA) axis signaling, and EtOH consumption (Hauger et al. 2006; Heilig and Koob 2007; Ryabinin and Weitemier 2006; Smith and Vale 2006). CRF and the Ucn peptides produce their effects by binding to the G protein-coupled CRF type 1 (CRF1R) and CRF type 2 (CRF2R) receptors. CRF binds to both receptors, but has greater affinity for the CRF1R (Bale and Vale 2004; Fekete and Zorrilla 2007; Hauger et al. 2006; Pioszak et al. 2008).
CRF1R and CRF2R are GPCRs that are predominantly positively linked to the activation of AC (Fig. 1), and recent reports also implicate other second messenger systems such as inositol triphosphate and PKC (Blank et al. 2003; Grammatopoulos et al. 2001). Using corticotrophins, Antoni et al. (2003) demonstrated a coupling of CRF1R to AC9 and AC7. The switch in coupling from AC9 to AC7 results in a more robust cAMP signal when CRF binds to the CRF1R (Antoni 2000; Antoni et al. 2003). It should be emphasized that AC7 is localized both postsynaptically (striatum, hippocampus) and presynaptically (nucleus accumbens, amygdala) (Mons et al. 1998a, b), and is anatomically positioned to receive signals from GPCRs on both dendrites and axon terminals.
Pharmacological and transgenic studies show that brain and pituitary CRF1Rs mediate many of the functional stress-like effects of the CRF system (Heinrichs and Koob 2004). CRF and the Ucn peptides have a wide distribution throughout the brain, but there are particularly high concentrations of cell bodies in the paraventricular nucleus of the hypothalamus, the basal forebrain (notably the extended amygdala), and the brainstem (Swanson et al. 1983). Ucn1 binds with equal affinity to CRF1R and CRF2R, and Ucn2 and Ucn3 are CRF2R agonists (Hauger et al. 2006; Pioszak et al. 2008). CRF and the Ucn peptides exert their behavioral and neuroendocrine actions through central hypothalamic and extrahypothalamic pathways (Hauger et al. 2006; Heilig and Koob 2007; Heinrichs and Koob 2004; Koob and Le Moal 2008).
Increasing evidence implicates CRF and its receptors in the synaptic effects of EtOH. EtOH induces release of CRF from the hypothalamus that initiates the activation of the HPA axis (Ogilvie et al. 1998). EtOH also modulates the extraneuroendocrine CRF system involved in behavioral stress responses, particularly in the amygdala. EtOH withdrawal induces an increase in CRF levels in the amygdala (Merlo Pich et al. 1995) and in the BNST (Olive et al. 2002).
The central administration of a CRF antagonist attenuates both EtOH self-administration and the anxiety-like response to stress observed during alcohol abstinence (Valdez et al. 2002) and administration of a CRFR antagonist into the CeA reverses the anxiogenic-like effect of alcohol (Rassnick et al. 1993). Rats tested 3–5 weeks post alcohol withdrawal showed an anxiogenic-like response provoked by a mild restraint stress only in rats with a history of alcohol dependence. This stress-induced anxiogenic-like response was reversed by a competitive CRF1R antagonist (Valdez et al. 2003). The increased self-administration of alcohol observed during protracted abstinence also was blocked by a competitive CRF1R antagonist (Valdez et al. 2003). Gehlert et al. (2007) also described that a novel CRF1R antagonist, the 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo[1,2-b]pyridazine (MTIP) has advantageous properties for both clinical development and in preclinical alcoholism models. MTIP dose-dependently reversed anxiogenic effects of EtOH withdrawal, and blocked excessive alcohol self-administration in Wistar rats with a history of dependence (Gehlert et al. 2007). CRF also contributes to increased alcohol consumption in dependent animals, because increased EtOH self-administration is reduced by CRF1R antagonists in dependent animals but not in non-dependent animals (Funk et al. 2007; Overstreet et al. 2004) and by CRF1R deletion (Chu et al. 2007; Sillaber et al. 2002). More recently, it has been reported that chronic CRF1R antagonist treatment blocked withdrawal-induced increases in alcohol drinking by dependent rats, and tempered moderate increases in alcohol consumption (Roberto et al. 2010). These results have led to the hypothesis that negative emotional states (including anxiety-like states) contribute to the compulsive alcohol intake associated with dependence via negative reinforcement mechanisms (Koob 2008).
A recent review (Lowery and Thiele 2010) provides a comprehensive overview of preclinical evidence from rodent studies that suggest a promising role for CRFR antagonists in the treatment of alcohol abuse disorders. CRFR antagonists protect against excessive EtOH intake resulting from EtOH dependence without influencing EtOH intake in non-dependent animals. Similarly, CRFR antagonists block excessive binge-like EtOH drinking in non-dependent mice but do not alter EtOH intake in mice drinking moderate amounts of EtOH (Lowery and Thiele 2010). CRFR antagonists also protect against increased EtOH intake and relapse-like behaviors precipitated by exposure to a stressful event. Additionally, CRFR antagonists attenuate the negative emotional responses associated with EtOH withdrawal. The protective effects of CRFR antagonists are modulated by CRF1R. Finally, recent evidence has emerged suggesting that CRF2R agonists may also be useful for treating alcohol abuse disorders for review see (Lowery and Thiele 2010).
Low CRF concentrations can influence neuronal properties in the CNS (see (Aldenhoff et al. 1983; Siggins et al. 1985). CRF decreases the slow after hyperpolarizing potential in hippocampus (Aldenhoff et al. 1983) and CeA (Rainnie et al. 1992), and enhances R-type voltage-gated calcium channels in rat CeA neurons (Yu and Shinnick-Gallagher 1998). These and other data (Liu et al. 2004; Nie et al. 2004, 2009; Roberto et al. 2010; Ungless et al. 2003) also suggest that CRF plays an important role in regulating synaptic transmission in CNS. For example, in VTA dopamine neurons, CRF potentiates NMDA-mediated synaptic transmission via CRF2 activation (Ungless et al. 2003), and we recently found that CRF augments GABAergic inhibitory transmission in mouse CeA neurons via CRF1 activation (Fig. 1).
3.1.1 Corticotropin-Releasing Factor Actions in the Ventral Tegmental Area
The ventral tegmental area (VTA) receives CRF inputs from a number of sources including the limbic forebrain and the paraventricular nucleus of the hypothalamus (Rodaros et al. 2007). These CRF inputs form symmetric and asymmetric synapses, mostly onto dendrites that co-release either GABA or glutamate, respectively (Tagliaferro and Morales 2008). VTA dopamine neurons express both types of CRF receptors, CRF1R and CRF2R (Ungless et al. 2003), and approximately 25% of VTA dopamine neurons express the CRF binding protein (CRF-BP) (Wang et al. 2005; Wang and Morales 2008). CRF regulates dopamine neurons through a subtle interplay of effects at CRF1R, CRF2R, and CRF-BP. CRF increases the action potential firing rate in VTA dopamine neurons via CRF1R and involves a PKC-dependent enhancement of Ih (a hyperpolarization-activated inward current) (Wanat et al. 2008). CRF enhanced the amplitude and slowed the kinetics of IPSCs following activation of D2-dopamine and GABAB receptors. This action is postsynaptic and dependent on the CRF1R. The enhancement induced by CRF was attenuated by repeated in vivo exposures to psychostimulants or restraint stress (Beckstead et al. 2009).
CRF can induce a slowly developing, but transient, potentiation of NMDAR-mediated synaptic transmission (Ungless et al. 2003). This effect involves the CRF2R and activation of the protein kinase C pathway and the requirement of CRF-BP. However, the effect of CRF is restricted to a subset of dopamine neurons expressing large Ih currents (Ungless et al. 2003).
In addition to fast, excitatory glutamate-mediated synaptic transmission, dopamine neurons also express metabotropic glutamate receptors (mGluRs) which mediate slower, inhibitory synaptic transmission (Fiorillo and Williams 1998). The rapid rise and brief duration of synaptically released glutamate in the extracellular space mediates a rapid excitation through activation of ionotropic receptors, followed by inhibition through the mGluR1 receptor (Fiorillo and Williams 1998). CRF can enhance these mGluRs via a CRF2R-PKA pathway that stimulates release of calcium from intracellular stores (Riegel and Williams 2008). The CRF modulation of VTA synaptic activity is very complex because CRF has diverse actions on dopamine neurons that are excitatory and inhibitory. In summary, the excitatory effects of CRF on dopamine neurons appear to affect fast events (e.g. action potential firing rate and NMDAR-mediated synaptic transmission), whereas the inhibitory effects involve slow forms of synaptic transmission. Another important aspect is that CRF1R-mediated effects do not involve interactions with the CRF-BP, whereas CRF2R-mediated effects do.
It is speculated that these effects on short-term plasticity phenomena may modulate longer lasting forms of plasticity. For example, NMDAR activation is required for the induction of long-term potentiation in VTA dopamine neurons (Bonci and Malenka 1999; Borgland et al. 2010).
3.1.2 Corticotropin-Releasing Factor Actions in the Central Amygdala
The central amygdala (CeA) contains CRF receptors and abundant CRF-containing fibers (De Souza et al. 1984) (Uryu et al. 1992); CRF itself is generally co-localized in CeA neurons together with GABA (Eliava et al. 2003) (Asan et al. 2005). Acute EtOH augments evoked GABAA receptor-mediated inhibitory postsynaptic currents (IPSCs) by increasing GABA release in both mouse (Bajo et al. 2008; Nie et al. 2004) and rat CeA neurons (Roberto et al. 2003, 2004).
CRF1Rs mediate the EtOH-induced augmentation of IPSCs in mouse CeA (Nie et al. 2004; Nie et al. 2009) via the PKCε signaling pathway (Bajo et al. 2008; Nie et al. 2004). Both CRF and EtOH augment evoked IPSCs in mice CeA neurons, and CRF1R (but not CRFR2) antagonists blocked both CRF and EtOH effects. In addition, CRF and EtOH augment IPSCs in wild-type and CRF2R knockout mice, but not in CRF1R knockout mice (Nie et al. 2004).
New electrophysiological data showed that CRF, like EtOH, also enhances GABAergic transmission in the rat CeA (Roberto et al. 2010). As in mice, CRF and EtOH actions involve presynaptic CRF1R activation at the CeA GABAergic synapses. Interestingly, the interactions between the CRF and GABAergic systems in the CeA may play an important role in alcohol reward and dependence (Roberto et al. 2010). These results suggest that the presynaptic effect of EtOH on GABA release in rodent CeA involves CRF1R and perhaps release of CRF itself. Thus, superfusion of CRF has an effect on GABA IPSCs equivalent to that of EtOH: an increase in IPSC amplitude of about 30–50%. Furthermore, both CRF and EtOH decreased PPF of IPSCs in mouse and rat neurons, and the effects of both were selectively blocked by CRF1R antagonists. In addition, both EtOH and CRF increase the frequency of GABAR-mediated mIPSCs, and this effect is blocked by CRF1R antagonists (Nie et al. 2004; 2009; Roberto et al. 2010). Thus, EtOH probably enhances the release of GABA by activating CRF1R on GABAergic terminals (Nie et al. 2009; Roberto et al. 2010). Conversely, CRF1R antagonists directly increased PPF of IPSCs and decreased mIPSC frequencies, consistent with decreased GABA release, thus opposing EtOH effects. Because GABA and CRF are often co-localized in CeA neurons, the EtOH-elicited GABA release may involve release of the CRF peptide itself, perhaps even from the terminals synapsing on autoreceptors on the same cell bodies or on collaterals from other GABAergic interneurons. Thus, this example raises the possibility of involvement of other, secondary messengers in EtOH effects on GABAergic terminals.
Chronic EtOH exposure produces functional adaptation of the CRF system in CeA (Hansson et al. 2006, 2007; Sommer et al. 2008; Weiss et al. 2001). In the study by Roberto et al. electrophysiological experiments were performed 2–8 h after preparation of CeA slices from EtOH-dependent or naïve control rats. Interestingly, in CeA of dependent rats, the ability of maximal (200 nM) and a submaximal (100 nM) concentrations of CRF to augment evoked IPSCs was significantly enhanced compared to naïve CeA. A greater effect of CRF1R antagonists on basal IPSCs of dependent rats was also reported. The greater effect of CRF and CRF1R antagonists may reflect increased tonic release of endogenous CRF, constitutive CRF1R activation, increased receptor number, and/or sensitization of CRF1R in CeA of dependent rats. These combined findings suggest an important EtOH–CRF interaction on GABAergic transmission in the CeA that markedly increases during development of EtOH dependence (Roberto et al. 2010).
CRF-related peptides serve as hormones and neuromodulators of the stress response and play a role in affective disorders. It has been shown that excitatory glutamatergic transmission is modulated by two endogenous CRF-related peptide ligands, CRF rat/human (r/h) and Ucn I, within the CeA and the lateral septum mediolateral nucleus (LSMLN) (Liu et al. 2004). Activation of these receptors exerts diametrically opposing actions on glutamatergic transmission in these nuclei. In the CeA, CRF(r/h) depressed excitatory glutamatergic transmission through a CRF1R-mediated postsynaptic action, whereas Ucn I facilitated synaptic responses through pre and postsynaptic CRF2R-mediated mechanisms. Conversely, in the lateral septum mediolateral nucleus (LSMLN), CRF induced a CRF1R-mediated facilitation of glutamatergic transmission via postsynaptic mechanisms, whereas Ucn I depressed EPSCs by postsynaptic and presynaptic CRF2R-mediated actions. Furthermore, antagonists of these receptors also affected glutamatergic neurotransmission, indicating a tonic endogenous modulation at these synapses (Liu et al. 2004). These data show that CRF receptors in CeA and LSMLN synapses exert and maintain a significant synaptic tone and thereby regulate excitatory glutamatergic transmission. The results also suggest that CRF receptors may provide novel targets in affective disorders and stress (Liu et al. 2004).
3.1.3 Corticotropin-Releasing Factor Actions in the Bed Nucleus of the Stria Terminalis
The bed nucleus of the stria terminalis (BNST), a brain region associated with anxiety, has enriched expression of CRF (Ju and Han 1989) and CRFRs (Van Pett et al. 2000). A component of the extended amygdala, the BNST is anatomically well-situated to integrate stress and reward-related processing in the CNS, regulating activation of the HPA axis and reward circuits. The BNST receives dense GABAergic and CRF input from the CeA (Sakanaka et al. 1986), suggesting that CRF regulation of function in the BNST is critical for shaping BNST output. Pharmacological studies suggest that CRF signaling in the BNST is involved in anxiety (Lee and Davis 1997) and stress-induced relapse to cocaine self-administration (Erb and Stewart 1999). Moreover, a stimulus that promotes anxiogenic responses, the withdrawal of rodents from chronic EtOH exposure, produces rises in extracellular levels of CRF in the BNST (Olive et al. 2002). Interactions between CRF and GABAergic transmission in BNST have been reported to play a role in regulating stress and anxiety (Kash and Winder 2006). In this study the actions of CRF on GABAergic transmission in the ventrolateral region of the BNST (vlBNST) were examined. This region projects to both the VTA (Georges and Aston-Jones 2002) and the PVN of the hypothalamus (Cullinan et al. 1993), thus providing a point of access to both reward and stress pathways. Using whole-cell recordings in a BNST slice preparation, Kash et al. found that CRF enhances GABAergic transmission. Their pharmacological and genetic experiments suggest that CRF and urocortin CRF enhance postsynaptic responses to GABA through activation of the CRF1R.
In the same laboratory, a recent study showed the action of dopamine on cellular and synaptic function in the BNST. Kash et al. (2008) directly assessed the ability of dopamine to modulate neuronal function in the BNST using an ex vivo slice preparation. These investigators demonstrated a rapid and robust dopamine-induced enhancement of excitatory transmission in the BNST. This enhancement is activity-dependent and requires the downstream action of CRF1R, suggesting that dopamine induces CRF release through a local network mechanism. Furthermore, it was found that both in vivo and ex vivo cocaine induced a dopamine receptor and CRF1R-dependent enhancement of a form of NMDA receptor-dependent short-term potentiation in the BNST. These data highlight a direct and rapid interaction between dopamine and CRF systems that regulate excitatory transmission and plasticity in a brain region key to reinforcement and reinstatement. Because a rise in extracellular dopamine levels in the BNST is a shared consequence of multiple classes of drugs of abuse, this suggests that the CRF1R-dependent enhancement of glutamatergic transmission in this region may be a common key action of substances of abuse (Kash et al. 2008).
Francesconi et al. (2009a, b) investigated the effects of protracted withdrawal from alcohol in the juxtacapsular nucleus of the anterior division of the BNST (jcBNST). The jcBNST receives robust glutamatergic projections from the Basolateral amygdal (BLA), the postpiriform transition area, and the insular cortex as well as dopamine inputs from the midbrain. In turn, the jcBNST sends GABAergic projections to the medial division of the central nucleus of the amygdala (CeAm) as well as other brain regions. These investigators described a form of long-term potentiation of the intrinsic excitability (LTP-IE) of neurons of the jcBNST in response to high-frequency stimulation (HFS) of the stria terminalis that was impaired during protracted withdrawal from alcohol (Francesconi et al. 2009b). Administration of the selective CRF1R antagonist (R121919), but not of the CRF2R antagonist (astressin 2B), normalized jcBNST LTP-IE in animals with a history of alcohol dependence (Francesconi et al. 2009b). In addition, repeated, but not acute, administration of CRF itself produced a decreased jcBNST LTP-IE. These investigators also showed that dopaminergic neurotransmission is required for the induction of LTP-IE of jcBNST neurons through dopamine D1 receptors (Francesconi et al. 2009b). Thus, activation of the central CRF stress system and altered dopaminergic neurotransmission during protracted withdrawal from alcohol and drugs of abuse may contribute to the disruption of LTP-IE in the jcBNST. Impairment of this form of intrinsic neuronal plasticity in the jcBNST could result in inadequate neuronal integration and reduced inhibition of the CeA, contributing to the negative affective state that characterizes protracted abstinence in postdependent individuals (Francesconi et al. 2009a, b).
3.1.4 Corticotropin-Releasing Factor Actions in the Basolateral Amygdala
Liu et al. (2004) demonstrated that CRF and its related family of peptides act differentially at CRF1 versus CRF2 synaptic receptors to facilitate or depress excitatory transmission in CeA and lateral septum mediolateral nucleus. Notably, the effects of CRF and its ligands occurred without any apparent direct action on membrane potential or membrane excitability, suggesting that the role of CRF at these limbic synapses is that of a ‘neuroregulator’. The investigators suggested pre and postsynaptic loci for CRF1 and CRF2 receptors within the glutamatergic CeA and LSMN synapses. Although both synapses exhibit a comparable pre and postsynaptic location of CRF1 and CRF2 receptors, their functions (facilitation versus depression of glutamatergic transmission) are opposite within each synapse (Gallagher et al. 2008). Liu et al. (2004) also demonstrated that endogenous CRF ligands induce a tonic effect on excitatory glutamatergic transmission at synapses within both of these nuclei since application of competitive, selective CRF1 or CRF2 receptor antagonists resulted in an enhancement or depression of glutamatergic EPCS. A similar tonic endogenous action of CRF ligands was not observed under control conditions in the medial prefrontal cortex (Orozco-Cabal et al. 2006). This latter result further emphasizes that CRF effects are different depending upon the CNS synapse being investigated. Most of these studies in the Gallagher group aimed to investigate the action of CRF on glutamatergic synapses in relation to cocaine administration. There is very poor data on EtOH–CRF–glutamate interaction.
Taken together these data suggest that a dysregulation of the extrahypothalamic CRF function is a major determinant of vulnerability to high alcohol intake and maintenance of alcohol and drug dependence.
3.1.5 Neuropeptide Y
Neuropeptide Y (NPY) is an inhibitory peptide produced in abundance in the hypothalamus, and phylogenetically conserved across species (Allen et al. 1986). NPY is involved in regulation of food and water intake. It has recently been ascribed its prominent role in the aversive aspects of alcohol withdrawal and relapse via their actions in the CeA. Endogenous NPY reduces anxiety via actions in the amygdala (Heilig et al. 1993; Sajdyk et al. 2002) and suppresses alcohol drinking in rats (Gilpin et al. 2003) via its actions in CeA (Gilpin et al. 2008a, b; Thorsell 2008). More specifically, NPY microinjection into the CeA exhibits an enhanced ability to suppress alcohol drinking in certain subpopulations of drinkers, including rats that are made dependent on alcohol via vapor inhalation.
NPY is generally co-localized with GABA in inhibitory interneurons. NPY mediates its actions by interacting with a family of G protein-coupled receptors (GPCRs), at least five of which have been cloned and designated Y1, Y2, Y4, Y5, and Y6. These receptors are widely distributed throughout the brain. NPY has also been shown to be a regulator of neuronal excitability in hippocampus, where its cellular actions have been most extensively studied (Colmers et al. 1991). In the amygdala, NPY has anxiolytic effects that are mediated via activation of Y1 receptors (Heilig et al. 1993). NPY neurons in the amygdala project to the BNST (Allen et al. 1984), which also contains Y1 receptors and Y1 and Y2 receptor mRNA. Further, the CeA receives NPYergic input from the nucleus of the solitary tract, arcuate nucleus, and the lateral septum (see (Kask et al. 2002) for a review). Y1, Y2, and Y5 receptors, and receptor mRNA are found in the amygdala, and each of these receptor subtypes has been implicated in anxiety (Kask et al. 2002). Y2 receptors are thought to act presynaptically as auto-receptors providing negative feedback to NPYergic nerve terminals, whereas Y1 receptors appear to act postsynaptically (Kask et al. 2002; Wolak et al. 2003).
Many in vivo studies point to the involvement of NPY in mediating some of the behavioral effects of EtOH (Caberlotto et al. 2001; Cippitelli et al. 2010; Rimondini et al. 2005). NPY KO mice show increased EtOH preference but blunted behavioral responses to EtOH, while NPY overexpressors show a lower preference and increased sensitivity to EtOH (Thiele et al. 1998). Likewise, increased NPY expression in the CeA was noted in two independent strains of alcohol-preferring rats (Hwang et al. 1999). There were increased levels of NPY in the paraventricular nucleus of the hypothalamus (PVN) and arcuate nucleus of EtOH-preferring rats and decreased NPY levels in the CeA of EtOH-preferring rats, suggesting an inverse relationship between NPY levels in the CeA and EtOH consumption. Additionally, alcohol-preferring rats show significant decreases in both cAMP-responsive element-binding protein (CREB) and NPY levels in the CeA and medial amygdala, but not the basolateral amygdala (Pandey et al. 2005). Further, virally mediated alterations in NPY levels in the CeA differentially affect EtOH consumption in rats with low and high basal levels of anxiety (Primeaux et al. 2006). Also, recent genetic and pharmacological evidence indicates that C57BL/6 J mice have low NPY levels in CeA compared to DBA/2 mice, suggesting that NPY contributes to the high EtOH consumption characteristic of C57BL/6 J mice (Hayes et al. 2005).
Electrophysiologic findings suggest that NPY and EtOH have a similar profile of actions (Ehlers et al. 1998a, b, 1999). Increased sensitivity to NPY and CRF was observed in cortex and amygdala after chronic EtOH exposure, as measured by EEG activity and event-related potentials (Slawecki et al. 1999). Modulation of amygdala EEGs by NPY differs in naïve P and NP rats, suggesting that NPY has different neuromodulatory effects in these two strains (Ehlers et al. 1998a). Furthermore, NPY antagonizes the effects of CRF in the amygdala. However, to date neither the cellular actions of NPY in neither the CeA nor its interactions with EtOH or CRF, have been fully characterized. Recent findings by Gilpin et al. (2009, 2011) show that NPY superfusion decreased baseline GABAergic transmission in CeA slices and blocked the alcohol-induced enhancement of inhibitory transmission in CeA via presynaptic Y2 receptors.
Recently, it has been shown that NPY and CRF have opposing effects on stress and anxiety as well as on synaptic activity in BNST (Heilig et al. 1994; Kash and Winder 2006). Kash and Winder found that NPY and CRF inhibit and enhance GABAergic transmission, respectively: NPY depresses GABAergic transmission through activation of the Y2 receptors, whereas CRF and urocortin enhance GABAergic transmission through activation of CRF1 receptors. Further, NPY appears to reduce GABA release, whereas CRF enhances postsynaptic responses to GABA, suggesting potential anatomic and cellular substrates for the robust behavioral interactions between NPY and CRF in the extended amygdala.
3.1.6 Orphanin FQ/nociceptin (OFQ/N)
Nociceptin (known also as orphanin FQ) is the most recently discovered member of the endogenous opioid peptide family, albeit nearly 15 years ago. Nociceptin mediates or influences many behavioral, psychological, and neurobiological processes, including memory, anxiety, stress, and reward (Economidou et al. 2008; Martin-Fardon et al. 2010; Murphy 2010). The hepta decapeptide nociceptin is the endogenous ligand of the nociceptin opioid receptor (NOR), previously referred to as opiate receptor-like1 (ORL1). NOR is a GPCR that belongs to the opioid receptor family (Mogil et al. 1996; Mogil and Pasternak 2001). In rodents, moderate to high levels of NOR mRNA are detected in cerebral cortex, nucleus accumbens, amygdala, dorsal raphe nucleus, and hippocampus (Harrison and Grandy 2000). Nociceptin has a high structural homology with opioid peptides, especially dynorphin A (Meunier et al. 1995; Reinscheid et al. 1995), but nociceptin does not bind to MOR, DOR or KOR (μ, δ and κ-opioid receptors) and opioid peptides do not bind NOR (Lachowicz et al. 1995; Reinscheid et al. 1995). Nociceptin inhibits forskolin-stimulated cAMP formation (see Harrison and Grandy 2000; Hawes et al. 2000), and protein kinase C (PKC), MAP kinases and phospholipase A2 have been linked to NOR (Fukuda et al. 1998; Hawes et al. 2000; Lou et al. 1998).
At the cellular level, nociceptin acts at NOR to augment K+ conductances in amygdalar (Meis and Pape 1998, 2001), hippocampal (Amano et al. 2000; Ikeda et al. 1997; Madamba et al. 1999; Tallent et al. 2001; Yu and Xie 1998) and thalamic neurons (Meis 2003; Meis et al. 2002), thus depressing cell excitability. Nociceptin has also been shown to decrease Ca2+ currents (Abdulla and Smith 1997; Calo et al. 2000; Connor et al. 1999; Henderson and McKnight 1997; Larsson et al. 2000) and reduce the amplitude of both non-NMDA receptor-mediated excitatory postsynaptic currents (EPSCs) and IPSCs in rat lateral amygdala (Meis et al. 2002).
Roberto and Siggins (2006) found that nociceptin did not significantly alter the resting membrane potential, input resistance, or spike amplitude, in accord with the results reported by others in CeA (Meis and Pape 1998) and for other brain regions (Ikeda et al. 1997; Madamba et al. 1999; Tallent et al. 2001). However, nociceptin dose-dependently reduced GABAA IPSCs. This inhibition of GABAergic transmission was reversible on washout (Roberto and Siggins 2006). Nociceptin also concomitantly increased the PPF of IPSCs, and decreased the frequency of mIPSCs, suggesting decreased GABA release. Thus, nociceptin decreases GABAergic transmission by reducing GABA release at CeA synapses (Roberto and Siggins 2006). Interestingly, nociceptin applied before EtOH completely prevented the EtOH-induced enhancement of GABAergic transmission in CeA. On the other hand, EtOH alone significantly increased both the evoked IPSCs and mIPSC frequencies, and decreased the PPF ratio; nociceptin in the presence of EtOH completely reversed these EtOH effects opposing the EtOH increase of GABA release (Roberto and Siggins 2006). These investigators also found that the nociceptin-induced decrease of GABAergic transmission was larger in EtOH-dependent rats and might reflect neuroadaptations associated with EtOH dependence.
The functional interactions of neuropeptides (CRF, NPY, nociceptin) with GABAergic and glutamatergic systems may play major roles in the acute effects of EtOH on GABAergic and glutamatergic transmission. Understanding the underlying mechanisms of these interactions may offer a possible avenue for restoring “normal” function following chronic drug exposure. The neuroadaptations induced by chronic EtOH on GABAergic and glutamatergic systems may represent homeostatic or compensatory mechanisms in response to the acute EtOH actions on these systems.
4 Conclusions
In this review we have focused on acute and chronic EtOH actions on synaptic transmission. It is not possible to cover all aspects of this topic, and thus we have focused on describing the best established EtOH actions. As the review attests, EtOH affects numerous aspects of synaptic transmission both directly and indirectly, to alter brain function and behavior. Acute exposure to EtOH generally increases the function of cys-loop ligand-gated ion channels, with prominent effects of GABAA and glycine receptors. These actions increase synaptic and extra-synaptic inhibition and are thought to contribute to sedation and other aspects of intoxication. Ionotropic glutamate and P2X receptors are generally inhibited by acute EtOH exposure, with some noted exceptions. The inhibitory effect on ionotropic glutamate receptors is most prominent at NMDARs and on NMDAR-mediated synaptic responses, and this inhibitory action is thought to contribute to cognitive impairment produced by EtOH. At present, the postsynaptic EtOH effects on neurotransmitter receptors appear to occur within the receptor molecules themselves, although more work is needed to elucidate the roles of post-translation modification. On the presynaptic side, acute EtOH generally potentiates GABA release, contributing to the enhanced neuronal inhibition produced by the drug. The molecular mechanisms involved in EtOH potentiation of GABA release remain to be fully explored. EtOH also alters other aspects of synaptic transmission involving amino acid transmitters and monoamines. The net result of the EtOH effects of transmission seems to be to dampen synaptic excitation in many brain regions and reduce most forms of synaptic plasticity (with noted exceptions).
Chronic exposure to EtOH, whether by forced administration or ingestion, generally enhances the function of NMDARs, most often those containing the NR2B subunit. Increases in glutamate release and responses to some other glutamate receptors are also observed following chronic exposure. The net effect of these increases in glutamatergic transmission appears to be a hyperexcitable CNS state during withdrawal that contributes to withdrawal symptoms and relapse. Excitotoxicity might be another result of this hyper-glutamatergic state. In general, acute EtOH effects on glutamate receptor function and glutamatergic transmission are intact even after subchronic or chronic EtOH exposure, suggesting that behavioral tolerance is not a simple function of loss of pharmacological effects at these synapses. At GABAergic synapses, chronic EtOH generally alters either the efficacy of inhibitory synaptic transmission or the types of receptors involved in transmission. Extrasynaptic GABAA receptor-mediated synaptic responses are also altered, leading to changes in tonic current in the postsynaptic neuron. The pattern of chronic EtOH effects on GABAergic transmission varies considerably across brain regions, making this subject a rich and important area for future investigation. The resultant alterations in patterns of GABAergic transmission in key brain regions may contribute to EtOH tolerance, dependence and drug intake. More work is needed to determine the exact pattern of changes in GABAergic inhibition across brain regions, and how these changes contribute to aspects of alcohol use disorders including tolerance, dependence, and escalating intake.
The modulatory effects of neuropeptides have become subjects of intense investigation in the alcohol research field. Neuropeptides implicated in stress responses, such as CRF, appear to contribute to stress–EtOH interactions as well as drinking and relapse. Acute EtOH exposure alters the release of some neuropeptides, while others alter synaptic transmission in ways that interfere with the actions of EtOH. Chronic EtOH exposure also appears to alter neuropeptide modulatory actions. In addition to providing tools for investigation of mechanisms involved in EtOH actions, the neuropeptides may also provide new avenues for pharmacotherapies that could be used in the treatment of alcohol use disorders. Researchers have just begun to explore the alcohol-related actions of a few of the many neuropeptides found in the brain. Thus, more work remains to fully define how peptides participate in the neural actions of alcohol.
Contributor Information
David M. Lovinger, Email: lovindav@mail.nih.gov, Laboratory for Integrative Neuroscience, NIAAA, 5625 Fishers Lane, Room TS-13A, Rockville, MD 20852, USA.
Marisa Roberto, Email: mroberto@scripps.edu, Committee on Neurobiology of Addictive Disorders, The Scripps Research Institute, 10550 N Torrey Pines Rd., SP30-1160, La Jolla, CA 92037, USA.
References
- Abdulla FA, Smith PA. Nociceptin inhibits T-type Ca2+ channel current in rat sensory neurons by a G-protein-independent mechanism. J Neurosci. 1997;17:8721–8728. doi: 10.1523/JNEUROSCI.17-22-08721.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguayo LG. Ethanol potentiates the GABAA-activated Cl- current in mouse hippocampal and cortical neurons. Eur J Pharmacol. 1990;187(1):127–130. doi: 10.1016/0014-2999(90)90349-b. [DOI] [PubMed] [Google Scholar]
- Aguayo LG, Tapia JC, Pancetti FC. Potentiation of the glycine-activated Cl-current by ethanol in cultured mouse spinal neurons. J Pharmacol Exp Ther. 1996;279(3):1116–1122. [PubMed] [Google Scholar]
- Aguayo LG, Peoples RW, Yeh HH, Yevenes GE. GABAA receptors as molecular sites of ethanol action. Direct or indirect actions? Curr Top Med Chem. 2002;2(8):869–885. doi: 10.2174/1568026023393426. [DOI] [PubMed] [Google Scholar]
- Akinshola BE. Straight-chain alcohols exhibit a cutoff in potency for the inhibition of recombinant glutamate receptor subunits. Br J Pharmacol. 2001;133(5):651–658. doi: 10.1038/sj.bjp.0704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinshola BE, Yasuda RP, Peoples RW, Taylor RE. Ethanol sensitivity of recombinant homomeric and heteromeric AMPA receptor subunits expressed in Xenopus oocytes. Alcohol Clin Exp Res. 2003;27(12):1876–1883. doi: 10.1097/01.ALC.0000098874.65490.52. [DOI] [PubMed] [Google Scholar]
- Aldenhoff JB, Gruol DL, Rivier J, Vale W, Siggins GR. Corticotropin releasing factor decreases postburst hyperpolarizations and excites hippocampal neurons. Science. 1983;221:875–877. doi: 10.1126/science.6603658. [DOI] [PubMed] [Google Scholar]
- Allan AM, Harris RA. Acute and chronic ethanol treatments alter GABA receptor-operated chloride channels. Pharmacol Biochem Behav. 1987;27:665–670. doi: 10.1016/0091-3057(87)90192-4. [DOI] [PubMed] [Google Scholar]
- Allen YS, Roberts GW, Bloom SR, Crow TJ, Polak JM. Neuropeptide Y in the stria terminalis: evidence for an amygdalofugal projection. Brain Res. 1984;321:357–362. doi: 10.1016/0006-8993(84)90193-8. [DOI] [PubMed] [Google Scholar]
- Allen YS, Bloom SR, Polak JM. The neuropeptide Y-immunoreactive neuronal system: discovery, anatomy and involvement in neurodegenerative disease. Hum Neurobiol. 1986;5:227–234. [PubMed] [Google Scholar]
- Amano T, Matsubayashi H, Tamura Y, Takahashi T. Orphanin FQ-induced outward current in rat hippocampus. Brain Res. 2000;853:269–274. doi: 10.1016/s0006-8993(99)02245-3. [DOI] [PubMed] [Google Scholar]
- Anderson NJ, Daunais JB, Friedman DP, Grant KA, McCool BA. Long-term ethanol self-administration by the nonhuman primate, Macaca fascicularis, decreases the benzodiazepine sensitivity of amygdala GABAA receptors. Alcohol Clin Exp Res. 2007;31:1061–1070. doi: 10.1111/j.1530-0277.2007.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni FA. Molecular diversity of cyclic AMP signaling. Front Neuroendocrinol. 2000;21:103–132. doi: 10.1006/frne.1999.0193. [DOI] [PubMed] [Google Scholar]
- Antoni FA, Sosunov AA, Haunso A, Paterson JM, Simpson J. Short-term plasticity of cyclic adenosine 3′, 5′-monophosphate signaling in anterior pituitary corticotrope cells: the role of adenylyl cyclase isotypes. Mol Endocrinol. 2003;17:692–703. doi: 10.1210/me.2002-0369. Epub 2003 Jan 16. [DOI] [PubMed] [Google Scholar]
- Ariwodola OJ, Weiner JL. Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABAB receptors. J Neurosci. 2004;24:10679–10686. doi: 10.1523/JNEUROSCI.1768-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariwodola OJ, Crowder TL, Grant KA, Daunais JB, Friedman DP, Weiner JL. Ethanol modulation of excitatory and inhibitory synaptic transmission in rat and monkey dentate granule neurons. Alcohol Clin Exp Res. 2003;27:1632–1639. doi: 10.1097/01.ALC.0000089956.43262.17. [DOI] [PubMed] [Google Scholar]
- Aryal P, Dvir H, Choe S, Slesinger PA. A discrete alcohol pocket involved in GIRK channel activation. Nat Neurosci. 2009;12(8):988–995. doi: 10.1038/nn.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asan E, Yilmazer-Hanke DM, Eliava M, Hantsch M, Lesch KP, Schmitt A. The corticotropin-releasing factor (CRF)-system and monoaminergic afferents in the central amygdala: investigations in different mouse strains and comparison with the rat. Neuroscience. 2005;131:953–967. doi: 10.1016/j.neuroscience.2004.11.040. [DOI] [PubMed] [Google Scholar]
- Bajo M, Cruz MT, Siggins GR, Messing R, Roberto M. Protein kinase C epsilon mediation of CRF- and ethanol-induced GABA release in central amygdala. Proc Natl Acad Sci USA. 2008;105:8410–8415. doi: 10.1073/pnas.0802302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Gantz SC, Ford CP, Stenzel-Poore MP, Phillips PE, Mark GP, Williams JT. CRF enhancement of GIRK channel-mediated transmission in dopamine neurons. Neuropsychopharmacology. 2009;34:1926–1935. doi: 10.1038/npp.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmeguenai A, Botta P, Weber JT, Carta M, De Ruiter M, De Zeeuw CI, Valenzuela CF, Hansel C. Alcohol impairs long-term depression at the cerebellar parallel fiber–Purkinje cell synapse. J Neurophysiol. 2008;100(6):3167–3174. doi: 10.1152/jn.90384.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork K, Rimondini R, Hansson AC, Terasmaa A, Hyytia P, Heilig M, Sommer WH. Modulation of voluntary ethanol consumption by beta-arrestin 2. FASEB J. 2008;22:2552–2560. doi: 10.1096/fj.07-102442. [DOI] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Grammatopoulos DK, Randeva HS, Hillhouse EW, Spiess J. Corticotropin-releasing factor receptors couple to multiple G-proteins to activate diverse intracellular signaling pathways in mouse hippocampus: role in neuronal excitability and associative learning. J Neurosci. 2003;23:700–707. doi: 10.1523/JNEUROSCI.23-02-00700.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Stoffel M, Chang SR, Harris RA. Potassium channels as targets for ethanol: studies of G-protein-coupled inwardly rectifying potassium channel 2 (GIRK2) null mutant mice. J Pharmacol Exp Ther. 2001;298(2):521–530. [PubMed] [Google Scholar]
- Blitzer RD, Gil O, Landau EM. Long-term potentiation in rat hippocampus is inhibited by low concentrations of ethanol. Brain Res. 1990;537(1–2):203–208. doi: 10.1016/0006-8993(90)90359-j. [DOI] [PubMed] [Google Scholar]
- Bonci A, Malenka RC. Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area. J Neurosci. 1999;19:3723–3730. doi: 10.1523/JNEUROSCI.19-10-03723.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Ungless MA, Bonci A. Convergent actions of orexin/hypocretin and CRF on dopamine neurons: emerging players in addiction. Brain Res. 2010;1314:139–144. doi: 10.1016/j.brainres.2009.10.068. [DOI] [PubMed] [Google Scholar]
- Botta P, Radcliffe RA, Carta M, Mameli M, Daly E, Floyd KL, Deitrich RA, Valenzuela CF. Modulation of GABAA receptors in cerebellar granule neurons by ethanol: a review of genetic and electrophysiologic studies. Alcohol. 2007;41(3):187–199. doi: 10.1016/j.alcohol.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Criswell HE, Carta M, Dodson PD, Hanchar HJ, Khisti RT, Mameli M, Ming Z, Morrow AL, Olsen RW, Otis TS, Parsons LH, Penland SN, Roberto M, Siggins GR, Valenzuela CF, Wallner M. Basis of the gabamimetic profile of ethanol. Alcohol Clin Exp Res. 2006;30:731–744. doi: 10.1111/j.0145-6008.2006.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckner MK, Rossner S, Arendt T. Differential changes in the expression of AMPA receptors genes in rat brain after chronic exposure to ethanol: an in situ hybridization study. J Hirnforsch. 1997;38:369–376. [PubMed] [Google Scholar]
- Buck KJ, Harris RA. Benzodiazepine agonist and inverse agonist actions on GABAA receptor-operated chloride channels II. Chronic effects of ethanol. J Pharmacol Exp Ther. 1990;253:713–719. [PubMed] [Google Scholar]
- Caberlotto L, Thorsell A, Rimondini R, Sommer W, Hyytia P, Heilig M. Differential expression of NPY and its receptors in alcohol-preferring AA and alcohol-avoiding ANA rats. Alcohol Clin Exp Res. 2001;25:1564–1569. [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- Calo G, Bigoni R, Rizzi A, Guerrini R, Salvadori S, Regoli D. Nociceptin/orphanin FQ receptor ligands. Peptides. 2000;21:935–947. doi: 10.1016/s0196-9781(00)00230-8. [DOI] [PubMed] [Google Scholar]
- Cardoso RA, Brozowski SJ, Chavez-Noriega LE, Harpold M, Valenzuela CF, Harris RA. Effects of ethanol on recombinant human neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1999;289(2):774–780. [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Woodward JJ, Chandler LJ. Chronic ethanol induces synaptic but not extra-synaptic targeting of NMDA receptors. J Neurosci. 2004;24:7859–7868. doi: 10.1523/JNEUROSCI.1902-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Olivera DS, Dettmer TS, Valenzuela CF. Ethanol withdrawal upregulates kainate receptors in cultured rat hippocampal neurons. Neurosci Lett. 2002;327:128–132. doi: 10.1016/s0304-3940(02)00399-3. [DOI] [PubMed] [Google Scholar]
- Cebere A, Cebers G, Liljequist S. Enhancement of NMDA-induced functional responses without concomitant NMDA receptor changes following chronic ethanol exposure in cerebellar granule cells. Naunyn Schmiedebergs Arch Pharmacol. 1999;360(6):623–632. doi: 10.1007/s002109900133. [DOI] [PubMed] [Google Scholar]
- Celentano JJ, Gibbs TT, Farb DH. Ethanol potentiates GABA- and glycine-induced chloride currents in chick spinal cord neurons. Brain Res. 1988;455(2):377–380. doi: 10.1016/0006-8993(88)90098-4. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Newsom H, Sumners C, Crews F. Chronic ethanol exposure potentiates NMDA excitotoxicity in cerebral cortical neurons. J Neurochem. 1993;60(4):1578–1581. doi: 10.1111/j.1471-4159.1993.tb03326.x. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Sutton G, Norwood D, Sumners C, Crews FT. Chronic ethanol increases N-methyl-D-aspartate-stimulated nitric oxide formation but not receptor density in cultured cortical neurons. Mol Pharmacol. 1997;51(5):733–740. doi: 10.1124/mol.51.5.733. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Harris RA, Crews FT. Ethanol tolerance and synaptic plasticity. Trends Pharmacol Sci. 1998;19:491–495. doi: 10.1016/s0165-6147(98)01268-1. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Norwood D, Sutton G. Chronic ethanol upregulates NMDA and AMPA, but not kainate receptor subunit proteins in rat primary cortical cultures. Alcohol Clin Exp Res. 1999;23:363–370. [PubMed] [Google Scholar]
- Charlton ME, Sweetnam PM, Fitzgerald LW, Terwilliger RZ, Nestler EJ, Duman RS. Chronic ethanol administration regulates the expression of GABAA receptor alpha 1 and alpha 5 subunits in the ventral tegmental area and hippocampus. J Neurochem. 1997;68:121–127. doi: 10.1046/j.1471-4159.1997.68010121.x. [DOI] [PubMed] [Google Scholar]
- Choi DS, Wei W, Deitchman JK, Kharazia VN, Lesscher HM, McMahon T, Wang D, Qi ZH, Sieghart W, Zhang C, Shokat KM, Mody I, Messing RO. Protein kinase Cdelta regulates ethanol intoxication and enhancement of GABA-stimulated tonic current. J Neurosci. 2008;28(46):11890–11899. doi: 10.1523/JNEUROSCI.3156-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B, Anantharam V, Treistman SN. Ethanol inhibition of recombinant heteromeric NMDA channels in the presence and absence of modulators. J Neurochem. 1995;65(1):140–148. doi: 10.1046/j.1471-4159.1995.65010140.x. [DOI] [PubMed] [Google Scholar]
- Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacol Biochem Behav. 2007;86:813–821. doi: 10.1016/j.pbb.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churn SB, Rana A, Lee K, Parsons JT, De Blas A, Delorenzo RJ. Calcium/calmodulin-dependent kinase II phosphorylation of the GABAA receptor alpha1 subunit modulates benzodiazepine binding. J Neurochem. 2002;82:1065–1076. doi: 10.1046/j.1471-4159.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Hansson AC, Singley E, Sommer WH, Eskay R, Thorsell A, Heilig M. Neuropeptide Y (NPY) suppresses yohimbine-induced reinstatement of alcohol seeking. Psychopharmacology. 2010;208:417–426. doi: 10.1007/s00213-009-1741-y. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Olsen RW, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacology. 2009;56(1):2–5. doi: 10.1016/j.neuropharm.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmers WF, Klapstein GJ, Fournier A, St-Pierre S, Treherne KA. Presynaptic inhibition by neuropeptide Y in rat hippocampal slice in vitro is mediated by a Y2 receptor. Br J Pharmacol. 1991;102:41–44. doi: 10.1111/j.1476-5381.1991.tb12129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M, Vaughan CW, Jennings EA, Allen RG, Christie MJ. Nociceptin, phe(1)psinociceptin( 1–13), nocistatin and prepronociceptin(154–181) effects on calcium channel currents and a potassium current in rat locus coeruleus in vitro. Br J Pharmacol. 1999;128:1779–1787. doi: 10.1038/sj.bjp.0702971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa ET, Soto EE, Cardoso RA, Olivera DS, Valenzuela CF. Acute effects of ethanol on kainate receptors in cultured hippocampal neurons. Alcohol Clin Exp Res. 2000;24:220–225. [PubMed] [Google Scholar]
- Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu JH, Ary AW, Obara I, Rahn A, Abou-Ziab H, Tyrrel B, Marini C, Yoneyama N, Metten P, Snelling C, Dehoff MH, Crabbe JC, Finn DA, Klugmann M, Worley PF, Szumlinski KK. Binge drinking upregulates accumbens mGluR5-Homer2-PI3 K signaling: functional implications for alcoholism. J Neurosci. 2009;29(27):8655–8668. doi: 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell HE, Breese GR. A conceptualization of integrated actions of ethanol contributing to its GABAmimetic profile: a commentary. Neuropsychopharmacology. 2005;30:1407–1425. doi: 10.1038/sj.npp.1300750. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Ming Z, Griffith BL, Breese GR. Comparison of effect of ethanol on N-methyl-d-aspartate- and GABA-gated currents from acutely dissociated neurons: absence of regional differences in sensitivity to ethanol. J Pharmacol Exp Ther. 2003;304:192–199. doi: 10.1124/jpet.102.041590. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Watson SJ. Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol. 1993;332:1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Effect of repeated ethanol withdrawal on glutamate microdialysate in the hippocampus. Alcohol Clin Exp Res. 1999;23:1698–1703. doi: 10.1111/j.1530-0277.1999.tb04063.x. [DOI] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Excitatory and inhibitory amino acid changes during repeated episodes of ethanol withdrawal: an in vivo microdialysis study. Eur J Pharmacol. 2003;459:171–178. doi: 10.1016/s0014-2999(02)02851-0. [DOI] [PubMed] [Google Scholar]
- Davies DL, Machu TK, Guo Y, Alkana RL. Ethanol sensitivity in ATP-gated P2X receptors is subunit dependent. Alcohol Clin Exp Res. 2002;26(6):773–778. [PubMed] [Google Scholar]
- Davies DL, Trudell JR, Mihic SJ, Crawford DK, Alkana RL. Ethanol potentiation of glycine receptors expressed in Xenopus oocytes antagonized by increased atmospheric pressure. Alcohol Clin Exp Res. 2003;27(5):743–755. doi: 10.1097/01.ALC.0000065722.31109.A1. [DOI] [PubMed] [Google Scholar]
- Davies DL, Kochegarov AA, Kuo ST, Kulkarni AA, Woodward JJ, King BF, Alkana RL. Ethanol differentially affects ATP-gated P2X(3) and P2X(4) receptor subtypes expressed in Xenopus oocytes. Neuropharmacology. 2005;49(2):243–253. doi: 10.1016/j.neuropharm.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Davis TJ, de Fiebre CM. Alcohol’s actions on neuronal nicotinic acetylcholine receptors. Alcohol Res Health. 2006;29(3):179–185. [PMC free article] [PubMed] [Google Scholar]
- Daws LC, Montañez S, Munn JL, Owens WA, Baganz NL, Boyce-Rustay JM, Millstein RA, Wiedholz LM, Murphy DL, Holmes A. Ethanol inhibits clearance of brain serotonin by a serotonin transporter-independent mechanism. J Neurosci. 2006;26(24):6431–6438. doi: 10.1523/JNEUROSCI.4050-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza EB, Perrin MH, Insel TR, Rivier J, Vale WW, Kuhar MJ. Corticotropin-releasing factor receptors in rat forebrain: autoradiographic identification. Science. 1984;224:1449–1451. doi: 10.1126/science.6328656. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Smith FD, Grayson DR, Morrow AL. Chronic ethanol consumption differentially alters the expression of gamma-aminobutyric acidA receptor subunit mRNAs in rat cerebral cortex: competitive, quantitative reverse transcriptase-polymerase chain reaction analysis. Mol Pharmacol. 1995;48:861–868. [PubMed] [Google Scholar]
- Devaud LL, Fritschy JM, Sieghart W, Morrow AL. Bidirectional alterations of GABAA receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. J Neurochem. 1997;69:126–130. doi: 10.1046/j.1471-4159.1997.69010126.x. [DOI] [PubMed] [Google Scholar]
- Diamond I, Gordon AS. The role of adenosine in mediating cellular and molecular responses to ethanol. Exs. 1994;71:175–183. doi: 10.1007/978-3-0348-7330-7_18. [DOI] [PubMed] [Google Scholar]
- Dildy JE, Leslie SW. Ethanol inhibits NMDA-induced increases in intracellular Ca2+ in dissociated brain cells. Brain Res. 1989;499:383–387. doi: 10.1016/0006-8993(89)90789-0. [DOI] [PubMed] [Google Scholar]
- Dildy-Mayfield JE, Harris RA. Comparison of ethanol sensitivity of rat brain kainate, dl-alpha-amino-3-hydroxy-5-methyl-4-isoxalone proprionic acid and N-methyl-d-aspartate receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1992;262:487–494. [PubMed] [Google Scholar]
- Dolphin AC. G protein modulation of voltage-gated calcium channels. Pharmacol Rev. 2003;55(4):607–627. doi: 10.1124/pr.55.4.3. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci USA. 1992;89(10):4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand D, Carlen PL. Impairment of long-term potentiation in rat hippocampus following chronic ethanol treatment. Brain Res. 1984;308(2):325–332. doi: 10.1016/0006-8993(84)91072-2. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, Kalant H, Koob GF, Li TK, Tabakoff B. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Economidou D, Hansson AC, Weiss F, Terasmaa A, Sommer WH, Cippitelli A, Fedeli A, Martin-Fardon R, Massi M, Ciccocioppo R, Heilig M. Dysregulation of nociceptin/orphanin FQ activity in the amygdala is linked to excessive alcohol drinking in the rat. Biol Psychiatry. 2008;64:211–218. doi: 10.1016/j.biopsych.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Berger AJ. Mechanisms for the modulation of native glycine receptor channels by ethanol. J Neurophysiol. 2004;91(6):2685–2695. doi: 10.1152/jn.00907.2003. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Li TK, Lumeng L, Hwang BH, Somes C, Jimenez P, Mathe AA. Neuropeptide Y levels in ethanol-naive alcohol-preferring and nonpreferring rats and in Wistar rats after ethanol exposure. Alcohol Clin Exp Res. 1998a;22:1778–1782. [PubMed] [Google Scholar]
- Ehlers CL, Somes C, Cloutier D. Are some of the effects of ethanol mediated through NPY? Psychopharmacology. 1998b;139:136–144. doi: 10.1007/s002130050698. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Somes C, Lumeng L, Li TK. Electrophysiological response to neuropeptide Y (NPY): in alcohol-naive preferring and non-preferring rats. Pharmacol Biochem Behav. 1999;63:291–299. doi: 10.1016/s0091-3057(99)00012-x. [DOI] [PubMed] [Google Scholar]
- Eliava M, Yilmazer-Hanke D, Asan E. Interrelations between monoaminergic afferents and corticotropin-releasing factor-immunoreactive neurons in the rat central amygdaloid nucleus: ultrastructural evidence for dopaminergic control of amygdaloid stress systems. Histochem Cell Biol. 2003;120:183–197. doi: 10.1007/s00418-003-0557-9. [DOI] [PubMed] [Google Scholar]
- Elmslie KS. Neurotransmitter modulation of neuronal calcium channels. J Bioenerg Biomembr. 2003;35(6):477–489. doi: 10.1023/b:jobb.0000008021.55853.18. [DOI] [PubMed] [Google Scholar]
- Enoch MA. The role of GABA(A) receptors in the development of alcoholism. Pharmacol Biochem Behav. 2008;90(1):95–104. doi: 10.1016/j.pbb.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici M, Nisticò R, Giustizieri M, Bernardi G, Mercuri NB. Ethanol enhances GABAB-mediated inhibitory postsynaptic transmission on rat midbrain dopaminergic neurons by facilitating GIRK currents. Eur J Neurosci. 2009;29(7):1369–1377. doi: 10.1111/j.1460-9568.2009.06700.x. [DOI] [PubMed] [Google Scholar]
- Fekete EM, Zorrilla EP. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: ancient CRF paralogs. Front Neuroendocrinol. 2007;28:1–27. doi: 10.1016/j.yfrne.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink K, Göthert M. Both ethanol and ifenprodil inhibit NMDA-evoked release of various neurotransmitters at different, yet proportional potency: potential relation to NMDA receptor subunit composition. Naunyn Schmiedebergs Arch Pharmacol. 1996;354(3):312–319. doi: 10.1007/BF00171062. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Williams JT. Glutamate mediates an inhibitory postsynaptic potential in dopamine neurons. Nature. 1998;394:78–82. doi: 10.1038/27919. [DOI] [PubMed] [Google Scholar]
- Fleming RL, Manis PB, Morrow AL. The effects of acute and chronic ethanol exposure on presynaptic and postsynaptic gamma-aminobutyric acid (GABA) neurotransmission in cultured cortical and hippocampal neurons. Alcohol. 2009;43:603–618. doi: 10.1016/j.alcohol.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd DW, Jung KY, McCool BA. Chronic ethanol ingestion facilitates N-methyl-d-aspartate receptor function and expression in rat lateral/basolateral amygdala neurons. J Pharmacol Exp Ther. 2003;307:1020–1029. doi: 10.1124/jpet.103.057505. [DOI] [PubMed] [Google Scholar]
- Floyd DW, Friedman DP, Daunais JB, Pierre PJ, Grant KA, McCool BA. Long-term ethanol self-administration by cynomolgus macaques alters the pharmacology and expression of GABAA receptors in basolateral amygdala. J Pharmacol Exp Ther. 2004;311:1071–1079. doi: 10.1124/jpet.104.072025. [DOI] [PubMed] [Google Scholar]
- Follesa P, Ticku MK. Chronic ethanol treatment differentially regulates NMDA receptor subunit mRNA expression in rat brain. Brain Res Mol Brain Res. 1995;29:99–106. doi: 10.1016/0169-328x(94)00235-7. [DOI] [PubMed] [Google Scholar]
- Francesconi W, Berton F, Koob GF, Sanna PP. Intrinsic neuronal plasticity in the juxtacapsular nucleus of the bed nuclei of the stria terminalis (jcBNST) Prog Neuropsychopharmacol Biol Psychiatry. 2009a;33:1347–1355. doi: 10.1016/j.pnpbp.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francesconi W, Berton F, Repunte-Canonigo V, Hagihara K, Thurbon D, Lekic D, Specio SE, Greenwell TN, Chen SA, Rice KC, Richardson HN, O’Dell LE, Zorrilla EP, Morales M, Koob GF, Sanna PP. Protracted withdrawal from alcohol and drugs of abuse impairs long-term potentiation of intrinsic excitability in the juxtacapsular bed nucleus of the stria terminalis. J Neurosci. 2009b;29:5389–5401. doi: 10.1523/JNEUROSCI.5129-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye GD, Fincher A. Sensitivity of postsynaptic GABAB receptors on hippocampal CA1 and CA3 pyramidal neurons to ethanol. Brain Res. 1996;735:239–248. doi: 10.1016/0006-8993(96)00579-3. [DOI] [PubMed] [Google Scholar]
- Frye GD, Fincher A. Sustained ethanol inhibition of native AMPA receptors on medial septum/diagonal band (MS/DB) neurons. Br J Pharmacol. 2000;129:87–94. doi: 10.1038/sj.bjp.0703039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye GD, McCown TJ, Breese GR. Differential sensitivity of ethanol withdrawal signs in the rat to gamma-aminobutyric acid (GABA)mimetics: blockade of audiogenic seizures but not forelimb tremors. J Pharmacol Exp Ther. 1983;226:720–725. [PubMed] [Google Scholar]
- Fujii S, Yamazaki Y, Sugihara T, Wakabayashi I. Acute and chronic ethanol exposure differentially affect induction of hippocampal LTP. Brain Res. 2008;1211:13–21. doi: 10.1016/j.brainres.2008.02.052. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Shoda T, Morikawa H, Kato S, Mima H, Mori K. Activation of phospholipase A2 by the nociceptin receptor expressed in Chinese hamster ovary cells. J Neurochem. 1998;71:2186–2192. doi: 10.1046/j.1471-4159.1998.71052186.x. [DOI] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher JP, Orozco-Cabal LF, Liu J, Shinnick-Gallagher P. Synaptic physiology of central CRH system. Eur J Pharmacol. 2008;583:215–225. doi: 10.1016/j.ejphar.2007.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Ciccocioppo R, Heilig M. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2, 6-dimethyl-imidazo [1, 2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman G, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is necessary for long-term depression in the striatum. Nat Neurosci. 2002;5(5):446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Murphy JM, Li TK, Badia-Elder NE. Neuropeptide Y reduces oral ethanol intake in alcohol-preferring (P) rats following a period of imposed ethanol abstinence. Alcohol Clin Exp Res. 2003;27:787–794. doi: 10.1097/01.ALC.0000065723.93234.1D. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Koob GF. Neuropeptide Y in the central nucleus of the amygdala suppresses dependence-induced increases in alcohol drinking. Pharmacol Biochem Behav. 2008a;90:475–480. doi: 10.1016/j.pbb.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Badia-Elder NE. Neuropeptide Y administration into the amygdala suppresses ethanol drinking in alcohol-preferring (P) rats following multiple deprivations. Pharmacol Biochem Behav. 2008b;90:470–474. doi: 10.1016/j.pbb.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin N, Misra K, Roberto M, Koob GF. Role of Neuropeptide Y (NPY) in the transition to alcohol dependence. Alcohol Clin Exp Res 277A. 2009;33:6. [Google Scholar]
- Gilpin NW, Misra K, Herman M, Cruz MT, Koob GF, Roberto M. Neuropeptide Y Opposes Alcohol Effects on GABA Release in Amygdala and Blocks the Transition to Alcohol Dependence. Biological Psychiatry. 2011;69(11):1091–1099. doi: 10.1016/j.biopsych.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givens B, McMahon K. Ethanol suppresses the induction of long-term potentiation in vivo. Brain Res. 1995;688(1–2):27–33. doi: 10.1016/0006-8993(95)00499-g. [DOI] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABAA receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10(1):40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103(2):121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gouaux E. Structure and function of AMPA receptors. J Physiol. 2004;554(Pt 2):249–253. doi: 10.1113/jphysiol.2003.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammatopoulos DK, Randeva HS, Levine MA, Kanellopoulou KA, Hillhouse EW. Rat cerebral cortex corticotropin-releasing hormone receptors: evidence for receptor coupling to multiple G proteins. J Neurochem. 2001;76:509–519. doi: 10.1046/j.1471-4159.2001.00067.x. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABAA receptors in the acute and chronic effects of ethanol. Psychopharmacology. 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Fritschy JM, Morrow AL. Chronic ethanol administration alters immunoreactivity for GABAA receptor subunits in rat cortex in a region-specific manner. Alcohol Clin Exp Res. 2000;24:1137–1144. [PubMed] [Google Scholar]
- Grover CA, Frye GD, Griffith WH. Acute tolerance to ethanol inhibition of NMDA-mediated EPSPs in the CA1 region of the rat hippocampus. Brain Res. 1994;642(1–2):70–76. doi: 10.1016/0006-8993(94)90906-7. [DOI] [PubMed] [Google Scholar]
- Grover CA, Wallace KA, Lindberg SA, Frye GD. Ethanol inhibition of NMDA currents in acutely dissociated medial septum/diagonal band neurons from ethanol dependent rats. Brain Res. 1998;782:43–52. doi: 10.1016/s0006-8993(97)01001-9. [DOI] [PubMed] [Google Scholar]
- Gulya K, Grant KA, Valverius P, Hoffman PL, Tabakoff B. Brain regional specificity and time-course of changes in the NMDA receptor-ionophore complex during ethanol withdrawal. Brain Res. 1991;547:129–134. [PubMed] [Google Scholar]
- Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. Alcohol-induced motor impairment caused by increased extrasynaptic GABAA receptor activity. Nat Neurosci. 2005;8(3):339–345. doi: 10.1038/nn1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L, Terasmaa A, Massi M, Heilig M, Ciccocioppo R. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci USA. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Ciccocioppo R, Heilig M. Region-specific down-regulation of Crhr1 gene expression in alcohol-preferring msP rats following ad lib access to alcohol. Addict Biol. 2007;12:30–34. doi: 10.1111/j.1369-1600.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- Harris RA. Ethanol actions on multiple ion channels: Which are important? Alcohol Clin Exp Res. 1999;23(10):1563–1570. [PubMed] [Google Scholar]
- Harrison LM, Grandy DK. Opiate modulating properties of nociceptin/orphanin FQ. Peptides. 2000;21:151–172. doi: 10.1016/s0196-9781(99)00185-0. [DOI] [PubMed] [Google Scholar]
- Haugbol SR, Ebert B, Ulrichsen J. Upregulation of glutamate receptor subtypes during alcohol withdrawal in rats. Alcohol Alcohol. 2005;40:89–95. doi: 10.1093/alcalc/agh117. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol Disord Drug Targ. 2006;5:453–479. doi: 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausser MA, Yung WH. Inhibitory synaptic potentials in guinea-pig substantia nigra dopamine neurones in vitro. J Physiol. 1994;479(Pt 3):401–422. doi: 10.1113/jphysiol.1994.sp020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes BE, Graziano MP, Lambert DG. Cellular actions of nociceptin: transduction mechanisms. Peptides. 2000;21:961–967. doi: 10.1016/s0196-9781(00)00232-1. [DOI] [PubMed] [Google Scholar]
- Hayes DM, Knapp DJ, Breese GR, Thiele TE. Comparison of basal neuropeptide Y and corticotropin releasing factor levels between the high ethanol drinking C57BL/6 J and low ethanol drinking DBA/2 J inbred mouse strains. Alcohol Clin Exp Res. 2005;29:721–729. doi: 10.1097/01.ALC.0000164375.16838.F3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, McLeod S, Brot M, Heinrichs SC, Menzaghi F, Koob GF, Britton KT. Anxiolytic-like action of neuropeptide Y: mediation by Y1 receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacology. 1993;8:357–363. doi: 10.1038/npp.1993.35. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF, Ekman R, Britton KT. Corticotropin-releasing factor and neuropeptide Y: role in emotional integration. Trends Neurosci. 1994;17:80–85. doi: 10.1016/0166-2236(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol. 2010;15(2):169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exp Ther. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- Henderson G, McKnight AT. The orphan opioid receptor and its endogenous ligand—nociceptin/orphanin FQ. Trends Pharmacol Sci. 1997;18:293–300. [PubMed] [Google Scholar]
- Hendricson AW, Miao CL, Lippmann MJ, Morrisett RA. Ifenprodil and ethanol enhance NMDA receptor-dependent long-term depression. J Pharmacol Exp Ther. 2002;301(3):938–944. doi: 10.1124/jpet.301.3.938. [DOI] [PubMed] [Google Scholar]
- Herring D, Huang R, Singh M, Robinson LC, Dillon GH, Leidenheimer NJ. Constitutive GABAA receptor endocytosis is dynamin-mediated and dependent on a dileucine AP2 adaptin-binding motif within the beta 2 subunit of the receptor. J Biol Chem. 2003;278:24046–24052. doi: 10.1074/jbc.M301420200. [DOI] [PubMed] [Google Scholar]
- Hoffman PL, Rabe CS, Moses F, Tabakoff B. N-methyl-d-aspartate receptors and ethanol: Inhibition of calcium flux and cyclic GMP production. J Neurochem. 1989;52:1937–1940. doi: 10.1111/j.1471-4159.1989.tb07280.x. [DOI] [PubMed] [Google Scholar]
- Hu XJ, Follesa P, Ticku MK. Chronic ethanol treatment produces a selective upregulation of the NMDA receptor subunit gene expression in mammalian cultured cortical neurons. Brain Res Mol Brain Res. 1996;36(2):211–218. doi: 10.1016/0169-328x(95)00223-f. [DOI] [PubMed] [Google Scholar]
- Hu M, Walker DW, Vickroy TW, Peris J. Chronic ethanol exposure increases 3H-GABA release in rat hippocampus by presynaptic muscarinic receptor modulation. Alcohol Clin Exp Res. 1999;23:1587–1595. [PubMed] [Google Scholar]
- Hwang BH, Zhang JK, Ehlers CL, Lumeng L, Li TK. Innate differences of neuropeptide Y (NPY) in hypothalamic nuclei and central nucleus of the amygdala between selectively bred rats with high and low alcohol preference. Alcohol Clin Exp Res. 1999;23:1023–1030. [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Kobayashi K, Kobayashi T, Ichikawa T, Kumanishi T, Kishida H, Yano R, Manabe T. Functional coupling of the nociceptin/orphanin FQ receptor with the G- protein-activated K ? (GIRK) channel. Brain Res Mol Brain Res. 1997;45:117–126. doi: 10.1016/s0169-328x(96)00252-5. [DOI] [PubMed] [Google Scholar]
- Iorio KR, Reinlib L, Tabakoff B, Hoffman PL. Chronic exposure of cerebellar granule cells to ethanol results in increased N-methyl-d-aspartate receptor function. Mol Pharmacol. 1992;41(6):1142–1148. [PubMed] [Google Scholar]
- Iorio KR, Tabakoff B, Hoffman PL. Glutamate-induced neurotoxicity is increased in cerebellar granule cells exposed chronically to ethanol. Eur J Pharmacol. 1993;248(2):209–212. doi: 10.1016/0926-6917(93)90045-r. [DOI] [PubMed] [Google Scholar]
- Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev. 2001;81(3):1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- Ives JH, Drewery DL, Thompson CL. Differential cell surface expression of GABAA receptor alpha1, alpha6, beta2 and beta3 subunits in cultured mouse cerebellar granule cells influence of cAMP-activated signaling. J Neurochem. 2002;80:317–327. doi: 10.1046/j.0022-3042.2001.00700.x. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Nagashima K, Murayama K, Zorumski CF. Acute effects of ethanol on hippocampal long-term potentiation and long-term depression are mediated by different mechanisms. Neuroscience. 2005;136(2):509–517. doi: 10.1016/j.neuroscience.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Jia F, Chandra D, Homanics GE, Harrison NL. Ethanol modulates synaptic and extrasynaptic GABAA receptors in the thalamus. J Pharmacol Exp Ther. 2008;326(2):475–482. doi: 10.1124/jpet.108.139303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Woodward JJ. Effects of 8 different NR1 splice variants on the ethanol inhibition of recombinant NMDA receptors. Alcohol Clin Exp Res. 2006;30(4):673–679. doi: 10.1111/j.1530-0277.2006.00079.x. [DOI] [PubMed] [Google Scholar]
- Jin C, Smothers CT, Woodward JJ. Enhanced ethanol inhibition of recombinant N-methyl-d-aspartate receptors by magnesium: role of NR3A subunits. Alcohol Clin Exp Res. 2008;32(6):1059–1066. doi: 10.1111/j.1530-0277.2008.00667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Mathews TA, Budygin EA. Effect of moderate ethanol dose on dopamine uptake in rat nucleus accumbens in vivo. Synapse. 2006;60(3):251–255. doi: 10.1002/syn.20294. [DOI] [PubMed] [Google Scholar]
- Ju G, Han ZS. Coexistence of corticotropin releasing factor and neurotensin within oval nucleus neurons in the bed nuclei of the stria terminalis in the rat. Neurosci Lett. 1989;99:246–250. doi: 10.1016/0304-3940(89)90454-0. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10(8):561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kang M, Spigelman I, Sapp DW, Olsen RW. Persistent reduction of GABAA receptor-mediated inhibition in rat hippocampus after chronic intermittent ethanol treatment. Brain Res. 1996;709:221–228. doi: 10.1016/0006-8993(95)01274-5. [DOI] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998a;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Kang MH, Spigelman I, Olsen RW. Alteration in the sensitivity of GABAA receptors to allosteric modulatory drugs in rat hippocampus after chronic intermittent ethanol treatment. Alcohol Clin Exp Res. 1998b;22:2165–2173. [PubMed] [Google Scholar]
- Kash TL, Winder DG. Neuropeptide Y and corticotropin-releasing factor bi-directionally modulate inhibitory synaptic transmission in the bed nucleus of the stria terminalis. Neuropharmacology. 2006;51:1013–1022. doi: 10.1016/j.neuropharm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Kash TL, Nobis WP, Matthews RT, Winder DG. Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1-dependent process. J Neurosci. 2008;28:13856–13865. doi: 10.1523/JNEUROSCI.4715-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Baucum AJ, II, Conrad KL, Colbran RJ, Winder DG. Alcohol exposure alters NMDAR function in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2009;34(11):2420–2429. doi: 10.1038/npp.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kask A, Harro J, von Horsten S, Redrobe JP, Dumont Y, Quirion R. The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci Biobehav Rev. 2002;26:259–283. doi: 10.1016/s0149-7634(01)00066-5. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8(11):844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kellenberger S, Malherbe P, Sigel E. Function of the alpha 1 beta 2 gamma 2S gamma-aminobutyric acid type A receptor is modulated by protein kinase C via multiple phosphorylation sites. J Biol Chem. 1992;267:25660–25663. [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR. Calcium release from presynaptic internal stores is required for ethanol to increase spontaneous gamma-aminobutyric acid release onto cerebellum Purkinje neurons. J Pharmacol Exp Ther. 2007;323(1):356–364. doi: 10.1124/jpet.107.126144. [DOI] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR. The role of protein kinase A in the ethanol-induced increase in spontaneous GABA release onto cerebellar Purkinje neurons. J Neurophysiol. 2008;100(6):3417–3428. doi: 10.1152/jn.90970.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm MK, Weinberg RJ, Criswell HE, Breese GR. The PLC/IP 3 R/PKC pathway is required for ethanol-enhanced GABA release. Neuropharmacology. 2010;58(7):1179–1186. doi: 10.1016/j.neuropharm.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Chen G, Kukhtina V, Vahedi-Faridi A, Gu Z, Tretter V, Smith KR, McAinsh K, Arancibia-Carcamo IL, Saenger W, Haucke V, Yan Z, Moss SJ. Regulation of synaptic inhibition by phospho-dependent binding of the AP2 complex to a YECL motif in the GABAA receptor gamma2 subunit. Proc Natl Acad Sci U S A. 2008;105:3616–3621. doi: 10.1073/pnas.0707920105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliethermes CL. Anxiety-like behaviors following chronic ethanol exposure. Neurosci Biobehav Rev. 2005;28:837–850. doi: 10.1016/j.neubiorev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ikeda K, Kojima H, Niki H, Yano R, Yoshioka T, Kumanishi T. Ethanol opens G protein activated inwardly rectifying K+ channels. Nat Neurosci. 1999;2:1091–1097. doi: 10.1038/16019. [DOI] [PubMed] [Google Scholar]
- Kokka N, Sapp DW, Taylor AM, Olsen RW. The kindling model of alcohol dependence: similar persistent reduction in seizure threshold to pentylenetetrazol in animals receiving chronic ethanol or chronic pentylenetetrazol. Alcohol Clin Exp Res. 1993;17:525–531. doi: 10.1111/j.1530-0277.1993.tb00793.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Krishek BJ, Xie X, Blackstone C, Huganir RL, Moss SJ, Smart TG. Regulation of GABAA receptor function by protein kinase C phosphorylation. Neuron. 1994;12:1081–1095. doi: 10.1016/0896-6273(94)90316-6. [DOI] [PubMed] [Google Scholar]
- Kumar S, Sieghart W, Morrow AL. Association of protein kinase C with GABAA receptors containing alpha1 and alpha4 subunits in the cerebral cortex: selective effects of chronic ethanol consumption. J Neurochem. 2002;82:110–117. doi: 10.1046/j.1471-4159.2002.00943.x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Kralic JE, O’Buckley TK, Grobin AC, Morrow AL. Chronic ethanol consumption enhances internalization of alpha1 subunit-containing GABAA receptors in cerebral cortex. J Neurochem. 2003;86:700–708. doi: 10.1046/j.1471-4159.2003.01894.x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Fleming RL, Morrow AL. Ethanol regulation of gamma-aminobutyric acid A receptors: genomic and nongenomic mechanisms. Pharmacol Ther. 2004;101:211–226. doi: 10.1016/j.pharmthera.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABAA receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology. 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner T, Schoepfer R, Korpi ER. Ethanol inhibits glutamate-induced currents in heteromeric NMDA receptor subtypes. Neuroreport. 1993;5(3):297–300. doi: 10.1097/00001756-199312000-00029. [DOI] [PubMed] [Google Scholar]
- Lachowicz JE, Shen Y, Monsma FJ, Jr, Sibley DR. Molecular cloning of a novel G-protein-coupled receptor related to the opiate receptor family. J Neurochem. 1995;64:34–40. doi: 10.1046/j.1471-4159.1995.64010034.x. [DOI] [PubMed] [Google Scholar]
- Lack AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic ethanol and withdrawal differentially modulate pre and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J Neurophysiol. 2007;98:3185–3196. doi: 10.1152/jn.00189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack AK, Ariwodola OJ, Chappell AM, Weiner JL, McCool BA. Ethanol inhibition of kainate receptor-mediated excitatory neurotransmission in the rat basolateral nucleus of the amygdala. Neuropharmacology. 2008;55:661–668. doi: 10.1016/j.neuropharm.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack AK, Christian DT, Diaz MR, McCool BA. Chronic ethanol and withdrawal effects on kainate receptor-mediated excitatory neurotransmission in the rat basolateral amygdala. Alcohol. 2009;43:25–33. doi: 10.1016/j.alcohol.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läck AK, Floyd DW, McCool BA. Chronic ethanol ingestion modulates proanxiety factors expressed in rat central amygdala. Alcohol. 2005;36(2):83–90. doi: 10.1016/j.alcohol.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson KP, Olsen UB, Hansen AJ. Nociceptin is a potent inhibitor of N-type Ca(2+) channels in rat sympathetic ganglion neurons. Neurosci Lett. 2000;296:121–124. doi: 10.1016/s0304-3940(00)01640-2. [DOI] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenders AG, Sheng ZH. Modulation of neurotransmitter release by the second messenger-activated protein kinases implications for presynaptic plasticity. Pharmacol Ther. 2005;105(1):69–84. doi: 10.1016/j.pharmthera.2004.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidenheimer NJ, McQuilkin SJ, Hahner LD, Whiting P, Harris RA. Activation of protein kinase C selectively inhibits the gamma-aminobutyric acidA receptor role of desensitization. Mol Pharmacol. 1992;41:1116–1123. [PubMed] [Google Scholar]
- LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence findings of animal studies. Biol Psychiatry. 1994;36:395–421. doi: 10.1016/0006-3223(94)91215-7. [DOI] [PubMed] [Google Scholar]
- Lewohl JM, Wilson WR, Mayfield RD, Brozowski SJ, Morrisett RA, Harris RA. G-protein-coupled inwardly rectifying potassium channels are targets of alcohol action. Nat Neurosci. 1999;2:1084–1090. doi: 10.1038/16012. [DOI] [PubMed] [Google Scholar]
- Li C, Aguayo L, Peoples RW, Weight FF. Ethanol inhibits a neuronal ATP-gated ion channel. Mol Pharmacol. 1993;44(4):871–875. [PubMed] [Google Scholar]
- Liang J, Cagetti E, Olsen RW, Spigelman I. Altered pharmacology of synaptic and extrasynaptic GABAA receptors on CA1 hippocampal neurons is consistent with subunit changes in a model of alcohol withdrawal and dependence. J Pharmacol Exp Ther. 2004;310(3):1234–1245. doi: 10.1124/jpet.104.067983. [DOI] [PubMed] [Google Scholar]
- Liang J, Suryanarayanan A, Abriam A, Snyder B, Olsen RW, Spigelman I. Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J Neurosci. 2007;27:12367–12377. doi: 10.1523/JNEUROSCI.2786-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Spigelman I, Olsen RW. Tolerance to sedative/hypnotic actions of GABAergic drugs correlates with tolerance to potentiation of extrasynaptic tonic currents of alcohol-dependent rats. J Neurophysiol. 2009;102:224–233. doi: 10.1152/jn.90484.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima-Landman MTR, Albuquerque EX. Ethanol potentiates and blocks NMDA-activated single-channel currents in rat hippocampal pyramidal cells. FEBS Lett. 1989;247:61–67. doi: 10.1016/0014-5793(89)81241-4. [DOI] [PubMed] [Google Scholar]
- Liu J, Yu B, Neugebauer V, Grigoriadis DE, Rivier J, Vale WW, Shinnick-Gallagher P, Gallagher JP. Corticotropin-releasing factor and Urocortin I modulate excitatory glutamatergic synaptic transmission. J Neurosci. 2004;24:4020–4029. doi: 10.1523/JNEUROSCI.5531-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo IA, Harris RA. GABAA receptors and alcohol. Pharmacol Biochem Behav. 2008;90(1):90–94. doi: 10.1016/j.pbb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou LG, Zhang Z, Ma L, Pei G. Nociceptin/orphanin FQ activates mitogen-activated protein kinase in Chinese hamster ovary cells expressing opioid receptor-like receptor. J Neurochem. 1998;70:1316–1322. doi: 10.1046/j.1471-4159.1998.70031316.x. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Ethanol potentiates 5-HT3 receptor-mediated ion current in NCB-20 neuroblastoma cells. Neurosci Lett. 1991;122:54–56. doi: 10.1016/0304-3940(91)90192-v. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. High ethanol sensitivity of recombinant AMPA-type glutamate receptors expressed in mammalian cells. Neurosci Lett. 1993;159:83–87. doi: 10.1016/0304-3940(93)90804-t. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Developmental decrease in ethanol inhibition of N-methyl-d-aspartate receptors in rat neocortical neurons: relation to the actions of ifenprodil. J Pharmacol Exp Ther. 1995;274:164–172. [PubMed] [Google Scholar]
- Lovinger DM. Alcohols and neurotransmitter gated ion channels: past, present and future. Naunyn–Schmiedeberg’s Archives of Pharmacology. 1997;356:267–282. doi: 10.1007/pl00005051. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Homanics GE. Tonic for what ails us? High affinity GABAA receptors and alcohol. Alcohol. 2007;41(3):139–143. doi: 10.1016/j.alcohol.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. NMDA receptor-mediated synaptic excitation selectively inhibited by ethanol in hippocampal slice from adult rat. J Neurosci. 1990;10:1372–1379. doi: 10.1523/JNEUROSCI.10-04-01372.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery EG, Thiele TE. Preclinical evidence that corticotropin-releasing factor (CRF) receptor antagonists are promising targets for pharmacological treatment of alcoholism. CNS Neurol Disord Drug Targ. 2010;9:77–86. doi: 10.2174/187152710790966605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SM, Yeh HH. Ethanol modulates AMPA-induced current responses of primary somatosensory cortical neurons. Neurochem Int. 1999;35(2):175–183. doi: 10.1016/s0197-0186(99)00059-5. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65(4):445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthin GR, Tabakoff B. Activation of adenylate cyclase by alcohols requires the nucleotide-binding protein. J Pharmacol Exp Ther. 1984;228(3):579–587. [PubMed] [Google Scholar]
- Macdonald RL. Ethanol, gamma-aminobutyrate type A receptors, and protein kinase C phosphorylation. Proc Natl Acad Sci U S A. 1995;92:3633–3635. doi: 10.1073/pnas.92.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machu TK, Harris RA. Alcohols and anesthetics enhance the function of 5-hydroxytryptamine3 receptors expressed in Xenopus laevis oocytes. J Pharmacol Exp Ther. 1994;271(2):898–905. [PubMed] [Google Scholar]
- Madamba SG, Schweitzer P, Siggins GR. Nociceptin augments K(+) currents in hippocampal CA1 neurons by both ORL-1 and opiate receptor mechanisms. J Neurophysiol. 1999;82:1776–1785. doi: 10.1152/jn.1999.82.4.1776. [DOI] [PubMed] [Google Scholar]
- Mahmoudi M, Kang MH, Tillakaratne N, Tobin AJ, Olsen RW. Chronic intermittent ethanol treatment in rats increases GABAA receptor alpha4-subunit expression: possible relevance to alcohol dependence. J Neurochem. 1997;68:2485–2492. doi: 10.1046/j.1471-4159.1997.68062485.x. [DOI] [PubMed] [Google Scholar]
- Maiya R, Buck KJ, Harris RA, Mayfield RD. Ethanol-sensitive sites on the human dopamine transporter. J Biol Chem. 2002;277(34):30724–30729. doi: 10.1074/jbc.M204914200. [DOI] [PubMed] [Google Scholar]
- Mameli M, Zamudio PA, Carta M, Valenzuela CF. Developmentally regulated actions of alcohol on hippocampal glutamatergic transmission. J Neurosci. 2005;25:8027–8036. doi: 10.1523/JNEUROSCI.2434-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalec W, Kurata Y, Hamilton BJ, Carter DB, Narahashi T. Selective effects of alcohols on gamma-aminobutyric acidA receptor subunits expressed in human embryonic kidney cells. J Pharmacol Exp Ther. 1994;269(1):157–163. [PubMed] [Google Scholar]
- Martin-Fardon R, Zorrilla EP, Ciccocioppo R, Weiss F. Role of innate and drug-induced dysregulation of brain stress and arousal systems in addiction: focus on corticotropin-releasing factor, nociceptin/orphanin FQ, orexin/hypocretin. Brain Res. 2010;1314:145–161. doi: 10.1016/j.brainres.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marutha Ravindran CR, Mehta AK, Ticku MK. Effect of chronic administration of ethanol on the regulation of the delta-subunit of GABAA receptors in the rat brain. Brain Res. 2007;1174:47–52. doi: 10.1016/j.brainres.2007.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascia MP, Mihic SJ, Valenzuela CF, Schofield PR, Harris RA. A single amino acid determines differences in ethanol actions on strychnine-sensitive glycine receptors. Mol Pharmacol. 1996;50(2):402–406. [PubMed] [Google Scholar]
- Masood K, Wu C, Brauneis U, Weight FF. Differential ethanol sensitivity of recombinant N-methyl-d-aspartate receptor subunits. Mol Pharmacol. 1994;45:324–329. [PubMed] [Google Scholar]
- Mathews TA, John CE, Lapa GB, Budygin EA, Jones SR. No role of the dopamine transporter in acute ethanol effects on striatal dopamine dynamics. Synapse. 2006;60(4):288–294. doi: 10.1002/syn.20301. 2006. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Devaud LL, Fritschy JM, Sieghart W, Morrow AL. Differential regulation of GABAA receptor gene expression by ethanol in the rat hippocampus versus cerebral cortex. J Neurochem. 1998;70:1160–1166. doi: 10.1046/j.1471-4159.1998.70031160.x. [DOI] [PubMed] [Google Scholar]
- Mayfield RD, Maiya R, Keller D, Zahniser NR. Ethanol potentiates the function of the human dopamine transporter expressed in Xenopus oocytes. J Neurochem. 2001;79(5):1070–1079. doi: 10.1046/j.1471-4159.2001.00656.x. [DOI] [PubMed] [Google Scholar]
- McCool BA, Frye GD, Pulido MD, Botting SK. Effects of chronic ethanol consumption on rat GABAA and strychnine-sensitive glycine receptors expressed by lateral/basolateral amygdala neurons. Brain Res. 2003;963(1–2):165–177. doi: 10.1016/s0006-8993(02)03966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCown TJ, Frye GD, Breese GR. Evidence for site specific ethanol actions in the CNS. Alcohol Drug Res. 1985;6:423–429. [PubMed] [Google Scholar]
- Meis S. Nociceptin/orphanin FQ: actions within the brain. Neuroscientist. 2003;9:158–168. doi: 10.1177/1073858403252231. [DOI] [PubMed] [Google Scholar]
- Meis S, Pape HC. Postsynaptic mechanisms underlying responsiveness of amygdaloid neurons to nociceptin/orphanin FQ. J Neurosci. 1998;18:8133–8144. doi: 10.1523/JNEUROSCI.18-20-08133.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meis S, Pape HC. Control of glutamate and GABA release by nociceptin/orphanin FQ in the rat lateral amygdala. J Physiol. 2001;532:701–712. doi: 10.1111/j.1469-7793.2001.0701e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meis S, Munsch T, Pape HC. Antioscillatory effects of nociceptin/orphanin FQ in synaptic networks of the rat thalamus. J Neurosci. 2002;22:718–727. doi: 10.1523/JNEUROSCI.22-03-00718.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcohol Clin Exp Res. 2005;29(3):326–333. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- Melis M, Camarini R, Ungless MA, Bonci A. Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. J Neurosci. 2002;22:2074–2082. doi: 10.1523/JNEUROSCI.22-06-02074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, Marzagull H, Vassart G, Parmentier M, Costentin J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Mhatre MC, Ticku MK. Chronic ethanol administration alters gamma-aminobutyric acidA receptor gene expression. Mol Pharmacol. 1992;42:415–422. [PubMed] [Google Scholar]
- Mihic JS, Harris RA. Alcohol actions at the GABAA receptor/choloride complex. In: Deitrich R, Erwin VG, editors. Pharmacological effects of ethanol on the nervous system. Boca Raton: CRC Press; 1995. pp. 51–72. [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL. Sites of alcohol and volatile anaesthetic action on GABAA and glycine receptors. Nature. 1997;389(6649):385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- Miller RJ. Presynaptic receptors. Ann Rev Pharmacol Toxicol. 1998;38:201–207. doi: 10.1146/annurev.pharmtox.38.1.201. [DOI] [PubMed] [Google Scholar]
- Minami K, Minami M, Harris RA. Inhibition of 5-hydroxytryptamine type 2A receptor-induced currents by n-alcohols and anesthetics. J Pharmacol Exp Ther. 1997a;281(3):1136–1143. [PubMed] [Google Scholar]
- Minami K, Vanderah TW, Minami M, Harris RA. Inhibitory effects of anesthetics and ethanol on muscarinic receptors expressed in Xenopus oocytes. Eur J Pharmacol. 1997b;339(2–3):237–244. doi: 10.1016/s0014-2999(97)01354-x. [DOI] [PubMed] [Google Scholar]
- Minami K, Gereau RW 4th, Minami M, Heinemann SF, Harris RA. Effects of ethanol and anesthetics on type 1 and 5 metabotropic glutamate receptors expressed in Xenopus laevis oocytes. Mol Pharmacol. 1998;53(1):148–156. doi: 10.1124/mol.53.1.148. [DOI] [PubMed] [Google Scholar]
- Ming Z, Criswell HE, Yu G, Breese GR. Competing presynaptic and postsynaptic effects of ethanol on cerebellar purkinje neurons. Alcohol Clin Exp Res. 2006;30(8):1400–1407. doi: 10.1111/j.1530-0277.2006.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mio K, Kubo Y, Ogura T, Yamamoto T, Sato C. Visualization of the trimeric P2X2 receptor with a crown-capped extracellular domain. Biochem Biophys Res Commun. 2005;337:998–1005. doi: 10.1016/j.bbrc.2005.09.141. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Yagi T, Kitazawa H, Yasuda M, Kawai N, Tsuboi K, Niki H. Fyn-kinase as a determinant of ethanol sensitivity: relation to NMDA-receptor function. Science. 1997;278(5338):698–701. doi: 10.1126/science.278.5338.698. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Pasternak GW. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol Rev. 2001;53:381–415. [PubMed] [Google Scholar]
- Mogil JS, Grisel JE, Zhangs G, Belknap JK, Grandy DK. Functional antagonism of mu-, delta- and kappa-opioid antinociception by orphanin FQ. Neurosci Lett. 1996;214:131–134. doi: 10.1016/0304-3940(96)12917-7. [DOI] [PubMed] [Google Scholar]
- Mohler H, Fritschy JM, Luscher B, Rudolph U, Benson J, Benke D. The GABAA receptors. From subunits to diverse functions. Ion Channels. 1996;4:89–113. [PubMed] [Google Scholar]
- Mons N, Decorte L, Jaffard R, Cooper DM. Ca2+-sensitive adenylyl cyclases, key integrators of cellular signaling. Life Sci. 1998a;62:1647–1652. doi: 10.1016/s0024-3205(98)00122-2. [DOI] [PubMed] [Google Scholar]
- Mons N, Yoshimura M, Ikeda H, Hoffman PL, Tabakoff B. Immunological assessment of the distribution of type VII adenylyl cyclase in brain. Brain Res. 1998b;788:251–261. doi: 10.1016/s0006-8993(98)00005-5. [DOI] [PubMed] [Google Scholar]
- Morrisett RA, Swartzwelder HS. Attenuation of hippocampal long-term potentiation by ethanol: a patch-clamp analysis of glutamatergic and GABAergic mechanisms. J Neurosci. 1993;13(5):2264–2272. doi: 10.1523/JNEUROSCI.13-05-02264.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, Suzdak PD, Karanian JW, Paul SM. Chronic ethanol administration alters gamma-aminobutyric acid, pentobarbital and ethanol-mediated 36Cl−uptake in cerebral cortical synaptoneurosomes. J Pharmacol Exp Ther. 1988;246:158–164. [PubMed] [Google Scholar]
- Morrow AL, Herbert JS, Montpied P. Differential effects of chronic ethanol administration on GABAA receptor alpha1 and alpha6 subunit mRNA levels in rat cerebellum. Mol Cell Neurosci. 1992;3:251–258. doi: 10.1016/1044-7431(92)90045-4. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Devaud LL, Bucci D, Smith FD. GABAA and NMDA receptor subunit mRNA expression in ethanol dependent rats. Alcohol Alcohol Suppl. 1994;2:89–95. [PubMed] [Google Scholar]
- Möykkynen T, Korpi ER, Lovinger DM. Ethanol inhibits alpha-amino-3-hydyroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor function in central nervous system neurons by stabilizing desensitization. J Pharmacol Exp Ther. 2003;306(2):546–555. doi: 10.1124/jpet.103.050666. [DOI] [PubMed] [Google Scholar]
- Möykkynen TP, Coleman SK, Keinänen K, Lovinger DM, Korpi ER. Ethanol increases desensitization of recombinant GluR-D AMPA receptor and TARP combinations. Alcohol. 2009;43(4):277–284. doi: 10.1016/j.alcohol.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkeen D, Anwyl R, Rowan MJ. Enhancement of long-term potentiation by the calcium channel agonist Bayer K8644 in CA1 of the rat hippocampus in vitro. Neurosci Lett. 1987;80:351–355. doi: 10.1016/0304-3940(87)90481-2. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9(5):967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- Murphy NP. The nociceptin/orphanin FQ system as a target for treating alcoholism. CNS Neurol Disord Drug Targ. 2010;9:87–93. doi: 10.2174/187152710790966713. [DOI] [PubMed] [Google Scholar]
- Netzeband JG, Trotter C, Caguioa JN, Gruol DL. Chronic ethanol exposure enhances AMPA-elicited Ca2+ signals in the somatic and dendritic regions of cerebellar Purkinje neurons. Neurochem Int. 1999;35:163–174. doi: 10.1016/s0197-0186(99)00058-3. [DOI] [PubMed] [Google Scholar]
- Netzeband JG, Schneeloch JR, Trotter C, Caguioa-Aquino JN, Gruol DL. Chronic ethanol treatment and withdrawal alter ACPD-evoked calcium signals in developing Purkinje neurons. Alcohol Clin Exp Res. 2002;26(3):386–393. [PubMed] [Google Scholar]
- Newton PM, Messing RO. Intracellular signaling pathways that regulate behavioral responses to ethanol. Pharmacol Ther. 2006;109:227–237. doi: 10.1016/j.pharmthera.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Woo NH. Regulation of hippocampal synaptic plasticity by cyclic AMP-dependent protein kinases. Prog Neurobiol. 2003;71(6):401–437. doi: 10.1016/j.pneurobio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Nie Z, Yuan X, Madamba SG, Siggins GR. Ethanol decreases glutamatergic synaptic transmission in rat nucleus accumbens in vitro: naloxone reversal. J Pharmacol Exp Ther. 1993;266:1705–1712. [PubMed] [Google Scholar]
- Nie Z, Madamba SG, Siggins GR. Ethanol enhances gamma-aminobutyric acid responses in a subpopulation of nucleus accumbens neurons: role of metabotropic glutamate receptors. J Pharmacol Exp Ther. 2000;293:654–661. [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303:1512–1514. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- Nie Z, Zorrilla EP, Madamba SG, Rice KC, Roberto M, Siggins GR. Presynaptic CRF1 receptors mediate the ethanol enhancement of GABAergic transmission in the mouse central amygdala. Sci World J. 2009;9:68–85. doi: 10.1100/tsw.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieber K, Poelchen W, Sieler D, Illes P. Inhibition by ethanol of excitatory amino acid receptors in rat locus coeruleus neurons in vitro. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:299–308. doi: 10.1007/pl00005171. [DOI] [PubMed] [Google Scholar]
- Nishio M, Narahashi T. Ethanol enhancement of GABA-activated chloride current in rat dorsal root ganglion neurons. Brain Res. 1990;518(1–2):283–286. doi: 10.1016/0006-8993(90)90982-h. [DOI] [PubMed] [Google Scholar]
- Obara I, Bell RL, Goulding SP, Reyes CM, Larson LA, Ary AW, Truitt WA, Szumlinski KK. Differential effects of chronic ethanol consumption and withdrawal on homer/glutamate receptor expression in subregions of the accumbens and amygdala of P rats. Alcohol Clin Exp Res. 2009;33(11):1924–1934. doi: 10.1111/j.1530-0277.2009.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie KM, Lee S, Rivier C. Divergence in the expression of molecular markers of neuronal activation in the parvocellular paraventricular nucleus of the hypothalamus evoked by alcohol administration via different routes. J Neurosci. 1998;18:4344–4352. doi: 10.1523/JNEUROSCI.18-11-04344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Hanchar HJ, Meera P, Wallner M. GABAA receptor subtypes: the “one glass of wine” receptors. Alcohol. 2007;41(3):201–209. doi: 10.1016/j.alcohol.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cabal L, Pollandt S, Liu J, Shinnick-Gallagher P, Gallagher JP. Regulation of synaptic transmission by CRF receptors. Rev Neurosci. 2006;17:279–307. doi: 10.1515/revneuro.2006.17.3.279. [DOI] [PubMed] [Google Scholar]
- Overstreet LS, Pasternak JF, Colley PA, Slater NT, Trommer BL. Metabotropic glutamate receptor mediated long-term depression in developing hippocampus. Neuropharmacology. 1997;36(6):831–844. doi: 10.1016/s0028-3908(97)00031-2. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Xu T. Deficits in amygdaloid cAMP-responsive element-binding protein signaling play a role in genetic predisposition to anxiety and alcoholism. J Clin Invest. 2005;115:2762–2773. doi: 10.1172/JCI24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazis NJ, West JR, Dai D. The nitric oxide-cyclic GMP pathway plays an essential role in both promoting cell survival of cerebellar granule cells in culture and protecting the cells against ethanol neurotoxicity. J Neurochem. 1998;70(5):1826–1838. doi: 10.1046/j.1471-4159.1998.70051826.x. [DOI] [PubMed] [Google Scholar]
- Papadeas S, Grobin AC, Morrow AL. Chronic ethanol consumption differentially alters GABA(A) receptor alpha1 and alpha4 subunit peptide expression and GABA(A) receptor-mediated 36 Cl(−) uptake in mesocorticolimbic regions of rat brain. Alcohol Clin Exp Res. 2001;25:1270–1275. [PubMed] [Google Scholar]
- Peris J, Eppler B, Hu M, Walker DW, Hunter BE, Mason K, Anderson KJ. Effects of chronic ethanol exposure on GABA receptors and GABAB receptor modulation of 3H-GABA release in the hippocampus. Alcohol Clin Exp Res. 1997;21:1047–1052. [PubMed] [Google Scholar]
- Perkins DI, Trudell JR, Crawford DK, Alkana RL, Davies DL. Molecular targets and mechanisms for ethanol action in glycine receptors. Pharmacol Ther. 2010;127(1):53–65. doi: 10.1016/j.pharmthera.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pioszak AA, Parker NR, Suino-Powell K, Xu HE. Molecular recognition of corticotropin-releasing factor by its G-protein-coupled receptor CRFR1. J Biol Chem. 2008;283:32900–32912. doi: 10.1074/jbc.M805749200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primeaux SD, Wilson SP, Bray GA, York DA, Wilson MA. Overexpression of neuropeptide Y in the central nucleus of the amygdala decreases ethanol self-administration in “anxious” rats. Alcohol Clin Exp Res. 2006;30:791–801. doi: 10.1111/j.1530-0277.2006.00092.x. [DOI] [PubMed] [Google Scholar]
- Qi ZH, Song M, Wallace MJ, Wang D, Newton PM, McMahon T, Chou WH, Zhang C, Shokat KM, Messing RO. Protein kinase C epsilon regulates gamma-aminobutyrate type A receptor sensitivity to ethanol and benzodiazepines through phosphorylation of gamma2 subunits. J Biol Chem. 2007;282(45):33052–33063. doi: 10.1074/jbc.M707233200. [DOI] [PubMed] [Google Scholar]
- Rabin RA, Molinoff PB. Activation of adenylate cyclase by ethanol in mouse striatal tissue. J Pharmacol Exp Ther. 1981;216(1):129–134. [PubMed] [Google Scholar]
- Rainnie DG, Fernhout BJ, Shinnick-Gallagher P. Differential actions of corticotropin releasing factor on basolateral and central amygdaloid neurones, in vitro. J Pharmacol Exp Ther. 1992;263:846–858. [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr, Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G-protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Prasad A. Ethanol enhances GABAA receptor-activated chloride currents in chick cerebral cortical neurons. Brain Res. 1991;564(1):138–142. doi: 10.1016/0006-8993(91)91363-6. [DOI] [PubMed] [Google Scholar]
- Riegel AC, Williams JT. CRF facilitates calcium release from intracellular stores in midbrain dopamine neurons. Neuron. 2008;57:559–570. doi: 10.1016/j.neuron.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimondini R, Thorsell A, Heilig M. Suppression of ethanol self-administration by the neuropeptide Y (NPY) Y2 receptor antagonist BIIE0246: evidence for sensitization in rats with a history of dependence. Neurosci Lett. 2005;375:129–133. doi: 10.1016/j.neulet.2004.10.084. [DOI] [PubMed] [Google Scholar]
- Roberto M, Siggins GR. Nociceptin/orphanin FQ presynaptically decreases GABAergic transmission and blocks the ethanol-induced increase of GABA release in central amygdala. Proc Natl Acad Sci USA. 2006;103:9715–9720. doi: 10.1073/pnas.0601899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Nelson TE, Ur CL, Gruol DL. Long-term potentiation in the rat hippocampus is reversibly depressed by chronic intermittent ethanol exposure. J Neurophysiol. 2002;87:2385–2397. doi: 10.1152/jn.2002.87.5.2385. [DOI] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004a;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. J Neurosci. 2004b;24:1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Bajo M, Crawford E, Madamba SG, Siggins GR. Chronic ethanol exposure and protracted abstinence alter NMDA receptors in central amygdala. Neuropsychopharmacology. 2006;31:988–996. doi: 10.1038/sj.npp.1300840. [DOI] [PubMed] [Google Scholar]
- Roberto M, Gilpin NW, O’Dell LE, Cruz MT, Morse AC, Siggins GR, Koob GF. Cellular and behavioral interactions of gabapentin with alcohol dependence. J Neurosci. 2008;28:5762–5771. doi: 10.1523/JNEUROSCI.0575-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH. Corticotropin releasing factor-induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010;67(9):831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Volz TJ, Schenk JO, Wightman RM. Acute ethanol decreases dopamine transporter velocity in rat striatum: invivo and in vitro electrochemical measurements. Alcohol Clin Exp Res. 2005;29:746–755. doi: 10.1097/01.alc.0000164362.21484.14. [DOI] [PubMed] [Google Scholar]
- Rodaros D, Caruana DA, Amir S, Stewart J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150:8–13. doi: 10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Rogers J, Wiener SG, Bloom FE. Long-term ethanol administration methods for rats: advantages of inhalation over intubation or liquid diets. Behav Neural Biol. 1979;27:466–486. doi: 10.1016/s0163-1047(79)92061-2. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S. Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur J Pharmacol. 1995;283:177–183. doi: 10.1016/0014-2999(95)00344-k. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Weitemier AZ. The urocortin 1 neurocircuit: ethanol-sensitivity and potential involvement in alcohol consumption. Brain Res Rev. 2006;52:368–380. doi: 10.1016/j.brainresrev.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Gehlert DR. Neuropeptide Y receptor subtypes in the basolateral nucleus of the amygdala modulate anxiogenic responses in rats. Neuropharmacology. 2002;43:1165–1172. doi: 10.1016/s0028-3908(02)00234-4. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res. 1986;382:213–238. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- Sanna E, Dildy-Mayfield JE, Harris RA. Ethanol inhibits the function of 5-hydroxytryptamine type 1c and muscarinic M1 G protein-linked receptors in Xenopus oocytes expressing brain mRNA: role of protein kinase C. Mol Pharmacol. 1994;45(5):1004–1012. [PubMed] [Google Scholar]
- Sapp DW, Yeh HH. Ethanol-GABAA receptor interactions: a comparison between cell lines and cerebellar Purkinje cells. J Pharmacol Exp Ther. 1998;284(2):768–776. [PubMed] [Google Scholar]
- Sebe JY, Eggers ED, Berger AJ. Differential effects of ethanol on GABAA and glycine receptor-mediated synaptic currents in brain stem motoneurons. J Neurophysiol. 2003;90(2):870–875. doi: 10.1152/jn.00119.2003. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Gruol D, Aldenhoff J, Pittman Q. Electrophysiologic actions of corticotropin-releasing factor in the central nervous system. Fed Proc. 1985;44:237–242. [PubMed] [Google Scholar]
- Siggins GR, Roberto M, Nie Z. The tipsy terminal: presynaptic effects of ethanol. Pharmacol Ther. 2005;107:80–98. doi: 10.1016/j.pharmthera.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Sillaber I, Rammes G, Zimmermann S, Mahal B, Zieglgansberger W, Wurst W, Holsboer F, Spanagel R. Enhanced and delayed stress-induced alcohol drinking in mice lacking functional CRH1 receptors. Science. 2002;296:931–933. doi: 10.1126/science.1069836. [DOI] [PubMed] [Google Scholar]
- Sinclair JG, Lo GF. Ethanol blocks tetanic and calcium-induced long-term potentiation in the hippocampal slice. Gen Pharmacol. 1986;17:231–233. doi: 10.1016/0306-3623(86)90144-8. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Somes C, Ehlers CL. Effects of chronic ethanol exposure on neurophysiologic responses to corticotropin-releasing factor and neuropeptide Y. Alcohol Alcohol. 1999;34:289–299. doi: 10.1093/alcalc/34.3.289. [DOI] [PubMed] [Google Scholar]
- Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8:383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smothers CT, Mrotek JJ, Lovinger DM. Chronic ethanol exposure leads to a selective enhancement of N-methyl-d-aspartate receptor function in cultured hippocampal neurons. J Pharmacol Exp Ther. 1997;283(3):1214–1222. [PubMed] [Google Scholar]
- Snell LD, Nunley KR, Lickteig RL, Browning MD, Tabakoff B, Hoffman PL. Regional and subunit specific changes in NMDA receptor mRNA and immunoreactivity in mouse brain following chronic ethanol ingestion. Brain Res Mol Brain Res. 1996;40:71–78. doi: 10.1016/0169-328x(96)00038-1. [DOI] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Stephens DN, Ripley TL, Borlikova G, Schubert M, Albrecht D, Hogarth L, Duka T. Repeated ethanol exposure and withdrawal impairs human fear conditioning and depresses long-term potentiation in rat amygdala and hippocampus. Biol Psychiatry. 2005;58:392–400. doi: 10.1016/j.biopsych.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Su LD, Sun CL, Shen Y. Ethanol acutely modulates mGluR1-dependent long-term depression in cerebellum. Alcohol Clin Exp Res. 2010;34(7):1140–1145. doi: 10.1111/j.1530-0277.2010.01190.x. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD, Klugmann M, Kippin TE. Accumbens Homer2 over-expression facilitates alcohol-induced neuroplasticity in C57BL/6 J mice. Neuropsychopharmacology. 2008;33:1365–1378. doi: 10.1038/sj.npp.1301473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliaferro P, Morales M. Synapses between corticotropin-releasing factor-containing axon terminals and dopaminergic neurons in the ventral tegmental area are predominantly glutamatergic. J Comp Neurol. 2008;506:616–626. doi: 10.1002/cne.21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallent MK, Madamba SG, Siggins GR. Nociceptin reduces epileptiform events in CA3 hippocampus via presynaptic and postsynaptic mechanisms. J Neurosci. 2001;21:6940–6948. doi: 10.1523/JNEUROSCI.21-17-06940.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terunuma M, Xu J, Vithlani M, Sieghart W, Kittler J, Pangalos M, Haydon PG, Coulter DA, Moss SJ. Deficits in phosphorylation of GABAA receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during status epilepticus. J Neurosci. 2008;28:376–384. doi: 10.1523/JNEUROSCI.4346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theile JW, Morikawa H, Gonzales RA, Morrisett RA. Ethanol enhances GABAergic transmission onto dopamine neurons in the ventral tegmental area of the rat. Alcohol Clin Exp Res 2008. 2008;32(6):1040–1048. doi: 10.1111/j.1530-0277.2008.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theile JW, Morikawa H, Gonzales RA, Morrisett RA. Role of 5-hydroxytryptamine2C receptors in Ca2+-dependent ethanol potentiation of GABA release onto ventral tegmental area dopamine neurons. J Pharmacol Exp Ther. 2009;329(2):625–633. doi: 10.1124/jpet.108.147793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Marsh DJ, Ste Marie L, Bernstein IL, Palmiter RD. Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature. 1998;396:366–369. doi: 10.1038/24614. [DOI] [PubMed] [Google Scholar]
- Thielen RJ, Morzorati SL, McBride WJ. Effects of ethanol on the dorsal raphe nucleus and its projections to the caudate putamen. Alcohol. 2001;23:131–139. doi: 10.1016/s0741-8329(01)00126-4. [DOI] [PubMed] [Google Scholar]
- Thorsell A. Central neuropeptide Y in anxiety- and stress-related behavior and in ethanol intake. Ann N Y Acad Sci. 2008;1148:136–140. doi: 10.1196/annals.1410.083. [DOI] [PubMed] [Google Scholar]
- Ticku MK, Burch T. Alterations in gamma-aminobutyric acid receptor sensitivity following acute and chronic ethanol treatments. J Neurochem. 1980;34:417–423. doi: 10.1111/j.1471-4159.1980.tb06612.x. [DOI] [PubMed] [Google Scholar]
- Tonner PH, Miller KW. Molecular sites of general anaesthetic action on acetylcholine receptors. Eur J Anaesthesiol. 1995;12(1):21–30. [PubMed] [Google Scholar]
- Tremwel MF, Hunter BE, Peris J. Chronic ethanol exposure enhances [3H]GABA release and does not affect GABAA receptor mediated 36Cl uptake. Synapse. 1994;17:149–154. doi: 10.1002/syn.890170302. [DOI] [PubMed] [Google Scholar]
- Trevisan L, Fitzgerald LW, Brose N, Gasic GP, Heinemann SF, Duman RS, Nestler EJ. Chronic ingestion of ethanol up-regulates NMDAR1 receptor subunit immunoreactivity in rat hippocampus. J Neurochem. 1994;62(4):1635–1638. doi: 10.1046/j.1471-4159.1994.62041635.x. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Uryu K, Okumura T, Shibasaki T, Sakanaka M. Fine structure and possible origins of nerve fibers with corticotropin-releasing factor-like immunoreactivity in the rat central amygdaloid nucleus. Brain Res. 1992;577:175–179. doi: 10.1016/0006-8993(92)90554-m. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, Roberts AJ, Koob GF. Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol abstinence. Alcohol. 2003;29:55–60. doi: 10.1016/s0741-8329(03)00020-x. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Machu TK, McKernan RM, Whiting P, VanRenterghem BB, McManaman JL, Brozowski SJ, Smith GB, Olsen RW, Harris RA. Tyrosine kinase phosphorylation of GABAA receptors. Brain Res Mol Brain Res. 1995;31:165–172. doi: 10.1016/0169-328x(95)00048-w. [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Bhave S, Hoffman P, Harris RA. Acute effects of ethanol on pharmacologically isolated kainate receptors in cerebellar granule neurons: comparison with NMDA and AMPA receptors. J Neurochem. 1998a;71:1777–1780. doi: 10.1046/j.1471-4159.1998.71041777.x. [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Cardoso RA, Wick MJ, Weiner JL, Dunwiddie TV, Harris RA. Effects of ethanol on recombinant glycine receptors expressed in mammalian cell lines. Alcohol Clin Exp Res. 1998b;22(5):1132–1136. [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154(2):299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majerksy LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA. Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcohol Clin Exp Res. 2001;25:1087–1097. [PubMed] [Google Scholar]
- Wan FJ, Berton F, Madamba SG, Francesconi W, Siggins GR. Low ethanol concentrations enhance GABAergic inhibitory postsynaptic potentials in hippocampal pyramidal neurons only after block of GABAB receptors. Proc Natl Acad Sci U S A. 1996;93:5049–5054. doi: 10.1073/pnas.93.10.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol. 2008;586:2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat MJ, Sparta DR, Hopf FW, Bowers MS, Melis M, Bonci A. Strain specific synaptic modifications on ventral tegmental area dopamine neurons after ethanol exposure. Biol Psychiatry. 2009;65:646–653. doi: 10.1016/j.biopsych.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Morales M. Corticotropin-releasing factor binding protein within the ventral tegmental area is expressed in a subset of dopaminergic neurons. J Comp Neurol. 2008;509:302–318. doi: 10.1002/cne.21751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Carnicella S, Phamluong K, Jeanblanc J, Ronesi JA, Chaudhri N, Janak PH, Lovinger DM, Ron D. Ethanol induces long-term facilitation of NR2B-NMDA receptor activity in the dorsal striatum: implications for alcohol drinking behavior. J Neurosci. 2007;27(13):3593–3602. doi: 10.1523/JNEUROSCI.4749-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner MJ, Armstrong DL, Polan-Curtain JL, Denny JB. Ethanol and diazepam inhibition of hippocampal LTP is mediated by angiotensin II and AT1 receptors. Peptides. 1993;14(3):441–444. doi: 10.1016/0196-9781(93)90129-5. [DOI] [PubMed] [Google Scholar]
- Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABAA receptors in hippocampal neurons. J Neurosci. 2004;24(38):8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: The view from the slice. Pharmacology & Therapeutics. 2006;111(3):533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Zhang L, Carlen PL. Potentiation of GABAA-mediated synaptic current by ethanol in hippocampal CA1 neurons: possible role of protein kinase C. J Pharmacol Exp Ther. 1994;268(3):1388–1395. [PubMed] [Google Scholar]
- Weiner JL, Gu C, Dunwiddie TV. Differential ethanol sensitivity of subpopulations of GABAA synapses onto rat hippocampal CA1 pyramidal neurons. J Neurophysiol. 1997;77:1306–13012. doi: 10.1152/jn.1997.77.3.1306. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Dunwiddie TV, Valenzuela CF. Ethanol inhibition of synaptically evoked kainate responses in rat hippocampal CA3 pyramidal neurons. Mol Pharmacol. 1999;56:85–90. doi: 10.1124/mol.56.1.85. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Ariwodola OJ, Bates WH, Davenport AT, Daunais JB, Grant KA, Friedman DP. The effect of long-term voluntary ethanol consumption on GABAergic and glutamatergic synaptic transmission in the monkey CNS. Alcohol Clin Exp Res. 2004;28(5):134A. [Google Scholar]
- Weiner JL, Ariwodola OJ, Bates WH, Bryant V, Silberman Y, Daunais JB, Grant KA, Friedman DP. Presynaptic mechanisms underlying ethanol actions at GABAergic synapses in rat and monkey hippocampus. Alcohol Clin Exp Res. 2005;29(5):187A. [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Weitlauf C, Egli RE, Grueter BA, Winder DG. High-frequency stimulation induces ethanol-sensitive long-term potentiation at glutamatergic synapses in the dorsolateral bed nucleus of the stria terminalis. J Neurosci. 2004;24(25):5741–5747. doi: 10.1523/JNEUROSCI.1181-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh BT, Goldstein BE, Mihic SJ. Single-channel analysis of ethanol enhancement of glycine receptor function. J Pharmacol Exp Ther. 2009;330(1):198–205. doi: 10.1124/jpet.109.154344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G, Lovinger DM, Weight FF. Ethanol inhibits NMDA-activated current but does not alter GABA-activated current in an isolated adult mammalian neuron. Brain Res. 1990;507(2):332–336. doi: 10.1016/0006-8993(90)90292-j. [DOI] [PubMed] [Google Scholar]
- Whittemore ER, Yang W, Drewe JA, Woodward RM. Pharmacology of the human gamma-aminobutyric acidA receptor alpha 4 subunit expressed in Xenopus laevis oocytes. Mol Pharmacol. 1996;50:1364–1375. [PubMed] [Google Scholar]
- Wickman K, Clapham DE. Ion channel regulation by G proteins. Physiol Rev. 1995;75(4):865–885. doi: 10.1152/physrev.1995.75.4.865. [DOI] [PubMed] [Google Scholar]
- Wirkner K, Eberts C, Poelchen W, Allgaier C, Illes P. Mechanism of inhibition by ethanol of NMDA and AMPA receptor channel functions in cultured rat cortical neurons. Naunyn Schmiedebergs Arch Pharmacol. 2000;362(6):568–776. doi: 10.1007/s002100000262. [DOI] [PubMed] [Google Scholar]
- Winkler A, Mahal B, Kiianmaa K, Zieglgänsberger W, Spanagel R. Effects of chronic alcohol consumption on the expression of different NR1 splice variants in the brain of AA and ANA lines of rats. Brain Res Mol Brain Res. 1999;72(2):166–175. doi: 10.1016/s0169-328x(99)00218-1. [DOI] [PubMed] [Google Scholar]
- Wolak ML, DeJoseph MR, Cator AD, Mokashi AS, Brownfield MS, Urban JH. Comparative distribution of neuropeptide Y Y1 and Y5 receptors in the rat brain by using immunohistochemistry. J Comp Neurol. 2003;464:285–311. doi: 10.1002/cne.10823. [DOI] [PubMed] [Google Scholar]
- Wu LG, Saggau P. Presynaptic calcium is increased during normal synaptic transmission and paired-pulse facilitation, but not in long-term potentiation in area CA1 of hippocampus. J Neurosci. 1994;14:645–654. doi: 10.1523/JNEUROSCI.14-02-00645.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Shao XM, Olive MF, Griffin WC, III, Li KY, Krnjević K, Zhou C, Ye JH. Ethanol facilitates glutamatergic transmission to dopamine neurons in the ventral tegmental area. Neuropsychopharmacology. 2009;34(2):307–318. doi: 10.1038/npp.2008.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaka R, Tang KC, Camarini R, Janak PH, Ron D. Fyn kinase and NR2B-containing NMDA receptors regulate acute ethanol sensitivity but not ethanol intake or conditioned reward. Alcohol Clin Exp Res. 2003;27:1736–1742. doi: 10.1097/01.ALC.0000095924.87729.D8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Marszalec W, Yeh JZ, Narahashi T. Effects of ethanol on tonic GABA currents in cerebellar granule cells and mammalian cells recombinantly expressing GABAA receptors. J Pharmacol Exp Ther. 2006;319(1):431–438. doi: 10.1124/jpet.106.106260. [DOI] [PubMed] [Google Scholar]
- Yan QS. Involvement of non-exocytotic mechanisms in ethanol-induced in vivo dopamine release: comparisons with cocaine. Eur J Pharmacol. 2003;477(1):37–44. doi: 10.1016/j.ejphar.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Yavich L, Tiihonen J. Ethanol modulates evoked dopaminerelease in mouse nucleus accumbens: dependence on social stress and dose. Eur J Pharmacol. 2000;401:365–373. doi: 10.1016/s0014-2999(00)00456-8. [DOI] [PubMed] [Google Scholar]
- Ye JH, Tao L, Ren J, Schaefer R, Krnjevic K, Liu PL, Schiller DA, McArdle JJ. Ethanol potentiation of glycine-induced responses in dissociated neurons of rat ventral tegmental area. J Pharmacol Exp Ther. 2001;296(1):77–83. [PubMed] [Google Scholar]
- Yevenes GE, Moraga-Cid G, Peoples RW, Schmalzing G, Aguayo LG. A selective G betagamma-linked intracellular mechanism for modulation of a ligand-gated ion channel by ethanol. Proc Natl Acad Sci U S A. 2008;105(51):20523–20528. doi: 10.1073/pnas.0806257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim HJ, Schallert T, Randall PK, Gonzales RA. Comparison of local and systemic ethanol effects on extracellular dopamine concentration in rat nucleus accumbens by microdialysis. Alcohol Clin Exp Res. 1998;22(2):367–374. [PubMed] [Google Scholar]
- Yin HH, Park BS, Adermark L, Lovinger DM. Ethanol reverses the direction of long-term synaptic plasticity in the dorsomedial striatum. Eur J Neurosci. 2007;25(11):3226–3232. doi: 10.1111/j.1460-9568.2007.05606.x. [DOI] [PubMed] [Google Scholar]
- Yu B, Shinnick-Gallagher P. Corticotropin-releasing factor increases dihydropyridine- and neurotoxin-resistant calcium currents in neurons of the central amygdala. J Pharmacol Exp Ther. 1998;284:170–179. [PubMed] [Google Scholar]
- Yu TP, Xie CW. Orphanin FQ/nociceptin inhibits synaptic transmission and long-term potentiation in rat dentate gyrus through postsynaptic mechanisms. J Neurophysiol. 1998;80:1277–1284. doi: 10.1152/jn.1998.80.3.1277. [DOI] [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM. Ethanol potentiates GABAergic synaptic transmission in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. J Neurophysiol. 2006;96(1):433–441. doi: 10.1152/jn.01380.2005. [DOI] [PubMed] [Google Scholar]
- Zhu W, Bie B, Pan ZZ. Involvement of non-NMDA glutamate receptors in central amygdala in synaptic actions of ethanol and ethanol-induced reward behavior. J Neurosci. 2007;27:289–298. doi: 10.1523/JNEUROSCI.3912-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Verdoorn TA, Lovinger DM. Alcohols potentiate the function of 5-HT3 receptor-channels on NCB-20 neuroblastoma cells by favouring and stabilizing the open channel state. J Physiol. 1998;507(2):335–352. doi: 10.1111/j.1469-7793.1998.335bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskind-Conhaim L, Gao BX, Hinckley C. Ethanol dual modulatory actions on spontaneous postsynaptic currents in spinal motoneurons. J Neurophysiol. 2003;89:806–813. doi: 10.1152/jn.00614.2002. [DOI] [PubMed] [Google Scholar]