Abstract
APP/PS1 double transgenic mice expressing human mutant amyloid precursor protein (APP) and presenilin-1 (PS-1) demonstrate robust brain Aβ peptide containing plaque deposition, increased markers of oxidative stress, behavioral dysfunction, and proinflammatory gliosis. On the other hand, lack of growth hormone, prolactin, and thyroid-stimulating hormone due to a recessive mutation in the Prop 1 gene (Prop1df) in Ames dwarf mice results in a phenotype characterized by potentiated anti-oxidant mechanisms, improved learning and memory, and significantly increased longevity in homozygous mice. Based upon this, we hypothesized that a similar hormone deficiency might attenuate disease changes in the brains of APP/PS1 mice. To test this idea, APP/PS1 mice were crossed to the Ames dwarf mouse line. APP/PS1, wild type, df/+, df/df, df/+/APP/PS1, and df/df/APP/PS1 mice were compared at 6 months of age through behavioral testing and assessing amyloid burden, reactive gliosis, and brain cytokine levels. df/df mice demonstrated lower brain growth hormone (GH) and insulin-like growth factor 1 (IGF-1) concentrations. This correlated with decreased astrogliosis and microgliosis in the df/df/APP/PS1 mice and, surprisingly, reduced Aβ plaque deposition and Aβ 1-40 and Aβ 1-42 concentrations. The df/df/APP/PS1 mice also demonstrated significantly elevated brain levels of multiple cytokines in spite of the attenuated gliosis. These data indicate that the df/df/APP/PS1 line is a unique resource in which to study aging and resistance to disease and suggest that the affected pituitary hormones may have a role in regulating disease progression.
Keywords: Neuroinflammation, cytokine, growth hormone, aging, dwarf, Alzheimer, IGF-1, amyloid
1. Introduction
Alzheimer’s disease (AD) is the 6th leading cause of death in the U.S. and is characterized by progressive cognitive deterioration and accumulation of amyloid β (Aβ) peptide containing plaques (Alzheimer's, 2014) . Another characteristic change of the AD brain is the accumulated evidence of increased production of reactive oxygen species (ROS) (Markesbery, 1997,Martins, et al., 1986,Subbarao, et al., 1990,Yan, et al., 1995). For example, mutations in the amyloid precursor protein (APP) and presenilin-1 (PS1) encoding genes are found in early-onset AD patients leading to APP processing to Aβ and Aβ deposition which are both associated with increased markers of oxidative stress in the AD brains (Guo, et al., 1999,Hensley, et al., 1996,Markesbery, 1997,Mattson and Pedersen, 1998,Multhaup, et al., 1997,Zhang, et al., 1997) . These oxidative damage associated changes can be partially modeled in APP/PS1 transgenic mice expressing human mutations for the amyloid precursor protein (APP) and human presenilin-1 (PS-1). These mice also have increased Aβ content in the brain and exhibit changes associated with enhanced oxidative stress (Jung, et al., 2014,Kanninen, et al., 2009,Liu, et al., 2014,Lv, et al., 2014)
On the other hand, Ames dwarf mice have an upregulated antioxidant defense system (Brown-Borg, et al., 1999,Brown-Borg and Rakoczy, 2000), significant life span extension (Brown-Borg, et al., 1996), enhanced cellular stress resistance (Bokov, et al., 2009,Brown-Borg, 2006,Salmon, et al., 2005) and improved learning and memory compared to their wild-type siblings (Kinney-Forshee, et al., 2004,Kinney, et al., 2001,Sharma, et al., 2010). These mice have a point mutation in the gene encoding Prop-1, a protein required for appropriate expression of PIT-1 to drive pituitary differentiation. The mutation results in deficiencies in circulating growth hormone (GH), prolactin (PRL), and thyroid stimulating hormone (TSH) (Sornson, et al., 1996). It has been previously demonstrated that GH and/or insulin-like growth factor (IGF)-1 are directly involved in regulating the antioxidant response and their loss provides, in large part, the protective benefits observed in these mice in terms of health span and longevity (Brown-Borg, et al., 2009). More importantly, Ames mice have been shown to resist Aβ toxicity (Brown-Borg, et al., 2009,Schrag, et al., 2008).
Ames dwarf mice, therefore, are a valuable tool for studying aging processes and lifespan while the APP/PS1 line of mice is a valuable resource for examining development of AD-like pathology. These particular animals exhibit unique characteristics of cellular defense and lifespan and disease that are associated with reduced GH signaling and the expression of mutant human APP/PS1 proteins, respectively. Based upon this knowledge, we hypothesized that crossing these two lines could either generate a line with a prolonged disease time-course to monitor and alter progression due to the APP/PS1 mutations or an attenuated disease progression based upon the protective, stress resistance and lifespan enhancing changes intrinsic to the hormone deficiency benefits of the dwarf mice. To test this hypothesis we crossed the Ames dwarf to the APP/PS1 mice to generate Ames dwarf (df/df), Ames dwarf x APP/PS1 (df/df/APP/PS1), dwarf heterozygotes (df/+) x APP/PS1 (df/+/APP/PS1), dwarf heterozygotes, hets (df/+), APP/PS1, and wild type (+/+) littermate controls to assess changes in markers of oxidative damage, cognition, gliosis, and Aβ burden.
2. Material and Methods
2.1 Antibodies and Reagents
Anti-β-Amyloid Precursor Protein (APP) and phospho-IR/IGF1R antibodies were purchased from Life Technologies (Grand Island, NY, USA). Anti-mouse IgM, anti-rabbit (goat), anti-goat (bovine), anti-rat (goat), and anti-mouse (bovine) horseradish peroxidase-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Goat Anti-rabbit IR Dye® 800CW secondary antibody was purchased from LI-COR (Lincoln, NE, USA). The α-tubulin antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Elite Vectastain ABC reagents, Vector VIP, biotinylated anti-rabbit, and anti-mouse antibodies were purchased from Vector Laboratories Inc (Burlingame, CA, USA). Anti-GFAP, PSD95, p-insulin receptor β, IGF1R, P-IGF-IRβ, insulin receptor β (for synaptosome blots), GSK3β, p-GSK3β, CDK5, BACE, PS1, pAKT, and AKT antibodies were purchased from Cell Signaling Technology Inc (Danvers, MA, USA). Anti-Aβ clone, 4G8, was purchased from Covance (Emeryville, CA, USA). Anti-synaptophysin, tau, and HNE adducts antibodies was purchased from EMD Millipore (Billerica, MA, USA). Anti-Iba-1 antibody was purchased from Wako Chemicals USA, Inc. (Richmond, VA, USA). BAM-10 was purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA). Anti-ERAB and insulin receptor β (for lysate blots) antibodies were purchased from Abcam Inc (Cambridge, MA, USA). PHF-1 (p-tau) antibody was a gift from Dr. Peter Davies.
2.2 Transgenic mice
C57BL6/ APP/PS1 (APP/PS1; APPswe/PS1dE9; Mo/Hu APPswe PS1dE9; Tg(APPswe,PSEN1dE9)85Dbo) transgenic mice were originally purchased from Jackson Labs and maintained as a colony at the University of North Dakota. The transgenic mice express the human APP, amyloid beta (A4) precursor protein and the human PSEN1, presenilin 1. The Ames dwarf (df/df) mice used in this study were derived from a closed colony at the University of North Dakota with a heterogeneous background (over 25 years). Dwarf mice were generated by mating either homozygous (df/df) or heterozygous (df/+) dwarf males with carrier females (df/+). Ames dwarf mice were bred with APP/PS1 mice to produce F1 offspring. The heterozygous F1 generation df/+/APP/PS1 mice were then bred to each other to produce F2 offspring consisting of APP/PS1, wild type (+/+), dwarf (df/df), dwarf het (df/+), dwarf het APP/PS1 (df/+/APP/PS1) and dwarf APP/PS1 (df/df/APP/PS1) all with the same genetic background and maintained at the University of North Dakota's Center for Biomedical Research under controlled conditions of photoperiod (12 h light:12 h dark) and temperature (22 ± 1 °C) with ad libitum access to food and water for 6 months. These F2 offspring were used for the studies described. At 6 months of age, mice were behaviorally tested and then euthanized followed by cardiac perfusion and the brains were collected. All procedures involving animals were reviewed and approved by the UND Institutional Animal Care and Use Committee.
2.3 Cell cultures
Cultured microglia and astrocytes were derived from postnatal day 0–2 (P0) Ames dwarf (df/df) and littermate controls (df/+) pups or C57BL/6 pups and isolated from mixed cultures at 14 days in vitro as previously described (Floden and Combs, 2011). Briefly, postnatal day 0 mice were killed via decapitation; cortices were isolated, finely minced, trypsinized, neutralized, and plated. The microglia layer was shaken off and collected and the cells were then counted and used immediately for experiments. The astrocyte layer was trypsinized, neutralized, counted and plated for 3 days prior to treatments.
2.4 Enzyme-Linked Immunosorbent Assay (ELISA)
Microglia were incubated overnight in serum free DMEM/F12 in the presence of 5μM Aβ (rPeptide, Bogart, GA, USA), 2.5 ng/mL LPS (Sigma Aldrich, St. Louis, MO, USA), or media alone with or without 10 ng/mL growth hormone REPORCIN Porcine Somatotropin (Zamira Life Sciences Pty Ltd, Knoxfield, VIC), 10 ng/mL IGF-1 (R&D systems, Minneapolis, MN, USA), 10 ng/mL prolactin (R&D systems, Minneapolis, MN, USA), or 1 ng/mL thyroxine (EMD Millipore, Billerica, MA, USA). The media was collected for TNFα ELISAs (R&D systems, Minneapolis, MN, USA).
Astrocytes were incubated overnight in serum free DMEM/F12 in the presence of 2.5 ng/mL LPS (Sigma Aldrich, St. Louis, MO, USA) or media alone. The media was collected for TNFα ELISAs (R&D systems, Minneapolis, MN, USA).
At the 6 month old collection, the brain and a portion of liver were removed and half the brain was isolated into regions. The hippocampus, frontal/temporal/parietal cortex, and liver portions were flash frozen, pulverized and lysed using ice cold radioimmunoprecipitation assay (RIPA) buffer (20mM Tris, pH 7.4, 150mM NaCl, 1mM Na3VO4, 10mM NaF, 1mM EDTA, 1mM EGTA, 0.2mM phenylmethylsulfonyl fluoride, 1% Triton, 0.1% SDS, and 0.5% deoxycholate) with protease inhibitors (AEBSF 1mM, Aprotinin 0.8 M, Leupeptin 21 M, Bestatin 36 M, Pepstatin A 15 M, E-64 14 M). To remove insoluble material, lysates were sonicated and centrifuged (14,000 rpm, 4°C, 10 min) and the supernatants were collected for soluble Aβ and cytokine/growth factor ELISA analysis. The parietal cortex pellet was re-suspended in 5M guanidine HCL/50mM Tris HCL, pH 8.0, samples were again sonicated and centrifuged (14,000 rpm, 4°C, 10 min) and the supernatant was removed and used to determine fibrillar Aβ concentrations. The Bradford method (Bradford, 1976) was used to quantify protein concentrations. The supernatants from both lysing methods were used for both Aβ 1-40 and 1-42 ELISAs (EMD Millipore, Billerica, MA, USA). The first soluble fraction from the RIPA lysing was also used for IGF-1, TNFα, IL-6, IL-10, IL-4, MCP-1, IL-1β (R&D systems, Minneapolis, MN, USA) and GH (EMD Millipore, Billerica, MA, USA) ELISAs.
2.5 Western Blotting
Concentrations of the isolated protein from the cortices were quantified using the Bradford method (Bradford, 1976). Proteins were resolved by 7 and 10% sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes (PVDF) for western blotting using anti-APP, PS1, BACE, HNE adduct, ERAB, PSD95, Synaptophysin, pAKT, AKT, pIR/IGF1R, pIR, IR, IGF1R, p-tau, tau, pGSK3β, GSK3β, pCDK5, CDK5 and α-tubulin (loading control) antibodies. Antibody binding was detected with enhanced chemiluminescence (GE Healthcare, Piscataway, NJ). In some instances, blots were stripped in 0.2 NaOH, 10 min, 25°C, for reprobing. Western blots were quantified using Adobe Photoshop software (Adobe Systems, San Jose, CA). Optical densities of bands were normalized against their respective loading controls and averaged (+/−SD).
For synaptosomes, the BCA method was used to prepare samples of equal protein concentration for western blotting. Proteins were resolved by 8% sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes for western blotting. Western blots were imaged using LI-COR Odyssey infrared imaging system (LI-COR, Lincoln, Nebraska), application software version 3.0.30. The density of each immunoreactive band was measured using Image J.
2.6 Synaptosome preparation
Synaptosomes containing both pre- and post-synaptic components were isolated from frozen frontal cortex tissue using SynPER reagent (Thermo Scientific, Rockford, IL). The final pellet was resuspend in a physiological buffer, HBK (143 mM NaCl, 4.7 mM KCl, 1.3 mM MgSO4, 1.2 mM CaCl2, 20 mM HEPES, 0.1 mM NaH2PO4 and 10 mM D-glucose, pH 7.4), to carry out the insulin stimulation experiment, and the samples were split into two fractions for insulin stimulation controls. After insulin stimulation, the samples were then pelleted, washed once in HBK buffer (143 mM NaCl, 4.7 mM KCl, 1.3 mM MgSO4, 1.2 mM CaCl2, 20 mM HEPES, 0.1 mM NaH2PO4 and 10 mM D-glucose, pH 7.4), pelleted again, and the final pellet was resuspended in 1X RIPA (75mM NaCl, 25mM Na2PO4, 1mM EDTA, 0.5% NP-40, and 0.5% TritonX-100) plus 1% protease inhibitor cocktail and phosphatase inhibitor cocktail to solubilize the proteins for western blot detection.
2.7 Immunohistochemistry
At collection, half of the brain was fixed in 4% paraformaldehyde and cryoprotected through two successive 30% sucrose changes. The brains were embedded in gelatin, serially sectioned (40μm) via freezing microtome and immunostained using anti-β-amyloid (4G8 and BAM-10), GFAP, IGF-1, and Iba-1 antibodies or respective secondary only antibodies. Antibody binding was visualized using the Vector VIP chromogen (Vector Laboratories, Burlingame, CA). Images were taken using an upright Leica DM1000 microscope and Leica DF320 digital camera system. Figures were made using Adobe Photoshop software.
2.8 Phagocytosis
Peptide phagocytosis was quantitated by measuring the uptake of FITC-conjugated Aβ 1–42 (rPeptide, Athens, GA). The peptide was fibrillized according to manufacturer protocol and as previously described (Floden and Combs, 2006). Briefly, the peptide was dissolved in 2mM NaOH at 1mg/mL, sonicated for 30 seconds and diluted with 10× PBS and water to a final concentration of 50μM Aβ. The peptide was allowed to fibrillize 24hr at 37 degree C before use. Cultured microglia were incubated with Aβ 1–42 fibril aggregates or FITC-E. coli bioparticles (positive control, 0.125mg/ml) in 96-well plates for 6 hours. To quench the FITC signal from extracellular peptide or bioparticles, medium was removed and the cells were rinsed with 0.25mg/ml trypan blue in PBS. Application of trypan serves to quench any remaining FITC signal on the plate as well as any bound to the external leaflet of the plasmalemma. Intracellular fluorescence was read (480 nm excitation and 520 nm emission) via fluorescent plate reader (Bio-Tek, Winooski, VT).
2.9 T-Maze
To address whether animals had an intact working memory, mice were allowed to explore a T shaped maze at their own discretion without any added stress such as lights, sound, food deprivation, etc. Mice were placed on the T maze starting platform and the entry door opened to allow them to walk down the stem and explore and choose an arm. Once all four feet were within an arm this was considered a choice and the mouse was placed back on the starting platform for 30 seconds. The door was then opened and the mouse was allowed to explore again and choose an arm. On this second trial the rodent tends to choose the arm not visited before, reflecting memory of the first choice. This is called ‘spontaneous alternation’. Spontaneous alternation is very sensitive to dysfunction of the hippocampus, which is the region of the brain where consolidation of short-term memory to long-term memory as well as spatial navigation occurs. The animals were allowed to make a total of 5-9 choices (trials) before the test was ended. The number of alternations were counted (defined as 2 consecutive entries into 2 different arms), and % alternation for each mouse was calculated as follows: # alternations/(total choices-1).
2.10 Y-Maze
As an additional means of addressing whether animals had an intact working memory, Y-Maze behavioral testing was also performed. Mice were allowed to explore a Y shaped maze at their own discretion without any added stress such as lights, sound, food deprivation, etc. Mice were placed in one of the identical arms of the Y maze and allowed to explore and choose an arm. Once all four feet were within an arm this was considered a choice, the mouse was allowed to explore the maze for 5 minutes, and the arm entries were recorded. The number of alternations were counted (defined as 3 consecutive entries into 3 different arms), and % alternation for each mouse was calculated as follows: # alternations/(total entries-2).
2.11 Genotyping
At P0 or 2-3 weeks of age, mice were ear or tail clipped and the clipping sample was used for genotyping. DNA was isolated by placing the sample in 150μL of NaOH solution (25mM NaOH, 0.2mM EDTA), heating samples at 95°C for 40 min followed by cooling at 4°C before adding 150uL Tris solution (40mM Tris-HCL), samples were then centrifuged at 1800×g for 3 minutes to separate DNA from contaminants (DNA in top layer). To genotype for the APP/PS1 mutation, PCR was performed using 3uL of DNA, 5μL of Quick-Load Taq2X Master Mix (New England BioLabs Inc, Ipswich, MA, USA), 25mM MgCl2, dH20 and the following primers (Life Technologies, Grand Island, NY, USA): 5′ AAT AGA GAA CGG CAG GAG CA 3′ and 5′ GCC ATG AGG GCA CTA ATC AT 3′ at 1.33μM concentration; 5′ CTA GGC CAC AGA ATT GAA AGA TCT 3′ and 5′ GTA GGT GGA AAT TCT AGC ATC ATC C 3′ at 1μM concentration per reaction. The PCR was run with the following settings step 1: 94°C for 3 min, step 2: 94°C for 30 sec, step 3: 54°C for 1 min, step 4: 72°C for 1 min, steps 2-4 were repeated for 35 cycles, step 5: 72°C for 2 min, step 6: 10°C hold till run on 1.5% agarose gel. Results yielded the transgene at ~608bp and internal positive control at 324bp. To genotype for the Dwarf mutation, sequencing was performed with pyromark PCR kit (QIAGEN) using the following primers: Forward Prop 1 5′ CCT TCA ACC CAG CAC AGC 3′ at 1μM concentration, Reverse Prop 1 5′ CTC TCG AGC CCA GAT GTC A 3′ at 1μM concentration, and Sequencing Prop 1 5′ GTT CCT CCC AAA GGC 3′ at 0.3μM concentration per reaction. DNA was Pyrosequenced using a PyroMark Q24 System (QIAGEN).
2.12 Statistical Analysis
The data were analyzed by two-way ANOVA with Holm-Sidak post hoc test with the exception of the microglia phagocytosis assay being analyzed by an unpaired two-tailed t-test and the C57BL6 microglia being analyzed by a one-way ANOVA with Holm-Sidak post hoc test.
3.0 Results
3.1 Ames dwarf (df/df) mice had attenuated brain growth hormone (GH) and insulin-like growth factor 1 (IGF-1) concentrations
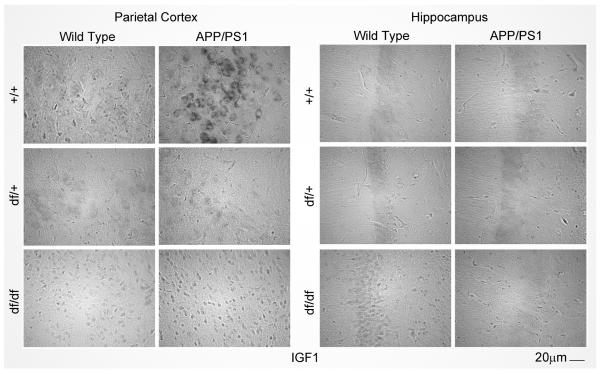
Ames dwarf mice have a well-documented deficiency of growth hormone (GH). Therefore, we first assessed brain levels of GH. In addition, we quantified levels of insulin-like growth factor (IGF)-1 since its peripheral expression and uptake into the brain is largely regulated by growth hormone stimulation (Armstrong, et al., 2000,Pan, et al., 2005,Reinhardt and Bondy, 1994). ELISA measurements were performed for GH and IGF-1 from the parietal cortex and hippocampus of wild type (+/+), APP/PS1, dwarf het (df/+), dwarf het APP/PS1 (df/+/APP/PS1), dwarf (df/df), and dwarf APP/PS1 (df/df/APP/PS1) mice. The df/df mice had attenuated IGF-1 and GH levels compared to their littermate controls, df/+ and wild type (+/+) mice (Fig. 1A, B, C, D). Similar to the dwarf mutation, the APP/PS1 mice also demonstrated reduced GH levels (Fig. 1A, B). On the other hand, APP/PS1 mice had increased brain IGF-1 levels compared to wild type (+/+) in only the parietal cortex and this was significantly attenuated in mice containing the dwarf mutation (df/+/APP/PS1 and df/df/APP/PS1) (Fig. 1C). The increased protein levels correlated with increased IGF-1 immunoreactivity in the APP/PS1 mice (Fig. 2). As expected, liver levels of IGF-1 were attenuated in the mice containing the dwarf mutation (df/df and df/df/APP/PS1) (Fig. 1E). However, IGF-1 levels in the livers of APP/PS1 mice were not different from wild type controls indicating that brains had a region selective increase in IGF-1 levels in these mice (Fig. 1E).
Fig. 1.
Ames dwarf (df/df) mice had attenuated brain growth hormone (GH) and insulin-like growth factor 1 (IGF-1) concentrations. Parietal cortices, hippocampi, and liver were collected from wild type (+/+), APP/PS1, dwarf het (df/+), dwarf het APP/PS1 (df/+/APP/PS1), dwarf (df/df), and dwarf APP/PS1 (df/df/APP/PS1) mice. The tissues were lysed and the brain supernatants were used for A, B, GH and C,D, IGF-1 ELISAs and the liver supernatants were used for E, only IGF-1 ELISAs. Hippocampi lysates were also used for eotaxin ELISA F. Data is from 8-13 mice in each group and displayed as mean +/− SD, *p<0.05.
Fig. 2.
APP/PS1 mice have increased IGF-1 in the parietal cortex. Brains from each mouse line were fixed in 4% paraformaldehyde and serially sectioned for staining with anti-IGF-1 antibody using vector VIP as the chromogen. Representative parietal cortex and hippocampus images from 8-13 mice in each group are shown.
3.2 df/df/APP/PS1 mice had attenuated hippocampal eotaxin concentrations
Eotaxin is an inflammatory cytokine that has been reported as increased in Alzheimer’s Disease patient serum (Choi, et al., 2008). Consistent with this notion, the APP/PS1 mice had increased eotaxin concentrations that were significantly decreased in the mice carrying the dwarf trait (Fig. 1F), suggesting a protective benefit due to the dwarf trait.
3.3 df/df/APP/PS1 mice had increased HNE protein adducts
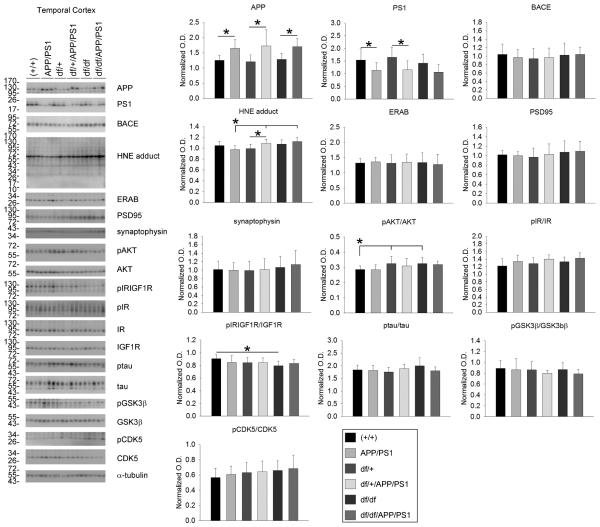
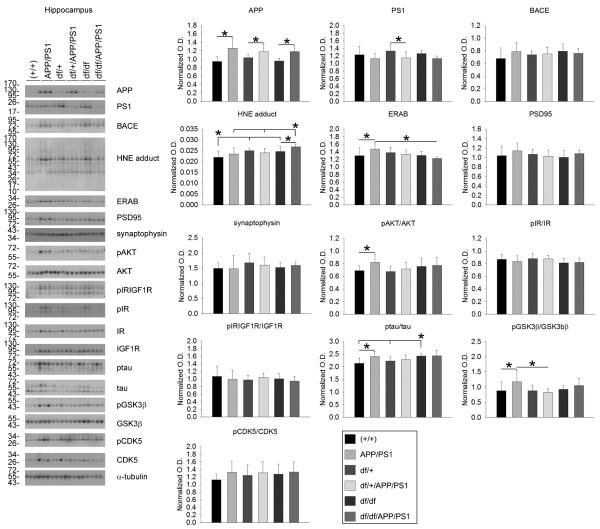
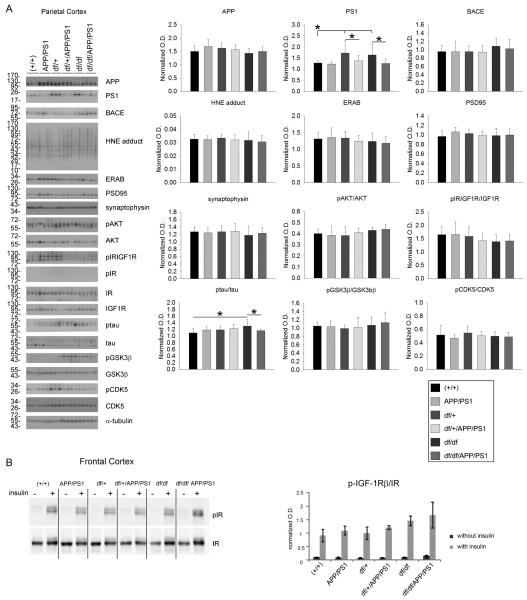
To validate expression of the APP/PS1 transgene and downstream effects, western blot analyses of cortical and hippocampal lysates were performed. As expected, exogenous mutant human APP was higher in the temporal cortex and hippocampus of APP/PS1, df/+/APP/PS1 and df/df/APP/PS1 lines with no difference between each group. This demonstrated that crossing the two lines of mice did not decrease expression of the APP/PS1 transgene (Fig. 3, 4). Since there were elevated levels of APP it is likely that there would also be elevated levels of Aβ in these mice. Therefore, as one assessment of neurotoxic effects attributed to Aβ and reactive oxygen intermediates, we examined, ERAB, the endoplasmic reticulum associated Aβ binding protein, since cells that overexpress ERAB are more sensitive to Aβ-mediated stress. Only in the hippocampus was there a significant increase in ERAB protein levels in the APP/PS1 compared to wild type (+/+) and df/df/APP/PS1 mice (Fig. 4). No differences across lines were noted for the synaptic markers, synaptophysin or PSD95 in any of the brain regions (Fig. 3, 4, 5). Surprisingly, the df/df/APP/PS1 and the df/+/APP/PS1 mice had slightly elevated levels of HNE-protein adducts, a ROS lipid oxidation marker, compared to the APP/PS1 line in the temporal cortex and the hippocampus but not in the parietal cortex (Fig. 3, 4, 5). Based upon the elevated IGF-1 protein levels and immunoreactivity observed, at least in the parietal cortex, we next assessed change in the IGF-1/IR signaling response. Somewhat unexpectedly, we did not observe any increase in APP/PS1 compared to wild type (+/+) mice in levels of IGF-1 receptor phosphorylation in temporal cortex, hippocampus, or parietal cortex using an antibody that recognizes both pIGF-1R and pIR (Fig. 3, 4, 5). Similarly, there were no differences in pIR detected in either the temporal cortex or the hippocampus comparing wild type (+/+) and APP/PS1 mice with no pIR even detected in the parietal cortex (Fig. 3, 4, 5). Based upon our inability to detect any differences in pIR levels across the mouse genotypes we elected to also compare changes in pIR from isolated frontal cortex synaptosomes from the mice. Similar to the tissue lysate western blots, we again found no changes in pIR levels in any mouse line suggesting that the tissue lysate western blots were sufficiently sensitive (Fig. 5). We also observed no differences in protein levels of pAkT or pGSK3β in the cortices while examining these two key kinases associated with IGF-1R signaling (Fig. 3, 4, 5). The hippocampus, on the other hand, did demonstrate a significant increase in pAkt and pGSK3β levels in the APP/PS1 mice that was attenuated by the dwarf mutation in df/+/APP/PS1 and df/df/APP/PS1 mice (Fig 4). Since GSK3β is also associated with tau phosphorylation we also assessed active, phosphorylated levels of another tau related kinase, CDK5. Although there were no differences in phosphorylated CDK5 in the hippocampus of APP/PS1 mice, we did detect a significant increase in p-tau protein levels in these mice compare to wild type (+/+) that correlated with the increased phospho-GSK3β levels but reduced by the df mutation (Fig. 4). This data demonstrated regional differences in protein levels of HNE adducts, ERAB, pAKT/AKT , p-tau/tau, and pGSK3β/GSK3β levels that resulted from the df/df mutation.
Fig. 3.
df/df/APP/PS1 mice had increased HNE-protein adducts in the temporal cortex. Temporal cortices were collected from wild type (+/+), APP/PS1, dwarf het (df/+), dwarf het APP/PS1 (df/+/APP/PS1), dwarf (df/df), and dwarf APP/PS1 (df/df/APP/PS1) mice. The tissue was lysed, resolved by 10% SDS-PAGE and western blotted using anti-APP, PS1, BACE, HNE protein adduct, ERAB, PSD95, synaptophysin, phospho-Akt, Akt (loading control), phospho-IR/IGF1R, phospho-IR, IR (loading control), IGF1R (loading control), ptau, tau (loading control), pGSK3β, GSK3β (loading control), pCDK5, CDK5 (loading control) or α-tubulin (loading control) antibodies. Antibody binding was visualized by chemiluminescence. Blots from 3 animals per group are shown. Optical densities of the western blotted proteins were normalized against their respective loading control and averaged (+/− SD) from 8-13 animals per each condition and graphed, *p<0.05.
Fig. 4.
df/df/APP/PS1 mice had increased HNE-protein adducts and decreased ERAB in the hippocampus. Hippocampi were collected from wild type (+/+), APP/PS1, dwarf het (df/+), dwarf het APP/PS1 (df/+/APP/PS1), dwarf (df/df), and dwarf APP/PS1 (df/df/APP/PS1) mice. A) The tissues were lysed, resolved by 10% SDS-PAGE and western blotted using anti-APP, PS1, BACE, HNE protein adduct, ERAB, PSD95, synaptophysin, phospho-Akt, Akt (loading control), phospho-IR/IGF1R, phospho-IR, IR (loading control), IGF1R (loading control), ptau, tau (loading control), pGSK3β, GSK3β (loading control), pCDK5, CDK5 (loading control) or α-tubulin (loading control) antibodies. Antibody binding was visualized by chemiluminescence. Blots from 3 animals per group are shown. Optical densities of the western blotted proteins were normalized against their respective loading control and averaged (+/− SD) from 8-13 animals per each condition and graphed, *p<0.05.
Fig. 5.
df/df/APP/PS1 mice were not significantly different from APP/PS1 mice in the parietal and frontal cortices. A) Parietal cortices were collected from wild type (+/+), APP/PS1, dwarf het (df/+), dwarf het APP/PS1 (df/+/APP/PS1), dwarf (df/df), and dwarf APP/PS1 (df/df/APP/PS1) mice. The tissues were lysed, resolved by 10% SDS-PAGE and western blotted using anti-APP, PS1, BACE, HNE protein adduct, ERAB, PSD95, synaptophysin, phospho-Akt, Akt (loading control), phospho-IR/IGF1R, phospho-IR, IR (loading control), IGF1R (loading control), ptau, tau (loading control), pGSK3β, GSK3β (loading control), pCDK5, CDK5 (loading control) or α-tubulin (loading control) antibodies. Antibody binding was visualized by chemiluminescence. Blots from 3 animals per group are shown. Optical densities of the western blotted proteins were normalized against their respective loading control and averaged (+/− SD) from 8-13 animals per each condition and graphed, *p<0.05. B) Synaptosomes were isolated from frontal cortices and insulin stimulated as described above. Insulin-receptor (IR) activation was performed with 0.333units/mL of insulin for 30 minutes at 37°C with 8mM ATP. Phosphorylation extent of IR was determined using western blots probing with anti-P-IGF-IRβ and IRβ (loading control) antibodies. A representative western blot from animal per group and synaptosomes from the same animal exposed or not exposed to insulin are shown. Optical densities of the phosphorylated form were normalized against the total amount of IR and averaged (+/− SE) from 5 animals per each condition and graphed.
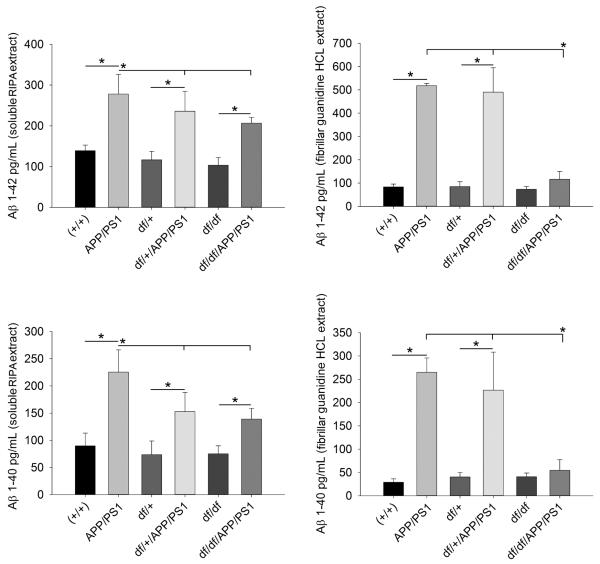
3.4 df/df/APP/PS1 mice had decreased levels of brain Aβ
Since APP was over-expressed in the df/+ APP/PS1 and df/df/APP/PS1 lines we next examined whether it was processed into elevated levels of Aβ. ELISA analysis of parietal cortices demonstrated that although the df/+/APP/PS1 and df/df/APP/PS1 mice still produced both Aβ1-40 and 1-42 in their brains, the values were significantly lower than the APP/PS1 line. In particular, the guanidine extracted, detergent insoluble fraction was the lowest with the df/df/APP/PS1 line dropping to control levels (Fig. 6).
Fig. 6.
df/df/APP/PS1 mice had decreased levels of brain Aβ. Parietal cortices were collected from wild type (+/+), APP/PS1, dwarf het (df/+), dwarf het APP/PS1 (df/+/APP/PS1), dwarf (df/df), and dwarf APP/PS1 (df/df/APP/PS1) mice. The tissues were lysed with RIPA buffer first to yield a detergent soluble supernatant extract and the pellet from this was lysed with guanidine/Tris HCL to yield the detergent insoluble, fibrillar supernatant extract. Both supernatants were used for Aβ 1-40 and Aβ 1-42 ELISAs. Data is from 8-13 mice in each group and displayed as mean +/− SD, *p<0.05.
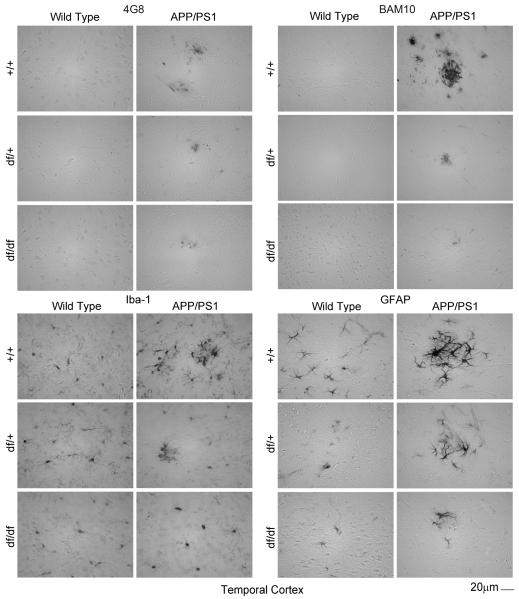
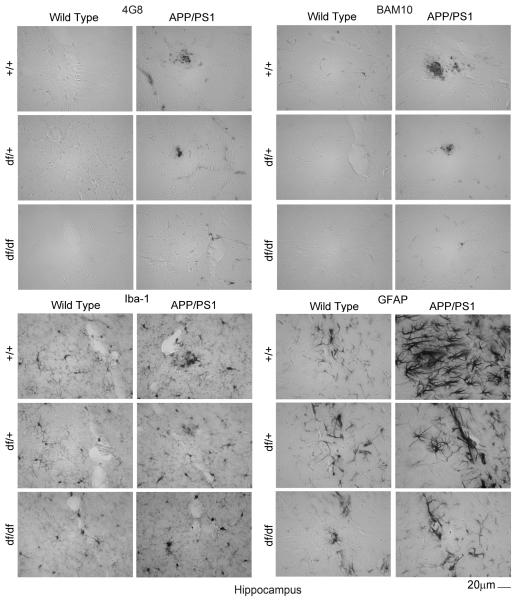
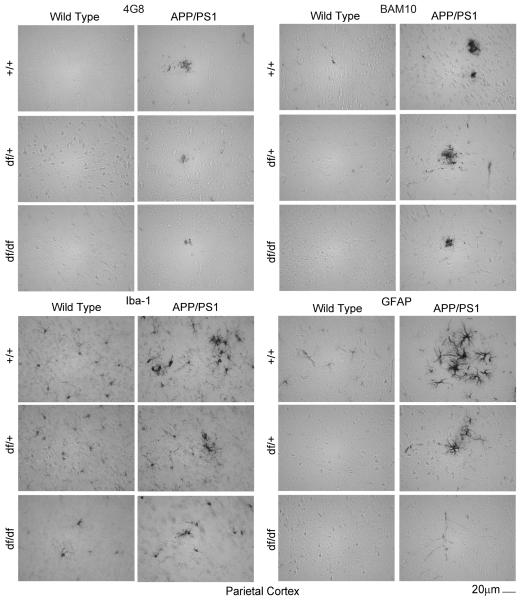
3.5 df/df/APP/PS1 mice had attenuated Aβ plaque deposition, microgliosis, and astrogliosis
To demonstrate this reduction of Aβ histologically, immunostaining of brain sections from all the mouse lines was performed using two different anti-Aβ antibodies, 4G8 and BAM-10. As predicted, APP/PS1 mice demonstrated the most dramatic plaque-like Aβ immunoreactivity with either antibody (Fig. 7, 8, 9). However, this staining was dramatically attenuated for both antibodies in both the df/+/APP/PS1 and the df/df/APP/PS1 mice (Fig. 7, 8, 9). This was consistent with the ELISA data demonstrating decreased Aβ levels with the dwarf mutation (Fig. 6). Based upon the dramatically attenuated Aβ levels and plaque deposition in mice carrying the dwarf mutation we hypothesized that these mice would also demonstrate decreased gliosis. The APP/PS1 mice had the characteristic robust staining expected for both activated microglia (Iba-1) and astrocytes (GFAP) particularly in a plaque-like distribution (Fig. 7, 8, 9). However, the df/+/APP/PS1 and df/df/APP/PS1 lines had dramatically less immunoreactivity for plaque-associated microglia (Iba-1) or astrocytes (GFAP) with the df/df/APP/PS1 mice reaching control levels (Fig. 5). This was consistent with the fact that these mice also demonstrated attenuated plaque load (Fig. 7, 8, 9).
Fig. 7.
df/df/APP/PS1 mice had attenuated Aβ plaque deposition and gliosis in the temporal cortex. Brains were collected from wild type (+/+), APP/PS1, dwarf het (df/+), dwarf het APP/PS1 (df/+/APP/PS1), dwarf (df/df), and dwarf APP/PS1 (df/df/APP/PS1) mice, fixed in 4% paraformaldehyde, serially sectioned, and immunostained. Tissue sections were immunostained using anti-Aβ 4G8 or BAM-10, anti-Iba-1 and anti-GFAP antibodies and antibody binding was visualized using Vector VIP as the chromogen. Representative temporal cortex images from 8-13 mice in each group are shown.
Fig. 8.
df/df/APP/PS1 mice had attenuated Aβ plaque deposition and gliosis in the hippocampus. Brains were collected from wild type (+/+), APP/PS1, dwarf het (df/+), dwarf het APP/PS1 (df/+/APP/PS1), dwarf (df/df), and dwarf APP/PS1 (df/df/APP/PS1) mice, fixed in 4% paraformaldehyde, serially sectioned, and immunostained. Tissue sections were immunostained using anti-Aβ 4G8 or BAM-10, anti-Iba-1 and anti-GFAP antibodies and antibody binding was visualized using Vector VIP as the chromogen. Representative hippocampus images from 8-13 mice in each group are shown.
Fig. 9.
df/df/APP/PS1 mice had attenuated Aβ plaque deposition and gliosis in the parietal cortex. Brains were collected from wild type (+/+), APP/PS1, dwarf het (df/+), dwarf het APP/PS1 (df/+/APP/PS1), dwarf (df/df), and dwarf APP/PS1 (df/df/APP/PS1) mice, fixed in 4% paraformaldehyde, serially sectioned, and immunostained. Tissue sections were immunostained using anti-Aβ 4G8 or BAM-10, anti-Iba-1 and anti-GFAP antibodies and antibody binding was visualized using Vector VIP as the chromogen. Representative parietal cortex images from 8-13 mice in each group are shown.
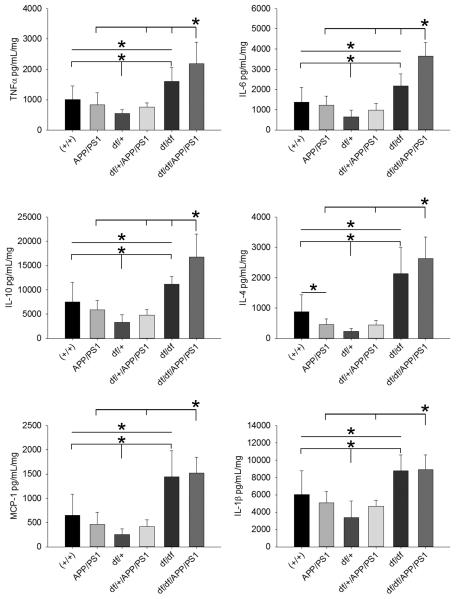
3.6 df/df and df/df/APP/PS1 mice had elevated levels of multiple cytokines
Based upon the lack of plaque deposition and attenuated histologic presentation of gliosis we expected that df/+/APP/PS1 and df/df/APP/PS1 mice would have attenuated brain cytokine levels compared to the APP/PS1 mice. We next quantified cytokines from the temporal cortex of mice to determine whether altered concentrations of these important immune signaling molecules were affected by the dwarf mutation. Surprisingly, we did not observe an increase in cytokine levels in the APP/PS1 mice compared to wild type mice (Fig. 10). Contrary to our expectations, both df/df and df/df/APP/PS1 mice had elevated levels of TNFα, IL-6, IL-10, IL-4, MCP-1 and IL-1β compared to their matched control mice (Fig. 10). This indicated that the dwarf mutation resulted in increased cytokine levels in the brain independent of expression of mutant APP/PS1. However only TNFα, IL-6 and IL-10 were increased in the df/df/APP/PS1 mice compared to the df/df mice (Fig. 10). This demonstrated that expression of the APP/PS1 transgene still resulted in elevated brain cytokines even in the context of the df/df background and in spite of attenuated gliosis and Aβ plaque deposition in the df/df/APP/PS1 mice.
Fig. 10.
df/df/APP/PS1 mice had elevated levels of multiple cytokines. Temporal cortices were collected from wild type (+/+), APP/PS1, dwarf het (df/+), dwarf het APP/PS1 (df/+/APP/PS1), dwarf (df/df), and dwarf APP/PS1 (df/df/APP/PS1) mice. Tissues were lysed with RIPA buffer and the supernatants were used for TNFα, IL-6, IL-10, IL-4, MCP-1, and IL-1β ELISAs. Data is from 8-13 mice in each group and displayed as mean +/− SD, *p<0.05.
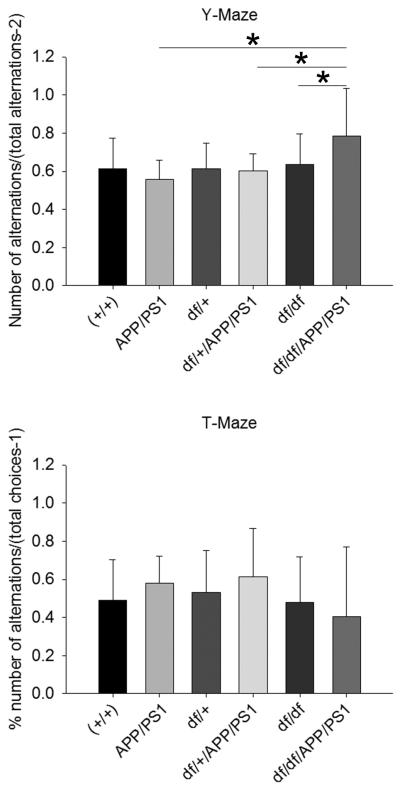
3.7 df/df/APP/PS1 mice demonstrated limited improvement in behavioral performance
Finally, to assess if this histological phenotype of elevated cytokines in spite of decreased Aβ deposition and attenuated gliosis improved behavioral performance, mice were assessed for intact working memory by both Y-Maze and T-Maze testing. The df/df/APP/PS1 mice had improved behavioral performance in the Y-Maze compared to the df/df, df/+/APP/PS1, and APP/PS1 mice consistent with their lack of plaques and gliosis (Fig. 11). However, there were no differences between any of the strains from the T-Maze testing due to the large variability in performance (Fig. 11). Surprisingly, there were no differences between the wild type and APP/PS1 lines in either test. This data suggested that the age of the mice at 6 months may not be sufficient to manisfest in any memory impairment in the APP/PS1 line and further work is required to accurately assess whether the df/df mutation improves memory performance (Savonenko, et al., 2005).
Fig. 11.
df/df/APP/PS1 mice demonstrated improved behavioral performance. At 6 months, wild type (+/+), APP/PS1, dwarf het (df/+), dwarf het APP/PS1 (df/+/APP/PS1), dwarf (df/df), and dwarf APP/PS1 (df/df/APP/PS1) mice were behaviorally tested in both the T-Maze and Y-Maze. Data are displayed as mean +/− SD, *p<0.05.
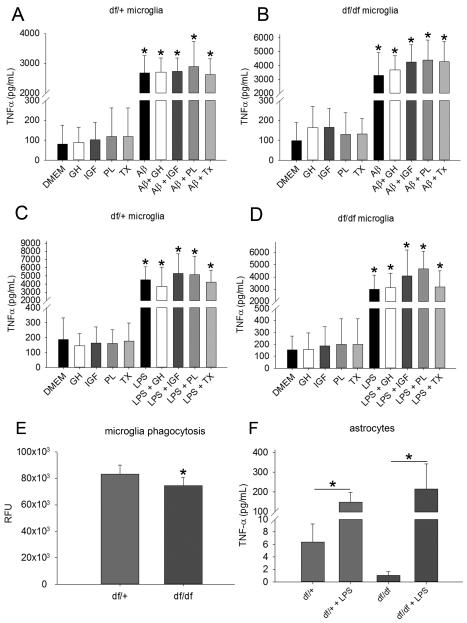
3.8 Aβ and LPS increased TNFα secretion in df/+ and df/df microglia and astrocytes
Since the immunohistochemical assessment indicated a decrease in gliosis but the ELISA analysis validated a significant increase in cytokine levels in the df/df and df/df/APP/PS1 lines, we hypothesized that the glia, particularly microglia, may be hypersecreting cytokines due to the dwarf mutation. It was possible that the cytokine changes in the cortex were the result of a glial difference due to the dwarf mutation affecting these cells via loss of stimulation by GH, IGF-1, PRL, or TSH. To test this idea, df/+ and df/df microglia and astrocytes were cultured and their media collected to measure TNFα secretion by ELISA. Microglia were stimulated in the presence of 5μM Aβ, 2.5 ng/mL LPS, or media alone with or without 10 ng/mL GH, 10 ng/mL IGF-1, 10 ng/mL PRL, or 1 ng/mL thyroxine and TNFα secretion was measured. Stimulation with GH, IGF-1, PRL and thyroxine had no basal effect on either the df/+ or df/df microglia (Fig. 12). However both Aβ and LPS were able to increase TNFα secretion similarly in both df/+ and df/df microglia (Fig. 12). LPS was also consistently able to increase TNFα secretion in both df/+ and df/df astrocytes (Fig. 12). This data suggested that, at least in vitro, there were no dramatic phenotype differences between df/+ and df/df microglia or astrocytes in their basal cytokine secretion or their ability to respond to a stimulus.
Fig. 12.
Aβ and LPS increased TNFα secretion from microglia and astrocytes. To demonstrate effects of the dwarf mutation on microglial phenotype, cultured A, C df/+ (control) and B, D df/df microglia were incubated overnight in serum free DMEM/F12 in the presence of 5μM Aβ, 2.5 ng/mL LPS, or media alone with or without 10 ng/mL growth hormone (GH), 10 ng/mL IGF-1, 10 ng/mL prolactin (PL), or 1 ng/mL thyroxine (TX). The media was collected for TNFα ELISAs. E, To determine whether the dwarf mutation affected phagocytic ability, df/+ (control) and df/df microglia were used to quantify phagocytic uptake of FITC-conjugated Aβ. Data is from 4 individual cultures in each group and displayed as mean +/− SD, *p<0.05. F, To determine whether the dwarf mutation affected astrocyte phenotype df/+ (control) and df/df astrocytes were stimulated with or without 2.5ng/mL LPS, overnight and the media was collected for quantifying secreted TNFα by ELISA. Data is from 4 individual cultures in each group and displayed as mean +/− SD, *p<0.05.
3.9 Ames dwarf (df/df) microglia had decreased phagocytic ability compared to littermate (df/+) controls
To test the hypothesis that the decreased apparent plaque load in the dwarf mice was due to increased microglial Aβ phagocytosis ability, we quantified df/+ and df/df microglial ability to phagocytose fluorescent Aβ in vitro. Somewhat surprisingly, df/df microglia had decreased phagocytic ability compared to the df/+ microglia (Fig. 12). This suggests that the attenuated Aβ levels and deposition we observed in the df/df/APP/PS1 mice was not a direct result of increased microglia clearance.
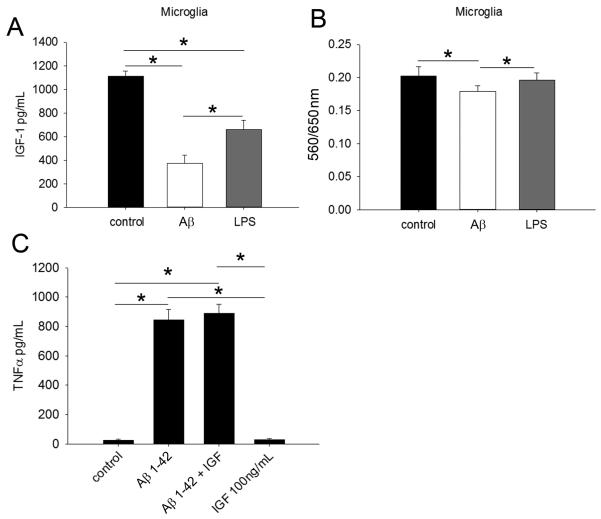
3.10 IGF-1 did not alter Aβ-stimulated TNFα secretion in C57BL6 cultures
Since microglia are capable of secreting IGF-1 (Butovsky, et al., 2006,Butovsky, et al., 2005,Rolls, et al., 2008,Skuljec, et al., 2011,Wang, et al., 2008) and it has immunomodulatory properties (Butovsky, et al., 2006,Butovsky, et al., 2005) it was possible that the high levels of brain IGF-1 in the APP/PS1 brains were, in part, due to microglia secretion and the attenuation of IGF-1 in the brains of the df/df/APP/PS1 mice might be responsible for altered microglial cytokine secretion. To answer this question we first used C57BL/6 microglia cultures to determine whether activation with Aβ might alter their IGF-1 secretion. Stimulation with both Aβ and LPS actually attenuated microglia secretion, however (Fig. 13). In addition, stimulation with IGF-1 did not alter the levels of TNFα secreted by Aβ-stimulated microglia suggesting that the elevated levels of brain IGF-1 do not robustly modulate microglial phenotype in the APP/PS1 brains (Fig. 13).
Fig. 13.
Aβ attenuated microglial IGF-1 secretion but IGF-1 did not alter Aβ-mediated TNFα secretion from microglia. A, In order to determine effects of microglial stimulation on IGF-1 secretion C57BL/6 microglia were stimulated with or without 1μM Aβ 1-42 or 2.5 ng/mL LPS overnight and media collected for IGF-1 ELISA. B, Cell viability was assessed via MTT reduction (560/650nm). Data is from 4 individual cultures in each group and displayed as mean +/− SD, *p<0.05. C, To evaluate the effect of IGF-1 on Aβ stimulation, C57BL/6 microglia were stimulated overnight with or without 1μM Aβ 1-42 and 100ng/mL IGF-1. Data is from 4 individual cultures in each group and displayed as mean +/− SD, *p<0.05.
4.0 Discussion
We observed that df/df mice have attenuated brain GH and IGF-1 concentrations. On the other hand, APP/PS1 mice have elevated IGF-1 levels and a surprising decrease in GH concentrations. Crossing the two lines resulted in attenuated IGF-1 levels in the brains of df/df/APP/PS1 mice. This correlated with decreased astrogliosis and microgliosis, increased cytokines, attenuated Aβ plaque deposition, improved behavior, and decreased and Aβ 1-40 and Aβ 1-42 concentrations.
Similar to our results, Liang and colleagues reported decreased whole brain protein levels of IGF-1 but also IGF-1R from Ames dwarf mice compared to wild type mice at 2 and 24 months, dramatically decreasing with age (Liang, et al., 2011). However, Sharma et al. found no differences in IGF1 mRNA in the hippocampus between dwarf and wild type mice at 3, 12, and 24 months (Sharma, et al., 2010). On the other hand, Sun et al. found no difference in IGF-1 mRNA levels, but increased GH, IGF-1, and pAKT protein levels in the hippocampus of Ames dwarf mice compared to wild type mice at 18-20 months (Sun, et al., 2005,Sun and Bartke, 2007). In an effort to provide a region specific analysis in our mice we compared hippocampus to parietal cortex and had findings somewhat consistent with prior work. We observed a decrease in both GH and IGF-1 protein levels in the hippocampus and the parietal cortex of df/df/ mice compared to wild type (+/+). This is consistent with prior work demonstrating whole brain decreases in IGF-1 levels in the df/df mice. We elected to examine protein changes in our study and did not examine mRNA levels and cannot compare our finding to prior reports of no changes in IGF-1 mRNA in the df/df line. Although we fully expected to find decreased GH/IGF-1 protein levels in all brain regions of the df/df mice, it is not clear why our results differ from those studies that identified elevated GH and IGF-1 levels in the hippocampi of df/df mice. It is possible that differences in tissue collection or brain perfusion before collection contribute to some of the variability. To help control for this, we PBS perfused all brains before collection and we compared to our littermate control wild type (+/+) mice throughout.
It is unclear why brain GH levels were attenuated and IGF-1 levels were increased in the parietal cortex but not hippocampus of APP/PS1 mice. There is reportedly no difference in GH levels between normal elderly subjects and subjects with AD suggesting the possibility that the alterations observed in the mice may be unrelated to sporadic human AD (Yin, et al., 2010). Although prior work has shown that increasing peripheral GH levels increases brain IGF-1 and not CSF IGF-1 (Liao, et al., 2015) , the attenuated GH but increased IGF-1 levels in the APP/PS1 mice suggests that a non GH-dependent mechanism is involved in increasing brain IGF-1 levels in this line. This dysregulation of GH-IGF-1 signaling is similar to findings derived from humans with the Swedish APP mutation who have no differences in peripheral GH or PRL levels but decreased IGF-1 validating that mutant APP expression regulates IGF-1 levels independent of GH and PRL (Mustafa, et al., 1999). Since liver IGF-1 levels in the APP/PS1 mice did not differ from wild type mice it is likely that local over-production is occurring in the brains of these mice.
Increased IGF-1 expression by neurons or other brain cells may be a compensatory response to attenuated IGF-1 receptor mediated signaling. The idea of attenuated IGF-1R mediated signaling in spite of elevated IGF-1 levels is supported by our inability to observe any change in IGF-1R phosphorylation in any brain region in these mice in spite of increased pAkt and pGSK3β levels in the hippocampus of APP/PS1 mice. Several studies have reached a similar conclusion of IGF-1 dysfunction in both mouse models and human AD brains either due to lower levels of brain or serum IGF-1 or attenuated receptor signaling (Alvarez, et al., 2007,Carro, et al., 2002,Cohen, et al., 2010,Duron, et al., 2012,Moloney, et al., 2010,Talbot, et al., 2012,Watanabe, et al., 2005,Westwood, et al., 2014,Zhang, et al., 2013). Moreover, rodent and human data support the fact that attenuated IGF-1R signaling is early in disease course correlating with stages of disease and even appearing first during mild cognitive impairment (Rivera, et al., 2005,Steen, et al., 2005,Talbot, et al., 2012,Trueba-Saiz, et al., 2013,Watanabe, et al., 2005).
Although our focus was primarily on IGF-1 levels and receptor signaling in this study it is important to note that changes in IR signaling have also been implicated in AD with similar observations of altered IR signaling in both rodent models of disease and human brains (Chua, et al., 2012,Freude, et al., 2009b,Frolich, et al., 1999,Jackson, et al., 2013,Kapogiannis, et al., 2015,Long-Smith, et al., 2013,Rivera, et al., 2005,Steen, et al., 2005,Talbot, et al., 2012,Yarchoan, et al., 2014). For example, recent work by Clarke and colleagues has demonstrated the ability of Aβ oligomer delivery to the mouse brain to increase cytokines such as TNFα to drive insulin resistance (Clarke, et al., 2015). In spite of using two different methods of assessing IR phosphorylation in our study, we did not observe any difference between wild type (+/+) and APP/PS1 mice or APP/PS1 and df/df/APP/PS1 mice. A more directed study providing acute in vivo stimulation with insulin or IGF-1 might better reveal a signaling difference across the brain regions and genotypes.
Effects of decreased IGF-1 in the brain are generally viewed as detrimental since an age-associated decrease in GH and IGF-1 levels occurs in humans and correlates with cognitive impairment (Deijen, et al., 2011). GH supplementation to increase serum IGF-1 levels in individuals with childhood onset GH deficiency (Arwert, et al., 2006,Deijen, et al., 1998) or control and mild cognitive impairment individuals (Baker, et al., 2012) is sufficient to improve cognitive performance. Similar results have been observed in rodents following intracerebroventricular, intrahippocampal, peripheral, or brain overexpression delivery of IGF-1 or IGF-2 (Carro, et al., 2006,Chen, et al., 2011,Lopez-Lopez, et al., 2007,Markowska, et al., 1998,Pascual-Lucas, et al., 2014). Mice with a liver specific knockdown of IGF-1 resulting in an 80-85% reduction in serum concentrations show decreased cognitive function at 15-18 months of age but not at 6 months supporting the notion that IGF-1 has a critical role in older brain homeostasis (Svensson, et al., 2006). IGF-1 may have other beneficial effects against AD including neuroprotection from Aβ toxicity (Dore, et al., 1997,Zhao, et al., 2009), attenuation of tau phosphorylation (Hong and Lee, 1997), altered processing of APP to minimize Aβ production (Adlerz, et al., 2007,Jacobsen, et al., 2010), and increased transport of Aβ from the brain to the blood. (Carro, et al., 2006,Carro, et al., 2002). APP/PS1 mice crossed to mice with reduced IGF-1 have increased plaques at 2 months further suggesting that IGF-1 normally reduces plaque load (Poirier, et al., 2012).
Another interpretation of our data is that elevated IGF-1 levels in the APP/PS1 mice were contributing to disease and reduction by the df/df mutation was responsible for the decreased plaque load, gliosis, Aβ levels, and improved behavior we observed. Multiple human studies support this contention by demonstrating increased serum or CSF IGF levels or IGF-1R activity with disease and higher levels, particularly at midlife, conferring increased risk (de Bruijn, et al., 2014,Johansson, et al., 2013,Salehi, et al., 2008,van Exel, et al., 2014,Vardy, et al., 2007). In fact, A SNP study examining the IGF-1 gene found that the rs972936 GG genotype, associated with higher serum IGF-1 levels, was significantly greater in AD versus control subjects and increased risk independent of ApoE genotype. (Vargas, et al., 2011). Rodent studies also support the detrimental contribution of IGF-1 to disease. Transgenic mice lacking one allele of the IGF-1R, in general or in a neuron selective fashion, crossed to individual AD lines have all demonstrated attenuated plaque accumulation or improved survival (Cohen, et al., 2009,Freude, et al., 2009a,Stohr, et al., 2013,Zemva and Schubert, 2014). In fact, IGF-1 receptor antagonists have demonstrated benefit in C. elegans offering protection from Aβ toxicity without decreasing their lifespan (El-Ami, et al., 2014). Consistent with our findings, these data support the idea that enhanced IGF-1 signaling contributes to disease progression and support continued effort for brain penetrant IGF-1R antagonist development (Yin, et al., 2010).
Our behavioral data was perplexing and did not follow our predictions. One possibility for the lack of significant differences between (+/+) and APP/PS1 mice is the fact that the mice used in our study were 6 months of age and too young to develop robust memory impairment, particularly with the Y and T maze tests we performed. This conclusion has been reported prior in this line of mice (Savonenko, et al., 2005). Due to our lack of observing any significant differences between the wild type and APP/PS1 mice and work from others demonstrating that significant memory impairment in this line requires ages beyond 6 months, we will need to perform different types of memory assessments at increased ages of mice to definitively determine whether any memory deficit exists in the APP/PS1 line compared to wild types that is attenuated by the df/df crossing (Savonenko, et al., 2005).
It is also important to point out that a recent study using growth hormone releasing hormone deficient mice crossed to an APP/PS1 line (lit/lit/APP/PS1) found no difference in Aβ pathology even though these mice are also deficient in both GH and IGF-1 levels (Liao, et al., 2015). It is interesting to speculate that the protective effects observed in the df/df/APP/PS1 mice may be more related to the decrease in prolactin or TSH in these mice. However, prior work by Pedros and colleagues has identified a decrease in prolactin receptor mRNA in the APP/PS1 line compared to wild type mice suggesting that attenuated prolactin-mediated signaling is a component of the disease process in the mice (Pedros, et al., 2015). Futher work will be required to determine whether a single or multiple hormone role can be attributed to the beneficial changes in AD-like pathology in the df/df/APP/PS1 mice. Independent of whether prolactin, TSH, or GH/IGF-1 stimulation is attenuating or potentiating the AD-like pathology in the mice, we were originally also interested in assessing whether the Ames dwarf mutation provided any anti-oxidant benefits against disease. However, our data demonstrated that both df/df/APP/PS1 and df/+/APP/PS1 had slightly increased levels of HNE protein adducts compared to APP/PS1 mice suggesting that the dwarf mutation did not enhance anti-oxidant protection in the brain, at least by this limited assessment.
Perhaps the most perplexing observation we made is the significant increase in brain levels of multiple cytokines in the df/df/APP/PS1 mice in spite of the attenuated gliosis. This was compounded by the lack of robust differences in secretory ability of df/df and df/+ microglia and astrocytes in vitro. One possibility for explaining the relatively high levels of brain cytokines could be that they are blood derived. Another possible explanation is that glia in the df/df/APP/PS1 mouse brains are hypersecreting cytokines independent of changes in the protein markers, Iba-1 and GFAP. Since the increase was independent of mutant APP/PS1 expression, one possible explanation is that loss of regulatory behaviors from GH/IGF-1, PRL, or TSH were responsible for the elevated cytokine levels. Although we did not observe any ability of these factors to modify microglial secretion in vitro, they are potent immunomodulators (Colaianni, et al., 2013,Costanza, et al., 2015,Spaziani, et al., 2014,van der Weerd, et al., 2014,Weigent, 2013,Wu, et al., 2014). Moreover, an increase in brain inflammatory cytokine levels, particularly IL-1β and TNFα, has been shown to have an ability to decrease Aβ plaque deposition in two different mouse models of AD (Chakrabarty, et al., 2011,Shaftel, et al., 2007). This supports the idea that some form of enhanced clearance or attenuated production of Aβ occurred in the df/df/APP/PS1 mice following enhanced cytokine secretion. Perhaps improved autophagy in the df/df mice also contributed to decreased plaque load since cells from these animals are more susceptible to autophagy induction (Wang and Miller, 2012).
We have demonstrated a protective effect the Ames dwarf mutation against Aβ plaque deposition and glial activation correlating with improved behavioral performance and attenuation of aberrant brain IGF-1 levels. At this point we are unable to determine whether disease is attenuated or simply delayed in the df/df/APP/PS1 mice due to their presumed longer life span. Future work will require a longitudinal collection of these mice across their lifespan for comparison to the APP/PS1 line. These mice demonstrate a unique resource to not only examine the biology of IGF-1 and GH in brain aging but also their contribution to AD. Future work assessing effects on longevity and specific, temporal manipulation of GH and IGF-1 levels will better determine the feasibility of therapeutically targeting these molecules or their receptors in AD.
Highlights.
A novel mouse model of AD was generated by crossing an APP/PS1 transgenic line to the Ames Dwarf line.
The longevity enhancing loss of GH, IGF-1, prolactin, and thyroid stimulating hormone characteristic of the Ames Dwarf mutation elevated brain cytokines in the APP/PS1 mice.
The Ames Dwarf mutation attenuated gliosis and Aβ levels in the APP/PS1 mice.
The Ames Dwarf mutation decreased the elevated IGF-1 levels in brains of APP/PS1 mice.
Acknowledgements
We are grateful to Dr. Lalida Rojanathammanee for her helpful suggestions during tissue sectioning. This work was supported by NIH 1P20RR17699, NIH 1R01AG026330, NIH 1R01AG042819 and NIH 1RO1AG034206.
Footnotes
Disclosure statement
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kendra L. Puig, Dept. of Basic Sciences University of North Dakota School of Medicine and Health Sciences 504 Hamline Street, Neuroscience Building Grand Forks, ND 58203 kendra.puig@med.und.edu
Joshua A. Kulas, Dept. of Basic Sciences University of North Dakota School of Medicine and Health Sciences 504 Hamline Street, Neuroscience Building Grand Forks, ND 58203 joshua.kulas@my.und.edu
Whitney Franklin, Dept. of Neuroscience and Cell Biology Dept. of Neurology University of Texas Medical Branch 301 University Blvd. Galveston, TX 77555 whfrankl@utmb.edu.
Sharlene G. Rakoczy, Dept. of Basic Sciences University of North Dakota School of Medicine and Health Sciences 501 North Columbia Road Grand Forks, ND 58203 sharlene.rakoczy@med.und.edu
Giulio Taglialatela, Dept. of Neurology University of Texas Medical Branch 301 University Blvd. Galveston, TX 77555 gtaglial@utmb.edu.
Holly M. Brown-Borg, Dept. of Basic Sciences University of North Dakota School of Medicine and Health Sciences 501 North Columbia Road Grand Forks, ND 58203 holly.brown.borg@med.und.edu
Colin K. Combs, Dept. of Basic Sciences University of North Dakota School of Medicine and Health Sciences 504 Hamline Street, Neuroscience Building Grand Forks, ND 58203
References
- Adlerz L, Holback S, Multhaup G, Iverfeldt K. IGF-1-induced processing of the amyloid precursor protein family is mediated by different signaling pathways. The Journal of biological chemistry. 2007;282(14):10203–9. doi: 10.1074/jbc.M611183200. 10.1074/jbc.M611183200. [DOI] [PubMed] [Google Scholar]
- Alvarez A, Cacabelos R, Sanpedro C, Garcia-Fantini M, Aleixandre M. Serum TNF-alpha levels are increased and correlate negatively with free IGF-I in Alzheimer disease. Neurobiology of aging. 2007;28(4):533–6. doi: 10.1016/j.neurobiolaging.2006.02.012. 10.1016/j.neurobiolaging.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Alzheimer's A. 2014 Alzheimer's disease facts and figures. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2014;10(2):e47–92. doi: 10.1016/j.jalz.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Armstrong CS, Wuarin L, Ishii DN. Uptake of circulating insulin-like growth factor-I into the cerebrospinal fluid of normal and diabetic rats and normalization of IGF-II mRNA content in diabetic rat brain. Journal of neuroscience research. 2000;59(5):649–60. doi: 10.1002/(SICI)1097-4547(20000301)59:5<649::AID-JNR8>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Arwert LI, Veltman DJ, Deijen JB, van Dam PS, Drent ML. Effects of growth hormone substitution therapy on cognitive functioning in growth hormone deficient patients: a functional MRI study. Neuroendocrinology. 2006;83(1):12–9. doi: 10.1159/000093337. 10.1159/000093337. [DOI] [PubMed] [Google Scholar]
- Baker LD, Barsness SM, Borson S, Merriam GR, Friedman SD, Craft S, Vitiello MV. Effects of growth hormone-releasing hormone on cognitive function in adults with mild cognitive impairment and healthy older adults: results of a controlled trial. Archives of neurology. 2012;69(11):1420–9. doi: 10.1001/archneurol.2012.1970. 10.1001/archneurol.2012.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokov AF, Lindsey ML, Khodr C, Sabia MR, Richardson A. Long-lived ames dwarf mice are resistant to chemical stressors. The journals of gerontology Series A, Biological sciences and medical sciences. 2009;64(8):819–27. doi: 10.1093/gerona/glp052. 10.1093/gerona/glp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical biochemistry. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM. Longevity in mice: is stress resistance a common factor? Age. 2006;28(2):145–62. doi: 10.1007/s11357-006-9003-y. 10.1007/s11357-006-9003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg HM, Bode AM, Bartke A. Antioxidative mechanisms and plasma growth hormone levels: potential relationship in the aging process. Endocrine. 1999;11(1):41–8. doi: 10.1385/ENDO:11:1:41. 10.1385/ENDO:11:1:41. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384(6604):33. doi: 10.1038/384033a0. 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG. Catalase expression in delayed and premature aging mouse models. Experimental gerontology. 2000;35(2):199–212. doi: 10.1016/s0531-5565(00)00079-6. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG, Sharma S, Bartke A. Long-living growth hormone receptor knockout mice: potential mechanisms of altered stress resistance. Experimental gerontology. 2009;44(1-2):10–9. doi: 10.1016/j.exger.2008.07.002. 10.1016/j.exger.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Landa G, Kunis G, Ziv Y, Avidan H, Greenberg N, Schwartz A, Smirnov I, Pollack A, Jung S, Schwartz M. Induction and blockage of oligodendrogenesis by differently activated microglia in an animal model of multiple sclerosis. The Journal of clinical investigation. 2006;116(4):905–15. doi: 10.1172/JCI26836. 10.1172/JCI26836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Talpalar AE, Ben-Yaakov K, Schwartz M. Activation of microglia by aggregated beta-amyloid or lipopolysaccharide impairs MHC-II expression and renders them cytotoxic whereas IFN-gamma and IL-4 render them protective. Molecular and cellular neurosciences. 2005;29(3):381–93. doi: 10.1016/j.mcn.2005.03.005. 10.1016/j.mcn.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Carro E, Trejo JL, Gerber A, Loetscher H, Torrado J, Metzger F, Torres-Aleman I. Therapeutic actions of insulin-like growth factor I on APP/PS2 mice with severe brain amyloidosis. Neurobiology of aging. 2006;27(9):1250–7. doi: 10.1016/j.neurobiolaging.2005.06.015. 10.1016/j.neurobiolaging.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Carro E, Trejo JL, Gomez-Isla T, LeRoith D, Torres-Aleman I. Serum insulin-like growth factor I regulates brain amyloid-beta levels. Nature medicine. 2002;8(12):1390–7. doi: 10.1038/nm1202-793. 10.1038/nm793. [DOI] [PubMed] [Google Scholar]
- Chakrabarty P, Herring A, Ceballos-Diaz C, Das P, Golde TE. Hippocampal expression of murine TNFalpha results in attenuation of amyloid deposition in vivo. Molecular neurodegeneration. 2011;6:16. doi: 10.1186/1750-1326-6-16. 10.1186/1750-1326-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DY, Stern SA, Garcia-Osta A, Saunier-Rebori B, Pollonini G, Bambah-Mukku D, Blitzer RD, Alberini CM. A critical role for IGF-II in memory consolidation and enhancement. Nature. 2011;469(7331):491–7. doi: 10.1038/nature09667. 10.1038/nature09667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C, Jeong JH, Jang JS, Choi K, Lee J, Kwon J, Choi KG, Lee JS, Kang SW. Multiplex analysis of cytokines in the serum and cerebrospinal fluid of patients with Alzheimer's disease by color-coded bead technology. Journal of clinical neurology. 2008;4(2):84–8. doi: 10.3988/jcn.2008.4.2.84. 10.3988/jcn.2008.4.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua LM, Lim ML, Chong PR, Hu ZP, Cheung NS, Wong BS. Impaired neuronal insulin signaling precedes Abeta42 accumulation in female AbetaPPsw/PS1DeltaE9 mice. Journal of Alzheimer's disease : JAD. 2012;29(4):783–91. doi: 10.3233/JAD-2012-111880. 10.3233/jad-2012-111880. [DOI] [PubMed] [Google Scholar]
- Clarke JR, Lyra ESNM, Figueiredo CP, Frozza RL, Ledo JH, Beckman D, Katashima CK, Razolli D, Carvalho BM, Frazao R, Silveira MA, Ribeiro FC, Bomfim TR, Neves FS, Klein WL, Medeiros R, LaFerla FM, Carvalheira JB, Saad MJ, Munoz DP, Velloso LA, Ferreira ST, De Felice FG. Alzheimer-associated Abeta oligomers impact the central nervous system to induce peripheral metabolic deregulation. EMBO molecular medicine. 2015;7(2):190–210. doi: 10.15252/emmm.201404183. 10.15252/emmm.201404183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Du D, Joyce D, Kapernick EA, Volovik Y, Kelly JW, Dillin A. Temporal requirements of insulin/IGF-1 signaling for proteotoxicity protection. Aging cell. 2010;9(2):126–34. doi: 10.1111/j.1474-9726.2009.00541.x. 10.1111/j.1474-9726.2009.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Paulsson JF, Blinder P, Burstyn-Cohen T, Du D, Estepa G, Adame A, Pham HM, Holzenberger M, Kelly JW, Masliah E, Dillin A. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell. 2009;139(6):1157–69. doi: 10.1016/j.cell.2009.11.014. 10.1016/j.cell.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaianni G, Cuscito C, Colucci S. FSH and TSH in the regulation of bone mass: the pituitary/immune/bone axis. Clinical & developmental immunology. 2013;2013:382698. doi: 10.1155/2013/382698. 10.1155/2013/382698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanza M, Binart N, Steinman L, Pedotti R. Prolactin: a versatile regulator of inflammation and autoimmune pathology. Autoimmunity reviews. 2015;14(3):223–30. doi: 10.1016/j.autrev.2014.11.005. 10.1016/j.autrev.2014.11.005. [DOI] [PubMed] [Google Scholar]
- de Bruijn RF, Janssen JA, Brugts MP, van Duijn CM, Hofman A, Koudstaal PJ, Ikram MA. Insulin-like growth factor-I receptor stimulating activity is associated with dementia. Journal of Alzheimer's disease : JAD. 2014;42(1):137–42. doi: 10.3233/JAD-140186. 10.3233/JAD-140186. [DOI] [PubMed] [Google Scholar]
- Deijen JB, Arwert LI, Drent ML. The GH/IGF-I Axis and Cognitive Changes across a 4-Year Period in Healthy Adults. ISRN endocrinology. 2011;2011:249421. doi: 10.5402/2011/249421. 10.5402/2011/249421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deijen JB, de Boer H, van der Veen EA. Cognitive changes during growth hormone replacement in adult men. Psychoneuroendocrinology. 1998;23(1):45–55. doi: 10.1016/s0306-4530(97)00092-9. [DOI] [PubMed] [Google Scholar]
- Dore S, Kar S, Quirion R. Insulin-like growth factor I protects and rescues hippocampal neurons against beta-amyloid- and human amylin-induced toxicity. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(9):4772–7. doi: 10.1073/pnas.94.9.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duron E, Funalot B, Brunel N, Coste J, Quinquis L, Viollet C, Belmin J, Jouanny P, Pasquier F, Treluyer JM, Epelbaum J, le Bouc Y, Hanon O. Insulin-like growth factor-I and insulin-like growth factor binding protein-3 in Alzheimer's disease. The Journal of clinical endocrinology and metabolism. 2012;97(12):4673–81. doi: 10.1210/jc.2012-2063. 10.1210/jc.2012-2063. [DOI] [PubMed] [Google Scholar]
- El-Ami T, Moll L, Carvalhal Marques F, Volovik Y, Reuveni H, Cohen E. A novel inhibitor of the insulin/IGF signaling pathway protects from age-onset, neurodegeneration-linked proteotoxicity. Aging cell. 2014;13(1):165–74. doi: 10.1111/acel.12171. 10.1111/acel.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floden AM, Combs CK. Beta-amyloid stimulates murine postnatal and adult microglia cultures in a unique manner. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(17):4644–8. doi: 10.1523/JNEUROSCI.4822-05.2006. 10.1523/JNEUROSCI.4822-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floden AM, Combs CK. Microglia demonstrate age-dependent interaction with amyloid-beta fibrils. Journal of Alzheimer's disease : JAD. 2011;25(2):279–93. doi: 10.3233/JAD-2011-101014. 10.3233/JAD-2011-101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freude S, Hettich MM, Schumann C, Stohr O, Koch L, Kohler C, Udelhoven M, Leeser U, Muller M, Kubota N, Kadowaki T, Krone W, Schroder H, Bruning JC, Schubert M. Neuronal IGF-1 resistance reduces Abeta accumulation and protects against premature death in a model of Alzheimer's disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2009a;23(10):3315–24. doi: 10.1096/fj.09-132043. 10.1096/fj.09-132043. [DOI] [PubMed] [Google Scholar]
- Freude S, Schilbach K, Schubert M. The role of IGF-1 receptor and insulin receptor signaling for the pathogenesis of Alzheimer's disease: from model organisms to human disease. Current Alzheimer research. 2009b;6(3):213–23. doi: 10.2174/156720509788486527. [DOI] [PubMed] [Google Scholar]
- Frolich L, Blum-Degen D, Riederer P, Hoyer S. A disturbance in the neuronal insulin receptor signal transduction in sporadic Alzheimer's disease. Annals of the New York Academy of Sciences. 1999;893:290–3. doi: 10.1111/j.1749-6632.1999.tb07839.x. [DOI] [PubMed] [Google Scholar]
- Guo Q, Sebastian L, Sopher BL, Miller MW, Ware CB, Martin GM, Mattson MP. Increased vulnerability of hippocampal neurons from presenilin-1 mutant knock-in mice to amyloid beta-peptide toxicity: central roles of superoxide production and caspase activation. Journal of neurochemistry. 1999;72(3):1019–29. doi: 10.1046/j.1471-4159.1999.0721019.x. [DOI] [PubMed] [Google Scholar]
- Hensley K, Butterfield DA, Hall N, Cole P, Subramaniam R, Mark R, Mattson MP, Markesbery WR, Harris ME, Aksenov M, et al. Reactive oxygen species as causal agents in the neurotoxicity of the Alzheimer's disease-associated amyloid beta peptide. Annals of the New York Academy of Sciences. 1996;786:120–34. doi: 10.1111/j.1749-6632.1996.tb39057.x. [DOI] [PubMed] [Google Scholar]
- Hong M, Lee VM. Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. The Journal of biological chemistry. 1997;272(31):19547–53. doi: 10.1074/jbc.272.31.19547. [DOI] [PubMed] [Google Scholar]
- Jackson HM, Soto I, Graham LC, Carter GW, Howell GR. Clustering of transcriptional profiles identifies changes to insulin signaling as an early event in a mouse model of Alzheimer's disease. BMC genomics. 2013;14:831. doi: 10.1186/1471-2164-14-831. 10.1186/1471-2164-14-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen KT, Adlerz L, Multhaup G, Iverfeldt K. Insulin-like growth factor-1 (IGF-1)-induced processing of amyloid-beta precursor protein (APP) and APP-like protein 2 is mediated by different metalloproteinases. The Journal of biological chemistry. 2010;285(14):10223–31. doi: 10.1074/jbc.M109.038224. 10.1074/jbc.M109.038224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson P, Aberg D, Johansson JO, Mattsson N, Hansson O, Ahren B, Isgaard J, Aberg ND, Blennow K, Zetterberg H, Wallin A, Svensson J. Serum but not cerebrospinal fluid levels of insulin-like growth factor-I (IGF-I) and IGF-binding protein-3 (IGFBP-3) are increased in Alzheimer's disease. Psychoneuroendocrinology. 2013;38(9):1729–37. doi: 10.1016/j.psyneuen.2013.02.006. 10.1016/j.psyneuen.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Jung YY, Lee YJ, Choi DY, Hong JT. Amelioration of Cognitive Dysfunction in APP/PS1 Double Transgenic Mice by Long-Term Treatment of 4-O-Methylhonokiol. Biomolecules & therapeutics. 2014;22(3):232–8. doi: 10.4062/biomolther.2014.030. 10.4062/biomolther.2014.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanninen K, Heikkinen R, Malm T, Rolova T, Kuhmonen S, Leinonen H, Yla-Herttuala S, Tanila H, Levonen AL, Koistinaho M, Koistinaho J. Intrahippocampal injection of a lentiviral vector expressing Nrf2 improves spatial learning in a mouse model of Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(38):16505–10. doi: 10.1073/pnas.0908397106. 10.1073/pnas.0908397106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapogiannis D, Boxer A, Schwartz JB, Abner EL, Biragyn A, Masharani U, Frassetto L, Petersen RC, Miller BL, Goetzl EJ. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer's disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29(2):589–96. doi: 10.1096/fj.14-262048. 10.1096/fj.14-262048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney-Forshee BA, Kinney NE, Steger RW, Bartke A. Could a deficiency in growth hormone signaling be beneficial to the aging brain? Physiology & behavior. 2004;80(5):589–94. doi: 10.1016/j.physbeh.2003.10.018. 10.1016/j.physbeh.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Kinney BA, Meliska CJ, Steger RW, Bartke A. Evidence that Ames dwarf mice age differently from their normal siblings in behavioral and learning and memory parameters. Hormones and behavior. 2001;39(4):277–84. doi: 10.1006/hbeh.2001.1654. 10.1006/hbeh.2001.1654. [DOI] [PubMed] [Google Scholar]
- Liang R, Khanna A, Muthusamy S, Li N, Sarojini H, Kopchick JJ, Masternak MM, Bartke A, Wang E. Post-transcriptional regulation of IGF1R by key microRNAs in long-lived mutant mice. Aging cell. 2011;10(6):1080–8. doi: 10.1111/j.1474-9726.2011.00751.x. 10.1111/j.1474-9726.2011.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao F, Zhang TJ, Mahan TE, Jiang H, Holtzman DM. Effects of growth hormone-releasing hormone on sleep and brain interstitial fluid amyloid-beta in an APP transgenic mouse model. Brain, behavior, and immunity. 2015;47:163–71. doi: 10.1016/j.bbi.2014.09.005. 10.1016/j.bbi.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Li JZ, Song JK, Zhou D, Huang C, Bai XY, Xie T, Zhang X, Li YJ, Wu CX, Zhang L, Li L, Zhang TT, Du GH. Pinocembrin improves cognition and protects the neurovascular unit in Alzheimer related deficits. Neurobiology of aging. 2014;35(6):1275–85. doi: 10.1016/j.neurobiolaging.2013.12.031. 10.1016/j.neurobiolaging.2013.12.031. [DOI] [PubMed] [Google Scholar]
- Long-Smith CM, Manning S, McClean PL, Coakley MF, O'Halloran DJ, Holscher C, O'Neill C. The diabetes drug liraglutide ameliorates aberrant insulin receptor localisation and signalling in parallel with decreasing both amyloid-beta plaque and glial pathology in a mouse model of Alzheimer's disease. Neuromolecular medicine. 2013;15(1):102–14. doi: 10.1007/s12017-012-8199-5. 10.1007/s12017-012-8199-5. [DOI] [PubMed] [Google Scholar]
- Lopez-Lopez C, Dietrich MO, Metzger F, Loetscher H, Torres-Aleman I. Disturbed cross talk between insulin-like growth factor I and AMP-activated protein kinase as a possible cause of vascular dysfunction in the amyloid precursor protein/presenilin 2 mouse model of Alzheimer's disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(4):824–31. doi: 10.1523/JNEUROSCI.4345-06.2007. 10.1523/JNEUROSCI.4345-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv C, Liu X, Liu H, Chen T, Zhang W. Geniposide attenuates mitochondrial dysfunction and memory deficits in APP/PS1 transgenic mice. Current Alzheimer research. 2014;11(6):580–7. doi: 10.2174/1567205011666140618095925. [DOI] [PubMed] [Google Scholar]
- Markesbery WR. Oxidative stress hypothesis in Alzheimer's disease. Free radical biology & medicine. 1997;23(1):134–47. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Mooney M, Sonntag WE. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience. 1998;87(3):559–69. doi: 10.1016/s0306-4522(98)00143-2. [DOI] [PubMed] [Google Scholar]
- Martins RN, Harper CG, Stokes GB, Masters CL. Increased cerebral glucose-6-phosphate dehydrogenase activity in Alzheimer's disease may reflect oxidative stress. Journal of neurochemistry. 1986;46(4):1042–5. doi: 10.1111/j.1471-4159.1986.tb00615.x. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Pedersen WA. Effects of amyloid precursor protein derivatives and oxidative stress on basal forebrain cholinergic systems in Alzheimer's disease. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 1998;16(7-8):737–53. doi: 10.1016/s0736-5748(98)00082-3. [DOI] [PubMed] [Google Scholar]
- Moloney AM, Griffin RJ, Timmons S, O'Connor R, Ravid R, O'Neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer's disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiology of aging. 2010;31(2):224–43. doi: 10.1016/j.neurobiolaging.2008.04.002. 10.1016/j.neurobiolaging.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Multhaup G, Ruppert T, Schlicksupp A, Hesse L, Beher D, Masters CL, Beyreuther K. Reactive oxygen species and Alzheimer's disease. Biochemical pharmacology. 1997;54(5):533–9. doi: 10.1016/s0006-2952(97)00062-2. [DOI] [PubMed] [Google Scholar]
- Mustafa A, Lannfelt L, Lilius L, Islam A, Winblad B, Adem A. Decreased plasma insulin-like growth factor-I level in familial Alzheimer's disease patients carrying the Swedish APP 670/671 mutation. Dementia and geriatric cognitive disorders. 1999;10(6):446–51. doi: 10.1159/000017188. doi:17188. [DOI] [PubMed] [Google Scholar]
- Pan W, Yu Y, Cain CM, Nyberg F, Couraud PO, Kastin AJ. Permeation of growth hormone across the blood-brain barrier. Endocrinology. 2005;146(11):4898–904. doi: 10.1210/en.2005-0587. 10.1210/en.2005-0587. [DOI] [PubMed] [Google Scholar]
- Pascual-Lucas M, Viana da Silva S, Di Scala M, Garcia-Barroso C, Gonzalez-Aseguinolaza G, Mulle C, Alberini CM, Cuadrado-Tejedor M, Garcia-Osta A. Insulin-like growth factor 2 reverses memory and synaptic deficits in APP transgenic mice. EMBO molecular medicine. 2014;6(10):1246–62. doi: 10.15252/emmm.201404228. 10.15252/emmm.201404228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedros I, Petrov D, Artiach G, Abad S, Ramon-Duaso C, Sureda F, Pallas M, Beas-Zarate C, Folch J, Camins A. Adipokine pathways are altered in hippocampus of an experimental mouse model of Alzheimer's disease. The journal of nutrition, health & aging. 2015;19(4):403–12. doi: 10.1007/s12603-014-0574-5. 10.1007/s12603-014-0574-5. [DOI] [PubMed] [Google Scholar]
- Poirier R, Fernandez AM, Torres-Aleman I, Metzger F. Early brain amyloidosis in APP/PS1 mice with serum insulin-like growth factor-I deficiency. Neuroscience letters. 2012;509(2):101–4. doi: 10.1016/j.neulet.2011.12.048. 10.1016/j.neulet.2011.12.048. [DOI] [PubMed] [Google Scholar]
- Reinhardt RR, Bondy CA. Insulin-like growth factors cross the blood-brain barrier. Endocrinology. 1994;135(5):1753–61. doi: 10.1210/endo.135.5.7525251. 10.1210/endo.135.5.7525251. [DOI] [PubMed] [Google Scholar]
- Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer's disease: link to brain reductions in acetylcholine. Journal of Alzheimer's disease : JAD. 2005;8(3):247–68. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- Rolls A, Shechter R, London A, Segev Y, Jacob-Hirsch J, Amariglio N, Rechavi G, Schwartz M. Two faces of chondroitin sulfate proteoglycan in spinal cord repair: a role in microglia/macrophage activation. PLoS medicine. 2008;5(8):e171. doi: 10.1371/journal.pmed.0050171. 10.1371/journal.pmed.0050171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi Z, Mashayekhi F, Naji M. Insulin like growth factor-1 and insulin like growth factor binding proteins in the cerebrospinal fluid and serum from patients with Alzheimer's disease. BioFactors. 2008;33(2):99–106. doi: 10.1002/biof.5520330202. [DOI] [PubMed] [Google Scholar]
- Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. American journal of physiology Endocrinology and metabolism. 2005;289(1):E23–9. doi: 10.1152/ajpendo.00575.2004. 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- Savonenko A, Xu GM, Melnikova T, Morton JL, Gonzales V, Wong MP, Price DL, Tang F, Markowska AL, Borchelt DR. Episodic-like memory deficits in the APPswe/PS1dE9 mouse model of Alzheimer's disease: relationships to beta-amyloid deposition and neurotransmitter abnormalities. Neurobiology of disease. 2005;18(3):602–17. doi: 10.1016/j.nbd.2004.10.022. 10.1016/j.nbd.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Schrag M, Sharma S, Brown-Borg H, Ghribi O. Hippocampus of Ames dwarf mice is resistant to beta-amyloid-induced tau hyperphosphorylation and changes in apoptosis-regulatory protein levels. Hippocampus. 2008;18(3):239–44. doi: 10.1002/hipo.20387. 10.1002/hipo.20387. [DOI] [PubMed] [Google Scholar]
- Shaftel SS, Kyrkanides S, Olschowka JA, Miller JN, Johnson RE, O'Banion MK. Sustained hippocampal IL-1 beta overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. The Journal of clinical investigation. 2007;117(6):1595–604. doi: 10.1172/JCI31450. 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Haselton J, Rakoczy S, Branshaw S, Brown-Borg HM. Spatial memory is enhanced in long-living Ames dwarf mice and maintained following kainic acid induced neurodegeneration. Mechanisms of ageing and development. 2010;131(6):422–35. doi: 10.1016/j.mad.2010.06.004. 10.1016/j.mad.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuljec J, Sun H, Pul R, Benardais K, Ragancokova D, Moharregh-Khiabani D, Kotsiari A, Trebst C, Stangel M. CCL5 induces a pro-inflammatory profile in microglia in vitro. Cellular immunology. 2011;270(2):164–71. doi: 10.1016/j.cellimm.2011.05.001. 10.1016/j.cellimm.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O'Connell SM, Gukovsky I, Carriere C, Ryan AK, Miller AP, Zuo L, Gleiberman AS, Andersen B, Beamer WG, Rosenfeld MG. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996;384(6607):327–33. doi: 10.1038/384327a0. 10.1038/384327a0. [DOI] [PubMed] [Google Scholar]
- Spaziani S, Imperlini E, Mancini A, Caterino M, Buono P, Orru S. Insulin-like growth factor 1 receptor signaling induced by supraphysiological doses of IGF-1 in human peripheral blood lymphocytes. Proteomics. 2014;14(13-14):1623–9. doi: 10.1002/pmic.201300318. 10.1002/pmic.201300318. [DOI] [PubMed] [Google Scholar]
- Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease--is this type 3 diabetes? Journal of Alzheimer's disease : JAD. 2005;7(1):63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- Stohr O, Schilbach K, Moll L, Hettich MM, Freude S, Wunderlich FT, Ernst M, Zemva J, Bruning JC, Krone W, Udelhoven M, Schubert M. Insulin receptor signaling mediates APP processing and beta-amyloid accumulation without altering survival in a transgenic mouse model of Alzheimer's disease. Age. 2013;35(1):83–101. doi: 10.1007/s11357-011-9333-2. 10.1007/s11357-011-9333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao KV, Richardson JS, Ang LC. Autopsy samples of Alzheimer's cortex show increased peroxidation in vitro. Journal of neurochemistry. 1990;55(1):342–5. doi: 10.1111/j.1471-4159.1990.tb08858.x. [DOI] [PubMed] [Google Scholar]
- Sun LY, Al-Regaiey K, Masternak MM, Wang J, Bartke A. Local expression of GH and IGF-1 in the hippocampus of GH-deficient long-lived mice. Neurobiology of aging. 2005;26(6):929–37. doi: 10.1016/j.neurobiolaging.2004.07.010. 10.1016/j.neurobiolaging.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Sun LY, Bartke A. Adult neurogenesis in the hippocampus of long-lived mice during aging. The journals of gerontology Series A, Biological sciences and medical sciences. 2007;62(2):117–25. doi: 10.1093/gerona/62.2.117. [DOI] [PubMed] [Google Scholar]
- Svensson J, Diez M, Engel J, Wass C, Tivesten A, Jansson JO, Isaksson O, Archer T, Hokfelt T, Ohlsson C. Endocrine, liver-derived IGF-I is of importance for spatial learning and memory in old mice. The Journal of endocrinology. 2006;189(3):617–27. doi: 10.1677/joe.1.06631. 10.1677/joe.1.06631. [DOI] [PubMed] [Google Scholar]
- Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. The Journal of clinical investigation. 2012;122(4):1316–38. doi: 10.1172/JCI59903. 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueba-Saiz A, Cavada C, Fernandez AM, Leon T, Gonzalez DA, Fortea Ormaechea J, Lleo A, Del Ser T, Nunez A, Torres-Aleman I. Loss of serum IGF-I input to the brain as an early biomarker of disease onset in Alzheimer mice. Translational psychiatry. 2013;3:e330. doi: 10.1038/tp.2013.102. 10.1038/tp.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Weerd K, van Hagen PM, Schrijver B, Heuvelmans SJ, Hofland LJ, Swagemakers SM, Bogers AJ, Dik WA, Visser TJ, van Dongen JJ, van der Lelij AJ, Staal FJ. Thyrotropin acts as a T-cell developmental factor in mice and humans. Thyroid : official journal of the American Thyroid Association. 2014;24(6):1051–61. doi: 10.1089/thy.2013.0396. 10.1089/thy.2013.0396. [DOI] [PubMed] [Google Scholar]
- van Exel E, Eikelenboom P, Comijs H, Deeg DJ, Stek ML, Westendorp RG. Insulin-like growth factor-1 and risk of late-onset Alzheimer's disease: findings from a family study. Neurobiology of aging. 2014;35(3):725, e7–10. doi: 10.1016/j.neurobiolaging.2013.08.014. 10.1016/j.neurobiolaging.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Vardy ER, Rice PJ, Bowie PC, Holmes JD, Grant PJ, Hooper NM. Increased circulating insulin-like growth factor-1 in late-onset Alzheimer's disease. Journal of Alzheimer's disease : JAD. 2007;12(4):285–90. doi: 10.3233/jad-2007-12401. [DOI] [PubMed] [Google Scholar]
- Vargas T, Martinez-Garcia A, Antequera D, Vilella E, Clarimon J, Mateo I, Sanchez-Juan P, Rodriguez-Rodriguez E, Frank A, Rosich-Estrago M, Lleo A, Molina-Porcel L, Blesa R, Gomez-Isla T, Combarros O, Bermejo-Pareja F, Valdivieso F, Bullido MJ, Carro E. IGF-I gene variability is associated with an increased risk for AD. Neurobiology of aging. 2011;32(3):556, e3–11. doi: 10.1016/j.neurobiolaging.2010.10.017. 10.1016/j.neurobiolaging.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Wang M, Miller RA. Augmented autophagy pathways and MTOR modulation in fibroblasts from long-lived mutant mice. Autophagy. 2012;8(8):1273–4. doi: 10.4161/auto.20917. 10.4161/auto.20917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li M, Song M, Xu X, Xiong J, Yang X, Tan J, Bai Y. Expression of OX40 ligand in microglia activated by IFN-gamma sustains a protective CD4+ T-cell response in vitro. Cellular immunology. 2008;251(2):86–92. doi: 10.1016/j.cellimm.2008.04.002. 10.1016/j.cellimm.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Miyazaki A, Katagiri T, Yamamoto H, Idei T, Iguchi T. Relationship between serum insulin-like growth factor-1 levels and Alzheimer's disease and vascular dementia. Journal of the American Geriatrics Society. 2005;53(10):1748–53. doi: 10.1111/j.1532-5415.2005.53524.x. 10.1111/j.1532-5415.2005.53524.x. [DOI] [PubMed] [Google Scholar]
- Weigent DA. Lymphocyte GH-axis hormones in immunity. Cellular immunology. 2013;285(1-2):118–32. doi: 10.1016/j.cellimm.2013.10.003. 10.1016/j.cellimm.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Westwood AJ, Beiser A, Decarli C, Harris TB, Chen TC, He XM, Roubenoff R, Pikula A, Au R, Braverman LE, Wolf PA, Vasan RS, Seshadri S. Insulin-like growth factor-1 and risk of Alzheimer dementia and brain atrophy. Neurology. 2014;82(18):1613–9. doi: 10.1212/WNL.0000000000000382. 10.1212/WNL.0000000000000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Sun M, Zhang HP, Chen T, Wu R, Liu C, Yang G, Geng XR, Feng BS, Liu Z, Liu Z, Yang PC. Prolactin mediates psychological stress-induced dysfunction of regulatory T cells to facilitate intestinal inflammation. Gut. 2014;63(12):1883–92. doi: 10.1136/gutjnl-2013-306083. 10.1136/gutjnl-2013-306083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SD, Yan SF, Chen X, Fu J, Chen M, Kuppusamy P, Smith MA, Perry G, Godman GC, Nawroth P, et al. Non-enzymatically glycated tau in Alzheimer's disease induces neuronal oxidant stress resulting in cytokine gene expression and release of amyloid beta-peptide. Nature medicine. 1995;1(7):693–9. doi: 10.1038/nm0795-693. [DOI] [PubMed] [Google Scholar]
- Yarchoan M, Toledo JB, Lee EB, Arvanitakis Z, Kazi H, Han LY, Louneva N, Lee VM, Kim SF, Trojanowski JQ, Arnold SE. Abnormal serine phosphorylation of insulin receptor substrate 1 is associated with tau pathology in Alzheimer's disease and tauopathies. Acta neuropathologica. 2014;128(5):679–89. doi: 10.1007/s00401-014-1328-5. 10.1007/s00401-014-1328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S, Girnita A, Stromberg T, Khan Z, Andersson S, Zheng H, Ericsson C, Axelson M, Nister M, Larsson O, Ekstrom TJ, Girnita L. Targeting the insulin-like growth factor-1 receptor by picropodophyllin as a treatment option for glioblastoma. Neuro-oncology. 2010;12(1):19–27. doi: 10.1093/neuonc/nop008. 10.1093/neuonc/nop008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemva J, Schubert M. The role of neuronal insulin/insulin-like growth factor-1 signaling for the pathogenesis of Alzheimer's disease: possible therapeutic implications. CNS & neurological disorders drug targets. 2014;13(2):322–37. doi: 10.2174/18715273113126660141. [DOI] [PubMed] [Google Scholar]
- Zhang B, Tang XC, Zhang HY. Alternations of central insulin-like growth factor-1 sensitivity in APP/PS1 transgenic mice and neuronal models. Journal of neuroscience research. 2013;91(5):717–25. doi: 10.1002/jnr.23201. 10.1002/jnr.23201. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhao B, Yew DT, Kusiak JW, Roth GS. Processing of Alzheimer's amyloid precursor protein during H2O2-induced apoptosis in human neuronal cells. Biochemical and biophysical research communications. 1997;235(3):845–8. doi: 10.1006/bbrc.1997.6698. 10.1006/bbrc.1997.6698. [DOI] [PubMed] [Google Scholar]
- Zhao WQ, Lacor PN, Chen H, Lambert MP, Quon MJ, Krafft GA, Klein WL. Insulin receptor dysfunction impairs cellular clearance of neurotoxic oligomeric a{beta} The Journal of biological chemistry. 2009;284(28):18742–53. doi: 10.1074/jbc.M109.011015. 10.1074/jbc.M109.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]