Abstract
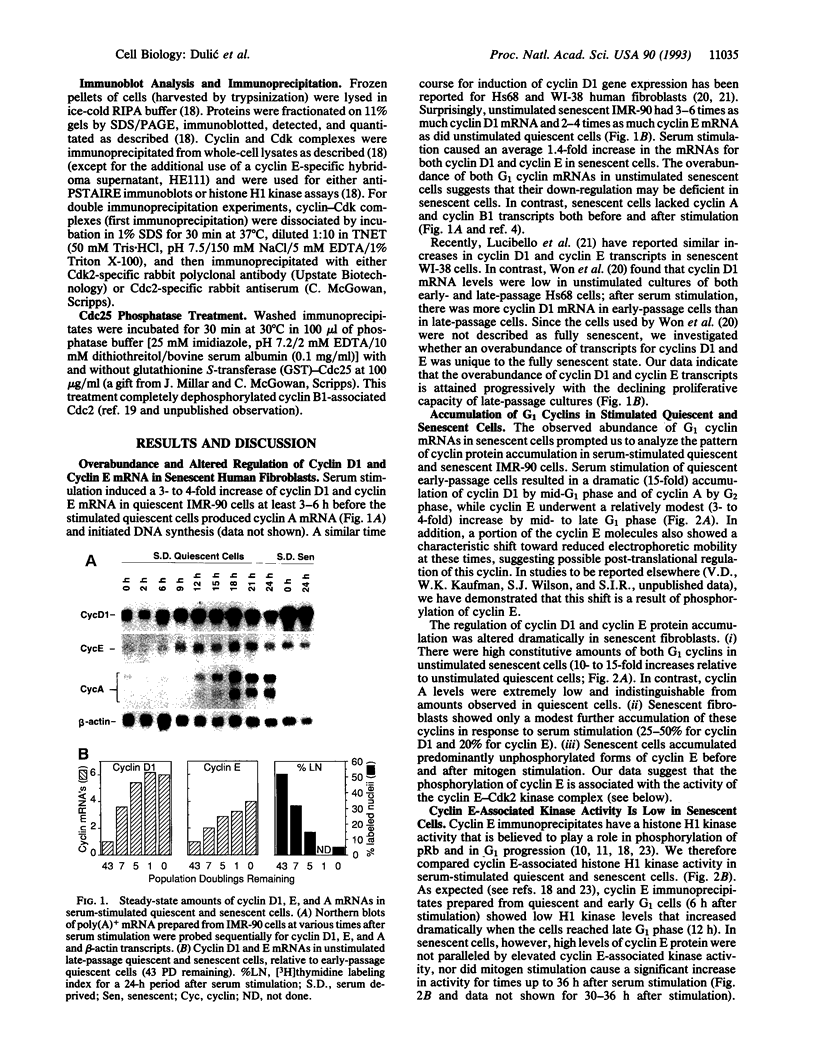
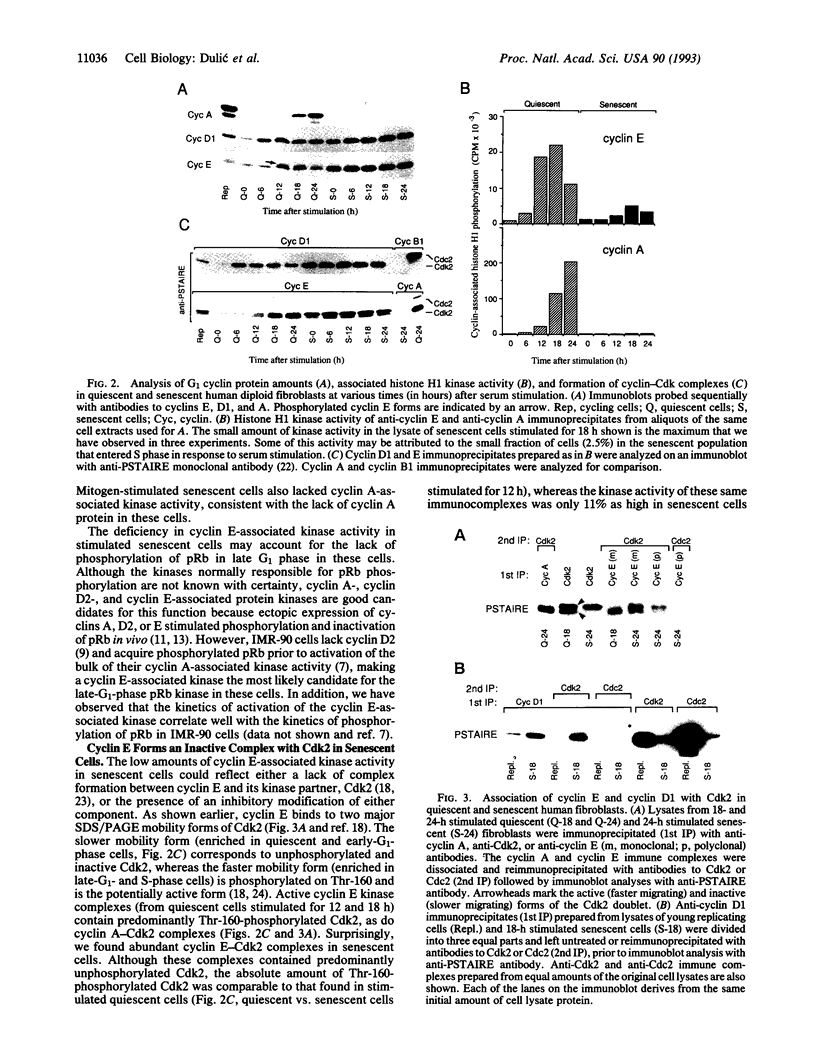
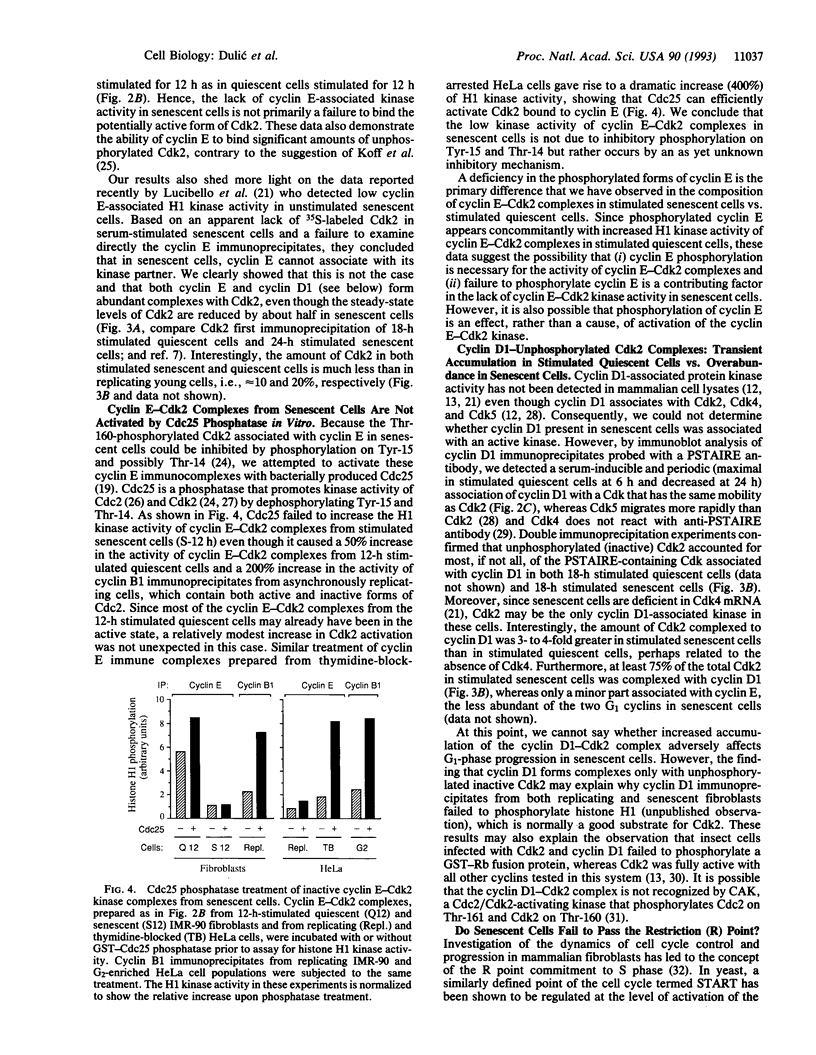
Senescent human diploid fibroblasts are unable to enter S phase in response to mitogenic stimulation. One of the key deficiencies in mitogen-stimulated senescent cells is their failure to phosphorylate the retinoblastoma protein, which acts as an inhibitor of entry into S phase in its unphosphorylated form. Recent data suggest that cyclin-dependent kinases (Cdks) regulated by G1 cyclins (D type and E) are responsible for the primary phosphorylation of the retinoblastoma protein prior to the G1/S boundary. Surprisingly, we found 10- to 15-fold higher constitutive amounts of both cyclin E and cyclin D1 in senescent cells compared to quiescent early-passage cells. Nevertheless, cyclin E-associated kinase activity in senescent cells was very low and did not increase significantly upon mitogenic stimulation even though cyclin E-Cdk2 complexes were abundant. In contrast to early-passage cells in late G1 phase, senescent cells contained mainly underphosphorylated cyclin E and proportionally more unphosphorylated and inactive Cdk2, perhaps accounting for the low kinase activity. We also show that a majority of the Cdk2 in senescent cells, but not in early-passage cells, was complexed with cyclin D1. Cyclin D1-Cdk2 complexes, severalfold enriched in senescent cells, contained exclusively unphosphorylated Cdk2. Amounts of cyclin A, which ordinarily accumulates in S and G2 phases, were extremely low in stimulated senescent cells. We suggest that the failure to activate cyclin E-Cdk2 kinase activity in senescent cells may account for the inability of these cells to phosphorylate the retinoblastoma protein in late G1 phase, which in turn may block the expression of late G1 genes such as cyclin A that are required for entry into S phase.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldin V., Lukas J., Marcote M. J., Pagano M., Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993 May;7(5):812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- Chang C. D., Phillips P., Lipson K. E., Cristofalo V. J., Baserga R. Senescent human fibroblasts have a post-transcriptional block in the expression of the proliferating cell nuclear antigen gene. J Biol Chem. 1991 May 15;266(14):8663–8666. [PubMed] [Google Scholar]
- Chang Z. F., Chen K. Y. Regulation of ornithine decarboxylase and other cell cycle-dependent genes during senescence of IMR-90 human diploid fibroblasts. J Biol Chem. 1988 Aug 15;263(23):11431–11435. [PubMed] [Google Scholar]
- Dowdy S. F., Hinds P. W., Louie K., Reed S. I., Arnold A., Weinberg R. A. Physical interaction of the retinoblastoma protein with human D cyclins. Cell. 1993 May 7;73(3):499–511. doi: 10.1016/0092-8674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- Dulić V., Lees E., Reed S. I. Association of human cyclin E with a periodic G1-S phase protein kinase. Science. 1992 Sep 25;257(5078):1958–1961. doi: 10.1126/science.1329201. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Kumagai A. The cdc25 protein contains an intrinsic phosphatase activity. Cell. 1991 Oct 4;67(1):189–196. doi: 10.1016/0092-8674(91)90582-j. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Sluss H. K., Sherr C. J., Matsushime H., Kato J., Livingston D. M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993 May 7;73(3):487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- Goldstein S. Replicative senescence: the human fibroblast comes of age. Science. 1990 Sep 7;249(4973):1129–1133. doi: 10.1126/science.2204114. [DOI] [PubMed] [Google Scholar]
- Gu Y., Rosenblatt J., Morgan D. O. Cell cycle regulation of CDK2 activity by phosphorylation of Thr160 and Tyr15. EMBO J. 1992 Nov;11(11):3995–4005. doi: 10.1002/j.1460-2075.1992.tb05493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwiger J. A., Wittenberg C., Richardson H. E., de Barros Lopes M., Reed S. I. A family of cyclin homologs that control the G1 phase in yeast. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6255–6259. doi: 10.1073/pnas.86.16.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley C. B., Futcher A. B., Greider C. W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990 May 31;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Hinds P. W., Mittnacht S., Dulic V., Arnold A., Reed S. I., Weinberg R. A. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992 Sep 18;70(6):993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- Koff A., Giordano A., Desai D., Yamashita K., Harper J. W., Elledge S., Nishimoto T., Morgan D. O., Franza B. R., Roberts J. M. Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science. 1992 Sep 18;257(5077):1689–1694. doi: 10.1126/science.1388288. [DOI] [PubMed] [Google Scholar]
- Koff A., Ohtsuki M., Polyak K., Roberts J. M., Massagué J. Negative regulation of G1 in mammalian cells: inhibition of cyclin E-dependent kinase by TGF-beta. Science. 1993 Apr 23;260(5107):536–539. doi: 10.1126/science.8475385. [DOI] [PubMed] [Google Scholar]
- Lew D. J., Dulić V., Reed S. I. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell. 1991 Sep 20;66(6):1197–1206. doi: 10.1016/0092-8674(91)90042-w. [DOI] [PubMed] [Google Scholar]
- Lucibello F. C., Sewing A., Brüsselbach S., Bürger C., Müller R. Deregulation of cyclins D1 and E and suppression of cdk2 and cdk4 in senescent human fibroblasts. J Cell Sci. 1993 May;105(Pt 1):123–133. doi: 10.1242/jcs.105.1.123. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Ewen M. E., Strom D. K., Kato J. Y., Hanks S. K., Roussel M. F., Sherr C. J. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell. 1992 Oct 16;71(2):323–334. doi: 10.1016/0092-8674(92)90360-o. [DOI] [PubMed] [Google Scholar]
- Meyerson M., Enders G. H., Wu C. L., Su L. K., Gorka C., Nelson C., Harlow E., Tsai L. H. A family of human cdc2-related protein kinases. EMBO J. 1992 Aug;11(8):2909–2917. doi: 10.1002/j.1460-2075.1992.tb05360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar J. B., McGowan C. H., Lenaers G., Jones R., Russell P. p80cdc25 mitotic inducer is the tyrosine phosphatase that activates p34cdc2 kinase in fission yeast. EMBO J. 1991 Dec;10(13):4301–4309. doi: 10.1002/j.1460-2075.1991.tb05008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992 Oct 16;258(5081):424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- Ohtsubo M., Roberts J. M. Cyclin-dependent regulation of G1 in mammalian fibroblasts. Science. 1993 Mar 26;259(5103):1908–1912. doi: 10.1126/science.8384376. [DOI] [PubMed] [Google Scholar]
- Pardee A. B. G1 events and regulation of cell proliferation. Science. 1989 Nov 3;246(4930):603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Poon R. Y., Yamashita K., Adamczewski J. P., Hunt T., Shuttleworth J. The cdc2-related protein p40MO15 is the catalytic subunit of a protein kinase that can activate p33cdk2 and p34cdc2. EMBO J. 1993 Aug;12(8):3123–3132. doi: 10.1002/j.1460-2075.1993.tb05981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch P. S. Regulation of human fibroblast growth rate by both noncycling cell fraction transition probability is shown by growth in 5-bromodeoxyuridine followed by Hoechst 33258 flow cytometry. Proc Natl Acad Sci U S A. 1983 May;80(10):2951–2955. doi: 10.1073/pnas.80.10.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. I. The role of p34 kinases in the G1 to S-phase transition. Annu Rev Cell Biol. 1992;8:529–561. doi: 10.1146/annurev.cb.08.110192.002525. [DOI] [PubMed] [Google Scholar]
- Rittling S. R., Brooks K. M., Cristofalo V. J., Baserga R. Expression of cell cycle-dependent genes in young and senescent WI-38 fibroblasts. Proc Natl Acad Sci U S A. 1986 May;83(10):3316–3320. doi: 10.1073/pnas.83.10.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian B., Kakizuka A., Hunter T. Cdc25M2 activation of cyclin-dependent kinases by dephosphorylation of threonine-14 and tyrosine-15. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3521–3524. doi: 10.1073/pnas.90.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri T., Campisi J. Repression of c-fos transcription and an altered genetic program in senescent human fibroblasts. Science. 1990 Jan 12;247(4939):205–209. doi: 10.1126/science.2104680. [DOI] [PubMed] [Google Scholar]
- Stein G. H., Beeson M., Gordon L. Failure to phosphorylate the retinoblastoma gene product in senescent human fibroblasts. Science. 1990 Aug 10;249(4969):666–669. doi: 10.1126/science.2166342. [DOI] [PubMed] [Google Scholar]
- Stein G. H., Drullinger L. F., Robetorye R. S., Pereira-Smith O. M., Smith J. R. Senescent cells fail to express cdc2, cycA, and cycB in response to mitogen stimulation. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11012–11016. doi: 10.1073/pnas.88.24.11012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G. H., Yanishevsky R. M., Gordon L., Beeson M. Carcinogen-transformed human cells are inhibited from entry into S phase by fusion to senescent cells but cells transformed by DNA tumor viruses overcome the inhibition. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5287–5291. doi: 10.1073/pnas.79.17.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won K. A., Xiong Y., Beach D., Gilman M. Z. Growth-regulated expression of D-type cyclin genes in human diploid fibroblasts. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9910–9914. doi: 10.1073/pnas.89.20.9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Zhang H., Beach D. D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell. 1992 Oct 30;71(3):505–514. doi: 10.1016/0092-8674(92)90518-h. [DOI] [PubMed] [Google Scholar]
- Yamashita M., Fukada S., Yoshikuni M., Bulet P., Hirai T., Yamaguchi A., Lou Y. H., Zhao Z., Nagahama Y. Purification and characterization of maturation-promoting factor in fish. Dev Biol. 1992 Jan;149(1):8–15. doi: 10.1016/0012-1606(92)90259-j. [DOI] [PubMed] [Google Scholar]
- Zambetti G., Dell'Orco R., Stein G., Stein J. Histone gene expression remains coupled to DNA synthesis during in vitro cellular senescence. Exp Cell Res. 1987 Oct;172(2):397–403. doi: 10.1016/0014-4827(87)90397-1. [DOI] [PubMed] [Google Scholar]