Abstract
Study Objectives:
To determine the effects of the nonbenzodiazepine sedative zopiclone on the threshold to arousal with increasing respiratory effort and genioglossus muscle activity and to examine potential physiological factors mediating disparate effects of zopiclone on obstructive sleep apnea (OSA) severity between patients.
Methods:
Twelve patients with OSA (apnea-hypopnea index = 41 ± 8 events/h) were studied during 2 single night sleep studies conducted approximately 1 w apart after receiving 7.5 mg of zopiclone or placebo according to a double-blind, placebo-controlled, randomized, crossover design. The respiratory arousal threshold (epiglottic pressure immediately prior to arousal during naturally occurring respiratory events), genioglossus activity and its responsiveness to pharyngeal pressure during respiratory events, and markers of OSA severity were compared between conditions. Genioglossus movement patterns and upper airway anatomy were also assessed via magnetic resonance imaging in a subset of participants (n = 7) during wakefulness.
Results:
Zopiclone increased the respiratory arousal threshold versus placebo (−31.8 ± 5.6 versus −26.4 ± 4.6 cmH2O, P = 0.02) without impairing genioglossus muscle activity or its responsiveness to negative pharyngeal pressure during respiratory events (−0.56 ± 0.2 versus −0.44 ± 0.1 %max/-cmH2O, P = 0.48). There was substantial interindividual variability in the changes in OSA severity with zopiclone explained, at least in part, by differences in pathophysiological characteristics including body mass index, arousal threshold, and genioglossus movement patterns.
Conclusions:
In a group of patients with predominantly severe OSA, zopiclone increased the arousal threshold without reducing genioglossus muscle activity or its responsiveness to negative pharyngeal pressure. These properties may be beneficial in some patients with OSA with certain pathophysiological characteristics but may worsen hypoxemia in others.
Clinical Trial Registration:
Australian New Zealand Clinical Trials Registry, http://www.anzctr.org.au, trial ID: ACTRN12614000364673.
Citation:
Carter SG, Berger MS, Carberry JC, Bilston LE, Butler JE, Tong BK, Martins RT, Fisher LP, McKenzie DK, Grunstein RR, Eckert DJ. Zopiclone increases the arousal threshold without impairing genioglossus activity in obstructive sleep apnea. SLEEP 2016;39(4):757–766.
Keywords: hypnotic, respiratory physiology, sedative, sleep disordered breathing, upper airway physiology
Significance.
Sedative use in obstructive sleep apnea (OSA) has traditionally been contraindicated due to concerns of upper-airway muscle relaxation and apnea prolongation leading to worsening hypoxemia. However, in accordance with recent advances in the understanding of OSA pathophysiology, some sedatives yield reductions in OSA severity in certain patients whereas other patients do indeed experience worsening hypoxemia. The current study represents the first investigation to examine the effects of any common sedative on pharyngeal muscle activity without continuous positive airway pressure. Contrary to previous beliefs, zopiclone increased the threshold for arousal without impairing genioglossus muscle activity. The detailed physiological assessments performed also provide insight into the key factors contributing to between-patient variability in OSA severity in response to sedatives such as zopiclone.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common sleep related respiratory disorder. Untreated OSA is associated with major adverse health and quality-of-life outcomes. In addition to anatomical vulnerability, there are several nonanatomical pathophysiological contributors to OSA, including a high propensity for arousal (low threshold to arousal with increasing respiratory effort), respiratory control instability (high loop gain), poor responsiveness of the upper airway dilator muscles to airway narrowing during sleep and inadequate or dysfunctional motion of the upper airway dilators.1,2 Anatomical and nonanatomical contributions to OSA vary widely between patients.1 Common sedatives may reduce OSA severity in some patients and worsen OSA in others.3–6 Differential responses likely depend on differences between patients in underlying pathophysiology including the threshold to arousal with increasing respiratory effort (respiratory arousal threshold), dilator muscle activity and movement patterns, upper airway anatomy, and responses to different drug classes.1–5,7,8
Sedative use is high in the general population, particularly among the elderly and obese.9 Aging and obesity are major risk factors for OSA.10 Accordingly, it is important to determine the effects of common sedatives on the key causes of OSA and to identify physiological characteristics to help differentiate between those in whom certain sedatives may be beneficial versus those in whom sedatives may be harmful. However, very few studies have systematically examined the effects of common sedatives on upper airway physiology or OSA severity. Trazo-done, a serotonin antagonist and reuptake inhibitor with sedative properties, triazolam, a benzodiazepine sedative, and eszopiclone, a nonbenzodiazepine sedative, increase the respiratory arousal threshold by 24% to 50% in patients with OSA.4,5,11,12 Increasing the respiratory arousal threshold can promote breathing stability for patients who wake up easily (low respiratory arousal threshold) but can prolong respiratory events and worsen hypoxemia in those with a high arousal threshold.3,5,11
A major concern regarding the use of sedatives in OSA is their potential to impair upper airway muscle activity. Indeed, benzodiazepine sedatives can reduce neural drive to the upper airway dilator muscles in cats13 and in healthy older individuals during CO2 rebreathing while awake.14 In contrast, a more recent study showed that systemic administration of zolpidem (a nonbenzodiazepine sedative) and lorazepam (a benzodiazepine sedative) can have excitatory influences on baseline activity of the largest upper airway dilator muscle, genioglossus, in rats.15 Similarly, recent studies in patients with OSA indicate that trazodone and zopiclone do not impair genioglossus electromyographic (EMG) activity.4,16 However, these studies were conducted while patients received continuous positive airway pressure (CPAP), which inhibits dilator muscle tone.17 No studies have examined the effects of any common sedative on upper airway muscle activity during sleep in the absence of CPAP in humans.
Current evidence indicates that standard doses of common sedatives do not systematically alter OSA severity in unselected patients as measured by the apnea-hypopnea index (AHI).3,4,11,18–21 However, there is wide interindividual variability. Extremely high doses or administration in patients with very severe OSA can worsen overnight hypoxemia.11,22 In contrast, a standard dose of eszopiclone (3 mg) reduces OSA severity without worsening hypoxemia in patients with OSA who have a low respiratory arousal threshold.5 Eszopiclone is the stereoisomer of zopiclone and 3 mg of eszopiclone is believed to be pharmacologically equivalent to 7.5 mg of zopi-clone. However, next-day performance following standard doses of eszopiclone may be better than zopiclone, suggesting overnight sedation effects may also differ.23 Whether standard doses of these two agents have equivalent effects on upper airway physiology, the respiratory arousal threshold, and OSA severity remains unknown. Indeed, no studies have examined the effects of zopiclone on the respiratory arousal threshold or OSA severity. Accordingly, the primary aim of this study was to determine the effects of a standard dose of zopiclone on the respiratory arousal threshold in patients with OSA. Secondary aims were to determine the effects of zopiclone on genioglossus muscle activity during sleep in the absence of CPAP and to explore potential physiological factors mediating disparate effects of zopiclone on OSA severity between patients.
METHODS
Participants
Fourteen otherwise healthy patients with OSA (≥ 5 respiratory events/h sleep) aged 18–65 y who responded to flyers to participate in sleep research were recruited. Patients were excluded if their overnight mean oxygen saturation (SaO2) nadir was ≤ 70% at baseline; were taking any medication that may affect breathing, sleep, or muscle activity; were pregnant or nursing mothers; or if they had any known allergy to zopiclone. Patients with no contraindications for magnetic resonance imaging (MRI) were invited to participate in the MRI component of the study. The study was conducted according to the principles of the Declaration of Helsinki, was approved by the University of New South Wales Human Research Ethics Committee, and was prospectively registered on the Australian New Zealand Clinical Trials Registry (ACTRN12614000364673).
Measurements and Equipment
Polysomnography
Electroencephalograms, electrooculograms, surface submentalis electromyograms, pulse oximetry, position (infrared video monitoring), airflow via a nasal mask attached to a pneumotachograph open to atmospheric air, and mask pressure via a differential pressure transducer were recorded for sleep staging and scoring cortical arousals and respiratory events. End-tidal carbon dioxide was assessed via a port in the nasal mask during wakefulness.
Upper Airway Physiology Measures
Nasal decongestant was applied to both nostrils. The less obstructed nostril was anesthetized with lignocaine and an epiglottic pressure catheter was advanced 1 to 2 cm intranasally below the base of the tongue.5
Bipolar EMG recordings of the genioglossus muscle were obtained from two fine-wire Teflon coated intramuscular electrodes. Following surface anesthesia with lignocaine, the electrodes were inserted 10 to 15 mm into the genioglossus muscle perorally via a 25-gauge needle.4 EMG data were acquired at a sample rate of 2,000 Hz and filtered at 20 to 500 Hz.
Magnetic Resonance Imaging
Spatial Modulation of Magnetization (SPAMM) was employed using a 3T MRI scanner in eligible participants to track genioglossus motion during quiet breathing while awake, similar to methodology described previously.2,7,8 Briefly, a grid of tags (saturated magnetization) was superimposed onto the upper airway structures in the midsagittal plane. This allowed for muscle displacement to be tracked during inspiration. Anatomical scans of the upper airway were also conducted to determine the cross sectional area of the velopharynx.
Study Design and Protocol
A randomized, double-blind, crossover design was utilized. Participants were studied overnight on two separate occasions approximately 1 w apart. Patients received either 7.5 mg of zopiclone or placebo orally immediately prior to sleep (Figure 1). For each visit, the patient arrived 3 to 4 h before lights-out and was fitted with the recording equipment. On one occasion, MRI was conducted shortly after arrival.
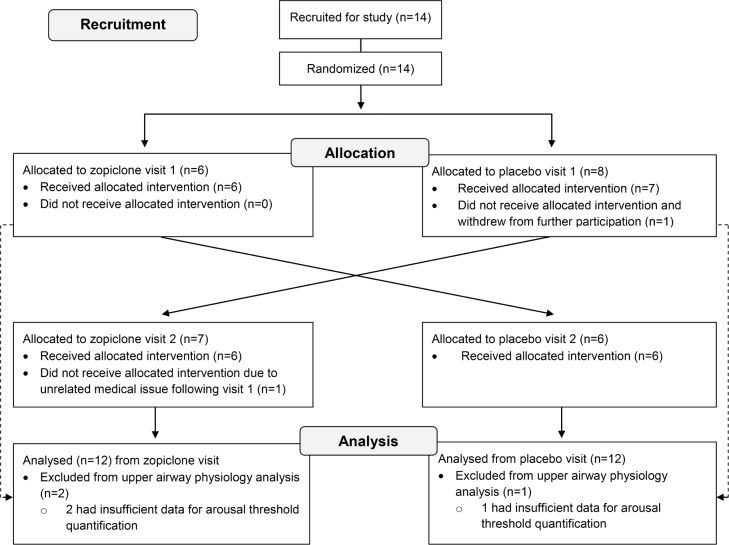
Figure 1.
CONSORT diagram indicating the recruitment, randomization, and analysis procedures for this double-blind, placebo-controlled, crossover study. Initially, patients with obstructive sleep apnea with a confirmed diagnosis responded to a research advertisement. Eligible patients (apnea-hypopnea index ≥ 5 events/h of sleep, overnight nadir arterial blood oxygen saturations > 70% who were otherwise healthy) were randomized to the allocation order (placebo first or 7.5 mg of zopiclone first). Participants attended two in-laboratory sleep studies at which time the allocated intervention was administered just prior to sleep during each visit. One of the 14 participants enrolled withdrew on the first night because of discomfort with the study equipment. Another participant discontinued involvement after their first sleep study due to an unrelated medical issue.
Prior to sleep, participants were also asked to perform maneuvers including swallows and tongue protrusions against the back of their top teeth under verbal encouragement in order to determine maximal genioglossus EMG.4 Genioglossus EMG activity and minute ventilation were also determined prior to receiving the study intervention during 5 min of quiet breathing awake immediately preceding lights out. Patients were then monitored overnight for 8 h. Participants were instructed to sleep on their back as much as possible. Sleepiness was assessed via the Karolinska Sleepiness Scale24 30 min after waking during each visit.
Data Analysis
All analyses were conducted blinded to the study intervention. Sleep staging for the overnight polysomnography recordings was performed by an experienced sleep technician. Respiratory events and the onset and duration of arousals were scored according to the American Academy of Sleep Medicine recommended criteria.25 Wakefulness data was analyzed on a breath-by-breath basis using custom semiautomated analysis scripts.4
A minimum of six and up to 20 arousals elicited by naturally occurring apneas or hypopneas throughout each overnight sleep study were selected at random for analysis in order to determine each patient's respiratory arousal threshold as described previously.5 Each patient's respiratory arousal threshold was quantified as the average nadir in epiglottic pressure immediately preceding arousal5 (Figure 2).
Figure 2.
Polysomnographic recordings in an individual patient demonstrating how the key upper airway physiology measures were obtained. This example shows an approximately 60-sec epoch of stage N2 sleep on zopiclone in which the 50-y-old male patient (apnea-hypopnea index = 77 events/h sleep; body mass index = 34 kg/m2) experienced an approximately 35-sec respiratory event that ended with an arousal from sleep (gray shaded portion of the electroencephalogram [EEG]). The respiratory arousal threshold is quantified as nadir epiglottic pressure (Pepi) on the effort immediately prior to arousal (in this example approximately −35 cmH2O). Note the increasing effort on Pepi up until arousal despite no airflow during the respiratory event (thick dashed line). The thin dashed line indicates increasing genioglossus muscle activity during the respiratory event. Boxes indicate the first and last efforts used for genioglossus muscle analysis. Nasal flow, nasal airflow measured via mask and pneumotachograph; Processed GG EMG, raw genioglossus EMG rectified and moving time averaged (100 msec); Raw GG EMG, unprocessed raw genioglossus EMG; Peak GG, maximum genioglossus EMG during inspiration; Tonic GG, nadir genioglossus EMG during expiration. Refer to the text for further details.
Genioglossus EMG activity was rectified, moving-time averaged (100 msec), and expressed as a percentage of maximal EMG during a tongue protrusion or swallow during wakefulness as described previously.4 Genioglossus EMG activity was also quantified in absolute units (μV) and as a percentage of baseline EMG during wakefulness. Peak (maximum during inspiration) and tonic EMG (nadir during expiration) were compared between conditions during the respiratory events selected for arousal threshold determination4 (Figure 2). Genioglossus EMG responsiveness to negative epiglottic pressure was quantified as the average slope between peak EMG versus nadir epiglottic pressure in each patient on the first versus last respiratory effort immediately prior to arousal for each respiratory event used for arousal threshold determination during N2 and N3 nonrapid eye movement (NREM) sleep4 (Figure 2).
Genioglossus muscle movement patterns and upper airway cross-sectional area during wakefulness were quantified using MRI according to previously published criteria.2,7,8 Briefly, genioglossus movement patterns were analyzed from three artefact-free inspiratory efforts. Tongue motion was measured by placing four to six markers (depending on tongue size) 8 mm apart in a vertical line approximately 8 mm anterior to the pharyngeal wall. The most superior marker was placed adjacent to the narrowest section of the velopharynx and the most inferior marker was placed adjacent to the superior tip of the epiglottis. For each individual, tongue movement was grouped into one of four different movement patterns based on previously described movement patterns.2 Briefly, tongue movement patterns included: (1) anterior movement – en bloc anterior movement > 1 mm, (2) oropharyngeal – minimal movement (< 1 mm) in the nasopharyngeal region with > 1 mm anterior movement in the oropharyngeal region, (3) counterproductive movement – posterior movement > 1 mm in either the nasopharynx or oropharynx, and (4) minimal movement – no movement > 1 mm. The nadir cross-sectional area of the velopharynx was quantified using Image J (National Institute of Mental Health, Bethesda, MD, USA).
Statistical Comparisons
The primary outcome was change in respiratory arousal threshold with zopiclone compared to placebo. Our target sample size of 12 was based on a between-night difference in the standard deviation of the arousal threshold of 4.5 cmH2O4,5 to detect a physiologically and clinically relevant change in arousal threshold of 4 cmH2O3,5 with > 80% power. For each outcome variable, where the distribution of the change between placebo and zopiclone was normally distributed (Shapiro– Wilk), statistical comparisons were performed using paired Student t-tests. Nonnormally distributed data were compared using a Wilcoxon signed-rank test. P < 0.05 was considered significant. Data are reported as mean ± standard error of the mean or medians with interquartile range (IQ range) for non-normally distributed variables unless otherwise stated.
RESULTS
Anthropometric Characteristics
Of the 14 participants recruited, 12 successfully completed both nights of the protocol (Figure 1). The mean age, body mass index, AHI, and Epworth Sleepiness Scale scores are reported in Table 1.
Table 1.
Patient anthropometric and sleep characteristics.

Upper Airway Physiology Measures
Two participants (one male) with mild OSA yielded insufficient arousal threshold and genioglossus muscle data during sleep for analysis (< 6 artefact-free events) in at least one of the study arms. Of the remaining 10 subjects, minute ventilation and genioglossus EMG activity during wakefulness prior to receiving the tablet were not different between placebo and zopi-clone (Table 2). The respiratory arousal threshold increased by 19 ± 8% during the zopiclone arm compared to placebo (Figure 3, P = 0.02). The respiratory arousal threshold was not different during the first compared to the second half of the night during the placebo (−25 ± 5 versus −28 ± 5, P = 0.14) or zopiclone nights (−32 ± 6 versus −32 ± 5, P = 0.86) but was higher during zopiclone versus placebo for both the first half (P = 0.01) and the second half of the night (P = 0.04).
Table 2.
Wakefulness minute ventilation and genioglossus activity during quiet breathing.

Figure 3.

Respiratory arousal threshold scatter plots representing each individual patient's arousal threshold during nonrapid eye movement sleep (NREM) during the placebo and zopiclone conditions (n = 10 patients with obstructive sleep apnea). Values and error bars presented adjacent to each condition are mean ± standard error of the mean. The asterisk indicates a statistically significant difference versus placebo.
Genioglossus muscle activity and responsiveness to increasing negative epiglottic pressure during respiratory events was not different between zopiclone and placebo conditions (Table 3, Figure 4). This was true for peak and tonic EMG on the first effort during respiratory events regardless of the units of measurement (Table 3). Similarly, peak genioglossus EMG activity during the effort immediately prior to arousal during respiratory events was not different between placebo versus zopiclone (Figure 5). Peak genioglossus EMG on the breath prior to arousal was higher compared to quiet breathing awake but also did not differ significantly between placebo and zopi-clone conditions (161.0 [IQ range 97.9 to 281.8] versus 204.5 [IQ range 128.0 to 675.6] % activity awake, P = 0.43).
Table 3.
Genioglossus muscle activity during the first effort of respiratory events.
Figure 4.

Genioglossus muscle responsiveness scatterplots representing each individual patient's slope of genioglossus muscle responsiveness (as a % of maximal activation) versus negative epiglottic pressure during respiratory events during placebo versus zopiclone (n = 10 patients with obstructive sleep apnea). n = 2 patients had values closer to zero that overlapped indicating poor muscle responsiveness. Values and error bars presented adjacent to each condition are mean ± standard error of the mean. Zopiclone did not impair genioglossus muscle responsiveness. Refer to the text for further details.
Figure 5.
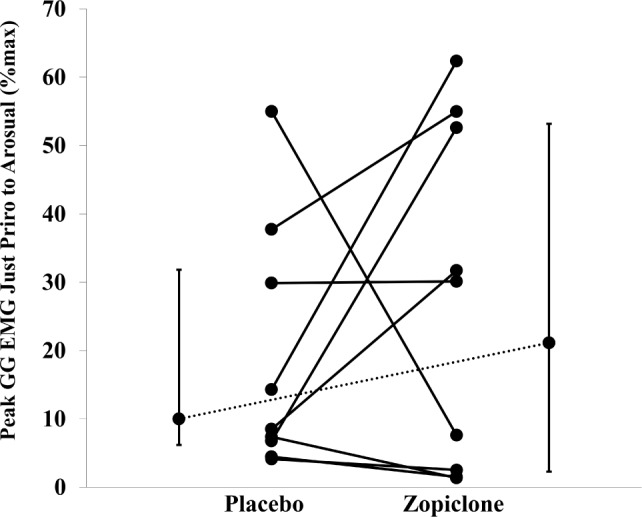
Peak genioglossus (GG) muscle activation (as a % of maximal activation) scatterplots representing each individual patient's muscle activation on the effort immediately prior to arousal during placebo versus zopiclone (n = 10 patients with obstructive sleep apnea). Values and error bars presented adjacent to each condition are median and interquartile range. Zopiclone did not impair genioglossus muscle activity immediately prior to arousal. EMG, electromyography. Refer to the text for further details.
OSA Severity and Sleep Parameters
Table 4 summarizes group polysomnographic data with and without zopiclone. Sleep onset latency was short and did not change with zopiclone. Sleep efficiency tended to increase with zopiclone, although this difference was not statistically significant compared to placebo. Patients tended to have proportionally more N2 and less N1 sleep with zopiclone, although these differences were not statistically significant. The percentage of rapid eye movement (REM) and N3 sleep was not different between placebo versus zopiclone.
Table 4.
Group polysomnography sleep parameters.

Zopiclone did not systematically alter the total AHI (Figure 6, P = 0.44), NREM, and REM AHI, the duration of respiratory events, or the arousal index. The obstructive apnea index during placebo versus zopiclone also was not different (33 ± 8 versus 33 ± 8, P = 0.94) nor was the hypopnea index (8 ± 3 versus 10 ± 3, P = 0.31). Participants spent the majority of the night sleeping supine with no difference between conditions or in the supine AHI. The AHI was not different during the first compared to the second half of the night during the placebo (43 ± 9 versus 39 ± 8, P = 0.47) or zopiclone nights (47 ± 9 versus 40 ± 7, P = 0.33) or between placebo and zopi-clone in the first half (P = 0.22) versus the second half of the night (P = 0.74). The NREM supine AHI was also not different during the first compared to the second half of the night during the placebo (46 ± 8 versus 42 ± 8, P = 0.21) or zopiclone nights (47 ± 10 versus 51 ± 9, P = 0.39) or between placebo and zopi-clone in the first half (P = 0.95) versus the second half of the night (P = 0.13). Mean cutaneous SaO2 during sleep decreased by approximately 1% with zopiclone. Nadir SaO2 tended to decrease and the percent sleep time spent below a SaO2 of 90% also tended to increase with zopiclone, although these differences were not statistically significant. Next-day sleepiness was low and not different between conditions (Table 4). Key individual patient polysomnographic and physiological parameters during placebo versus zopiclone are shown in Table 5.
Figure 6.
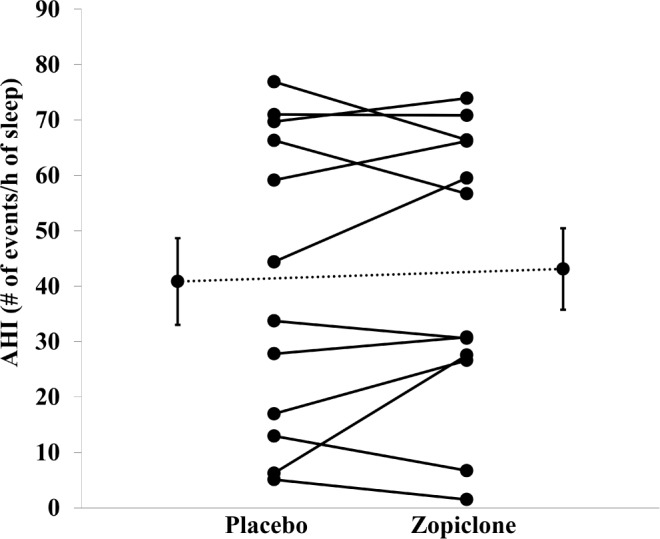
Apnea-hypopnea index (AHI) scatterplots representing each individual patient's value during placebo versus zopiclone (n = 12 patients with obstructive sleep apnea). Values and error bars presented adjacent to each condition are mean ± standard error of the mean. Zopiclone did not systematically alter the AHI.
Table 5.
Individual patient polysomnographic and physiology parameters during placebo versus zopiclone.

Magnetic Resonance Imaging
Of the 12 participants who took part in both arms of the study, seven completed the MRI protocol. Three were excluded due to MRI contraindications. Two participants had suspected metal shards in their eyes, another had a body habitus that was not compatible with the MRI scanner and two additional participants declined to undergo the MRI. Of the seven participants who were imaged, five had minimal genioglossus movement, one had counterproductive movement, and one had advantageous movement during inspiration. The velopharyngeal cross-sectional areas were small in all participants (Table 5).
DISCUSSION
The main findings of this detailed physiology study are that, on average, 7.5 mg of zopiclone taken immediately prior to sleep increases the respiratory arousal threshold by over 5 cmH2O without impairing genioglossus muscle activity or its responsiveness to increasing negative pharyngeal pressure during sleep in unselected patients with predominantly severe OSA. Furthermore, there is substantial between-patient variability in the response to zopiclone explained, at least in part, by differences in upper airway physiology as discussed in the next paragraphs.
Respiratory Arousal Threshold
An approximately 20% increase in the respiratory arousal threshold with 7.5 mg of zopiclone is comparable to 3 mg of its stereoisomer, eszopiclone, in which an approximately 30% increase occurred.5 Thus, standard doses of these two drugs yield similar increases in the respiratory arousal threshold. An increase in arousal threshold of this magnitude is also similar to that observed with standard doses of other commonly used sedatives.3 In patients with very severe OSA, triazolam increases the respiratory arousal threshold by approximately 20%11 and trazodone by approximately 30% in patients with OSA selected on the basis of a low arousal threshold.4
Genioglossus Muscle Activity
The current study is the first to examine the effects of any common sedative on upper airway muscle activity in humans during sleep without CPAP. This is important because administration of CPAP minimizes negative pharyngeal pressure and substantially reduces upper airway muscle activity.17,26 The findings of no impairment in either genioglossus EMG or its responsiveness to increasing negative pharyngeal pressure during respiratory events, even in patients with severe OSA, is consistent with a lack of effect on OSA severity. It is also consistent with prior studies of zopiclone and trazodone in patients on therapeutic CPAP in response to transient reductions in CPAP.4,16 Indeed, genioglossus muscle activity tended to increase rather than decrease with zopiclone in the current study. However, a larger sample size is required to determine if this is the case definitively. Genioglossus activity during wakefulness prior to the administration of the study intervention was similar between conditions in the current study. Thus, the absence of muscle impairment with zopiclone cannot be attributed to between-night physiological differences in muscle recordings.
OSA Severity and Sleep Parameters: Group Findings
This is the first study to examine the effects of zopiclone on OSA severity. Consistent with prior studies investigating the effects of common sedatives on OSA severity in unselected OSA patients,11,18–22 a single 7.5-mg dose of zopiclone does not systematically alter the AHI compared to placebo. Indeed, the majority of polysomnographic parameters used to assess sleep also do not systematically change with zopiclone. Consistent with these findings, perceived next-day sleepiness measured by the Karolinska Sleepiness Scale was low on placebo in the current study and did not increase with zopiclone. Although most prior sedative studies in OSA did not assess sleepiness, the current findings are consistent with the lack of next-day residual effects on alertness and concentration with the melatonin receptor agonist ramelteon20 or perceived sleepiness with the benzodiazepine nitrazepam.18
Similar to prior sedative studies in OSA,5,18,21 zopiclone tends to increase sleep efficiency with a reduction in N1 and an increase in N2 sleep. However, zopiclone caused a modest approximately 1% reduction in mean SaO2 during sleep, which may have been caused by mild hypoventilation. However, the magnitude of this effect is unlikely to be clinically important in most patients with OSA in the absence of confounding disease pathology (e.g., chronic obstructive pulmonary disease or obesity hypoventilation syndrome). Of the previous sedative studies that report mean SaO2, a similar magnitude decrease occurred at a very high dose of zolpidem and with flurazepam.22 Conversely, eszopiclone, trazodone, and ramelteon do not significantly alter mean SaO2.4,5,20 Thus, at a group level, common sedatives do not appear to cause major changes in overnight SaO2 in OSA. However, caution is warranted. Although the current study involved a comparable or larger number of participants to many of the prior sedative studies on OSA,4,11,18,22 it was not designed to assess the effects of zopiclone on AHI or other polysomnographic variables at the group level for which a larger sample size is required. Nonetheless, as discussed in the next paragraphs, there is wide interindividual variability on the AHI and other sleep parameters in response to zopiclone.
Individual Differences
In accordance with the high degree of between-patient variability in changes in AHI and hypoxia in prior sedative studies,3–5,11,18–22 we anticipated that individual responses to zopiclone would also vary widely and that varied responses may be explained by differences in certain underlying anthropometric and physiological characteristics. An earlier study indicated that the AHI decreases invariably with eszopiclone in patients with OSA who have a low arousal threshold (≥ −15 cmH2O).5 Only two patients in the current study had a low arousal threshold (Patients 1 and 9, Table 5), but they had contrasting changes in AHI (Patient 1 decreased, Patient 9 increased).
Patient 1 had severe OSA and a modest ∼3 cmH2O increase in arousal threshold with zopiclone. Nonetheless, his arousal threshold remained low after administration of zopiclone (∼ −15 cmH2O). The 9 events/h sleep reduction in OSA severity that occurred with zopiclone in this patient was likely driven by the 50% decrease in time spent supine versus placebo. Indeed, body position is a key determinant of OSA severity.27,28 Upper airway collapsibility increases by 2.2 cmH2O from lateral to supine.29 Given that impaired upper airway anatomy/increased collapsibility is the dominant cause of OSA,30 changes in AHI due to changes in sleeping posture may outweigh any smaller effects of altering nonanatomical factors such as the respiratory arousal threshold.
Despite Patient 9 having the lowest arousal threshold, this patient went from having clinically mild to almost severe OSA with zopiclone. However, this patient spent 14% more time supine during the zopiclone night. This may have been facilitated, at least in part, by the 9% increase in sleep efficiency with zopiclone in this individual. Zopiclone also had no quantifiable effect on the respiratory arousal threshold, which was very low at ∼8 cmH2O. Additionally, genioglossus movement patterns during quiet breathing awake have been shown to relate to OSA as measured via the AHI.2 This patient had large counterproductive genioglossus movement during inspiration, leading to airway narrowing during wakefulness. This may be caused by non-uniform neuropathy of the genioglossus, leading to uncoordinated tongue movement.2 Increased neural drive to genioglossus combined with counterproductive genioglossus motion during wakefulness is predicted to promote velopharyngeal closure rather than airway dilatation during sleep.31
Thus, arousal threshold alone does not explain between-patient variability in response to zopiclone. Rather, as indicated in Table 5, multiple other physiological modifiers of OSA severity and anatomical factors and the extent to which zopiclone alters each of these traits also likely contribute. Differences in individual absorption rates of zopiclone may contribute to the observed between-patient variability in response to zopiclone. Zopiclone pharmacokinetics may lead to time-of-night differences in sleep and the upper airway physiology parameters measured in this study, although arousal threshold and AHI were not systematically different during the first half compared to the second half of the night.
Typically, the use of a sedative in a patient with very severe OSA would be contraindicated. In Patient 10, one of three patients we studied who had very severe OSA (≥ 70 events/h sleep), zopiclone appeared to have no effect on OSA severity or any of the traits measured. This was despite Patient 10 having a narrow velopharyngeal airway and minimal genioglossus movement awake. Patient 12 had a very modest four events/h sleep increase in AHI and a high arousal threshold that increased further with zopiclone. However, muscle responsiveness was poor at baseline and remained poor with zopiclone. This combination would not be expected to reduce OSA severity with zopiclone because increasing the arousal threshold in the absence of effective dilator muscle recruitment is unlikely to be effective in restoring airflow. Despite Patient 11 experiencing an 11 events/h sleep reduction in AHI with zopiclone, mean overnight SaO2 decreased by 5% and nadir SaO2 decreased considerably. An increase of an already high arousal threshold with zopiclone likely mediated the considerable increase in respiratory event duration (27 to 41 sec) and consequently more pronounced hypoxemia. This is consistent with previous findings in patients with severe OSA with triazolam.11 Other distinguishing factors for this patient were that he had the lowest nadir SaO2 at baseline and the highest BMI of the very severe group, the latter being a key factor mediating susceptibility to nocturnal hypoxemia in OSA.32
Limitations
Participants were instructed to sleep supine. However, if participants moved to the lateral position during the night we did not intervene because we did not want to disrupt sleep. Accordingly, some participants spent considerably more time on their back in one condition than the other. This was likely a key determinant mediating some of the between-night variability in OSA severity. Nonetheless, on average, participants spent 90% or more of the night supine during both conditions. Thus, systematic differences in body position are unlikely to explain the observed differences in our primary outcome measure, arousal threshold.
The majority of the participants had severe OSA and only two had a low respiratory arousal threshold. Thus, the effects of zopiclone in patients with mild OSA and in those with a low arousal threshold require further investigation. Finally, as stated, although the current number of participants studied is similar to many of the prior sedative studies in OSA,4,11,18,22 a larger sample size is required to assess the effects of zopiclone on OSA severity definitively. Nonetheless, the current findings represent the first study to carefully examine the effects of zopiclone on upper airway physiology and OSA severity. The detailed physiological investigations performed also provide novel insight into key factors contributing to between-patient differences in OSA severity with zopiclone that will help to inform future sedative studies in OSA and clinical decision making.
CONCLUSIONS
Zopiclone increases the respiratory arousal threshold without any evidence of systematic impairment in genioglossus muscle activity or its responsiveness to negative pharyngeal pressure during sleep or next day sleepiness, even in patients with predominantly severe OSA. However, zopiclone decreases mean overnight SaO2 by 1%, with much larger reductions in certain individuals. Consistent with the heterogeneity of OSA patho-physiology, the between-patient variability in response to zopi-clone reinforces the need to develop simple, accurate tools to predict which patients with OSA respond favorably and those whose condition is likely to worsen with a sedative. These findings challenge previous notions and provide novel insight into the role of sedatives on upper airway physiology and OSA severity and the potential mediating factors. Given the high rates of sedative use in the community, especially in the elderly and those with obesity, these findings have important implications.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was funded by the National Health and Medical Research Council (NHRMC) of Australia (1042493), an Australasian Sleep Association Rob Pierce Grant-in-Aid, and an Australian Lung Foundation/Ludwig Engel Grant-in-Aid for Physiological Research. S.G.C. is supported by a NHMRC NeuroSleep CRE top-up scholarship (1060992). Dr. Eckert is supported by a NHMRC R.D. Fellowship (1049814). The authors have indicated no financial conflicts of interest. The work was performed at the Neuroscience Research Australia (NeuRA), Randwick, Sydney, New South Wales, Australia.
ACKNOWLEDGMENTS
The authors thank Professors Simon Gandevia and Robert Herbert for coordinating the randomization process for the study. Author Contributions: Dr. Eckert was responsible for the study conception and experimental design. Ms. Carter, Mr. Berger, and Dr. Eckert contributed to data collection, analysis, and interpretation. Mr. Tong and Dr. Carberry contributed to data collection and analysis. Dr. Martins contributed to data collection. Drs. Butler and Grunstein contributed to data interpretation. Ms. Fisher contributed to data collection, analysis, and participant recruitment. Dr. McKenzie contributed to participant recruitment and data interpretation. Dr. Bilston oversaw design of the MRI component of the study and contributed to data analysis and interpretation. Dr. Eckert, Ms. Carter, and Mr. Berger wrote the draft version of the manuscript. All authors contributed to the final version.
REFERENCES
- 1.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown EC, Cheng S, McKenzie DK, Butler JE, Gandevia SC, Bilston LE. Respiratory movement of upper airway tissue in obstructive sleep apnea. Sleep. 2013;36:1069–76. doi: 10.5665/sleep.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckert DJ, Younes MK. Arousal from sleep: implications for obstructive sleep apnea pathogenesis and treatment. J Appl Physiol. 2014;116:302–13. doi: 10.1152/japplphysiol.00649.2013. [DOI] [PubMed] [Google Scholar]
- 4.Eckert DJ, Malhotra A, Wellman A, White DP. Trazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal threshold. Sleep. 2014;37:811–9. doi: 10.5665/sleep.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckert DJ, Owens RL, Kehlmann GB, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond) 2011;120:505–14. doi: 10.1042/CS20100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smales ET, Edwards BA, Deyoung PN, et al. Trazodone Effects on Obstructive Sleep Apnea and Non-REM Arousal Threshold. Ann Am Thorac Soc. 2015;12:758–64. doi: 10.1513/AnnalsATS.201408-399OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng S, Brown EC, Hatt A, Butler JE, Gandevia SC, Bilston LE. Healthy humans with a narrow upper airway maintain patency during quiet breathing by dilating the airway during inspiration. J Physiol. 2014;592:4763–74. doi: 10.1113/jphysiol.2014.279240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng S, Butler JE, Gandevia SC, Bilston LE. Movement of the tongue during normal breathing in awake healthy humans. J Physiol. 2008;586:4283–94. doi: 10.1113/jphysiol.2008.156430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vozoris NT, Leung RS. Sedative medication use: prevalence, risk factors, and associations with body mass index using population-level data. Sleep. 2011;34:869–74. doi: 10.5665/SLEEP.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013–6. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]
- 11.Berry RB, Kouchi K, Bower J, Prosise G, Light RW. Triazolam in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151:450–4. doi: 10.1164/ajrccm.151.2.7842205. [DOI] [PubMed] [Google Scholar]
- 12.Heinzer RC, White DP, Jordan AS, et al. Trazodone increases arousal threshold in obstructive sleep apnoea. Eur Respir J. 2008;31:1308–12. doi: 10.1183/09031936.00067607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonora M, St John WM, Bledsoe TA. Differential elevation by protriptyline and depression by diazepam of upper airway respiratory motor activity. Am Rev Respir Dis. 1985;131:41–5. doi: 10.1164/arrd.1985.131.1.41. [DOI] [PubMed] [Google Scholar]
- 14.Leiter JC, Knuth SL, Krol RC, Bartlett D., Jr The effect of diazepam on genioglossal muscle activity in normal human subjects. Am Rev Respir Dis. 1985;132:216–9. doi: 10.1164/arrd.1985.132.2.216. [DOI] [PubMed] [Google Scholar]
- 15.Park E, Younes M, Liu H, Liu X, Horner RL. Systemic vs. central administration of common hypnotics reveals opposing effects on genioglossus muscle activity in rats. Sleep. 2008;31:355–65. doi: 10.1093/sleep/31.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loewen AH, Ostrowski M, Laprairie J, Maturino F, Hanly PJ, Younes M. Response of genioglossus muscle to increasing chemical drive in sleeping obstructive apnea patients. Sleep. 2011;34:1061–73. doi: 10.5665/SLEEP.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saboisky JP, Jordan AS, Eckert DJ, et al. Recruitment and rate-coding strategies of the human genioglossus muscle. J Appl Physiol. 2010;109:1939–49. doi: 10.1152/japplphysiol.00812.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoijer U, Hedner J, Ejnell H, Grunstein R, Odelberg E, Elam M. Nitrazepam in patients with sleep apnoea: a double-blind placebo-controlled study. Eur Respir J. 1994;7:2011–5. [PubMed] [Google Scholar]
- 19.Wang D, Marshall NS, Duffin J, et al. Phenotyping interindividual variability in obstructive sleep apnoea response to temazepam using ventilatory chemoreflexes during wakefulness. J Sleep Res. 2011;20:526–32. doi: 10.1111/j.1365-2869.2011.00931.x. [DOI] [PubMed] [Google Scholar]
- 20.Kryger M, Wang-Weigand S, Roth T. Safety of ramelteon in individuals with mild to moderate obstructive sleep apnea. Sleep Breath. 2007;11:159–64. doi: 10.1007/s11325-006-0096-4. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg R, Roach JM, Scharf M, Amato DA. A pilot study evaluating acute use of eszopiclone in patients with mild to moderate obstructive sleep apnea syndrome. Sleep Med. 2007;8:464–70. doi: 10.1016/j.sleep.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Cirignotta F, Mondini S, Zucconi M, Gerardi R, Farolfi A, Lugaresi E. Zolpidem-polysomnographic study of the effect of a new hypnotic drug in sleep apnea syndrome. Pharmacol Biochem Behav. 1988;29:807–9. doi: 10.1016/0091-3057(88)90212-2. [DOI] [PubMed] [Google Scholar]
- 23.Boyle J, Groeger JA, Paska W, et al. A method to assess the dissipation of the [corrected] residual effects of [corrected] hypnotics: eszopiclone versus zopiclone. J Clin Psychopharmacol. 2012;32:704–9. doi: 10.1097/JCP.0b013e3182664eec. [DOI] [PubMed] [Google Scholar]
- 24.Kaida K, Takahashi M, Akerstedt T, et al. Validation of the Karolinska sleepiness scale against performance and EEG variables. Clin Neurophysiol. 2006;117:1574–81. doi: 10.1016/j.clinph.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fogel RB, Trinder J, White DP, et al. The effect of sleep onset on upper airway muscle activity in patients with sleep apnoea versus controls. J Physiol. 2005;564:549–62. doi: 10.1113/jphysiol.2005.083659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joosten SA, O'Donoghue FJ, Rochford PD, et al. Night-to-night repeatability of supine-related obstructive sleep apnea. Ann Am Thorac Soc. 2014;11:761–9. doi: 10.1513/AnnalsATS.201309-306OC. [DOI] [PubMed] [Google Scholar]
- 28.Joosten SA, O'Driscoll DM, Berger PJ, Hamilton GS. Supine position related obstructive sleep apnea in adults: pathogenesis and treatment. Sleep Med Rev. 2014;18:7–17. doi: 10.1016/j.smrv.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Ong JS, Touyz G, Tanner S, Hillman DR, Eastwood PR, Walsh JH. Variability of human upper airway collapsibility during sleep and the influence of body posture and sleep stage. J Sleep Res. 2011;20:533–7. doi: 10.1111/j.1365-2869.2011.00925.x. [DOI] [PubMed] [Google Scholar]
- 30.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bilston LE, Gandevia SC. Biomechanical properties of the human upper airway and their effect on its behavior during breathing and in obstructive sleep apnea. J Appl Physiol. 2014;116:314–24. doi: 10.1152/japplphysiol.00539.2013. [DOI] [PubMed] [Google Scholar]
- 32.Peppard PE, Ward NR, Morrell MJ. The impact of obesity on oxygen desaturation during sleep-disordered breathing. Am J Respir Crit Care Med. 2009;180:788–93. doi: 10.1164/rccm.200905-0773OC. [DOI] [PMC free article] [PubMed] [Google Scholar]