Abstract
Study Objectives:
Exposure to hypoxia elevates chemosensitivity, which can lead to periodic breathing. Exercise impacts gas exchange, altering chemosensitivity; however, interactions between sleep, exercise and chronic hypoxic exposure have not been examined. This study investigated whether exercise exacerbates sleep-related periodic breathing in hypoxia.
Methods:
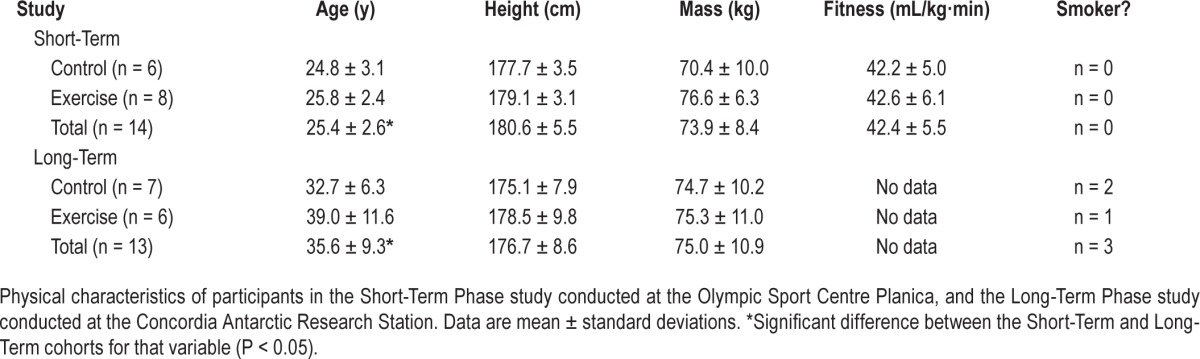
Two experimental phases. Short-Term Phase: a laboratory controlled, group-design study in which 16 active, healthy men (age: 25 ± 3 y, height: 1.79 ± 0.06 m, mass: 74 ± 8 kg) were confined to a normobaric hypoxic environment (FIO2 = 0.139 ± 0.003, 4,000 m) for 10 days, after random assignment to a sedentary (control, CON) or cycle-exercise group (EX). Long-Term Phase: conducted at the Concordia Antarctic Research Station (3,800 m equivalent at the Equator) where 14 men (age: 36 ± 9 y, height: 1.77 ± 0.09 m, mass: 75 ± 10 kg) lived for 12–14 months, continuously confined. Participants were stratified post hoc based on self-reported physical activity levels. We quantified apnea-hypopnea index (AHI) and physical activity variables.
Results:
Short-Term Phase: mean AHI scores were significantly elevated in the EX group compared to CON (Night1 = CON: 39 ± 51, EX: 91 ± 59; Night10 = CON: 32 ± 32, EX: 92 ± 48; P = 0.046). Long-Term Phase: AHI was correlated to mean exercise time (R2 = 0.4857; P = 0.008) and the coefficient of variation in night oxyhemoglobin saturation (SpO2; R2 = 0.3062; P = 0.049).
Conclusions:
Data indicate that exercise (physical activity) per se affects night SpO2 concentrations and AHI after a minimum of two bouts of moderate-intensity hypoxic exercise, while habitual physical activity in hypobaric hypoxic confinement affects breathing during sleep, up to 13+ months' duration
Citation:
Tellez HF, Morrison SA, Neyt X, Mairesse O, Piacentini MF, Macdonald-Nethercott E, Pangerc A, Dolenc-Groselj L, Eiken O, Pattyn N, Mekjavic IB, Meeusen R. Exercise during short-term and long-term continuous exposure to hypoxia exacerbates sleep-related periodic breathing. SLEEP 2016;39(4):773–783.
Keywords: Antarctica, sleep apnea, confinement, cycling, high altitude, oxygen saturation
Significance.
This investigation was interested in determining the longer-term, interactive effects hypoxia and habitual physical activity exert on nocturnal sleep-breathing disturbances. After running two experiments in confined, hypoxic conditions (a 10-day, normobaric hypoxic study [4,000 m], and a 13-month, hypobaric hypoxic study at the Concordia Antarctic Research Station [3,800 m]), we established that performing physical activity can exacerbate the number of nocturnal apneas and hypopneas experienced compared to matched controls. The Antarctic cohort demonstrated positive correlations in breathing disturbances and mean exercise duration across the 13-months, suggesting that periodic breathing remains problematic in lowlanders who remain at high altitude, even after one year duration. The dose-response relationship between exercise intensity and duration on sleep and breathing remains to be established.
INTRODUCTION
At altitudes above 2,500 meters, ventilation in healthy individuals commonly exhibits an oscillatory behavior, with alternating periods of hyperventilation followed by central apneas or hypopneas1 during both wake and nocturnal sleep. While this quantity of periodic breathing may be reduced with continued hypoxia exposure,2 more recent evidence supports its persistence over time. Consistent with the results from previous studies, Fernandez Tellez et al.3 report that throughout a 13-month confinement and high altitude exposure, periodic breathing prevails for the major part of sleeping time; these data were observed in the absence of acute mountain sickness (AMS) and concomitant with partial restoration of SpO2 levels. Indeed, periodic breathing, and its adaptation to hypoxia has been examined in the exercise literature, but with equivocal results. Namely, the magnitude, time-course and the altitude at which periodic breathing occurs, as well as its persistence over successive nights, differ greatly across studies. Some observe an increase in periodic breathing at various altitudes,1 while others have reported decreases2 or indeed, no changes4 in incidence over time. Continued exposure to hypoxia induces a series of physiological changes in the human body that improve its ability to tolerate persistently lower partial pressures of O2.5 A major aspect of the ventilatory acclimatization to hypoxia is the gradual elevation of minute ventilation despite a continuously increasing arterial PO2 and concomitant fall in arterial PCO2.6 Although the mechanisms underlying periodic breathing at high altitude are not clearly elucidated,5 it is widely accepted that acclimatization to hypoxia enhances chemoreceptor reactivity, which constitutes a critical factor inducing periodic breathing.7
Aspects of the mechanisms involved in the control of respiration during exercise also remain relatively unresolved, although it is generally accepted that exercise enhances chemoreactivity.8 Periodic breathing is exacerbated during hypoxic exercise (20% to 40% maximal aerobic power) in awake individuals, evidenced by a breathing pattern which demonstrates a period lengthening between 11.1 to 12.0 s, determined via spectral analysis of the breath-by-breath ventilation signal, O 2 saturation, and end-tidal PCO2.9 High altitude expeditions are characterized by strenuous physical demands which can alter both chemoreactivity and the control of respiration during exercise,10 which together, may translate to greater development of periodic breathing during sleep than in response to a hypoxic stimulus alone. Furthermore, during exercise, pulmonary vasoconstriction can lead to pulmonary hypertension, while hypoxia is known to independently impact pulmonary circulation, such that tissue constriction becomes even more pronounced during exercise.11 Although still contentious, it is possible that while performing exercise even at sea level, one can develop pulmonary edema,12 and with the increased resistance, possibly drive higher incidences of periodic breathing,13 especially during sleep.
Thus, the purpose of the present investigation was to determine to what extent regular physical activity per se may affect nocturnal periodic breathing during prolonged hypoxic confinement. Two experiments were conducted at roughly equivalent altitudes: (1) a 10-day group-design normobaric hypoxic confinement study to observe the effects of moderate exercise and shorter-term hypoxic exposure, and (2) a 13-month hypobaric hypoxic confinement study to observe the longer-term effects of hypoxia and habitual physical activity on sleep-breathing disturbances.
METHODS
All experimental protocols and procedures were performed according to the Declaration of Helsinki. The Short-Term Phase was approved by The National Committee for Medical Ethics, Ministry of Health of the Republic of Slovenia, and the Long-Term Phase was approved by the Ethics Committee of the Free University of Brussels. Written, informed consent was obtained from each subject prior to participation in either of the two studies. All participants in both studies were native lowlanders, none of whom were exposed to high altitude environments before entry into either study.
Short-Term Phase: Normobaric Hypoxia Group Design Study
All participants (n = 16) were nonsmokers, with no history of any known cardiorespiratory, musculoskeletal, or circulatory disease. All testing was performed in the hypoxic facility at the Olympic Sport Centre Planica (Rateče, Slovenia) situated at an altitude of 940 m above sea level. The study was conducted during the northern hemisphere spring, and was part of a larger group-design study to investigate the effects of physical activity and hypoxia on various aspects of human physiology, including: metabolic adaptations, oxidative stress, and cold-induced vasodilation, among others.14–16 Since these investigations were performed by many other scientific teams and fall outside the scope of the present paper, they are not discussed here.
Participants arrived at the facility for baseline data collection three nights prior to entering the hypoxic confinement condition. During the 10-day experimental period, participants were allowed to move freely between their own rooms, the common hallway, and living area (∼200 m2). The normobaric hypoxic condition on the floor and in the rooms was maintained using a Vacuum Pressure Swing Adsorption (VPSA) system (b-Cat, Tiel, The Netherlands), described in detail elsewhere.15 The mean environmental values throughout the 10-day protocol were: FIO2 = 0.139 ± 0.003, PIO2 = 88.2 ± 0.6 mm Hg, 23.1 ± 1.0°C, 56% ± 8% relative humidity.
Peak oxygen consumption was determined using an incremental-load test on a cycle ergometer (Ergo Bike Premium, Daum electronics, Germany) to volitional exhaustion on two occasions. On one occasion, the test was conducted in normobaric normoxia, and on another occasion, in normobaric hypoxia, on both occasions using standard, validated methodology.15 Participants were then randomly assigned to either the exercise (EX, n = 8) or control (CON, n = 8) group after they had completed a familiarization weekend at the facility. The EX participants were required to complete moderate intensity cycling exercise for two 1-hour sessions per day (morning: 10:00–11:00, afternoon: 15:00–16:00). All exercise took place in the normobaric hypoxic laboratory. Exercise intensity was adjusted so that heart rate was maintained at 50% of their individual hypoxic peak power output. Participants in the CON group were not allowed to perform any kind of static or dynamic exercise during the course of the study.
All subjects completed 2 nights of full polysomnography (PSG) sleep recordings, with the first recordings taking place after they spent at least 2 nights in the facility, and following their familiarization weekend. The second recording was conducted on the last night of the intervention (Night 10). The PSG recordings (Nicolet One, Viasys, USA) included electroencephalography (EEG), electrooculography (EOG), chin surface electromyography (EMG), electrocardiography (ECG), nasal pressure (nasal pressure cannula), respiratory movements (chest and abdominal belts), and oxyhemoglobin saturation (SpO2) following protocols described elsewhere.17 Acute mountain sickness (AMS) in participants was assessed via Lake Louise Score (LSS),18 with the questionnaire being administered each evening at 17:00 and with the score serving as an index for both occurrence and severity of AMS.
Long-Term Phase: Long-Duration Field-Based Hypobaric Hypoxia Study
Thirteen members of the 14 all-male crew at the Antarctic Concordia Station participated in the experiment. The Concordia Station is a permanent international research station located on the high plateau of the Antarctic mainland at 75° 06' S, 123° 23' E, approximately 1,100 km inland from the French coastal station Dumont d'Urville and ∼1,200 km inland from the Mario Zucchelli Station at Terra Nova Bay. It resides at an altitude of 3,100 m above sea level, where average air pressure is 645 hPa (equivalent to an altitude of 3,800 m at the Equator); thus, the base allows for long-duration, confined stay in hypobaric hypoxia. Participants arrived during the preceding Antarctic summer, and remained isolated on-site for an average of 13 months. Upon arrival at the Concordia Station, participants' acclimatization progress was monitored weekly via physical examination, SpO2 readings, and an assessment of AMS via Lake Louise Score,18 was obtained throughout the first 3 weeks only.
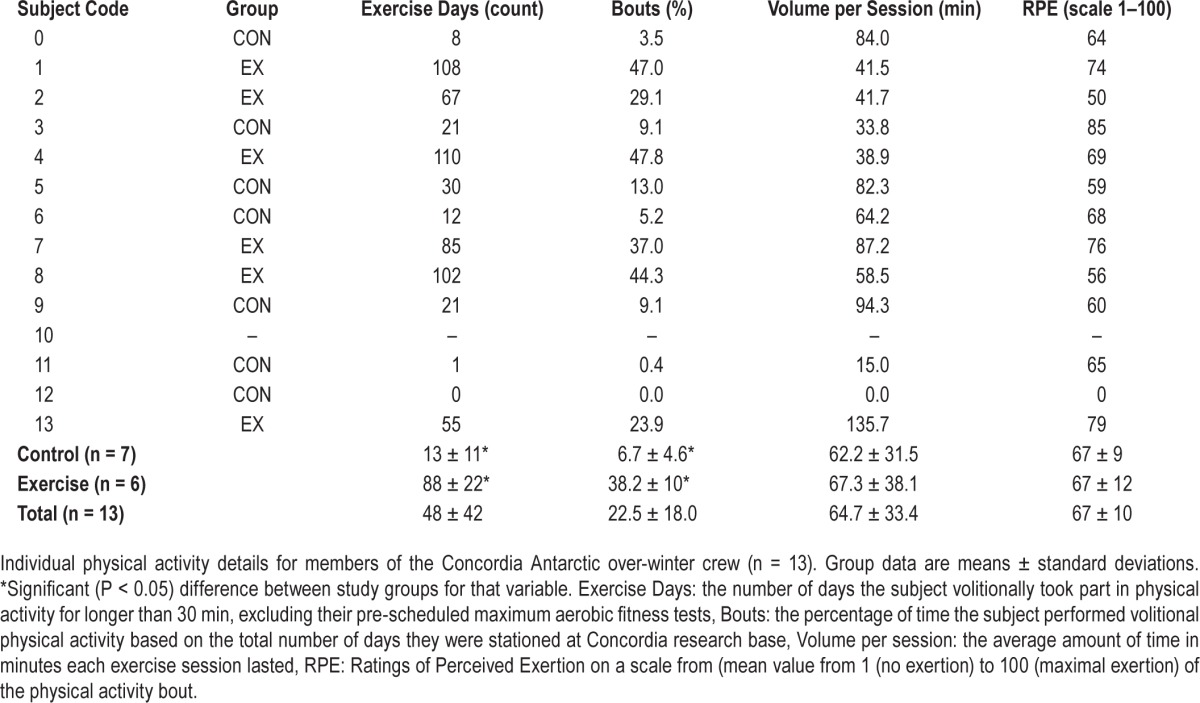
Individual physical activity sessions were recorded by the participants who logged the exercise mode, duration (min), and subjective exercise intensity via ratings of perceived exertion (RPE), scored on a 10-point visual analogue scale (VAS).19 The common-area gymnasium was equipped with a treadmill, a cycle ergometer, a stepping machine, and various free weights. Subjects were assigned to the exercise group (EX) post hoc, if: (1) they completed regular physical activity (of any mode) for ≥ 24% of the days they were stationed at the base (range: 24% to 48%), and (2) for an average ≥ 30 min duration per exercise bout. The rationale for this cutoff was that the definition loosely corresponds to international physical activity guidelines advocated for active, healthy adults, generally considered to be at minimum 30 min of physical activity, at least 4-d per week.20 In this way, we can consider these people as being “consistently physically active”; no small feat (both psychologically and physically) when considering these individuals are confined to a small living area for an entire calendar year, with no possibility of spontaneously leaving the protected environment of the research station. The remaining subjects were designated as controls (CON).
PSG sleep data were recorded over 7 testing nights measured across the entire 13-month overwinter campaign. The PSG recordings (BioRadio, Clevemed Inc., USA) included EEG, EOG, EMG, ECG, respiratory movements (chest and abdominal belts), and SpO2 following established protocols.3 Measurement testing times were variable throughout the year, and depended on the person's arrival to the research base. PSG testing occurred every ∼6 weeks, comprising roughly 8 data collection epochs over the course of 13+ months.
Data Analysis
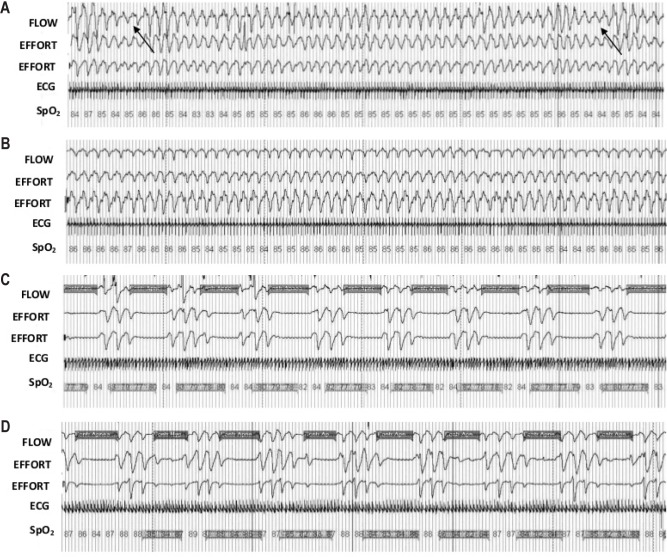
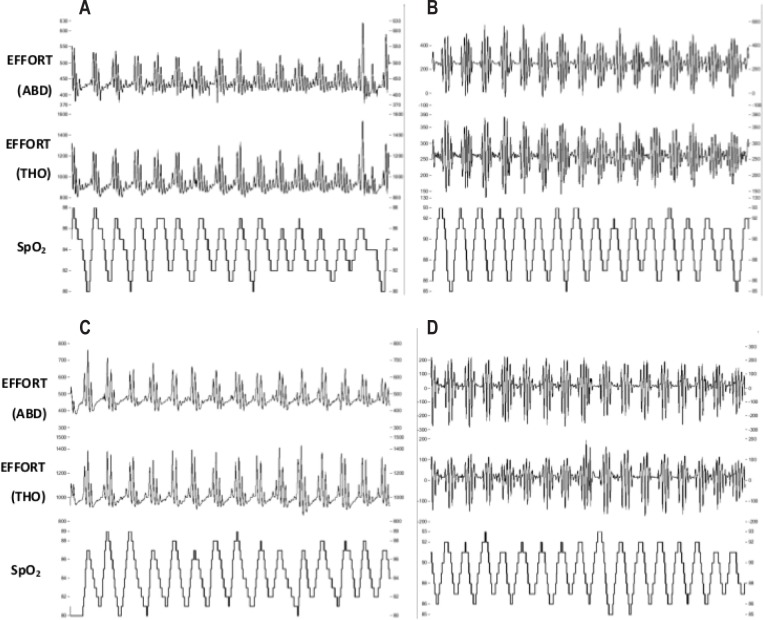
Apneas and hypopneas were defined according to international standards.21 The Short-Term Phase incorporated nasal airflow pressure to determine respiratory restriction(s) and central apneas in addition to effort (chest, abdomen; Figure 1). In the Long-Term Phase, discrimination between central and obstructive apneas was assessed by testing the existence of paradoxical breathing, i.e., breathing movements caused by obstruction in which the chest wall moves in reverse to the abdominal wall. The majority of respiratory effects were central events as a result of periodic breathing at high altitude (Figure 2), previously validated using the estimated amplitude modulation index (eAMI), a mathematical tool for detection and qualification of periodic breathing.22 On all Short-Term Phase demographic data, an independent t-test was conducted to determine potential differences between groups. On the dependent variables, a mixed-model ANOVA was conducted with one within-subjects' factor (time) and one between-subjects' factor (group) to determine any differences between the Night 1 (acute, 24-h) and Night 10 (longer-term, 240-h) hypoxia exposure, and the exercise intervention. Provided data was normally distributed and there were no significant differences by training group, significant data were pooled and paired t-tests were employed post hoc (Bonferroni correction). In some cases, the Long-Term Phase data sampling epochs (1–8) have been pooled and averaged with the next consecutive testing block; therefore we report data either as one of 8 testing time-points conducted throughout the 13-month campaign, or pooled by season: 1+2 = late summer, 3+4 = early winter, 5+6 = late winter, 7+8 = early summer. Total exercise data was obtained from self-reported physical activity logs which were averaged over the 8 data collection epochs. Correlations to various sleep-breathing parameters (2-tailed, bivariate correlations) were completed using a statistical package (SPSS v.17.0, Chicago, IL, USA). Data are expressed as means ± standard deviations, with 95% confidence intervals (CI) for effects of interest, at P < 0.05 level of significance.
Figure 1.
Representative nocturnal respiration and oxyhemoglobin saturation traces from two individuals in the Short-Term study. Respiratory effort was measured via piezoelectric bands at two locations (EFFORT; chest, abdomen). Capillary oxyhemoglobin saturation (SpO2) readings show desaturations throughout the screenshot. Nasal airflow (FLOW) was measured via nasal cannula pressure. (A) CON subject, first night at simulated altitude of 4,175 m. Note the arrows indicating central hypopnea (s). (B) the same CON subject, night ten, (C) EX subject, night one, (D) the same EX subject, night ten. Highlighted areas on FLOW channel in traces C, D indicate central sleep apneas; highlighted sections on SpO2 channel indicate desaturations. Time epoch of screen shots was set at 240-s to better display periodic breathing rhythmicity.
Figure 2.
Representative nocturnal respiration and oxyhemoglobin saturation traces from two individuals in the Long-Term study. Respiratory effort was measured via piezoelectric bands at two locations (EFFORT, ABD, abdominal, THO, thoracic). Capillary oxyhemoglobin saturation (SpO2) readings show oscillations throughout the screenshot. (A) CON subject, first epoch in Concordia (B) the same CON subject, the 8th and final epoch (C) EX subject, 1st epoch (D) the same EX subject, 8th epoch. Time epoch of screen shots was set at 300-s to better display periodic breathing rhythmicity.
RESULTS
Short-Term Phase
During the hypoxic confinement period, 2 participants withdrew from the study, one due to adaptation problems, the other due to acute appendicitis; data from these participants are excluded from further analysis. All participants assigned to the EX group were able to complete all training sessions. Physical characteristics of both EX and CON groups are described in Table 1. There were no differences between the Short-Term intervention groups in terms of age, aerobic fitness, or anthropometry measures. After the hypoxia confinement protocol, the EX group had a significant increase in peak power output (EX: 260 ± 34 v 276 ± 34 W, P = 0.0001), which was not observed in the control group (CON: 244 ± 37 v 247 ± 41 W, P = 0.414). The EX group also had decreases in measured percent body fat (EX: 22.4 ± 6.2 v 21.2% ± 5.8%; P = 0.0001), whereas no change was observed in the controls (CON: 21.9 ± 4.7 v 21.3% ± 4.8%; P = 0.103). Lake Louise Scores were comparable between groups throughout the campaign; clinically significant AMS scores were noted in 7 participants from both groups on Day 1 (EX = 4, CON = 3). Scores were significantly lower on Day 9 than Day 1 (P < 0.05), with an average rating (range) of 1.5 (0–6) and 1.4 (0–7) for both EX and CON groups, respectively.
Table 1.
Physical characteristics.
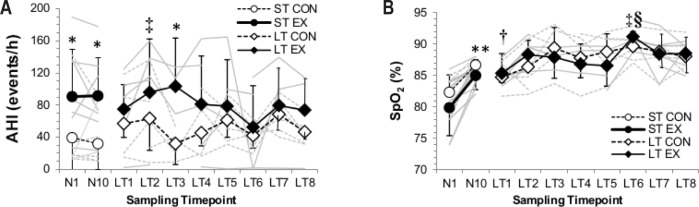
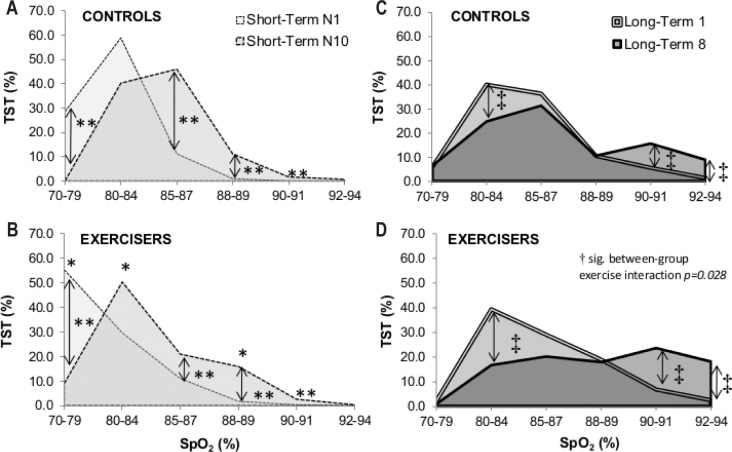
Night AHI scores were significantly elevated in the EX group on Night 1 (CON: 39 ± 51, EX: 91 ± 59) and Night 10 (CON: 32 ± 32, EX: 92 ± 48; P = 0.046, Figure 3) compared to CON. The periodic breathing cycle was ∼4 s longer after 10 days in hypoxia (P = 0.014), regardless of group. The EX group spent a greater proportion of their night at a lower given SpO2 concentration than their CON counterparts (Figure 4; significant 3-way interaction (time × SpO2 × exercise status; P = 0.013). For example, on N1 the EX spent 55% of their total sleep time at SpO2 concentrations between 70% and 75%, whereas the CON spent only 29% of TST at such concentrations (95% CI: 29 to 82%; P = 0.013). Mean night SpO2 concentrations were 5.8% higher after 10-day hypoxia exposure (95% CI: 2.9 to 6.6%; P = 0.0001), regardless of training group (Table 2). The overall increase in mean night SpO2 was not significantly related to the increase in time spent in REM sleep, which also increased in both groups (Figure 5, Table 2, R2 = 0.373, P = 0.189).
Figure 3.
Individual data. (A) Apnea-hypopnea index (AHI), expressed in events per hour, and (B) average night pulsed-oxygen saturation (SpO2) of the Short-Term (ST) and Long-Term (LT) studies. Mean group data with standard deviation bars are represented by black lines for the control (CON, open symbols) and exercise (EX, closed symbols) cohorts; individual responses included as gray background lines. *Significant difference between training groups. **Significant difference between Short-Term night one (N1) and Short-Term night 10 (N10). †Significantly different from all other Long-Term time-points. ‡Significantly different from Long-Term testing epoch 1 only. §Significantly different from Long-Term Phase testing epochs 2 and 6. Significance defined as P < 0.05.
Figure 4.
Proportion of total sleep time (TST) spent at a given SpO2 concentration. (A) Short-Term control (ST CON), (B) Short-Term exerciser (ST EX), (C) Long-Term control (LT CON), and (D) Long-Term exerciser (LT EX) cohorts. *Significantly different between training groups for that SpO2 concentration. **Significant difference between Short-Term night one (N1) and Short-Term night 10 (N10). ‡Significantly different from Long-Term testing epoch 1. Significance defined as P < 0.05.
Table 2.
Night respiratory patterns, total sleep time and oxygenation values in hypoxic confinement.
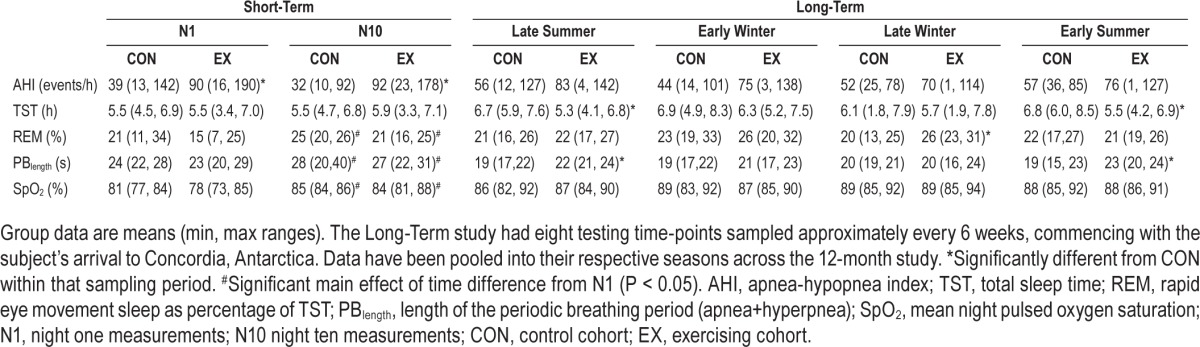
Figure 5.
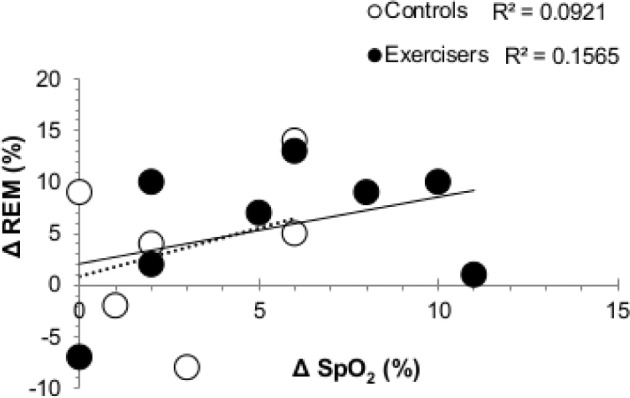
Bivariate correlations for the change in mean night SpO2 concentrations and versus the change in proportion of total sleep time spent in REM from Night 1 to Night 10 for exercisers (EX, closed circles) and matched controls (CON, open circles) of the Short-Term study. Linear regression equations are labeled in solid (EX) and dashed (CON) lines.
Long-Term Phase
Of all participants in the overwintering crew, 6/13 were assigned to the exercise group (EX), while 7/13 were grouped as controls (CON). Individual physical activity details are outlined in Table 3. AMS scores were calculated during the first 3 weeks on arrival to the Concordia Station. Symptoms of AMS did not reach clinical significance for any subject (median AMS score after 3 weeks was 1 [range, 0–1.5]).
Table 3.
Physical activity details.
Individuals' mean AHI was determined across the entire 13-month campaign, with the vast majority of the respiratory events happening during periodic breathing (95%). The AHI value was significantly correlated to participants' mean exercise duration (R2 = 0.4857, P = 0.008, Figure 6), including significant correlations between the AHI value and the mean coefficient of variation for night SpO2 concentrations (R2 = 0.3062, P = 0.049), calculated as an indirect marker of breathing instability in ventilatory drive. Additionally, the periodic breathing cycle was ∼2 s longer for EX than for CON during the early summer (95% CI: 1 to 5 s; P = 0.006) and late summer (95% CI: 0 to 7 s; P = 0.048) time epochs (Table 2). Total sleep time was also reduced in EX compared to in CON by between −23 to −150 min during the early summer (P = 0.012) and −1 to −163 min in late summer (P = 0.047).
Figure 6.
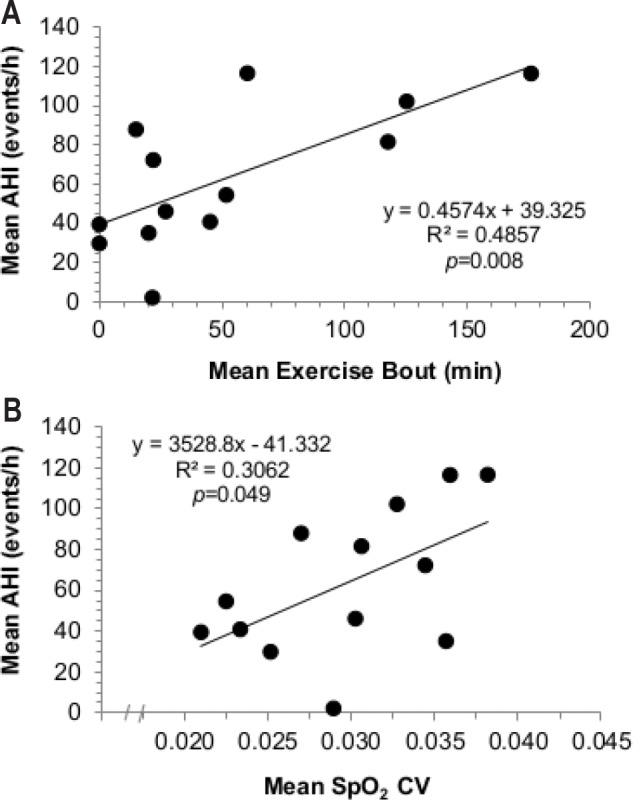
Bivariate correlations. (A) Correlation between apneahypopnea index (AHI) and average exercise time (min) in the Long-Term Concordia participants (P < 0.05), and between (B) AHI and the mean coefficient of variation for pulsed-oxygen saturation (mean SpO2 CV; an indirect measure of altered ventilatory drive) in the Concordia over-winter participants.
Finally, there were significant between-subject interactions observed in mean night SpO2 concentrations between EX and CON from the first testing epoch compared to the last epoch of the 13-month over-winter campaign (95% CI: 2.7 to 37.3%; P = 0.028). There was also a 2-way interaction effect of time × SpO2 concentration at a given percentage of total sleep time (P = 0.009, Figure 4C, 4D). This relationship was not observed in the REM data, which demonstrated only one significant difference between groups, during late winter (Table 2), when EX spent ∼33% greater proportion of the time in REM than did CON (95% CI: 1.9 to 12.0% absolute increase in REM as proportion of total sleep time; P = 0.013).
DISCUSSION
The principal finding of this joint, international investigation is that a direct, positive correlation exists between physical activity duration and severity of periodic breathing in long-term hypobaric hypoxia. Our data indicate that physical activity per se increases the incidence of periodic breathing, measured via clinical gold standard AHI scores and period length of the breathing disruption. We observed augmented AHI-exercise relationships in both hypobaric and normobaric hypoxic environments, regardless of habituation.
Previous studies have reported an increase in the amount of periodic breathing during acclimatization to hypoxia,1 whereas others have reported decreases2 or no change.4 In awake, healthy humans, periodic breathing severity is associated with physical exertion under acute, hypoxic exposure.9 The influence of long-term hypoxic exposure on nocturnal sleep and breathing remains a concern when the exposure is inevitable (military scenarios, research expeditions, future planetary habitats), especially considering that prolonged exposure negatively affects brain function, performance, cognition and subjective alertness following poor sleep during high altitude sojourns.23 Sleep-related periodic breathing may be influenced by regular physical activity via:
Chemoreceptor sensitivity: Several lines of evidence suggest that chemoreceptors play an important role in acclimatization to hypoxia.24 It has been observed in anesthetized goats that adaptive processes within peripheral chemoreceptors are of sufficient magnitude to explain ventilatory acclimatization to hypoxia25 and generally, that carotid bodies are needed to induce full acclimatization.26 Peripheral mechanisms may also contribute to the initial enhanced exercise-hyperpnoea observed upon exposure to hypoxia.27 The underlying mechanisms proposed so far include metabolic acidosis, especially during heavy exercise28 and hyperkalemia.29 Although a role for carotid bodies in exercise-induced hyperventilation is plausible,30 some animal models are not fully consistent with this mechanism.31 More recent evidence suggests that central and peripheral chemoreceptors are not functionally separate, but that central stimulation modifies the reactivity of peripheral chemoreceptors.24 Thus, exercise-related central chemoreactivity may also impact nocturnal periodic breathing. During heavy exercise, the respiratory drive is affected by chemosensors located within the working muscles32,33 and, according to Subudhi et al.34 during dynamic leg exercise, hypoxia-induced increases in peripheral chemoreceptor activation leads to marked reductions in PaCO2 and concomitant reductions in cerebral blood flow and cerebral oxygenation. Exercise-evoked sympathetic activation also induces small, but significant increases in chemoreceptor discharges that can persist up to 6 d after exercise is completed.35 The Long-Term study's significant correlations between habitual exercisers' activity time and increased AHI are consistent with this theory.
Alterations to cerebral and pulmonary structure and function: Rupp et al.36 found that prolonged moderate-intensity hypoxic exercise can accentuate the effects of hypoxia, increasing total brain volume (by up to ∼2%). During hypoxia, mild exercise induces cerebral vasodilation, increases cerebral blood flow, and augments cerebral oxygenation, suggesting that the exaggerated hyperpneas, which arise during high-intensity exercise, coincide with decreased overall cerebral blood flow. These findings are consistent with work demonstrating attenuation of middle cerebral artery flow velocity and cerebral oxygenation during hypoxic exercise after acclimation to intermittent or continuous hypoxia, resulting in augmented exercise hyperpnea.8 More recent research confirms that the ventilatory responses to exercise in hypoxia are augmented such that periodic breathing is positively related to both cardiac output and the ventilatory response to CO2.9 Whether pulmonary edema develops during exercise at sea level remains unresolved. Several studies have identified that pulmonary edema can follow exercise training.12 This systematic review of the subject found that ∼50% of all studies scrutinized reported evidence of exercise-induced extravascular lung fluid, while the remaining papers reported inconclusive results, or no changes.12 Periodic breathing at high altitude has been hypothesized to be more frequent, and SpO2 desaturation more severe during sleep, in subjects who develop high altitude pulmonary edema (HAPE), and/ or AMS13 in part due to the impaired gas exchange. Clinically, pulmonary edema is identified using radiographic imaging, which, for obvious logistic limitations, was not possible here. Although the possibility of severe pulmonary edema remains unlikely, especially given the low LLS scores reported in both cohorts, it is possible that mild cases of pulmonary edema did occur, especially in the Short-Term Phase in which seven participants from both groups (EX = 4, CON = 3) reported AMS symptoms on Day 1. The fact that there were no systematic discrepancies between groups rules out any significant correlational value of the LLS to explaining the differences in AHI and night SpO2 concentrations.
Night Oxyhemoglobin Saturations
In the Short-Term Phase study, mean night oxyhemoglobin saturations were lower in EX than CON. It appears initially counterintuitive that mean night SpO2 should be lower in the EX than the Con group, especially on the tenth night of the Short-Term study, since during exposure to hypoxia in healthy subjects, SpO2 during sleep is typically higher in subjects exhibiting period breathing than in those who do not.5 On the other hand, it is also true that hypoxic exercise enhances sympathetic nerve activity and exercise-induced hypoxemia, which is consistent with the present observation of a lower SpO2 in the EX group.37 In summary, although other factors might have played a role, we believe that the combination of lower mean night SpO2 in the EX group is compatible with exercise-induced exacerbation of sympathetic activation and/ or hypoxemia. It is also possible that any difference(s) in SpO2 between EX and CON may be attributed, purely by chance, to changes in body position or a greater time spent in a given sleep stage (REM vs. NREM sleep). As there were no systematic between-group interactions in REM within the 10-day protocol, this thesis remains unlikely. Since this relationship was not reflected in the Long-Term study, it suggests that in this condition the ventilatory control of periodic breathing may have reached a constant threshold, maintained for the duration of their hypoxic exposure,3 especially since increased SpO2 due to hypoxic acclimation (over many months) was not influenced by the presence or absence of exercise habits.
Effect of Season
The present study observed evidence that seasonal sleep effects exist in the Antarctica EX cohort, at least regarding total sleep time and the length of periodic breathing response. Comments on possible mechanisms are further discussed in Collet et al.,38 who reported higher sleep fragmentation and night energy expenditures in summer than in winter at two Antarctic research stations. They propose a circadian de-synchronization effect, induced by melatonin secretion delays from constant light exposure in summer months as a plausible explanation for the sleep fragmentation observed. Nevertheless, it should be noted that the literature is divergent on this aspect, with others reporting longer wake after sleep onset (WASO) and more sleep fragmentation in winter compared to summer months.39 The present study lends additional evidence to the fact that total sleep time is reduced in summer, but only in habitual exercisers.
Normobaric versus Hypobaric Hypoxic Exposures
A final point worth mentioning is the possible difference(s) between hypobaric and normobaric hypoxic stressors, and whether the testing environment could have impacted breathing and ventilatory drive parameters independent of exposure duration between investigations. On the one hand, Richard et al.40 concluded that 6 h of hypoxic exposure was sufficient to lower peripheral and central CO2 thresholds, but they did not find evidence that it induced any other cardiorespiratory or AMS differences between hypobaric and normobaric hypoxic exposures. This is in line with Mounier et al.41 who argue that up-to-date studies have not provided strong enough evidence to support the theory that (patho)-physiologic responses may differ between chronic hypobaric and normobaric hypoxia. Furthermore, a recent consensus for working in hypoxia published by the “Medical Commission of the Union Internationale des Associations d'Alpinisme”42 concludes that the physiologic differences between normobaric and hypobaric hypoxia are too insignificant for clinical relevance. On the other hand, Millet et al.43 have pointed out several works supporting the notion that hypobaric hypoxia is a more severe environmental stressor which can induce dissimilar responses to normobaric hypoxia, and thus, diverse physiological adaptations as well. Considering the nature of our stated aims and hypotheses, we do not believe there to be significant differences in the physiological outcomes observed in either cohort of the Short-Term or Long-Term studies in the present investigation.
Study Considerations
Study Design
Baseline fitness and personality characteristics could have influenced one's adherence to performing regular physical activity in Concordia, Antarctica, which may also have affected the distribution and characteristics of the two groups unbeknown to the researchers. We acknowledge that a fully repeated-measures crossover design would have been ideal to tease out the physiological mechanisms driving our observations in both studies. Due to the extreme nature of testing environments, multinational project scheduling, budget considerations, and other factors, this was not feasible.
Exercise Stratifications
Indeed, 30 min of physical activity at least 4 days per week is inherently a different physical stimulus than 2 hours of structured, moderate-intensity cycling per day, which is why the two studies were not directly compared to each other, statistically speaking. Of note, individuals were stationed at Concordia for a variety of reasons, none of which involved acting primarily as research subjects (e.g., cooks, mechanics, and medical personnel). Thus, subjects were not a priori divided into exercise intervention groups in the Long-Term study. Although the dose-response relationship between exercise and AHI in the Long-Term study is apparent in Figure 6, both mentioned Study Design and Exercise Stratifications, together with other environmental (e.g., cold, light) and psychological (e.g., intense isolation) variables present in the Long-Term study could explain why differences between EX and CON groups were only significant in 2 of the periods (Figure 5). Therefore, the primary goal of the Short-Term study was not to duplicate (or replicate) results observed from Concordia, but to limit possible between-group differences and the number of co-factors from Antarctica. We therefore feel confident in any differences between experiments, i.e., the binary distribution of EX versus CON in the Short-Term study, versus the self-paced, externally valid individual variations in the Long-Term study.
Hypoxic Sleep Studies
We do not know what the AHI and mean night oxyhemoglobin saturations were for either group prior to hypoxic exposure. AHI values vary markedly when individuals ascend to high altitude; however, Concordia subjects were not allowed to be stationed at the base until they passed a rigorous physical examination. Night recordings in all cases were screened before data analysis by a certified sleep professional to ensure there were no systematic obstructive apneas, no periodic limb movements, or any other discernable pathophysiological reason why a subject could not be included in the present investigation. Night recordings from the Long-Term study did not include nasal air pressure; thus respiratory events were characterized by identifying paradoxical breathing patterns, a gross measure of obstruction which may underestimate certain respiratory events or hypopneas during sleep. The issue of whether the AHI is mainly due to OSA or CSA is crucial, thus we further investigated whether respiratory events during the Long-Term study, where due to high altitude-induced periodic breathing using a validated mathematical model (eAMI22), confirming it is unlikely other respiratory events would have influenced these results. Indeed, the pattern of periodic breathing at high altitude is very characteristic and can be easily discernable from the effort band traces depicted in Figure 1 herein.
CONCLUSIONS
Mean apnea-hypopnea index scores were positively correlated to both mean exercise time and the coefficient of variation in mean night pulsed oxygen saturations in those who lived and worked in hypobaric hypoxia for up to one year in duration. This relationship was confirmed in a Short-Term study in which AHI indexes were significantly elevated in an exercise group compared to matched controls.
DISCLOSURE STATEMENT
This was not an industry supported study. Support for the Short-Term Phase Planica study was provided, in part, by the Slovene National Research Agency (ARRS: L3-4328 & L3-3654, Prof. Mekjavic; ARRS P3-0338, Dr. Dolenc-Grošelj). Dr. Morrison was partially supported by a Michael Smith Foundation for Health Research Fellowship (ST-PDF-03269 (11-1) CLIN). Support for the Long-Term Phase Concordia study was provided, in part, by the PRODEX program of the European Space Agency (ESA) AIIESA17, managed through the Belgian Science Policy Office (BELSPO). The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors express their gratitude to the participants of both studies, for altruistically dedicating their time and energies to these time-consuming and logistically challenging projects. The authors also express their gratitude to the European Space Agency for allowing us to conduct the study at the Concordia station. We thank the reviewers for their time in reviewing and critically commenting on this work.
REFERENCES
- 1.Bloch KE, Latshang TD, Turk AJ, et al. Nocturnal periodic breathing during acclimatization at very high altitude at Mount Muztagh Ata (7,546 m) Am J Respir Crit Care Med. 2010;182:562–8. doi: 10.1164/rccm.200911-1694OC. [DOI] [PubMed] [Google Scholar]
- 2.Berssenbrugge A, Dempsey J, Iber C, Skatrud J, Wilson P. Mechanisms of hypoxia-induced periodic breathing during sleep in humans. J Physiol-London. 1983;343:507–24. doi: 10.1113/jphysiol.1983.sp014906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez Tellez H, Mairesse O, Macdonald-Nethercott E, Neyt X, Meeusen R, Pattyn N. Sleep-related periodic breathing does not acclimatize to chronic hypobaric hypoxia: a 1-year study at high altitude in Antarctica. Am J Respir Crit Care Med. 2014;190:114–6. doi: 10.1164/rccm.201403-0598LE. [DOI] [PubMed] [Google Scholar]
- 4.Koziej M, Mańkowski M, Sarybaev AS, et al. [Quality of sleep and periodic breathing during sleep in healthy persons at a height of 3200 meters] Pneumonol Alergol Pol. 1996;64:651–7. [PubMed] [Google Scholar]
- 5.Ainslie PN, Lucas SJ, Burgess KR. Breathing and sleep at high altitude. Respir Physiol Neurobiol. 2013;188:233–56. doi: 10.1016/j.resp.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 6.Chiodi H. Respiratory adaptations to chronic high altitude hypoxia. J Appl Physiol. 1957;10:81–7. doi: 10.1152/jappl.1957.10.1.81. [DOI] [PubMed] [Google Scholar]
- 7.Khoo MCK. Determinants of ventilatory instability and variability. Respir Physiol. 2000;122:167–82. doi: 10.1016/s0034-5687(00)00157-2. [DOI] [PubMed] [Google Scholar]
- 8.Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1473–95. doi: 10.1152/ajpregu.91008.2008. [DOI] [PubMed] [Google Scholar]
- 9.Hermand E, Pichon A, Lhuissier FJ, Richalet JP. Periodic breathing in healthy humans at exercise in hypoxia. J Appl Physiol. 2015;118:115–23. doi: 10.1152/japplphysiol.00832.2014. [DOI] [PubMed] [Google Scholar]
- 10.Mateika JH, Duffin J. A review of the control of breathing during exercise. Eur J Appl Physiol Occup Physiol. 1995;71:1–27. doi: 10.1007/BF00511228. [DOI] [PubMed] [Google Scholar]
- 11.Reeves JT, Groves BM, Sutton JR, et al. Operation Everest-II -Preservation of cardiac-function at extreme altitude. J Appl Physiol. 1987;63:531–9. doi: 10.1152/jappl.1987.63.2.531. [DOI] [PubMed] [Google Scholar]
- 12.Hodges ANH, Mayo JR, McKenzie DC. Pulmonary oedema following exercise in humans. Sports Med. 2006;36:501–12. doi: 10.2165/00007256-200636060-00004. [DOI] [PubMed] [Google Scholar]
- 13.Eichenberger U, Weiss E, Riemann D, Oelz O, Bartsch P. Nocturnal periodic breathing and the development of acute high altitude illness. Am J Respir Crit Care Med. 1996;154:1748–54. doi: 10.1164/ajrccm.154.6.8970365. [DOI] [PubMed] [Google Scholar]
- 14.Debevec T, Simpson EJ, Macdonald IA, Eiken O, Mekjavic IB. Exercise training during normobaric hypoxic confinement does not alter hormonal appetite regulation. PLoS One. 2014;9:e98874. doi: 10.1371/journal.pone.0098874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Debevec T, Pialoux V, Mekjavic IB, Eiken O, Mury P, Millet GP. Moderate exercise blunts oxidative stress induced by normobaric hypoxic confinement. Med Sci Sports Exerc. 2014;46:33–41. doi: 10.1249/MSS.0b013e31829f87ef. [DOI] [PubMed] [Google Scholar]
- 16.Keramidas ME, Kölegård R, Mekjavic IB, Eiken O. Hand temperature responses to local cooling after a 10-day confinement to normobaric hypoxia with and without exercise. Scand J Med Sci Sports. 2015;25:650–60. doi: 10.1111/sms.12291. [DOI] [PubMed] [Google Scholar]
- 17.Rojc B, Morrison SA, Eiken O, Mekjavic IB, Dolenc-Groselj L. The separate and combined effects of hypoxia and sustained recumbency/inactivity on sleep architecture. Eur J Appl Physiol. 2014;114:1973–81. doi: 10.1007/s00421-014-2909-7. [DOI] [PubMed] [Google Scholar]
- 18.Roach RC, Bartsch P, Hackett PH, et al. The Lake Louise Acute Mountain-Sickness Scoring System. Hypoxia Mol Med. 1993:272–4. [Google Scholar]
- 19.Minganti C, Capranica L, Meeusen R, Amici S, Piacentini MF. The validity of sessionrating of perceived exertion method for quantifying training load in teamgym. J Strength Cond Res. 2010;24:3063–8. doi: 10.1519/JSC.0b013e3181cc26b9. [DOI] [PubMed] [Google Scholar]
- 20.Warburton DER, Katzmarzyk PT, Rhodes RE, Shephard RJ. Evidence-informed physical activity guidelines for Canadian adults. Appl Physiol Nutr Metab. 2007;32:S16–68. [PubMed] [Google Scholar]
- 21.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tellez HF, Pattyn N, Mairesse O, et al. eAMI: a qualitative quantification of periodic breathing based on amplitude of oscillations. Sleep. 2015;38:381–9. doi: 10.5665/sleep.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.West JB. Commuting to high altitude: value of oxygen enrichment of room air. High Alt Med Biol. 2002;3:223–35. doi: 10.1089/15270290260131948. [DOI] [PubMed] [Google Scholar]
- 24.Smith CA, Forster HV, Blain GM, Dempsey JA. An interdependent model of central/peripheral chemoreception: evidence and implications for ventilatory control. Respir Physiol Neurobiol. 2010;173:288–97. doi: 10.1016/j.resp.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Busch MA, Bisgard GE, Forster HV. Ventilatory acclimatization to hypoxia is not dependent on arterial hypoxemia. J Appl Physiol. 1985;58:1874–80. doi: 10.1152/jappl.1985.58.6.1874. [DOI] [PubMed] [Google Scholar]
- 26.Smith CA, Bisgard GE, Nielsen AM, et al. Carotid-bodies are required for ventilatory acclimatization to chronic hypoxia. J Appl Physiol. 1986;60:1003–10. doi: 10.1152/jappl.1986.60.3.1003. [DOI] [PubMed] [Google Scholar]
- 27.Dempsey JA, Reddan WG, Rankin J, et al. Control of exercise hyperpnea under varying durations of exposure to moderate hypoxia. Respir Physiol. 1972;16:213. doi: 10.1016/0034-5687(72)90052-7. [DOI] [PubMed] [Google Scholar]
- 28.Wasserman K, Whipp BJ, Koyal SN, Cleary MG. Effect of carotid-body resection on ventilatory and acid-base control during exercise. J Appl Physiol. 1975;39:354–8. doi: 10.1152/jappl.1975.39.3.354. [DOI] [PubMed] [Google Scholar]
- 29.Paterson DJ. Potassium and ventilation in exercise. J Appl Physiol. 1992;72:811–20. doi: 10.1152/jappl.1992.72.3.811. [DOI] [PubMed] [Google Scholar]
- 30.Prabhakar NR, Peng YJ. Peripheral chemoreceptors in health and disease. J Appl Physiol. 2004;96:359–66. doi: 10.1152/japplphysiol.00809.2003. [DOI] [PubMed] [Google Scholar]
- 31.Forster HV, Pan LG. The role of the carotid chemoreceptors in the control of breathing during exercise. Med Sci Sports Exerc. 1994;26:328–36. [PubMed] [Google Scholar]
- 32.Eiken O. Responses to dynamic leg exercise in man as influenced by changes in muscle perfusion-pressure. Acta Physiol Scand. 1987;131:5–37. [PubMed] [Google Scholar]
- 33.Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. Comprehensive physiology. John Wiley & Sons, Inc. 2010 [Google Scholar]
- 34.Subudhi AW, Lorenz MC, Fulco CS, Roach RC. Cerebrovascular responses to incremental exercise during hypobaric hypoxia: effect of oxygenation on maximal performance. Am J Physiol Heart Circul Physiol. 2008;294:H164–71. doi: 10.1152/ajpheart.01104.2007. [DOI] [PubMed] [Google Scholar]
- 35.Eyzaguirre C, Lewin J. The effect of sympathetic stimulation on carotid nerve activity. J Physiol. 1961;159:251–67. doi: 10.1113/jphysiol.1961.sp006806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rupp T, Jubeau M, Lamalle L, et al. Cerebral volumetric changes induced by prolonged hypoxic exposure and whole-body exercise. J Cereb Blood Flow Metab. 2014;34:1802–9. doi: 10.1038/jcbfm.2014.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dempsey JA, Morgan BJ. Humans in hypoxia: a conspiracy of maladaptation?! Physiology. 2015;30:304–16. doi: 10.1152/physiol.00007.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collet G, Mairesse O, Cortoos A, et al. Altitude and seasonality impact on sleep in Antarctica. Aerosp Med Hum Perform. 2015;86:392–6. doi: 10.3357/AMHP.4159.2015. [DOI] [PubMed] [Google Scholar]
- 39.Bhattacharyya M, Pal MS, Sharma YK, Majumdar D. Changes in sleep patterns during prolonged stays in Antarctica. Int J Biometeorol. 2008;52:869–79. doi: 10.1007/s00484-008-0183-2. [DOI] [PubMed] [Google Scholar]
- 40.Richard NA, Sahota IS, Widmer N, Ferguson S, Sheel AW, Koehle MS. Acute mountain sickness, chemosensitivity, and cardiorespiratory responses in humans exposed to hypobaric and normobaric hypoxia. J Appl Physiol. 2014;116:945–52. doi: 10.1152/japplphysiol.00319.2013. [DOI] [PubMed] [Google Scholar]
- 41.Mounier R, Brugniaux JV. Counterpoint: hypobaric hypoxia does not induce different responses from normobaric hypoxia. J Appl Physiol. 2012;112:1784–6. doi: 10.1152/japplphysiol.00067.2012a. [DOI] [PubMed] [Google Scholar]
- 42.Kuepper T, Milledge JS, Hillebrandt D, et al. Work in Hypoxic Conditions-Consensus Statement of the Medical Commission of the Union Internationale des Associations d'Alpinisme (UIAA MedCom) Ann Occup Hygiene. 2011;55:369–86. doi: 10.1093/annhyg/meq102. [DOI] [PubMed] [Google Scholar]
- 43.Millet GP, Faiss R, Pialoux V. Point: hypobaric hypoxia induces different physiological responses from normobaric hypoxia. J Appl Physiol. 2012;112:1783–4. doi: 10.1152/japplphysiol.00067.2012. [DOI] [PubMed] [Google Scholar]