Abstract
Study Objectives:
Despite mounting evidence for the overuse of prescription sleep aids (PSA), reliable data on PSA use among insomniacs are unavailable. Current studies focus on trends in PSA use at the general population level, and thus do not distinguish between transient sleep disturbance and insomnia disorder. Therefore, we prospectively examined the prevalence and predictors of baseline and chronic PSA use in a well-defined sample of individuals with insomnia.
Methods:
We analyzed longitudinal data from an urban, community-based cohort of 649 adults (48.1 ± 11.6 y; 69.3% female) with Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)–based insomnia disorder. Participants completed standardized measures of sleep disturbance, daytime alertness, depression, and anxiety at baseline and follow-up 1 y later. They also reported whether and with what frequency they used PSA at both time points.
Results:
Approximately 19% of the sample used PSA at baseline, the majority (69.4%) of whom continued use 1 y later. Anxiety and daytime alertness were the only independent predictors of both acute and chronic PSA use. An increase of 1 standard deviation (SD) in alertness was associated with a 33% increase in the odds of chronic PSA use (χ2 = 4.98; odds ratio [OR] = 1.33; 95% confidence interval [CI]: 1.04–1.72; P < 0.05), and a 1-SD increase in anxiety was associated with a 41% increase (χ2 = 6.95; OR = 1.41; 95% CI: 1.09–1.82; P < 0.05). Chronic PSA users did not report any significant improvements in sleep from baseline to follow-up relative to nonusers.
Conclusions:
Hyperarousal, as indexed by daytime alertness and anxiety, is a strong determinant of PSA use among individuals with insomnia. These findings are consistent with emerging data showing that insomnia is not just a nocturnal sleep disorder, but one characterized by 24-h arousal. Though current research targets sleep disturbance, this study highlights the role of the arousal system in pharmacological treatment seeking.
Citation:
Pillai V, Cheng P, Kalmbach DA, Roehrs T, Roth T, Drake CL. Prevalence and predictors of prescription sleep aid use among individuals with DSM-5 insomnia: the role of hyperarousal. SLEEP 2016;39(4):825–832.
Keywords: alertness, anxiety, hyperarousal, insomnia, prescription sleep aids
Significance.
Though daytime impairment is an important symptom of insomnia disorder, clinical trials for pharmacologic treatments of insomnia focus primarily on its nocturnal symptoms. In addition to quantifying the prevalence of prescription sleep aid use, this study showed that daytime hyperarousal is a more reliable predictor of both current and future prescription sleep aid use among insomniacs than nocturnal sleep disturbance. This finding raises important questions: should clinicians consider daytime hyperarousal as a treatment target? Further, as insomniacs vary in the level of hyperarousal, should choice of treatment differ as a function of hyperarousal? Future research along these lines will help clinicians develop individualized treatment options for insomnia.
INTRODUCTION
In a climate of heightened concerns about the side effects and abuse liability1–3 of prescription sleep aids (PSA), identifying determinants of medication use among insomniacs is critical. However, extant studies adopt indirect techniques to address this issue. The majority of reports4–6 analyze population-level prevalence data on medications commonly used for insomnia, instead of focusing specifically on the medication use habits of individuals with insomnia. Extrapolating these findings to individuals with insomnia is especially problematic given that prescription rates for the most common sleep aids4 have grown five times more rapidly in the past 15 y than have insomnia diagnoses.7 A second set of studies8–11 are based on data from outpatient physician consultations, primarily from surveys such as the National Ambulatory Medical Care Survey and the National Disease and Therapeutic Index. Again, sampled cases in these studies are not restricted to consultations in which an insomnia diagnosis is made, but also include cases where any sleep-related difficulty is reported or an insomnia medication is prescribed. As the majority of individuals with insomnia do not consult a physician,12 these data also fail to account for shared use of PSA.13 Finally, as all aforementioned studies rely on cross-sectional data, questions about long-term PSA use remain unanswered, which is a significant limitation given the chronicity of insomnia disorder.14
To the best of our knowledge, only a single prior study examined chronic PSA use among insomniacs. Takaesu et al.15 found that approximately 55% of individuals with insomnia taking PSA at baseline continued use at a 6-mo follow-up, and further that the severity of baseline sleep disturbance was significantly associated with follow-up use. Though this study called attention to the high prevalence of chronic PSA use in individuals with insomnia, it suffered from a number of limitations. The sample was relatively small (n = 140), recruited from a single sleep clinic, and only included individuals with insomnia who were already taking prescription medications at baseline. Further, important determinants of treatment seeking, including elevated daytime alertness16 and comorbid psychopathology17 were not assessed.
The current study sought to quantify the prevalence of PSA use among individuals with insomnia, and to identify salient predictors of both baseline and chronic PSA use. We analyzed longitudinal data from a large cohort of adults with a Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)18 based insomnia disorder. PSA use was assessed at baseline and at a 1-y follow-up. Participants also reported sleep parameters, such as sleep onset latency (SOL), wake time after sleep onset (WASO), and total sleep time (TST), and completed empirically validated measures of anxiety, depression, daytime alertness, and insomnia severity at both time points. Finally, to distinguish PSA from other sleep aids, we also assessed use of over-the-counter (OTC) medications and alcohol for sleep.
METHODS
Setting and Participants
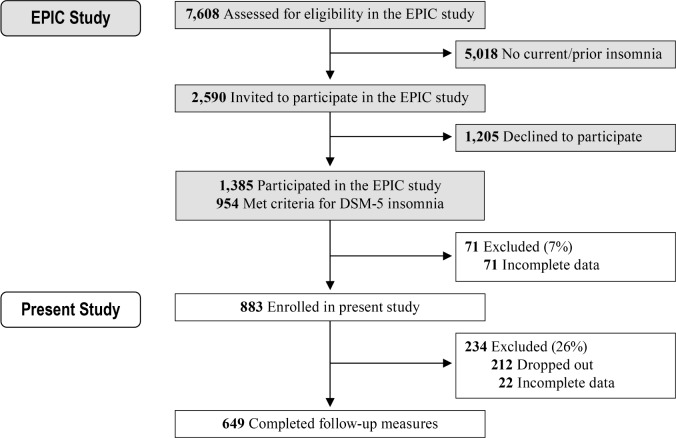
The current sample derives from the Evolution of Pathways to Insomnia Cohort (EPIC) study, a large National Institute of Mental Health-funded prospective investigation of individuals with current/previous insomnia. A detailed description of recruitment strategies and sample characteristics appears in a previous report.19 Briefly, a total of 7,608 individuals were randomly selected from a major statewide Health Maintenance Organization database, and completed a web-delivered eligibility survey that assessed for insomnia history (Figure 1). Approximately 34% (n = 2,590) of this sample met diagnostic criteria for current/prior insomnia and were invited to participate, 46% (n = 1,205) of whom declined. The remaining 1,385 individuals participated in the EPIC study. Here we present data from EPIC study participants who met the following criteria: met DSM-5 criteria for current insomnia (n = 954); completed measures of sleep disturbance, daytime sleepiness, anxiety, and depression (n = 883). A total of 649 participants from this initial sample completed follow-up measures 1 y later (retention rate: 74%).
Figure 1.
Flow of participants through the study.
Measures
Participants completed each of the following measures at both baseline and follow-up.
Insomnia
Per DSM-5, participants received a diagnosis of insomnia if they reported one or more sleep complaints (e.g., “difficulty falling asleep”; “difficulty staying asleep”) at least 3 nights a week for 3 mo or longer. Further, they had to endorse sleep related daytime distress as measured by the question, “to what extent do you consider your sleep problems to interfere with your daily functioning (e.g., ability to function at work/daily chores, concentration, memory, mood, etc.)?” Responses were coded on a 5-point scale ranging from 0 (not at all) to 4 (very much), such that participants who reported a score of 2 (somewhat) or higher were considered positive on diagnostic criteria. Participants also reported the extent of sleep disturbance during the previous month, i.e., SOL, WASO, and TST. Finally, they completed the Insomnia Severity Index (ISI), a validated index of insomnia symptom severity. Scores on the ISI range from 0 to 28, with scores ≥ 15 suggesting clinically significant insomnia symptoms.20
Use of Sleep Aids
PSA use was assessed using the following question: “in the past month, have you taken any prescription medications to help you sleep.” For each endorsed item, participants reported the frequency of use: “on average, how many times per month did you take this medication.” Participants also reported whether they had used any OTC medication or alcohol as a sleep aid, and, if so, the monthly frequency.
Daytime Impairment
Participants completed the Epworth Sleepiness Scale (ESS), the Beck Anxiety Inventory (BAI), and the 16-item Quick Inventory of Depressive Symptomatology (QIDS). The ESS is a validated measure of sleepiness and alertness.21 Overall scores on the ESS range from 0 to 24, such that high scores (> 10) indicate excessive sleepiness, whereas low scores (< 6) indicate alertness and are associated with increased sympathetic activity.22 The BAI is a psychometrically valid index of subjective anxiety and physiological hyperarousal, with an overall range of 0 to 63.23,24 Scores ≥ 16 are considered clinically significant.25 Finally, the possible range of scores on the QIDS is 0 to 27, with total scores ≥ 11 indicating moderate depression.26
Comorbid Sleep Disorders
The Berlin Apnea Questionnaire (BAQ) was used to assess risk for sleep apnea.27 Restless legs syndrome (RLS) risk was examined using an empirically validated questionnaire developed by the International RLS Study Group.28
Statistical Analysis
Chi-square tests of independence were used for univariate between-group comparisons for categorical outcomes. Logistic regression (dichotomous outcome) and analysis of covariance (ANCOVA) models (continuous outcome) were used as appropriate for estimating more complex models involving multiple covariates. Given the extensive array of variables, covariates were selected for inclusion in omnibus models if they were related to the dependent variable (DV) in univariate analyses at a significance level of P < 0.20.29 Differences between users and nonusers in sleep parameter changes from baseline to follow-up were examined using analysis of variance (ANOVA) models with change scores as the DVs. Though ANCOVA models with postscores as the DV are commonly used, recent simulation studies suggest that comparing change scores is more appropriate for designs with naturally occurring groups and two time- points.30
RESULTS
Baseline Characteristics
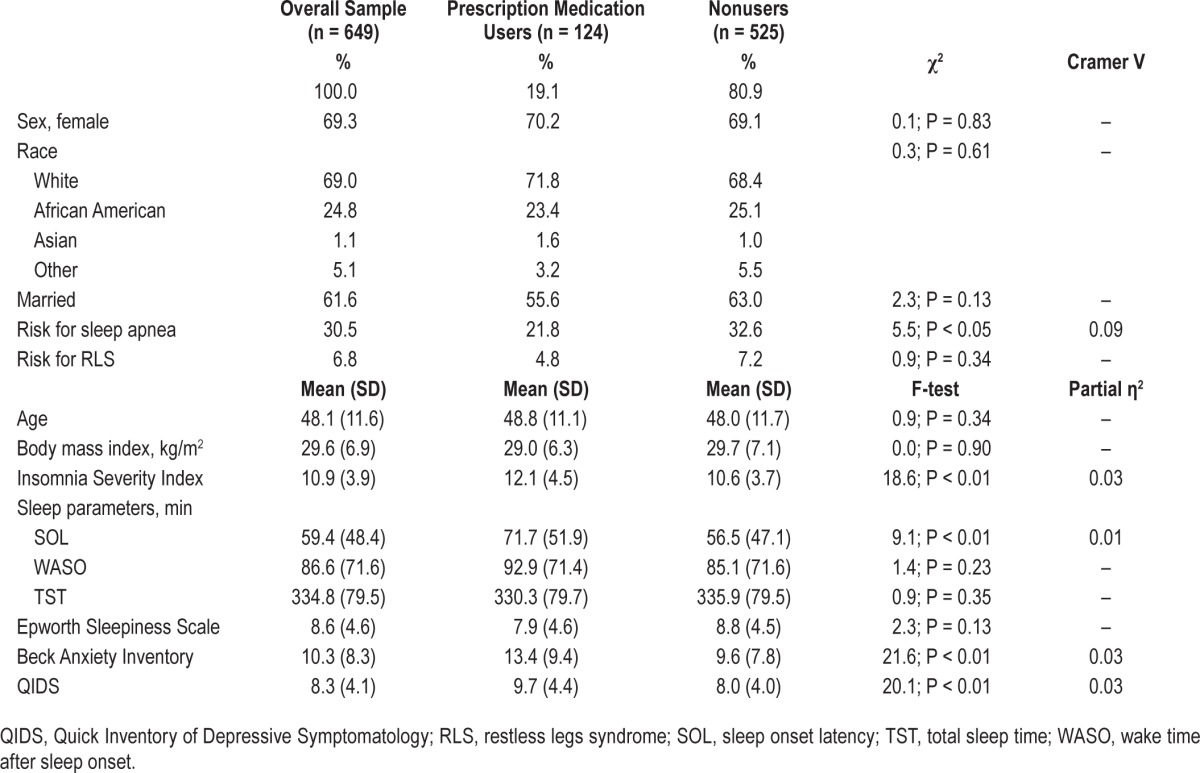
Baseline demographic and clinical characteristics appear in Table 1. Participants were middle-aged (48.14 ± 11.61 y), and, in terms of race, were 69% White, 24.8% African American, 1.1% Asian, and 5.5% other. Women accounted for 69% of the sample, reflecting the known sex prevalence of insomnia disorder.31 ESS scores (mean = 8.64 ± 4.56) were consistent with prior insomnia studies.32 Finally, mean BAI (10.33 ± 8.27) and QIDS (8.34 ± 4.11) scores indicated mild levels of anxiety and depressive symptoms.
Table 1.
Baseline sample characteristics stratified by prescription medication use.
PSA Use
At baseline, 124 participants (19.1%) endorsed PSA use, and there were 35 new cases (5.4%) at follow-up. The average baseline user reported PSA use for roughly 13 nights per month (mean = 13.08 ± 11.03), with 50% reporting more than 8 nights of use per month and 24% reporting nightly use. Further, 69.4% of baseline users (13.3% of the overall sample) engaged in chronic PSA use (i.e., endorsed PSA use at both the baseline and the follow-up assessments; chronic PSA use does not imply, however, that the same medication was used at both time points). Finally, 34.7% of PSA users reported concurrent use of OTC sleep aids at baseline, and 31.5% did so at follow-up.
Baseline Differences between Users and Nonusers
Sex, race, marital status, and risk for RLS were unrelated to PSA use. A chi-square test of independence between PSA and apnea risk was statistically significant (χ2 = 5.52, P < 0.05), such that those at risk for apnea were less likely to use (13.6%) than the remainder of the sample (21.5%). Given the aforementioned disparity in apnea risk, ANCOVA models examining between-group differences between PSA users and nonusers on all continuous variables included apnea risk as a covariate.
The overall ANCOVA model (Table 1) estimating group differences in ISI scores between users and non-users was statistically significant (Omnibus F = 13.42; Adj. R2 = 0.04; P < 0.05), with users scoring significantly higher than nonusers (F = 18.62; P < 0.05; partial η2 = 0.03). SOL differences were also statistically significant (Omnibus F = 6.11; Adj. R2 = 0.02; P < 0.01), such that users reported significantly longer SOLs than did nonusers (F = 9.06; P < 0.01; partial η2 = 0.01). ANCOVA models estimating group differences in BAI (Omnibus F = 10.91; Adj. R2 = 0.03; P < 0.01) and QIDS (Omnibus F = 15.60; Adj. R2 = 0.04; P < 0.01) scores were both statistically significant. Users scored significantly higher on both the BAI (F = 21.61; P < 0.01; partial η2 = 0.03) and the QIDS (F = 20.07; P < 0.01; partial η2 = 0.03). There were no significant differences in WASO, TST, or ESS scores.
Determinants of Baseline PSA Use
To examine the independent effects of study variables by accounting for shared variance, we fit a logistic regression model with prescription medication use (yes/no) as the DV, and ISI, SOL, OSA risk, ESS, BAI, and QIDS as independent variables (IV); these variables were related to PSA use at P < 0.20 in univariate analyses. Age was also included in this model, given prior research on increased PSA use among older adults.8 A test of the model with all predictors against a constant-only model was statistically significant (χ2 = 45.47; P < 0.01), indicating that the model reliably distinguished between PSA users and nonusers. The Hosmer-Lemeshow test revealed that this model fit the data well (χ2 = 6.22; P = 0.62). SOL (P = 0.20), ISI scores (P = 0.06), and QIDS scores (P = 0.14) scores were not significantly associated with use. However, a 1-SD increase on the BAI was associated with a 28% increase in the odds of PSA use (χ2 = 4.46; OR = 1.28; 95% CI: 1.02–1.62; P < 0.05). ESS scores had a significant inverse effect (χ2 = 3.96; OR = 1.25; 95% CI: 1.00–1.55; P < 0.05), such that a 1-SD decrease in ESS scores (i.e., a 1-SD increase in alertness) was associated with a 25% increase in the odds of PSA use. Finally, age (χ2 = 4.25; OR = 1.02; 95% CI: 1.00–1.04; P < 0.05) and OSA risk (χ2 = 6.00; OR = 0.53; 95% CI: 0.32–0.88; P < 0.05) were also significantly associated with PSA use.
Determinants of Chronic PSA Use
We fit a logistic regression model with the same predictors to estimate chronic PSA use (i.e., endorsed prescription medication use at both baseline and follow-up). The overall model was statistically significant (χ2 = 38.38; P < 0.01), and the Hosmer-Lemeshow test indicated a good fit (χ2 = 12.22; P = 0.14). BAI scores were significantly associated with chronic use, such that a 1-SD increase on the BAI was associated with a 41% increase in the odds of chronic PSA use (χ2 = 6.95; OR = 1.41; 95% CI: 1.09–1.82; P < 0.05). Further, a 1-SD decrease in ESS scores was associated with a 33% increase in the odds of chronic PSA use (χ2 = 4.98; OR = 1.33; 95% CI: 1.04–1.72; P < 0.05). Age (P = 0.10), SOL (P = 0.47), QIDS (P = 0.24), OSA risk (P = 0.12), and ISI (P = 0.11) were not significantly related to chronic PSA use.
Changes in Sleep Disturbance among Users and Nonusers
There were no significant differences between users and non-users in SOL (P = 0.91), TST (P = 0.99), WASO (P = 0.14), or ISI (P = 0.47) changes from baseline to follow-up. Similarly, there were no differences between chronic PSA users and non-users in SOL (P = 0.99), TST (P = 0.96), WASO (P = 0.65), or ISI (P = 0.24) changes.
ESS Scores
To further explore the association between prescription use and ESS scores, we examined the distribution of this variable. Though low on average, ESS scores were normally distributed and occupied the full range of the instrument (median = 8; range = 0–23; bottom tertile: 6; top tertile: 10). The sample was therefore divided into three groups: hyperaroused individuals with insomnia (ESS ≤ 6); alert individuals with insomnia (ESS: 7 through 10); and sleepy individuals with insomnia (ESS ≥ 11). The following analyses involved group comparisons between the sleepy and hyperaroused individuals with insomnia. A chi-square test of independence revealed a significant association between ESS scores and PSA use (χ2 = 4.57; P < 0.05), such that hyperaroused individuals with insomnia were significantly more likely to use (23.9%) than were sleepy individuals with insomnia (15.7%); see Figure 2. Proportion of chronic PSA use was also significantly greater (χ2 = 4.51; P < 0.05) among hyperaroused individuals with insomnia (17.4%) than sleepy individuals with insomniacs (10.3%).
Figure 2.
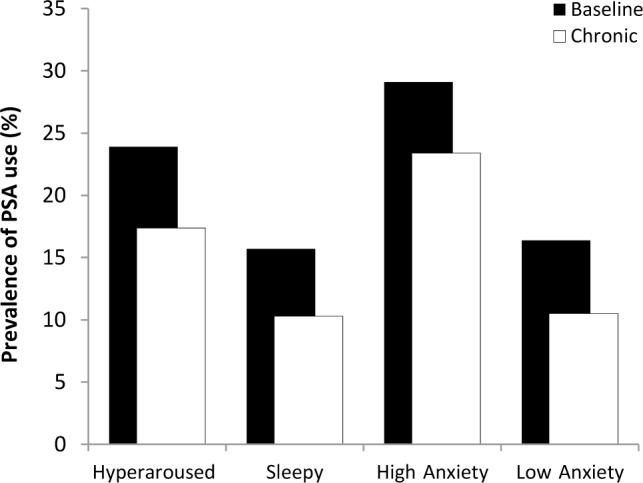
Prevalence of baseline and chronic prescription sleep aid use by daytime sleepiness and anxiety. Prevalence of both baseline and chronic use were significantly higher in the hyperaroused and high anxiety groups than in the sleepy and low anxiety groups respectively; all P values < 0.05.
BAI Scores
As noted previously, BAI scores were significantly higher among users than nonusers. To examine the clinical utility of this finding, we examined whether likelihood of usage varied significantly per clinical cutoffs for the BAI.25 Accordingly, the sample was divided into high- (BAI ≥ 16) and low anxiety (BAI < 16) groups. A chi-square test of independence between anxiety and PSA use was statistically significant (χ2 = 11.51; P < 0.01), such that the prevalence of use in the high anxiety group (29.1%) was significantly higher than in the low anxiety group (16.4%). The association between anxiety and chronic PSA use was also statistically significant (χ2 = 16.08; P < 0.01), such that the proportion of chronic PSA users in the high anxiety group (23.4%) was more than twice that in the low anxiety group (10.5%).
OTC Medication Use
At baseline, 172 participants (26.5%) endorsed the use of OTC medications as a sleep aid, 59.9% of whom also endorsed use at the follow-up assessment. Importantly, 25% of baseline OTC medication users also endorsed concurrent PSA use. Frequency of OTC medication use had a mean of 10.71 ± 9.69 days/mo, with a median of 6.5 days/mo. Sex was significantly associated with OTC medication use (χ2 = 7.02; P < 0.01), such that the prevalence of use was higher among women (29.6%) than among men (19.6%). None of the other study variables were significantly associated with likelihood of use. With respect to frequency of use, an OLS regression model with sex, race, WASO, ISI scores, and QIDS scores was statistically significant (Omnibus F = 3.92; Adj. R2 = 0.13; P < 0.01). ISI scores were significantly associated with frequency of OTC medication use (β = 0.23; t = 2.57; P < 0.05), such that higher ISI scores were associated with higher frequency. Further, frequency of OTC medication use was significantly higher (β = 0.23; t = 2.93; P < 0.01) among White participants (11.72 ± 9.93 days/mo) than among African-Americans (6.54 ± 7.21 days/mo). Note that given the low prevalence of Asian and other race in the current sample, all analyses on the race variable were conducted for its two most prevalent categories (White and African-American). Sex (P = 0.40), QIDS scores (P = 0.49), and OSA risk (P = 0.19) were unrelated to frequency.
Changes in Sleep Disturbance among Users and Nonusers
To examine differences between users and nonusers in sleep parameter changes from baseline to follow-up, we ran ANCOVA models with change scores as the DVs, OTC medication use as the IV, and sex as a covariate. There were no significant differences between user and non-users in SOL (P = 0.80), TST (P = 0.89), WASO (P = 0.23), and ISI (P = 0.17) changes. We also examined differences in sleep parameter changes between chronic OTC medication users and nonusers. ANCOVA model with change scores as the DV, and sex as a covariate revealed no difference between chronic OTC medication users and non-users in SOL (P = 0.98), TST (P = 0.74), WASO (P = 0.67), or ISI (P = 0.62) changes.
Alcohol Use
At baseline, 81 participants (12.5%) endorsed alcohol use as a sleep aid, 22% of whom also endorsed concurrent PSA use. Frequency of use had a mean of 6.59 ± 7.08 days/mo with a median of 4 days/mo. Of the baseline users, 53.1% (n = 43) reported continued use at follow-up. A chi-square test of independence (χ2 = 9.82; P < 0.01) indicated that the prevalence of alcohol use among men (18.6%) was significantly higher than that among women (9.8%). None of the other study variables were significantly associated with alcohol use as a sleep aid.
Changes in Sleep Disturbance among Users and Nonusers
To examine differences between users and nonusers in sleep parameter changes from baseline to follow-up, we ran ANCOVA models with change scores as the DVs, alcohol use as the IV, and sex as a covariate. There were no significant differences between users and nonusers in SOL (P = 0.31), TST (P = 0.85), WASO (P = 0.78), and ESS (P = 0.81) changes. Further, there were no differences between chronic alcohol users and nonusers in SOL (P = 0.62), WASO (P = 0.98), TST (P = 0.45) changes. However, non-users reported a significantly higher improvement in ESS scores (F = 5.08; P < 0.05; partial η2 = 0.01) from baseline (mean = 10.24 ± 3.44) to follow-up (mean = 7.87 ± 3.54) than did chronic alcohol users (baseline: mean = 11.11 ± 4.18; follow-up: mean = 10.89 ± 4.29).
DISCUSSION
Pharmacotherapy for insomnia has been in flux over the past few decades due to: mounting concerns about over-use of PSAs;7 increased access to nonpharmacological treatment options;33 and changes in the prescribing habits of physicians.4,5,7,11 Nearly all current studies focus on trends in PSA use at the population level, and do not distinguish insomnia disorder from transient sleep disturbance. The current study is the first to prospectively examine the prevalence and predictors of acute and chronic PSA use directly among insomniacs as defined by the newly established and more empirically robust DSM-5 criteria.18,34 Baseline prevalence of PSA use in the current sample of individuals with insomnia was 19%, which, as expected, was considerably higher than the general population prevalence estimate of 3%.4 Notably, almost 70% of this group continued PSA use at the 1-y follow-up, suggesting high rates of chronic PSA use. Chronic PSA users did not show any significant improvements in sleep from baseline to follow-up relative to nonusers.
Apnea risk was significantly lower among baseline PSA users (22%) than nonusers (33%). This finding is understandable given that the debate on the safety of PSA, especially benzodiazepines, in patients with OSA has not been adequately resolved.35,36 Therefore, physicians likely avoid PSA in the treatment of insomnia patients with comorbid OSA. Though baseline differences in SOL, insomnia severity, depressive and anxiety symptoms between users and nonusers were also statistically significant, effect sizes were small. However, baseline anxiety and daytime alertness were robust, independent predictors of both baseline and chronic PSA use. Together, these data point to hyperarousal,37,38 as manifested by anxiety and elevated alertness, as a reliable determinant of PSA use in insomnia.
Hyperarousal and PSA Use
Elevated Alertness
There is growing consensus that elevated alertness as evidenced by low scores on the ESS is not only an important clinical feature of insomnia disorder19,39 but also an index of physiological hyperarousal.22,40 In this study, elevated alertness was a strong determinant of PSA use. The prevalence of both baseline and chronic PSA use was significantly higher in the top tertile of alertness (ESS ≤ 6) than in the bottom tertile (ESS ≥ 11). Alertness was significantly associated with both baseline and chronic PSA use, even after controlling for insomnia severity as well as depressive and anxiety symptoms. Indeed, a 1-SD increase in alertness was associated with a 33% increase in the odds of chronic PSA use.
These findings are consistent with research on alertness in insomnia as operationalized by the multiple sleep latency test (MSLT). In an 8-mo randomized clinical trial of zolpidem versus placebo, insomniacs with elevated MSLTs at baseline evidenced similar elevations on the MSLT at the 8-mo follow-up regardless of group (treatment vs. placebo) membership.41 Alertness thus appeared to be a stable, traitlike feature of insomnia, and was largely independent of nocturnal sleep disturbance. Importantly, insomniacs with high MSLTs in both the placebo and active treatment groups were significantly more likely to increase self-administration of “medication.”42 Together, these data suggest that insomnia characterized by elevated alertness is a phenotype that is significantly associated with PSA use, and is also a potential risk factor for PSA abuse. Though further research is needed to fully address this hypothesis, the current study makes a significant contribution by demonstrating that a self-report instrument can capture alertness as it relates to PSA use.
Anxiety
Anxiety was significantly associated with PSA use, such that a 1-SD increase on the BAI was associated with a 41% increase in the odds of chronic PSA use. Further, the prevalence of chronic PSA use among individuals with insomnia (∼23%) with clinically significant anxiety levels was more than double that in the remainder of the sample (∼11%). Depressive symptoms, by contrast, were not related to the likelihood or frequency of PSA use. These findings are highly intriguing in light of the substantial comorbidity between insomnia, depression, and anxiety.43,44
The empirically based tripartite model of anxiety and depression offers a framework within which these results may be reconciled.45 This model attributes the significant overlap between depression and anxiety to a shared diagnostic factor called “general distress,” which captures the negative affect states complicit in both disorders. The model, however, discriminates between anhedonia, which is germane to depression, and physiological hyperarousal, which is more akin to anxiety. Substantial literature now supports the BAI as an index of such hyperarousal.23,24,46 The current data suggest that hyperarousal may be a unique predictor of PSA use. Preliminary support for this finding may be gleaned from recent research on “anxiety sensitivity,” a transdiagnostic construct referring to heightened awareness of and aversion to somatic hyperarousal.47 Studies show that anxiety sensitivity can impede sleep onset by directing attention toward somatic sensations believed to be incompatible with sleep,48 thus triggering further arousal in a manner consistent with the cognitive model of insomnia.49,50 Similarly, during the daytime, anxiety sensitivity can lead to a preoccupation with fatigue and other perceived signs of sleep loss,51 thereby providing an impetus for treatment seeking.
Alcohol and OTC Medication Use
Characteristics of alcohol use as a sleep aid among insomniacs in this study were strikingly similar to that observed in a prior general population survey of the Detroit metropolitan area.6 At approximately 13%, the overall prevalence of alcohol use as a sleep aid was identical among both the insomniacs and the general population. Sex significantly moderated use in both studies such that the prevalence in men was nearly double that found in women. Finally, insomniacs in the current study who abstained from alcohol experienced a normalization of daytime sleepiness from baseline (mean ESS = 10.24 ± 3.44) to follow-up (mean ESS = 7.87 ± 3.54); this reduction in ESS scores was significantly greater than that for chronic alcohol users whose scores remained largely the same. Alcohol users in the general population also had significantly higher levels of daytime sleepiness than abstainers. These findings offer ecological support for laboratory data that show that alcohol can disrupt sleep maintenance and that tolerance to the hypnotic effects develops quickly.52,53
Use of OTC medications as sleep aids was quite high, with 27% of the sample reporting use, almost 60% of whom engaged in chronic OTC medication use. Further, over 30% of PSA users engaged in concurrent use of OTC medications at both baseline and follow-up, thus supporting concerns raised in prior studies about polypharmacy in insomnia.4 Prevalence of OTC medication use was significantly higher among women than men, whereas frequency of use was significantly higher among White participants than among African-Americans. These findings are intriguing given that there were no sex or race effects on PSA use; most studies report a higher prevalence of both OTC and prescription sleep aids among female and White participants.4,6 However, these studies were population based and not specific to insomnia per se.
Limitations and Future Directions
A primary limitation of this study is that participants did not indicate which specific PSAs they were taking or the associated dose. Further, all PSA use data were self-reported and lacked the benefit of objective verification from medical records. It was therefore unclear whether PSA use was reflective of physician guidelines or self-directed by patients. These limitations notwithstanding, the identification in the current study of hyperarousal as a determinant of PSA use is an innovative paradigm shift from previous research that has focused primarily on sleep disturbance. There is now considerable evidence that insomnia is not just a nocturnal disorder, but one characterized by 24-h arousal.54 Should clinicians consider hyperarousal as a treatment target in insomnia?
The current report is part of a small but consistent literature that highlights daytime hyperarousal as a predictor of treatment seeking in insomnia.16,17,55 Stepanski et al.16 compared insomniacs who sought treatment at a sleep clinic with insomniacs who volunteered for research. Though the groups did not differ on any nocturnal sleep parameters, insomniacs from the sleep clinic were significantly more hyperaroused as evidenced by high MSLT means. In another study that examined daytime and nighttime self-administration of a hypnotic, hyperarousal was a significant predictor of daytime hypnotic self-administration.55 Specifically, insomniacs with high baseline MSLT means were significantly more likely to engage in daytime hypnotic self-administration. Importantly, daytime hypnotic use significantly lowered MSLT means among hyperaroused insomniacs, suggesting that their daytime self-administration was reflective of therapy seeking. Together, these findings indicate that insomniacs may seek treatment not just for nocturnal sleep disturbance, but also for daytime hyperarousal.
DISCLOSURE STATEMENT
This study was supported by an NIMH Grant (R01 MH082785) and an investigator-initiated research award from Merck, both to Dr. Drake. Dr. Roth has served as consultant for Abbott, Accadia, AstraZenca, Aventis, AVER, Bayer, BMS, Cypress, Ferrer, Glaxo Smith Kline, Impax, Intec, Jazz, Johnson and Johnson, Merck, Neurocrine, Novartis, Proctor and Gamble, Pfizer, Purdue, Shire, Somaxon, Transcept; has received research support from Cephalon, Merck, and Transcept; and has served on speakers bureau for Purdue. Dr. Roehrs has consulted for Elan, Sanofi- Aventis, and Sepracor and has participated in speaking engagements for Sanofi-Aventis and Sepracor. Dr. Drake has served as consultant for Teva; has received research support from Merck and Teva; and has served on speakers bureau for Jazz, Merck, and Teva. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22:1335–50. doi: 10.1007/s11606-007-0251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hajak G, Muller WE, Wittchen HU, Pittrow D, Kirch W. Abuse and dependence potential for the non-benzodiazepine hypnotics zolpidem and zopiclone: a review of case reports and epidemiological data. Addiction (Abingdon, England) 2003;98:1371–8. doi: 10.1046/j.1360-0443.2003.00491.x. [DOI] [PubMed] [Google Scholar]
- 3.Rush CR, Baker RW, Wright K. Acute behavioral effects and abuse potential of trazodone, zolpidem and triazolam in humans. Psychopharmacology. 1999;144:220–33. doi: 10.1007/s002130050997. [DOI] [PubMed] [Google Scholar]
- 4.Bertisch SM, Herzig SJ, Winkelman JW, Buettner C. National use of prescription medications for insomnia: NHANES 1999-2010. Sleep. 2014;37:343–9. doi: 10.5665/sleep.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roehrs T, Roth T. ‘Hypnotic’ prescription patterns in a large managed-care population. Sleep Med. 2004;5:463–6. doi: 10.1016/j.sleep.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Johnson EO, Roehrs T, Roth T, Breslau N. Epidemiology of alcohol and medication as aids to sleep in early adulthood. Sleep. 1998;21:178–86. doi: 10.1093/sleep/21.2.178. [DOI] [PubMed] [Google Scholar]
- 7.Moloney ME, Konrad TR, Zimmer CR. The medicalization of sleeplessness: a public health concern. Am J Public Health. 2011;101:1429–33. doi: 10.2105/AJPH.2010.300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balkrishnan R, Rasu RS, Rajagopalan R. Physician and patient determinants of pharmacologic treatment of sleep difficulties in outpatient settings in the United States. Sleep. 2005;28:715–9. doi: 10.1093/sleep/28.6.715. [DOI] [PubMed] [Google Scholar]
- 9.Ford ES, Wheaton AG, Cunningham TJ, Giles WH, Chapman DP, Croft JB. Trends in outpatient visits for insomnia, sleep apnea, and prescriptions for sleep medications among US adults: findings from the National Ambulatory Medical Care survey 1999-2010. Sleep. 2014;37:1283–93. doi: 10.5665/sleep.3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai LL, Tan MH, Lai YC. Prevalence and factors associated with off-label antidepressant prescriptions for insomnia. Drug Healthc Patient Saf. 2011;3:27–36. doi: 10.2147/DHPS.S21079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh JK, Schweitzer PK. Ten-year trends in the pharmacological treatment of insomnia. Sleep. 1999;22:371–5. [PubMed] [Google Scholar]
- 12.Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999;22(Suppl 2):S347–53. [PubMed] [Google Scholar]
- 13.Goldsworthy RC, Schwartz NC, Mayhorn CB. Beyond abuse and exposure: framing the impact of prescription-medication sharing. Am J Public Health. 2008;98:1115–21. doi: 10.2105/AJPH.2007.123257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janson C, Lindberg E, Gislason T, Elmasry A, Boman G. Insomnia in men--a 10-year prospective population based study. Sleep. 2001;24:430. doi: 10.1093/sleep/24.4.425. [DOI] [PubMed] [Google Scholar]
- 15.Takaesu Y, Komada Y, Asaoka S, Kagimura T, Inoue Y. Factors associated with long-term use of hypnotics among patients with chronic insomnia. PloS One. 2014;9:e113753. doi: 10.1371/journal.pone.0113753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stepanski E, Koshorek G, Zorick F, Glinn M, Roehrs T, Roth T. Characteristics of individuals who do or do not seek treatment for chronic insomnia. Psychosomatics. 1989;30:421–7. doi: 10.1016/S0033-3182(89)72248-9. [DOI] [PubMed] [Google Scholar]
- 17.Davidson JR, Aime A, Ivers H, Morin CM. Characteristics of individuals with insomnia who seek treatment in a clinical setting versus those who volunteer for a randomized controlled trial. Behav Sleep Med. 2009;7:37–52. doi: 10.1080/15402000802577769. [DOI] [PubMed] [Google Scholar]
- 18.American Psychological Association. 5th ed. Arlington, VA: American Psychiatric Publishing Inc.; 2013. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 19.Pillai V, Roth T, Drake CL. The nature of stable insomnia phenotypes. Sleep. 2015;38:127–38. doi: 10.5665/sleep.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–8. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 22.Taranto Montemurro L, Floras JS, Millar PJ, et al. Inverse relationship of subjective daytime sleepiness to sympathetic activity in patients with heart failure and obstructive sleep apnea. Chest. 2012;142:1222–8. doi: 10.1378/chest.11-2963. [DOI] [PubMed] [Google Scholar]
- 23.Brown TA, McNiff J. Specificity of autonomic arousal to DSM-IV panic disorder and posttraumatic stress disorder. Behav Res Ther. 2009;47:487–93. doi: 10.1016/j.brat.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joiner TE, Jr, Steer RA, Beck AT, Schmidt NB, Rudd MD, Catanzaro SJ. Physiological hyperarousal: construct validity of a central aspect of the tripartite model of depression and anxiety. J Abnorm Psychol. 1999;108:290–8. doi: 10.1037//0021-843x.108.2.290. [DOI] [PubMed] [Google Scholar]
- 25.Beck AT, Steer RA. San Antonia, TX: Psychological Corporation; 1993. Beck Anxiety Inventory Manual. [Google Scholar]
- 26.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 27.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 28.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 29.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–37. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 30.Jamieson J. Analysis of covariance (ANCOVA) with difference scores. Int J Psychophysiology. 2004;52:277–83. doi: 10.1016/j.ijpsycho.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69:592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 32.Sanford SD, Lichstein KL, Durrence HH, Riedel BW, Taylor DJ, Bush AJ. The influence of age, gender, ethnicity, and insomnia on Epworth sleepiness scores: a normative US population. Sleep Med. 2006;7:319–26. doi: 10.1016/j.sleep.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Harvey AG, Tang NK. Cognitive behaviour therapy for primary insomnia: can we rest yet? Sleep Med Rev. 2003;7:237–62. doi: 10.1053/smrv.2002.0266. [DOI] [PubMed] [Google Scholar]
- 34.Morin CM, Leblanc M, Ivers H, et al. Monthly fluctuations of insomnia symptoms in a population-based sample. Sleep. 2014;37:319–26. doi: 10.5665/sleep.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atwood CW, Jr, Strollo PJ, Jr, Givelber R. Medical therapy for obstructive sleep apnea. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 5th ed. Philadelphia: WB Saunders; 2011. pp. 1219–32. [Google Scholar]
- 36.Hanly P, Powles P. Hypnotics should never be used in patients with sleep apnea. J Psychosom Res. 1993;37(Suppl 1):59–65. [PubMed] [Google Scholar]
- 37.Fernandez-Mendoza J, Shaffer ML, Olavarrieta-Bernardino S, et al. Cognitive-emotional hyperarousal in the offspring of parents vulnerable to insomnia: a nuclear family study. J Sleep Res. 2014;23:489–98. doi: 10.1111/jsr.12168. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Vgontzas AN, Fernandez-Mendoza J, et al. Insomnia with physiological hyperarousal is associated with hypertension. Hypertension. 2015;65:644–50. doi: 10.1161/HYPERTENSIONAHA.114.04604. [DOI] [PubMed] [Google Scholar]
- 39.Severson CA, Tsai WH, Ronksley PE, Pendharkar SR. Identification of insomnia in a sleep center population using electronic health data sources and the insomnia severity index. J Clin Sleep Med. 2013;9:655–60. doi: 10.5664/jcsm.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taranto Montemurro L, Floras JS, Picton P, et al. Relationship of heart rate variability to sleepiness in patients with obstructive sleep apnea with and without heart failure. J Clin Sleep Med. 2014;10:271–6. doi: 10.5664/jcsm.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roehrs TA, Randall S, Harris E, Maan R, Roth T. MSLT in primary insomnia: stability and relation to nocturnal sleep. Sleep. 2011;34:1647–52. doi: 10.5665/sleep.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roehrs T, Randall S, Roth T. Chronic hypnotic self-administration and hyperarousal in insomnia. Sleep. 2012;35:A219. (Abstract Suppl) [Google Scholar]
- 43.Papadimitriou GN, Linkowski P. Sleep disturbance in anxiety disorders. Int Rev Psychiatry (Abingdon, England) 2005;17:229–36. doi: 10.1080/09540260500104524. [DOI] [PubMed] [Google Scholar]
- 44.Staner L. Comorbidity of insomnia and depression. Sleep Med Rev. 2010;14:35–46. doi: 10.1016/j.smrv.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 45.Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. J Abnorm Psychol. 1995;104:15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
- 46.Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther. 1995;33:335–43. doi: 10.1016/0005-7967(94)00075-u. [DOI] [PubMed] [Google Scholar]
- 47.Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav Res Ther. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- 48.Babson KA, Trainor CD, Bunaciu L, Feldner MT. An examination of anxiety sensitivity as a moderator of the relation between sleep anticipatory anxiety and sleep onset latency. J Cogn Psychother. 2008;22:258–70. [Google Scholar]
- 49.Espie CA. Understanding insomnia through cognitive modelling. Sleep Med. 2007;8:70002–9. doi: 10.1016/S1389-9457(08)70002-9. [DOI] [PubMed] [Google Scholar]
- 50.Harvey AG, Tang NK, Browning L. Cognitive approaches to insomnia. Clin Psychol Rev. 2005;25:593–611. doi: 10.1016/j.cpr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Short NA, Allan NP, Raines AM, Schmidt NB. The effects of an anxiety sensitivity intervention on insomnia symptoms. Sleep Med. 2015;16:152–9. doi: 10.1016/j.sleep.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Prinz PN, Roehrs TA, Vitaliano PP, Linnoila M, Weitzman ED. Effect of alcohol on sleep and nighttime plasma growth hormone and cortisol concentrations. J Clin Endocrinol Metab. 1980;51:759–64. doi: 10.1210/jcem-51-4-759. [DOI] [PubMed] [Google Scholar]
- 53.Roehrs T, Roth T. Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Med Rev. 2001;5:287–97. doi: 10.1053/smrv.2001.0162. [DOI] [PubMed] [Google Scholar]
- 54.Bonnet MH, Arand DL. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med. 1998;60:610–5. doi: 10.1097/00006842-199809000-00017. [DOI] [PubMed] [Google Scholar]
- 55.Roehrs T, Bonahoom A, Pedrosi B, Zorick F, Roth T. Nighttime versus daytime hypnotic self-administration. Psychopharmacology. 2002;161:137–42. doi: 10.1007/s00213-002-1041-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.