Abstract
Study Objectives:
Spontaneous saliva swallows (SS) appear especially during sleep. The rate of SS was rarely investigated in all-night sleep in patients with Parkinson disease (PD). Dysphagia is a frequent symptom in PD, but the rate of SS was never studied with an all-night sleep electroencephalogram (EEG).
Methods:
A total of 21 patients with PD and 18 age-matched healthy controls were included in the study. Frequencies of SS and coughing were studied in all-night sleep recordings of patients with PD and controls. During all-night sleep, video-EEG 12-channel recording was used including the electromyography (EMG) of the swallowing muscles, nasal airflow, and recording of vertical laryngeal movement using a pair of EEG electrodes over the thyroid cartilage.
Results:
The total number of SS was increased while the mean duration of sleep was decreased in PD when compared to controls. Sialorrhea and clinical dysphagia, assessed by proper questionnaires, had no effect in any patient group. The new finding was the so-called salvo type of consecutive SS in one set of swallowing. The amount of coughing was significantly increased just after the salvo SS.
Conclusions:
In PD, the rate of SS was not sufficient to demonstrate the swallowing disorder, such as oropharyngeal dysphagia, but the salvo type of SS was quite frequent. This is a novel finding and may contribute to the understanding of swallowing problems in patients with dysphagic or nondysphagic PD.
Citation:
Uludag IF, Tiftikcioglu BI, Ertekin C. Spontaneous swallowing during all-night sleep in patients with Parkinson disease in comparison with healthy control subjects. SLEEP 2016;39(4):847–854.
Keywords: cough, dysphagia, Parkinson disease, sleep, swallowing
Significance.
Investigations of spontaneous saliva swallowing during all night sleep can be a valuable tool in the diagnosis of dysphagia in neurologic disorders. This is the first study to assess all-night swallowing in patients with Parkinson's disease (PD) and control subjects using multimodal electrophysiologic recording methods. We define a novel type of swallowing pattern we termed salvo swallowing, which is characterized by a burst of four or more consecutive spontaneous swallows within a single swallow event. We showed that salvo swallows are increased in PD. Future studies are needed to determine whether salvo swallows are an indication of silent dysphagia in PD and other neurologic disorders.
INTRODUCTION
There are two types of swallowing: voluntary swallowing (VS), and spontaneous saliva swallowing (SS). SS is usually an unconscious activity mainly occurring in times other than mealtimes, especially during sleep.1 In fact, SS can be considered as a reflex mechanism protecting the respiratory tract from aspiration of saliva and food residue.1,2 Surprisingly, very few studies have investigated SS despite its critical importance.
The rate of SS for certain time periods during wakefulness was reported as 1 per minute.3–11 There are few studies that have investigated the frequency of SS during sleep.9,12 However, investigation of SS frequency during all-night sleep has attracted much less attention.13–15 Nonetheless, the previous sleep studies were conducted on normal controls and continuous video-electroencephalography (EEG) monitoring was not used. All-night sleep and SS features in patients with neurological disorders have been rarely reported, until now.
Evaluation of SS during sleep deserves more attention in Parkinson disease (PD). We hypothesized that in PD, the increased saliva accumulation in the oral cavity in a patient in the supine position at night increases the frequency of SS and leads to silent and/or apparent aspiration to the airway, which trigger the cough reflex afterward. Therefore, studying all-night sleep EEG with continuous video monitoring and evaluation of frequency and distribution of SS in the different sleep stages, together with cough and its relationship with possible aspiration, would substantially increase our understanding of dysphagia in patients with PD. In order to demonstrate the early involvement of subclinical oropharyngeal dysphagia in PD, we evaluated SS and its relation to all-night sleep, not including the PD patients with severe dysphagia. In addition, we compared these findings with those obtained from age-matched normal control subjects (NC).
METHODS
Study Subjects
Eighteen healthy adult participants (14 female and 4 male) without any diseases affecting the oropharyngeal or respiratory system or any sleep disorders such as sleep apnea or bruxism were investigated. NC in whom the presence of sleep bruxism and sleep apnea was suspected were excluded from the study. Their mean age was 63.5 y (range, 51–74 y).
Twenty-one patients with PD (8 female, 13 male) were investigated. Their mean age was 68.0 y (range, 51–80 y). Diagnosis of PD was based on UK Brain Bank Criteria.12,16 The clinical picture of the disease is described with the unified PD rating scale.17 Their clinical disability was measured according to the Hoehn-Yahr scale criteria.18
Sialorrhea severity was scored according to the Drooling Severity and Frequency Scale (DSFS-P), which has been adapted and validated for use in patients with PD.19 Severity was scored as follows: 1 = no complaints; 2 = feeling of increased saliva in the mouth, but no drooling; 3 = loss of saliva in the corners of the mouth or the chin; 4 = saliva also on clothes; 5 = saliva on clothes and also on books or the floor. Frequency was scored as follows: 1 = never or less than once a day; 2 = once or twice a day; 3 = 2–5 times a day; 4 = 6–10 times a day; 5 = almost constantly. The summarized score ranges from 2 to 10.
Degree of dysphagia was graded according to Ertekin et al.20 as follows: grade 1 = No complaint and no clinical findings of dysphagia; grade 2 = Dysphagia was suspected by patients complaints; however, the clinical examination did not support the presence of dysphagia; grade 3 = Dysphagia was clinically evident, but the patient could manage the swallowing by measures other than nonoral feeding; grade 4 = Obvious clinical dysphagia including aspiration and nonoral feeding. Dysphagia was clinically examined in detail by one of the examiners (CE).
This study was approved by the local ethics committee and informed consent was obtained from each study participant.
Electrophysiological Recording
In all-night sleep recording, normal control subjects and patients with PD were hospitalized for 1 night from 20:00 to 08:00. Nocturnal video-polygraphic recordings were collected using the Grass-Telefactor Beehive Millennium Video EEG Monitoring system (Grass Products, Natus Neurology, Middleton, WI). We simultaneously recorded EEG, electrooculogram (EOG), respiration (nasal airflow), electrocardiogram (ECG), electrodermal activity (EDA), vertical laryngeal movement, and electromyography (EMG) activities of the orbicularis oris (OR), masseter (MS) and suprahyoid/submental muscle group (SM). Silver cup electrodes of 10-mm diameter were used for EEG, EOG, and EMG recordings. EEG was obtained with electrodes placed over C3, C4, O1, O2, A1, and A2 according to the international 10–20 system. Laryngeal movement is recorded by two electrodes placed 2 cm apart vertically over the thyroid cartilage. The up and down movement of the larynx during swallowing can easily be measured using vertically oriented pairs of EEG electrodes. In addition, fixation and stability of electrodes during sleep were satisfactory to record the laryngeal movement during swallowing. The time scale was 30 mm/sec. The data were recorded to external memory. These values were changed in the offline analysis.
Sleep stages were scored epoch by epoch by a sleep technologist and one of us (IFU or BIT). The standard method for identifying and scoring sleep stages was used.21 Sleep variables including total sleep time (TST), sleep latency, sleep efficiency, duration of nonrapid eye movement (NREM) and rapid eye movement (REM) stages, REM sleep latency were calculated. In addition, numbers of awakenings, arousals, sleep stage shifts, masseter activity, breathing, and apnea were counted. Sleep latency was defined as the time until the first minute of stage N1 (NREM 1 sleep) followed by at least 10 min of this stage, not interrupted by awakenings. Sleep efficiency was defined as TST/time in bed (TIB). REM sleep latency was defined as the time from the onset of sleep until onset of the first REM sleep period. Arousal was defined as an abrupt change in sleep from a deeper stage to a lighter stage. Awakening was defined as a change from sleep to wakefulness.
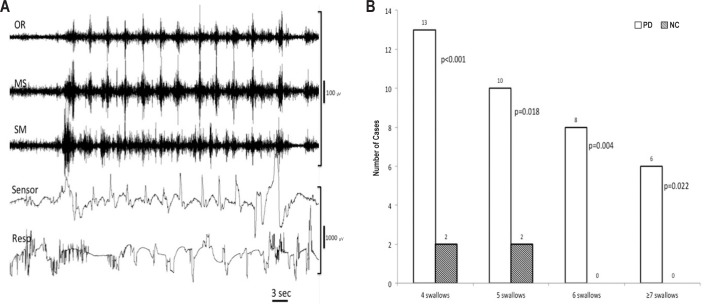
The SSs were counted visually during the offline mode. We used the criteria of SS as described previously7: (1) The OR, MS, and SM muscles had to fire together in a burst that lasted 1–2 sec. The SM-EMG was often highest in amplitude during this activity. (2) All three aforementioned muscles had to be recorded in parallel with a synchronous, laryngeal deflexion, indicating vertical movements of the larynx during swallowing. (3) During the recording one EEG-sleep technician ensured the upward and downward movement of thyroid cartilage and swallowing movement beside the video of the subjects. (4) In all three muscles, the response amplitudes for deglutition had to be at least four times higher than baseline. (5) During deglutitional activity, all swallowing muscle should be synchronously activated by the swallowing apnea obtained from the cessation of respiratory rhythm in the nasal airflow recording (Figure 1).
Figure 1.
Spontaneous swallowing (SS) simultaneously recorded from scalp, respiration, orbicularis oris (OR), masseter (MS), suprahyoid/ submental muscle group (SM), nasal airflow, and electrocardiogram (ECG) electrodes. Note the apnea period in respiration (RESP.) and up and down movements signs of larynx in “sensor”.
One of the major finding that differentiate patients from NCs was the increased number of peculiar type of swallowing, which was triggered with four or more swallows consecutively, bursting in one set of swallowing. This was called salvo type swallowings. Usually, NCs have solitary or two consecutive swallows (double swallow) in one trigger.7 The number of salvo type swallowings was also counted.
Because all the data were recorded to the external memory, the values could be changed in the offline analysis. For instance, the amplitude of each channel, the time base of all channels, or the size of video images could be altered. This allowed us to carefully compare the SS and coughs by looking at the swallowing muscles together with laryngeal and respiratory activities. In the offline mode, each swallow or the group of swallows could be analyzed by two directions of the recordings (backward and forward) together with the video monitoring images.
Coughing was also important for both of the groups investigated. All coughs were counted throughout the whole sleep recordings and video monitoring in combination. In particular, the coughs just after the swallows were counted separately because of their penetration through the larynx or anywhere in the airway.
The movements of other parts of the body including head, neck, eye, facial muscles, and limbs were also noted during swallowing movements in all cases. The SS and coughs when the subjects were awake were not analyzed.
Statistical Analysis
Statistical analysis was performed using SPSS version 15.0 for Windows (Statistical Package for Social Sciences, SPSS Inc., Chicago, IL). Categorical data were analyzed by means of chi-square test or Fisher exact test, where appropriate. Descriptive data, including degree of dysphagia, presence of drooling and salvo swallows, were analyzed as categorical data. The Kolmogorov-Smirnov (K-S) test was used to determine whether continuous variables followed a normal distribution. Data were presented as mean ± standard deviation (SD) together with minimum and maximum values (range). Given the small sample size, group means were compared using the Mann–Whitney U test. In all analyses, values of P < 0.05 were considered significant.
RESULTS
The characteristics of patients with PD and data regarding the degree of dysphagia and drooling are presented in the Table S1 (supplemental material). Only five patients with PD had grade 2 dysphagia (mild dysphagia) and the remaining were normal swallowers clinically categorized as grade 1.
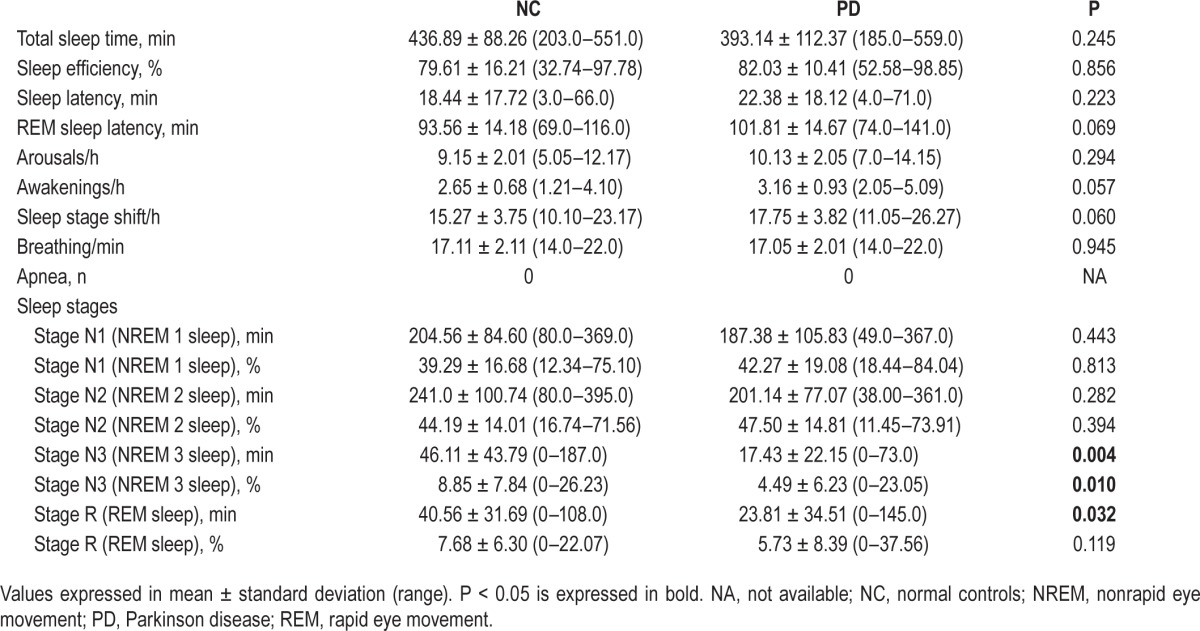
The sleep variables are documented in Table 1. Patients with PD were similar to NCs regarding TST, sleep efficiency, sleep latency, REM sleep latency, and the number of awakenings, arousals, and sleep stage shifts. None of the patients in PD group or healthy controls experienced sleep apnea or bruxism during the recordings.
Table 1.
Sleep variables of patients with Parkinson disease and normal controls.
Amount of Swallowing during Sleep
The mean number of total SS during sleep was significantly increased in patients with PD compared to the controls (201.3 ± 157.5 and 119.1 ± 110.0, respectively; Table 2). However, the mean duration of sleep of the patients and controls were not different, although there was a trend toward shorter sleep in PD (393.1+112.4 min) compared with age-matched NCs (436.9+88.3 min). It was not possible to formulate SS neither in patients with PD nor in NCs regardless of sleep stages because the occurrence of SS was highly variable and differed greatly among individuals.
Table 2.
Data of cough and swallows during all-night sleep recordings in normal controls, patients with Parkinson disease, and patients with Parkinson disease with or without dysphagia.
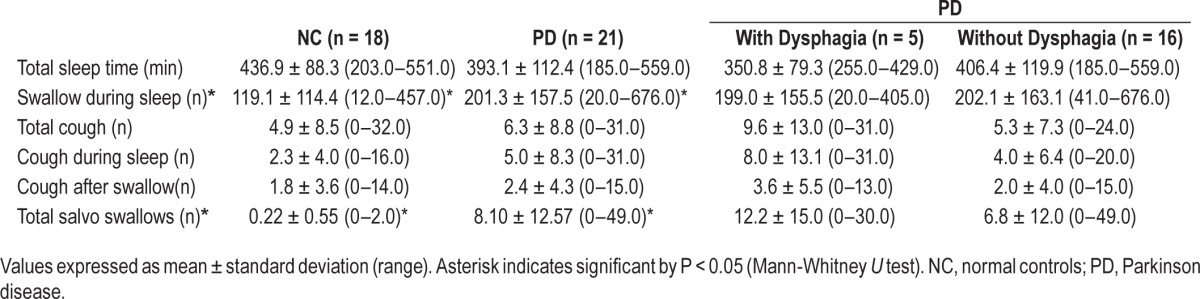
Two factors were investigated to evaluate the increased mean number of SS in patients with PD compared to the age-matched controls. The first was sialorrhea. Nine patients in the PD group did not have sialorrhea and 12 patients had sialorrhea and drooling. The mean number of SS during sleep was 174.3 ± 119.6 in patients with sialorrhea and 245.1 ± 206.0 in patients without sialorrhea, but the difference was not statistically significant. On the contrary, the mean number of SS was higher in patients without sialorrhea. According to these results, we could suggest that sialorrhea did not cause an increase in SS numbers during sleep. Certainly, we could assume a role for the increased SDs due to the substantial individual variations.
The number of patients with dysphagia was only 5 in our patient group. In the other 16 patients, no symptoms of dysphagia were present. However, no statistically significant difference was found between these two groups of patients (Table 2).
Salvo Type Swallowing
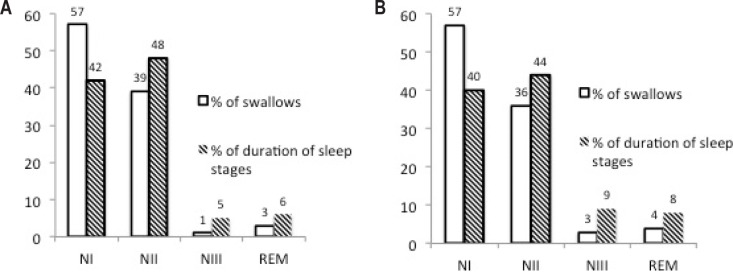
The salvo type swallowings of patients with PD are shown in Figure 2A. The characteristic salvo type swallowings was detected in only two NCs who were older than 70 y and the number of salvo type swallowings in all-night recording was only four.
Figure 2.
(A) Salvo type swallowing recorded in a patient with Parkinson disease (PD), showing a minimum of 13 consecutive swallowings. Note the three times slower paper speed (2A) than that depicted in Figure 1. (B) Comparison of the PD and normal control groups according to the number of cases with salvo type of swallows.
The salvo type swallowings were frequent in 15 of 21 patients with PD. The number of consecutive SSs in one salvo could vary in between 4 to 19. The mean number of swallows in one salvo swallowing was 8.1 ± 12.5 in patients with PD, whereas this number was 0.22 ± 0.5 in NCs, which means a major feature in patients with PD was the high number of recurrence of SS in salvo swallowings (P < 0.0001) (Figure 2B). Both single and salvo type swallows were related to a short arousal and there was a clear swallowing apnea (Figure 1). In addition, longer apnea periods were encountered in the salvo type of swallowing (Figure 2A).
Coughing
Nine of 18 NCs experienced coughing episodes during sleep identified by careful combination of video monitoring and EEG findings (range: 3–32). The total number of coughs was 89. On the contrary, 14 patients with PD had coughing ranging between 1 and 31. The total number of coughs in patients with PD was 114. The occurrence of cough was going on in three different situations:
Cough during sleep
Coughing occurred apart from the swallowings (i.e., the interval between the swallowing and cough were equal or more than 2 min). This type of coughing was present in 9 of NC and 14 of PDs; the difference was not statistically significant.
Cough following a swallowing
The maximum time interval between the end of swallowing and the onset of cough was 20 sec. This type of cough was present in seven NCs and nine patients in the PD group. The total number of coughs in the NC group (n = 7) was 32, whereas the nine PD patients had a total of 50 coughs. Although the total number of coughs in PD patients was higher than that in the NCs, this difference did not reach statistical significance.
Cough following a salvo type swallowing
This type of cough was not observed in any of the NCs in all-night recordings. However, 12 patients with PD (57%) experienced cough just following a salvo type swallowing. The difference between the groups was highly significant (P < 0.0001). The total number of this type of coughing in 12 patients was 30.
Sialorrhea
Sialorrhea was present in 13 patients with PD. The mean score of DSFS-P in the PD group was 3.1 ± 1.04 (2.0–5.0). The mean number of SS in TST in patients with sialorrhea was 174.0 ± 119.6 and 245.0 ± 206.0 in patients without sialorrhea. Interestingly, swallowings were more frequent in patients without sialorrhea. Nevertheless, the difference was not significant. This could possibly be an effect of high SDs in both groups. Similarly, the mean number of SS within the salvos in PD patients with sialorrhea was 6.3 ± 7.4, and 11.0 ± 18.4 in PD patients without sialorrhea. Still, the SDs were high in both groups and no significance was found statistically. Thus, the presence or absence of sialorrhea was not a contributing factor to change the frequency of swallows.
Presence of Clinical Dysphagia
Dysphagia was present in only five patients with PD according to clinical dysphagia scoring. In the other 16 patients, no symptoms of dysphagia were detected. In two of five patients with dysphagia the clinical score was “2” and in the other 3 patients the score was “3.” When the patients with or without dysphagia were compared according to the mean number of swallowings during sleep, the number of coughs, and the number of salvo swallowings, no significant differences were found. Although the number of patients with clinical dysphagia is small, the effects in patients with dysphagia on the swallowing features during sleep were not significant (Table 2).
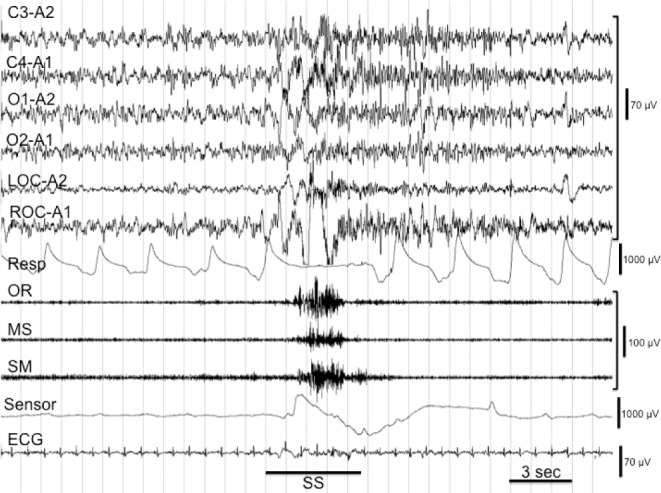
Sleep Stages and Swallowing
Duration of each sleep stage is shown in Table 1. Patients with PD had significantly shorter stage N3 (NREM 3 sleep) and stage R (REM sleep) when compared to the NCs. However, the percentage of stage R was not statistically different between the two groups. The sleep stages and the percentages of swallowings accordingly are presented in Figure 3. In addition, swallow quantities were decreased in stage N3 and stage R and increased in stages N1 and N2 in patients with PD. Certainly stages N1 and N2 were active for the occurrence of swallows, whereas stage N3 and stage R had significantly reduced SSs in both PD and NC patients.
Figure 3.
The comparison of the percentage of swallows and duration of sleep stages in all-night sleep recordings in Parkinson disease D (A) and normal control (B) groups. REM, rapid eye movement.
Finally, we can summarize our findings for discussion as follows: SS during sleep was significantly increased in patients with PD compared to age-matched control subjects (201 in patients with PD versus 119 in NCs; P < 0.05). Sialorrhea did not cause any significant increase in rate of SS in patients with PD during sleep. Clinically determined dysphagic (n = 5) and nondysphagic (n = 16) patients did not differ electrophysiologically from all respect during all-night sleep. The salvo type of consecutive swallowing in PD (Figure 2A) has never been described before and was found in 15 of 21 patients with PD (71.4%) (P < 0.0001). Coughing after the SS was also higher in patients with PD than in NCs (50 versus 32) but did not reach significance. However, coughs after the salvo type of swallows were significantly increased in patients with PD than NCs (P < 0.001). The accumulation of the SS in different stages of sleep was different. Stages N1 and N2 showed high frequency of SS, whereas the frequency of SS in stages N3 and R was considerably less (Figure 3).
DISCUSSION
In PD, because of the oromotor dysfunction that occurs during the oral phase of swallowing, food residues and saliva accumulate in the oral cavity, in spite of the normal secretion of saliva, and subsequently leaks out of the mouth (drooling).1,10,22,23 During sleep, this accumulation most likely increases the frequency of SS, which in turn can lead to airway aspiration and several other disorders. We can compare this hypothesis to the decreased spontaneous blinking pattern that occurs in patients with PD. The automatic movement pattern or SS could have been changed in a manner similar to that in which the spontaneous blinking pattern decreases, which helps to clean the cornea.10,24,25 Sleep problems, both nocturnal and diurnal changes to the physiologic sleep pattern, can present at any stage during the course of disease. Sleep disturbances included in an important group of nonmotor symptoms of PD may precede several years before the development of motor symptoms.26,27 Nonmotor symptoms in PD including several sleep disorders are basically different than the nigral dopaminergic insufficiency. They may be present in early involvement of the lower brainstem in patients with PD.27 This type of earlier degeneration than the motor symptomatology of PD could demonstrate two types of symptom complex, which are neurochemically different from each other. Nonmotor symptomatology including sleep disorders and even gastrointestinal and sensory disorders are attributed to the α-synucleinopathy that attack the lower brainstem nuclei and the vagal nerves.28–34 The nonmotor symptoms of PD such as sleep disorders and dysphagia may have greater effect on the quality-of-life measures than motor symptoms.29,35–37
The all-night sleep recording plus the SS rate relationship was reported previously in normal subjects.13–15 Video recording was not used; therefore, sleep EEG recording was not very reliable to observe some SSs. However, the rate of SS distribution among the different sleep stages was compatible with our results. It is interesting that the increase of SS in sleep in PD patients compared to NCs has not been described before. Previous all-night sleep recordings did not investigate the SS behavior and frequency in all-night sleep.
It is well known that saliva production in patients with PD is normal and even lower than in control patients,38,39 and the drooling and the increased accumulation of the saliva in the oropharyngeal cavity is related to the diurnal effect of disturbed saliva swallowing difficulties.19 Similarly, the sialorrhea in our patients with PD did not cause any significant increase in the frequency of swallowing during all-night sleep. Therefore, the daily drooling and the increase of SS at night could not be linked to saliva production or saliva accumulation.8,19 Indeed, the SS in sleep varies from case to case in both normal patients and patients with PD, apart from the proper distribution in different stages of sleep.
The sleep stages of somnography have demonstrated the different distribution of SS throughout the sleep cycles in both normal subjects with older age and patients with PD.14,15 The sleep stages N1 and N2 are more superficial and the number of SS were very high. On the contrary, with the N3 and REM stages, the frequency of SS is quite low. Such a very stable distribution of frequency of SS is not evident in all NCs and those with PD. There must be a strong inhibition on the brainstem-cranial and spinal motor neurons in REM sleep.40 For instance, the tonic EMG activity is often reduced,40 human soleus H-reflex was suppressed,41 and jaw opening reflex was also demonstrated to be suppressed.40 Similarly, the SS as a reflex deglutition movement is also expected to be suppressed during REM sleep. However, it should be noted that in almost all SS the electrographic and some clinical arousal was observed in both groups, but arousal is a more general feature of SS during all stages of sleep, as was also observed previously.7,14
The novel finding of the study is to record the salvo type of SS consecutively in 71.4% of patients with PD. This has not been described previously, but only briefly mentioned in a review.22 Though, this does not seem to be disease-specific for PD; because we also observed a similar phenomenon in 2 normal elderly subjects but with a less number of SSs in a set. The salvo type of SS could also be recorded in patients with brainstem infarcts.42 The salvo type of PD may be a nonspecific pattern related to subclinical or clinical dysphagia appearing during sleep stages N1 and N2. It may be proposed that such periodic movements recorded at the swallowing muscles synchronously may be different types of movements nonrelated to swallowing problems, such as sleep bruxism. Indeed, during sleep, patients with sleep bruxism could exhibit rhythmic masticatory muscle activity episodes.43 Even in the patients with sleep bruxism, 68% of swallowing events occur during rhythmic masticatory muscle episodes. Bruxism often occurred before the swallowing event in sleep. We can differentiate the bruxism from salvo type SS because the swallowing apnea could only be observed in SS events and bruxism could appear clinically in video-EEG recordings. Furthermore, we have not included the patients with sleep bruxism in our patient group. In all recordings from normal control to PD patients, we did not find any activity similar to bruxism described by others.43
The salvo types of SS are probably missed because the swallowing studies have been performed for VS and in the daytime. Even the previous SS studies have been done during the day. As a result, the salvo types of SS observed in sleep recordings could not be demonstrated previously. What is the mechanism of salvo type of SS? If we can focus on the pathophysiological mechanism of dysphagia in PD, we can find some clue for the mechanism. Logemann44 was the first to describe the oscillating anterior-posterior movements of the tongue and these movements may repeat many times before the swallow that is triggered by the sufficient tongue-driving force. Excessive tongue pumping movements have also been described by Murry and Carrau in 2001.45 They reported that these motor behaviors were more prevalent in liquid than in semisolids and solids. Weak pharyngeal contraction/squeeze and impaired pharyngeal peristalsis have been reported along with delays in triggering of the pharyngeal phase of swallowing that resulted in spillage and pooling of the bolus in valleculae or piriform muscle. Leow46 reported that individuals in PD may repetitively pump the tongue to advance the bolus from anterior to posterior in an attempt to trigger sensory receptors for pharyngeal swallowing. Patients with PD had sensory loss at the base of the tongue in comparison with their healthy counterparts and it was concluded that tongue-pumping behavior seen in patients with PD may also be related to sensory loss.46,47 Recently, the pharyngeal sensory nerves and branches of glossopharyngeal and vagal nerves are directly affected by the pathological process (α-synucleopathy) in PD.48,49 Thus, insufficient sensory input in the oropharyngeal mucosae may be the reason for the salvo type swallow of saliva that accumulated in the mouth and the pharyngeal spaces. However, this type of explanation cannot answer all of the remaining questions.
In patients with PD, coughing was encountered more commonly than in age-matched NCs. However, it was not statistically significant except for the coughing following a salvo type of consecutive SS in 57% of patients with PD (P < 0.001). A higher incidence of coughing may indicate that the swallowing material can be transmitted into the airway during deglutition.46,50,51 Dysphagia plus frequent coughing can occur in all stages of PD, including subclinical dysphagia, as we have demonstrated in our patients with PD without any clinical signs of dysphagia.52,53 However, many individuals with PD can have silent aspiration without awareness, especially in sleep, and there is no cough response to airway aspiration, probably because of a reduced sensitivity in the laryngopharynx.46,54–56 α-synucleopathic neuropathy in other parts of the nervous system has been demonstrated to exist in vagal and glossopharyngeal nerve branches in patients with PD.48
We determined that SS during sleep, single and salvo type swallowing, and cough after salvo swallowing were all significantly increased in patients with PD. It should be noted that these events are always associated with swallowing apnea and arousal with or without coughing.7,22,42 Hence, it may be expected that apneic events in sleep are increased in PD as has been identified by some studies.29 We have excluded patients with PD and sleep apnea syndrome from the study and only apneas were found during spontaneous swallowing processes in the sleep (see Table 1 and Table 2).
The poor correlation with the clinical stage of PD and swallowing disorders encountered in our patients may be coupled with the entity of silent aspiration, and could produce aspiration pneumonia, which was demonstrated with high incidence in pulmonary compromised individuals with PD.57,58
Some qualitative but not quantitative SS changes have been encountered in a minority of elderly NCs. Swallowing function starts to decrease as early as age 45 y and by age 70 y, the total duration of oropharyngeal swallowing times are significantly slower compared with those of younger patients.59,60 However, these findings do not necessarily indicate oropharyngeal dysphagia. Instead, dysphagia is more often the direct result of a pathological condition, such as PD, that may occur more commonly in elderly persons.61
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: Dr. Uludag - organization, execution and statistical analysis, writing of the first draft of manuscript; Dr. Tiftikcioglu -organization, execution and statistical analysis, writing of the first draft of manuscript; and, Dr. Ertekin - research project, conception, review and critique of the manuscript.
REFERENCES
- 1.Ertekin C. Voluntary versus spontaneous swallowing in man. Dysphagia. 2011;26:183–92. doi: 10.1007/s00455-010-9319-8. [DOI] [PubMed] [Google Scholar]
- 2.Nishino T. The swallowing reflex and its significance as an airway defensive reflex. Front Physiol. 2012;3:489. doi: 10.3389/fphys.2012.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afkari S. Measuring frequency of spontaneous swallowing. Australas Phys Eng Sci Med. 2007;30:313–7. [PubMed] [Google Scholar]
- 4.Crary MA, Carnaby Mann GD, Groher ME. Biomechanical correlates of surface electromyography signals obtained during swallowing by healthy adults. J Speech Lang Hear Res. 2006;49:186–93. doi: 10.1044/1092-4388(2006/015). [DOI] [PubMed] [Google Scholar]
- 5.Crary MA, Carnaby Mann GD, Groher ME. Identification of swallowing events from sEMG signals obtained from healthy adults. Dysphagia. 2007;22:94–9. doi: 10.1007/s00455-006-9059-y. [DOI] [PubMed] [Google Scholar]
- 6.Crary MA, Sura L, Carnaby G. Validation and demonstration of an isolated acoustic recording technique to estimate spontaneous swallow frequency. Dysphagia. 2013;28:86–94. doi: 10.1007/s00455-012-9416-y. [DOI] [PubMed] [Google Scholar]
- 7.Ertekin C, Eryasar G, Gurgor N, Arici S, Secil Y, Kurt T. Orbicularis oculi muscle activation during swallowing in humans. Exp Brain Res. 2013;224:79–91. doi: 10.1007/s00221-012-3290-6. [DOI] [PubMed] [Google Scholar]
- 8.Kalf JG, Borm GF, de Swart BJ, Bloem BR, Zwarts MJ, Munneke M. Reproducibility and validity of patient-rated assessment of speech, swallowing, and saliva control in Parkinson's disease. Arch Phys Med Rehabil. 2011;92:1152–8. doi: 10.1016/j.apmr.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Lear CS, FLANAGAN JB, Jr., Moorrees CF. The frequency of deglutition in man. Arch Oral Biol. 1965;10:83–100. doi: 10.1016/0003-9969(65)90060-9. [DOI] [PubMed] [Google Scholar]
- 10.Pehlivan M, Yuceyar N, Ertekin C, et al. An electronic device measuring the frequency of spontaneous swallowing: digital phagometer. Dysphagia. 1996;11:259–64. doi: 10.1007/BF00265212. [DOI] [PubMed] [Google Scholar]
- 11.Rudney JD, Ji Z, Larson CJ. The prediction of saliva swallowing frequency in humans from estimates of salivary flow rate and the volume of saliva swallowed. Arch Oral Biol. 1995;40:507–12. doi: 10.1016/0003-9969(95)00004-9. [DOI] [PubMed] [Google Scholar]
- 12.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lichter I, Muir RC. The pattern of swallowing during sleep. Electroencephalogr Clin Neurophysiol. 1975;38:427–32. doi: 10.1016/0013-4694(75)90267-9. [DOI] [PubMed] [Google Scholar]
- 14.Sato K, Nakashima T. Human adult deglutition during sleep. Ann Otol Rhinol Laryngol. 2006;115:334–9. doi: 10.1177/000348940611500503. [DOI] [PubMed] [Google Scholar]
- 15.Sato K, Umeno H, Chitose S, Nakashima T. Deglutition and respiratory patterns during sleep in younger adults. Acta Otolaryngol. 2011;131:190–6. doi: 10.3109/00016489.2010.522595. [DOI] [PubMed] [Google Scholar]
- 16.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51:745–52. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–70. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 18.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 19.Kalf JG, Munneke M, Engel-Hoek L, et al. Pathophysiology of diurnal drooling in Parkinson's disease. Mov Disord. 2011;26:1670–6. doi: 10.1002/mds.23720. [DOI] [PubMed] [Google Scholar]
- 20.Ertekin C, Aydogdu I, Yuceyar N, Kiylioglu N, Tarlaci S, Uludag B. Pathophysiological mechanisms of oropharyngeal dysphagia in amyotrophic lateral sclerosis. Brain. 2000;123(Pt 1):125–40. doi: 10.1093/brain/123.1.125. [DOI] [PubMed] [Google Scholar]
- 21.Silber MH, Ancoli-Israel S, Bonnet MH, et al. The visual scoring of sleep in adults. J Clin Sleep Med. 2007;3:121–31. [PubMed] [Google Scholar]
- 22.Ertekin C. Electrophysiological evaluation of oropharyngeal dysphagia in Parkinson's disease. J Mov Disord. 2014;7:31–56. doi: 10.14802/jmd.14008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leopold NA, Kagel MC. Prepharyngeal dysphagia in Parkinson's disease. Dysphagia. 1996;11:14–22. doi: 10.1007/BF00385794. [DOI] [PubMed] [Google Scholar]
- 24.Agostino R, Bologna M, Dinapoli L, et al. Voluntary, spontaneous, and reflex blinking in Parkinson's disease. Mov Disord. 2008;23:669–75. doi: 10.1002/mds.21887. [DOI] [PubMed] [Google Scholar]
- 25.Korosec M, Zidar I, Reits D, Evinger C, Vanderwerf F. Eyelid movements during blinking in patients with Parkinson's disease. Mov Disord. 2006;21:1248–51. doi: 10.1002/mds.20930. [DOI] [PubMed] [Google Scholar]
- 26.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology. 1996;46:388–93. doi: 10.1212/wnl.46.2.388. [DOI] [PubMed] [Google Scholar]
- 27.Braak H, Del TK, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 28.Videnovic A, Golombek D. Circadian and sleep disorders in Parkinson's disease. Exp Neurol. 2013;243:45–56. doi: 10.1016/j.expneurol.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peeraully T, Yong MH, Chokroverty S, Tan EK. Sleep and Parkinson's disease: a review of case-control polysomnography studies. Mov Disord. 2012;27:1729–37. doi: 10.1002/mds.25197. [DOI] [PubMed] [Google Scholar]
- 30.Schulte EC, Winkelmann J. When Parkinson's disease patients go to sleep: specific sleep disturbances related to Parkinson's disease. J Neurol. 2011;258(Suppl 2):S328–35. doi: 10.1007/s00415-011-5933-0. [DOI] [PubMed] [Google Scholar]
- 31.Schrempf W, Brandt MD, Storch A, Reichmann H. Sleep disorders in Parkinson's disease. J Parkinsons Dis. 2014;4:211–21. doi: 10.3233/JPD-130301. [DOI] [PubMed] [Google Scholar]
- 32.Lima MM. Sleep disturbances in Parkinson's disease: the contribution of dopamine in REM sleep regulation. Sleep Med Rev. 2013;17:367–75. doi: 10.1016/j.smrv.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Diederich NJ, McIntyre DJ. Sleep disorders in Parkinson's disease: many causes, few therapeutic options. J Neurol Sci. 2012;314:12–9. doi: 10.1016/j.jns.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 34.Bruin VM, Bittencourt LR, Tufik S. Sleep-wake disturbances in Parkinson's disease: current evidence regarding diagnostic and therapeutic decisions. Eur Neurol. 2012;67:257–67. doi: 10.1159/000335078. [DOI] [PubMed] [Google Scholar]
- 35.Aarsland D, Alves G, Larsen JP. Disorders of motivation, sexual conduct, and sleep in Parkinson's disease. Adv Neurol. 2005;96:56–64. [PubMed] [Google Scholar]
- 36.Karlsen KH, Larsen JP, Tandberg E, Maeland JG. Influence of clinical and demographic variables on quality of life in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 1999;66:431–5. doi: 10.1136/jnnp.66.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shulman LM, Taback RL, Bean J, Weiner WJ. Comorbidity of the nonmotor symptoms of Parkinson's disease. Mov Disord. 2001;16:507–10. doi: 10.1002/mds.1099. [DOI] [PubMed] [Google Scholar]
- 38.Tumilasci OR, Cersosimo MG, Belforte JE, Micheli FE, Benarroch EE, Pazo JH. Quantitative study of salivary secretion in Parkinson's disease. Mov Disord. 2006;21:660–7. doi: 10.1002/mds.20784. [DOI] [PubMed] [Google Scholar]
- 39.Bagheri H, Damase-Michel C, Lapeyre-Mestre M, et al. A study of salivary secretion in Parkinson's disease. Clin Neuropharmacol. 1999;22:213–5. [PubMed] [Google Scholar]
- 40.Okura K, Kato T, Montplaisir JY, Sessle BJ, Lavigne GJ. Quantitative analysis of surface EMG activity of cranial and leg muscles across sleep stages in human. Clin Neurophysiol. 2006;117:269–78. doi: 10.1016/j.clinph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu A, Yamada Y, Yamamoto J, Fujiki A, Kaneko Z. Pathways of descending influence on H reflex during sleep. Electroencephalogr Clin Neurophysiol. 1966;20:337–47. doi: 10.1016/0013-4694(66)90002-2. [DOI] [PubMed] [Google Scholar]
- 42.Ertekin C. Electrophysiological techniques to evaluate swallowing in central and peripheral nervous system disorders. J Clin Neurophysiol. 2015;32:314–23. doi: 10.1097/WNP.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 43.Miyawaki S, Lavigne GJ, Pierre M, Guitard F, Montplaisir JY, Kato T. Association between sleep bruxism, swallowing-related laryngeal movement, and sleep positions. Sleep. 2003;26:461–5. [PubMed] [Google Scholar]
- 44.Logemann JA. San Diego, CA: College-Hill Press; 1983. Evaluation and treatment of swallowing disorders. [Google Scholar]
- 45.Murry T, Carrau RL. San Diego, CA: Singular Thomson Learning; 2001. Clinical manual for swallowing disorders. [Google Scholar]
- 46.Leow LP. Mechanisms of airway protection in ageing and Parkinson's disease a thesis submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy in Speech and Language Therapy in the University of Canterbury. 2007 [Google Scholar]
- 47.Aviv JE, Martin JH, Jones ME, et al. Age-related changes in pharyngeal and supraglottic sensation. Ann Otol Rhinol Laryngol. 1994;103:749–52. doi: 10.1177/000348949410301001. [DOI] [PubMed] [Google Scholar]
- 48.Mu L, Sobotka S, Chen J, et al. Parkinson disease affects peripheral sensory nerves in the pharynx. J Neuropathol Exp Neurol. 2013;72:614–23. doi: 10.1097/NEN.0b013e3182965886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mu L, Sobotka S, Chen J, et al. Altered pharyngeal muscles in Parkinson disease. J Neuropathol Exp Neurol. 2012;71:520–30. doi: 10.1097/NEN.0b013e318258381b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Addington WR, Stephens RE, Gilliland K, Rodriguez M. Assessing the laryngeal cough reflex and the risk of developing pneumonia after stroke. Arch Phys Med Rehabil. 1999;80:150–4. doi: 10.1016/s0003-9993(99)90112-0. [DOI] [PubMed] [Google Scholar]
- 51.Ebihara S, Saito H, Kanda A, et al. Impaired efficacy of cough in patients with Parkinson disease. Chest. 2003;124:1009–15. doi: 10.1378/chest.124.3.1009. [DOI] [PubMed] [Google Scholar]
- 52.Ertekin C, Tarlaci S, Aydogdu I, et al. Electrophysiological evaluation of pharyngeal phase of swallowing in patients with Parkinson's disease. Mov Disord. 2002;17:942–9. doi: 10.1002/mds.10240. [DOI] [PubMed] [Google Scholar]
- 53.Wintzen AR, Badrising UA, Roos RA, Vielvoye J, Liauw L, Pauwels EK. Dysphagia in ambulant patients with Parkinson's disease: common, not dangerous. Can J Neurol Sci. 1994;21:53–6. doi: 10.1017/s0317167100048770. [DOI] [PubMed] [Google Scholar]
- 54.Robbins J. Normal swallowing and aging. Semin Neurol. 1996;16:309–17. doi: 10.1055/s-2008-1040989. [DOI] [PubMed] [Google Scholar]
- 55.Daggett A, Logemann J, Rademaker A, Pauloski B. Laryngeal penetration during deglutition in normal subjects of various ages. Dysphagia. 2006;21:270–4. doi: 10.1007/s00455-006-9051-6. [DOI] [PubMed] [Google Scholar]
- 56.Pitts T, Bolser D, Rosenbek J, Troche M, Sapienza C. Voluntary cough production and swallow dysfunction in Parkinson's disease. Dysphagia. 2008;23:297–301. doi: 10.1007/s00455-007-9144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fall PA, Saleh A, Fredrickson M, Olsson JE, Granerus AK. Survival time, mortality, and cause of death in elderly patients with Parkinson's disease: a 9-year follow-up. Mov Disord. 2003;18:1312–6. doi: 10.1002/mds.10537. [DOI] [PubMed] [Google Scholar]
- 58.Wang X, You G, Chen H, Cai X. Clinical course and cause of death in elderly patients with idiopathic Parkinson's disease. Chin Med J. 2002;115:1409–11. [PubMed] [Google Scholar]
- 59.Robbins J, Hamilton JW, Lof GL, Kempster GB. Oropharyngeal swallowing in normal adults of different ages. Gastroenterology. 1992;103:823–9. doi: 10.1016/0016-5085(92)90013-o. [DOI] [PubMed] [Google Scholar]
- 60.Aydogdu I, Yuceyar N, Kiylioglu N, et al. Physiological changes in oropharyngeal swallowing with age: an electrophysiological study. J Neurol Sci (Turkish) 2007;24:144–54. [Google Scholar]
- 61.Sonies BC. Oropharyngeal dysphagia in the elderly. Clin Geriatr Med. 1992;8:569–77. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.