Abstract
Study Objectives:
Sleep disordered breathing (SDB) is common in older adults and is strongly associated with cognitive decline, with increasing evidence suggesting that it may represent a risk factor for dementia. Given that SDB is characterized by intermittent episodes of hypoxemia during sleep, it is possible that cognitive impairment may relate to cerebral oxidative stress. This study aimed to examine the relationship between nocturnal markers of hypoxemia and proton magnetic resonance spectroscopy (1H-MRS) markers of oxidative stress within the anterior cingulate cortex (ACC) of the brain.
Methods:
Twenty-four older adults (mean age = 67.9 y) at-risk for dementia were recruited from our Healthy Brain Ageing Research Clinic. At-risk was defined as participants seeking help for assessment and/or intervention for cognitive decline, including those with subjective and/or objective cognitive complaints. This could occur in the context of prior depression or risk factors (e.g., vascular) for dementia. All participants underwent psychiatric, medical and neuropsychological assessment followed by overnight polysomnography. In addition, participants underwent 1H-MRS to derive levels of ACC metabolite glutathione (GSH) reported as a ratio to creatine (GSH/Cr).
Results:
Increased levels of GSH/Cr were associated with lower oxygen desaturation (r = −0.54, P = 0.007) and more severe apnea-hypopnea index scores during rapid eye movement sleep (r = 0.42, P = 0.050). In addition, ACC GSH/Cr correlated with poorer executive functioning (i.e., response inhibition: r = −0.49, P = 0.015; set shifting: r = −0.43, P = 0.037).
Conclusions:
Markers of nocturnal hypoxemia and SDB are associated with cerebral oxidative stress in older people at-risk for dementia, suggesting a potential mechanism by which SDB may contribute to brain degeneration, cognitive decline, and dementia. Further work focused on utilizing this biomarker for the early identification and treatment of this possible modifiable risk factor in older persons is now warranted.
Citation:
Duffy SL, Lagopoulos J, Terpening Z, Lewis SJG, Grunstein R, Mowszowski L, Cross N, Hermens DF, Hickie IB, Naismith SL. Association of anterior cingulate glutathione with sleep apnea in older adults at-risk for dementia. SLEEP 2016;39(4):899–906.
Keywords: anterior cingulate cortex, executive function, glutathione, oxidative stress, oxygen desaturation, proton magnetic resonance spectroscopy, sleep apnea
Significance.
This study shows for the first time, preliminary in vivo evidence of a possible neurobiological mechanism underpinning the relationship between sleep disordered breathing, cognitive decline and dementia. Specifically, this study demonstrates associations been markers of nocturnal hypoxemia, executive dysfunction and the brain's antioxidant system. From a clinical perspective, the findings of this study further highlight the need for early diagnosis and intervention strategies for sleep disordered breathing in older adults. In addition, further investigations of interventions that directly address oxidative stress are warranted. To facilitate the development of targeted interventions for dementia prevention, future research examining the interrelationship between oxidative stress and cellular processes including inflammation, mitochondrial dysfunction and the deposition of neurofibrillary plaques and tangles are now required.
INTRODUCTION
Sleep disordered breathing (SDB) and obstructive sleep apnea (OSA) occur in approximately 60% and 20% of older adults, respectively, and are etiologically linked to cognitive decline.1 Of significance, up to 30% of elderly patients with OSA meet criteria for mild cognitive impairment (MCI)1 and increasing evidence suggests that OSA may be an independent risk factor for dementia.2,3 Indeed, several studies have demonstrated that dementia is up to six times more likely to develop within 5 years in patients with OSA (depending on age and sex).3 Importantly, executive functioning is posited to be the cognitive domain most affected by OSA. In a model suggested by Beebe and Gozal,4 damage to the prefrontal cortex and subsequent executive dysfunction is theorized to underpin much of the daytime dysfunction associated with OSA. Thus, it is not surprising that OSA is emerging as an important area to target in both primary and secondary dementia prevention efforts.
Cross-sectional and intervention studies have provided initial insights into the association between OSA and neuro-psychological functioning5–7; however, these studies have not yet delineated the mechanisms by which OSA may exert deleterious effects on brain integrity. Neuropsychological studies suggest that both sleep fragmentation and intermittent hypoxia are relevant and have differential effects on cognition.8 Indeed, a recent meta-review demonstrates that OSA is associated with impairment in various cognitive domains, including attention, executive functions (including response inhibition, set-shifting, working memory, and problem solving), processing speed, and episodic memory.5 Importantly, although continuous positive airway pressure (CPAP) treatment has been shown to improve cognitive deficits in attention,5,6 deficits in working memory, phonemic fluency, and psychomotor speed persist, suggesting that OSA may cause irreversible damage to cortical and sub-cortical brain structures and/or networks.5,6,9
A corpus of research has examined the relationship between intermittent hypoxia induced by OSA and changes in markers of inflammation and oxidative stress, in the context of cardiovascular disease (CVD).10 CVD is one of the key comorbidities associated with OSA and evidence hitherto suggests that intermittent hypoxia, as opposed to sleep fragmentation, may have the greatest effect on oxidative stress in vivo.10 Importantly, animal models of intermittent hypoxia demonstrate increased lipid peroxidation and elevated levels of isoprostanes, both markers of oxidative stress.10 Furthermore, these changes have also been associated with impaired spatial learning in rats.10 In humans, recent studies have shown abnormal lipid peroxidation profiles, greater reactive oxygen species (ROS) concentration, and reduced antioxidant capacity in patients with OSA, compared to healthy controls.11,12 However, the relationship between hypoxemia and oxidative stress during sleep has never before been studied in biologically active, in vivo brain tissue in humans. This can be achieved using proton magnetic resonance spectroscopy (1H-MRS), a neuroimaging technique that facilitates resolution of the metabolite glutathione (GSH), the brain's primary antioxidant and a marker of oxidative stress.13 This line of inquiry is critical, particularly in light of recent work demonstrating strong links between oxidative stress, depressive symptoms,14 and cognitive decline.15 In addition, more detailed investigation is pertinent given the known associations between vascular risk factors and incident dementia.16
The majority of research examining the relationship between hypoxemia and oxidative stress to date has been conducted in middle-aged adults or animal models of OSA and as such, it is unclear how this process may be influenced or exacerbated by aging. Indeed, both the incidence of OSA and accumulation of neurodegenerative pathology (including beta-amyloid and tau deposition, as well as ischemic vascular change in individuals vulnerable to CVD) increases with age,17 and it is possible that the neurobiological changes resulting from OSA either underpin or expedite these neurodegenerative processes. Given the potential for some cognitive effects of OSA to be alleviated with CPAP treatment, studies examining the mechanisms underpinning cognitive change in this group may offer important insights for secondary neurodegenerative disease prevention strategies. In this regard, examining patients in the preclinical phase of neurodegenerative disease manifestation, where subjective or objective cognitive change is evident (but prior to the functional decline associated with established dementia), will be most informative and of greatest benefit for targeted intervention.
In this study we aimed to examine the relationship between in vivo GSH concentration in the anterior cingulate cortex (ACC) and measures of hypoxemia, specifically minimum oxygen desaturation (O2-desaturation) and apnea-hypopnea index (AHI), in older adults at-risk for dementia. We chose to examine the ACC over other possible regions of interest for several reasons: (1) our prior work in “at-risk” cohorts has demonstrated relationships between cognitive dysfunction and GSH in the ACC15; (2) the known involvement of the ACC in executive functions18 (the cognitive domain most affected by OSA)9; and (3) the ACC is well suited to 1H-MRS because its position within the brain facilitates voxel placement without close proximity to bone, sinuses, or cerebrospinal fluid, thereby maximizing spectroscopic signal quality. Based on our prior work and that of the extant literature, we hypothesized that increased GSH concentration in the ACC would be associated with increasing OSA severity, including O2-desaturation and AHI. Although exploratory in nature, we further hypothesized that measures of hypoxemia would correlate with executive dysfunction, which would be mediated by GSH concentration as measured by 1H-MRS.
METHODS
Participants
Twenty-four older adults (mean age = 67.9 y; standard deviation [SD] = 6.3 y) “at-risk” for dementia with subjective sleep complaints (including snoring, poor sleep efficiency, sleep latency, and wake after sleep onset) were recruited from the Healthy Brain Ageing (HBA) Clinic at the Brain and Mind Centre, Sydney, Australia. As described previously,19 “at-risk” was defined as those who are seeking help for assessment and/ or intervention for cognitive decline, including those with subjective and/or objective cognitive complaints. This could occur in the context of prior depression or risk factors (e.g., vascular) for dementia. Exclusion criteria included age younger than 50 y at time of testing, established dementia, Mini-Mental State Examination (MMSE) score less than 24,20 history of neurological or psychotic illness, head injury (with loss of consciousness for more than 30 min), other medical conditions known to affect cognition (e.g. stroke, transient ischemic attack), substance misuse, or medical contraindications to magnetic resonance imaging. This study was approved by the University of Sydney Institutional Human Research Ethics Committee. All participants provided written informed consent prior to participation.
Clinical Assessment
Using a semistructured interview, a psychiatrist specializing in the treatment of older adults recorded a full medical, clinical, psychiatric, and medication history. For each participant, the psychiatrist conducted the Structured Clinical Interview for DSM-IV Disorders21 to assess for lifetime and current major depression.
In total, four participants met criteria for major depression at the time of assessment and 10 participants reported current antidepressant medication use. Of these, five participants were taking a selective serotonin reuptake inhibitor, four were taking a serotonin and norepinephrine reuptake inhibitor, and one was taking a tricyclic antidepressant. In addition, two participants reported current benzodiazepine use and two participants were taking an atypical antipsychotic.
As detailed elsewhere,22 a clinical neuropsychologist conducted a standardized neuropsychological assessment for each participant. The assessment covered a range of cognitive domains including attention, working memory, processing speed, verbal and visual learning and memory, language, visuospatial skills, and executive functioning. For reporting purposes, the MMSE was administered and premorbid intellect was estimated using the Wechsler Test of Adult Reading.23
Due to known associations with the ACC, in this study we examined the following executive functions in relation to GSH:
Polysomnography
As described previously,7 participants underwent polysomnography (PSG) in the Chronobiology and Sleep Laboratory at the Brain and Mind Centre. Nocturnal PSG recordings were collected using an ambulatory recording system (Compumedics Siesta, Melbourne, Victoria, Australia) using a six-channel electroencephalographic (EEG) montage (C3-M2, O2-M1, Fz-M1, Pz-M2, Fpz and Cz); two electro-oculographic (EOG) channels (left and right outer canthi) and electromyogram (EMG; submentalis). In addition, nasal airflow was recorded using a nasal pressure transducer or thermistor and respiratory effort was assessed using thoracic and abdominal bands, enabling AHI to be calculated. Blood oxygenation was recorded using pulse oximetry. EEG data were sampled at 250 Hz. Frequencies less than 0.3 Hz and greater than 70 Hz were digitally filtered and a notch filter of 50 Hz was used. Sleep architecture stages were scored manually in 30-sec epochs by an experienced sleep technician using Rechtschaffen and Kales standardized scoring criteria,26 with modifications for older participants.27 While in the laboratory, participants were monitored physiologically and behaviorally at all times under controlled conditions, with fixed light levels (< 50 lux during waking; < 1 lux during scheduled sleep periods) and ambient temperature (24 ± 1°C). Patients were required to maintain their usual bedtime and wake-up schedule during the study, and were asked to abstain from caffeinated beverages for the 8 h prior to and during PSG data collection. Primary outcome measures for this study were: (1) O2-desaturation (minimum level recorded throughout the night); and (2) AHI (total number of apneas + hypopneas) which was calculated across total sleep (AHI), rapid eye movement sleep (AHI-REM), and non-rapid eye movement sleep (AHI-NREM). In addition, for descriptive purposes, we report: sleep onset and offset times (hh:mm); total sleep time (TST; min); wake after sleep onset (WASO; min); and sleep efficiency (TST / time in bed × 100).
Proton Magnetic Resonance Spectroscopy (1H-MRS)
As detailed elsewhere,15 imaging was conducted within 8 w of clinical assessment and took place at the Brain and Mind Imaging Centre on a 3-Tesla GE Discovery MR750 scanner (GE Medical Systems, Milwaukee, WI) using an eight-channel phased array head coil. The following images were acquired in order: (1) three-dimensional sagittal whole-brain scout for orientation and positioning of subsequent scans; (2) T1-weighted magnetization prepared rapid gradient-echo (MPRAGE) sequence producing 196 sagittal slices (repetition time [TR] = 7.2 msec; echo time [TE] = 2.8 msec; flip angle = 10°; matrix 256 × 256; 0.9-mm isotropic voxels; scan time = 4 min, 24 sec) to aid in the anatomical localization of sampled voxels; and (3) single-voxel 1H-MRS using Point RESolved Spectroscopy (PRESS) acquisition, with two chemical shift-selective imaging pulses for water suppression (scan time = 5 min, 4 sec). Spectra were shimmed to achieve full-width half maximum of less than 13 Hz.
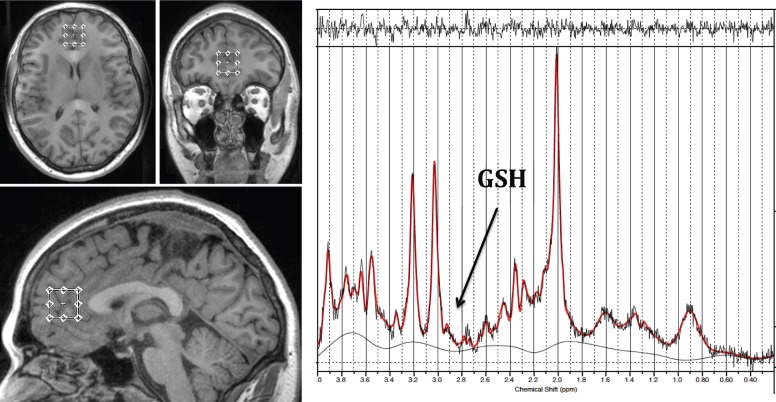
For each participant, a spectra was acquired from a voxel measuring 20 × 20 × 20 mm placed midline in the ACC (Figure 1, TE = 35 msec, TR = 2000 msec and 128 averages). Voxel size was selected to maximize the ACC volume being sampled and thus optimize signal-to-noise ratio, without including a large volume of surrounding structures. Anatomical localisation of voxel placement was based on the Talairach brain atlas28 and positioning was guided by the T1 MPRAGE image.
Figure 1.
Diagram demonstrating the anterior cingulate cortex voxel placement and LCModel output for one participant. The GSH metabolite peak is resolved at 2.95 ppm and is labelled in the water suppressed spectra. GSH, glutathione.
Following MRS acquisition, data were transferred offline for postprocessing using the LCModel software package29 and metabolite concentrations were determined as a relative ratio to creatine (Cr) to reduce subject specific variation. All spectra were quantified using a PRESS TE = 35 basis set of 15 metabolites, which included GSH and incorporated macromolecule and baseline fitting routines. The spectra were visually inspected separately by two different raters to ensure consistent spectra and poorly fitted metabolite peaks as reflected by large Cramer–Rao lower bounds (greater than 20%) were excluded from further analysis. From an imaging perspective, GSH is often resolved using specialized pulse sequences such as MEGAPRESS or double quantum coherence filtering techniques. However, we have previously shown using phantom data that GSH/Cr is also able to be reliably resolved using an optimized PRESS acquisition with higher order shimming.15
Statistical Analysis
Data were analyzed using the Statistical Package for the Social Sciences (IBM Corp, Version 20 for Mac). Pearson coefficients were used for all correlations unless otherwise stated. All analyses were 2-tailed and used an alpha level of 0.05. Partial correlations were used to assess whether the unique variance between two measures was confounded by a third variable.
RESULTS
Sample Characteristics
At the time of assessment, no participant was currently receiving treatment for OSA. Four participants reported a prior diagnosis of OSA; however, these data were not available for all study participants. Only one participant reported CPAP use and ceased use approximately 3 y before the PSG assessment date for the current study. Sixteen participants had an AHI > 10; for these participants, the mean AHI = 22.8, SD = 12.1.
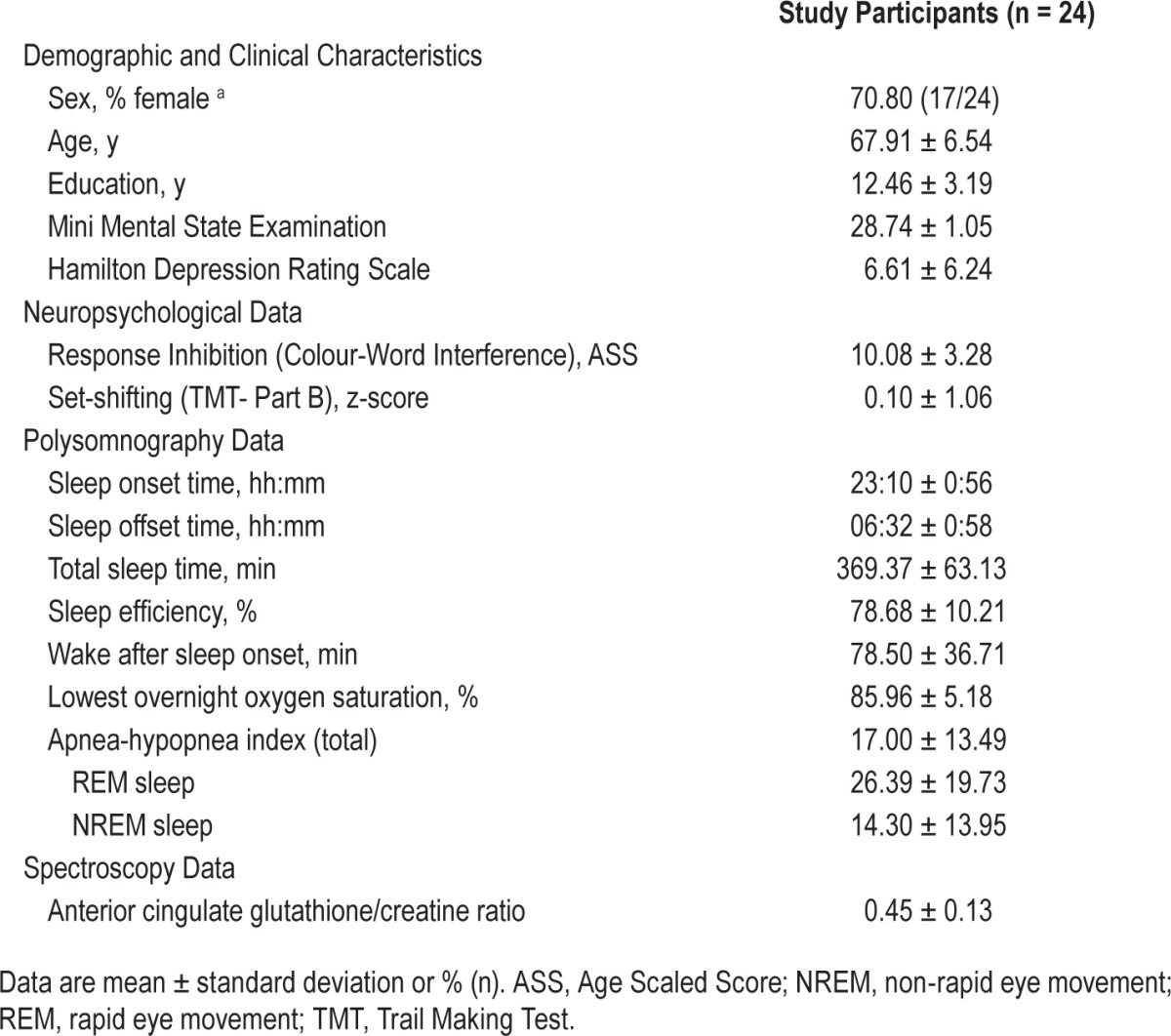
Table 1 displays the demographic, clinical, and sleep data for the sample. On average, participants scored within the normal range for global cognition (i.e., MMSE > 24) and reported only mild depressive symptoms. Compared to normative data,30 participants recorded below-average sleep duration and on average, met criteria for moderate OSA.31
Table 1.
Demographic, clinical, sleep, and spectroscopy data for study participants.
Relationship between OSA Markers and Oxidative Stress
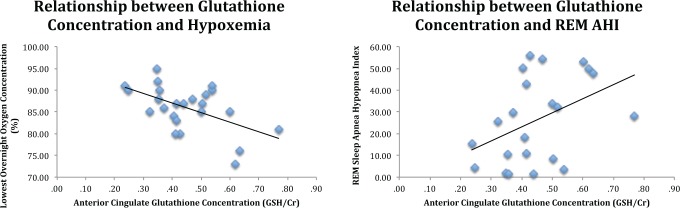
As shown in Table 2 and Figure 2, lower O2-desaturation and more severe AHI in REM sleep were associated with increased ACC GSH/ Cr. Importantly, the correlation between ACC GSH/Cr and O2-destauration remained significant after controlling for age (partial r = −0.57, P = 0.007). However, in this small clinical sample, there was only a trend association between REM AHI and ACC GSH after controlling for age (partial r = 0.43, P = 0.055), though notably the correlation coefficient was of moderate magnitude.
Table 2.
Correlations between anterior cingulate glutathione concentration and participants' demographic and neuropsychological data.
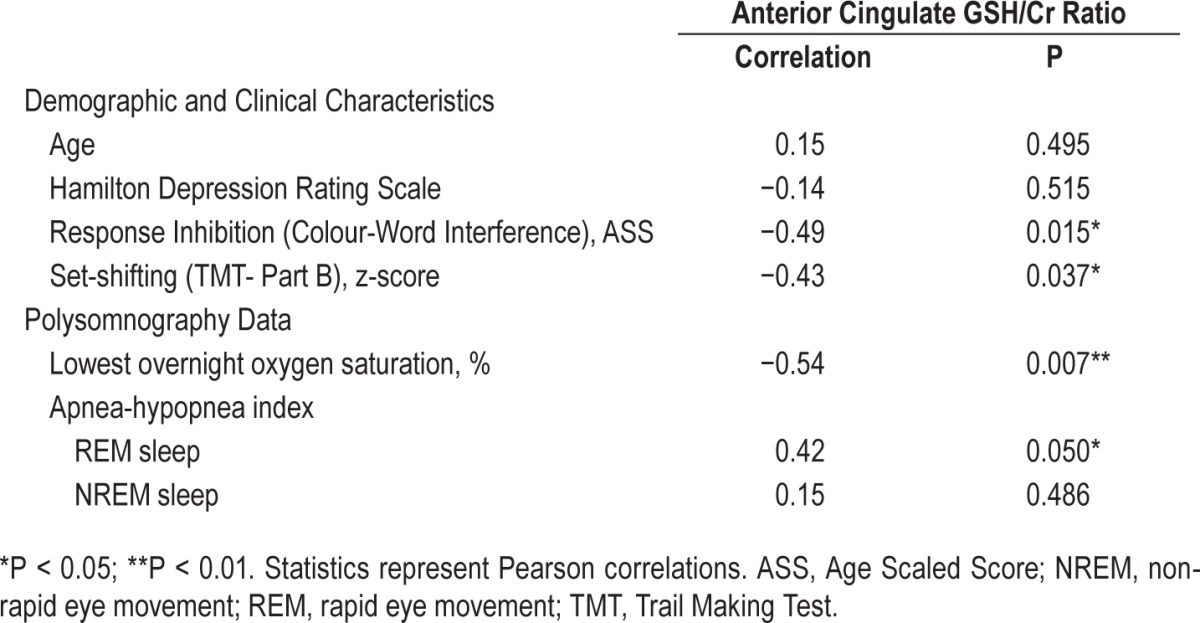
Figure 2.
Graphs demonstrating correlations between glutathione (GSH/Cr) and measures of hypoxemia and rapid eye movement sleep apnea-hypopnea index in patients at-risk for dementia. AHI, apnea-hypopnea index; REM, rapid eye movement.
Associations between Markers of SDB, Oxidative Stress, and Cognition
Confirming prior work linking the ACC with cognition, higher levels of ACC GSH/Cr were significantly correlated with poorer performance on set-shifting and response inhibition tasks (see Table 2). However, there were no significant correlations between measures of executive functioning and hypoxemia (set shifting: r = 0.14, P = 0.505; response inhibition: r = 0.20, P = 0.350) or sleep fragmentation (AHI; set shifting: r = −0.38, P = 0.073; response inhibition: r = −0.22, P = 0.323) in this sample. As such, no further exploration of the relationship between ACC GSH/Cr, measures of SDB, and executive functioning were conducted.
In order to confirm that depressive symptoms were not mediating the aforementioned associations, we also analyzed the relationship between depressive symptoms and hypoxemia/ sleep fragmentation, set-shifting/response inhibition (data not presented) and oxidative stress (Table 2), with all resulting findings being nonsignificant.
DISCUSSION
This study demonstrates for the first time that SDB and associated nocturnal hypoxemia are linked to cerebral oxidative stress in the ACC in older adults at-risk for dementia. These results were evident despite, on average, only moderate OSA severity in this patient group. These findings are aligned with prior work in CVD samples, which show a robust association between intermittent hypoxemia and oxidative stress.10 Importantly, however, our research extends previous work to an aging sample at-risk for dementia, and specifically shows an associations between oxidative stress and hypoxemia as well as poorer executive functioning, respectively.
Although the contribution of oxidative stress to cognitive decline and neurodegeneration is being increasingly recognized,32–34 only a few recent studies have examined in vivo brain GSH in humans in relation to cognitive decline and dementia. Of these, our own studies have shown strong associations between increased levels of GSH and both cognitive decline and increased depressive symptoms.14,15 In the current study, we focused on the ACC, on the basis of this work, but also due to the clear role of the ACC in executive functioning, an area of cognition implicated consistently in OSA.5,8 Interestingly, we demonstrated independent associations between ACC GSH and hypoxemia as well as executive functioning. Although we did not find a direct correlation between hypoxemia and executive function in this cohort, it is important to note that on average, performance was within the normal range on all cognitive tasks and as such, GSH may be an important marker of subtle cognitive change in this cohort. Although 1H-MRS does not facilitate further exploration of the neurobiological mechanisms underpinning the associations between hypoxemia, oxidative stress, and executive functioning demonstrated in this study, it is possible that multiple cellular processes are at play including inflammation, mitochondrial dysfunction, and the deposition of neurofibrillary plaques and tangles. Briefly, although mitochondria are the main source of intracellular free radical production (via oxidative phosphorylation),35 their lipid membrane is particularly vulnerable to oxidative damage. An increase in intracellular oxidative stress can result in mitochondrial permeability transition and culminate in apoptotic cell death.36,37 Increasing mitochondrial oxidative stress can also result in dysfunctional protein behavior, which can lead to additional ROS production and accelerated neurodegeneration.38 Alternatively, the interrelationship between inflammation and oxidative stress may be an important consideration. Although oxidative damage is an important cause of neuroinflammation in brain tissue, the activation of ROS producing microglia via increased proinflammatory cytokine production also results in increased oxidative stress.39,40 Importantly, chronic low-grade inflammation has been implicated in the pathogenesis of several psychiatric conditions including major depression as well as CVD and Alzheimer's disease. Interestingly, inflammation is a key process involved in the clearance of Alzheimer's disease pathology, in which microglia degrade soluble and aggregated forms of beta-amyloid.41 However, activated microglia are an important source of ROS, generating the free radical super-oxide41 and increasing oxidative stress. This may, in turn, lead to further beta-amyloid deposition and the progression of neurodegeneration. Although we cannot determine the interrelationship between these cellular processes and oxidative stress within the context our study, GSH plays a key role in the neutralization of ROS in the brain13 and it is clear that further investigation is now warranted.
Of interest, the findings of this study suggest that hypoxemia, particularly during REM sleep, has the most pronounced relationship with oxidative stress. It is well established that OSA during REM sleep is associated with greater apnea length and consequently, greater hypoxemia than OSA during NREM periods.42,43 Furthermore, previous research has suggested that REM sleep OSA is more common when severity is milder, in female patients and in individuals who are not morbidly obese,42,43 characteristics consistent with the demographic and clinical profile of our sample. In addition, our finding is consistent with prior literature showing the importance of the ACC during REM sleep. Indeed, functional neuroimaging studies have shown increased cerebral blood flow to the ACC during REM sleep and downregulation during the NREM period.44 A large proportion of ROS are produced as a result of energy production via oxidative phosphorylation in brain tissue.45 In combination with the known association between OSA and REM sleep, and the role of the ACC, our data demonstrating a significant correlation between hypoxemia and oxidative stress is thus consistent with the literature.
From a clinical perspective, the identification of SDB and associated hypoxemia as a pathophysiological mechanism underpinning cognitive decline has important implications for early detection and intervention. In this regard, interventions that directly address either SDB or oxidative stress are worthy of pursuit. For instance, N-acetyl cysteine46 and omega-347 may counteract oxidative stress in the brain, and in an older at-risk sample, we have previously demonstrated that omega-3 fatty acid supplementation over a 3-mo period stabilized oxidative stress levels in the thalamus.47 Similarly, further investigation of the effect of other OSA treatments on markers of oxidative stress is now warranted. In this regard, both mandibular advancement splint therapy48 and CPAP treatment may offer cognitive benefits. Although there appears to be some heterogeneity in responsiveness, CPAP in particular may improve performance across multiple cognitive domains49–51 and interestingly, has been shown to reduce oxidative stress markers in blood plasma.52–54 This study also highlights the importance of early diagnosis of SDB in older adults. Although it is not logistically or economically feasible to conduct overnight PSG studies on all at-risk older persons, and because screening questions for cognitively impaired older adults require further empiric development and validation,55 the incorporation of objective, home-based diagnostic modalities such as single-channel nasal airflow monitoring may be an appropriate alternative.56 In this regard, the addition of home-based monitoring of SDB for older at-risk persons may increase the efficiency and number of cases of OSA detected, in turn, enabling earlier intervention strategies.19
This study has a number of strengths including the incorporation of detailed clinical and neuropsychological assessment of patients, gold-standard objective PSG measures of sleep as well as sophisticated in vivo brain imaging of GSH. However, when interpreting the findings, some limitations are also worth considering. Firstly, GSH is reported as a ratio to Cr, and while we have previously validated this signal using phantom scanning techniques,15 replication using absolute GSH concentration is required. In addition, although this clinical cohort specifically targets individuals in a clear at-risk dementia period, such samples are by their very nature heterogeneous. Indeed, although increasing evidence suggests that individuals with subjective and/or objective cognitive complaints are at greater risk of progression to MCI or dementia,57–59 their ultimate clinical trajectory is unknown. However, this faithfully represents the epitome of a preclinical disease group and prospective longitudinal analysis of at-risk samples are now required to more closely map the interrelationships between GSH and SDB in various brain regions in relation to the time course and profile of cognitive decline, and investigate the effect of OSA treatment on neurometabolite and cognitive outcomes.
Overall, this study suggests that SDB may be an important mechanism contributing to oxidative stress and neurodegeneration in older people. Identification of modifiable risks such as SDB is highly significant given that rates of dementia will quadruple by 2050, with current efforts focused on the early identification of modifiable risks factors.60 Given that there are many options for early identification and treatment of these important markers, further research using larger samples with prospective or interventional designs is now required.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Duffy is supported by a Postdoctoral Fellowship awarded through the National Health and Medical Research Council Centre of Research Excellence ‘Neurosleep’ (1060992). Prof. Naismith is supported by a National Health and Medical Research Council Career Development Award (1008117). Prof. Grunstein is supported by a National Health and Medical Research Council Practitioner Fellowship (1022730). Prof. Lewis is supported by a National Health and Medical Research Council Practitioner Fellowship (1003007). Dr. Mowszowski is supported by an Alzheimer's Australia Dementia Research Foundation Postdoctoral Fellowship. Dr. Hermens has received honoraria for educational seminars from Janssen-Cilag and Eli Lilly. Prof. Hickie is supported by a National Health and Medical Research Council Senior Principle Research Fellowship (1046899). He is the Co-Executive Director of the Brain and Mind Centre, at the University of Sydney, which operates two early-intervention youth services under contract to headspace, the national youth mental health foundation. He is a commissioner of the Australian National Mental Health Commission and was previously the Chief Executive Officer of beyondblue: the national depression initiative. Previously, Prof. Hickie has led or participated in a range of community-based and pharmaceutical industry-supported depression awareness, education and training programs (supported by BMS, Eli-Lilly, Janssen, Servier, and Pfizer). He has led depression and other mental health research service evaluation or investigator-initiated research projects that have been supported by a variety of pharmaceutical partners (including BMS, Servier and Pfizer). A current investigator-initiated study of circadian rhythm abnormalities in depression is supported by Servier (manufacturers of agomelatine). He has received honoraria for his contributions to professional educational seminars related to depression, youth mental health and circadian-rhythms research and he has received travel support from Servier to attend scientific meetings related specifically to circadian rhythm disorders. Dr. Duffy, A/Prof. Lagopoulos, Dr. Terpening, Prof. Lewis, Dr. Mowszowski, Prof. Grunstein, Mr. Cross and Prof. Naismith have no conflicts of interest to declare.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the generosity of the patients who undertook this research. We also thank Dr. Matt Paradise and Dr. Louisa Norrie for their assistance with clinical assessments. We thank Prof. Naomi Rogers and Mr. Tony Ip for their contribution to data collection.
REFERENCES
- 1.Gagnon K, Baril AA, Gagnon JF, et al. Cognitive impairment in obstructive sleep apnea. Pathol Biol (Paris) 2014;62:233–40. doi: 10.1016/j.patbio.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 2.Blackwell T, Yaffe K, Laffan A, et al. Associations between sleep-disordered breathing, nocturnal hypoxemia, and subsequent cognitive decline in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2015;63:453–61. doi: 10.1111/jgs.13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan W, Kastin AJ. Can sleep apnea cause Alzheimer's disease? Neurosci Biobehav Rev. 2014;47:656–69. doi: 10.1016/j.neubiorev.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11:1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- 5.Bucks RS, Olaithe M, Eastwood P. Neurocognitive function in obstructive sleep apnoea: a meta-review. Respirology. 2013;18:61–70. doi: 10.1111/j.1440-1843.2012.02255.x. [DOI] [PubMed] [Google Scholar]
- 6.Kushida CA, Nichols DA, Holmes TH, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the Apnea Positive Pressure Long-term Efficacy Study (APPLES) Sleep. 2012;35:1593–602. doi: 10.5665/sleep.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terpening Z, Lewis SJ, Yee B, Grunstein R, Hickie IB, Naismith SL. Association between sleep-disordered breathing and neuropsychological performance in older adults with mild cognitive impairment. J Alzheimers Dis. 2015;46:157–65. doi: 10.3233/JAD-141860. [DOI] [PubMed] [Google Scholar]
- 8.Naismith S, Winter V, Gotsopoulos H, Hickie I, Cistulli P. Neurobehavioral functioning in obstructive sleep apnea: differential effects of sleep quality, hypoxemia and subjective sleepiness. J Clin Exp Neuropsychol. 2004;26:43–54. doi: 10.1076/jcen.26.1.43.23929. [DOI] [PubMed] [Google Scholar]
- 9.Saunamaki T, Jehkonen M. A review of executive functions in obstructive sleep apnea syndrome. Acta Neurol Scand. 2007;115:1–11. doi: 10.1111/j.1600-0404.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki YJ, Jain V, Park AM, Day RM. Oxidative stress and oxidant signaling in obstructive sleep apnea and associated cardiovascular diseases. Free Radic Biol Med. 2006;40:1683–92. doi: 10.1016/j.freeradbiomed.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.May AM, Mehra R. Obstructive sleep apnea: role of intermittent hypoxia and inflammation. Semin Respir Crit Care Med. 2014;35:531–44. doi: 10.1055/s-0034-1390023. [DOI] [PubMed] [Google Scholar]
- 12.Yang Q, Wang Y, Feng J, Cao J, Chen B. Intermittent hypoxia from obstructive sleep apnea may cause neuronal impairment and dysfunction in central nervous system: the potential roles played by microglia. Neuropsychiatr Dis Treat. 2013;9:1077–86. doi: 10.2147/NDT.S49868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–71. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 14.Duffy SL, Lagopoulos J, Cockayne N, Hermens DF, Hickie IB, Naismith SL. Oxidative stress and depressive symptoms in older adults: a magnetic resonance spectroscopy study. J Affect Disord. 2015;180:29–35. doi: 10.1016/j.jad.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Duffy SL, Lagopoulos J, Hickie IB, et al. Glutathione relates to neuropsychological functioning in mild cognitive impairment. Alzheimers Dement. 2014;10:67–75. doi: 10.1016/j.jalz.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Hasnain M, Vieweg WVR. Possible role of vascular risk factors in Alzheimer's disease and vascular dementia. Curr Pharm Des. 2014;20:6007–13. doi: 10.2174/1381612820666140314153440. [DOI] [PubMed] [Google Scholar]
- 17.Hughes TM, Kuller LH, Barinas-Mitchell EJ, et al. Arterial stiffness and beta-amyloid progression in nondemented elderly adults. JAMA Neurol. 2014;71:562–8. doi: 10.1001/jamaneurol.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci. 1999;10:49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- 19.Naismith SL, Glozier N, Burke D, Carter PE, Scott E, Hickie IB. Early intervention for cognitive decline: is there a role for multiple medical or behavioural interventions? Early Interv Psychiatry. 2009;3:19–27. doi: 10.1111/j.1751-7893.2008.00102.x. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.First M, Spitzer R, Gibbon M, Williams J. New York, NY: Biometrics Research Department; 2007. Structured clinical interview for DSM-IV-TR axis I disorders - patient edition (SCID-I/P, 1/2007 Revision) [Google Scholar]
- 22.Naismith SL, Redoblado-Hodge MA, Lewis SJ, Scott EM, Hickie IB. Cognitive training in affective disorders improves memory: a preliminary study using the NEAR approach. J Affect Disord. 2010;121:258–62. doi: 10.1016/j.jad.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 23.Wechsler D. San Antonio: TX: The Psychological Corporation; 1997. Manual for the Wechsler adult intelligence scale - third edition. [Google Scholar]
- 24.Tombaugh TN. Trail making test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19:203–14. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 25.Delis DC, Kaplan E, Kramer JH. San Antonio, TX: Harcourt Assessment Company; 2001. Delis-Kaplan executive function system: technical manual. [Google Scholar]
- 26.Rechtschaffen A, Kales A. Los Angeles, CA: Brain Information Service/Brain Research Institute, University of California; 1968. A manual of standardized terminology, techniques and scoring system of sleep stages in human subjects. [Google Scholar]
- 27.Webb WB, Dreblow LM. A modified method for scoring slow wave sleep of older subjects. Sleep. 1982;5:195–9. doi: 10.1093/sleep/5.2.195. [DOI] [PubMed] [Google Scholar]
- 28.Talairach J, Tournoux P. New York, NY: Thieme; 1987. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system. An approach to cerebral imaging (Translated from French by Mark Raport) [Google Scholar]
- 29.Provencher SW. Estimation of metabolite concentrations from localized in-vivo proton NMR-spectra. Magnet Reson Med. 1993;30:672–9. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 30.Ohayon MM, Vecchierini MF. Normative sleep data, cognitive function and daily living activities in older adults in the community. Sleep. 2005;28:981–9. [PubMed] [Google Scholar]
- 31.The Report of American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 32.Nunomura A, Perry G, Aliev G, et al. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–67. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 33.Fatokun AA, Stone TW, Smith RA. Oxidative stress in neurodegeneration and available means of protection. Front Biosci. 2008;13:3288–311. doi: 10.2741/2926. [DOI] [PubMed] [Google Scholar]
- 34.Ansari MA, Scheff SW. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J Neuropathol Exp Neurol. 2010;69:155–67. doi: 10.1097/NEN.0b013e3181cb5af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paradies G, Petrosillo G, Paradies V, Ruggiero FM. Mitochondrial dysfunction in brain aging: role of oxidative stress and cardiolipin. Neurochem Int. 2011;58:447–57. doi: 10.1016/j.neuint.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 36.Gardner A, Boles RG. Beyond the serotonin hypothesis: mitochondria, inflammation and neurodegeneration in major depression and affective spectrum disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:730–43. doi: 10.1016/j.pnpbp.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 37.Mancuso M, Coppede F, Migliore L, Siciliano G, Murri L. Mitochondrial dysfunction, oxidative stress and neurodegeneration. J Alzheimers Dis. 2006;10:59–73. doi: 10.3233/jad-2006-10110. [DOI] [PubMed] [Google Scholar]
- 38.Glade MJ. Oxidative stress and cognitive longevity. Nutrition. 2010;26:595–603. doi: 10.1016/j.nut.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 39.Maes M, Yirmiya R, Noraberg J, et al. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis. 2009;24:27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- 40.Moylan S, Maes M, Wray NR, Berk M. The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry. 2013;18:595–606. doi: 10.1038/mp.2012.33. [DOI] [PubMed] [Google Scholar]
- 41.Dumont M, Beal MF. Neuroprotective strategies involving ROS in Alzheimer disease. Free Radic Biol Med. 2011;51:1014–26. doi: 10.1016/j.freeradbiomed.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gillman A, Roebuck T, Ho S, van Braak E, Naughton MT. Comparison of supine-only and REM-only obstructive sleep apnoea. Sleep Med. 2012;13:875–8. doi: 10.1016/j.sleep.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 43.Mokhlesi B. REM-related obstructive sleep apnea: to treat or not to treat? J Clin Sleep Med. 2012;8:249–50. doi: 10.5664/jcsm.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maquet P, Laureys S, Peigneux P, et al. Experience-dependent changes in cerebral activation during human REM sleep. Nat Neurosci. 2000;3:831–6. doi: 10.1038/77744. [DOI] [PubMed] [Google Scholar]
- 45.Fatokun AA, Stone TW, Smith RA. Oxidative stress in neurodegeneration and available means of protection. Front Biosci. 2008;13:3288–311. doi: 10.2741/2926. [DOI] [PubMed] [Google Scholar]
- 46.Das P, Tanious M, Fritz K, et al. Metabolite profiles in the anterior cingulate cortex of depressed patients differentiate those taking N-acetyl-cysteine versus placebo. Aust N Z J Psychiatry. 2013;47:347–54. doi: 10.1177/0004867412474074. [DOI] [PubMed] [Google Scholar]
- 47.Duffy SL, Lagopoulos J, Cockayne N, et al. The effect of 12-week omega-3 fatty acid supplementation on in-vivo thalamus glutathione concentration in patients ‘at-risk’ for major depression. Nutrition. 2015;31:1247–54. doi: 10.1016/j.nut.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 48.Naismith SL, Winter VR, Hickie IB, Cistulli PA. Effect of oral appliance therapy on neurobehavioral functioning in obstructive sleep apnea: a randomized controlled trial. J Clin Sleep Med. 2005;1:374–80. [PubMed] [Google Scholar]
- 49.Canessa N, Castronovo V, Cappa SF, et al. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med. 2011;183:1419–26. doi: 10.1164/rccm.201005-0693OC. [DOI] [PubMed] [Google Scholar]
- 50.Lau EY, Eskes GA, Morrison DL, Rajda M, Spurr KF. Executive function in patients with obstructive sleep apnea treated with continuous positive airway pressure. J Int Neuropsychol Soc. 2010;16:1077–88. doi: 10.1017/S1355617710000901. [DOI] [PubMed] [Google Scholar]
- 51.Matthews EE, Aloia MS. Cognitive recovery following positive airway pressure (PAP) in sleep apnea. Prog Brain Res. 2011;190:71–88. doi: 10.1016/B978-0-444-53817-8.00004-9. [DOI] [PubMed] [Google Scholar]
- 52.Celec P, Hodosy J, Behuliak M, et al. Oxidative and carbonyl stress in patients with obstructive sleep apnea treated with continuous positive airway pressure. Sleep Breath. 2012;16:393–8. doi: 10.1007/s11325-011-0510-4. [DOI] [PubMed] [Google Scholar]
- 53.Murri M, Garcia-Delgado R, Alcazar-Ramirez J, et al. Continuous positive airway pressure therapy reduces oxidative stress markers and blood pressure in sleep apnea-hypopnea syndrome patients. Biol Trace Elem Res. 2011;143:1289–301. doi: 10.1007/s12011-011-8969-1. [DOI] [PubMed] [Google Scholar]
- 54.Oyama J, Yamamoto H, Maeda T, Ito A, Node K, Makino N. Continuous positive airway pressure therapy improves vascular dysfunction and decreases oxidative stress in patients with the metabolic syndrome and obstructive sleep apnea syndrome. Clin Cardiol. 2012;35:231–6. doi: 10.1002/clc.21010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilson G, Terpening Z, Wong K, et al. Screening for sleep apnoea in mild cognitive impairment: the utility of the multivariable apnoea prediction index. Sleep Disord. 2014;2014:945287. doi: 10.1155/2014/945287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rofail LM, Wong KK, Unger G, Marks GB, Grunstein RR. The utility of single-channel nasal airflow pressure transducer in the diagnosis of OSA at home. Sleep. 2010;33:1097–105. doi: 10.1093/sleep/33.8.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr Scand. 2014;130:439–51. doi: 10.1111/acps.12336. [DOI] [PubMed] [Google Scholar]
- 58.Mendonça MD, Alves L, Bugalho P. From subjective cognitive complaints to dementia: who is at risk: a systematic review. Am J Alzheimers Dis Other Dement. 2015 Jul 3; doi: 10.1177/1533317515592331. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet. 2006;367:1262–70. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 60.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9:63–75. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]