Abstract
Genetic and epigenetic factors may predispose women to polycystic ovary syndrome (PCOS), a common heritable disorder of unclear etiology. Here we investigated differences in genome-wide gene expression and DNA methylation in adipose tissue from 64 women with PCOS and 30 controls. In total, 1720 unique genes were differentially expressed (Q < 0.05). Six out of twenty selected genes with largest expression difference (CYP1B1, GPT), genes linked to PCOS (RAB5B) or type 2 diabetes (PPARG, SVEP1), and methylation (DMAP1) were replicated in a separate case-control study. In total, 63,213 sites (P < 0.05) and 440 sites (Q < 0.15) were differently methylated. Thirty differentially expressed genes had corresponding changes in 33 different DNA methylation sites. Moreover, a total number of 1913 pairs of differentially expressed “gene-CpG” probes were significantly correlated after correction for multiple testing and corresponded with 349 unique genes. In conclusion, we identified a large number of genes and pathways that are affected in adipose tissue from women with PCOS. We also identified specific DNA methylation pathways that may affect mRNA expression. Together, these novel findings show that women with PCOS have multiple transcriptional and epigenetic changes in adipose tissue that are relevant for development of the disease.
Polycystic ovary syndrome (PCOS) is the most common endocrine and metabolic disorder in women, with a worldwide prevalence of 7–15%1. The reproductive phenotype of PCOS includes hyperandrogenism, ovulatory disturbances, and polycystic ovarian morphology. The metabolic phenotype includes hyperinsulinemia with pancreatic β-cell dysfunction, insulin resistance, and obesity. These metabolic abnormalities often precede type 2 diabetes (T2D)2,3. Compensatory hyperinsulinemia stimulates androgen production and secretion by thecal cells and reduces the level of sex hormone binding globulin, which increases free androgens and further exacerbates PCOS symptoms2. Thus, there is a strong association between hyperinsulinemia and hyperandrogenemia that drives a specific vicious circle. Despite the detrimental effect of PCOS on women’s health, the etiology is not well understood. Genetic and environmental factors have been implicated in its development, but studies examining the impact of epigenetics on PCOS remain scarce4,5,6.
The considerable overlap between the reproductive and metabolic features of PCOS implicates adipose tissue dysfunction as a key feature of the disease7,8,9,10. Importantly, both lean and obese women with PCOS have aberrant adipose tissue morphology and function, as evidenced by enlarged subcutaneous adipocytes and decreased secretion of adiponectin—two factors that are strongly associated with insulin resistance9. Further, subcutaneous adipocytes from women with PCOS are resistant to insulin-stimulated glucose uptake and display inhibited lipolysis11,12. Moreover, altered adipose tissue expression of genes such as PPARG, LEPR, TWIST1, CCL2 that may be important in PCOS pathophysiology have been identified13,14,15,16,17,18. These findings indicate that adipose tissue dysfunction may contribute to the pathogenesis of PCOS including insulin resistance and hyperandrogenism.
Emerging evidence from women with PCOS and from prenatally androgenized animal models suggests that maternal androgen excess predisposes offspring to PCOS19. An unfavorable intrauterine environment may lead to epigenetic changes that alter gene expression and increase risk of disease in adulthood20. Several lines of evidence support a role for epigenetic mechanisms in metabolic disturbances. For example, differential DNA-methylation in adipose tissue from subjects with T2D is associated with differential expression of genes involved in oxidative phosphorylation, mitochondrial function, and the metabolism of carbohydrates, amino acids, and lipids, all of which are relevant to the development of disease21. The possibility of an epigenetic component of PCOS was only recently explored4,5,6. Although hampered by minimal sample size22,23, these studies revealed differential DNA methylation and gene expression profiles in ovarian tissue within pathways related to the pathogenesis of PCOS. Also, epigenetic modification of PPARGC1A has been reported in granulosa cells from women with PCOS24.
Nevertheless, knowledge concerning the role of epigenetics in PCOS is limited. The genome-wide DNA methylation pattern has not been explored in human adipose tissue from women with and without PCOS, and genome-wide expression profiles have not been linked to the epigenome until just recently25. Therefore, our aim was to investigate genome-wide gene differences in expression and DNA methylation patterns in adipose tissue from 64 women with PCOS and 30 controls. In a separate case-control study, key findings were replicated in adipose tissue from 42 unrelated women.
Results
Clinical Characteristics
The characteristics of women in the two cohorts are presented in Table 1. In cohort 1, women with PCOS were older (30.0 ± 4.4 vs 27.6 ± 3.54) and had a higher BMI. (28.4 ± 7.1 vs 24.8 ± 5.0) Therefore, all analyzes in cohort 1 were adjusted for age and BMI including clinical characteristics, genome-wide gene expression and methylation. Women with PCOS had a higher HOMA-IR (homeostatic model assessment of insulin resistance) and higher levels of HbA1c and circulating testosterone than controls, and a lower glucose disposal rate (GDR) as measured by euglycemic hyperinsulinemic clamp. In cohort 2 replication was performed in cases and controls that were overweight or obese and matched for age (±5 years), weight (±4 kilogram), and body mass index (BMI) (±2) with controls. Women with PCOS in cohort 2 had higher HOMA-B (reflecting insulin secretion) and circulating testosterone levels and larger adipocytes than controls (Table 1).
Table 1. Clinical characteristics of women with polycystic ovary syndrome and controls in cohorts 1 and 2.
| Cohort 1 | Controls (n = 30) | PCOS (n = 64) | P valuea |
|---|---|---|---|
| Age (years) | 27.6 ± 3.54 | 30.0 ± 4.4 | NA |
| Anthropometry | |||
| BMI (kg/m2) | 24.8 ± 5.0 | 28.4 ± 7.1 | NA |
| Waist-to-Hip-Ratio | 0.79 ± 0.06 | 0.84 ± 0.07 | 0.027 |
| PCOS characteristics, n (%) | |||
| Oligo/amenorrhea | 0 | 76% (55) | NA |
| Hirsutism | 0 | 75% (54) | NA |
| Acne (yes/no) | 0 | 64% (46) | NA |
| Hormones | |||
| Estradiol (pg/ml) | 38.9 ± 18.2 | 73.9 ± 40.3 | < 0.000 |
| Testosterone (ng/ml) | 0.21 ± 0.08 | 0.45 ± 0.18 | < 0.000 |
| SHBG (nmol/l) | 67.8 ± 29.6 | 39.3 ± 21.3 | < 0.000 |
| Metabolic measures | |||
| HOMA-IR | 1.22 ± 0.82 | 2.48 ± 2.16 | 0.008 |
| HOMA-B | 152.5 ± 113.2 | 203.3 ± 159.6 | 0.522 |
| GDR (mg/kg x min) | 12.58 ± 3.45 | 9.69 ± 3.16 | 0.005 |
| Hba1c (mmol/mol) | 28.73 ± 5.94 | 31.56 ± 3.07 | 0.016 |
| Adipocyte size (μm) | 93.2 ± 9.87 | 100.2 ± 11.45 | 0.153 |
| Cohort 2 | Controls (n = 21) | PCOS (n = 21) | P valueb |
| Age (years) | 29.8 ± 6.4 | 31.2 ± 5.6 | 0.520 |
| Anthropometry | |||
| BMI (kg/m2) | 30.4 ± 3.62 | 31.2 ± 4.12 | 0.534 |
| Waist-to-Hip-Ratio | 0.82 ± 0.05 | 0.85 ± 0.08 | 0.155 |
| PCOS characteristics, n (%) | |||
| Oligo/amenorrhea | 0 | 85% (18) | NA |
| Hirsutism | 0 | 67% (14) | NA |
| Acne (yes/no) | 0 | 43% (9) | NA |
| Hormones | |||
| Estradiol (pg/ml) | 57.3 ± 41.8 | 87.12 ± 63.2 | 0.080 |
| Testosterone (ng/ml) | 0.25 ± 0.09 | 0.49 ± 0.30 | < 0.001 |
| SHBG (nmol/l) | 39.5 ± 25.0 | 38.9 ± 16.2 | 0.819 |
| Metabolic measures | |||
| HOMA-IR | 2.20 ± 1.11 | 3.18 ± 2.05 | 0.061 |
| HOMA-B | 121.5 ± 62.9 | 196.9 ± 132.7 | 0.020 |
| GDR (mg/kg x min) | 6.73 ± 2.84 | 6.17 ± 3.11 | 0.549 |
| Hba1c (mmol/mol) | 31.8 ± 2.91 | 31.6 ± 2.55 | 0.911 |
| Adipocyte size (μm) | 110.5 ± 8.03 | 116.7 ± 9.78 | 0.030 |
Values are mean ± standard deviation (SD). BMI, body mass index; SHBG, sex hormone binding globulin; LH, luteinizing hormone; FSH, follicle-stimulating hormone; HOMA, homeostatic model assessment; GDR, glucose disposal rate.
aANCOVA with adjustment for age and BMI.
bFisher’s permutation test.
Phenotypic presentation
In cohort 1, 49 of the 64 women with PCOS met all three criteria for PCOS: hyperandrogenism, irregular cycles, and PCO morphology; 15 had hyperandrogenemia and PCO morphology. In cohort 2, 13 of the 21 women with PCOS met all three PCOS criteria; two had hyperandrogenemia and PCO morphology, one had hyperandrogenemia and irregular cycles, and five had irregular cycles and PCO morphology.
Differential expression in adipose tissue
To investigate differential gene expression in adipose tissue, we used Illumina HumanHT-12 v4 Expression BeadChips to analyze subcutaneous adipose tissue from the 64 women with PCOS and the 30 controls in cohort 1. Each chip has 47,000 probes that represent well-characterized genes and unknown splice variants. A false-discovery rate (FDR) < 5% (Q < 0.05) was used to correct for multiple testing. After correction, 1899 probe sets, representing 1769 annotated transcripts and 1720 unique genes (Q < 0.05), were differentially expressed in adipose tissue from women with PCOS and controls (Table S1). Of the 1720 unique genes, 912 were down-regulated (by up to 57%) and 808 were up-regulated (by up to 58%) in women with PCOS (Table S1).
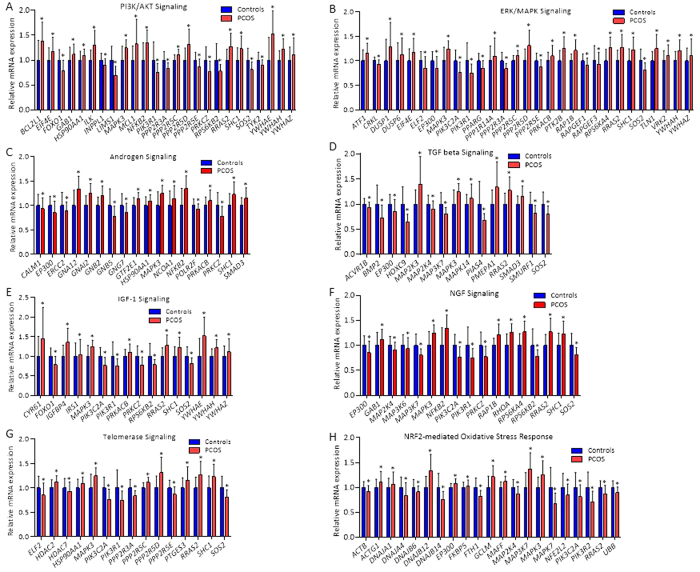
Next, all differentially expressed genes (P < 0.05) were analyzed with Ingenuity Pathway Analysis software to identify biological canonical pathways. All significantly up- and down-regulated gene sets are presented in Table 2. A selection of significantly up- and down-regulated canonical pathways relevant to PCOS and T2D—PI3K/AKT, ERK/MAPK-, androgen, TGF-β, IGF-1, NGF, telomerase, and NRF2-mediated oxidative stress response signaling pathways—are shown in Fig. 1A–H. All genes contributing to significantly up-regulated and down-regulated canonical pathways are presented in Tables S2 and S3, respectively.
Table 2. Significant gene sets with differential gene expression in adipose tissue from women with PCOS versus controls. IPA analyses with Q < 0.05.
| Pathway | Regulated/total | Ratio | Z-score | P value | Signaling Pathway Category | Top Function & Diseases |
|---|---|---|---|---|---|---|
| Up-regulated pathways | ||||||
| PI3K/AKT Signaling | 25/113 | 0.221 | 2.132 | 1.72E-5 | Cancer; Cellular Growth, Proliferation and Development; Intracellular and Second Messenger Signaling | Cell Death and Survival; Cancer; Cell Cycle |
| ILK Signaling | 28/152 | 0.184 | 2.887 | 1.79E-4 | Cellular Growth, Proliferation and Development | Cellular Movement; Cancer; Cardiovascular System Development and Function |
| ERK/MAPK Signaling | 29/167 | 0.174 | 1.347 | 3.92E-4 | Cancer; Intracellular and Second Messenger Signaling | Cancer; DNA Replication, Recombination, and Repair; Cell Cycle |
| Cholecystokinin/Gastrin-mediated Signaling | 19/93 | 0.204 | 4.359 | 5.03E-4 | Neurotransmitters and Other Nervous System Signaling | DNA Replication, Recombination, and Repair; Developmental Disorder; Cellular Development |
| ERK5 Signaling | 14/62 | 0.226 | 3.162 | 9.60E-4 | Intracellular and Second Messenger Signaling | DNA Replication, Recombination, and Repair; Cancer; Developmental Disorder |
| p70S6K Signaling | 20/107 | 0.187 | 1.698 | 1.18E-3 | Cellular Growth, Proliferation and Development; Cellular Stress and Injury | Cellular Function and Maintenance; Cell Death and Survival; Post-Translational Modification |
| Role of NFAT in Cardiac Hypertrophy | 26/160 | 0.163 | 3.545 | 2.06E-3 | Cardiovascular Signaling; Disease-Specific Pathways | Cardiac Hypertrophy; Cardiovascular Disease; Developmental Disorder |
| Cardiac Hypertrophy Signaling | 29/191 | 0.152 | 4.707 | 3.35E-3 | Cardiovascular Signaling; Disease-Specific Pathways | Cardiac Hypertrophy; Cardiovascular Disease; Developmental Disorder |
| Androgen Signaling | 18/103 | 0.175 | 2.333 | 4.35E-3 | Nuclear Receptor Signaling | ene Expression; Cell Death and Survival; Cellular Development |
| TGF-β-Signaling | 15/80 | 0.188 | 1.508 | 4.51E-3 | Cellular Growth, Proliferation and Development; Growth Factor Signaling; Ingenuity Toxicity List Pathways | Gene Expression; Embryonic Development; Organismal Development |
| IGF-1 Signaling | 16/92 | 0.174 | 2.53 | 7.27E-3 | Cellular Growth, Proliferation and Development; Growth Factor Signaling | Cancer; Cellular Development; Hematological System Development and Function |
| VEGF Signaling | 15/85 | 0.176 | 3.606 | 8.03E-3 | Cellular Growth, Proliferation and Development; Growth Factor Signaling | Cardiovascular System Development and Function; Cell Cycle; Cell Death and Survival |
| Phospholipase C Signaling | 29/207 | 0.14 | 4.69 | 1.04E-2 | Intracellular and Second Messenger Signaling | Cell Signaling; Molecular Transport; Vitamin and Mineral Metabolism |
| Acute Myeloid Leukemia Signaling | 13/72 | 0.181 | 3.051 | 1.08E-2 | Cancer; Disease-Specific Pathways | Cellular Development; Cellular Growth and Proliferation; Hematological System Development and Function |
| Cardiac β-adrenergic Signaling | 18/113 | 0.159 | 0.302 | 1.14E-2 | Cardiovascular Signaling | Skeletal and Muscular System Development and Function; DNA Replication, Recombination, and Repair; Molecular Transport |
| NGF Signaling | 17/105 | 0.162 | 4.123 | 1.18E-2 | Growth Factor Signaling; Neurotransmitters and Other Nervous System Signaling | Cancer; Cellular Growth and Proliferation; Cellular Development |
| Gαq Signaling | 20/131 | 0.153 | 4.243 | 1.26E-2 | Intracellular and Second Messenger Signaling | Post-Translational Modification; Lipid Metabolism; Small Molecule Biochemistry |
| Integrin Signaling | 25/175 | 0.143 | 4.379 | 1.31E-2 | Cell Cycle Regulation; Cellular Growth, Proliferation and Development; Intracellular and Second Messenger Signaling | Cell Morphology; Cellular Assembly and Organization; Cellular Function and Maintenance |
| AMPK Signaling | 20/133 | 0.15 | 0.832 | 1.47E-2 | Cellular Growth, Proliferation and Development; Intracellular and Second Messenger Signaling | Energy Production; Lipid Metabolism; Small Molecule Biochemistry |
| Telomerase Signaling | 15/91 | 0.165 | 1.155 | 1.48E-2 | Apoptosis; Cancer | Cellular Assembly and Organization; Cellular Function and Maintenance; Cancer |
| Ceramide Signaling | 13/75 | 0.173 | 2.496 | 1.51E-2 | Apoptosis; Cell Cycle Regulation | Cell Death and Survival; Cell Signaling; Renal Necrosis/Cell Death |
| mTOR Signaling | 24/170 | 0.141 | 2.183 | 1.70E-2 | Cellular Growth, Proliferation and Development | Cell Morphology; Protein Synthesis; Cellular Function and Maintenance |
| fMLP Signaling in Neutrophils | 16/102 | 0.157 | 3.742 | 1.89E-2 | Cellular Immune Response; Cytokine Signaling | Cell-To-Cell Signaling and Interaction; Cellular Function and Maintenance; Inflammatory Response |
| CXCR4 Signaling | 19/128 | 0.148 | 4 | 1.94E-2 | Cellular Immune Response; Cytokine Signaling | Cell Morphology; Cell-To-Cell Signaling and Interaction; Cellular Function and Maintenance |
| Melanocyte Development and Pigmentation Signaling | 13/79 | 0.165 | 3.606 | 2.26E-2 | Cellular Growth, Proliferation and Development; Growth Factor Signaling | Cellular Development; Tissue Development; Hair and Skin Development and Function |
| Colorectal Cancer Metastasis Signaling | 28/211 | 0.133 | 5 | 2.27E-2 | Cancer; Disease-Specific Pathways | Cell Death and Survival; Cell Cycle; Cellular Development |
| Relaxin Signaling | 17/115 | 0.148 | 3 | 2.70E-2 | Growth Factor Signaling; Organismal Growth and Development | Cellular Development; Cellular Growth and Proliferation; Organ Development |
| Neurotrophin/TRK Signaling | 11/65 | 0.169 | 2.714 | 2.85E-2 | Growth Factor Signaling; Neurotransmitters and Other Nervous System Signaling | Cellular Growth and Proliferation; Cell Cycle; Cell Morphology |
| SAPK/JNK Signaling | 14/90 | 0.156 | 2–496 | 2.87E-2 | Apoptosis | Cell Signaling; Post-Translational Modification; Cell Death and Survival |
| Role of CHK Proteins in Cell Cycle Checkpoint Control | 9/50 | 0.18 | 1.134 | 3.17E-2 | Cell Cycle Regulation; Cellular Stress and Injury | Cell Cycle; DNA Replication, Recombination, and Repair; Cell Death and Survival |
| Regulation of eIF4 and p70S6K Signaling | 19/135 | 0.141 | 1.155 | 3.20E-2 | Cell Cycle; DNA Replication, Recombination, and Repair; Cell Death and Survival | Protein Synthesis; Cell Cycle; Cancer |
| IL-1 Signaling | 18/83 | 0.157 | 3.162 | 3.26-2 | Cytokine Signaling | Cell-mediated Immune Response; Cellular Development; Cellular Function and Maintenance |
| Role of NFAT in Regulation of the Immune Response | 21/154 | 0.136 | 3.873 | 3.41E-2 | Cellular Immune Response; Humoral Immune Response; Intracellular and Second Messenger Signaling | Cellular Development; Hematological System Development and Function; Hematopoiesis |
| Sphingosine-1-phosphate Signaling | 14/93 | 0.151 | 3.606 | 3.68E-2 | Intracellular and Second Messenger Signaling | Cellular Development; Embryonic Development; Organismal Development |
| VEGF Family Ligand-Receptor Interactions | 11/69 | 0.159 | 3.162 | 4.20E-2 | Cellular Growth, Proliferation and Development; Growth Factor Signaling | Cell Cycle; Organismal Development; Embryonic Development |
| FLT3 Signaling in Hematopoietic Progenitor Cells | 11/70 | 0.157 | 3.317 | 4.60E-2 | Cytokine Signaling | Cell Cycle; Cellular Development; Hematological System Development and Function |
| p38 MAPK Signaling | 15/105 | 0.143 | 2.673 | 4.70E-2 | Cellular Immune Response; Cellular Stress and Injury; Cytokine Signaling; Humoral Immune Response; Intracellular and Second Messenger Signaling | Gene Expression; Cell Death and Survival; Cellular Development |
| Melatonin Signaling | 10/62 | 0.161 | 2.828 | 4.77E-2 | Neurotransmitters and Other Nervous System Signaling | Cell Signaling; Molecular Transport; Small Molecule Biochemistry |
| GDNF Family Ligand-Receptor Interactions | 10/62 | 0.161 | 3 | 4.77E-2 | Growth Factor Signaling; Neurotransmitters and Other Nervous System Signaling | Cellular Development; Cellular Growth and Proliferation; Nervous System Development and Function |
| Production of Nitric Oxide and Reactive Oxygen Speaces in Macrophages | 21/160 | 0.131 | 2.236 | 4.85E-2 | Cellular Immune Response | Cell Signaling; Small Molecule Biochemistry; Free Radical Scavenging |
| CNTF Signaling | 8/46 | 0.174 | 2.828 | 4.96E-2 | Cellular Growth, Proliferation and Development; Cytokine Signaling; Neurotransmitters and Other Nervous System Signaling | Cellular Development; Cellular Growth and Proliferation; Hematological System Development and Function |
| Tec Kinase Signaling | 19/142 | 0.134 | 3.742 | 4.98E-2 | Intracellular and Second Messenger Signaling | Cell-To-Cell Signaling and Interaction; Cellular Function and Maintenance; Inflammatory Response |
| Down-regulated pathways | ||||||
| HIPPO Signaling | 18/77 | 0.234 | −1,069 | 1.20E-4 | Organismal Growth and Development | Cancer; Organismal Injury and Abnormalities; Cell Cycle |
| NRF2-mediated Oxidative Stress Response | 23/136 | 0.141 | −3,606 | 1.92E-2 | Cellular Stress and Injury; Ingenuity Toxicity List Pathways | Cell Death and Survival; Post-Translational Modification; Organismal Survival |
| PPARα/RXRα Activation | 21/152 | 0.138 | −2,357 | 3.01E-2 | Ingenuity Toxicity List Pathways; Nuclear Receptor Signaling | Energy Production; Lipid Metabolism; Small Molecule Biochemistry |
Figure 1. Gene sets contributing to selected significant top canonical pathways, identified by Ingenuity Pathway Analysis, of possible relevance to PCOS and T2D.
Up-regulated canonical pathways: (A) PI3K/AKT Signaling pathway, (B) ERK/MAPK signaling pathway, (C) Androgen receptor signaling pathway, (D) TGF-β signaling pathway, (E) IGF1-1 signaling pathway, (F) NGF signaling pathway, and (G) Telomerase signaling pathway. Down-regulated canonical pathway: (H) NRF2-mediated oxidative stress response signaling pathway. Values are mean ± SD. *P < 0.05.
Fifty genes whose expression in adipose tissue differed the most between women with PCOS and controls (Q < 0.05) are presented in Table 3. Among the most up-regulated genes (expression range 34.8% to 57.8%) we found genes associated with hormone/aromatase activity and metabolic disturbances (e.g., CD74, APOLD1, CYP1B1, and UCP2). Among the most down-regulated genes (expression range –28.7% to –57.4%) were those involved in metabolic disturbance, including T2D, glycerol and lipid processes (e.g., SLC7A10, UGP2 and GPT).
Table 3. Fifty individual genes with the largest mRNA expression differences in adipose tissue between PCOS (n = 64) and controls (n = 30) (Q < 0.05) including uncharacterized (LOC) genes.
| Up-regulated genes | ||||||
|---|---|---|---|---|---|---|
| Symbol | Gene Description | Controls mean ± SD | PCOS mean ± SD | Difference (%) | p value | Qvalue |
| CD74 | CD74 molecule, major histocompatibility complex, class II invariant chain | 59.4 ± 1.4 | 93.7 ± 1.5 | 57.8 | 8E-06 | 1E-03 |
| SNORD48 | small nucleolar RNA, C/D box 48 | 11.2 ± 1.4 | 17.5 ± 1.5 | 56.4 | 3E-07 | 1E-04 |
| APOLD1 | apolipoprotein L domain-containing 1 | 217.2 ± 1.6 | 330.9 ± 1.6 | 52.3 | 9E-06 | 1E-03 |
| IDO1 | indoleamine 2,3-dioxygenase 1 | 18 ± 1.5 | 26.9 ± 1.6 | 49.6 | 9E-04 | 2E-02 |
| MYL9 | myosin, light chain 9, regulatory | 267.2 ± 1.3 | 397.2 ± 1.3 | 48.6 | 2E-07 | 7E-05 |
| NRCAM | neuronal cell-adhesion molecule | 102.3 ± 1.5 | 151.2 ± 1.5 | 47.8 | 4E-04 | 1E-02 |
| CYP1B1 | cytochrome P450, family 1, subfamily B, polypeptide 1 | 61.3 ± 1.4 | 89.8 ± 1.4 | 46.3 | 1E-05 | 1E-03 |
| CYR61 | cysteine-rich, angiogenic inducer, 61 | 169.7 ± 1.5 | 248 ± 1.7 | 46.1 | 3E-04 | 1E-02 |
| AZIN1 | antizyme inhibitor 1 | 33.5 ± 1.6 | 48.9 ± 1.7 | 45.7 | 8E-04 | 2E-02 |
| STC1 | stanniocalcin 1 | 28 ± 1.5 | 40.4 ± 1.6 | 44.3 | 3E-03 | 4E-02 |
| NEK7 | NIMA-related kinase 7 | 40.8 ± 1.4 | 58 ± 1.4 | 42.2 | 1E-05 | 1E-03 |
| UCP2 | uncoupling protein 2 (mitochondrial, proton carrier) | 352.9 ± 1.3 | 499.3 ± 1.4 | 41.5 | 6E-06 | 8E-04 |
| C1S | complement component 1, s subcomponent | 33.7 ± 1.4 | 47.5 ± 1.4 | 41.0 | 1E-04 | 6E-03 |
| PLEKHO2 | pleckstrin homology domain containing, family O member 2 | 138 ± 1.4 | 193.4 ± 1.3 | 40.1 | 8E-05 | 4E-03 |
| SNX10 | sorting nexin 10 | 148.6 ± 1.6 | 206.7 ± 1.5 | 39.1 | 5E-04 | 1E-02 |
| C1R | complement component 1, r subcomponent | 97.4 ± 1.4 | 134.2 ± 1.4 | 37.8 | 2E-03 | 3E-02 |
| SEMA3C | sema domain, immunoglobulin domain (Ig), short basic domain, secreted, (semaphorin) 3C | 176.5 ± 1.4 | 242.5 ± 1.4 | 37.4 | 1E-04 | 6E-03 |
| LOC100132793 | chromosome 8 open reading frame 88 | 12.1 ± 1.3 | 16.5 ± 1.3 | 36.2 | 8E-08 | 4E-05 |
| SIK1 | salt-inducible kinase 1 | 25.7 ± 1.4 | 35 ± 1.4 | 36.1 | 3E-05 | 2E-03 |
| LOC100133511 | uncharacterized LOC100131581 | 77 ± 1.4 | 104.6 ± 1.4 | 35.9 | 2E-04 | 7E-03 |
| MYL9 | myosin, light chain 9, regulatory | 405.4 ± 1.3 | 549.5 ± 1.3 | 35.6 | 1E-05 | 1E-03 |
| EFEMP1 | EGF containing fibulin-like extracellular matrix protein 1 | 321 ± 1.4 | 434.9 ± 1.4 | 35.5 | 2E-03 | 3E-02 |
| GSTT2B | glutathione S-transferase theta 2 | 21.3 ± 1.5 | 28.8 ± 1.4 | 35.3 | 8E-04 | 2E-02 |
| CXCR4 | chemokine (C-X-C motif) receptor 4 | 24.4 ± 1.6 | 32.9 ± 1.5 | 34.9 | 2E-03 | 4E-02 |
| GSTT2 | glutathione S-transferase theta 2 | 16.8 ± 1.5 | 22.7 ± 1.4 | 34.8 | 1E-03 | 2E-02 |
| Upregulated genes | ||||||
| LOC100132761 | Uncharacterized | 104.1 ± 2.1 | 44.3 ± 2.7 | −57.4 | 3E-05 | 2E-03 |
| SLC7A10 | Solute carrier family 7 (neutral amino acid transporter light chain, asc system), member 10 | 655.1 ± 1.5 | 391.6 ± 1.9 | −40.2 | 3E-03 | 4E-02 |
| PKD1L2 | polycystic kidney disease 1-like 2 | 203.3 ± 1.5 | 123.9 ± 1.7 | −39.1 | 2E-03 | 3E-02 |
| LOC389342 | maltase-glucoamylase (alpha-glucosidase) | 2047.4 ± 1.3 | 1253.2 ± 1.6 | −38.8 | 2E-05 | 2E-03 |
| MYOC | myocilin, trabecular meshwork inducible glucocorticoid response | 126 ± 1.5 | 79.6 ± 1.6 | −36.9 | 1E-03 | 2E-02 |
| C21orf81 | ankyrin repeat domain 20 family, member A11, pseudogene | 98.1 ± 1.5 | 63.2 ± 1.6 | −35.6 | 6E-04 | 1E-02 |
| LOC729222 | liprin-beta-1-like | 49 ± 1.3 | 31.5 ± 1.3 | −35.6 | 3E-11 | 5E-07 |
| COL8A1 | collagen, type VIII, alpha 1 | 456 ± 1.6 | 297.7 ± 1.5 | −34.7 | 2E-04 | 7E-03 |
| SYT17 | synaptotagmin XVII | 33 ± 1.5 | 21.6 ± 1.6 | −34.4 | 4E-03 | 5E-02 |
| ANKRD20A1 | ankyrin repeat domain 20 family, member A4 | 417.4 ± 1.4 | 276.5 ± 1.4 | −33.8 | 4E-06 | 6E-04 |
| LOC100128888 | uncharacterized | 34.2 ± 1.3 | 22.8 ± 1.3 | −33.4 | 1E-10 | 5E-07 |
| LOC654342 | lymphocyte-specific protein 1 pseudogene | 55.1 ± 1.3 | 36.7 ± 1.4 | −33.4 | 4E-07 | 1E-04 |
| PPFIBP1 | PTPRF interacting protein, binding protein 1 (liprin beta 1) | 43.4 ± 1.3 | 29.1 ± 1.3 | −32.9 | 7E-07 | 2E-04 |
| TSPAN18 | tetraspanin 18 | 162.5 ± 1.3 | 109.2 ± 1.3 | −32.8 | 1E-08 | 1E-05 |
| NBPF20 | neuroblastoma breakpoint family, member 15 | 1226.5 ± 1.3 | 826.3 ± 1.5 | −32.6 | 8E-05 | 4E-03 |
| FAM13A | family with sequence similarity 13, member A | 568.1 ± 1.4 | 395.2 ± 1.5 | −30.4 | 3E-04 | 1E-02 |
| NFIC | nuclear factor I/C (CCAAT-binding transcription factor) | 308.9 ± 1.2 | 215.9 ± 1.3 | −30.1 | 9E-11 | 5E-07 |
| FAM126B | family with sequence similarity 126, member B | 146.6 ± 1.3 | 103.2 ± 1.4 | −29.6 | 7E-05 | 4E-03 |
| UGP2 | UDP-glucose pyrophosphorylase 2 | 32.6 ± 1.5 | 23 ± 1.5 | −29.5 | 2E-04 | 7E-03 |
| MOGAT1 | monoacylglycerol O-acyltransferase 1 | 33.7 ± 1.4 | 23.9 ± 1.4 | −29.2 | 3E-04 | 1E-02 |
| LOC100129905 | uncharacterized LOC100128905 | 38.2 ± 1.3 | 27 ± 1.4 | −29.2 | 2E-05 | 2E-03 |
| FAM129A | family with sequence similarity 129, member A | 162.1 ± 1.4 | 115.1 ± 1.5 | −29.0 | 5E-06 | 8E-04 |
| LOC100128899 | uncharacterized LOC100128979 | 172.3 ± 1.4 | 122.4 ± 1.4 | −29.0 | 1E-03 | 2E-02 |
| GPT | glutamic-pyruvate transaminase (alanine aminotransferase) | 161.1 ± 1.3 | 114.6 ± 1.5 | −28.9 | 2E-03 | 4E-02 |
| C1orf152 | profilin 1 pseudogene 2 | 30.1 ± 1.3 | 21.4 ± 1.3 | −28.7 | 6E-06 | 8E-04 |
Next we investigated whether genes linked to PCOS, T2D, and obesity in published GWAS (P < 5 × 105; http://www.ebi.ac.uk/gwas, accessed on August 20, 2015) were differently expressed in adipose tissue from women with PCOS versus controls (Q < 0.05). We found one out of nineteen PCOS candidate genes, RAB5B26, expressed at a lower level in adipose tissue from women with PCOS (Table 4). Additionally, 18 out of 597 T2D candidate genes (e.g., PPARG27, SVEP128, and IRS129) and five out of 1238 obesity candidate genes (e.g. RTN430 and LEPR31) were differentially expressed in adipose tissue from women with PCOS (Table 4).
Table 4. Genes previously linked to PCOS, type 2 diabetes (T2D) and obesity in published GWAS with differential expression in adipose tissue from women with PCOS (n = 64) compared with controls (n = 30) (cohort 1).
| Symbol | Illumina ID | Controls mean ± SD | PCOS mean ± SD | Difference (%) | pvalues | Qvalues |
|---|---|---|---|---|---|---|
| PCOS | ||||||
| RAB5B (Rabaptin5 β or member RAS oncogene family) | 2850626 | 1260.2 ± 1 | 1195.5 ± 1.1 | –5.14 | 0.000 | 0.009 |
| Diabetes | ||||||
| C2orf79 (peptidyl–tRNA hydrolase domain containing 1) | 3170725 | 64.3 ± 1.1 | 61.2 ± 1.1 | –4.80 | 0.002 | 0.034 |
| CCDC102A (coiled–coil domain containing 102A) | 1660681 | 76.3 ± 1.2 | 70 ± 1.2 | –8.21 | 0.000 | 0.009 |
| CLEC16A (C-type lectin domain family 16, member A) | 160224 | 134.4 ± 1.1 | 122.1 ± 1.1 | –9.19 | 0.000 | 0.001 |
| IKZF4 (IKAROS family zinc finger 4 (Eos)) | 5550608 | 29.6 ± 1.2 | 24.8 ± 1.2 | –16.23 | 0.001 | 0.024 |
| IRS1 (Insulin receptor substrate) | 1710091 | 240.4 ± 1.3 | 257.3 ± 1.4 | 7.03 | 0.001 | 0.024 |
| MEG3 (maternally expressed 3 (non-protein coding)) | 3360113 | 310.6 ± 1.3 | 235.4 ± 1.4 | –24.22 | 0.001 | 0.021 |
| PHTF1 (putative homeodomain transcription factor 1) | 6900521 | 70.6 ± 1.1 | 79.6 ± 1.1 | 12.77 | 0.000 | 0.011 |
| POMC (proopiomelanocortin) | 5080072 | 34.6 ± 1.3 | 28.9 ± 1.3 | –16.42 | 0.003 | 0.044 |
| PPARG (peroxisome proliferator–activated receptor gamma) | 830019 | 2866 ± 1.1 | 2478 ± 1.2 | –13.54 | 0.001 | 0.025 |
| RBMS1 (RNA binding motif, single stranded interacting protein 1) | 4150639 | 526.1 ± 1.1 | 586.7 ± 1.1 | 11.52 | 0.000 | 0.009 |
| SGCG (8sarcoglycan. gamma (35kDa dystrophin-associated glycoprotein) | 5670082 | 138.5 ± 1.2 | 115.6 ± 1.2 | –16.50 | 0.000 | 0.009 |
| SPRY2 (sprouty homolog 2 (Drosophila)) | 6590575 | 284.4 ± 1.2 | 304.7 ± 1.2 | 7.12 | 0.000 | 0.010 |
| SVEP1 (sushi. von Willebrand factor type A, EGF and pentraxin domain containing 1) | 6180554 | 932.8 ± 1.2 | 837 ± 1.4 | –10.27 | 0.000 | 0.001 |
| TNRC6A (trinucleotide repeat containing 6A) | 130386 | 19.5 ± 1.2 | 17.2 ± 1.2 | –12.15 | 0.000 | 0.014 |
| TRAFD1 (TRAF-type zinc finger domain containing 1) | 1570129 | 90.4 ± 1.1 | 82.3 ± 1.1 | –8.93 | 0.001 | 0.019 |
| TYK2 (tyrosine kinase 2) | 6900424 | 404.9 ± 1.1 | 380 ± 1.1 | –6.17 | 0.003 | 0.045 |
| WWOX (WW domain containing oxidoreductase) | 6450189 | 34.5 ± 1.2 | 29 ± 1.2 | –15.92 | 0.000 | 0.001 |
| Obesity | ||||||
| ASAH1 (N-acylsphingosine amidohydrolase [acid ceramidase] 1) | 5420332 | 17.7 ± 1.2 | 19.9 ± 1.2 | 12.38 | 0.000 | 0.012 |
| LEPR (leptin receptor) | 6480348 | 25.7 ± 1.2 | 30.2 ± 1.2 | 17.63 | 0.002 | 0.030 |
| RTN4 (reticulon 4) | 780402 | 1202.6 ± 1.3 | 1468 ± 1.2 | 22.07 | 0.000 | 0.001 |
| RTN4 (reticulon 4) | 2230161 | 5505.2 ± 1.1 | 5988.2 ± 1.1 | 8.77 | 0.003 | 0.040 |
| TSEN34 (TSEN34 tRNA splicing endonuclease subunit) | 5890497 | 268.1 ± 1.1 | 282.9 ± 1.1 | 5.53 | 0.004 | 0.049 |
Replication of differential mRNA expression
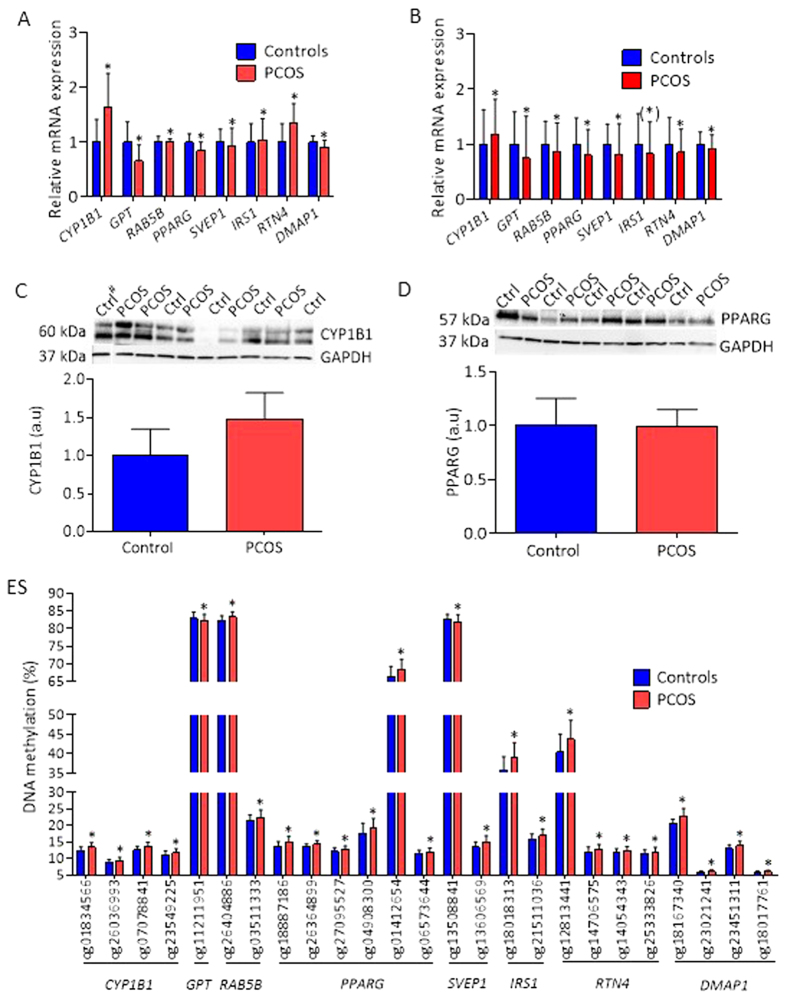
We proceeded to replicate differential gene expression in cohort 2. Selected differentially expressed genes were (1) among those with largest fold change e.g.: CYP1B132, which was up-regulated and is involved in metabolism of sex steroids and adipogenesis, and GPT 33, which was down-regulated and is involved in glucose and amino acid metabolism; (2) among candidate genes identified in GWAS studies of PCOS26 (RAB5B, involved in regulation of intracellular vesicle transport), T2D27,28,29 (PPARG, SVEP1, and IRS1, involved in fatty acid storage and glucose metabolism), and obesity30 (RTN4, a multifunctional gene linked to extreme obesity); and (3) among genes with overlapping with methylation (DMAP1), which is involved in DNA methylation and regulation of obesity-related inflammation and cancer34, all with a Q < 0.05 (Fig. 2A, Table S4).
Figure 2. Selected genes relevant to PCOS that were differentially expressed in cohort 1 and replicated in cohort 2.
(A) Expression data of selected genes from the Illumina HumanHT- 12 v4 Expression BeadChip array (cohort 1). (B) Replication of gene expression data from the array by qPCR in adipose tissue from 21 unrelated women with PCOS and 21 controls (cohort 2). Protein expression of CYP1B1 (C) and PPARG (D) in control (n=5) and PCOS (n=5) are presented. (E) Differential DNA methylation of biologically validated genes. Values for gene expression are mean ± SD and for proteins mean ± SEM. *P < 0.05 (*) P = 0.056. # Sample run on a different membrane.
Six of the 20 selected genes were successfully replicated in cohort 2, as shown by significant expression differences in the same direction as in cohort 1 (Fig. 2B, Table S4). The mRNA expression of CYP1B1 was higher and the mRNA expression of GPT, RAB5B, PPARG, SVEP1, and DMAP1 was significantly lower in adipose tissue of women with PCOS. IRS1 and RTN4 were differentially expressed but in directions opposite to those in cohort 1. The expression of CD74, the gene with the largest increase in expression in cohort 1, was upregulated in cohort 2, although not significant (P = 0.061), and the expression of ADIPOR2 was down regulated (P = 0.069) (Table S4).
Next we preformed western blot (WB) analyses as protein expression is more relevant for biological functions. Protein expression of CYP1B1 was higher in adipose tissue of women with PCOS compared with controls (Fig. 2C) reflecting differential gene expression obtained from BeadChip gene expression array and in the replication cohort 2 (Fig. 2A,B). Protein expression of PPARG displayed a week trend to be lower (Fig. 2D). The WB analyses are hampered by small sample size and big variations.
Genome-wide DNA methylation analysis in adipose tissue from women with PCOS
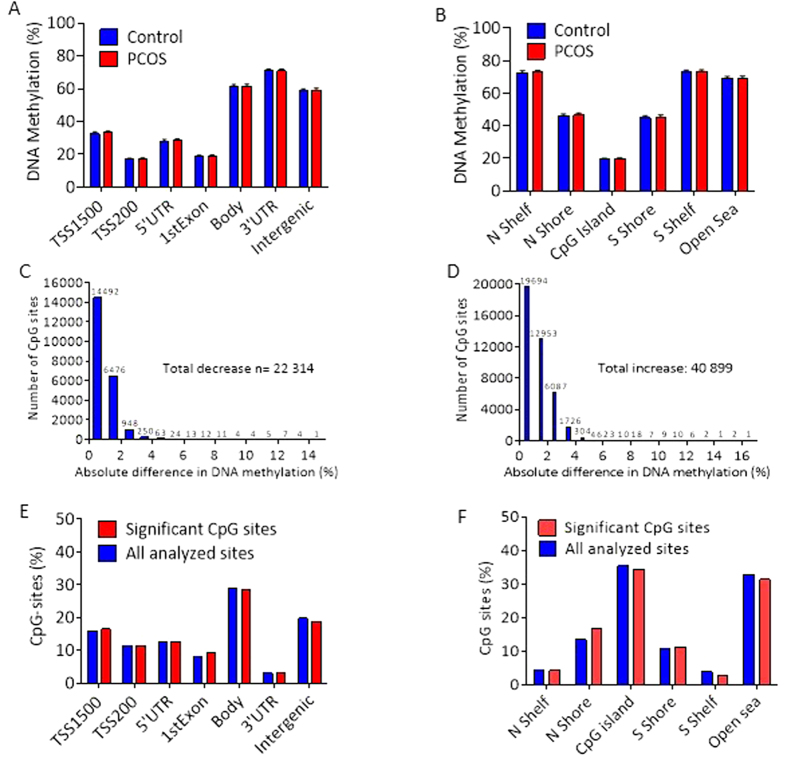
Next we used Illumina Infinium HumanMethylation450k array BeadChips to evaluate the global DNA methylation pattern in adipose tissue from women in cohort 1. After quality control and filtering, methylation data was obtained in 483,317 CpG sites. The average levels of DNA methylation for these sites were grouped based on their location in relation to the nearest gene (Fig. 3A) or in relation to CpG islands (Fig. 3B). We found no differences in average adipose tissue DNA methylation between women with PCOS and controls.
Figure 3. Effect of PCOS on global DNA methylation in human adipose tissue.
Global DNA methylation was calculated as the average DNA methylation of all CpG sites in each annotated region on the Infinium Human Methylation 450 BeadChip presented for (A) the nearest gene region and (B) nearest CpG island region (mean ± SD). Absolute difference in DNA methylation of 63,213 individual sites divided into (C) sites with less methylation and (D) sites with more methylation in 64 women with PCOS than in 30 controls (cohort 1). Distribution of significant sites compared with all analyzed sites in relation to nearest gene region (E) and nearest CpG island region (F). TSS, proximal promotor defined as 200 or 1500 bp upstream of the transcription site; Shore, flanking region of CpG island (0–2000 bp); Shelf, regions flanking island shores (2000–4000 bp from the CpG island).
Of the 483,317 CpG sites, 63,213 (13%) were differentially methylated in adipose tissue from women with PCOS and controls (P < 0.05) (Table S5). This is 2.6 times more than the expected number with a P < 0.05 (a P value threshold of 5% may yield a false positive rate of 5% in the dataset) and significantly more than would be expected by chance (chi-square test P < 0.0001). After FDR correction, 440 CpG sites were differentially methylated (Q < 0.15), Table S6. The most significant CpG site was cg13496119, annotated to LOC100129345 (Q = 0.046, P < 0.000). This uncharacterized locus of unknown function belongs to long noncoding RNAs associated with chromatin remodeling and transcriptional regulation. As judged from absolute difference in DNA methylation between cases and controls, methylation decreased at 22,314 sites (Fig. 3C) and increased at 40,899 sites (Fig. 3D). The majority of differentially methylated sites were in the gene body, intergenic regions, CpG islands, and “open sea” regions (Fig. 3E,F).
To assess the biological relevance of differently methylated genes in adipose tissue from women with PCOS and controls, we used Ingenuity Pathway Analysis software to analyze the 48,426 transcripts annotated to the 63,213 differentially methylated CpG sites (P < 0.05). All significantly up- and down-regulated gene sets with differential methylation are presented in (Table S7). Significantly up-regulated canonical pathways relevant to PCOS and T2D are the Wnt/β-catenin and planar cell polarity signaling pathways, which regulate glucose transporters, mitochondrial biogenesis, and adipogenesis35; and oxidative stress and reactive oxygen species in the macrophage signaling pathway36, all involved in glucose regulation, energy metabolism, and obesity. A significantly down-regulated pathway of importance is the NRF-2-mediated oxidative stress response pathway, which helps regulate a wide range of genes in response to environmental stress37. Importantly, the NRF-2-mediated oxidative stress response pathway was among the significant gene sets for both differential gene expression and methylation. Differentially methylated genes contributing to up-regulated and down-regulated top canonical pathways are presented in Tables S8 and S9, respectively.
Next we investigated whether genes previously linked to PCOS, T2D, and obesity in published GWAS (P < 5 × 105; http://www.ebi.ac.uk/gwas, accessed on August 20, 2015) were differently methylated in adipose tissue from women with PCOS and controls (Q < 0.15). This analysis identified four out of 597 T2D candidate genes (ARF538, GALNTL439, R3HDML40, and PSMD640) and one obesity candidate gene out of 1238 (PROX141) (Table 5), but no PCOS candidate gene. DNA methylation (P < 0.05) in the replicated genes (Fig. 2A,B) is shown in Fig. 2E.
Table 5. Genes previously linked to type 2 diabetes and obesity in published GWAS with differential methylation (Q < 0.15) in adipose tissue from women with PCOS (n = 64) versus controls (n = 30) (cohort 1).
| Symbol (name) | Illumina ID | Controls mean ± SD | PCOS mean ± SD | Difference (%) | Pvalues | Qvalues |
|---|---|---|---|---|---|---|
| Diabetes | ||||||
| ARF5 (ADP-ribosylation factor 5) | cg15802323 | 17.4 ± 1.4 | 19.1 ± 1.9 | 1.69 | 7.8E-05 | 0.15 |
| GALNTL4 (polypeptide N-acetylgalactosaminyltransferase 18) | cg04807025 | 88.6 ± 0.7 | 87.9 ± 1.1 | -0.78 | 7.1E-06 | 0.11 |
| R3HDML (R3H domain containing-like) | cg17692403 | 15.3 ± 1.7 | 17.6 ± 2.4 | 2.26 | 9.2E-05 | 0.15 |
| PSMD6 (26S proteasome non-ATPase regulatory subunit 6) | cg07662771 | 77.5 ± 2 | 74.7 ± 3.4 | -2.78 | 1.2E-04 | 0.15 |
| Obesity | ||||||
| PROX1 (prospero homeobox 1) | cg22489498 | 9.2 ± 0.8 | 10 ± 0.8 | 0.79 | 1.5E-05 | 0.11 |
Finally, based on data by Chen et al.42, potentially cross-reactive probes among our differentially methylated CpG sites with P < 0.05 are presented in Table S10.
Overlap and associations between gene expression and DNA methylation
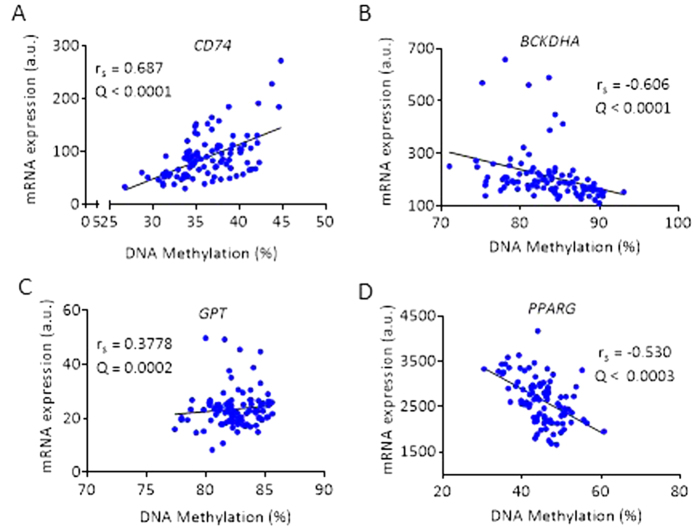
Since mRNA expression can be regulated by epigenetic modifications, we next investigated whether genes whose mRNA expression in adipose tissue differed in women with PCOS and controls also differed in DNA methylation. For this analysis, we merged the mRNA expression data and the DNA methylation data (cohort 1). All genes that were differentially expressed (P < 0.05) were linked to corresponding methylated probes. In total, in 30 individual genes with differential mRNA expression (Q < 0.05), we found a corresponding change in 33 DNA methylation sites (Table S11). One of these genes, DMAP1, was replicated in cohort 2 and is involved in DNA methylation and regulation of obesity-related inflammation and developing cancer34,42; its mRNA expression was also replicated in cohort 2. Of note, a large number of DNA methyltransferases (DNMTs) genes, a family of enzymes catalyzing the transfer of a methyl group to DNA, were differentially methylated in women with PCOS compared with controls (Table S12). Next, to investigate direct correlations between gene expression and DNA methylation, we performed Spearman’s with correction for multiple testing. We included mRNA expression of the 5453 annotated transcripts differentially expressed in adipose tissue from women with PCOS (P < 0.05), and DNA methylation of CpG probes located within the cis distance 500 kb upstream and 100 kb downstream of the genes. We identified 1913 pairs of “gene-CpG probes” that were significantly correlated after correction for multiple testing (Q < 0.05) that corresponded to 349 unique genes (Table S13). 1057 of these correlations were positive (Rho = 0.37–0.61) and 856 were negative (Rho = −0.37–0.69).
Several probes of CD74, the gene with the highest increase in expression difference, showed a strong positive correlation with DNA methylation (Rho = 0.611–0.687; Q < 0.05) (Table S13). The CD74 molecule is associated with class II major histocompatibility complex (MHC) class II invariant chain that is involved in regulation of adipogenesis and inflammation43. The gene with the strongest negative correlation with DNA methylation was the branched chain keto acid dehydrogenase E1 alpha polypeptide provided (BCKDHA) gene (Table S13). This gene is involved in metabolic signaling and insulin resistance44. Representative correlation between mRNA expression and DNA methylation of CD74, BCKDHA, GPT, and PPARG are shown in Fig. 4A–D.
Figure 4. Representative illustrations of correlations of gene expression with DNA methylation in adipose tissue from cohort 1.
Expression CD74 correlated positively with DNA methylation (A); BCKDHA correlated negatively with DNA methylation (B); GPT correlated positively with DNA methylation (C); and PPARG correlated negatively with DNA methylation (D). Spearman rank correlation with FDR corrections.
Associations between hyperandrogenemia, glucose homeostasis, or adipocyte size and gene expression or DNA methylation in adipose tissue
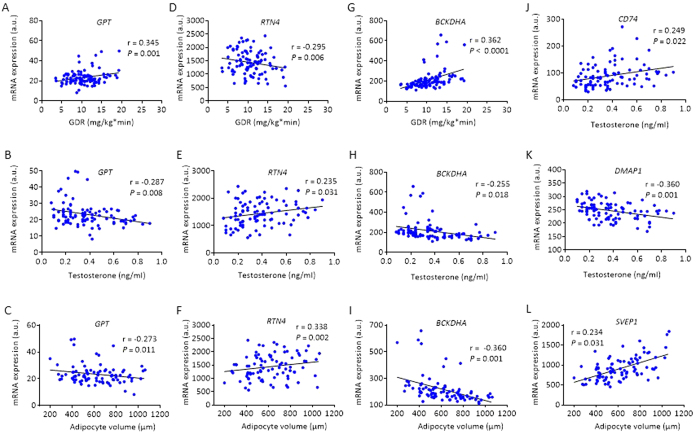
Hyperandrogenemia and insulin resistance are the strongest clinical features of PCOS, and adipocytes size is a strong marker for insulin resistance in women with PCOS9. Therefore, we tested whether the GDR measured by euglycemic hyperinsulinemic clamp, circulating testosterone measured by LC-MS/MS, or adipocyte size was associated with (1) genes with largest fold changes CD74, CYP1B1 and GPT; (2) candidate genes identified in GWAS studies of PCOS (RAB5B), T2D (PPARG, SVEP1 and BCKDHA), and obesity (RTN4); and, (3) a gene overlapping with methylation (DMAP1). With Spearman’s we found a significantly positive correlation between GDR and the expression of RAB5B (rs = 0.211), PPARG (rs = 0.426), and BCKDHA (rs = 0.561), and negative correlation with CD74 (rs = −0.281), SVEP1 (rs = −0.421), and CYP1B1 (rs = −0.325). Circulating testosterone correlated positively with the expression of CD74 (rs = 0.356), CYP1B1 (rs = 0.219) and RTN4 (rs = 0.261), and negatively with the expression of GPT (rs = −0.317), PPARG (rs = −0.269), BCKDHA (rs = −0.463), and DMAP1 (rs = −0.339). Adipocyte size was positively correlated with CD74 (rs = 0.425), SVEP1 (rs = 0.525), RTN4 (rs = 0.217), and CYP1B1 (rs = 0.284), and negatively associated with PPARG (rs = −0.449) and BCKDHA (rs = −0.587). Correlations that remained significant after adjustments for BMI are presented in Fig. 5A–L.
Figure 5. Correlations of GDR, adipocyte size, and circulating testosterone level with gene expression in adipose tissue from cohort 1.
Expression of GPT correlated positively with GDR (A), negatively with circulating testosterone (B), and with adipocyte volume (C); RTN4 correlated negatively with GDR (D), positively with testosterone (E), and with adipocyte volume (F); BCKDHA correlated positively with GDR (G), negatively with circulating testosterone (H), and with adipocyte volume (I); CD74 correlated positively with circulating testosterone (J); DMPA1 correlated negatively with circulating testosterone (K); and SVEP1 correlated positively with adipocyte volume (L). Pearson’s partial correlation.
Discussion
This study shows that genome-wide mRNA expression in subcutaneous adipose tissue is altered in women with PCOS and that several genes are associated with insulin resistance, adipocyte size, and hyperandrogenemia. Among the top up- and down-regulated canonical pathways relevant to PCOS and T2D, we found gene sets representing the PI3K/AKT, ERK/MAPK, androgen, TGF-β, telomerase, and NRF2-mediated oxidative stress response signaling pathways. We also demonstrate for the first time that genome-wide DNA methylation alterations in adipose tissue were larger than expected. However, correction for multiple testing reduced the number of differentially methylated sites to 440. Also, we found more than 1900 pairs of “gene-CpG probes” that were significantly correlated after correction for multiple testing and corresponded to 349 unique genes. Since PCOS is a polygenic disease, the combined effect of several modest changes in DNA methylation might contribute to the pathogenesis of PCOS. This speculation is supported by other studies demonstrating a relative modest difference (0.13% to 11%) in DNA methylation in different target tissues45. Also, an absolute change of a few percent could make a relatively large difference, as we found in a recent study in which the fold change in DNA methylation ranged from 0.54 to 1.84 between the groups45. Consistent with these observations, differences of DNA methylation in the present study ranged from 0.24 to 6.28.
We identified several genes with differential mRNA expression and corresponding changes in DNA methylation, indicating that altered DNA methylation may have the potential to influence the expression of corresponding genes. Genes with the largest expression differences e.g. CD74, CYP1B1, and GPT were strongly associated with DNA methylation after FDR corrections. These genes are involved in adipogenesis and inflammatory response (CD74)46, in metabolism of sex steroids and adipogenesis (CYP1B1)47, and in glucose and amino acid metabolism (GPT)33. In candidate genes for PCOS (RAB5B), T2D (PPARG and SVEP1), and methylation (DMAP1) we found that a decrease in mRNA expression was paralleled with an increase in DNA methylation, consistent with previous functional in vitro studies demonstrating that an increase in DNA methylation reduces transcriptional activity48,49. Many of the up-regulated genes had a parallel increase in DNA methylation. One possible explanation is that these CpG sites are in the gene body region, as evidenced by the over-representation of differentially methylated sites we found in this region. Importantly, DNA methylation in the gene body positively affects gene expression50.
Although changes in mRNA expression were more pronounced than changes in DNA methylation, we found a large number of differentially expressed genes and methylated CpG sites that were significantly correlated after FDR correction. Of note, changes in methylation can be targets for other kind of transcriptional regulation, microRNAs, or histone modifications. There may also be an interaction between nongenetic, epigenetic, and genetic factors affecting gene expression and subsequent development of polygenic diseases such as PCOS, T2D, and obesity51,52,53,54. Support for a genetic effect comes from a study demonstrating that T2D-related single nucleotide polymorphisms (SNPs) are CpG-SNPs, which affect DNA methylation and gene expression in human pancreatic cells55, and from studies of multiple tissues from twins56,57. Of note, studies in twins indicate that genetic influences explain more than 70% of PCOS pathogenesis58. Nevertheless, recent GWAS have not identified SNPs that could explain this estimated heritability26,59, which therefore might reflect contributions of epigenetic variations.
The first GWAS on Han Chinese populations identified more than 10 susceptibility loci for PCOS, including RAB5B26,59. This gene encodes the Ras-related protein RAB5B. Although the functional role of this PCOS susceptible gene in adipose tissue is unknown, RAB5B expression was down-regulated both in the discovery cohort and in the replication cohort and was accompanied by an up-regulation in DNA methylation. Genes acting through Rab5b-dependent post-translational regulation of β1/β2 integrins60, indicates a functional role of RAB5B in a target tissue.
A very recent GWAS of women of European ancestry who had a classic PCOS phenotype (hyperandrogenism and irregular menstruation) identified two novel genetic susceptibility loci that mapped to chr 8p32.1 and chr 11p14.161. Interestingly, we found differential DNA methylation in all four genes that mapped to the chr 8p32.1 PCOS candidate locus (NEIL2, FDFT1, GATA4, and CTSB) and differential expression of FDF1 and CTSB. Further, we found differential gene expression and DNA methylation of one of the most promising functional candidate genes—ARL14EP, which maps to chr 11p14.1—but no changes in the FSHB expression or methylation in adipose tissue from women with PCOS. In the GWAS study mentioned above, there was no evidence of transcriptional activation and microarray analysis revealed low levels in adipocytes.
We also found that two T2D susceptibility genes, PPARG27 and SVEP128, followed the same pattern of decreased expression and increased DNA methylation, and both showed a strong negative correlation between “gene-CpG probes” after multiple corrections. PPARG, a master regulator of adipocyte differentiation, directly controls the expression of genes involved in lipid transport and metabolism, adipokine production, and insulin signaling62; it is a target for insulin-sensitizing drugs such as glitazones, which improve plasma glucose maintenance in patients with T2D63. Previously we found higher levels of DNA methylation of PPARG in adipose tissue in subjects with T2D than in controls21. PPARG also protects against vascular calcification by inducing the expression of secreted frizzled-related protein-2, a Wnt5a antagonist. Targeting the Wnt/β-catenin signaling pathway may have clinical implications in the context of common complications of atherosclerosis, T2D and obesity64,65. Of interest, we found DNA methylation activated gene sets in the Wnt/β-catenin signaling pathway in adipose tissue in the present study. Previous studies have implicated that Wnt signaling activation of β-catenin, a negative co-activator, and PPARG might dysregulate adipogenesis66. PPARG gene expression was positively associated with insulin sensitivity (GDR) and negatively associated with adipocyte size and testosterone, although these associations did not survive after adjustment for BMI. Further, many of the significant canonical pathways included gene sets with differentially methylated genes or with differential gene expression in human adipose tissue that are similar to pathways detected in adipose tissue of prenatal androgenized rhesus monkeys67 (e.g., the Wnt/β-catenin and TGF-β signaling pathways). These findings support the hypothesis that maternal androgen excess causes an unfavorable intrauterine environment and leads to epigenetic changes that contribute to the development of insulin resistance and altered molecular signaling pathways in adipose tissue of women with PCOS.
SVEP1, another gene associated with T2D28, encodes a cell-adhesion molecule expressed by cells related to skeletal tissues. Hypermethylation of SVEP1 decreases its expression68, consistent with our finding of decreased mRNA expression and increased DNA methylation of SVEP1 in adipose tissue in women with PCOS; however, SVEP1 mRNA expression correlated negatively with adipocyte volume after adjustment for BMI.
IRS1, a substrate of insulin receptor tyrosine kinase, have a central role in the insulin stimulated signaling and is a candidate gene of T2D69. We were not able to replicate the expression found in cohort 1. Instead there was a trend towards decreased mRNA expression of IRS1 was (P = 0.058) in cohort 2. Of note, cohort 1 includes normal, overweight and obese subjects and all these analyses were corrected for BMI. The decrease in IRS1 mRNA expression may reflect that the replication cohort was overweight – obese. This is further supported by the fact that when dividing cohort 1 into lean vs overweight – obese, the IRS1 mRNA expression was lower among the overweight – obese women (mean ± SD) 318.2 ± 113.4 vs 232.4 ± 71.8, P < 0.001). This phenomenon has previously been demonstrated in e.g. Pima Indians there IRS1 mRNA expression is approximately 1.8 fold lower in adipocytes from obese subjects compared with normal weight subjects70. The results in the present study is in agreement with a recent study demonstrating that the insulin signaling pathway in subcutaneous adipose tissues is not the major contributor to the pathogenesis of PCOS71.
DNA methylation is important in many biological processes, such as regulation of transcription, genomic imprinting, chromatin structure, and silencing of repetitive DNA elements72. Abnormal DNA methylation is common in human cancers and contributes to tumorigenesis73. DNA methylation in mammalian cells is carried out by DNMTs. DNMT1-associated protein (DMAP1) has an intrinsic repressive activity and helps maintain DNA methylation in a heritable manner74. Interestingly, a large number of DNMTs were differentially methylated in adipose tissue form women with PCOS. These finding further support the hypothesis that maternal androgen excess75 can induce epigenetic changes57.
Adipose tissue is composed of many cell types, and changes in cell composition may have contributed to the differences in gene expression and DNA methylation we observed. Previously, we showed that there is no major impact of cellular composition or inflammatory response on observed associations in DNA methylation in adipose tissue and BMI and HbA1c53. Although adipocyte isolation per se may affect gene expression and DNA methylation, it would be of interest to investigate genome-wide gene expression and DNA methylation in adipocytes from different fat depots. Future studies should also investigate the role of additional epigenetic mechanisms, including miRNAs and histone modifications which may be of importance in PCOS. Analyzes in the present study is limited to subcutaneous adipose tissue. Visceral fat accumulation is an independent risk factor of T2D76. The visceral fat depot is drained by the portal venous system leading to a direct supply of free fatty acids, and may therefore be considered as more metabolic active77. However, different genes may have different functions in the two depots; e.g. PPARG have a twofold higher mRNA expression in subcutaneous than in visceral adipose tissue in lean/overweight subjects78. In contrast, in obese subjects, mRNA expression of PPARG was similar in the two depots. Of note, in the present study including both lean and overweight/obese women with PCOS displayed lower PPARG expression independent of BMI. Women with PCOS display a “male like” fat distribution with abdominal (subcutaneous and visceral) fat accumulation79. Few studies have investigated differences between gene expression in subcutaneous and visceral fat depots in women with PCOS with no major differences15,80. Another limitation is the cross sectional design and that we have only 30 controls compared with 64 cases, although to date this study is the largest investigating genome wide DNA methylation and expression in women with PCOS.
In conclusion, we identified a large number of genes and pathways that are affected in adipose tissue from women with PCOS. We also identified specific DNA methylation pathways that may affect mRNA expression. Together, these novel findings show that women with PCOS have multiple transcriptional and epigenetic changes in adipose tissue that are relevant for development of the disease.
Material and Methods
Study participants
Cohort 1 consisted of 64 women with PCOS and 30 controls who had successful subcutaneous adipose tissue biopsies and were previously described in detail9. Case-control cohort 2 consisted of 21 women with PCOS and 21 controls matched pairwise for age, weight, and BMI were included for replication of the findings in cohort 1.
All women provided oral and written informed consent. The two studies were conducted at the Sahlgrenska Academy, University of Gothenburg and at Sahlgrenska University Hospital, Gothenburg, Sweden, in accordance with the Declaration of Helsinki and were approved by the Regional Ethical Review Board of the University of Gothenburg. After all relevant clinical information was obtained, samples were coded and anonymized.
Subject recruitment, exclusion and inclusion criteria are described in detail in Supplementary Material and Methods.
Clinical examination
Body weight and height were measured in subjects wearing light clothing; body mass index was calculated as kg/m2. Waist circumference was measured between the lower rib and iliac crest. In controls, blood samples were obtained in the morning after an overnight fast during the early follicular phase (days 1–7 of the menstrual cycle) to match the hormonal milieu of PCOS subjects and to avoid the preovulatory estrogen rise. Fasting blood samples were collected independently of cycle day in women with PCOS as the majority had oligo/anovulation. Subcutaneous abdominal adipose tissue biopsies were obtained under local anesthesia and immediately isolated for measurement of adipocyte size9 or snap frozen in liquid nitrogen and stored at −80 °C. Whole-body glucose homeostasis was measured by euglycemic hyperinsulinemic clamp with calculation of GDR, and by calculation of HOMA-IR and HOMA-B9.
Biochemical analyses
Plasma glucose was measured at 37 °C with an enzymatic photometric method (Roche Diagnostics, Mannheim, Germany) in cohort 1 and by One Touch Ultra2 (LifeScan) in cohort 2. Serum insulin was measured with an immunometric two-step sandwich method and chemiluminescence (Advia Centaur Insulin ReadyPack; Bayer HealthCare). Estrogen and testosterone were measured by gas chromatography-tandem mass spectrometry, and SHBG was analyzed by chemiluminescent microparticle immunoassay as described10,81.
RNA and DNA Extraction from Adipose Tissue
For gene expression array studies, RNA was extracted with the RNeasy Lipid tissue Mini Kit (Qiagen). For methylation array studies, DNA was isolated with the QIAamp DNA Mini Kit (Qiagen). Nucleic acid concentrations and purity were estimated with a NanoDrop spectrophotometer (Thermo Scientific). DNA integrity was also checked by gel electrophoresis, and RNA quality was determined with an automated electrophoresis station (Experion, Bio-Rad).
Expression Arrays and quantitative real-time PCR
To assess the global mRNA expression profile in adipose tissue, we analyzed isolated mRNA with high abundance with microarray HumanHT-12 v4 Expression BeadChip (Illumina). cRNA synthesis, including biotin labeling, was carried out using Illumina TotalPrep RNA Amplification Kit (Life Technologies & Invitrogen) according to manufacturer’s recommendations. Biotin-cRNA complex was then fragmented and hybridized to the probes on the Illumina BeadChip array. Probes were hybridized and stained with streptavidin-Cy3 before visualization with Illuminas HiScan fluorescence camera. The Oligo package from Bioconductor was used to compute Robust Multichip Average expression measures82.
In cohort 2, quantitative real-time PCR analysis of selected genes (Table S4) was validated with custom TaqMan gene expression array micro fluid cards (Life Technologies) i.e. the replication cohort. Samples were run in duplicate; the amount of cDNA in each loading port was equivalent to 100 ng of mRNA. The arrays were run according to the manufacturer’s protocol with a QuantStudio 7 Flex Real-time PCR System and QuantStudio 7 software (Life Technologies). Candidate reference genes (GAPDH, LRP10, and CLN3) were validated with NormFinder algorithm; from this algorithm, the combination of LRP10 and CLN3 was used as the reference control. Gene expression values were calculated with the ΔΔCq method (i.e., RQ = 2−ΔΔCq).
Immunoblot Analysis
To analyze protein expression in adipose tissue homogenates we used antibodies against CYP1B1 (AV51761, Sigma-Aldrich, Stockholm Sweden) and PPARG (ab191407, Abcam, Cambride, UK). Briefly, samples were centrifuged at 13,000 g for 10 min at 4 °C and protein concentration was determined with a Direct Detect™ spectrometer (Millipore, Billerica, USA). 50 μg of total protein was loaded on Criterion™ TGX (Tris-Glycine eXtended) Stain-Free™ precast gels (Bio-Rad) and transferred to nitrocellulose midi-membrane in the Trans-Blot® Turbo™ Transfer System (Bio-Rad). GAPDH was used as a loading control and for normalization.
DNA Methylation Arrays
Genome-wide DNA methylation in adipose tissue was analyzed with Illumina Infinium HumanMethylation450k array BeadChips. The array contains 485,577 cytosine probes covering 21,231 (99%) RefSeq genes83. A Zymo Methylation Kit (D5001-D5002, Zymo Research) was used to convert genomic DNA to the bisulfite-modified DNA. Briefly, gDNA (500 ng) of high quality was fragmented and hybridized on the BeadChip, and the intensities of the signal were measured with a HiScanQ scanner (Illumina). The methylation values for each CpG site are presented as a β-value ranging from 0 (unmethylated) to 100% (completely methylated).
The bioinformatics analyses were performed as described21,53. In brief, Y chromosome probes, rs-probe, and probes with average detection p-value > 0.01 were removed. After quality control and filtering, methylation data was obtained in 483,317 CpG sites. Beta-values were converted to M-values (M = log2 (β/(1 − β), which were used for all data analyses. Data were then quantile normalized and batch corrected with COMBAT84. A linear regression model was used to identify differences in DNA methylation between women with PCOS and controls. To improve interpretation, after all the preprocessing steps, data were reconverted to beta-values, which are presented in tables and figures.
Ingenuity pathway analysis
Ingenuity Pathway Analysis (Qiagen) was used for core analyses of data from the gene expression array and DNA methylation array so we could interpret the outcome and make predictions based on known libraries. Fold change and Q- and P-values were calculated before data entry, and thereafter uploaded for Ingenuity Pathway Analysis. Q < 0.05 was used as the cut-off for gene expression and P < 0.05 for DNA methylation. Top canonical pathways are based on the P-value overlap of genes in our dataset with molecules in the canonical pathway.
Statistics
Differences between clinical characteristics in cohort 1 were based on ANCOVA with adjustment for age and BMI. Fisher’s permutation test was used for comparisons of clinical characteristics in cohort 2; no adjustments for age and BMI were needed, as the cases and controls were matched for age, weight, and BMI. DNA methylation differences between cases and controls in cohort 1 were based on linear regression including batch, age and BMI. The Mann-Whitney U test was used for expression analyses. FDR was used to correct for multiple testing in the analyses of gene and methylation arrays. The chi-square test was used to calculate whether the differentially methylated sites were more than the expected number by chance. Also, differentially expressed genes with a Q < 0.05 were merged with corresponding probes in the methylation data. For these analyses, a CpG site was annotated to the nearest gene. Correlations in women with PCOS plus controls (cohort 1) between DNA methylation and gene expression of selected genes and GDR and testosterone were analyzed by Spearman´s correlation with correction for multiple testing. Data are presented as mean ± SD.
Additional Information
How to cite this article: Kokosar, M. et al. Epigenetic and Transcriptional Alterations in Human Adipose Tissue of Polycystic Ovary Syndrome. Sci. Rep. 6, 22883; doi: 10.1038/srep22883 (2016).
Supplementary Material
Acknowledgments
The work was supported by the Swedish Medical Research Council Project No. 2014-2775 (ESV) and 2013-3018 (CL), Jane and Dan Olsson Foundation (ESV), Wilhelm and Martina Lundgrens’s Science Fund (ESV), Hjalmar Svensson Foundation (ESV), Adlerbert Research Foundation (ESV), and the Swedish federal government under the LUA/ALF agreement ALFGBG-429501 (ESV) and ALF Lund 2014-354 (CL), Novo Nordisk Foundation (ESV CL), Swedish Diabetes Foundation (CL), and Påhlsson Foundation (CL), and Swedish Pharmaceutical Society (MK). We thank the Genomics Core Facility at the Sahlgrenska Academy, University of Gothenburg, for the use of technical equipment and support.
Footnotes
Author Contributions A.B., C.L. and E.S.-V. conceived and designed the study. M.K., A.B., R.F., A.P., M.M., C.J.B. and A.S. performed experiments and statistical analyses. M.K., R.F., E.N., A.B., C.O., C.L. and E.S.-V. contributed to the analysis and interpretation of data. M.K. and E.S.-V. wrote the manuscript. All of the authors revised critically and approved the manuscript.
References
- Norman R. J., Dewailly D., Legro R. S. & Hickey T. E. Polycystic ovary syndrome. Lancet 370, 685–697 (2007). [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E. & Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 33, 981–1030 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. S., Davies M. J., Norman R. J. & Moran L. J. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 18, 618–637 (2012). [DOI] [PubMed] [Google Scholar]
- Hickey T. E., Legro R. S. & Norman R. J. Epigenetic modification of the X chromosome influences susceptibility to polycystic ovary syndrome. J Clin Endocrinol Metab 91, 2789–2791 (2006). [DOI] [PubMed] [Google Scholar]
- Moran L. J., Noakes M., Clifton P. M., Norman R. J. & Fenech M. F. Genome instability is increased in lymphocytes of women with polycystic ovary syndrome and is correlated with insulin resistance. Mutat Res 639, 55–63 (2008). [DOI] [PubMed] [Google Scholar]
- Xu N., Azziz R. & Goodarzi M. O. Epigenetics in polycystic ovary syndrome: A pilot study of global DNA methylation. Fertil Steril 94, 781–783 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manneras-Holm L. et al. Coagulation and fibrinolytic disturbances in women with polycystic ovary syndrome. J Clin Endocrinol Metab 96, 1068–1076 (2011). [DOI] [PubMed] [Google Scholar]
- Manneras-Holm L., Benrick A. & Stener-Victorin E. Gene expression in subcutaneous adipose tissue differs in women with polycystic ovary syndrome and controls matched pair-wise for age, body weight, and body mass index. Adipocyte 3, 190–196 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manneras-Holm L. et al. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab 96, E304–311 (2011). [DOI] [PubMed] [Google Scholar]
- Stener-Victorin E. et al. Are there any sensitive and specific sex steroid markers for polycystic ovary syndrome? J Clin Endocrinol Metab 95, 810–819 (2010). [DOI] [PubMed] [Google Scholar]
- Ciaraldi T. P., Aroda V., Mudaliar S., Chang R. J. & Henry R. R. Polycystic ovary syndrome is associated with tissue-specific differences in insulin resistance. J Clin Endocrinol Metab 94, 157–163 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek I., Arner P., Bergqvist A., Carlstrom K. & Wahrenberg H. Impaired adipocyte lipolysis in nonobese women with the polycystic ovary syndrome: a possible link to insulin resistance? J Clin Endocrinol Metab 82, 1147–1153 (1997). [DOI] [PubMed] [Google Scholar]
- Jones M. R. et al. Steroidogenic regulatory factor FOS is underexpressed in polycystic ovary syndrome (PCOS) adipose tissue and genetically associated with PCOS susceptibility. J Clin Endocrinol Metab 97, E1750–1757 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corton M. et al. Differential Gene Expression Profile in Omental Adipose Tissue in Women with Polycystic Ovary Syndrome. J Clin Endocrinol Metab 92, 328–337 (2007). [DOI] [PubMed] [Google Scholar]
- Carmina E. et al. Subcutaneous and omental fat expression of adiponectin and leptin in women with polycystic ovary syndrome. Fertil Steril 9, 642–648 (2008). [DOI] [PubMed] [Google Scholar]
- Tan B. K. et al. Omentin-1, a novel adipokine, is decreased in overweight insulin-resistant women with polycystic ovary syndrome: ex vivo and in vivo regulation of omentin-1 by insulin and glucose. Diabetes 57, 801–808 (2008). [DOI] [PubMed] [Google Scholar]
- Tan B. K. et al. Upregulation of adiponectin receptor 1 and 2 mRNA and protein in adipose tissue and adipocytes in insulin-resistant women with polycystic ovary syndrome. Diabetologia 49, 2723–2728 (2006). [DOI] [PubMed] [Google Scholar]
- Chazenbalk G. et al. Abnormal expression of genes involved in inflammation, lipid metabolism, and Wnt signaling in the adipose tissue of polycystic ovary syndrome. J Clin Endocrinol Metab 97, E765–770 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott D. H., Barnett D. K., Bruns C. M. & Dumesic D. A. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update 11, 357–374 (2005). [DOI] [PubMed] [Google Scholar]
- Waterland R. A. Is epigenetics an important link between early life events and adult disease? Hormone research 71 Suppl 1, 13–16 (2009). [DOI] [PubMed] [Google Scholar]
- Nilsson E. et al. Altered DNA methylation and differential expression of genes influencing metabolism and inflammation in adipose tissue from subjects with type 2 diabetes. Diabetes 63, 2962–2976 (2014). [DOI] [PubMed] [Google Scholar]
- Wang X. X. et al. Genome-wide DNA methylation and gene expression patterns provide insight into polycystic ovary syndrome development. Oncotarget 5, 6603–6610 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. Y. et al. Genome-wide screen of ovary-specific DNA methylation in polycystic ovary syndrome. Fertil Steril S0015–0282(15)00249-6 (2015). [DOI] [PubMed] [Google Scholar]
- Qu F. et al. A molecular mechanism underlying ovarian dysfunction of polycystic ovary syndrome: hyperandrogenism induces epigenetic alterations in the granulosa cells. J Mol Med (Berl) 90, 911–923 (2012). [DOI] [PubMed] [Google Scholar]
- Jones M. R. et al. Systems Genetics Reveals the Functional Context of PCOS Loci and Identifies Genetic and Molecular Mechanisms of Disease Heterogeneity. PLoS genetics 11, e1005455 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. et al. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat Genet 44, 1020–1025 (2012). [DOI] [PubMed] [Google Scholar]
- Replication D. I. G. et al. Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat Genet 46, 234–244 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale L. R. et al. Exploring genome-wide - dietary heme iron intake interactions and the risk of type 2 diabetes. Frontiers in genetics 4, 7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voight B. F. et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet 42, 579–589 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsapas C. et al. Common body mass index-associated variants confer risk of extreme obesity. Human molecular genetics 18, 3502–3507 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler E. et al. Genome-wide SNP and CNV analysis identifies common and low-frequency variants associated with severe early-onset obesity. Nat Genet 45, 513–517 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellero S. et al. Xenobiotic-metabolizing cytochromes p450 in human white adipose tissue: expression and induction. Drug Metab Dispos 38, 679–686 (2010). [DOI] [PubMed] [Google Scholar]
- Inoue K., Matsumoto M., Miyoshi Y. & Kobayashi Y. Elevated liver enzymes in women with a family history of diabetes. Diabetes Res Clin Pract 79, e4–7 (2008). [DOI] [PubMed] [Google Scholar]
- Kamei Y. et al. Increased expression of DNA methyltransferase 3a in obese adipose tissue: studies with transgenic mice. Obesity (Silver Spring) 18, 314–321 (2010). [DOI] [PubMed] [Google Scholar]
- Yoon J. C. et al. Wnt signaling regulates mitochondrial physiology and insulin sensitivity. Genes Dev 24, 1507–1518 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Lay S., Simard G., Martinez M. C. & Andriantsitohaina R. Oxidative stress and metabolic pathologies: from an adipocentric point of view. Oxid Med Cell Longev 2014, 908539 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagishita Y. et al. Nrf2 protects pancreatic beta-cells from oxidative and nitrosative stress in diabetic model mice. Diabetes 63, 605–618 (2014). [DOI] [PubMed] [Google Scholar]
- Ma R. C. et al. Genome-wide association study in a Chinese population identifies a susceptibility locus for type 2 diabetes at 7q32 near PAX4. Diabetologia 56, 1291–1305 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer N. D. et al. A genome-wide association search for type 2 diabetes genes in African Americans. PLoS One 7, e29202 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y. S. et al. Meta-analysis of genome-wide association studies identifies eight new loci for type 2 diabetes in east Asians. Nat Genet 44, 67–72 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. J. et al. Combined linkage and association analyses identify a novel locus for obesity near PROX1 in Asians. Obesity (Silver Spring) 21, 2405–2412 (2013). [DOI] [PubMed] [Google Scholar]
- Cheng J. et al. Molecular mechanism for USP7-mediated DNMT1 stabilization by acetylation. Nature communications 6, 7023 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B. S., Pallua N., Bernhagen J. & Bucala R. The macrophage migration inhibitory factor protein superfamily in obesity and wound repair. Experimental & molecular medicine 47, e161 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch C. J. & Adams S. H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol 10, 723–736 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall E. et al. Effects of palmitate on genome-wide mRNA expression and DNA methylation patterns in human pancreatic islets. BMC Med 12, 103 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B. S. et al. Macrophage Migration Inhibitory Factor in Acute Adipose Tissue Inflammation. PLoS One 10, e0137366 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. et al. CYP1B1 deficiency ameliorates obesity and glucose intolerance induced by high fat diet in adult C57BL/6J mice. American journal of translational research 7, 761–771 (2015). [PMC free article] [PubMed] [Google Scholar]
- Yang B. T. et al. Increased DNA methylation and decreased expression of PDX-1 in pancreatic islets from patients with type 2 diabetes. Mol Endocrinol 26, 1203–1212 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitert M. D. et al. Impact of an Exercise Intervention on DNA Methylation in Skeletal Muscle From First-Degree Relatives of Patients With Type 2 Diabetes. Diabetes 61, 3322–3332 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. A. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet 13, 484–492 (2012). [DOI] [PubMed] [Google Scholar]
- Ling C. et al. Genetic and epigenetic factors are associated with expression of respiratory chain component NDUFB6 in human skeletal muscle. J Clin Invest 117, 3427–3435 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A. H. et al. Genome-wide associations between genetic and epigenetic variation influence mRNA expression and insulin secretion in human pancreatic islets. PLoS genetics 10, e1004735 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronn T. et al. Impact of age, BMI and HbA1c levels on the genome-wide DNA methylation and mRNA expression patterns in human adipose tissue and identification of epigenetic biomarkers in blood. Human molecular genetics 24, 3792–3813 (2015). [DOI] [PubMed] [Google Scholar]
- Dayeh T. et al. Genome-wide DNA methylation analysis of human pancreatic islets from type 2 diabetic and non-diabetic donors identifies candidate genes that influence insulin secretion. PLoS genetics 10, e1004160 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayeh T. A. et al. Identification of CpG-SNPs associated with type 2 diabetes and differential DNA methylation in human pancreatic islets. Diabetologia 56, 1036–1046 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky Z. A. et al. DNA methylation profiles in monozygotic and dizygotic twins. Nat Genet 41, 240–245 (2009). [DOI] [PubMed] [Google Scholar]
- Ollikainen M. et al. DNA methylation analysis of multiple tissues from newborn twins reveals both genetic and intrauterine components to variation in the human neonatal epigenome. Human molecular genetics 19, 4176–4188 (2010). [DOI] [PubMed] [Google Scholar]
- Vink J. M., Sadrzadeh S., Lambalk C. B. & Boomsma D. I. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab 91, 2100–2104 (2006). [DOI] [PubMed] [Google Scholar]
- Chen Z. J. et al. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet 43, 55–59 (2011). [DOI] [PubMed] [Google Scholar]
- Taniguchi Ishikawa E. et al. Klf5 controls bone marrow homing of stem cells and progenitors through Rab5-mediated beta1/beta2-integrin trafficking. Nature Communications 4, 1660 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes G. M. et al. Genomewide Association of Polycystic Ovary Syndrome Implicates Alterations in Gonadotropin Secretion in European Ancestry Populations. Nature Communication 6, 7502 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrke M. & Lazar M. A. The many faces of PPARgamma. Cell 123, 993–999 (2005). [DOI] [PubMed] [Google Scholar]
- Hughes T. S. et al. An alternate binding site for PPARgamma ligands. Nature Communications 5, 3571 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta J. R. et al. Increased fat cell size: a major phenotype of subcutaneous white adipose tissue in non-obese individuals with type 2 diabetes. Diabetologia, doi: 10.1007/s00125-015-3810-6 (2015). [DOI] [PubMed] [Google Scholar]
- Woldt E. et al. The nuclear hormone receptor PPARgamma counteracts vascular calcification by inhibiting Wnt5a signalling in vascular smooth muscle cells. Nature Communications 3, 1077 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarstedt A. et al. WISP2 regulates preadipocyte commitment and PPARgamma activation by BMP4. Proc Natl Acad Sci USA 110, 2563–2568 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N. et al. Epigenetic mechanism underlying the development of polycystic ovary syndrome (PCOS)-like phenotypes in prenatally androgenized rhesus monkeys. PLoS One 6, e27286 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glait-Santar C. & Benayahu D. SVEP1 promoter regulation by methylation of CpG sites. Gene 490, 6–14 (2011). [DOI] [PubMed] [Google Scholar]
- Kwon H., Lee J., Jeong K., Jang D. & Pak Y. Fatty acylated caveolin-2 is a substrate of insulin receptor tyrosine kinase for insulin receptor substrate-1-directed signaling activation. Biochim Biophys Acta 1853, 1022–1034 (2015). [DOI] [PubMed] [Google Scholar]
- Kovacs P. et al. The role of insulin receptor substrate-1 gene (IRS1) in type 2 diabetes in Pima Indians. Diabetes 52, 3005–3009 (2003). [DOI] [PubMed] [Google Scholar]
- Xu N. et al. Comprehensive assessment of expression of insulin signaling pathway components in subcutaneous adipose tissue of women with and without polycystic ovary syndrome. J Clin Transl Endocrinol 2, 99–104 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Z. D. & Meissner A. DNA methylation: roles in mammalian development. Nat Rev Genet 14, 204–220 (2013). [DOI] [PubMed] [Google Scholar]
- Baylin S. B. & Jones P. A. A decade of exploring the cancer epigenome - biological and translational implications. Nature reviews. Cancer 11, 726–734 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rountree M. R., Bachman K. E. & Baylin S. B. DNMT1 binds HDAC2 and a new co-repressor, DMAP1, to form a complex at replication foci. Nat Genet 25, 269–277 (2000). [DOI] [PubMed] [Google Scholar]
- Maliqueo M. et al. Placental steroidogenesis in pregnant women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 166, 151–155 (2013). [DOI] [PubMed] [Google Scholar]
- Ohlson L. O. et al. The influence of body fat distribution on the incidence of diabetes mellitus. 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes 34, 1055–1058 (1985). [DOI] [PubMed] [Google Scholar]
- Bjorntorp P. “Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis 10, 493–496 (1990). [PubMed] [Google Scholar]
- Lefebvre A. M. et al. Depot-specific differences in adipose tissue gene expression in lean and obese subjects. Diabetes 47, 98–103 (1998). [DOI] [PubMed] [Google Scholar]
- Oh J. Y., Sung Y. A. & Lee H. J. The Visceral Adiposity Index as a Predictor of Insulin Resistance in Young Women with Polycystic Ovary Syndrome. Obesity 21, 1690–1694 (2013). [DOI] [PubMed] [Google Scholar]
- Martinez-Garcia M. A. et al. Evidence for masculinization of adipokine gene expression in visceral and subcutaneous adipose tissue of obese women with polycystic ovary syndrome (PCOS). J Clin Endocrinol Metab 98, E388–396 (2013). [DOI] [PubMed] [Google Scholar]
- Nilsson M. E. et al. Measurement of a comprehensive sex steroid profile in rodent serum by high-sensitive gas chromatography-tandem mass spectrometry. Endocrinology, en20141890 (2015). [DOI] [PubMed] [Google Scholar]
- Bolstad B. M., Irizarry R. A., Astrand M. & Speed T. P. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19, 185–193 (2003). [DOI] [PubMed] [Google Scholar]
- Bibikova M. et al. High density DNA methylation array with single CpG site resolution. Genomics 98, 288–295 (2011). [DOI] [PubMed] [Google Scholar]
- Johnson W. E., Li C. & Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 8, 118–127 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.