Abstract
A culture independent method based on qPCR was developed for the detection and quantification of two fungal inoculants in soil. The aim was to adapt a genotyping approach based on SSR (Simple Sequence Repeat) marker to a discriminating tracing of two different species of bioinoculants in soil, after their in-field release. Two entomopathogenic fungi, Beauveria bassiana and B. brongniartii, were traced and quantified in soil samples obtained from field trials. These two fungal species were used as biological agents in Poland to control Melolontha melolontha (European cockchafer), whose larvae live in soil menacing horticultural crops. Specificity of SSR markers was verified using controls consisting of: i) soil samples containing fungal spores of B. bassiana and B. brongniartii in known dilutions; ii) the DNA of the fungal microorganisms; iii) soil samples singly inoculated with each fungus species. An initial evaluation of the protocol was performed with analyses of soil DNA and mycelial DNA. Further, the simultaneous detection and quantification of B. bassiana and B. brongniartii in soil was achieved in field samples after application of the bio-inoculants. The protocol can be considered as a relatively low cost solution for the detection, identification and traceability of fungal bio-inoculants in soil.
In the last decade the application of biological control agents (BCA) and biofertilizers has started to be considered a feasible and effective alternative for pest control or to improve plant nutrition efficacy1,2,3. Different microorganisms, belonging to several species of bacteria and fungi, started to be used as inoculants4. However, despite the huge amount of studies and findings on beneficial strains, the application of microbial-based fertilizers and BCAs in agricultural practice is still hindered by several factors. These include the standardization of the products, whose quality can affect the inoculant viability and persistence in soil, as well as the possibility of tracing the inoculant in the environment5,6.
The assessment of inoculants persistence and traceability in soil is a key task to improve their efficacy, since it is the basis for understanding their behaviour in the crop environment, and to fine-tune their application method7,8. Verifying and monitoring their presence can thus provide knowledge of the functioning and robustness of the bio-inoculant itself. So far, the fate and persistence of target microorganisms in environmental samples has been assessed with a variety of culture-dependent and independent methods9,10,11,12,13,14,15. Among the various approaches used for tracing and quantifying mycorrhizal and pathogenic fungi in the environment, PCR-based methods have been the most used to address some of the key issues in the assessment of the microorganism persistence in soil16,17,18,19,20. These key issues regard also the monitoring methods that can properly identify the inoculated strains, distinguishing them from the native populations resident in soil.
There are several DNA fingerprinting methods used to probe inoculated strain/s, but they are mainly qualitative and not quantitative19,20,21,22,23,24. Culture-independent DNA-based detection of strains directly in soil-extracted DNA could reduce the time and costs of monitoring the persistence and amount of bio-inoculants in soil after their application. Among the available culture-independent methods, different approaches can be used to investigate soil microbial communities25,26,27,28. Such analyses have been performed based on universal primers, which, however, have not yet been used to discriminate between non-native and native soil microorganisms, nor between two different bio-inoculants.
To overcome such technical gaps, we have developed a culture-independent method based on simple sequence repeat (SSR or microsatellites) markers and qPCR for the direct, discriminant and simultaneous detection and quantification of two bio-inoculants in soil. Multilocus simple SSR genotyping is a technique used in the characterization of cultivated fungi based on PCR amplification of multiple loci29. Several fungal SSR markers have been reported to be species specific, with a polymorphic character, thus producing a highly discriminant fingerprint22,30. The qPCR with probes targeting genes of interest allows to quantify their copy number, providing information on the relative abundance within the microbial community31, so it could be used to monitor the dynamic of the presence of the specific inoculant after its application in the field. The aim of our study was to adapt a genotyping approach based on SSR markers to the discriminating tracing of two different species of bio-inoculants in soil, after their field application as biocontrol agents. The proposed method was tested for tracing and quantifying in soil two entomopathogenic fungi, Beauveria bassiana (Bals.-Criv.) Vuill. and B. brongniartii (Sacc.) Petch. used as BCAs of Melolontha melolontha L. (European cockchafer) in field trials.
Materials and Methods
Fungal strains and preparation of the bio-inocula
The strain of B. brongniartii was isolated on a selective medium32 from the soil of a potato field highly infested by M. melolontha. The soil samples were collected in May 2012 in Romanów locality (Lublin voivodship, Eastern Poland). The strain was deposited in the Fungal Collection of the Department of Plant Protection and Breeding, Siedlce University of Natural Sciences and Humanities. The sequence has been deposited in the GenBank database and can be accessed to ID KT932309.
The strain of B. bassiana was selected from rhizospheric soil of an apple orchard located in Valle d’Aosta by the company CCS Aosta, (Aosta, Italy) and named BB59. The sequence has been deposited in the GenBank database and can be accessed to ID KT932307.
Both bio-inocula were grown in a liquid medium based on malt extract and glucose and formulated as a wettable powder into a carrier material made of a mixture of corn fibres and zeolite (1:10 w-w). The concentration of each of the two fungi in the inoculum was about 1 · 107 CFU · g−1.
Field application of the B. brongniartii and B. bassiana strains and soil sampling
Two experiments were established on different field plots, each designed with 3 replications in a split plot design, located near Brzostówka (Lublin, Poland). Both fields (about 1 ha) were highly infested by M. melolontha larvae. The first trial (A) was carried out on an established strawberry plantation. The second trial (B) was on a bare field, on which, testing an integrated effort to control the white grubs of the cockchafer, buckwheat was previously grown in spring for green plowing (June) and soil disinfection was carried out (at the beginning of August) on half of the plots by active steaming33,34,35.
The inocula of B. bassiana or B. brongniartii were applied as an aqueous suspension, after overnight incubation of the bio-inocula in water containing about 10% of a stillage from yeast production (organic fertilizer) to foster the fungal growth. Each inoculum was applied singly at a dose of 15 kg·ha−1 to the soil, splitted into two applications with three weeks interval. When the two species were applied together, 7.5 kg·ha−1 of each inoculum was distributed with the same application schedule. In case of the strawberry plantation (trial A), the applications were carried out in June, near the plants row, with a sprayer having nozzles of large diameter, without a fan, to reduce the risk of damage of the fungal cells. In case of the bare field trial (B) the bio-inocula were distributed the day after the soil disinfection treatment with active steam and again after three weeks. After each application, the soil was mixed on the surface with a light hand hoeing.
Soil samples were collected three weeks after the last application. Ten sub-samples of about 50 g each were gathered from each plot and then they were mixed to obtain a composite sample used for the DNA analyses. They were collected either in the vicinity of the plant roots or in the inter-row. Soil samples were also collected from non-inoculated parts of the field as a negative control. However, since other strains of B. brongniartii or B. bassiana could naturally occur in the soil considered as negative control, the proposed method should have been able to trace both these and the inoculated strains.
Extraction of genomic soil DNA
DNA was extracted in duplicate from 0.6 g of three bulk soil (from ten sub-samples) using the PowerSoil DNA Isolation Kit (Mo Bio Laboratories, Carlsbad, CA) according to the manufacturer’s protocol. Duplicates were then pooled for downstream analyses. Nucleic acids were eluted in 100 μl of elution buffer (MoBio). The concentration of DNA crude extracts was checked by Qubit® 2.0 Fluorometer following manufacturer’s instructions kit and stored to −20 °C for PCR analysis.
DNA isolation of Beauveria brongniartii and B. bassiana strains
B. brongniartii and B. bassiana strains were cultured on fresh PDA agar (Oxoid S.p.A., Milan, Italy) for 10 days at 25 °C. Mycelium and spores were harvested aseptically from each culture using a sterile scalpel. The fungal biomass used for DNA isolation was harvested from about 1 cm2 of agar surface, and was approximately the same for each of the two strains. Genomic DNA was extracted from 0.5 g fresh mycelium by using PowerSoil DNA Isolation Kit (Mo Bio Laboratories, Carlsbad, CA) as described above, and quantified using Qubit® 2.0 Fluorometer kit following manufacturer’s instructions.
Discriminant and quantitative analysis of SSR markers in soil DNA
Three B. brongniartii and three B. bassiana SSR primers pairs were tested for species specificity and performance in soil DNA extracts and in mycelia DNA extracts. For B. brongniartii SSR marker detection the following primers were tested: i) Bb1F4, Bb2A3, Bb4H922. The three primers were evaluated according to the method of Enkerli et al.22 and were used to amplify B. brongniartii and B. bassiana genomic DNA, and soil DNA samples, in order to test the specificity and the traceability of B. brongniartii in soil.
For the detection of B. bassiana SSR marker, the following primers were tested: i) Ba01, Ba02, Ba0330. The three loci were selected according to the method of Rehner and Buckley30, which was applied to both fungal genomic DNAs, to the DNA extracted from the soils investigated in our experiments, and to a selection of soil fungal isolates, in order to test the specificity and the traceability of B. bassiana in soil. However, the specificity of these SSR primers was already broadly confirmed in the above mentioned studies by Enkerli et al.22 and Rehner and Buckley30. Both sensitivity and specificity in discriminating the two Beauveria species by the chosen SSR loci were thus tested by PCR and checked on 2% agarose gel with ethidium bromide staining36. The method was then adapted to qPCR Real Time conditions.
A calibration towards the sensitivity of the applied protocol was performed using liquid dilutions of fungal spores’ suspension with a known concentration of spores. The suspensions were prepared from B. brongniartii and B. bassiana cultures grown on Potato Dextrose Agar (Oxoid S.p.A., Milan, Italy), after a 10-day incubation at 25 °C, aseptically harvesting the spores from the surface of the colonies, by a sterile cotton swab, and suspending them in a three-fold diluted Czapek broth (Oxoid S.p.A., Milan, Italy). Spores’ concentrationwas measured in each suspension by counting them with a hemacytometer (Thoma Chamber) according to Smith et al.37. The initial concentrations in the two fungal samples were: 26.5 · 104 and 27.25 · 104 spores · ml−1 for B. brongniartii and B. bassiana, respectively. Four sequential dilutions in sterile distilled water of the initial suspensions were then prepared (from 104 to 101 spores · ml−1).
The dilutions were used as such and also added to soil samples. The soil used as basis for obtaining the microcosms with a known number of fungal spores was a control soil, not previously treated in the field with the fungal inocula. One ml of each spores’ dilutions was added to 0.6 g of soil and gently mixed, in order to obtain soil microcosms with a known, decreasing, concentration of fungal spores from the two fungal strains. The microcosms obtained were thus used to establish a kind of standard curve in order to compare the efficiency, sensitivity and specificity of the SSR genotyping approach for fungal bio-inoculants traceability in soil.
DNA was extracted from the microcosms within 48 h after the addition of the fungal spores, in duplicate as described above. The concentration of DNA crude extracts was checked by Qubit® 2.0 Fluorometer kit, following manufacturer’s instructions, and stored at −20 °C for further PCR analysis.
Standard curves were created, for each targeted gene, using soil samples containing a known concentration of fungal spores. The qPCR reactions were performed in duplicate within each DNA template.
PCR products were purified after the qPCR reaction with a Qiaquick PCR purification kit (Qiagen Inc., Chatsworth, CA, USA), then quantified by Qubit® 2.0 Fluorometer kit, following manufacturer’s instructions and diluted in order to minimize the PCR bias26. The gene copy number was calculated using the following equation (http://scienceprimer.com/copy-number-calculator-for-realtime-pcr):
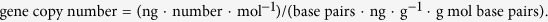 |
The standards were created using triplicate 10-fold dilution series covering six orders of magnitude from 102 to 108 gene copies per qPCR reaction during each run. B. brongniartii and B. bassiana SSR copy numbers were expressed per g−1 soil (dry weight).
All qPCR reactions were carried out in 25 μl reactions containing 10 μl of template DNA (2ng/μl), 12.5μl of Quanti Fast Sybr Green PCR Master Mix (Qiagen), 1.2 μM of primer, and PCR-grade water up to 15 μl. The reactions were performed in a Stratagene Mx3000P qPCR (Agilent Technologies). In order to protect soil DNA and mycelial DNA extracts from potentially present PCR-inhibitory substances, bovine serum albumin (BSA) was added to the SYBR green mix (Qiagen). Experiments were performed in duplicate. The results were processed using the program of the instrument Stratagene Mx3000P qPCR (Agilent Technologies). The absence of primers dimers in amplification products was evaluated analysing the melting curves of the products considering the fluorescence range 50–99 °C. Moreover, qPCR products were screened for purity and molecular weight in 1% agarose gel. Cycling condition was optimized running the qPCR at a different number of cycles, and selecting the protocol resulting in the best curves. Selected cycling conditions consisted of a 10 min initial denaturation at 95 °C and forty-two PCR cycles of 1 min at 95 °C, 40 s at 58 °C, and 30 s at 72 °C. The template quantities in soil samples were also compared with the template quantities in mycelia samples.
Specificity of SSR markers
The fungal strains used in this study to further test the specificity of SSR markers are listed in Table 1. All fungal isolates were grown on potato dextrose agar (PDA, Oxoid S.p.A., Milan, Italy) at 25 °C. Genomic DNA was extracted from 0.5 g fresh mycelium by using PowerSoil DNA Isolation Kit (Mo Bio Laboratories, Carlsbad, CA) as described above, and quantified using Qubit® 2.0 Fluorometer kit following manufacturer’s instructions. The amplification of fungal DNA was performed with the primers ITS1/ITS4, and the conditions used in PCR reactions were as follows: initial denaturation at 95 °C for 5 minutes; 34 cycles with denaturation at 94 °C for 1 minute, annealing at 60 °C for 1 minute, extension at 72 °C for 2 minutes; and a final extension of 8 minutes at 72 °C.
Table 1. List of fungal strains used in this study to test specificity of SSR marker.
| Species | Isolate | Id |
|---|---|---|
| Engyodontium album (Limber) de Hoog | From soil | KU686684 |
| Aspergillus tubingensis Mosseray | From saline soil | KU686682 |
| Eurotium rubrum Jos. König, E. Spieckermann & W. Bremer | From saline soil | KU686683 |
| Stilbella Lindau | From forest soil | KU686685 |
| Penicillium pancosmium Houbraken, Frisvad & Samson | From forest soil | KU686681 |
| Engyodontium arenarum (Cavara) W. Gams | From spider | KU686686 |
| Beauveria Beauveria Vuill. | From soil | – |
| Pochonia Bat. & O.M. Fonseca | From adult butterfly | KU686687 |
The quality of amplification products was checked by 1.5% agarose gels and ethidium bromide staining. PCR products resulting from three replicate reactions were pooled and purified with a Qiaquick PCR purification kit (Qiagen Inc. Chatsworth, CA, USA). Purified products were quantified by Qubit® 2.0 Fluorometer following manufacturer’s instructions kit. Specificity was tested by PCR following the instructions reported above in the section 2.5. SSR marker detection for the locus Bb4H9 was performed according to the method of Enkerli et al.22 and was applied to all selected species (Table 1). SSR marker detection for the locus Ba01 was performed according Rehner S. A., and Buckley E. P.30.
Statistical analyses
Data of qPCR quantification of DNA were obtained from soil samples where B. brongniartii and B. bassiana spores were added. A control consisting of soil without the inoculums was also included. The data were expressed as numbers of cells g−1 dry weight soil. Separated values of average number of copies of genes· g−1 dry soil are presented. qPCR results were analysed using one way ANOVA (α = 0.05), and the means within each treatment were compared for statistical significance of the differences by using Fisher’s Least Significant Difference (LSD) test at a significance level for p ≤ 0.05.
Data obtained from qPCR runs were plotted and compared using Origin 6.0 (Microcal, OriginLab Northampton, MA 01060 USA) software.
Results
Specificity of B. brongniartii and B. bassiana SSR markers detection
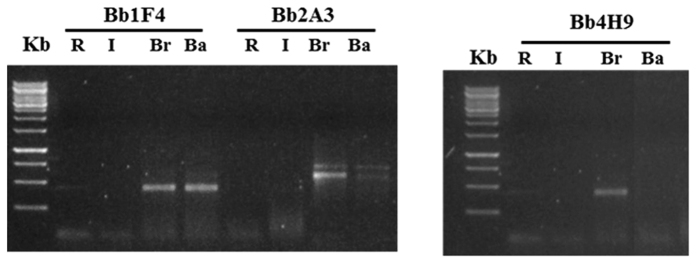
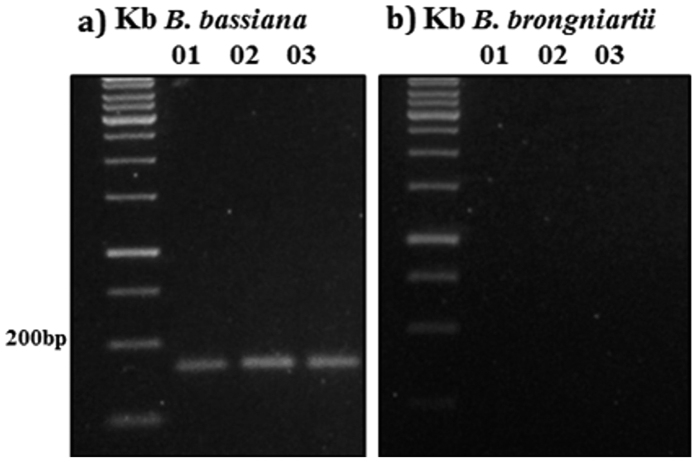
Three B. bassiana and three B. brongniartii SSR primer pairs were evaluated for specificity in DNA detection of our fungal strains in soil, as well as for species identification from mycelia. Only the primer Bb4H9, among those used for B. brongniartii, resulted capable of discriminating the fungal species, and in particular allowed a highly specific detection of B. brongniartii in soil (Fig. 1). Specific PCR amplification of DNA from B. bassiana with SSR primers was obtained for all the three considered loci. However, the primer Ba01 was selected for the following analyses because it amplified better than the other two. Amplification of the locus Bb4H9 in soil samples inoculated with B. brongniartii resulted in only one product, while, as expected, no amplification was obtained with the primer for the locus specific for B. bassiana. PCR amplification of the locus Ba01 showed only one product in soil samples inoculated with B. bassiana and no amplification for the locus specific for B. brongniartii (Fig. 2). Gel analysis revealed amplification product from B. brongniartii fresh mycelium (Fig. 3b), and B. bassiana fresh mycelium (Fig. 3c).
Figure 1. Gel electrophoretic analyses of PCR products derived from the B. brongniartii SSR marker amplification.
The bands represent the B. brongniartii (Br) fungal PCR products for SSR loci Bb1F4, Bb2A3, Bb4H9 separated in 1.5% agarose. R: soil near the strawberry roots; I: soil from the inter rows; Ba. Soil inoculated with B. bassiana.
Figure 2. Gel electrophoretic analyses of PCR products derived from the B. bassiana SSR marker amplification.
The bands shows the B. bassiana (a) and the B. brongniartii (b) fungal PCR product for SSR loci Ba01, Ba02, Ba03 separated in 1.5% agarose.
Figure 3. Agarose gel electrophoresis showing the PCR products amplified using the SSR primer sets from the fungal isolates listed in Table 1.
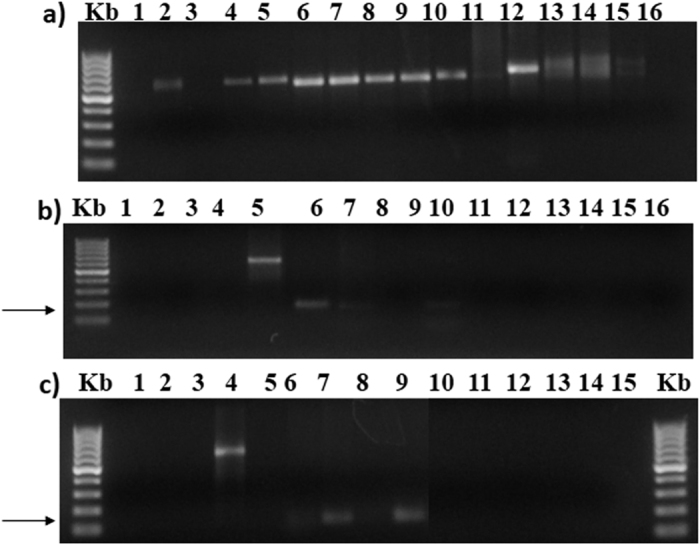
. PCR products derived from (a) ITS1/TS4 amplification, (b) Beauveria brongniartii SSR amplification, (c) B. bassiana SSR amplification. Kb: 100 bp DNA ladder; 1: Engyodontium album; 2: Eurotium rubrum; 3: Aspergillus tubingensis; 4: Penicillium pancosmium; 5: Beauveria sp.; 6: B. brongniartii (DNA from fresh mycelium); 7: B. bassiana (DNA from fresh mycelium); 8: soil inoculated with Beauveria brongniartii; 9: soil inoculated with B. bassiana; 10: UPH/019; 11: UPH/018; 12: Pochonia sp.; 13: Soil DNA; 14: soil DNA; 15: soil DNA; 16: negative control, addition of sterile water to the PCR mix.
To further assure the specificity of these two SSR markers, the DNA of eight fungal isolates frequently present in agricultural soils or characterised by entomopathogenic ability were was amplified separately with Bb4H9 and Ba01 primers. All fungi were also amplified with ITS primers to check both their identity and the quality of extracted DNA. All the strains generated PCR products of 500 to 650 bp corresponding to the ribosomal DNA (ITS1, ITS2, and. 5.8 S rRNA) (Fig. 3a), while none of the eight strains have resulted in PCR products with neither Bb4H9 nor Ba01 primers (Fig. 3b,c). The size of the amplification product was ~200 bp and ~140 bp, from B. brongniartii and B. bassiana fresh mycelia (Fig. 3b,c).
Sensitivity of cultivation-independent SSR detection
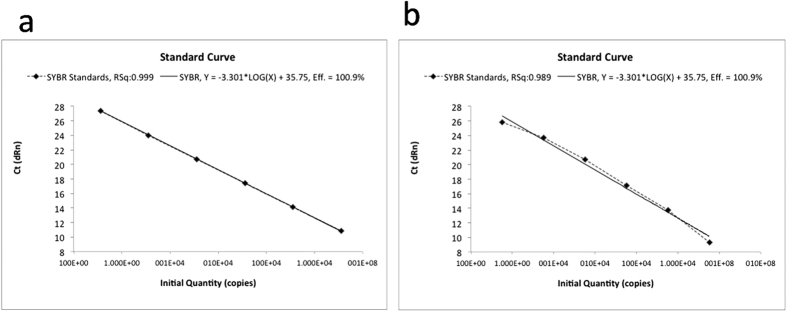
The sensitivity for the discriminant detection of B. brongniartii and B. bassiana in bulk soil DNA was tested using 50 ng of bulk soil DNA extract from Beauveria-free samples (control) untreated or singly spiked with different quantities of genomic DNA of each fungal species. The locus Bb4H9 has been reported to be highly polymorphic, while Bb1F4, and Bb2A3 were not. The absence of any amplification products in the spiking experiments with B. bassiana (as reported above) further confirmed the specificity of the primers. The detection sensitivities of markers were adjusted according to the total amount of genomic DNA extracted from soil samples, and considering the relative Ct values (Fig. 4).
Figure 4. q-PCR assay: Standard curve established between Log of DNA concentration vs. cycle threshold (CT) obtained in Sybr green assay.
The plots represents serially diluted (1:10) soil genomic DNA inoculated with 26.5·104 and 27.25·104 spores ·ml−1 for B. brongniartii (a) and B. bassiana (b), respectively.
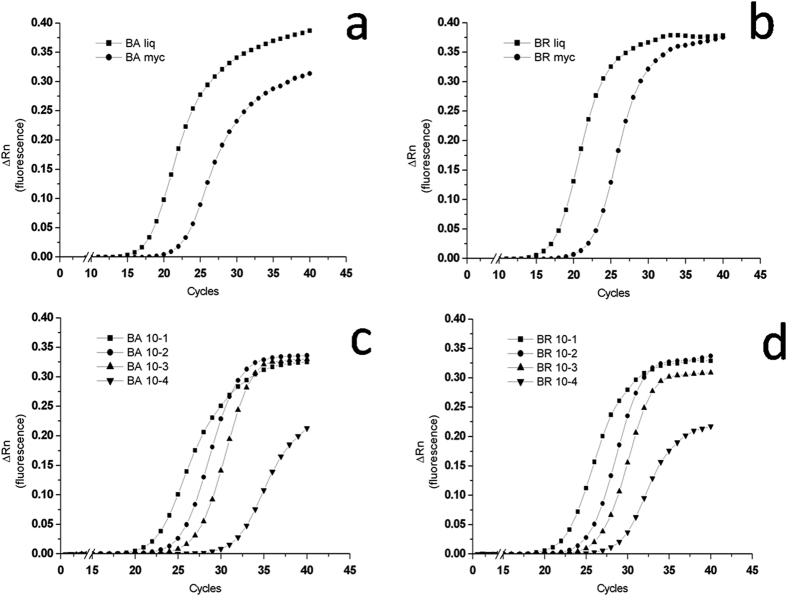
The qPCR was highly specific when tested with the two loci on fungal DNA from the fungal pure cultures of both B. brongniartii and B. bassiana strains (Fig. 5). The qPCR results obtained from the fungal spores’ suspensions and the mycelium grown on solid agar are showed in Fig. 5a,b for B. bassiana and B. brongniartii, respectively. The results obtained from in vitro soil dilutions of B. bassiana and B. brongniartii spores from the qPCR are shown in Fig. 5c,d, respectively. The relation between the number of PCR cycles and the amount of PCR product obtained was similar for both Beauveria species (Fig. 5). The reduction in spores’ concentrations obtained with the first three dilutions (10−1, 10−2 and 10−3) resulted in a decreasing amount of DNA template for both fungal species, thus requiring more amplification cycles for the fluorescence signal to rise above background in the most diluted samples. After 30 cycles the fluorescence signal of the first three dilutions (10−1, 10−2 and 10−3) raised at comparable intensities in both fungi. The last dilution (10−4) resulted in a qPCR product amount that plotted separately from the others, with a very delayed Ct, confirming that a small amount of template was present at the start of the reaction (Fig. 5c,d). Moreover, more amplification cycles were required for both fungal species with the 10−4 dilution samples for the fluorescence signal to rise above background, resulting also in a plateau of the curve with intensities sensibly lower than those attained by the other dilutions. These data indicate that the method allows to detect a very small number of gene copies per g of soil (Table 2).
Figure 5.
On the top, plots of PCR cycle number against PCR product amount obtained from the liquid fungal spores’ suspensions (liq) and the mycelium grown on solid agar (myc) for (a) B. bassiana and (b) B. brongniartii respectively. On the bottom, plots of PCR cycle number against PCR product amount obtained from in vitro soil dilutions of spores. The highest yields in the two series of samples, corresponding to the 10−1 dilution of the initial suspensions, were: 26.5 · 103 and 27.25 · 103 spores for respectively (c) B. bassiana and (d) B. brongniartii; the other curves in the plots correspond to three further ten-fold sequential dilutions of the fungal spores’ suspension (from 10−2 to 10−4).
Table 2. Data obtained in PCR amplification of target DNA from the two fungi in soil samples of trial “A” from the plants’ roots (Root) or from inter-rows soil (Inter), and in soil samples of trial “B” where two treatments were considered: soil treated with active steam (Steam) or not treated/control (Cntr).
| Trial A (Strawberry field) | |||
|---|---|---|---|
|
B. bassiana |
B. brogniartii |
||
| Gene copy number g−1dry soil | Gene copy number g−1 dry soil | ||
| Inter-BA | 21.64 ± 10.86a | Inter-BR | 0.00 ± 0.00b |
| Inter-cntr | 6.35 ± 2.96b | Inter-cntr | 0.01 ± 0.00a |
| Root-BA | 13.84 ± 6.95ab | Root-BR | 0.00 ± 0.00b |
| Root-cntr | 11.30 ± 6.64ab | Root-cntr | 0.01 ± 0.01a |
| Trial B (Bare field) | |||
| Cntr | 26.37 ± 10.88a | Cntr | 0.03 ± 0.01a |
| Cntr + BA | 20.06 ± 1.47a | Cntr + BR | 0.00 ± 0.00b |
| Steam | 30.23 ± 10.57a | Steam | 0.03 ± 0.01a |
| Steam + BA | 22.80 ± 3.74a | Steam + BR | 0.00 ± 0.00b |
Detection of genotype-specific alleles of B. brongniartii (BA) and B. bassiana (BR) in samples of soil treated with 50 ng·g−1 (dry soil) of B. brongniartii and B. bassiana inocula. Means of 4 laboratory replicates ± SDfrom one-way ANOVA of real time quantification data (Average copy number/gram dry soil). Different letters indicate a significant difference (p < 0.05) after Fisher test.
Presence and yield of B. brongniartii and B. bassiana in the treated soils
When applied to the field situation, namely the detection and quantification of the entomopathogenic fungi B. brongniartii and B. bassiana in previously inoculated soils under field conditions, the qPCR analysis showed very low values (Table 2). Despite the very low numbers of gene copies obtained from all the trials (with B. brongniartii close to zero in all the trials), the data were highly repeatable and able to show statistic differences between the agronomical treatments, as in Trial A (Strawberry field) where soil samples from the root zone (Root-BA) showed a higher amount of B. bassiana than in the untreated soil (Root). The results however did not indicate the persistence of the fungi in the inoculated soils. In general, the B. bassiana gene copy values were higher than those of B. brongniartii in all the treatments.
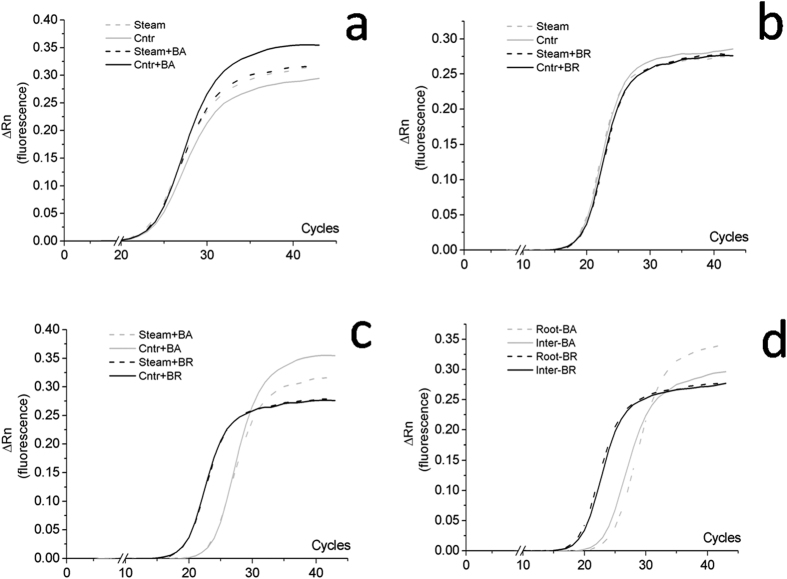
The relation between PCR cycle number and the amount of PCR product obtained in the trials A and B, for the two fungal inoculants, is presented in Fig. 6.
Figure 6.
Plots of PCR cycle number against PCR product amount obtained in the trials B (plots a–c) and A (plot d), for the two fungal inoculants. Plot (a) soil inoculated with B. bassiana previously treated (Steam + BA) or not treated (Steam) with active steam, compared with the relevant controls (Cntr and Cntr + BA). Plot (b) soil inoculated with B. brongniartii previously treated (Steam + BR) or not treated (Steam) with active steam, compared with the relevant controls (Cntr and Cntr + BR). Plot (c) soil co-inoculated with B. bassiana and B. brongniartii and previously treated, or not, with active steam. Plot (d) soil inoculated with B. bassiana or B. brongniartii sampled near the strawberry roots (R) and from the inter rows (I).
In particular, in Fig. 6a raw results obtained from soil samples inoculated with B. bassiana and previously treated (Steam + BA) or not treated (Cntr + BA) with active steam were compared with the relevant not inoculated control samples (Steam and Cntr) consisting of not inoculated soil samples. qPCR using SSR marker as primer allowed to detect and quantify the inoculated B. bassiana in the soil previously disinfected or not with active steam (Fig. 6a), showing that these differences were not significant when reported as the average copy number/gram dry soil (Table 2).
In Fig. 6b are reported the results obtained from soil samples inoculated with B. brongniartii and previously treated (Steam + BR) or not treated (Cntr + BR) with active steam, compared with the relevant controls (Steam and Cntr). The proposed method applied to the soil samples collected from the field plots where B. brongniartii was applied showed that this fungus was not present or in an amount lower than the detection limit of the method (Fig. 6b), and with an amplification plateau due to lack of DNA template lower than that of B. bassiana (Fig. 6c).
In Fig. 6d results obtained from soil samples inoculated with B. bassiana or B. brongniartii and taken from the strawberry plants’ roots (Roots) and from the plants’ inter rows (Inter) are compared. The results from the analysis of soil samples deriving from plots co-inoculated with the two fungi showed the persistence of B. bassiana in those collected from the root zone (Roots-BA) and not in the samples taken from inter rows (Inter-BA), even after a significant number of PCR cycles (Fig. 6d). B. brongniartii was not detected in any sample irrespective of the collection sites.
Discussion
In our study, we have coupled highly discriminant species-specific SSR primers with a real-time qPCR using the same SSR markers as primers. Such an approach allowed us to define: a) the primers that were able to detect B. brongniartii and B. bassiana in soil samples, b) that the technique could be used to monitor with sensitivity and specificity their persistence in soil, c) that the qPCR based on SSR could be used to quantify single fungal species biomass in soil.
Microsatellite markers22 showed a good discrimination power for filamentous fungi, and over the last few years various studies have been carried out to develop SSR markers in Beauveria spp.22,29,30. Such approach allowed to reveal fungus genetic diversity for a given species or genera, and more generally made possible the cultivation-independent detection of fungal strains in soil samples29,30. The polymorphic character of SSRs is highly discriminating in fungi, more than other DNA loci (i.e. ITS sequences or functional genes), and may represent a promising option for culture-independent detection and identification of fungal strains in environmental samples, especially if coupled with cloning and sequencing.
Specificity for a target organism is a critical element in the design or use of any PCR and qPCR real time assay. Species specificity was demonstrated in our study by the discriminant presence or absence of SSR amplification products from the two fungi using specific primers (Bb4H9 for B. brongniartii and Ba01 for B. bassiana). However, some of the tested SSR primer pairs (Bb1F4 and Bb2A3) detected both species (Figs 1 and 2). Therefore, detection specificity of SSR primers needs to be validated case by case prior to being applied to complex soil DNA samples29.
Protocol efficacy was firstly tested with in vitro fungal spores’ dilutions in soil samples, thus performing a sort of calibration and validation of the method using microcosms where a known amount of fungal reproductive units was purposely added before the DNA extraction. We assumed that if a large amount of template DNA was present at the start of the reaction, relatively few amplification cycles would be required to accumulate enough product to give a fluorescence signal above background. A higher amount of DNA template was present in the DNA extracts obtained from spores’ suspensions (liq) than in mycelia (myc) harvested from solid cultures for both Beauveria species. In B. bassiana the fluorescence signal obtained from the mycelia DNA never reached the level obtained with the spores DNA template. In B. brongniartii the DNA template from mycelia at the start of the reaction was lower, but at the end of the PCR cycles the fluorescence signal obtained was comparable to that obtained from spores’ template. This passage proved to be determinant for defining the threshold of fungal detection as well as for evaluating the presence of interferences in DNA recovery and amplification, due to soil components. The results obtained from microcosms and pure fungal cultures have been confirmed when analysing soil samples gathered from field experimental trials. The presence of a number of gene copies different from zero in the non-inoculated treatments, in the case of both B. bassiana and B. brongniartii plots could indicate the presence of a local, natural, population of the fungus. Being the soils naturally infested by M. melolontha it is reasonable that its natural parasites are also present. It is worth of notice that in both the B experiments without the steam treatment, the control showed a significant higher presence of the fungus respect to the inoculated trials, which could indicate that the added inoculums, although too low to establish in soil, had the effect of depressing the native strains of Beauveria.
Schwarzenbach et al.29 reported for the first time the specificity of SSR primers within bulk soil. In this work we are reporting for the first time the SSR species specificity also in soil close to the plants root system, where a higher microbial diversity is usually present, thus being theoretically a more difficult environment to trace single species. To our best knowledge, this is also the first report where this double approach (SSR and real time qPCR) has been applied for the simultaneous detection and traceability of inoculants in soil.
The marketing of microorganisms as soil inoculants for biocontrol or biofertilization requires risk assessment for regulatory and technical purposes5. The registration of a microbial-based product obliges the producer to present several data about the efficacy and safety of the inoculum to the competent authorities. Both efficacy and safety are related with the methods of application of the product, which can affect the viability of the inoculant as well as its establishment in the soil. Indeed, recovery of a single inoculated strain in soil microcosms for a plant growth promoting bacteria was limited to 30–40 days after inoculation38 and changing the sprayer technical parameters deeply affected the viability of the strain applied39. The method allowed to assess not only the effect of the treatment in association with other agronomical practices, but also to point out the need of increasing the dose of the inoculum, particularly of B. brongniartii, or to change its application method, which could explain the limited biocontrol activity recorded in the trial40.
Furthermore, farmers like to utilize “multifunctional” products and manufacturers prefer to market products with several activities (nutritional and plant strengtheners) because they are more likely to have effects and attract users. It appear thus evident that there is a perceived and real need for accurate and standard methods, to support the registration process and to fine tune the bio-product application strategy, suitable to monitor the persistence of the different inoculated strains and to evaluate the success of inoculation. Detection of microorganisms released in the environment with molecular methods based on PCR techniques that use natural genome polymorphism, such as the one described in this report, can largely facilitate and allow the discrimination at the strain level of natural and introduced organisms, increasing the safety of bio-inoculants for the environment and the time and economic efforts of farmers41,42,43.
Conclusions
Cultivation-independent multilocus genotyping, combined with the qPCR (qPCR-SSR analysis), allowed detecting with high specificity and sensitivity the fungal inoculants even when present in a very low concentration in soil. It was possible to trace and quantify B. bassiana in soil after field inoculation. The lack of detection of B. brongniartii after the application of the bio-inoculum under field conditions showed the potentiality of the method to evaluate the efficacy of biocontrol treatments to soil. The results suggest that it represents an attractive method for reliable and flexible, as well as cost- and time-effective, detection and dosing of inoculants in soil.
Additional Information
How to cite this article: Canfora, L. et al. Development of a method for detection and quantification of B. brongniartii and B. bassiana in soil. Sci. Rep. 6, 22933; doi: 10.1038/srep22933 (2016).
Acknowledgments
This work was supported by the Polish Ministry of Agriculture and Rural Development, Sadownictwo metodami ekologicznymi – Określenie dobrych praktyk ochrony przed szkodnikami i chorobami w uprawach sadowniczych” (Grant nr. HORre-029-31-29/14(103)) through funding of equipment and other expenses. We thank Dr. Anna Benedetti for sharing laboratory facilities.
Footnotes
Author Contributions L.C., E.M. and F.P. designed the research and wrote the manuscript. L.C., E.M., C.T., M.T. and B.H.L. established the field experiment, E.M., C.T., M.T. and B.H.L. collected the soil samples data, T.C. provided Beauveria brongniartii, L.C. and F.P. performed statistical analysis, L.C. and F.P. performed the experiments. All the authors revised the manuscript and approved the final version.
References
- Adesemoye A. O., Torbert H. A. & Kloepper J. W. Enhanced plant nutrient use efficiency with PGPR and AMF in an integrated nutrient management system. Can. J. Microbiol. 54, 876–886 (2008). [DOI] [PubMed] [Google Scholar]
- Mazid S., Rajkhowa R. C. & Kalita J. C. A review on the use of biopesticides in insect pest management. Int. J. of Sc. and Advanc. Technol. 1(7), 169–178 (2011). [Google Scholar]
- Richardson A. E., Barea J. M., McNeill A. M. & Prigent-Combaret C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant & Soil 321, 305–339 (2009). [Google Scholar]
- Malusá E., Pinzari F. & Canfora L. Efficacy of biofertilizers: challenges to improve crop production In Microbial Inoculants in Sustainable Agricultural Productivity, Functional Applications Vol 2 (eds Singh D. P. et al.) ISBN978-81-322-2642-0 (Springer, India, In Press, 2015). [Google Scholar]
- Malusá E. & Vassilev N. A contribution to set a legal framework for biofertilizers. Appl. Microbiol. and Biotechnol. 98, 6599–6607 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashan Y., de-Bashan L. E., Prabhu S. R. & Hernandez J.-P. Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998–2013). Plant & Soil 378(1–2), 1–3 (2014). [Google Scholar]
- Torsvik V. & Ovreas L. Microbial diversity and function in soil: from genes to ecosystems. Curr. Opin. Microbiol. 5, 240–245 (2002). [DOI] [PubMed] [Google Scholar]
- Fierer N., Schimel J. P. & Holden P. A. Variations in microbial community composition through two soil depth profiles. Soil Biol. & Biochem. 35, 167–176 (2003). [Google Scholar]
- Bonaterra A., Badosa E., Cabrefiga J., Francés J. & Montesinos E. Prospects and limitations of microbial pesticides for control of bacterial and fungal pomefruit tree diseases. Trees 26, 215–226 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filion M., St-Amaud M. & Jabaji-Hare Suha h. Direct quantification of fungal DNA from soil substrate using real-time PCR. J. of Microbiol. Meth. 53, 67–76 (2003). [DOI] [PubMed] [Google Scholar]
- Babu B. K., Mesapogu S., Sharma A., Somasani S. R. & Arora D. K. Quantitative real-time PCR assay for rapid detection of plant and human pathogenic Macrophomina phaseolina from field and environmental samples. Mycologia, 103(3), 466–473 (2011). [DOI] [PubMed] [Google Scholar]
- Guo Y., Li W., Sun H., Wang N., Yu H. & Chen H. Detection and quantification of Rhizoctonia cerealis in soil using real-time PCR. J. Gen. Plant Pathol 78, 247–254 (2012). [Google Scholar]
- Lievens B., Brower M., Vanachter C. R. C. A., Cammue B. P. A. & Thomma B. PH. J. Real-Time PCR for detection and quantification of fungal and oomycete tomato pathogens in plant and soil samples. Plant Sc. 171, 155–165 (2006). [Google Scholar]
- Xiang M. C., Xiang P. A., Jiang X. Z., Duan W. J. & Liu X. Z. Detection and quantification of the nematophagous fungus Hirsutella minnesotensis in soil with real-time PCR. Appl. Soil Ecol. 44, 170–175 (2010). [Google Scholar]
- Zeng Q. Y., Westermark S. O., Rasmuson-Lestander A. & Wang X. R. Detection and quantification of Wallemia sebi in Aerosols by real-time PCR, conventional PCR, and cultivation. Appl. and Environm. Microbiol. 70(12), 7295–7302 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkan N., Gadkar V., Coburn J., Yarden O. & Kapilnik Y. Quantification of the arbuscular mycorrhizal fungus Glomus intraradices in host tissue using real-time polymerase chain reaction. New Phytol. 161, 877–885 (2003). [DOI] [PubMed] [Google Scholar]
- Atkins S. D., Clark I. M., Sosnowska D., Hirsch P. R. & Kerry B. R. Detection and quantification of Plectosphaerella cucumerina, a potential biological control agent of potato cyst nematodes, by using conventional PCR, Real-time PCR, Selective Media, and Baiting. Appl. and Environm. Microbiol. 69(8), 4788–4793 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillo L. A., Thomsen L., Juneja P. & Hajek A. E. Detection and quantification of Entomophaga maimaiga resting spores in forest soil using real-time PCR. Mycol. Res. III, 324–331 (2007). [DOI] [PubMed] [Google Scholar]
- Savazzini F., Oliveira Longa C. M. & Pertot I. Real-time PCR for detection and quantification of the biocontrol agent Trichoderma atroviride strain SC1 in soil. J. of Microbiol. Meth. 73, 185–194 (2008). [DOI] [PubMed] [Google Scholar]
- Lopez-Mondéjar R., Raidi S., Ros M. & Pascual J. A. Quantification of the biocontrol agent Trichoderma harzianum with real-time TaqMan PCR and its potential extrapolation to the hyphal biomass. Bioresour Technol. 101, 2888–2891 (2010). [DOI] [PubMed] [Google Scholar]
- Douglas Inglis G., Goettel M. S., Butt T. M. & Strasser H. Use of Hyphomycetous fungi for managing insect pests, in Fungi as Biocontrol Agents (eds Butt T. M. et al.) 23–69 (CAB International, Wallingford, Oxon, UK, 2001). [Google Scholar]
- Enkerli J., Widmer F., Gessler C. & Keller S. Strain-specific microsatellite markers in entomopathogenic fungus Beauveria brongnartii. Mycol. Res. 105(9), 1079–1087 (2001). [Google Scholar]
- Warrior P. Prospects and challenges - biocontrol agents. Growing biocontrol markets challenge research and development, in Proceedings 9th European Meeting of the IOBC/WPRS Working Group, 23–29 May, 113 (Schloss Salzau, Germany, 2003).
- Herrmann L. & Lesueur D. Challenges of formulation and quality of biofertilizers for successful inoculation. Appl. Microbiol. Biotechnol. 97, 8859–8873 (2013). [DOI] [PubMed] [Google Scholar]
- Trabelsi D. & Mhamdi R. Microbial inoculants and their impact on soil microbial communities: A review. Biomed. Res. Int. 863240, doi: 10.1155/bmri863240 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwieger F. & Tebbe C. Effect of field inoculation with Sinorhizobium meliloti L33 on the composition of bacterial communities in rhizospheres of a target plant (Medicago sativa) and a non-target plant (Chenopodium album) - linking of 16S rRNA gene-based single-strand conformation polymorphism community profiles to the diversity of cultivated bacteria. Appl. and Environ. Microbiol. 66(8), 3556–3565 (2000). [DOI] [PMC free article] [PubMed]
- Hirsch P. R., Mauchline T. H. & Clark I. M. Culture independent molecular techniques for soil microbial ecology. Soil Biol. & Biochem. 42(6), 878–887 (2010). [Google Scholar]
- Han I. et al. Improved detection of microbial risk of releasing genetically modified bacteria in soil by using massive sequencing and antibiotic resistance selection. J. Hazard. Mat. 227–228, 172–178 (2012). [DOI] [PubMed] [Google Scholar]
- Schwarzenbach K., Widmer F. & Enkerli J. Cultivation-independent analysis of fungal genotypes in soil by using Simple Sequence Repeat Markers. Appl. and Environ. Microbiol. 73(20), 6519–6525 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehner S. A. & Buckley E. P. Isolation and characterization of microsatellite loci from the entomopathogenic fungus Beauveria bassiana (Ascomycota: Hypocreales). Mol. ecol. notes 3, 409–411 (2003). [Google Scholar]
- Babić K. H. et al. Influence of different Sinorhizobium meliloti inocula on abundance of genes involved in nitrogen transformations in the rhizosphere of alfalfa (Medicago sativa L). Environ. Microbiol. 10, 2922–2930 (2008). [DOI] [PubMed] [Google Scholar]
- Strasser H., Forrer A. & Schinner F. Development of media for the selective isolation and maintenance of virulence of Beauveria brongniartii, In Microbial control of soil dwelling pests (eds Jackson T. A. & Glare T. R.) 125–130 (AgResearch, Lincoln, New Zeland 1996). [Google Scholar]
- Tesi R. et al. A. Soil disinfection with steam alone or combined with CaO in a greenhouse radish crop. Adv. Hort. Sci. 21, 75–82 (2007). [Google Scholar]
- Malusá E. et al. Biological control of soil pests in organic strawberry plantations: the need of a holistic approach. Proceedings International Conference. Innovative Technologies in Organic Horticultural Production”. Skierniewice, Poland. October 22–24, 133–134 (2014).
- Meszka B. & Malusá E. Effects of soil disinfection on health status, growth and yield of strawberry stock plants. Crop Protection 63, 113–119 (2014). [Google Scholar]
- Maniatus T., Fritsch E. F. & Sambrook J. Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory (1989). [Google Scholar]
- Smith C. S. et al. Sources of Variability in the Measurement of Fungal Spore Yields. Appl. and Environ. Microbiol. 54(6), 1430–1435 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashan Y. et al. Survival of Azospirillum brasilense in the bulk soil and rhizosphere of 23 soil types. Appl. Environ. Microbiol. 61, 1938–1945 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Świechowski W., Doruchowski G. & Trzciński P. Effect of spray application parameters on viability of bacterium Pseudomonas fluorescens used as bio-pesticide in organic fruit production, In Proc. II Int. Congress on Organic Fruit Research Symposium, ISHS (eds Granatstein D. & Andrews P.) 9, (Leavenworth WA, USA, 2012). [Google Scholar]
- Łabanowska B. H. et al. Szkodliwość i próba zwalczania pędraków chrabąszcza majowego (Melolontha melolontha) w ekologicznych uprawach truskawki. Proceedings 58 Ogólnopolska Konferencja Ochrony Roślin Sadowniczych, Warszawa: 99–101 (19 February 2015).
- Öpik M. et al. The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol. 188, 223–241 (2010). [DOI] [PubMed] [Google Scholar]
- Stockinger H., Krüger M. & Schüßler A. DNA barcoding of arbuscular mycorrhizal fungi. New Phytol. 187, 461–474 (2010). [DOI] [PubMed] [Google Scholar]
- Sýkorová Z. et al. Long-term tracing of Rhizophagus irregularis isolate BEG140 inoculated on Phalaris arundinacea in a coal mine spoil bank, using mitochondrial large subunit rDNA markers. Mycorrhiza 22, 69–80 (2012). [DOI] [PubMed] [Google Scholar]