Abstract
The enamel matrix derivative (EMD) contains hundreds of peptides in different levels of proteolytic processing that may provide a range of biological effects of importance in wound healing. The aim of the present study was to compare the effect of EMD and its fractions on the cytokine profiles from human gingival fibroblasts in vitro and in gingival crevicular fluid (GCF) in a randomized controlled split-mouth clinical study (n = 12). Levels of cytokines in cell culture medium and in GCF were measured by Luminex over a 2-week period. In the clinical study, levels of pro-inflammatory cytokines and chemokines were increased, whereas the levels of transforming growth factor-α (TGF-α) and platelet-derived growth factor-BB (PDGF-BB) were reduced. The in vitro study showed that EMD and its high and low molecular weight fractions reduced the secretion of pro-inflammatory cytokines and chemokines compared to untreated cells. EMD had an effect on levels of cytokines related to fibroplasia, angiogenesis, inflammation and chemotaxis both in vitro and in vivo, however, the anti-inflammatory effect induced by EMD observed in the in vitro study could not be confirmed clinically.
Several studies have demonstrated that enamel matrix proteins, in particular amelogenin, applied to the root surface during periodontal surgery promote periodontal regeneration in pre-clinical1 and in clinical studies2. Clinical studies on enamel matrix proteins have focussed on healing periods varying between 6 months and 3 years2,3. Early healing events are critical and dictate the type of tissue that will develop later1,4,5. Histological studies6 have suggested that enamel matrix proteins are detectable on the denuded tooth root surface for 2–4 weeks following surgery. This seems to be a sufficiently long period of time to permit recolonization of periodontal ligament cells, initiate regenerative pathways and inhibit the down growth and proliferation of epithelial cells4. It is crucial to understand early healing mechanisms and use the knowledge gained to develop improved therapeutic strategies to promote regrowth of periodontal tissues lost due to disease. Moreover, clinical reports have suggested that enamel matrix proteins lead to improved early soft tissue wound healing7,8. Based on these clinical observations, it has been suggested that EMD may have an effect on gingival fibroblasts; relevant cells in soft tissue wound healing9. The gingival connective tissue is the oral equivalent to the dermis of the skin, and the main residing fibroblast is the gingival fibroblast10. Fibroblasts have a central role in wound healing as they do not only produce and organize the ECM11,12,13 but are also able to regulate inflammation through chemokine and cytokine expression14, angiogenesis15, and re-epithelialization16,17. EMD-induced cytokine release on gingival fibroblasts during the early healing period contribute to a possible favourable early wound healing effect18 or even play a role in the subsequent EMD-mediated regeneration of the tissues.
Several in vitro studies have provided valuable information on the molecular mediators induced by EMD19,20. In periodontal ligament (PDL) fibroblasts, EMD increased interleukin-6 (IL-6), TGF-β1 and PDGF-AB production21 and reduced IL-4 gene expression22. In an extensive analyses of cytokines secreted from PDL cells23, higher molecular weight fractions of EMD were found to induce an increase in vascular endothelial growth factor (VEGF) and IL-6 secretion, whereas lower molecular weight fractions enhanced cell proliferation and secretion of IL-8 and monocyte chemoattractant protein-1 (MCP-1) and reduced IL-4 release.
In vitro studies are often used as models to indicate and evaluate molecular mechanisms of clinical treatment strategies. GCF components have been studied with the aim of using them as predictors of periodontal disease progression and healing after therapy24, but with little success24. It is likely that a diagnostic model utilising a broader spectrum of GCF components would have a greater predictive power compared to models based on few components.
The aim of the present study was to assess and compare the effect of enamel matrix derivative (EMD) on the cytokine profiles from human gingival fibroblasts in vitro and, clinically, in gingival crevicular fluid during early periodontal wound healing. In addition, the bioactivity of various fractions of EMD was evaluated in vitro to identify active components.
Results
Clinical study
An overview of the cytokine profile induced by EMD and control groups is shown in Table 1. There were no adverse events or discomforts associated to the EMD or the control group. There were no significant differences between EMD- and control-treated sites at baseline for any of the cytokines measured.
Table 1. Cytokine profile induced by EMD- and controlled-operated sites during early periodontal wound healing.
| Day 7 | Range day 7 | Day 14 | Range day 14 | |
|---|---|---|---|---|
| EMD | ↑Total protein | 5227.14 | ↑Total protein | 4362.86 |
| ↓TGF-α# | 0.03 | ↓TGF-α# | 0.05 | |
| ↓IFN-γ | 0.01 | ↑IFN-γ | 0.01 | |
| ↑MDC | 0.01 | ↑MDC | 0.20 | |
| ↓IL-4 | 0.03 | ↑IL-4 | 0.03 | |
| ↑IL-6# | 0.40 | ↑IL-6# | 0.21 | |
| ↑IL-8# | 3.80 | ↑IL-8#,* | 1.18 | |
| ↑PDGF-AA | 0.02 | ↑PDGF-AA | 0.06 | |
| ↓PDGF-BB | 0.12 | ↓PDGF-BB* | 0.12 | |
| ↓IP-10 | 0.03 | ↑IP-10 | 0.14 | |
| ↑MCP-1 | 0.05 | ↑MCP-1 | 0.06 | |
| ↑TNF-α# | 0.02 | ↑TNF-α | 0.08 | |
| ↓VEGF | 0.15 | =VEGF | 0.38 | |
| CONTROL | ↑Total protein | 6400.00 | ↑Total protein | 5531.43 |
| ↓TGF-α# | 0.05 | ↓TGF-α# | 0.06 | |
| ↓IFN-γ | 0.01 | ↑IFN-γ | 0.04 | |
| ↑MDC | 0.02 | ↑MDC | 0.21 | |
| ↓IL-4# | 0.03 | ↑IL-4 | 0.10 | |
| ↑IL-6# | 0.51 | ↑IL-6# | 0.11 | |
| ↑IL-8# | 2.12 | ↑IL-8#,* | 0.94 | |
| ↑PDGF-AA | 0.05 | ↑PDGF-AA | 0.16 | |
| ↓PDGF-BB | 0.10 | ↑PDGF-BB* | 0.25 | |
| ↓IP-10# | 0.04 | ↑IP-10 | 0.27 | |
| ↑MCP-1 | 0.03 | ↑MCP-1 | 0.03 | |
| ↑TNF-α# | 0.08 | ↑TNF-α# | 0.03 | |
| ↓VEGF | 0.24 | ↑VEGF | 0.71 |
Cytokines after symbol ↑, ↓ or = mean that they are increased, decreased or constant respectively based on mean values of fold changes compared to baseline cytokine levels. Total protein values are given in μg ml−1. Cytokine levels are given in pg cytokine μg total protein−1. The range is calculated from changes with respect to baseline levels. Only statistically significant changes marked with * or # define whether a cytokine is changed. #Significant intra-group differences compared to baseline cytokine levels (p < 0.05). *Significant inter-group differences at day 7 or day 14 (p < 0.05) (n = 12 patients).
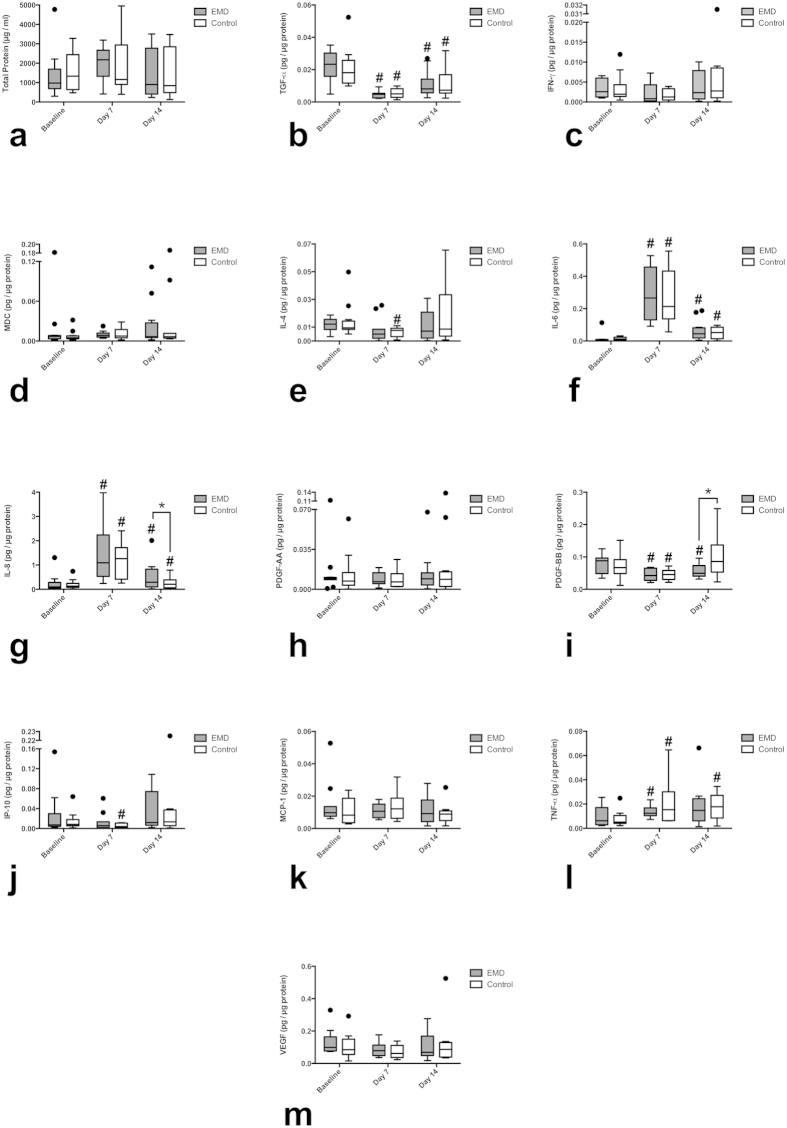
TGF-α was significantly lower in both control (p = 0.0001) and EMD (p = 0.0001) at day 7 compared to baseline TGF-α levels. At day 14, TGF-α values were also significantly reduced in both the control (p = 0.022) and EMD (p = 0.003) groups compared to baseline values. There were no significant differences between the groups at any time point studied (Fig. 1b).
Figure 1. Box plot of GCF normalized cytokine levels (pg cytokine/μg total protein) at baseline, day 7 and day 14.
(a) Total protein, (b) TGF-α, (c) IFN-γ, (d) MDC, (e) IL-4, (f) IL-6, (g) IL-8, (h) PDGF-AA, (i) PDGF-BB, (j) IP-10, (k) MCP-1, (l) TNF-α, (m) VEGF. #Significant intra-group differences in cytokine levels between baseline and day 7 or day 14 (p < 0.05). *Significant inter-group differences in cytokine levels between control and EMD sites (p < 0.05). Generalized estimating equations (GEE) (n = 12 patients). Box plots show the median, the 25th and 75th percentiles, Tukey whiskers (median ± 1.5 times interquartile range), and outliers (•).
IL-4 levels in GCF were reduced in both the test and control group at day 7 compared to baseline values, however, it was only significantly changed (p = 0.014) for the control group. There were no significant differences between the two groups at day 7 and day 14. (Fig. 1e).
IL-6 was enhanced 50-fold in EMD-treated sites (p < 0.0001) and 25 fold in the control group (p < 0.0001) compared to baseline values at day 7. The difference between groups did not reach statistical significance. At day 14, IL-6 was also significantly increased 12-fold and 5-fold in EMD (p = 0.009) and control groups (p < 0.0001) respectively, compared to baseline values. There were no significant differences between the groups at any time point (Fig. 1f).
Interestingly, EMD-treated sites presented significantly increased levels of IL-8, around 25-fold (p < 0.0001) at day 7 and 5-fold (p = 0.006) at day 14 compared to baseline values. The control group also presented significantly increased levels of IL-8, around 11-fold (p < 0.0001) at day 7 and 2-fold (p < 0.0001) at day 14 compared to baseline values. Significant inter-group differences were found at day 14 with EMD-treated sites having a more pronounced effect on IL-8 release than in the control group (p = 0.030) (Fig. 1g).
PDGF-BB levels in GCF were significantly decreased in EMD both at day 7 (p = 0.001) and day 14 (p = 0.021) compared to baseline levels. By contrast, PDGF-BB levels in control sites were significantly reduced at day 7 (p = 0.001) and were increased twice at day 14, although this increase was not significant. EMD-treated sites significantly decreased PDGF-BB levels at day 14 compared to the control sites (p = 0.042) (Fig. 1i).
TNF-α release was significant at day 7 in the EMD and control groups, with an increase of 3-fold (p = 0.044) and 4-fold (p = 0.021) respectively, compared to baseline values. The release of TNF-α was also significantly enhanced 3-fold in the control group (p < 0.0001) at day 14 compared to baseline values. There were no differences between groups at any time point (Fig. 1l).
IP-10 release was unchanged at day 7 in both groups. A significant intra-group difference was found at day 7 in the control group compared to baseline values (p = 0.013). There were no significant differences between groups at any time point (Fig. 1j).
There were no significant intra-group or inter-group differences for total protein, IFN-γ, MDC, PDGF-AA, MCP-1 and VEGF at any of the time points assessed.
In vitro study
An overview of changes in cytokine levels for the different fractions is given in Table 2. In contrast to clinical findings, EMD reduced the IL-6, IL-8 and VEGF secretion compared to untreated gingival fibroblasts during the first week of the experiment.
Table 2. Cytokine levels in cell culture medium from gingival fibroblasts incubated with EMD and EMD fractions (F1–F13) relative to untreated control at the same timepoint.
| Gingival fibroblasts | Day 1 | Day 3 | Day 7 | Day 14 |
|---|---|---|---|---|
| EMD | ↓IL6** | ↓VEGF* | ↓IL8** | |
| F1 | ↓IL8**, VEGF** | ↓IL6***, IL8** | ↓IL6**, IL8**, MCP1**, VEGF* | |
| F2 | ↓IL6**, MCP1**, VEGF*** | ↓IL6**, IL8**, MCP1** | ||
| F3 | ↓VEGF** | ↓VEGF*** | ↓MCP1** | |
| F4 | ↓IL6***, IL8**, MCP1***, VEGF*** | |||
| F5 | ↓IL6**, IL8**, MCP1**, VEGF** | ↓IL6**, VEGF** | ↓IL6***, MCP1** | |
| F6 | ↓IL6** | ↓IL6***, IL8**, VEGF*** | ↓IL6** | ↓IL6***, IL8***, MCP1***, VEGF** |
| F7 | ↓IL6**, IL8***, MCP1**, VEGF*** | ↓IL8**, MCP1** | ↓IL6** | |
| F8 | ↓VEGF** | |||
| F9 | ↓IL6***, IL8***, MCP1*** | ↓IL6**, IL8**, MCP1***, VEGF** | ||
| F10 | ↓VEGF* | |||
| F11 | ||||
| F12 | ↓VEGF* | |||
| F13 |
EMD: enamel matrix derivative. IL-6: interleukin-6; IL-8: interleukin-8; MCP-1: monocyte chemoattractant protein-1; VEGF: vascular endothelial growth factor. ↓ mean that cytokine levels are significantly reduced compared to the control group. Fractions 1–6 shared components above or around 20 KDa, whereas fractions 7–13 contained components below 5 kDa. *p < 0.05, **p < 0.01, ***p < 0.001 (n = 3).
Similarly, both high and low molecular weight fractions of EMD reduced IL-6, IL-8, VEGF and MCP-1 secretion compared to untreated cells over the 2-week study period. No differences between high and low molecular weight fractions were observed in terms of inducing cytokine secretion.
The anti-inflammatory cytokine profile obtained by the various fractions in the in vitro study could not be confirmed clinically.
While IL-4, IP-10, TNF-α and IFN-γ levels could be detected in GCF in the clinical study, these cytokines were below the detection limit for the assay and for all treatment groups at all time points in the in vitro study. Other cytokines that were below the detection limit in the in vitro study were: G-CSF, IL-7 and RANTES.
Discussion
The observed effect of EMD on the various cytokines secreted from gingival fibroblasts in vitro was not replicated in GCF from treated periodontal patients. The discrepancy between the in vitro and clinical results may be related to contribution of factors from more than one cell type in GCF. In addition, the EMD used in the clinical study was from a different lot number than the EMD used in vitro and being a biologically derived product, the content of EMD might vary between different batches. Differences among lots of EMD will have to be determined.
TGF-α levels in GCF were reduced in both control and EMD treated sites compared to baseline values over the 2-week study period. TGF-α plays an important role in the proliferative phase of wound healing as it stimulates epidermal regrowth, angiogenesis and granulation tissue formation25,26,27. Nonetheless, TGF-α has been shown to be significantly reduced in patients with periodontal disease compared to healthy patients28. Altered TGF-α secretion in periodontitis patients may affect periodontal wound healing.
Among all cytokines analyzed, the pro-inflammatory cytokines IL-6 and IL-8 were the cytokines most influenced by periodontal surgery in both EMD and control sites. Levels of these two cytokines are elevated during the inflammatory phase of wound healing29,30. Engelhardt et al.29 demonstrated enhanced IL-8 expression in the superficial wound bed that correlated spatially and temporally with neutrophil infiltration. Galluci et al.30 identified IL-6 expression in the epithelial wound edge and in macrophages and fibroblasts of the dermis.
In the in vitro study, EMD and its fractions induced a reduced secretion of pro-inflammatory factors. This observation is in line with Nokhbehsaim et al.31 who found that EMD downregulated IL-1β, IL-6 and IL-8 expression in PDL cells. A reduction of IL-6, IL-8 and MCP-1 secretion during the early phase of healing might lead to a reduced migration of inflammatory cells at the wound site32,33,34. In contrast, EMD and its fractions have been found to enhance the secretion of IL-6, IL-8 and MCP-1 in PDL fibroblasts23. This discrepancy might be explained by the difference in characteristics and functions of these cells35. EMD contains hundreds of peptides in different levels of proteolytic processing that may provide a range of biological effects of importance in wound healing23. It is possible that the effect of EMD in wound healing and regeneration may be related to a single component or, on the other hand, its effect may be caused by its composition as a whole, or a range of molecular weight components. This question remains unanswered in the literature. There are difficulties to characterize EMD because, upon size fractionation, the same proteins may be found in several fractions23. There is the possibility that a purified bioactive component can be characterized and thus an improved EMD formulation may be developed.
Reports from clinicians have claimed that early wound healing is characterized by a reduced inflammation in the gingival soft tissue7,8. The observed increase in pro-inflammatory cytokines in GCF may indicate contribution from other sources than gingival fibroblasts.
The total GCF protein content was increased, however, this did not reach significance compared to baseline values. Total protein has been shown to increase with gingival inflammation36,37. Giannopoulou et al.38 showed that after non-surgical periodontal therapy, total protein significantly decreased. This was accompanied with a decrease in clinical signs of inflammation38. Thus, it is expected that the total protein would increase during the early inflammatory phase of wound healing.
Villa et al.23 found that most enamel matrix derived fractions induced a decreased secretion of IL-4 in PDL fibroblasts compared to untreated cells. In the present clinical study, IL-4 secretion was decreased in both EMD and control groups compared to baseline levels, but only reached significance in the control group.
PDGF-AA levels were increased in both the control and EMD groups over the study period, although none of these effects were significant neither in the intra-group nor in the inter-group analyses. By contrast, PDGF-BB levels were significantly reduced by EMD compared to baseline values. This pattern was not found for the control group. It has been suggested that PDGF-A chains might play a role in periodontal wound healing during the first days of healing and PDGF-B chains might become more relevant in the wound bed later, correlating with fibroblast induced collagen synthesis that takes place after week 139.
Examples where no significant changes in cytokine levels between test and control groups were observed may be related to high variation between individuals40 and the relatively low number of patients. Another issue to take into account is that some of the inflammatory cytokines analyzed might be constitutively overproduced in periodontitis lesions41. Thus, high levels of these cytokines might already be present at baseline and also during wound healing. This might explain why some cytokines might not show differences over time or between groups. It is generally accepted that the intra- and inter-assay variation should be below 15%42. 3 cytokines presented an inter-assay variation above 15%: IP-10 (15.3%), PDGF-AA (16.7%) and IL-6 (18.3%). Thus, the results have to be interpreted cautiously.
This study had a split-mouth design. An important drawback of such a design is the possibility of a carry-across effect43. The results have to be interpreted carefully as this type of design can lead to reduced treatment differences, and estimates of the treatment effect from split-mouth and parallel designs may not be the same43.
The most preferred strategy for collecting GCF is by use of paper strips. In the present study paper points were used. To our knowledge there is no evidence to support the use of paper strips over paper points to collect GCF. In one study44, paper strips showed higher levels of cytokines and a higher recovery rate than paper points. Nonetheless, these factors also depend on paper composition45 and binding properties of different cytokines which may be influenced by factors such as hydrophobicity, charge and structure44.
A common problem in GCF analyses is the low volume collected, which precludes the analyses of multiple cytokines46,47. Modern multiplexing methods such as Luminex require low sample volumes, are highly sensitive, and allow the analyses of up to 100 analytes simultaneously in one sample, a great advantage compared to methods measuring individual analytes (e.g. ELISA). Identification of cytokine profiles may increase the predictability of regenerative procedures. Future studies should establish which cytokine profiles are associated with regenerative outcomes and which profiles are related to unfavourable outcomes.
To the best of our knowledge this is the first report that simultaneously assesses cytokine secretion from human gingival fibroblasts and gingival crevicular fluid after the administration of EMD. In this report we are suggesting some mechanisms underlying the possible early healing effect of EMD. The assessment of postoperative healing with an adequate healing index3 and a cytokine profile might predict regenerative outcomes.
Conclusions
From the findings of the present study and previously published work23, EMD may promote improved early wound healing with reduced gingival fibroblasts induced inflammation. The regenerative capabilities of EMD might be mediated through its influence on PDL fibroblasts. The anti-inflammatory cytokine profile observed in vitro could not be confirmed clinically. In addition, EMD affects growth factors and cytokines related to fibroplasia, angiogenesis, inflammation and chemotaxis both in vitro and in vivo.
Material and Methods
Clinical study
The clinical study was designed as a randomised controlled split-mouth clinical trial. The study protocol was approved by the Regional Committee for Research Ethics, Oslo, Norway (2013/1827/REK sør-øst C), and patients signed an (approved) informed consent form before entering the study. The investigation was performed according to the principles of the Declaration of Helsinki on experiments involving human subjects. The presence of biological markers in the GCF was assessed at baseline, and one and two weeks postoperatively.
Patients
Subjects undergoing elective periodontal treatment at a private periodontal specialist clinic were included in the present study. Fifteen patients aged 25–75 years suffering from generalized severe chronic periodontitis were recruited during the first trimester of 2014 and treated during the period between April and August of 2014. Three subjects that required grafting procedures were excluded from the study and one patient did not attend the surgery for the control site, leaving a total number of 11 sites for the control group and 12 sites for the EMD treated group. Adverse events were evaluated and recorded at each visit. All patients had undergone an initial causative treatment phase consisting of patient motivation, oral hygiene instruction and supra-, subgingival scaling and root planing. Patients were re-evaluated six weeks after scaling and root planning and scheduled for surgery as appropriate. Baseline measurements at the test and control sites were obtained on the day of surgery. Baseline defect characteristics are presented in Table 3.
Table 3. Baseline characteristics of the periodontal defects.
| Variable | EMD (n = 12) | CONTROL (n = 11) | P value |
|---|---|---|---|
| PPD at deepest defect (mm) | 7.0 (6, 8) | 7.0 (6, 8) | n.s. |
| CAL at deepest defect (mm) | 6.0 (5, 8) | 6.0 (6, 7) | n.s. |
| Deepest infrabony defect (mm) | 2.5 (0, 8) | 2.0 (0, 5) | n.s. |
| Total protein (μg ml−1) | 975.57 (4475.71) | 1307 (2802.86) | n.s |
| TGF-α (pg ml−1) | 28.04 (94.25) | 22.3 (64.03) | n.s. |
| IFN-γ (pg ml−1) | 2.47 (4.65) | 3.2 (10.1) | n.s. |
| MDC (pg ml−1) | 7.39 (116.75) | 8.3 (14.08) | n.s. |
| IL-4 (pg ml−1) | 12.04 (17.88) | 15.16 (29.32) | n.s. |
| IL-6 (pg ml−1) | 7.61 (50.66) | 13.46 (37.29) | n.s. |
| IL-8 (pg ml−1) | 96 (1581.8) | 186 (422.67) | n.s. |
| PDGF-AA (pg ml−1) | 9.6 (73.35) | 11.84 (30.13) | n.s. |
| PDGF-BB (pg ml−1) | 77.09 (189.93) | 80.74 (186.79) | n.s. |
| IP-10 (pg ml−1) | 7.63 (96.26) | 11.7 (31.81) | n.s. |
| MCP-1 (pg ml−1) | 12.64 (40.02) | 11.37 (26.95) | n.s. |
| TNF-α (pg ml−1) | 6.28 (39.09) | 7.18 (22.48) | n.s. |
| VEGF (pg ml−1) | 116.02 (819.13) | 131.92 (193.36) | n.s. |
Clinical data given as median (min, max). Cytokine baseline levels data given as median (range). Cytokine levels were normalized to total protein (pg cytokine μg total protein−1). PPD: probing pocket depth. CAL: clinical attachment level. Comparisons between EMD and control treated sites. Clinical parameters analysed by Mann-Whitney U test. Cytokine parameters analysed by GEE; n.s. (non- significant).
Inclusion criteria and exclusion criteria for participation in the study
The inclusion criteria were based on the presence of two similar periodontal defects in different quadrants with clinical attachment loss of at least 5 mm, probing pocket depth of 6 mm or more, horizontal and/or vertical bone loss as demonstrated by the probing measurement and radiographic assessments following the initial phase of periodontal treatment. Experimental teeth must either have exhibited a vital pulp or, if subjected to root canal treatment, be asymptomatic.
Exclusion criteria were smoking, patients having used antibiotics in the 6 months prior to treatment, patients with disorders that compromise wound healing, such as diabetes mellitus, cancer, HIV, chronic high dose steroid therapy, radiation or immune-suppressive therapy and patients with acute infectious lesions in the area of intended therapy.
Surgical intervention
Clinical measurements were obtained at baseline. Full-thickness mucoperiosteal flaps were elevated on the buccal, lingual and interproximal aspects of the involved teeth. Granulation tissue adherent to the alveolar bone was removed. Remaining subgingival calculus was removed with curettes. Following open flap debridement, teeth were randomly assigned to receive either the test or the control treatment by the tossing of a coin. In the test sites, EMD in propylene glycol alginate (Emdogain; Straumann, Basel, Switzerland) was directly applied to the exposed alveolar bone as well as onto the root surfaces. Flaps were repositioned and tension-free primary closure of the wound was achieved by means of single non-resorbable sutures (Propylen 5.0; Medipac, Kilkis, Greece). Control sites received the same surgical procedure but without the application of Emdogain. Sutures were removed after 14 days. Patients were advised to rinse twice daily with a 0.2% solution of chlorhexidine digluconate (Corsodyl; GlaxoSmithKline, Münchenbuchsee, Germany) for 2 weeks. No antibiotics were given48,49, and mechanical oral hygiene was not allowed in the surgical areas during this period. Analgesics were taken by the patients on an as needed basis.
Gingival crevicular fluid (GCF) sampling
GCF samples were obtained from both test and control sites at baseline, day 7 and day 14 post-surgery as previously described50. Multiplex analyses of the level of cytokines in GCF was performed as previously described50. The experimental sites were isolated with cotton rolls, and GCF was collected using endodontic paper points ISO size 50 (Beutel rock VDW; München, Germany) in the gingival sulcus for 10 seconds at the experimental sites of control and test teeth. The paper points were transferred to Eppendorf microtubes (Axygen, Tewksbury, MA, USA) and were coded with a unique identifier. Samples were immediately frozen at −20 °C after collection, and transferred and stored at minus 80 °C until analysis.
Protein quantification from gingival crevicular fluid
All laboratory analyses were performed blind with respect to sample identification, visit and patient information. Tris-buffered saline (TBS; 200 μl) was added to each tube containing GCF samples. Multianalyte profiling of the level of cytokines was performed on the Luminex-200 system (Luminex, Austin, TX, USA). The levels were monitored related to the amount of total protein in each sample. The amount of TGF-α, interferon-γ (IFN-γ), human macrophage-derived chemokine (MDC/CCL22), PDGF-AA, PDGF-BB, IL-4, IL-6, IL-8, interferon-γ induced protein (IP-10), MCP-1, tumor necrosis factor-α (TNF-α), and VEGF, was measured using the human cytokine/chemokine kit (Milliplex human cytokine MPXHCYTO-60k, Millipore, Billerica, MA, USA). All analyses were performed according to the manufacturers’ protocols. The intra-assay and inter-assay variation for the cytokines analyzed ranged between 1.5 and 18.3%.
The total protein content in GCF was determined using the BCA protein assay kit (Pierce, Rockford, IL, USA) following the manufacturer’s kit instructions.
In vitro study
Cell cultures
Commercially available human gingival fibroblasts (ATCC, Manassas, VA, USA) were grown in Dulbecco’s modified Eagle’s medium (High Glucose 4.5 g/l, PAA laboratories, Linz, Austria) supplemented with 10% fetal bovine serum and 100 U/ml penicillin and 100 μg/ml Streptomycin (PAA laboratories). Cells were subcultured at 37 °C in a humidified atmosphere of 10% CO2 according to the manufacturer’s instructions.
EMD fractions
Fractions were obtained as described previously23. Briefly, EMD (Biora, Malmö, Sweden) was subjected to size-exclusion chromatography using a 90 × 1.6-cm column of Bio Gel P10 (Bio-Rad, Hemel Hempstead, UK). Proteins were eluted using 0.125 M formic acid and the column eluent was monitored at 280 nm and 5-ml fractions were collected and freeze dried. The cultured cells were treated with either EMD or the various fractions reconstituted in cell culture medium at molar concentrations equivalent to their content in 10 μg ml−1 of whole EMD. Untreated cells were used as controls. Three replicate experiments were conducted for every fraction, EMD and the controls. Cell culture media was harvested after 1, 3, 7 and 14 days. The fractions were characterized by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and liquid chromatography electrospray ionization–tandem mass spectrometry as previously described23.
Protein quantification in cell culture medium
Multianalyte profiling of the level of cytokines in concentrated cell culture medium of gingival fibroblasts was performed on the Luminex-200 system (Luminex) as previously described23. The amount of granulocyte colony-stimulating factor (G-CSF), IL-4, IP-10, TNF-α, IFN-γ, IL-6, IL-7, IL-8, MCP-1, regulated upon activation of normal T-cell expressed and secreted (RANTES) and VEGF was measured using the human cytokine/chemokine kit (Milliplex human cytokine MPXHCYTO-60k; Millipore). The intra-assay variation for the analyzed cytokines varied between 1.5 and 4.3%. The inter-assay variation varied between 3.5% and 18.3%.
Statistical analysis
Sample size calculation was not performed, as this study was a pilot to assess the effect of EMD on multiple cytokines after periodontal surgical therapy. For the clinical study, cytokine concentrations were normalized to total protein (pg of cytokine μg total protein−1) and served as the primary outcome. Generalized estimating equations were used to adjust for clustering within subjects in a split-mouth design and to investigate the interaction between GCF cytokine levels (dependent variable) and time or treatment group (independent factors). Thus, inter-group comparisons in cytokine levels were made at each time point and intra-group comparisons in cytokine levels were made to baseline values at day 7 and day 14. This statistical analyses was performed with SPSS for Mac (version 21; SPSS, Chicago, IL, USA) (Supplementary Dataset).
For the in vitro study, statistical comparison between treatment groups and controls was performed using parametric one-way analysis of variance (ANOVA) and post-hoc Dunnet’s test with multiple comparisons towards the untreated control group. When the normality test failed, ANOVA on ranks (Kruskal Wallis test) and post-hoc Dunn’s test was performed. (SigmaStat software; Systat, San Jose, CA, USA). P ≤ 0.05 was considered significant.
Additional Information
How to cite this article: Villa, O. et al. EMD in periodontal regenerative surgery modulates cytokine profiles: A randomised controlled clinical trial. Sci. Rep. 6, 23060; doi: 10.1038/srep23060 (2016).
Supplementary Material
Acknowledgments
This work was supported by the Norwegian Research Council (grant 231530/F20). The authors are specially thankful for the excellent technical assistance from Aina Maria Lian (Oral Research Laboratory, Institute of Clinical Dentistry, University of Oslo, Norway).
Footnotes
Author Contributions Conception and design: O.V., J.C.W., O.C.K., S.P.L., A.M.A. and J.E.R. Cell experiments and protein quantification: O.V. Surgical procedure: J.C.W. Chromatography analyses: S.J.B. Analysis and interpretation of data: O.V. and J.E.R. Drafting the article: O.V. Review the article: all authors
References
- Hammarstrom L. Enamel matrix, cementum development and regeneration. J. Clin. Periodontol. 24, 658–668 (1997). [DOI] [PubMed] [Google Scholar]
- Esposito M., Grusovin M. G., Papanikolaou N., Coulthard P. & Worthington H. V. Enamel matrix derivative (Emdogain(R)) for periodontal tissue regeneration in intrabony defects. Cochrane Database Syst. Rev. CD003875 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtel H. et al. Microsurgical access flap and enamel matrix derivative for the treatment of periodontal intrabony defects: a controlled clinical study. J. Clin. Periodontol. 30, 496–504 (2003). [DOI] [PubMed] [Google Scholar]
- Gestrelius S. et al. Formulation of enamel matrix derivative for surface coating. Kinetics and cell colonization. J. Clin. Periodontol. 24, 678–684 (1997). [DOI] [PubMed] [Google Scholar]
- Gestrelius S., Andersson C., Lidström D., Hammarström L. & Somerman M. In vitro studies on periodontal ligament cells and enamel matrix derivative. J. Clin. Periodontol. 24, 685–692 (1997). [DOI] [PubMed] [Google Scholar]
- Sculean A. et al. Presence of an enamel matrix protein derivative on human teeth following periodontal surgery. Clin. Oral Investig. 6, 183–187 (2002). [DOI] [PubMed] [Google Scholar]
- Wennström J. L. & Lindhe J. Some effects of enamel matrix proteins on wound healing in the dento-gingival region. J. Clin. Periodontol. 29, 9–14 (2002). [DOI] [PubMed] [Google Scholar]
- Tonetti M. S. et al. Healing, post-operative morbidity and patient perception of outcomes following regenerative therapy of deep intrabony defects. J. Clin. Periodontol. 31, 1092–1098 (2004). [DOI] [PubMed] [Google Scholar]
- Weinreb M. & Nemcovsky C. E. In vitro models for evaluation of periodontal wound healing/regeneration. Periodontol. 2000 68, 41–54 (2015). [DOI] [PubMed] [Google Scholar]
- Fournier B. P. J. et al. Multipotent Progenitor Cells in Gingival Connective Tissue. Tissue Eng. Part A 16, 2891–2899 (2010). [DOI] [PubMed] [Google Scholar]
- Roberts A. B. et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc. Natl. Acad. Sci. USA 83, 4167–4171 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignotz R. A. & Massague J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J. Biol. Chem. 261, 4337–4345 (1986). [PubMed] [Google Scholar]
- Schor S. L. et al. Subpopulations of fetal-like gingival fibroblasts: characterisation and potential significance for wound healing and the progression of periodontal disease. Oral Dis. 2, 155–166 (1996). [DOI] [PubMed] [Google Scholar]
- Smith R. S., Smith T. J., Blieden T. M. & Phipps R. P. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. The American journal of pathology 151, 317–322 (1997). [PMC free article] [PubMed] [Google Scholar]
- Martin T. A., Harding K. & Jiang W. G. Matrix-bound fibroblasts regulate angiogenesis by modulation of VE-cadherin. Eur. J. Clin. Invest. 31, 931–938 (2001). [DOI] [PubMed] [Google Scholar]
- el-Ghalbzouri A., Gibbs S., Lamme E., van Blitterswijk C. A. & Ponec M. Effect of fibroblasts on epidermal regeneration. The British journal of dermatology 147, 230–243 (2002). [DOI] [PubMed] [Google Scholar]
- Tsuboi R. & Rifkin D. B. Recombinant basic fibroblast growth factor stimulates wound healing in healing-impaired db/db mice. J. Exp. Med. 172, 245–251 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa O. et al. A Proline-Rich Peptide Mimic Effects of EMD in Rat Oral Mucosal Incisional Wound Healing. J. Periodontol. 86, 1386–1395 (2015). [DOI] [PubMed] [Google Scholar]
- Bosshardt D. D. Biological mediators and periodontal regeneration: a review of enamel matrix proteins at the cellular and molecular levels. J. Clin. Periodontol. 35, 87–105 (2008). [DOI] [PubMed] [Google Scholar]
- Grandin H. M. Enamel matrix derivative: a review of cellular effects in vitro and a model of molecular arrangement and functioning. Tissue Eng. Part B Rev. 18, 181–202 (2011). [DOI] [PubMed] [Google Scholar]
- Lyngstadaas S. P., Lundberg E., Ekdahl H., Andersson C. & Gestrelius S. Autocrine growth factors in human periodontal ligament cells cultured on enamel matrix derivative. J. Clin. Periodontol. 28, 181–188 (2001). [DOI] [PubMed] [Google Scholar]
- Parkar M. H. & Tonetti M. Gene expression profiles of periodontal ligament cells treated with enamel matrix proteins in vitro: analysis using cDNA arrays. J. Periodontol. 75, 1539–1546 (2004). [DOI] [PubMed] [Google Scholar]
- Villa O. et al. Subfractions of enamel matrix derivative differentially influence cytokine secretion from human oral fibroblasts. J. Tissue Eng. 6, 1–11 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannobile W. V., Al-Shammari K. F. & Sarment D. P. Matrix molecules and growth factors as indicators of periodontal disease activity. Periodontol. 2000 31, 125–134 (2003). [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J. & Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science 241, 708–712 (1988). [DOI] [PubMed] [Google Scholar]
- Schreiber A. B., Winkler M. E. & Derynck R. Transforming growth factor-alpha: a more potent angiogenic mediator than epidermal growth factor. Science 232, 1250–1253 (1986). [DOI] [PubMed] [Google Scholar]
- Schultz G. et al. Epithelial wound healing enhanced by transforming growth factor-alpha and vaccinia growth factor. Science 235, 350–352 (1987). [DOI] [PubMed] [Google Scholar]
- Mogi M. et al. Interleukin 1β, interleukin 6, β2-microglobulin, and transforming growth factor-α in gingival crevicular fluid from human periodontal disease. Arch. Oral Biol. 44, 535–539 (1999). [DOI] [PubMed] [Google Scholar]
- Engelhardt E. et al. Chemokines IL-8, GROalpha, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am. J. Pathol. 153, 1849–1860 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallucci R. M. et al. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. FASEB J. 14, 2525–2531 (2000). [DOI] [PubMed] [Google Scholar]
- Nokhbehsaim M. et al. Anti-inflammatory effects of EMD in the presence of biomechanical loading and interleukin-1beta in vitro. Clin. Oral Investig. 16, 275–283 (2012). [DOI] [PubMed] [Google Scholar]
- Liechty K. W., Adzick N. S. & Crombleholme T. M. Diminished interleukin 6 (IL-6) production during scarless human fetal wound repair. Cytokine 12, 671–676 (2000). [DOI] [PubMed] [Google Scholar]
- Liechty K. W., Crombleholme T. M., Cass D. L., Martin B. & Adzick N. S. Diminished interleukin-8 (IL-8) production in the fetal wound healing response. J. Surg. Res. 77, 80–84 (1998). [DOI] [PubMed] [Google Scholar]
- Gillitzer R. & Goebeler M. Chemokines in cutaneous wound healing. J. Leukoc. Biol. 69, 513–521 (2001). [PubMed] [Google Scholar]
- Ogata Y., Niisato N., Sakurai T., Furuyama S. & Sugiya H. Comparison of the characteristics of human gingival fibroblasts and periodontal ligament cells. J. Periodontol. 66, 1025–1031 (1995). [DOI] [PubMed] [Google Scholar]
- Hattingh J. & Ho E. The concentration of proteins in human gingival crevicular fluid. J. Periodontal Res. 15, 90–95 (1980). [DOI] [PubMed] [Google Scholar]
- Pinheiro M. L. B. et al. Quantification and localization of platelet-derived growth factor in gingiva of periodontitis patients. J. Periodontol. 74, 323–328 (2003). [DOI] [PubMed] [Google Scholar]
- Giannopoulou C., Andersen E., Brochut P., Plagnat D. & Mombelli A. Enamel matrix derivative and systemic antibiotics as adjuncts to non-surgical periodontal treatment: biologic response. J. Periodontol. 77, 707–713 (2006). [DOI] [PubMed] [Google Scholar]
- Green R. J., Usui M. L., Hart C. E., Ammons W. F. & Narayanan A. S. Immunolocalization of platelet-derived growth factor A and B chains and PDGF-α and β receptors in human gingival wounds. J. Periodontal Res. 32, 209–214 (1997). [DOI] [PubMed] [Google Scholar]
- Becerik S., Öztürk V. Ö., Atmaca H., Atilla G. & Emingil G. Gingival crevicular fluid and plasma acute-phase cytokine levels in different periodontal diseases. J. Periodontol. 83, 1304–1313 (2012). [DOI] [PubMed] [Google Scholar]
- Dongari-Bagtzoglou A. I. & Ebersole J. L. Increased presence of interleukin-6 (IL-6) and IL-8 secreting fibroblast subpopulations in adult periodontitis. J. Periodontol. 69, 899–910 (1998). [DOI] [PubMed] [Google Scholar]
- Christiansson L. et al. The use of multiplex platforms for absolute and relative protein quantification of clinical material. EuPA Open Proteomics 3, 37–47 (2014). [Google Scholar]
- Lesaffre E., Philstrom B., Needleman I. & Worthington H. The design and analysis of split-mouth studies: what statisticians and clinicians should know. Stat. Med. 28, 3470–3482 (2009). [DOI] [PubMed] [Google Scholar]
- Guentsch A. et al. Comparison of gingival crevicular fluid sampling methods in patients with severe chronic periodontitis. J. Periodontol. 82, 1051–1060 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. B., Streckfus C. F., Dai X. & Tucci M. A. Protein recovery from several paper types used to collect gingival crevicular fluid. J. Periodontal Res. 34, 283–289 (1999). [DOI] [PubMed] [Google Scholar]
- Griffiths G. S. Formation, collection and significance of gingival crevice fluid. Periodontol. 2000 31, 32–42 (2003). [DOI] [PubMed] [Google Scholar]
- Goodson J. M. Gingival crevice fluid flow. Periodontol. 2000 31, 43–54 (2003). [DOI] [PubMed] [Google Scholar]
- Sculean A. et al. The effect of postsurgical antibiotics on the healing of intrabony defects following treatment with enamel matrix proteins. J. Periodontol. 72, 190–195 (2001). [DOI] [PubMed] [Google Scholar]
- Herrera D., Alonso B., León R., Roldán S. & Sanz M. Antimicrobial therapy in periodontitis: the use of systemic antimicrobials against the subgingival biofilm. J. Clin. Periodontol. 35, 45–66 (2008). [DOI] [PubMed] [Google Scholar]
- Wohlfahrt J. C., Aass A. M., Granfeldt F., Lyngstadaas S. P. & Reseland J. E. Sulcus fluid bone marker levels and the outcome of surgical treatment of peri-implantitis. J. Clin. Periodontol. 41, 424–431 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.