Abstract
Repeated stressful events are associated with the onset of major depressive disorder (MDD). We previously showed oligodendrocyte (OL)-specific activation of the serum/glucocorticoid-regulated kinase (SGK)1 cascade, increased expression of axon-myelin adhesion molecules, and elaboration of the oligodendrocytic arbor in the corpus callosum of chronically stressed mice. In the current study, we demonstrate that the nodes and paranodes of Ranvier in the corpus callosum were narrower in these mice. Chronic stress also led to diffuse redistribution of Caspr and Kv 1.1 and decreased the activity in white matter, suggesting a link between morphological changes in OLs and inhibition of axonal activity. OL primary cultures subjected to chronic stress resulted in SGK1 activation and translocation to the nucleus, where it inhibited the transcription of metabotropic glutamate receptors (mGluRs). Furthermore, the cAMP level and membrane potential of OLs were reduced by chronic stress exposure. We showed by diffusion tensor imaging that the corpus callosum of patients with MDD exhibited reduced fractional anisotropy, reflecting compromised white matter integrity possibly caused by axonal damage. Our findings suggest that chronic stress disrupts the organization of the nodes of Ranvier by suppressing mGluR activation in OLs, and that specific white matter abnormalities are closely associated with MDD onset.
Major depressive disorder (MDD) is a multifactorial disease arising from both environmental and genetic factors; however, the responsible genes and mechanisms of pathogenesis are not fully known. Among many potential environmental factors, repeated stressful events have been linked to the onset of MDD1. Recently, brain imaging and postmortem evaluations of human brain tissue revealed that patients with MDD have white matter or oligodendrocyte (OL) abnormalities2. In addition, rodent models of stress have found decreased numbers of cortical and amygdalar OLs and neural/glial antigen (NG)2-positive OL precursor cells (OPCs) in these individuals3,4, suggesting potential links between disturbed myelination and mood disorders.
We previously reported that chronically stressed mice with elevated plasma levels of corticosterone exhibit increased serum/glucocorticoid-inducible kinase (SGK)1 phosphorylation in OLs located in nerve fiber bundles such as the corpus callosum5,6,7. We also found that the expression of adhesion molecules such as N-cadherin and α- and β-catenin was upregulated and that OL morphology was altered by chronic stress5,6,7. Conduction velocity has been shown to be affected by glutamate-induced modulation of mature OL activity8, and that the resting membrane potential of OLs is regulated by cyclic (c)AMP level9. These findings suggest that SGK1 phosphorylation is associated with the regulation of OL metabotropic glutamate receptor (mGluR) activity, which is known to regulate cAMP levels.
In order to clarify the relationship between excessive arborization of oligodendrocytic processes in rodent models of MDD and the molecular pathogenesis of this disorder in humans, in this study, we investigated the mechanisms underlying myelin-axon interactions at nodes and paranodes of the corpus callosum. To this end, we analyzed the expression of several adhesion molecules and channels identified in the nodes of Ranvier in mice by immunohistochemistry10,11,12. We also examined the corpus callosum of MDD patients by diffusion tensor imaging (DTI) to determine whether white matter abnormalities similar to those in mice exist in humans. Our results suggest that axonal damage due to dysregulation of OLs and nodes of Ranvier is closely associated with the development of MDD.
Results
Chronic stress disrupts the morphology and region-specific distribution of proteins at the nodes and paranodes of Ranvier in the corpus callosum
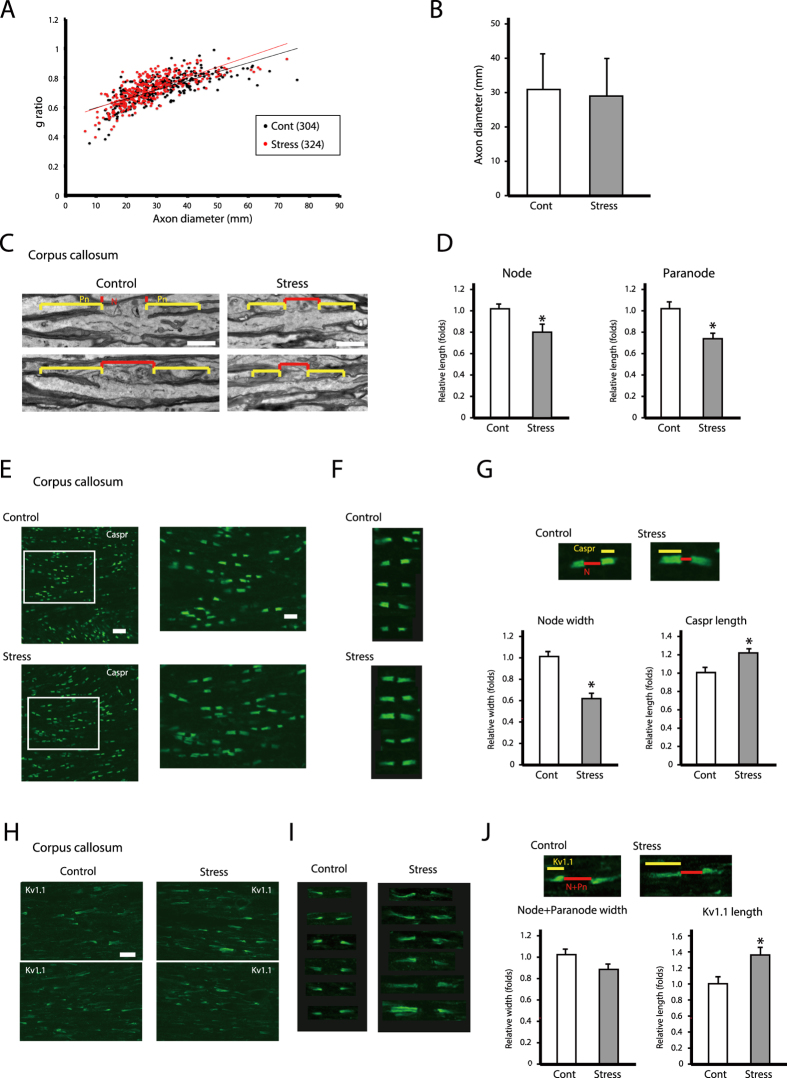
We previously reported that in the corpus callosum of mice exposed to chronic stress, oligodendrocytic processes are thicker, more numerous, and longer5,6,7. We hypothesized that these morphological changes affect the morphology and function of nodes of Ranvier and axonal projections. Firstly, to confirm the validity of our brain samples for structural analysis, we conducted g ratio analyses and measured the diameter of nerve fibers in the corpus callosum. There were no significant changes in the g ratio or average diameter of the nerve fibers in the corpus callosum of stress-exposed mice relative to the controls (Fig. 1A,B). We therefore carried out comparative ultrastructural analyses of the nodes of Ranvier at the same brain levels in chronically stressed and control mice. Chronic stress reduced the lengths of axon nodes and paranodes in the corpus callosum by approximately 20% relative to those in control mice (Fig. 1C,D). We did not find any additional lesions such as hypo- or demyelination in the corpus callosum of either group (Fig. 1A–D). These results indicated that decreases of nodal and paranodal lengths in the corpus callosum might be induced by chronic stress exposure.
Figure 1. Chronic stress exposure causes morphological alterations in nodes and paranodes of Ranvier of the corpus callosum.
(A) Axon size and myelin thickness measured by g ratio (the ratio of the inner axonal diameter to the total outer diameter). The distribution of the g ratio is plotted as a function of axonal diameter. The results are expressed as the mean of six images (Cont; 304 axons, Stress; 324 axon) obtained from three independent experiments. *P < 0.05, Student’s t test. Cont; non-stressed control mice data, Stress; chronic stress-exposed mice data. (B) The distribution of the axon diameters of the corpus callosum in the control and repeated WIRS-exposed mice was assessed. Results are expressed as the mean of six images (300 nodes of Ranvier) obtained from three independent experiments. The results are expressed as the mean ± SEM. *P < 0.05, Student’s t test. (C) Electron micrographs of nodes (N) and paranodes (Pn) of Ranvier in longitudinal sections of the corpus callosum from control (left panels) and chronically stressed (right panels) mice. Scale bars, 1 μm. (D) Measurement of node width and paranode length. Results are expressed as mean ± SEM of six measurements from three independent experiments. *P < 0.05, Student’s t test. (E,H) Immunohistochemical analyses of Caspr (E) and K v1.1 (H) expression in longitudinal sections of the corpus callosum from control (upper panels) and chronically stressed (lower panels) mice. Scale bar, 20 μm. (F,I) Representative images of Caspr (F) and K v1.1 (I) expression. Scale bar, 5 μm. (G,J) Measurement of node width and the length of Caspr-labeled regions at paranodes (G), and node and paranode width and length of K v1.1-labeled regions at juxtaparanodes (J). Results are expressed as the mean of six images (300 nodes of Ranvier) obtained from three independent experiments. Cas, Caspr-labeled region; N, node region; K v, K v1.1-labeled region; N + Pn, node and paranode region. *P < 0.05, Student’s t test.
Previous studies have reported several region-specific adhesion molecules and channels at the nodes of Ranvier10,11,12. To investigate their roles in the morphological changes induced by chronic stress exposure, we examined the expression of contactin-associated protein (Caspr) and voltage-dependent potassium channel (K v)1.1 (paranodal and juxtaparanodal protein, respectively) in the corpus callosum.
Areas of Caspr immunoreactivity in the paranodes of Ranvier were smaller in chronically stressed as compared to control mice (Fig. 1E,F). Node width, defined as the length of Caspr-positive structures, was reduced by 55% in stressed as compared to control mice (Fig. 1G, Node width). However, the distribution of the Caspr-positive area was more diffuse in the stress group than in controls (Fig. 1G, Caspr length).
Similar to the distribution of Caspr, areas of K v1.1 immunoreactivity in juxtaparanodes of Ranvier were smaller in chronically stressed mice than in control mice (Fig. 1H,I): the nodes and paranodes of Ranvier in the former group were approximately 0.85 times the length of the same regions in the latter group (Fig. 1J; Node + Paranode width). However, the distribution of K v1.1—that is, the K v1.1 cluster—was significantly more diffuse in stressed as compared to control mice (Fig. 1J; K v.1.1 length).
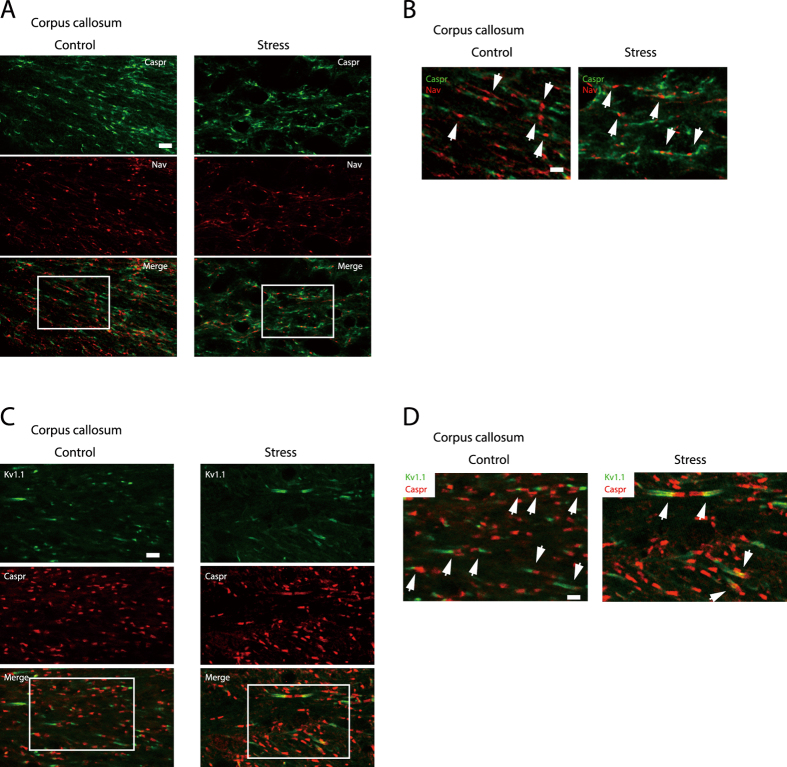
Caspr and Kv1.1 expression domains overlap in corpus callosum boundary regions in chronically stressed mice
To determine whether narrowing of the nodal regions affects Nav localization, we examined voltage-dependent sodium channel (Nav) and Caspr expression in the nodal and paranodal regions, respectively, by immunocytochemistry12,13,14. Nav localization was distinct from Caspr clusters in the corpus callosum of both control and chronically stressed mice (Fig. 2A,B).
Figure 2. Increase in the length of Caspr- and K v1.1-immunoreactive regions at paranodes and juxtaparanodes of the corpus callosum upon chronic stress exposure.
(A,C) Immunohistochemical analyses of Nav and Caspr (A), and K v1.1 and Caspr (C) expression in longitudinal sections of the corpus callosum from control (left panels) and chronically stressed (right panels) mice. Scale bar, 10 μm. (B,D) Enlargement of the areas enclosed by squares in (A,C). Scale bar, 5 μm. Arrows indicate Nav- and Caspr-positive (B) and K v1.1- and Caspr-positive (D) boundary regions.
To clarify the relationship between the narrowing of paranodal regions and the diffuse distributions of Caspr and K v1.1, the expression of these two molecules in the paranodal and juxtaparanodal regions was examined by immunocytochemistry11,13,15. In the corpus callosum of control mice, Caspr and K v1.1 were expressed in distinct domains (Fig. 2C,D; Control); however, in stressed mice, the distribution of the two proteins overlapped at paranode/juxtaparanode boundaries (Fig. 2C,D; Stress).
Adhesion molecule expression levels of adjacent nodes of Ranvier are altered after chronic stress exposure
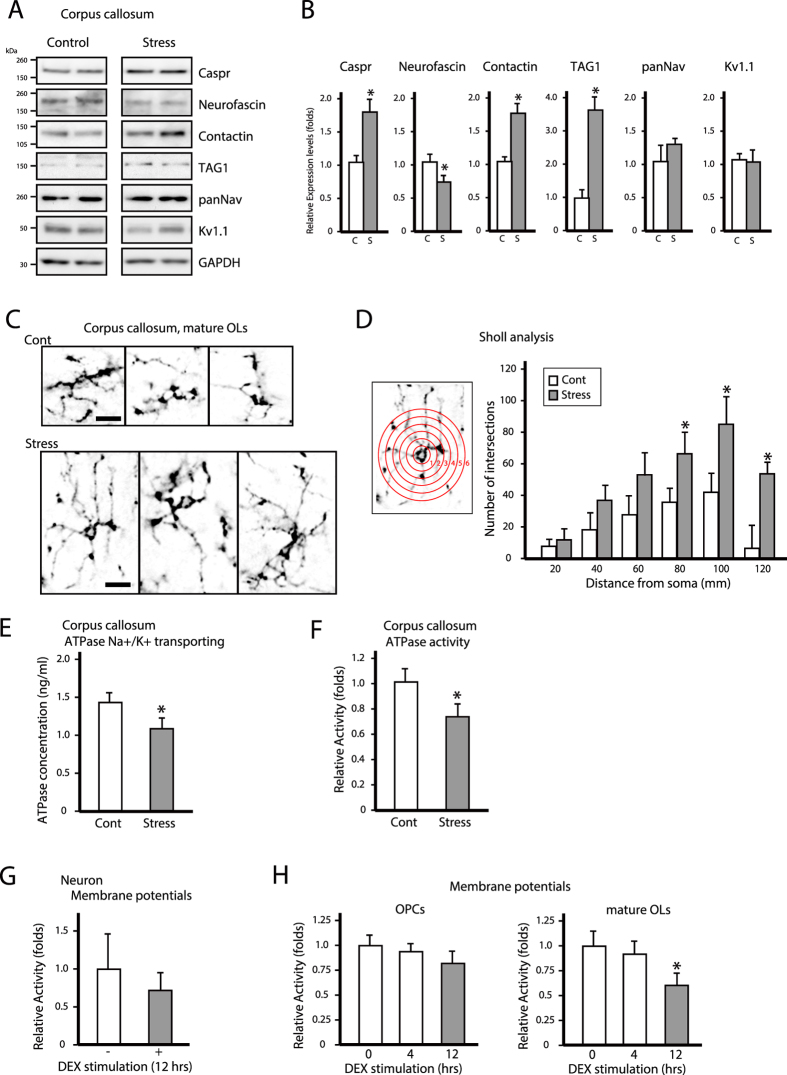
We next investigated the expression levels of adhesion molecules required for axon-myelin interactions13,16,17,18,19,20. We obtained protein samples not only from the corpus callosum, but also from the frontal cortex and hippocampus for western blotting using a brain slicer. The expression levels of 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) and N-myc downstream regulated (NDRG)1 (myelin and OL markers, respectively) in the corpus callosum were significantly higher than in the frontal cortex and hippocampus (Supplementary Fig. S1A,B). Furthermore, neuronal nuclei (a neural marker) expression was scarcely detected in corpus callosum samples (Supplementary Fig. S1A,B), confirming that they were suitable for examining protein expression levels in specific regions of the corpus callosum.
Nav and K v1.1 expression was unaltered in the corpus callosum, whereas levels of the paranodal proteins Caspr and contactin as well as the juxtaparanodal protein transient axonal glycoprotein 1 (TAG1) were upregulated and that of total neurofascin (155 and 186 isoforms) was downregulated in response to chronic stress (Fig. 3A,B). These results indicate that chronic stress disrupts normal axon-myelin adhesion.
Figure 3. Chronic stress exposure leads to upregulation of adhesion molecules at adjacent nodes of Ranvier, causes morphological changes in mature oligodendrocytes, and decreases axonal activity in the corpus callosum.
(A) Western blot analysis of the expression of adhesion molecules (Caspr, total neurofascin, contactin, TAG1) and channels (Nav and K v1.1) at adjacent nodes of Ranvier in the corpus callosum of control and chronically stressed mice. (B) Quantification of protein bands from (A). Data are expressed as mean ± SEM of at least three independent experiments. *P < 0.05, Student’s t test. (C) Representative images of the processes of oligodendrocytes in control (Cont) and chronic stress exposed (Stress) mice. Scale bar, 40 μm. (F) Process complexity and branching are indicated by Sholl analysis. Quantification of intersection numbers within each radius (1–6). Results are the mean of six images obtained from three independent experiments and are expressed as the mean ± SEM. *P < 0.05, Student’s t test. (E,F) Na+/K+ transporting ATPase levels (E) and Na+/K+-ATPase activity (F) in the corpus callosum of control and chronically stressed mice. Data are expressed as mean ± SEM of at least three independent experiments. *P < 0.05, Student’s t test. (G,H) Time course of membrane potential activities of OPCs and mature OLs primary cultures (G) and membrane potential activities of 12 h DEX administration primary neurons (H) after 100 μM (G) or 10 μM (H) dexamethasone (DEX) administration. Data are expressed as mean ± SEM of at least three independent experiments. *P < 0.05, Student’s t test.
Chronic stress induces morphological changes in mature OLs decreases axonal activity of the corpus callosum
We sought to determine whether oligodendrocytic processes induced the axonal branching that was observed following chronic stress exposure. Immature OLs or OPCs were distinguished from mature OLs by the expression of NG2 and adenomatous polyposis coli (APC), respectively21,22. The morphological complexity of OL processes was quantified by Sholl analysis23,24. APC-positive OL processes in the corpus callosum of stressed mice were more numerous and thicker than those in control mice (Fig. 3C,D). Furthermore, APC-labeled processes extended longer distances in stressed mice than in controls (Fig. 3C,D and Supplementary Fig. S2A; APC columns). However, chronic stress exposure had no effect on the number or morphology of NG2-labeled OPCs of the corpus callosum (Supplementary Fig. S2A; NG2 columns). These results indicate that chronic stress affects the structure of mature but not immature OLs. Moreover, chronic stress did not lead to microglia activation nor did it increase the number astrocytes in the corpus callosum (Supplementary Fig. S2B).
Since axonal integrity is essential for action potential propagation, any disruption in axon structure is likely to affect its activity. As a measure of axonal integrity, we investigated adenosine triphosphatase (ATPase) concentrations (Na+/K+ transporting) and ATPase activity in the corpus callosum25,26,27,28,29. Chronic stress exposure significantly decreased both Na+/K+-ATPase concentrations and activity in the fiber tract of the corpus callosum (Fig. 3E,F). Furthermore, to investigate the axonal membrane activity, we examined the membrane potentials of the differentiated neuronal cells by using fluorescent dye30. The membrane potentials of differentiated neuronal cells decreased after 12 hrs of chronic stress, mimicked by treatment with the synthetic glucocorticoid dexamethasone (DEX) (Fig. 3G). We further examined membrane potentials in the undifferentiated OPC cells and mature OLs. The undifferentiated OPC cells membrane potentials did not show significant change after 12 hrs of DEX stimulation (Fig. 3H). In contrast, membrane potentials of differentiated OLs were significantly decreased after 12 hrs of DEX stimulation (Fig. 3H). These results suggested that chronic stress exposure induced morphological changes in the processes of mature OLs and compromised mature OL membrane function in the corpus callosum. Furthermore, these structural and functional problems in mature OLs might affect axonal activities.
SGK1 nuclear translocation reduces OL activity by suppressing mGluR expression
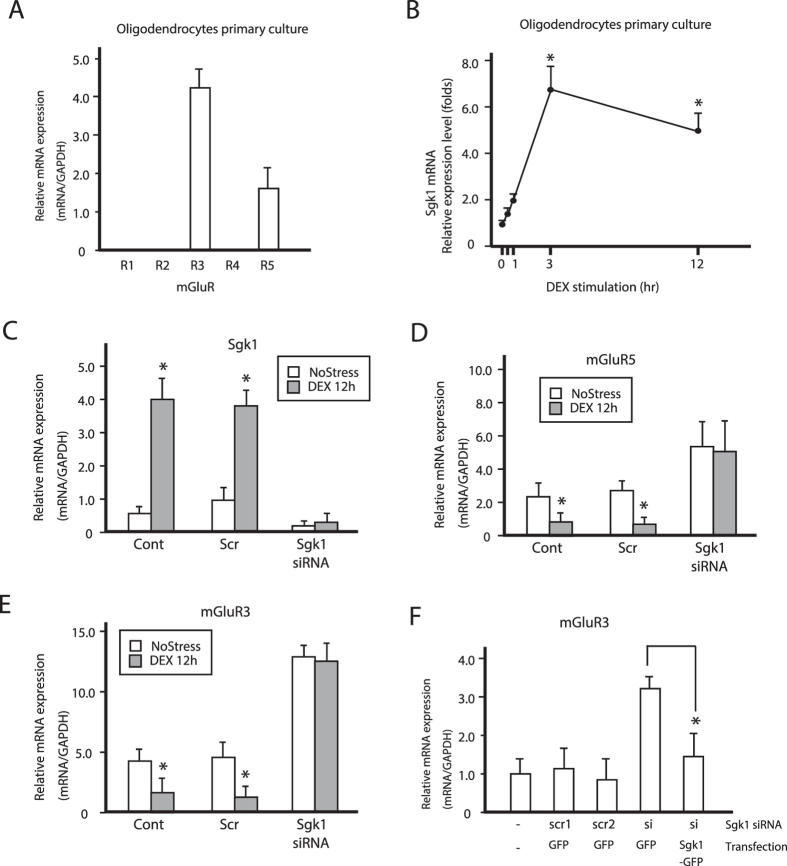
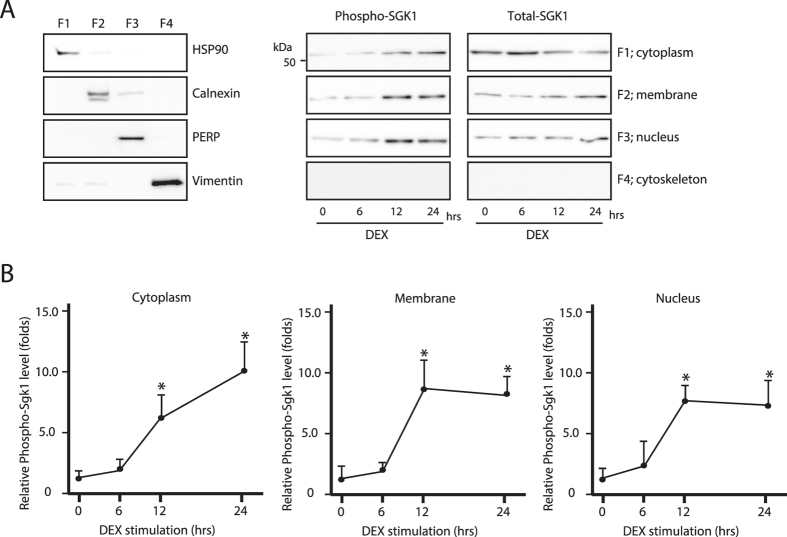
Glutamate is a major excitatory neurotransmitter in the mammalian central nervous system and modulates mature OL activity8,31. Previous reports showed that mGluRs are therapeutic targets for the treatment of mood disorders31. Several subtypes of mGluRs are associated with adenylate cyclase activity and cAMP levels and are responsible for signal propagation in the central nervous system31,32,33. The regulation of cAMP level is linked to the resting membrane potential of OLs9. To determine how morphological changes in OLs modulate neuronal activity, we examined the expression of mGluRs, which are known to regulate cAMP levels in OLs34. mGluR3 and 5 mRNA was expressed in primary cultured OLs (Fig. 4A); transcript levels decreased after chronic DEX administration (Fig. 4D,E). In contrast, Sgk1 mRNA expression increased after chronic stress exposure (Fig. 4B,C). Chronic stress has been shown to induce the phosphorylation and activation of SGK1 in OLs5,6,7. Upon DEX treatment, SGK1 was phosphorylated and translocated to the nucleus (Fig. 5A,B). Nuclear SGK1 repressed the transcription of mGluR3 and 5, since Sgk1 knockdown resulted in the upregulation of both transcripts (Fig. 4D,E), an effect that was reversed by SGK1 overexpression (Fig. 4F). In addition, the phosphorylation level of NDRG1 in the corpus callosum was increased after chronic stress exposure, while mGluR3 protein expression was downregulated in the corpus callosum (Fig. 4G,H).
Figure 4. Chronic stress suppresses mGluR3 and 5 expressions in primary oligodendrocytes.
(A) mGluR1 to 5 mRNA expression in oligodendrocytes. Data are expressed as mean ± SEM of at least three independent experiments. *P < 0.05, Student’s t test. (B) Time course of Sgk1 mRNA expression in oligodendrocytes after 100 μM DEX administration. Data are expressed as mean ± SEM of at least three independent experiments. *P < 0.05, Student’s t test. (C–F) Real-time PCR analysis of Sgk1 (C), mGluR5 (D), and mGluR3 (E,F) mRNA expression in primary oligodendrocytes. Data are expressed as mean ± SEM of at least three independent experiments. *P < 0.05, Student’s t test.
Figure 5. Chronic stress induces nuclear translocation of phospho-SGK1.
(A) Western blot analysis of total and phosphorylated SGK1 protein levels in cytoplasmic (F1), membrane (F2), nuclear (F3), and cytoskeletal (F4) fractions of primary oligodendrocytes after treatment with 100 μM DEX. (B) Quantification of protein bands from (A). Data are expressed as mean ± SEM of at least three independent experiments. *P < 0.05, Student’s t test.
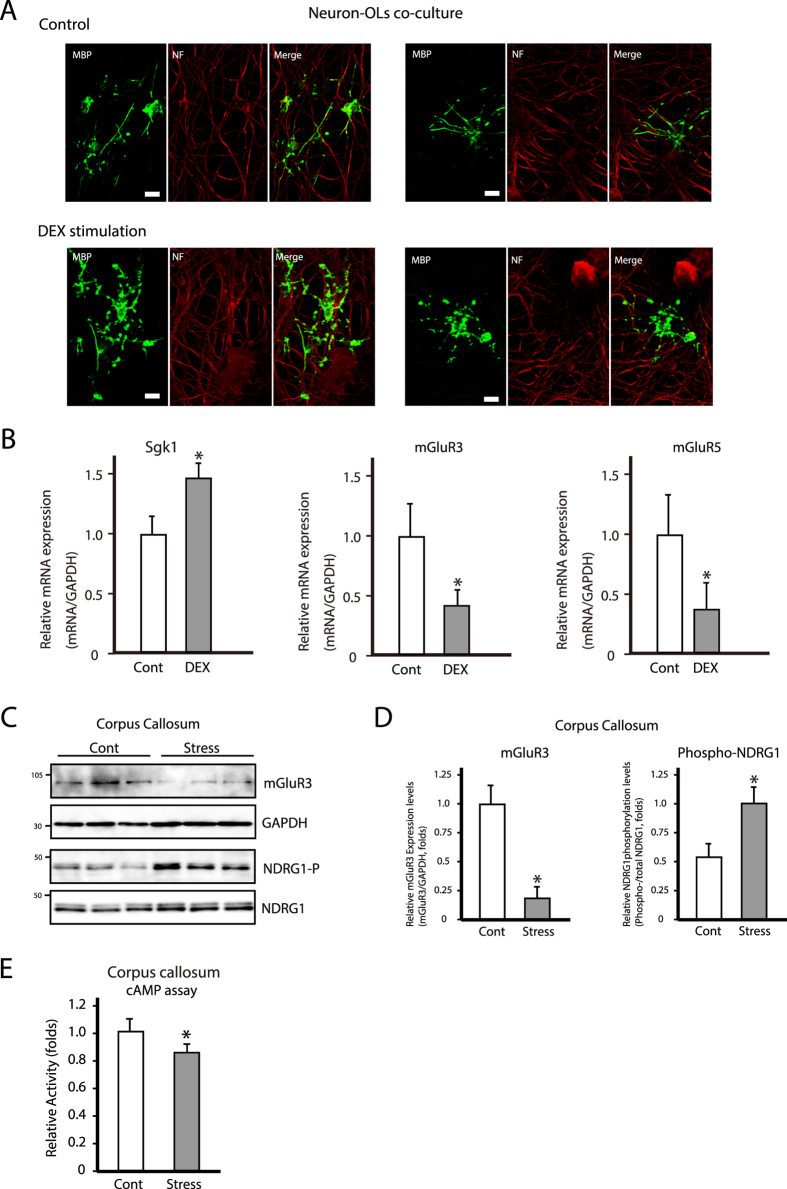
To investigate the relationship between neuronal axons and mature OLs, we established a DRG neuron and OL co-culture system35. We investigated mature OL processes and myelin-like sheath formations with and without chronic DEX stimulation. Control mature OLs had several branched processes and myelin-like sheath formations (Fig. 6A; control columns). However, after chronic DEX administration, mature OL processes were more complex and longer than those in control conditions, and the formation levels of myelin-like sheath in chronic stressed conditions decreased in comparison with those in control conditions (Fig. 6A; DEX stimulation columns). We further examined mature OL stress levels by measuring Sgk1, mGluR3, and -5 mRNA expression levels in co-culture conditions. Chronic DEX administration increased Sgk1 mRNA expression in these co-cultures, while both mGluR3 and -5 mRNA levels were decreased, as observed in primary OL cultures exposed to chronic stress (Fig. 6B).
Figure 6. Chronic stress decreases mGluR3 and 5 expressions in a co-culture system and in the corpus callosum, and cAMP level in the corpus callosum.
(A) Representative images of immunocytochemical analysis of Neurofilament (NF) and MBP in oligodendrocyte and DRG-neuron co-culture system after treatment with (Cont) or without (DEX stimulations) 10 μM DEX for 12 hrs. Scale bar = 20 μm. (B) Sgk1, mGluR3, and -5 mRNA expression in co-culture system. Data are expressed as mean ± SEM of at least three independent experiments. *P < 0.05, Student’s t test. (C) Western blot analysis of mGluR3 and phosphorylated NDRG1 (oligodendrocyte stress marker) in the corpus callosum in control (Cont) and chronic stress-exposed (Stress) mice. (D) Quantification of protein bands from (C). Data are expressed as mean ± SEM of at least three independent experiments. *P < 0.05, Student’s t test. (E) cAMP levels in the corpus callosum of control (Cont) and chronically stressed (Stress) mice.
We next investigated the relationship between mGluRs protein expression and cAMP levels in the corpus callosum. NDRG1 phosphorylation was increased in the corpus callosum after chronic stress exposure in mice (Fig. 6C,D)5 while mGluR3 and cAMP levels were decreased (Fig. 6C–E). These results suggest that chronic stress can suppress OL activity in the corpus callosum by downregulating mGluR expression, and might correspondingly change axon-mature OL interactions.
MDD patients exhibit axonal white matter abnormalities in the corpus callosum
Our findings in the mouse model indicated that microstructural damage to white matter tracts may predispose humans to depressive symptoms. To investigate this possibility, we used DTI to examine the microstructure of fiber tracts in the corpus callosum of MDD patients2,36,37. DTI combines a conventional magnetic resonance imaging sequence with additional magnetic field gradients to quantify water diffusion by fractional anisotropy (FA), which is a measure of how diffusion is directionally hindered; this allows an assessment of brain white matter integrity.
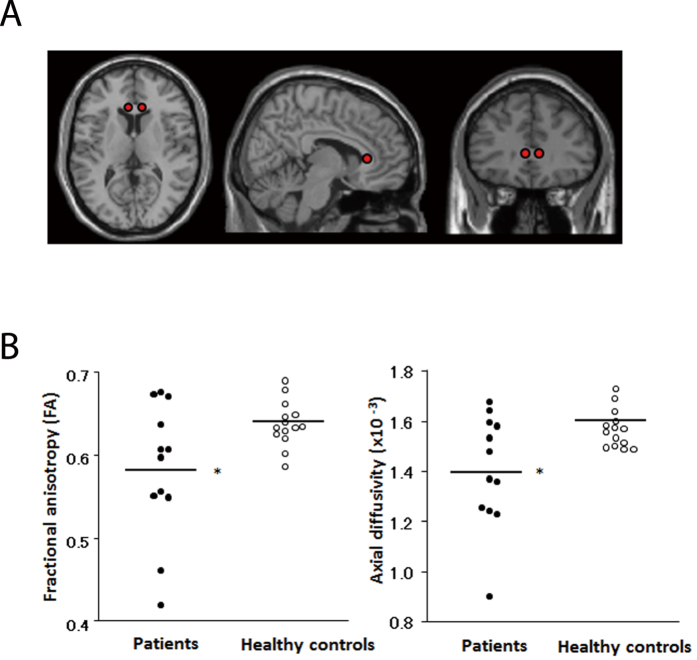
In the voxel-based analysis of FA values, there was no difference between patients and healthy controls (P < 0.001, uncorrected). However, by analyzing volumes of interest (VOI) on FA maps of the anterior corpus callosum—where stress-induced abnormalities were observed in mice—we found significantly lower FA values in MDD patients. We also detected reduced axial diffusivity in treatment-naive MDD patients (Fig. 7, Table 1). Taken together, our findings suggest that chronic stress can lead to axonal abnormalities that may underlie the development of MDD.
Figure 7. Lower fractional anisotropy (FA) values are observed in patients with MDD compared to healthy control subjects by DTI.
(A) Spherical voxels of interest (VOIs) placed on the anterior genu of the corpus callosum and (B) scatter plots of FA and axial diffusivity values in this region are shown for patients with MDD and controls. *Significantly lower FA and axial diffusivity values were observed in patients than in controls. Values were adjusted to the mean values of age and gender.
Table 1. Differences in values of FA and axial/radial diffusivity in anterior genu of corpus callosum between patients and healthy control subjects.
| FA and axial/radial diffusivity | Patients (n = 12) | Healthy controls (n = 14) | Analysis of Covariancea |
|
|---|---|---|---|---|
| F (1, 22) | P | |||
| FA | 0.58 ± 0.08 | 0.64 ± 0.03 | 6.79 | 0.02 * |
| Axial diffusivity (×10−3) | 1.41 ± 0.21 | 1.57 ± 0.08 | 5.35 | 0.007 ** |
| Radial diffusivity (×10−3) | 0.54 ± 0.06 | 0.52 ± 0.07 | 1.16 | 0.29 |
Data are mean ± sd. *p < 0.05, **p < 0.01.
aAge and gender are entered as covariates.
Discussion
Many diseases of the nervous system attack non-neuronal cells and indirectly affect the function and integrity of neurons. In the current study, we found that exposure to chronic stress leads to morphological alterations in myelinating OLs of the corpus callosum in mice. Altered oligodendrocytic activity and structure disrupted axon-myelin adhesion at paranodes and nodes of Ranvier, as evidenced by the diffuse distribution of key proteins and reduced axonal activity. Patients with MDD also showed white matter abnormalities in their corpus callosum. We therefore propose that chronic stress undermines axon-myelin interactions and reduces axonal activity, possibly triggering disorders such as MDD.
Neurofascin-deficient mice showed disruption of node and paranode complexes and reduced neural function38. Chronic stress induced abnormalities at paranode/juxtaparanode of Ranvier boundary regions in the corpus callosum, although the functional significance of these findings is unclear39,40. Caspr- and contactin-deficient mice showed junctional disruptions13,16,17,18,19,20. Despite the decrease in the width of nodes of Ranvier in chronically stressed mice, the structure of nodal regions and Nav clusters were preserved by the adherence of paranodal loops12,13,14,16,17,18,19,20. The upregulation of Caspr and contactin may account for the maintenance of nodal boundaries following chronic stress. The diffuse distribution of Caspr and K v1.1 in these boundary regions may alter neuronal activity and axonal conduction velocity, although the latter is reportedly unaffected by diffuse K v1.1 distribution at internodes11,15,41. We observed an overlap between Caspr and K v1.1 expression domains in boundary regions. Hence, it can be supposed that Caspr upregulation and alterations in the boundary regions modulate axonal conduction velocity or the regulation of neuronal activity by OLs.
Active signal transduction between myelinated axons and the myelin sheath of mature OLs has only recently been recognized8,42,43. Up to 60 axons can be myelinated simultaneously by a single mature OL44, which can thus potentially regulate neural transmission in a number of axons within the same fiber assembly. Several recent studies have shown that in animal models of major depression, stressful experiences decreased OL proliferation in the frontal cortex and amygdala3,4. Interestingly, OLs are reportedly a target of plasma corticosterone4,45,46,47. Sgk1 is a glucocorticoid receptor-regulated gene; SGK1 phosphorylation in OLs resulted in its nuclear translocation and negative regulation of mGluR transcription, resulting in decreased cAMP levels in OLs after stress exposure8,9. Previous reports have suggested mGluR2/3 and 5 as novel therapeutic targets for mood disorders such as MDD and schizophrenia, among others34,48,49,50. In various animal models of MDD, mGluR level has been linked to disease pathogenesis and symptoms51,52; for instance, mGluR deficiency had adverse effects on depressive symptoms49,53. The results of the present study demonstrate the critical importance of OL mGluR2/3 and 5 function in the treatment of depression, although the detailed molecular mechanisms of how they regulate cAMP levels and thereby contribute to the onset of MDD remain to be elucidated.
Given our observation that axon-myelin structure is disturbed in chronically stressed mice, we investigated whether patients with MDD—a disorder typically arising from repeated bouts of stress—have similarly compromised white matter integrity. As proposed by a previous study54, we used DTI to examine microstructural abnormalities in the white matter2,36,37 and found that compared to control subjects, patients with MDD showed reduced FA values in the anterior corpus callosum, as well as a trend of decreased axial but not radial diffusivity. Axonal damage markedly decreases axial diffusivity, while demyelination increases radial diffusivity55. Therefore, our findings suggest that chronic stress and MDD do not result in demyelination but rather in alterations to axonal structure, possibly caused by chronic stress-induced dysregulation of nodes of Ranvier and white matter disturbance.
The anterior genu of the corpus callosum is the major commissure connecting homologous regions of the left and right prefrontal, anterior cingulate, and insular cortices. Both the anterior cingulate cortex and insula have connections with limbic structures such as the amygdala, and are involved in affective processing and cognitive functioning56. Given that the corpus callosum connects brain regions in the two hemispheres that are important for emotional and cognitive functions, microstructural abnormalities in this region related to the loss of white matter integrity may negatively impact interhemispheric communication and trigger the onset of mood disorders.
In summary, the results of the present study highlight important parallels between abnormalities in fiber projections of patients with MDD and alterations in axonal structure, OL activity, and neural transmission in the chronic stress mouse model of MDD. We demonstrated that Na+/K+-ATPase activity in axons and cAMP level in OLs were decreased in mice subjected to chronic stress, and that treatment-naive patients with MDD had microstructural abnormalities in fiber projections. We also showed that disturbances in nodes, paranodes, and juxtaparanodes of Ranvier were not linked to demyelinating pathologies. A primary goal of future research will be to clarify the functional implications of these novel findings in the fiber tracts of stressed mice and patients with MDD.
Methods
Animals
All animal care and handling procedures were approved by the Institutional Animal Care and Use Committee of Kinki University (no. KAME-24-021). All animal experiments were carried out in accordance with the guidelines set forth in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA).
Chronic stress exposure
Mice were exposed to chronic stress as previously described5 (details are provided in the Supplementary Information).
Immunohistochemistry
Immunohistochemical analysis was performed as previously described5,57. Briefly, sections were immersed in pre-warmed 0.1 M citrate buffer (pH 6.0) and boiled for 5 min at 95 °C–100 °C, then allowed to cool for 20 min. The sections were then rinsed with phosphate-buffered saline (PBS) for 30 min and incubated at 4 °C for 24 h with anti-Caspr, anti-K v1.1, anti-pan-Na, anti-CD11, anti-GFAP, anti-CC1, and anti-NG2 diluted 1:500 in PBS. The sections were then rinsed with phosphate-buffered saline (PBS) for 30 min and incubated at room temperature for 1 h with Alexa Fluor 488-conjugated goat anti-rabbit or anti-mouse IgG (Life Technologies, Carlsbad, CA, USA) diluted 1:500 in PBS. After a 1-h wash with PBS, sections were mounted on slides using PermaFluor (Thermo Scientific, Waltham, MA, USA) and visualized by confocal microscopy (LSM-510; Carl Zeiss, Oberkochen, Germany) under a 63× objective.
Electron microscopy
Mice were anesthetized and transcardially perfused with 4% paraformaldehyde and 2% glutaraldehyde (v/v) in 0.1 M PBS. The brain was dissected and dehydrated in a graded series of ethanol (50–100%), then embedded in Epon. Semi-thin sections (0.9 mm) were cut and stained with Toluidine Blue for examination by light microscopy. Ultra-thin sections (80 nm) were cut and stained with 2% uranyl acetate (v/v; Watson’s modified method) and Reynold’s lead citrate, and visualized using a Hitachi H-7650 transmission electron microscope (Tokyo, Japan). Digital images of coronal and longitudinal serial sections were acquired and analyzed using Image Pro Plus 3.0 software (Media Cybernetics, Rockville, MD, USA). The width of nodes and paranodes of Ranvier were counted in 300–1,000 fibers in corpus callosum sections. The g ratio was calculated from the obtained diameters of fibers and axons. Statistical analyses of the measured g ratios were performed using GraphPad Prism 6 (GraphPad, La Jolla, CA, USA).
DTI study
After providing a complete description of the study, written, informed consent was obtained from each subject. The study was approved by the medical ethics committee of the Osaka University Medical School and the National Cerebral and Cardiovascular Center in Japan, and has been performed in accordance with ethical standards outlined by the Declaration of Helsinki. Additional details are provided in the Supplementary Information.
Additional Information
How to cite this article: Miyata, S. et al. Association between chronic stress-induced structural abnormalities in Ranvier nodes and reduced oligodendrocyte activity in major depression. Sci. Rep. 6, 23084; doi: 10.1038/srep23084 (2016).
Supplementary Material
Acknowledgments
We thank Drs. K. Ikenaka, Y. Shigeyoshi, S. Shiosaka, S. Yoshida, and Y. Ishikawa for their valuable comments. We thank Ms. A. Matsumura, A. Kawakami, E. Hisamatsu, E. Kashima for their technical assistance. We would like to thank the members of the MRI facility of the Department of Investigative Radiology, National Cerebral and Cardiovascular Center in Japan for carrying out the acquisition of MRI data and caring for the subjects during the MRI procedures. This work was supported in part by the Japan Society for the Promotion of Science, a Grant-in-Aid for Scientific Research on Innovative Areas (KAKENHI; grant 26117519), a Grant-in-Aid for Scientific Research (C) (KAKENHI; grant 25430079, 15K06790), the Sakamoto Research Institute of Psychopathology, and the Global COE Program from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
Author Contributions S.M. and M.T. designed experiments, S.M., M.T., Y.K., F.Y., A.Y., H.I. and T.K. performed experiments, S.M., S.S., T.T., A.M. and T.K. analyzed data, and S.M. wrote the manuscript with input from all coauthors. S.M. oversaw the experimental process.
References
- Lupien S. J., McEwen B. S., Gunnar M. R. & Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 10, 434–445 (2009). [DOI] [PubMed] [Google Scholar]
- Tham M. W., Woon P. S., Sum M. Y., Lee T. S. & Sim K. White matter abnormalities in major depression: evidence from post-mortem, neuroimaging and genetic studies. J. Affect. Disord. 132, 26–36 (2011). [DOI] [PubMed] [Google Scholar]
- Czéh B. et al. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology 32, 1490–1503 (2007). [DOI] [PubMed] [Google Scholar]
- Banasr M. et al. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol. Psychiatry 62, 496–504 (2007). [DOI] [PubMed] [Google Scholar]
- Miyata S. et al. Plasma corticosterone activates SGK1 and induces morphological changes in oligodendrocytes in corpus callosum. PLoS one 6, e19859 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S., Hattori T., Shimizu S., Ito A. & Tohyama M. Disturbance of Oligodendrocyte Function Plays a Key Role in the Pathogenesis of Schizophrenia and Major Depressive Disorder. Biomed Res Inter 2015, 492367 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohyama M., Miyata S., Hattori T., Shimizu S. & Matsuzaki S. Molecular basis of major psychiatric diseases such as schizophrenia and depression. Anat Sci Int In press (2015). [DOI] [PubMed] [Google Scholar]
- Yamazaki Y. et al. Modulatory effects of oligodendrocytes on the conduction velocity of action potentials along axons in the alveus of the rat hippocampal CA1 region. Neuron Glia Biol 3, 325–334 (2007). [DOI] [PubMed] [Google Scholar]
- Bolton S. & Butt A. M. Cyclic AMP-mediated regulation of the resting membrane potential in myelin-forming oligodendrocytes in the isolated intact rat optic nerve. Exp Neurol 202, 36–43 (2006). [DOI] [PubMed] [Google Scholar]
- Nave K. A. Myelination and support of axonal integrity by glia. Nature 468, 244–252 (2010). [DOI] [PubMed] [Google Scholar]
- Poliak S. & Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nat. Rev. Neurosci. 4, 968–980 (2003). [DOI] [PubMed] [Google Scholar]
- Edgar N. & Sibille E. A putative functional role for oligodendrocytes in mood regulation. Transl. Psychiatry 2, e109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat M. A. et al. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron 30, 369–383 (2001). [DOI] [PubMed] [Google Scholar]
- Craner M. J. et al. Molecular changes in neurons in multiple sclerosis: altered axonal expression of Nav1.2 and Nav1.6 sodium channels and Na+/Ca2+ exchanger. Proc. Natl. Acad. Sci. USA 101, 8168–8173 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre-Sarrailh C. & Devaux J. J. Neuro-glial interactions at the nodes of Ranvier: implication in health and diseases. Front Cell Neurosci 7, 196 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M. E. et al. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron 30, 385–397 (2001). [DOI] [PubMed] [Google Scholar]
- Sherman D. L. et al. Neurofascins are required to establish axonal domains for saltatory conduction. Neuron 48, 737–742 (2005). [DOI] [PubMed] [Google Scholar]
- Pillai A. M. et al. Spatiotemporal ablation of myelinating glia-specific neurofascin (Nfasc NF155) in mice reveals gradual loss of paranodal axoglial junctions and concomitant disorganization of axonal domains. J. Neurosci. Res. 87, 1773–1793 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell O. W. et al. Disruption of neurofascin localization reveals early changes preceding demyelination and remyelination in multiple sclerosis. Brain 129, 3173–3185 (2006). [DOI] [PubMed] [Google Scholar]
- Rasband M. N. & Shrager P. Ion channel sequestration in central nervous system axons. J. Physiol. 525, 63–73 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. et al. Notch1 signaling plays a role in regulating precursor differentiation during CNS remyelination. Proc. Natl. Acad. Sci. USA 106, 19162–19167 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J. M., Reynolds R. & Fawcett J. W. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 24, 39–47 (2001). [DOI] [PubMed] [Google Scholar]
- Murtie J. C., Macklin W. B. & Corfas G. Morphometric analysis of oligodendrocytes in the adult mouse frontal cortex. J. Neurosci. Res. 85, 2080–2086 (2007). [DOI] [PubMed] [Google Scholar]
- Rajasekharan S. et al. Netrin 1 and Dcc regulate oligodendrocyte process branching and membrane extension via Fyn and RhoA. Development 136, 415–26 (2009). [DOI] [PubMed] [Google Scholar]
- McCarren M. & Alger B. E. Sodium–potassium pump inhibitors increase neuronal excitability in the rat hippocampal slice: role of a Ca2+-dependent conductance. J. Neurophysiol. 57, 496–509 (1987). [DOI] [PubMed] [Google Scholar]
- Tsuchiya Y., Hosokawa T. & Kasuya Y. Influence of ouabain on the cholinergic neurotransmission in the canine trachea. Jpn. J. Pharmacol. 53, 301–309 (1990). [DOI] [PubMed] [Google Scholar]
- Mulkey R. M. & Zucker R. S. Posttetanic potentiation at the crayfish neuro-muscular junction is dependent on both intracellular calcium and sodium ion accumulation. J. Neurosci. 12, 4327–4336 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. et al. Na, K-ATPase activity regulates AMPA receptor turnover through proteasome-mediated proteolysis. J. Neurosci. 29, 4498–4511 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames A. 3rd. CNS energy metabolism as related to function. Brain Res. Brain Res. Rev. 30, 42–68 (2000). [DOI] [PubMed] [Google Scholar]
- Fairless R. et al. Membrane potential measurements of isolated neurons using a voltage-sensitive dye. PLoS One 8, e58260 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson C. J. et al. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat. Rev. Drug Discov. 4, 131–144 (2005). [DOI] [PubMed] [Google Scholar]
- Ferraguti F. & Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 326, 483–504 (2006). [DOI] [PubMed] [Google Scholar]
- Ritter S. L. & Hall R. A. Fine-tuning of GPCR activity by receptor-interacting proteins. Nat. Rev. Mol. Cell Biol. 10, 819–830 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matosin N. et al. Metabotropic glutamate receptor mGluR2/3 and mGluR5 binding in the anterior cingulate cortex in psychotic and nonpsychotic depression, bipolar disorder and schizophrenia: implications for novel mGluR-based therapeutics. J. Psychiatry Neurosci. 39, 407–416 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen L. S., Chan C. W. & Ffrench-Constant C. Translation of myelin basic protein mRNA in oligodendrocytes is regulated by integrin activation and hnRNP-K. J. Cell Biol. 192, 797–811 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L. et al. Prefrontal white matter abnormalities in young adult with major depressive disorder: a diffusion tensor imaging study. Brain Res. 1168, 124–128 (2007). [DOI] [PubMed] [Google Scholar]
- Basser P. J. & Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. B. 111, 209–219 (1996). [DOI] [PubMed] [Google Scholar]
- Zonta B. et al. Glial and neuronal isoforms of Neurofascin have distinct roles in the assembly of nodes of Ranvier in the central nervous system. J. Cell Biol. 181, 1169–1177 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S., Eftekharpour E. & Fehlings M. G. Temporal and spatial patterns of Kv1.1 and Kv1.2 protein and gene expression in spinal cord white matter after acute and chronic spinal cord injury in rats: implications for axonal pathophysiology after neurotrauma. Eur. J. Neurosci. 19, 577–589 (2004). [DOI] [PubMed] [Google Scholar]
- Coman I. et al. Nodal, paranodal and juxtaparanodal axonal proteins during demyelination and remyelination in multiple sclerosis. Brain 129, 3186–3195 (2006). [DOI] [PubMed] [Google Scholar]
- Traka M. et al. Association of TAG-1 with Caspr2 is essential for the molecular organization of juxtaparanodal regions of myelinated fibers. J. Cell Biol. 162, 1161–1172 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micu I. et al. Real-time measurement of free Ca2+ changes in CNS myelin by two-photon microscopy. Nat. Med. 13, 874–879 (2007). [DOI] [PubMed] [Google Scholar]
- Tanaka H. et al. Mice with altered myelin proteolipid protein gene expression display cognitive deficits accompanied by abnormal neuron-glia interactions and decreased conduction velocities. J. Neurosci. 29, 8363–8371 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. H. Regulation of oligodendrocyte development in the vertebrate CNS. Prog. Neurobiol. 67, 451–67 (2002). [DOI] [PubMed] [Google Scholar]
- Cheng J. D. & de Vellis J. Oligodendrocytes as glucocorticoids target cells: functional analysis of the glycerol phosphate dehydrogenase gene. J. Neurosci. Res. 59, 436–445 (2000). [DOI] [PubMed] [Google Scholar]
- McEwen B. S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 87, 873–904 (2007). [DOI] [PubMed] [Google Scholar]
- Alonso G. Prolonged corticosterone treatment of adult rats inhibits the proliferation of oligodendrocyte progenitors present throughout white and gray matter regions of the brain. Glia 31, 219–231 (2000). [DOI] [PubMed] [Google Scholar]
- Nicoletti F. et al. Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology 60, 1017–1041 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matosin N. & Newell K. A. Metabotropic glutamate receptor 5 in the pathology and treatment of schizophrenia. Neurosci. Biobehav. Rev. 37, 256–268 (2013). [DOI] [PubMed] [Google Scholar]
- Newell K. A., Matosin N. & Lum J. S. Metabotropic glutamate receptors in the pathophysiology and treatment of schizophrenia and major depression. Metabotropic glutamate receptors: molecular mechanisms, role in neurological disorders and pharmacological effects. Hauppauge; NY, USA. (2014). [Google Scholar]
- Kovačević T., Skelin I., Minuzzi L., Rosa-Neto P. & Diksic M. Reduced metabotropic glutamate receptor 5 in the Flinders Sensitive Line of rats, an animal model of depression: an autoradiographic study. Brain Res. Bull. 87, 406–412 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierońska J. M. et al. Changes in the expression of metabotropic glutamate receptor 5 (mGluR5) in the rat hippocampus in an animal model of depression. Pol. J. Pharmacol. 53, 659–662 (2001). [PubMed] [Google Scholar]
- Vinson P. N. & Conn J. P. Metabotropic glutamate receptors as therapeutic targets for schizophrenia. Neuropharmacology 62, 1461–1472 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. et al. Improved in vivo diffusion tensor imaging of human cervical spinal cord. Neuroimage 67, 64–76 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S. K. et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 26, 132–140 (2005). [DOI] [PubMed] [Google Scholar]
- Dalgleish T. Interfacing basic science with clinical practice: a Festschrift special issue for John Teasdale. Behav. Res. Ther. 42, 971–974 (2004). [DOI] [PubMed] [Google Scholar]
- Ishikawa T. et al. Transient expression of Xpn, an XLMR protein related to neurite extension, during brain development and participation in neurite outgrowth. Neuroscience 214, 181–191 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.