Abstract
Aims
To compare the outcomes of neovascular glaucoma (NVG) treated with and without intravitreal bevacizumab in a large case comparison study.
Methods
The study is a retrospective, comparative, case series of 163 eyes of 151 patients with NVG, including 99 treated without and 64 treated with intravitreal bevacizumab. Medical and surgical treatments for NVG were assessed. The main outcome measures were visual acuity (VA) and intraocular pressure (IOP).
Results
At the time of NVG diagnosis, the median VA was count fingers (CF) in the non-bevacizumab group and 2/300 in the bevacizumab group. IOP (mean±SD) was 43.1±13.0 mm Hg in the non-bevacizumab group and 40.8±11.5 mm Hg in the bevacizumab group. IOP (mean±SD) decreased to 18.3±13.8 mm Hg in the non-bevacizumab group and 15.3±8.0 mm Hg in the bevacizumab group, and the median VA was CF in both treatment groups at a mean follow-up of 12 months. Panretinal photocoagulation (PRP) substantially reduced the need for glaucoma surgery (P<0.001) in bevacizumab treated NVG eyes.
Conclusions
Although bevacizumab delayed the need for glaucoma surgery, PRP was the most important factor that reduced the need for surgery. Vision and IOP in eyes with NVG treated with bevacizumab showed no long-term differences when compared with eyes that were not treated with bevacizumab. Thus, intravitreal bevacizumab serves as an effective temporizing treatment, but is not a replacement for close monitoring and definitive treatment of NVG. PRP remains the treatment modality that affects the course of NVG in terms of decreasing the need for surgery to control IOP.
Introduction
Neovascular glaucoma (NVG) is an aggressive type of glaucoma that complicates a number of systemic and ocular disorders. NVG is typically characterized by significantly elevated intraocular pressure (IOP) in the setting of neovascularization of the iris (NVI) and the anterior chamber angle, with ensuing glaucomatous optic neuropathy and vision loss. NVG progression leads to extensive peripheral anterior synechiae (PAS) formation with flattening and effacement of the anterior surface of the iris by a confluent fibrovascular membrane with a subsequent further IOP rise and exacerbation of glaucomatous damage.1 The prognosis of NVG is typically poor with devastating visual consequences.2 Common causes of NVG include central retinal vein occlusion (CRVO), diabetic retinopathy, and carotid artery occlusive disease, among many other ischemic diseases.2, 3, 4 These conditions share a common underlying initiating mechanism as a predisposition for developing NVG—retinal ischemia. As the presenting signs and symptoms of NVG are usually a late manifestation of the causative disease processes, NVG poses a great challenge for ophthalmologists and is often devastating for patients.
Treatment of NVG has two main components: (1) management of IOP elevation and (2) reduction of the ischemic drive, traditionally through panretinal photocoagulation (PRP).5 If applied early, PRP can induce regression of both anterior and posterior segment neovascularization.6 However, the response to adequate PRP is often incomplete,7, 8 and effective laser treatment may be hampered by the presence of cloudy media secondary to corneal edema, hyphema, cataract, and/or vitreous hemorrhage. Moreover, the effects of PRP often takes several weeks to take effect;9 during this window, angle closure and further ocular damage due to continually elevated IOP can occur.
Retinal ischemia has been shown to upregulate the expression of vascular endothelial growth factor (VEGF),10, 11, 12, 13 which triggers an angiogenic signaling cascade that promotes the development of NVI and anterior chamber angle.14 The level of VEGF expression in the aqueous humor is closely correlated to the extent of neovascularization, and inhibition of VEGF through intravitreal injection of anti-VEGF monoclonal antibodies in adult non-human primate eyes was found to prevent NVI associated with retinal ischemia.14, 15
Bevacizumab is an anti-VEGF recombinant humanized monoclonal antibody that has been approved by the US Food and Drug Administration (FDA) for the treatment of certain forms of cancer such as metastatic colorectal cancer.16 Although intravitreal injection of bevacizumab has not received FDA approval, bevacizumab has been widely used off-label to treat VEGF-mediated ocular conditions such as choroidal neovascularization secondary to age-related macular degeneration, diabetic macular edema, CRVO-associated macular edema and as an adjunctive agent in glaucoma surgery.17, 18, 19, 20, 21 The outcomes of off-label treatment of NVG with intravitreal bevacizumab have been reported.22, 23, 24, 25, 26, 27, 28, 29, 30 We have previously published a retrospective interventional case series of NVG eyes describing the natural history of NVG treated with intravitreal bevacizumab.22 However, conclusions regarding the exact role, efficacy, and drawbacks of intravitreal bevacizumab injection in eyes with NVG could not be drawn, since no comparison to eyes that did not receive this method of treatment was performed.
The purpose of this current large case comparison study is to compare the outcomes of intravitreal bevacizumab in patients with NVG with the outcomes previously achieved in the absence of bevacizumab. This study also evaluates the relative efficacy of this widely used adjunctive modality compared to that of other outcome modifiers such as PRP and glaucoma drainage implant (GDI) surgery in NVG. Our results demonstrate PRP was the most important factor that reduced the need for GDI surgery for lowering IOP, regardless of intravitreal bevacizumab administration. The role of intravitreal bevacizumab is that of a temporizing treatment that temporarily delayed the need for surgical intervention to lower IOP. Eyes with NVG should receive PRP whenever possible to prevent ocular complications and for IOP control.
Materials and methods
This study is a retrospective chart review that compares patients who received with those who did not receive intravitreal bevacizumab for NVG at the Bascom Palmer Eye Institute (BPEI). Approval from the University of Miami's Institutional Review Board was received. Patients with NVG from October 2005 (when the first patient was injected with intravitreal bevacizumab for NVG at BPEI) to March 2007 were treated with intravitreal bevacizumab. Chart review did not reveal patients with NVG (in the treatment group) who were excluded from intravitreal bevacizumab for any reason. This treatment group was compared with a random sample of NVG patients who were not treated with intravitreal bevacizumab from January 1997 to April 2005, which is a period during which intravitreal bevacizumab was not available as a treatment for NVG. Control patients received standard treatment measures including a variable combination of IOP lowering medications, PRP, and GDI as necessitated by the severity of the disease and response to treatment. Medical records identified with the ICD-9 code 365.63 (glaucoma associated with a vascular disorder) were reviewed. Thus, no treatment bias in the decision to use bevacizumab existed. The other treatment options for NVG such as PRP and GDI surgery remained unchanged after the advent of intravitreal bevacizumab.
Intravitreal bevacizumab (1.25 mg/0.05 ml) was injected under sterile conditions as described previously.17 Anterior chamber paracentesis was performed with a 30-gauge needle under sterile conditions at the discretion of the treating physician either before or after bevacizumab injection based on the extent of IOP elevation.
For the purposes of this study, NVG was defined as IOP>21 mm Hg and associated with neovascularization of the iris and/or anterior chamber angle. Eyes were excluded from the study if bevacizumab was injected strictly for reasons other than NVG, if IOP never exceeded 21 mm Hg, or if follow-up was <1 month after initial treatment. The following data were collected: date of birth, sex, past ocular history, previous ocular procedures, and the cause of NVG. The following data were collected at the time of diagnosis of NVG and at the time of each treatment administered for NVG: date, visual acuity, IOP, number of glaucoma medications, and type of treatment administered. The outcomes of visual acuity, IOP, and number of glaucoma medications were also collected at the following time intervals after the administration of each treatment: 1 month, 3 months, 6 months, 1 year, 1.5 years, and last follow-up visit. Visual outcomes, as well as nature and timing of complications related to the treatment of NVG, were recorded.
Results
Retrospective chart review identified 64 eyes of 58 patients with a diagnosis of NVG that received intravitreal bevacizumab between May 2005 and April 2007. These cases were compared with 99 eyes of 93 patients with NVG between January 1997 and April 2005 that did not receive bevacizumab. The demographic characteristics of the two groups were similar (Table 1), and are reflective of ethnic demographics in South Florida. The causes of NVG in the two groups were not significantly different (P=0.41). NVG eyes receiving intravitreal bevacizumab were more likely to have undergone cataract extraction (P<0.001) and pars plana vitrectomy (PPV; P=0.026), and were treated with fewer glaucoma medications before the diagnosis of NVG relative to the comparison group (P=0.027).
Table 1. Patient characteristics at diagnosis.
| Bevacizumab group (n=64) | Non-bevacizumab Group (n=99) | P-value | |
|---|---|---|---|
| Age (years), mean (SD) | 66.1 (14.2) | 63.7 (14.4) | 0.33a |
| Male gender, n (%) | 27 (47) | 58 (62) | 0.090b |
| Ethnicity, n (%) | 0.78c | ||
| Caucasian | 10 (18) | 11 (15) | |
| Hispanic | 27 (49) | 44 (60) | |
| African ancestry | 15 (27) | 15 (21) | |
| Other | 1 (2) | 3 (4) | |
| Causes of NVG, n (%) | 0.41c | ||
| DM type 2 | 29 (46) | 39 (42) | |
| CRVO | 16 (25) | 37 (39) | |
| DM type 1 | 5 (8) | 6 (6) | |
| OIS | 3 (5) | 3 (3) | |
| Other | 10 (16) | 9 (10) | |
| Visual acuity | |||
| Median (range) | 3/200 (20/40-LP) | CF (20/25-NLP) | 0.38d |
| HM or better acuity, n (%) | 55 (86) | 79 (80) | 0.40b |
| IOP, mean (SD) | 40.8 (11.5) | 43.1 (13.0) | 0.25a |
| Glaucoma meds, mean (SD) | 0.9 (1.4) | 1.4 (1.5) | 0.027a |
| Prior ocular surgeries, n (%) | |||
| Cataract extraction | 35 (56) | 18 (18) | <0.001b |
| Trabeculectomy/GDI | 1 (2) | 3 (3) | 1.00b |
| PRP | 22 (34) | 28 (28) | 0.49b |
| PPV | 15 (23) | 10 (10) | 0.026b |
Abbreviations: CF, count fingers; CRVO, central retinal vein occlusion; DM, diabetes mellitus; GDI, glaucoma drainage implant; HM, hand motion; IOP, intraocular pressure; LP, light perception; Meds, medications; OIS, ocular ischemic syndrome; PPV, pars plana vitrectomy; PRP, panretinal photocoagulation.
Two-sample t-test.
Fisher exact test.
χ2 test.
Two-sample Wilcoxon test.
Initial treatments performed in the two groups before the diagnosis of NVG (and before injection of bevacizumab) are compared in Table 2. Of the 99 eyes in the non-bevacizumab group, 70 eyes (71%) received their first treatment for neovascular glaucoma at the time of NVG diagnosis. This was not significantly different from the 50 eyes (78%) in the bevacizumab group that received a treatment at diagnosis that was not bevacizumab injection (P=0.36). Of the 64 eyes that did receive an injection of bevacizumab, 38 eyes (59%) received their first injection at the time of NVG diagnosis. The most commonly performed treatment was IOP lowering via medications in eyes that received both an injection of bevacizumab and another treatment at the time of NVG diagnosis. Eyes in the non-bevacizumab group were more likely to have been treated with panretinal photocoagulation (P=0.025) after the development of a retinal ischemia-related event, and before the development (and diagnosis) of NVG. Sixteen eyes (16%) in the non-bevacizumab group underwent conjunctival cutting surgeries (trabeculectomy, GDI with or without PPV) as their initial treatment compared with 2 (3%) in the bevacizumab group, since bevacizumab and/or glaucoma medications were the initial treatment in this group. On average, the non-bevacizumab group had 2.7 times more PRP sessions per month of follow-up than the bevacizumab group, but the difference in rates was not statistically significant (P=0.24).
Table 2. Initial treatments.
| Bevacizumab group (n=64) | Non-bevacizumab group (n=99) | P-valuea | |
|---|---|---|---|
| Glaucoma medications | 39 (61) | 45 (46) | 0.057 |
| PRP | 23 (36) | 54 (55) | 0.025 |
| Trabeculectomy or GDI (no PPV) | 0 | 4 (4) | 0.16 |
| CPC | 0 | 3 (3) | 0.28 |
| PPV with GDI | 2 (3) | 12 (12) | 0.050 |
Abbreviations: CPC, cyclophotocoagulation; GDI, glaucoma drainage implant; PPV, pars plana vitrectomy; PRP, panretinal photocoagulation.
Data are presented as number (percentage).
Fisher exact test.
The mean IOP and number of glaucoma medications in both groups were analyzed (Table 3). At 1 month after diagnosis, the bevacizumab group had a lower average intraocular pressure than the non-bevacizumab group (P=0.012), but this difference did not persist with longer follow-up. The non-bevacizumab group, despite being treated with a greater average number of glaucoma medications than the bevacizumab group at the time of NVG diagnosis, tended to require fewer medications on short-term follow-up. This difference was only statistically significant at the 6-month follow-up time point.
Table 3. Intraocular pressure and glaucoma medical therapy.
| Bevacizumab group (n=64) | Non-bevacizumab group (n=99) | P-valuea | |
|---|---|---|---|
| 1 month | |||
| N | 60 | 87 | 0.012 |
| IOP (mm Hg), mean (SD) | 24.4 (12.6) | 30.9 (17.0) | 0.80 |
| Glaucoma meds, mean (SD) | 2.3 (1.7) | 2.3 (1.5) | |
| 3 months | |||
| N | 50 | 74 | 0.38 |
| IOP (mm Hg), mean (SD) | 20.5 (11.7) | 22.8 (15.3) | 0.60 |
| Glaucoma meds, mean (SD) | 2.4 (1.7) | 2.2 (1.6) | |
| 6 months | |||
| N | 37 | 51 | 0.67 |
| IOP (mm Hg), mean (SD) | 19.4 (10.3) | 20.7 (16.7) | 0.022 |
| Glaucoma meds, mean (SD) | 2.1 (1.6) | 1.3 (1.5) | |
| 12 months | |||
| N | 22 | 43 | 0.36 |
| IOP (mm Hg), mean (SD) | 15.3 (8.0) | 18.3 (13.8) | 0.10 |
| Glaucoma meds, mean (SD) | 1.68 (1.49) | 1.1 (1.4) | |
Abbreviations: IOP, intraocular pressure; Meds, medications.
Two-sample t-test.
Bevacizumab treatment did not improve visual acuity outcomes over the comparison group (Table 4A). Three months following diagnosis, the comparison group was more likely to retain visual acuities of hand motions or better (P=0.013), but this effect did not persist at 6 or 12 months.
Table 4A. Median visual acuity and eyes retaining hand motions or greater vision after NVG diagnosis.
| Bevacizumab group (n=64) | Non-bevacizumab group (n=99) | P-value | |
|---|---|---|---|
| 1 month | |||
| Median (range) | CF (20/30, LP) | CF (20/30, NLP) | 0.38a |
| N (%) | 48 (44) | 61 (56) | 0.23b |
| 3 months | |||
| Median (range) | 2/200 (20/20, NLP) | HM (20/30, NLP) | 0.28a |
| N (%) | 40 (46) | 48 (55) | 0.013b |
| 6 months | |||
| Median (range) | 3/250 (20/50, NLP) | HM (20/25, NLP) | 0.48a |
| N (%) | 27 (47) | 31 (53) | 0.79b |
| 12 months | |||
| Median (range) | CF (20/30, NLP) | CF (20/30, NLP) | 0.93a |
| N (%) | 15 (35) | 28 (65) | 0.48b |
Abbreviations: CF, count fingers; HM, hand motion; NLP, no light perception.
Two-sample Wilcoxon test.
Fisher exact test.
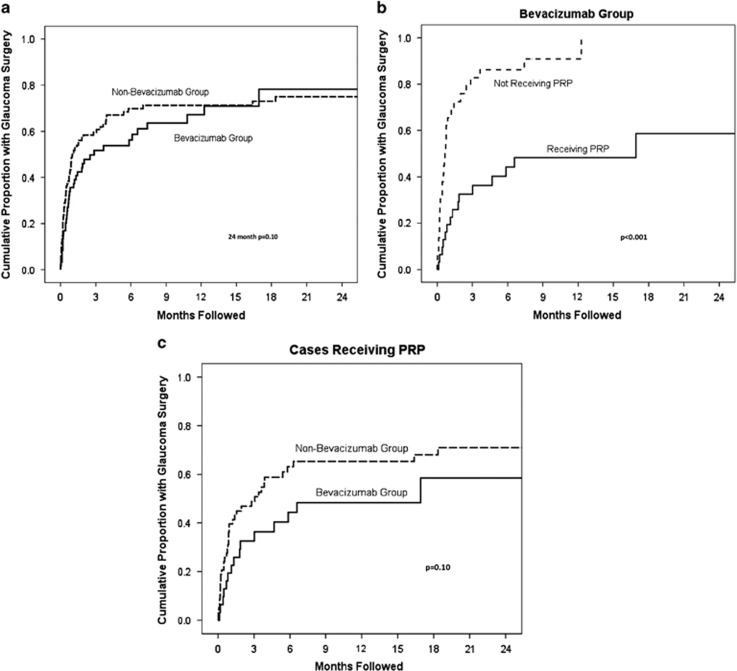
The cumulative proportion of eyes receiving a GDI over time from NVG diagnosis for each of the two treatment groups was analyzed with Kaplan–Meier survival analysis (Figure 1a). Within the first 6 months, the bevacizumab group had a lower cumulative proportion of eyes receiving a GDI compared with the non-bevacizumab group, but this difference was not statistically significant after 2 years of follow-up (P=0.38).
Figure 1.
(a) Kaplan–Meier Survival Analysis of NVG Eyes Receiving GDIs. The figure shows that cumulative proportion of eyes receiving a GDI over time from NVG diagnosis in the bevacizumab group was lower compared with the non-bevacizumab group within the first 6 months. This difference was not statistically significant after 2 years of follow-up (P=0.38). (b) Kaplan–Meier survival analysis of patients receiving bevacizumab. The figure shows the cumulative proportion of eyes receiving bevacizumab with and without PRP that required GDI surgery and the time to glaucoma surgery. PRP substantially reduced the rates of glaucoma surgery (P<0.001). (c) Kaplan–Meier survival analysis of patients receiving PRP. The figure compares time to glaucoma surgery between cases with and without bevacizumab. The reduction in incidence of glaucoma surgery in the bevacizumab group did not achieve statistical significance (P=0.10).
The cumulative proportion of eyes receiving bevacizumab with and without PRP that required GDI surgery (Figure 1b) was analyzed by Kaplan–Meier survival analysis. PRP had a substantial beneficial effect on reducing rates of glaucoma surgery (P<0.001) in bevacizumab treated NVG eyes. The cumulative proportion of eyes receiving PRP with and without bevacizumab therapy was also analyzed with survival analysis (Figure 1c). Among the cases that received PRP, the reduction in incidence of glaucoma surgery in the bevacizumab group did not achieve statistical significance (P=0.10). These findings suggest that PRP significantly delays and decreases the need for IOP control by means of GDI surgery, regardless of bevacizumab administration.
The main causes of vision loss in these patients included retinal ischemia, glaucomatous optic neuropathy, and proliferative diabetic retinopathy/tractional retinal detachment. Vision loss factors were essentially equivalent in both study groups. All eyes were presumed to have retinal ischemia contributing to vision loss because neovascularization was present.
Ocular complications observed in both groups were compared (Table 4B). The most common complication was hyphema, which occurred with significantly greater frequency in the bevacizumab group. None of the hyphemas were complete (that is, ‘eight ball'). Among the eight eyes that did not receive intravitreal bevacizumab, two cases had a documented hyphema without or before GDI/PPV. Another five cases had hyphema noted within a month of GDI/PPV and one case had a delayed hyphema noted 3 months after GDI/PPV. Of note, eyes with no light perception (NLP) vision initially were not excluded from the study, since another of the outcome measures was IOP, and NLP vision is not uncommon in the setting of NVG, although this may have made visual acuity outcome on average worse. In addition, progression to NLP vision was not considered a complication, given that severe vision loss is often part of the natural history of NVG.
Table 4B. Complications of treatment.
| Bevacizumab group (n=64) | Non-bevacizumab group (n=99) | P-valuea | |
|---|---|---|---|
| Retinal detachment | 2 (3%) | 0 | 0.15 |
| Cataract | 0 | 3 (3%) | 0.28 |
| Hyphema | 19 (30%) | 8 (8%) | <0.001 |
| Tube erosion | 0 | 1 (1%) | 1.00 |
Data are presented as number (percentage).
Two-sample Wilcoxon test.
Discussion
NVG is an aggressive form of glaucoma resulting from retinal ischemia. The disease typically starts with the development of rubeotic vessel tufts at the pupillary border and/or anterior chamber angle. The growth of fibrovascular tissue over the trabecular meshwork obstructs aqueous outflow and elevates IOP.31 At this stage, the anterior chamber angle is partially open in various regions of the angle. This fibrovascular tissue eventually grows and contracts causing progressive synechial angle closure and ectropion uveae. Blood leaking from these incompetent neovascular growths can cause hyphema, and inflammation can develop during any stage, exacerbating IOP elevation.
Treatment of NVG depends on the stage of the disease. Early during the course of the disease, the goal is to ablate ischemic retina, thereby decreasing the ischemic drive and reducing the amount of VEGF released and neovessel formation. PRP appears to be effective in inducing regression of NVI and preventing the development of NVG if administered before the development of IOP elevation and if the amount of neovascularization over the angle is minimal.32 Once IOP is elevated and a significant degree of angle closure is present, additional treatment modalities aimed at reducing IOP are typically needed. IOP lowering treatment modalities may include a combination of topical drops, filtering surgery, GDIs, and cyclodestructive procedures. These modalities have variable success rates depending on the intensity of IOP elevation, degree of ischemia-induced neovascularization, status of the angle and extent of glaucomatous damage incurred.5
The role of antiangiogenic factors such as bevacizumab in the management of NVG is becoming increasingly popular and better established. We present here a case comparison study to examine the efficacy of intravitreal bevacizumab in eyes with NVG relative to eyes treated under similar circumstances but did not receive intravitreal bevacizumab. The administration of bevacizumab has been shown to induce rapid regression of NVI.23 We have observed by slit lamp examination and by iris angiography that iris neovascularization is completely regressed within 3 days of intravitreal bevacizumab injection. However, bevacizumab, although rapidly-acting, induces temporary antiangiogenic effects with recurrence of neovascularization,28, 33 due to its short duration of action.34, 35 On the other hand, the effects of PRP appear to be more long lasting, suggesting that eyes with NVG can benefit from both the early-onset regression of neovascularization of bevacizumab and the long duration of action of PRP.36
In this study, although the treatment group tended to require fewer antiglaucoma medications on short-term follow-up, the authors think that this difference, although statistically significant, did not influence the ultimate outcome in terms of the need for glaucoma surgery, since this difference was only statistically significant at the 6-month follow-up time point, and consensus exists that antiglaucoma medications may only serve a temporizing role in an aggressive type of glaucoma such as NVG. Nearly 80% of all the eyes in this study subsequently received a GDI, which was the principal glaucoma surgery performed. Trabeculectomies alone were not performed in our study owing to a prevailing view that GDIs are less likely to fail than trabeculectomy with or without adjuvant antifibrotic agents in the setting of NVG, especially in terms of long-term IOP control success.37 Of note, the tube versus trabeculectomy study excluded eyes with NVG due to the challenging nature of management of this condition.38 Shen et al39 found similar IOP reduction and surgical success rate in NVG eyes treated with augmented trabeculectomy versus GDI. However, the retrospective nature of the study could have resulted in significant selection bias. In the present study, treatment with bevacizumab did not significantly reduce the rates of GDI surgery over the long term. The cumulative proportion of eyes receiving a GDI was initially lower in eyes receiving intravitreal bevacizumab compared with those eyes not treated with bevacizumab; however, the cumulative proportions were similar with longer term follow-up. This illustrates the importance of both initiating definitive treatment and instituting long-term follow-up in those eyes injected with bevacizumab, regardless of initial iris and angle neovascularization status and initial IOP lowering. The majority of NVG patients, if followed long enough, frequently require surgical intervention to lower IOP, especially if the ischemic drive for neovascularization is not ablated.
Our findings suggest that PRP significantly delays the need for glaucoma drainage implantation in both study groups, irrespective of bevacizumab administration. This is consistent with the findings of Ehlers et al36 in their study of combination intravitreal bevacizumab and PRP vs PRP alone in the treatment of NVG. Although they showed a trend towards greater surgical interventions in the PRP only group, it was not statistically significant, and the mean initial IOP was lower in the combination group, which might have enhanced the apparent response to treatment. In another retrospective review by Wakabayashi et al,30 repeat intravitreal injections of bevacizumab as an adjunctive modality to PRP appeared to reduce the rate of surgical interventions in eyes with open angles, although this did not reduce the rate of such interventions in eyes with closed angles.
Our results also demonstrate that with long-term follow-up, no significant difference in visual acuity or IOP was observed between both groups, which correlates with results reported by Wittström et al.40 In another prospective study, a significant improvement in IOP but not visual acuity was observed in eyes receiving adjunctive intravitreal bevacizumab.41 In another retrospective study, IOP was significantly lower in patients who received combination intravitreal bevacizumab/PRP than in patients who received PRP alone.36 However, the number of eyes in our study is significantly larger than in these studies and we assessed the role of surgery on outcomes including use of medications after treatment (including with or without PRP, GDIs, and anti-VEGF).
Taken together, these results suggest that the role of bevacizumab in NVG is that of a temporizing rather than a definitive treatment, and eyes with NVG should uniformly receive PRP to treat ischemia, regardless of prior intravitreal bevacizumab injection(s). These observations are important, because they remind us as clinicians that we must not overlook the necessity of other important treatment modalities even when one observes initial dramatic regression of neovascularization with intravitreal bevacizumab. Without ablation of the ischemic drive for new vessel formation, neovascularization will recur after regression with initial anti-VEGF therapy.
Another important clinical impression of treating NVG with intravitreal bevacizumab has been that eyes requiring subsequent incisional surgery were often less inflamed and had less intraoperative or postoperative hemorrhage.23, 24 In this study, no intraoperative bleeding complications were recorded. However, non-sight threatening hyphema was a significant complication associated with bevacizumab treatment of NVG. This may relate to increased frequency of paracentesis in this subgroup, although a number of hyphemas occurred asynchronously with bevacizumab injection as a late manifestation and may be owing to the nature of the incompetent, leaky neovessels.
Several limitations are inherently associated with a retrospective study. Patients in our study were lost to follow-up even at early time points, likely because many were initially evaluated in an emergency setting. The response of neovascularization to bevacizumab or the reason for repeat bevacizumab were not always noted in the chart, although the indication for repeated injection was presumably recurrence of neovascularization. Although intravitreal bevacizumab has been shown to induce regression of anterior segment neovascularization, the documentation of gonioscopy did not allow for sufficient analysis of the effect of bevacizumab on progressive angle closure in the setting of NVG. Another relevant concern is the lack of assessment of the percentage of the retina covered by PRP spots, although most patients who were able to receive PRP in both groups underwent a full treatment, unless lost to follow-up. Finally, no established protocol dictating the sequence or timing of the various treatments was followed; this was left to the discretion of the managing physicians and therefore reflects real world clinical practice patterns.
As treatment with intravitreal bevacizumab is now part of the standard of care for NVG at many institutions, a prospective randomized clinical trial (RCT) to definitively establish the efficacy and safety of adjunctive intravitreal bevacizumab in NVG would be difficult to undertake. Case series and retrospective studies have reported only short-term reduction in neovascularization, inflammation and IOP in eyes receiving anti-VEGF agents.24, 28, 30, 33, 36, 42, 43, 44, 45 A systematic review of literature in 2013 concluded that no compelling evidence exists from which reliable conclusions could be drawn comparing the effects of anti-VEGF agents either alone or as an adjunctive modality to other forms of treatment but no anti-VEGF agents in NVG.46 The review identified two RCTs evaluating intravitreal bevacizumab injection in NVG.40, 41 Wittström et al40 randomized 19 eyes with NVG secondary to ischemic CRVO to either intravitreal bevacizumab and PRP (10 eyes) or PRP alone (9 eyes). Although resolution of iris and angle neovascularization was faster in the combined intravitreal bevacizumab/PRP group, results showed significant reduction of a-wave amplitudes of combined rod-cone response on electroretinogram, suggesting that bevacizumab may reduce photoreceptor function in eyes with NVG. Yazdani et al. randomized 26 eyes with NVG to either 3 intravitreal bevacizumab injections 4 weeks apart (14 eyes) or subconjunctival sham injections at similar time intervals (12 eyes) in addition to conventional NVG treatment. Unlike results from our present study, their results showed a significant reduction of IOP in the intravitreal bevacizumab group. However, the heterogeneity and uncontrolled assignment of adjunctive treatment modalities, as well as the small number of participants were major drawbacks of these RCTs.46
As we previously recommended,22 the standard of care for NVG at BPEI includes (1) administering intravitreal bevacizumab at the time of NVG diagnosis or before glaucoma surgery; (2) administering PRP if an adequate view of the posterior pole exists, or applying endolaser during PPV (if indicated with or without glaucoma surgery); and (3) lowering IOP medically and via placement of a GDI as necessary, or, if the vision is not considered useful, cyclophotocoagulation. Based on our experience with managing these challenging cases, we have also proposed a treatment algorithm for NVG.5
In summary, intravitreal bevacizumab is now a frequently used adjunct for the treatment of NVG. Bevacizumab is an important temporizing measure, used to bridge the patient to definitive treatment, including PRP and GDIs as needed. In a minority of cases with minimal neovascularization and early NVG, administration of bevacizumab may prevent permanent angle closure by PAS and thus preclude GDI surgery. However, it is rare for patients to present for treatment early enough to prevent permanent angle closure since most patients present to emergency rooms with advanced neovascularization with high IOP, severe pain, and vision loss. Most importantly, patients with NVG require close follow-up on diagnosis and after treatment. NVG can recur owing to recurrent retinal ischemia that can lead to elevation in IOP.
Footnotes
PJR received research support from Acucela, Apellis, Genentech/Roche, GlaxoSmithKline, Neurotech, Ocata Therapeutics, and Tyrogenex. He is a consultant for Achillion, Acucela, Alcon, Bayer, Chengdu Kanghong Biotech, CoDa Therapeutics, Genentech/Roche, Healios K.K., Merck, Regeneron, Stealth and Tyrogenex. LCO is on the Scientific Advisory Board for: Alcon surgical, ScienceBased Health (none of them relevant to the published work). The other authors have no financial interests in any of the products discussed in this article. The Bascom Palmer Eye Institute is supported by NIH Center Core Grant P30EY014801 and a Research to Prevent Blindness Unrestricted Grant.
Author contributions
RKL, LCO, ALM, MSS, PJR and SJG participated in the conception and design of this study, analysis, and interpretation of data. WS and WJF participated in the statistical analysis and interpretation of the data. RKL, LCO, and MSS participated in drafting the article and revising it critically for important intellectual content and final approval of the version to be published. Ethics approval was provided by the University of Miami Miller School of Medicine Institutional Review Board.
References
- John T, Sassani JW, Eagle RC Jr. The myofibroblastic component of rubeosis iridis. Ophthalmology 1983; 90(6): 721–728. [DOI] [PubMed] [Google Scholar]
- Shazly TA. Latina MA. Neovascular glaucoma: etiology, diagnosis and prognosis. Semin Ophthalmol 2009; 24(2): 113–121. [DOI] [PubMed] [Google Scholar]
- Gartner S, Henkind P. Neovascularization of the iris (rubeosis iridis). Surv Ophthalmol 1978; 22(5): 291–312. [DOI] [PubMed] [Google Scholar]
- Brown GC, Magargal LE, Schachat A, Shah H. Neovascular glaucoma. Etiologic considerations. Ophthalmology 1984; 91(4): 315–320. [DOI] [PubMed] [Google Scholar]
- Olmos LC, Lee RK. Medical and surgical treatment of neovascular glaucoma. Int Ophthalmol Clin 2011; 51(3): 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashwell LF, Marks WP. Panretinal photocoagulation in the management of neovascular glaucoma. South Med J 1988; 81(11): 1364–1368. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Klugman MR, Podhajsky P, Servais GE, Perkins ES. Argon laser panretinal photocoagulation in ischemic central retinal vein occlusion. A 10-year prospective study. Graefes Arch Clin Exp Ophthalmol 1990; 228(4): 281–296. [DOI] [PubMed] [Google Scholar]
- Sivak-Callcott JA, O'Day DM, Gass JD, Tsai JC. Evidence-based recommendations for the diagnosis and treatment of neovascular glaucoma. Ophthalmology 2001; 108(10): 1767–1776. [DOI] [PubMed] [Google Scholar]
- Doft BH, Blankenship G. Retinopathy risk factor regression after laser panretinal photocoagulation for proliferative diabetic retinopathy. Ophthalmology 1984; 91(12): 1453–1457. [DOI] [PubMed] [Google Scholar]
- Adamis AP, Miller JW, Bernal MT, D'Amico DJ, Folkman J, Yeo TK et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol 1994; 118(4): 445–450. [DOI] [PubMed] [Google Scholar]
- Malecaze F, Clamens S, Simorre-Pinatel V, Mathis A, Chollet P, Favard C et al. Detection of vascular endothelial growth factor messenger RNA and vascular endothelial growth factor-like activity in proliferative diabetic retinopathy. Arch Ophthalmol 1994; 112(11): 1476–1482. [DOI] [PubMed] [Google Scholar]
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994; 331(22): 1480–1487. [DOI] [PubMed] [Google Scholar]
- Pe'er J, Folberg R, Itin A, Gnessin H, Hemo I, Keshet E. Upregulated expression of vascular endothelial growth factor in proliferative diabetic retinopathy. Br J Ophthalmol 1996; 80(3): 241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd SR, Zachary I, Chakravarthy U, Allen GJ, Wisdom GB, Cree IA et al. Correlation of increased vascular endothelial growth factor with neovascularization and permeability in ischemic central vein occlusion. Arch Ophthalmol 2002; 120(12): 1644–1650. [DOI] [PubMed] [Google Scholar]
- Adamis AP, Shima DT, Tolentino MJ, Gragoudas ES, Ferrara N, Folkman J et al. Inhibition of vascular endothelial growth factor prevents retinal ischemia-associated iris neovascularization in a nonhuman primate. Arch Ophthalmol 1996; 114(1): 66–71. [DOI] [PubMed] [Google Scholar]
- Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) plus Carboplatin and Paclitaxel as first-line treatment of advanced/metastatic recurrent nonsquamous non-small cell lung cancer. Oncologist 2007; 12(6): 713–718. [DOI] [PubMed] [Google Scholar]
- Rich RM, Rosenfeld PJ, Puliafito CA, Dubovy SR, Davis JL, Flynn HW Jr et al. Short-term safety and efficacy of intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Retina 2006; 26(5): 495–511. [DOI] [PubMed] [Google Scholar]
- Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, Sanchez JG, Wu L, Maia M et al. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology 2007; 114(4): 743–750. [DOI] [PubMed] [Google Scholar]
- Costa RA, Jorge R, Calucci D, Melo LA Jr, Cardillo JA, Scott IU. Intravitreal bevacizumab (avastin) for central and hemicentral retinal vein occlusions: IBeVO study. Retina 2007; 27(2): 141–149. [DOI] [PubMed] [Google Scholar]
- Kiddee W, Orapiriyakul L, Kittigoonpaisan K, Tantisarasart T, Wangsupadilok B. Efficacy of adjunctive subconjunctival bevacizumab on the outcomes of primary trabeculectomy with mitomycin C: a prospective randomized placebo-controlled trial. J Glaucoma 2015; 24(8): 600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bergen T, Vandewalle E, Moons L, Stalmans I. Complementary effects of bevacizumab and MMC in the improvement of surgical outcome after glaucoma filtration surgery. Acta Ophthalmol 2015; 93: 667–678. [DOI] [PubMed] [Google Scholar]
- Moraczewski AL, Lee RK, Palmberg PF, Rosenfeld PJ, Feuer WJ. Outcomes of treatment of neovascular glaucoma with intravitreal bevacizumab. Br J Ophthalmol 2009; 93(5): 589–593. [DOI] [PubMed] [Google Scholar]
- Davidorf FH, Mouser JG, Derick RJ. Rapid improvement of rubeosis iridis from a single bevacizumab (Avastin) injection. Retina 2006; 26(3): 354–356. [DOI] [PubMed] [Google Scholar]
- Iliev ME, Domig D, Wolf-Schnurrbursch U, Wolf S, Sarra GM. Intravitreal bevacizumab (Avastin) in the treatment of neovascular glaucoma. Am J Ophthalmol 2006; 142(6): 1054–1056. [DOI] [PubMed] [Google Scholar]
- Kahook MY, Schuman JS, Noecker RJ. Intravitreal bevacizumab in a patient with neovascular glaucoma. Ophthalmic Surg Lasers Imaging 2006; 37(2): 144–146. [PubMed] [Google Scholar]
- Mason JO 3rd, Albert MA Jr., Mays A, Vail R. Regression of neovascular iris vessels by intravitreal injection of bevacizumab. Retina 2006; 26(7): 839–841. [DOI] [PubMed] [Google Scholar]
- Silva Paula J, Jorge R, Alves Costa R, Rodrigues Mde L, Scott IU. Short-term results of intravitreal bevacizumab (Avastin) on anterior segment neovascularization in neovascular glaucoma. Acta Ophthalmol Scand 2006; 84(4): 556–557. [DOI] [PubMed] [Google Scholar]
- Gheith ME, Siam GA, de Barros DS, Garg SJ, Moster MR. Role of intravitreal bevacizumab in neovascular glaucoma. J Ocul Pharmacol Ther 2007; 23(5): 487–491. [DOI] [PubMed] [Google Scholar]
- Chilov MN, Grigg JR, Playfair TJ. Bevacizumab (Avastin) for the treatment of neovascular glaucoma. Clin Experiment Ophthalmol 2007; 35(5): 494–496. [DOI] [PubMed] [Google Scholar]
- Wakabayashi T, Oshima Y, Sakaguchi H, Ikuno Y, Miki A, Gomi F et al. Intravitreal bevacizumab to treat iris neovascularization and neovascular glaucoma secondary to ischemic retinal diseases in 41 consecutive cases. Ophthalmology 2008; 115(9): 1571–1580 1580.e1–e3. [DOI] [PubMed] [Google Scholar]
- Anderson DM, Morin JD, Hunter WS. Rubeosis iridis. Can J Ophthalmol 1971; 6(3): 183–188. [PubMed] [Google Scholar]
- Duker JS, Brown GC. The efficacy of panretinal photocoagulation for neovascularization of the iris after central retinal artery obstruction. Ophthalmology 1989; 96(1): 92–95. [DOI] [PubMed] [Google Scholar]
- Kotecha A, Spratt A, Ogunbowale L, dell'Omo R, Kulkarni A, Bunce C et al. Intravitreal bevacizumab in refractory neovascular glaucoma: a prospective, observational case series. Arch Ophthalmol 2011; 129(2): 145–150. [DOI] [PubMed] [Google Scholar]
- Beer PM, Wong SJ, Hammad AM, Falk NS, O'Malley MR, Khan S. Vitreous levels of unbound bevacizumab and unbound vascular endothelial growth factor in two patients. Retina 2006; 26(8): 871–876. [DOI] [PubMed] [Google Scholar]
- Bakri SJ, Snyder MR, Reid JM, Pulido JS, Singh RJ. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology 2007; 114(5): 855–859. [DOI] [PubMed] [Google Scholar]
- Ehlers JP, Spirn MJ, Lam A, Sivalingam A, Samuel MA, Tasman W. Combination intravitreal bevacizumab/panretinal photocoagulation versus panretinal photocoagulation alone in the treatment of neovascular glaucoma. Retina 2008; 28(5): 696–702. [DOI] [PubMed] [Google Scholar]
- Tsai JC, Feuer WJ, Parrish RK 2nd, Grajewski AL. 5-Fluorouracil filtering surgery and neovascular glaucoma. Long-term follow-up of the original pilot study. Ophthalmology 1995; 102(6): 887–892. [DOI] [PubMed] [Google Scholar]
- Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL et al. Treatment outcomes in the tube versus trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol 2012; 153(5): 789–803 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen CC, Salim S, Du H, Netland PA. Trabeculectomy versus Ahmed Glaucoma Valve implantation in neovascular glaucoma. Clin Ophthalmol 2011; 5: 281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittstrom E, Holmberg H, Hvarfner C, Andreasson S. Clinical and electrophysiologic outcome in patients with neovascular glaucoma treated with and without bevacizumab. Eur J Ophthalmol 2012; 22(4): 563–574. [DOI] [PubMed] [Google Scholar]
- Yazdani S, Hendi K, Pakravan M, Mahdavi M, Yaseri M. Intravitreal bevacizumab for neovascular glaucoma: a randomized controlled trial. J Glaucoma 2009; 18(8): 632–637. [DOI] [PubMed] [Google Scholar]
- Costagliola C, Cipollone U, Rinaldi M, della Corte M, Semeraro F, Romano MR. Intravitreal bevacizumab (Avastin) injection for neovascular glaucoma: a survey on 23 cases throughout 12-month follow-up. Br J Clin Pharmacol. 2008; 66(5): 667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisanti S, Biester S, Peters S, Tatar O, Ziemssen F, Bartz-Schmidt KU et al. Intracameral bevacizumab for iris rubeosis. Am J Ophthalmol 2006; 142(1): 158–160. [DOI] [PubMed] [Google Scholar]
- Martinez-Carpio PA, Bonafonte-Marquez E, Heredia-Garcia CD, Bonafonte-Royo S. [Efficacy and safety of intravitreal injection of bevacizumab in the treatment of neovascular glaucoma: systematic review]. Arch Soc Esp Oftalmol 2008; 83(10): 579–588. [DOI] [PubMed] [Google Scholar]
- Oshima Y, Sakaguchi H, Gomi F, Tano Y. Regression of iris neovascularization after intravitreal injection of bevacizumab in patients with proliferative diabetic retinopathy. Am J Ophthalmol 2006; 142(1): 155–158. [DOI] [PubMed] [Google Scholar]
- Simha A, Braganza A, Abraham L, Samuel P, Lindsley K. Anti-vascular endothelial growth factor for neovascular glaucoma. Cochrane Database Syst Rev 2013; 10: CD007920. [DOI] [PMC free article] [PubMed] [Google Scholar]