Abstract
Background and objectives
Monoclonal gammopathies (MGs) with renal involvement can lead to ESRD caused by myeloma cast nephropathy (MCN), immunoglobulin light chain amyloidosis (ALA), or light–chain deposition disease (LCDD). Few studies have focused on the prognosis of patients with MG on chronic dialysis. We evaluated the outcomes of patients with MG incident on chronic dialysis in France.
Design, setting, participants, & measurements
All incident patients registered in the Renal Epidemiology and Information Network Registry between 2002 and 2011 with ESRD caused by ALA, LCDD, or MCN were included. Patient’s survival, censored for renal transplantation, renal recovery, and loss to follow-up, as well as renal outcomes were analyzed and compared with a control group. Risk factors and causes of death were analyzed.
Results
We included 1459 patients, comprising 265 (18%) patients with ALA, 334 (23%) patients with LCDD, and 861 (59%) patients with MCN. Median age was 72 years, and 56% were men. Median follow-up was 13.1 months. Renal recovery was observed in 9.1% of patients and more frequent after 2006. Kidney transplantation was rare in this population (2.3%). Among 1272 patients who remained on dialysis, 67% died. Median survival on dialysis was 18.3 months. Main causes of death were malignancies (34.4%), cardiovascular diseases (18%), infections (13.3%), and cachexia (5.2%). Independent risk factors of death were age (hazard ratio [HR], 1.03 per year increase; 95% confidence interval [95% CI], 1.02 to 1.03), frailty (HR, 1.93; 95% CI, 1.58 to 2.36), congestive heart failure (HR, 1.54; 95% CI, 1.23 to 1.93), and dialysis initiation on a central catheter (HR, 1.40; 95% CI, 1.11 to 1.75). Factors associated with a lower risk of death were year of dialysis initiation (HR, 0.95 per year increase; 95% CI, 0.91 to 0.99) and high BP (HR, 0.80; 95% CI, 0.67 to 0.97).
Conclusions
Survival of patients with ALA, LCDD, or MCN on chronic dialysis is poor but has improved over time. Progressive malignancy is the main cause of death in this population. Renal recovery has increased since 2006.
Keywords: chronic kidney disease, end stage kidney disease, light chain deposition disease, monoclonal gammopathy, kidney transplantation, amyloidosis, humans, multiple myeloma, paraproteinemias, renal dialysis
Introduction
Renal involvement of monoclonal gammopathies (MGs) can lead to ESRD. The most frequent causes of ESRD in this setting are myeloma cast nephropathy (MCN), immunoglobulin light chain amyloidosis (ALA), and light–chain deposition disease (LCDD). Multiple myeloma (MM) accounts for 1% of malignant diseases and 12%–15% of hematologic malignancies in Europe, with a three to five per 100,000 annual incidence. Incidence rates increase with age (1) and have remained stable in the last decades (2). ESRD caused by MM represented 1.5% of patients on RRT in the 1986–2005 European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) Registry (3). Dialysis dependence is associated with poor survival in MM (3–5). Recent use of efficacious low–toxicity chemotherapy, such as bortezomib, may have improved the outcome of patients with MM on dialysis. However, although bortezomib increased median time to disease progression of 45% in the general population of patients with MM (6,7), large studies failed to show a benefit in patients on chronic dialysis (3,4). Bortezomib was approved in France in 2004 (8) for patients with MM or ALA and renal failure.
Few studies have addressed specifically the outcome of patients with ALA or LCDD on chronic dialysis. Estimated incidence of ALA was 6–10 per 1 million per year in 2008 in the United Kingdom (9). ESRD occurs in one quarter of patients with renal ALA (10), whose survival depends on cardiac involvement. LCDD, characterized by monoclonal light–chain granular tissue deposition (11,12), is distinct from MM or ALA (13), but outcomes of patients with LCDD have rarely been studied specifically (14).
We report here the characteristics and outcomes of the national cohort of patients with ALA, LCDD, or MCN incident on chronic dialysis registered in the Renal Epidemiology and Information Network (REIN) Registry over a 10-year period (2002–2011).
Materials and Methods
Patient Population, Data Collection, and Definitions
All incident patients with ESRD related to ALA, LCDD, or MCN registered in the REIN between January 1, 2002 and December 31, 2011 were included. The REIN was founded in 2001 as a tool to provide public health decision support, evaluation, and research related to RRT for ESRD (15). Data are collected at the initiation of RRT (i.e., hemodialysis [HD], peritoneal dialysis, or kidney transplantation [KTR]) for all patients in France and updated annually. Only patients considered as having ESRD by their attending nephrologist are included in the REIN, not patients requiring RRT for AKI. Patients may be included from their first day of RRT if they had previous severe CKD or a posteriori in case of absence of recovery after 3 months on dialysis. In both cases, the first day on dialysis is recorded in the registry. Thirty-six dedicated clinical research assistants coordinate data collection and quality control. Eight types of events are reported in the REIN from the first day of RRT: (1) KTR, (2) registration on the kidney transplant waiting list, (3) changes in dialysis setting, (4) changes in type of dialysis, (5) recovery of renal function, (6) loss of follow-up, (7) therapy withdrawal, and (8) death. If death occurs, the main cause of death is recorded. The National Commission for Information Technology and Privacy approved the data collection conducted by the REIN, and the REIN Scientific Committee approved this study.
Major comorbidities, including frailty (inability to walk without help), and demographic information were collected as well as the following information relative to RRT: dialysis start in emergency, dialysis start on a catheter, primary renal disease, and dialysis settings. Dialysis start in emergency was defined as unplanned first dialysis for a vital risk linked to hyperkalemia, overhydration, acidosis, pericarditis, or severe anemia. Primary renal diseases were coded according to the thesaurus of the French language Society of Nephrology on the basis of the 10th revision of the International Classification of Disease.
Any events were registered from the first day of dialysis to the study’s end point on November 13, 2013. The primary outcome examined was the survival of patients on dialysis censored for recovery of renal function, KTR, loss to follow-up, or the end of the study.
Patients with MG were divided in three groups according to primary renal disease codes: ALA, LCDD, and MCN. Patients with other amyloidosis (nonimmunoglobulin light chain) were excluded.
A control population of incident patients with ESRD was selected randomly without any matching from all patients without MG included in the REIN during the same period of time (three or four control patients per one patient with MG).
Aims of the Study
The main objective was to evaluate the survival of patients with MG (ALA, LCDD, or MCN) on chronic dialysis and identify risk factors of death.
Additional objectives were to determine causes of death, disease incidence over time, rates of renal recovery over time, trends in mortality over time, and access to the transplantation waiting list and KTR.
We also compared survival and causes of death between patients with ALA, LCDD, or MCN and between patients with MG and control patients.
Statistical Analyses
For descriptive analyses, results were expressed as frequencies and percentages for categorical variables. Means and SDs or medians and interquartile ranges (IQRs) were used for continuous variables with asymmetric distributions. Groups of patients with MG were compared using chi-squared tests (or Fisher exact tests) for frequencies and ANOVA for continuous variables. Patients with MG and control patients were compared using chi-squared tests for frequencies and t tests for continuous variables. Survival probabilities on dialysis were estimated using the Kaplan–Meier method and compared using the log-rank test for equality of the survival curves. Cumulative incidences of death on dialysis was also analyzed using the competing risks analysis method. Death was the main event. Transplantation and renal recovery were considered as competing events. Equality across groups was tested with Fine and Gray analysis.
Risk factors associated with death were explored with the multivariate Cox model adjusted on mortality risk factor. Age and risk factors with a P value <0.25 in the univariate analysis were included in the Cox model.
For survival analysis comparison between patients with MG and control patients on dialysis, a multivariate Cox analysis was performed after adjustment for age and the risk factors of mortality identified.
In patients with multiple events, the first event was registered. Additional events were only considered in descriptive analysis of subgroups.
Statistical analysis was performed using R and SPSS (SPSS Inc., Chicago, IL) software. P values <0.05 were considered statistically significant.
Results
Characteristics of Patients with MG at the Initiation of RRT
Among 63,349 incident patients registered in the REIN during the study period, 1462 (2.3%) had ESRD related to MG: ALA in 267 (18%), LCDD in 334 (23%), and MCN in 861 (59%). Prevalence of patients with MG was stable (2%–3%) over time.
Patients’ characteristics are detailed in Table 1. Sex ratio of men to women was 1.26, and median age was 72.3 years. Dialysis was initiated as an emergency for 45.6% of patients, and 72% started HD on a central venous catheter. Most patients (95%) were treated with HD, mainly in hospital centers (92.6%); 5% were treated with peritoneal dialysis, and there was no preemptive KTR. Comorbid conditions were hypertension (50.1%), congestive heart failure (CHF; 15.9%), diabetes mellitus (11.6%), coronary artery disease (10.8%), chronic lung disease (8%), peripheral vascular disease (5.8%), and cerebrovascular disease (5.6%). Frailty was reported in 22% of patients.
Table 1.
Baseline characteristics of patients with monoclonal gammopathy and controls at the initiation of RRT in France between 2002 and 2011
| Characteristic | ALA (n=267) | LCDD (n=334) | MCN (n=861) | P Value | Patients with MG (n=1462) | Controls on Dialysis (n=5036) | P Value |
|---|---|---|---|---|---|---|---|
| Age (yr), median ± IQR | 70.6±14.6 | 72.3±16 | 72.9±14.3 | <0.001 | 72.3±15 | 70.9±22.2 | <0.001 |
| Men (%) | 172 (64.4) | 176 (52.7) | 467 (54.2) | <0.01 | 815 (55.7) | 3100 (61.6) | <0.001 |
| Hemodialysis started on catheter (%) | 156 (58.4) | 226 (67.7) | 673 (78.2) | <0.001 | 1055 (72.2) | 2225 (44.2) | <0.001 |
| Missing | 19 (7.1) | 23 (6.9) | 50 (5.8) | 92 (6.3) | 609 (12.1) | ||
| Dialysis start in emergency (%) | 86 (32.2) | 133 (39.8) | 447 (51.9) | <0.001 | 666 (45.6) | 1310 (26) | <0.001 |
| Missing | 20 (7.5) | 24 (7.2) | 65 (7.5) | 109 (7.5) | 581 (11.5) | ||
| First treatment modality (%) | |||||||
| Hemodialysis | 254 (95.1) | 312 (93.4) | 823 (95.6) | 0.30 | 1389 (95) | 4324 (85.9) | <0.001 |
| Peritoneal dialysis | 13 (4.9) | 22 (6.6) | 38 (4.4) | 73 (5) | 565 (11.2) | ||
| Transplantation | 0 | 0 | 0 | 0 | 144 (2.9) | ||
| Diabetes (%) | 23 (8.6) | 34 (10.2) | 113 (13.1) | 0.09 | 170 (11.6) | 1937 (38.5) | <0.001 |
| Missing | 17 (6.4) | 21 (6.3) | 48 (5.6) | 86 (5.9) | 279 (5.5) | ||
| High BP (%) | 132 (49.4) | 176 (52.7) | 424 (49.2) | 0.43 | 732 (50.1) | 3647 (72.4) | <0.001 |
| Missing | 20 (7.5) | 26 (7.8) | 59 (6.9) | 105 (7.2) | 442 (8.8) | ||
| Ever smoker (%) | |||||||
| Never | 135 (50.6) | 173 (51.8) | 470 (54.6) | 0.25 | 778 (53.2) | 2503 (49.7) | <0.001 |
| Current | 20 (7.5) | 19 (5.7) | 39 (4.5) | 78 (5.3) | 436 (8.7) | ||
| Former | 48 (18) | 53 (15.9) | 127 (14.8) | 228 (15.6) | 1082 (21.5) | ||
| Missing | 64 (24) | 89 (26.6) | 225 (26.1) | 378 (25.9) | 1015 (20.2) | ||
| Heart failure (%) | 72 (27) | 48 (14.4) | 112 (13) | <0.001 | 232 (15.9) | 1256 (24.9) | <0.001 |
| Missing | 26 (9.7) | 31 (9.3) | 61 (7.1) | 118 (8.1) | 478 (9.5) | ||
| Peripheral vascular disease (%) | 24 (9) | 15 (4.5) | 46 (5.3) | 0.03 | 85 (5.8) | 961 (19.1) | <0.001 |
| Missing | 29 (10.9) | 30 (9) | 68 (7.9) | 127 (8.7) | 509 (10.1) | ||
| Coronary artery disease (%) | 40 (15) | 30 (9) | 88 (10.2) | 0.04 | 158 (10.8) | 1155 (22.9) | <0.001 |
| Missing | 22 (8.2) | 31 (9.3) | 62 (7.2) | 115 (7.9) | 495 (9.8) | ||
| Cerebrovascular disease (%) | 16 (6) | 13 (3.9) | 53 (6.2) | 0.36 | 82 (5.6) | 503 (10) | <0.001 |
| Missing | 26 (9.7) | 37 (11.1) | 63 (7.3) | 126 (8.6) | 538 (10.7) | ||
| Chronic lung disease (%) | 30 (11.2) | 26 (7.8) | 61 (7.1) | 0.09 | 117 (8) | 584 (11.6) | <0.001 |
| Missing | 21 (7.9) | 31 (9.3) | 68 (7.9) | 120 (8.2) | 505 (10) | ||
| BMI (kg/m2), mean±SD | 23.2±3.9 | 23.9±4.5 | 24.1±4.4 | 0.07 | 23.9±4.4 | 25.8±5.6 | <0.001 |
| BMI<20 kg/m2 (%) | 38 (14) | 39 (12) | 92 (11) | 0.63 | 169 (12) | 469 (9.3) | <0.01 |
| Missing | 66 (25) | 97 (29) | 286 (33) | 449 (31) | 1497 (29.7) | ||
| Albumin (g/dl), mean±SD | 2.5±0.9 | 3.1±0.7 | 3.2±0.7 | <0.001 | 3.0±0.8 | 3.3±0.7 | <0.001 |
| Albumin <3.0 g/dl (%) | 115 (43.1) | 76 (22.8) | 179 (20.8) | <0.001 | 370 (25.3) | 703 (14) | <0.001 |
| Missing | 105 (39.3) | 157 (47) | 382 (44.4) | 644 (44) | 2402 (47.7) | ||
| Hemoglobin (g/dl), mean±SD | 10.5±2.1 | 9.7±1.8 | 9.3±1.7 | <0.001 | 9.6±1.9 | 10.3±1.8 | <0.001 |
| Missing (%) | 75 (28) | 97 (29) | 224 (26) | 396 (27) | 1352 (26.8) | ||
| Frailty (%) | 49 (18.4) | 64 (19.2) | 209 (24.3) | 0.02 | 322 (22) | 768 (15.3) | <0.001 |
| Missing | 50 (18.7) | 57 (17.1) | 174 (20.2) | 281 (19.2) | 1113 (22.1) |
ALA, immunoglobulin light chain amyloidosis; LCDD, light–chain deposition disease; MCN, myeloma cast nephropathy; MG, monoclonal gammopathy; IQR, interquartile range; BMI, body mass index.
Baseline characteristics differed between the groups (Table 1): patients with ALA were younger, had lower albumin levels, and suffered more frequently from CHF, peripheral vascular disease, and coronary artery disease; patients with MCN had lower hemoglobin levels, were more likely to be frail, and started HD more often in emergency on a central catheter.
Comparisons between Patients with MG and Control Patients on Chronic Dialysis
The control group comprised 5036 patients, and their characteristics differed from those of patients with MG (Table 1). Median follow-up was 27 months (IQR, 10–45). Compared with patients with MG (Table 2), more controls received KTR after a shorter dialysis duration. Thus, fewer controls remained on dialysis, despite a lower rate of renal recovery. Among control patients who remained on dialysis, median survival was better (51.6 months; 95% confidence interval [95% CI], 49.6 to 54.1) than in patients with MG (P<0.001). Multivariate analysis showed an adjusted hazard ratio (HR) of death of 2.18 [95% CI, 1.93 to 2.46 (P<0.001)] in patients with MG compared with controls.
Table 2.
Outcomes of patients with monoclonal gammopathy and control patients on chronic dialysis
| Events | ALA (n=267) | LCDD (n=334) | MCN (n=861) | P Value | Patients with MG (n=1462) | Controls on Dialysis (n=5036) | P Value |
|---|---|---|---|---|---|---|---|
| Remained on dialysis (%) | 239 (89.5) | 290 (86.8) | 743 (86.3) | 0.39 | 1272 (87) | 3656 (72.6) | <0.001 |
| Death among patients on dialysis (%) | 173 (72.4) | 218 (72.2) | 591 (79.5) | 0.67 | 982 (77.2) | 2171 (59.4) | <0.001 |
| Renal recovery (%) | 8 (3) | 28 (8.4) | 97 (11.3) | <0.001 | 133 (9.1) | 246 (4.9) | <0.001 |
| Waiting list registration (%) | 20 (7.5) | 15 (4.5) | 11 (1.3) | <0.001 | 46 (3.1) | 1447 (28.7) | <0.001 |
| Kidney transplantation (%) | 17 (6.4) | 11 (3.3) | 6 (0.7) | <0.001 | 34 (2.3) | 1065 (21.1) | <0.001 |
| Loss of follow-up (%) | 3 (1.1) | 5 (1.5) | 15 (1.7) | 0.88 | 23 (1.6) | 69 (1.4) | 0.56 |
ALA, immunoglobulin light chain amyloidosis; LCDD, light–chain deposition disease; MCN, myeloma cast nephropathy; MG, monoclonal gammopathy.
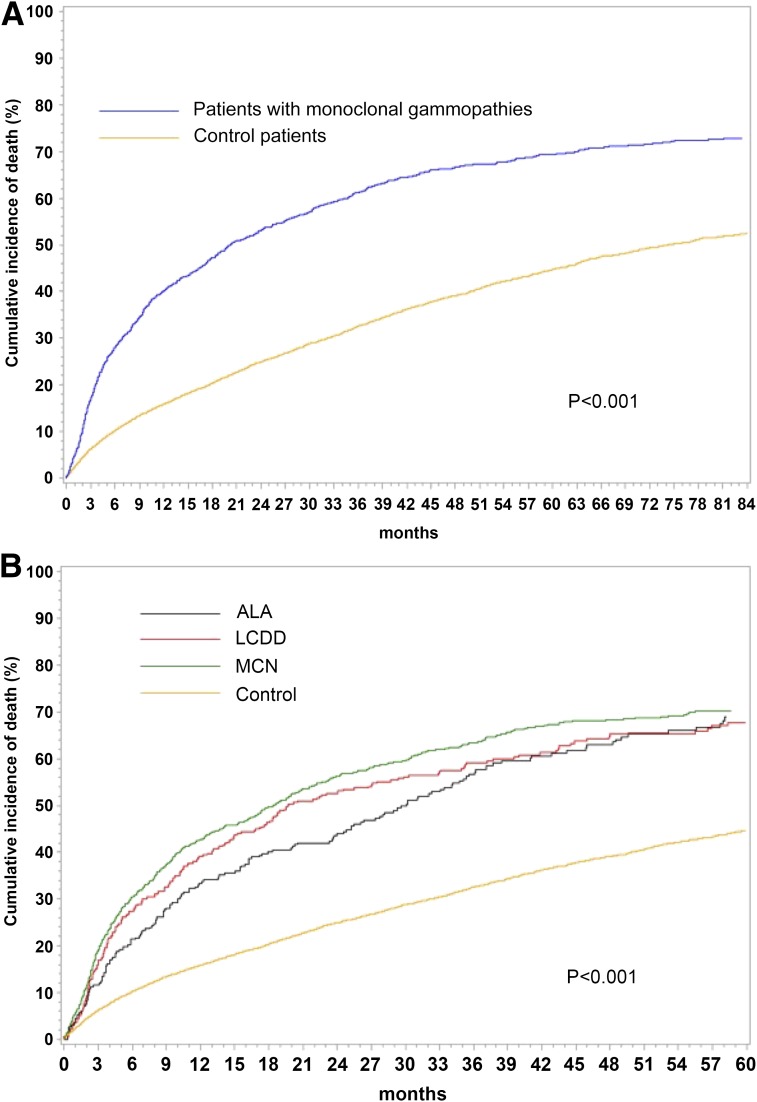
Similarly, cumulative incidence of death using competing risks analysis was lower in control patients than in patients with MG (P<0.001).
Cardiovascular mortality was more frequent in the control group, whereas malignancy-related mortality caused by hematologic malignancies and also, solid tumors was less frequent (Table 4).
Table 4.
Causes of death in patients with monoclonal gammopathy and control patients on chronic dialysis
| Causes of Death | ALA (n=173) | LCDD (n=218) | MCN (n=591) | P Value | Patients with MG (n=982) | Controls on Dialysis (n=2171) | P Value |
|---|---|---|---|---|---|---|---|
| Cardiovascular disease (%) | 53 (30.6) | 33 (15.1) | 91 (15.4) | <0.001 | 177 (18) | 773 (35.6) | <0.001 |
| Sudden cardiac death | 20 (11.6) | 12 (5.5) | 42 (7.1) | 74 (7.5) | 239 (11) | ||
| Cerebrovascular disease | 7 (4) | 7 (3.2) | 17 (2.9) | 31 (3.2) | 98 (4.5) | ||
| Congestive heart failure | 12 (6.9) | 4 (1.8) | 10 (1.7) | 26 (2.6) | 128 (5.9) | ||
| Myocardial infarction | 4 (2.3) | 3 (1.4) | 8 (1.4) | 15 (1.5) | 122 (5.6) | ||
| Cardiac arrhythmia | 3 (1.7) | 3 (1.4) | 4 (0.7) | 10 (1) | 41 (1.9) | ||
| Pulmonary embolism | 0 | 1 (0.5) | 1 (0.2) | 2 (0.2) | 12 (0.6) | ||
| Others | 7 (4) | 3 (1.4) | 9 (1.5) | 19 (1.9) | 133 (6.1) | ||
| Infectious disease (%) | 20 (11.6) | 33 (15.1) | 78 (13.2) | 0.58 | 131 (13.3) | 247 (11.4) | 0.11 |
| Septicemia | 15 (8.7) | 17 (7.8) | 41 (6.9) | 73 (7.4) | 146 (6.7) | ||
| Pulmonary infection | 2 (1.2) | 8 (3.7) | 22 (3.7) | 32 (3.3) | 56 (2.6) | ||
| Peritonitis | 0 | 2 (0.9) | 4 (0.7) | 6 (0.6) | 12 (0.6) | ||
| Others | 3 (1.7) | 6 (2.8) | 11 (1.9) | 20 (2) | 33 (1.5) | ||
| Malignancy (%) | 21 (12.1) | 71 (32.6) | 246 (41.6) | <0.001 | 338 (34.4) | 188 (8.7) | <0.001 |
| Solid malignancy | 20 (11.6) | 50 (22.9) | 105 (17.8) | 175 (17.8) | 180 (8.3) | ||
| Hematologic disease | 1 (0.6) | 21 (9.6) | 141 (23.9) | 163 (16.6) | 8 (0.4) | ||
| Others (%) | |||||||
| Cachexia | 15 (8.7) | 7 (3.2) | 29 (4.9) | 0.05 | 51 (5.2) | 144 (6.6) | 0.12 |
| All-causes bleedings | 1 (0.6) | 4 (1.8) | 7 (1.2) | 0.05 | 12 (1.2) | 44 (2) | 0.11 |
| Hyperkaliemia | 0 | 2 (0.9) | 5 (0.8) | 0.65 | 7 (0.7) | 17 (0.8) | 0.83 |
| Others known causes | 29 (16.8) | 21 (9.6) | 56 (9.5) | 0.02 | 106 (10.8) | 295 (13.6) | 0.03 |
| Unknown causes | 34 (19.7) | 47 (21.6) | 79 (13.4) | <0.01 | 160 (16.3) | 462 (21.3) | 0.001 |
ALA, immunoglobulin light chain amyloidosis; LCDD, light–chain deposition disease; MCN, myeloma cast nephropathy; MG, monoclonal gammopathy.
Survival of Patients with MG
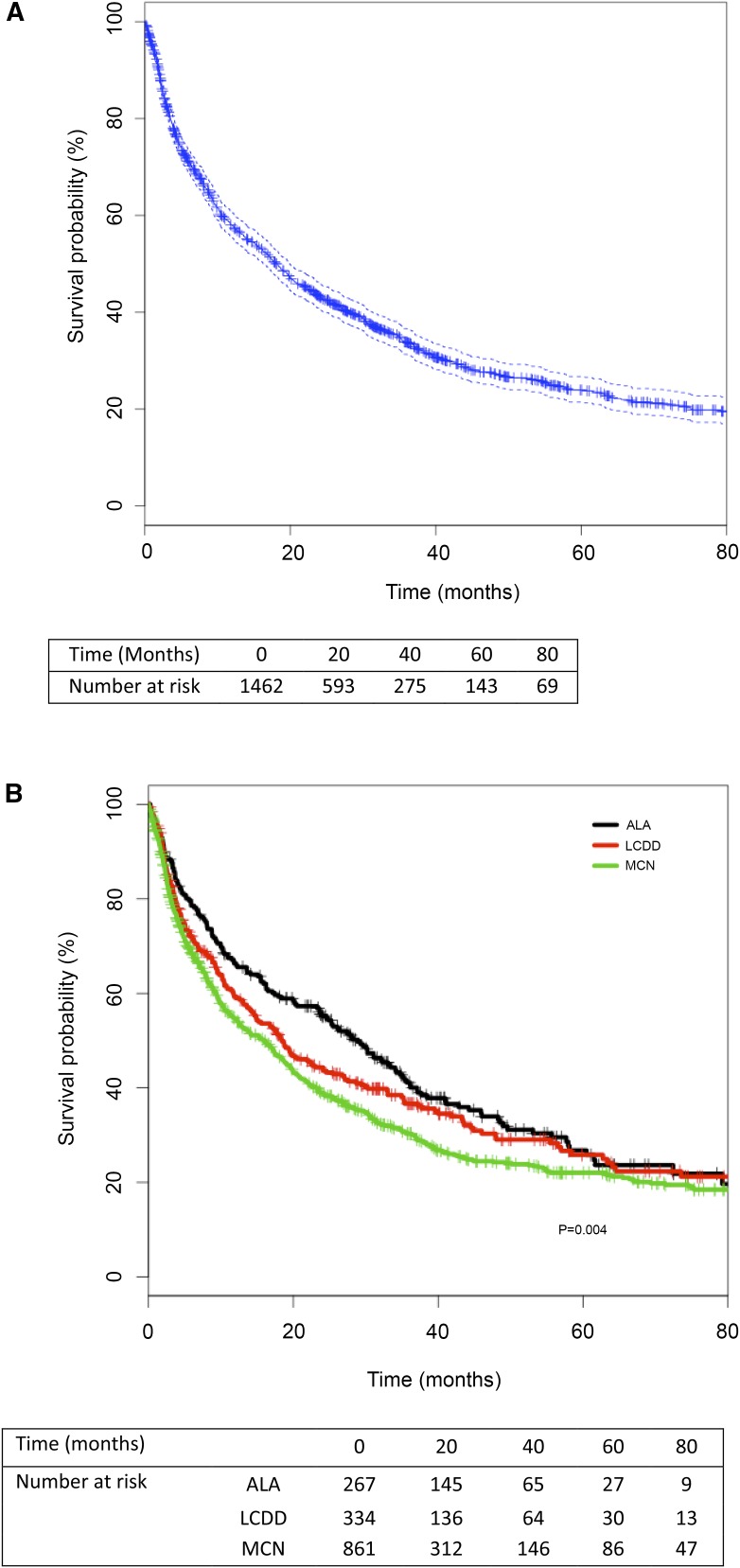
Median follow-up was 13.1 months (IQR, 4–33). Overall, 1272 (87%) patients remained on chronic dialysis, among whom 982 (77.2%) died after median dialysis duration of 8.8 months (IQR, 3–30). Death occurred in 173 (72.4%) patients with ALA, 218 (75.2%) patients with LCDD, and 591 (79.5%) patients with MCN (Table 2). Median survival of patients with MG who remained on dialysis was 18.3 months (95% CI, 16.3 to 19.9). Survival rates at 1, 3, 5, and 8 years were 58%, 34%, 24%, and 18%, respectively (Figure 1A). Survival censored for KTR, renal recovery, loss of follow-up, or end of the study period was better in patients with ALA than in patients with MCN (P=0.004 between the three groups) (Figure 1B). Median survival was 28.9 months (95% CI, 23.7 to 34.8) in ALA, 18.4 months (95% CI, 14.9 to 24.1) in LCDD, and 16 months (95% CI, 12.5 to 18.3) in patients with MCN. Survival rates at 1, 3, and 5 years were 66%, 41%, and 26%, respectively, in ALA; 59%, 37%, and 26%, respectively, in LCDD; and 55%, 30%, and 22% in MCN, respectively.
Figure 1.
Kaplan–Meier survival analyses of patients with monoclonal gammopathies. (A) Overall survival probability of patients with monoclonal gammopathies (censored for kidney transplantation, renal recovery, and loss of follow-up or end of the study period). (B) Survival probabilities of patients with immunoglobulin light chain amyloidosis (ALA), light–chain deposition disease (LCDD), or myeloma cast nephropathy (MCN) (censored for kidney transplantation, renal recovery, and loss of follow-up or end of the study period).
Cumulative incidences of death were also analyzed considering transplantation and renal recovery as competing risks (Figure 2). Similarly, the risk of death was higher in patients with MCN than in patients with ALA (P=0.04).
Figure 2.
Cumulative incidence of death is higher in patients with monoclonal gammopathies than in control patients. Cumulative incidence of death (considering transplantation and renal recovery as competing risks) in (A) patients with monoclonal gammopathies and control patients on chronic dialysis and (B) patients with AL amyloidosis (ALA), light–chain deposition disease (LCDD), or myeloma cast nephropathy (MCN) and control patients on chronic dialysis.
Incidence of Renal Recovery
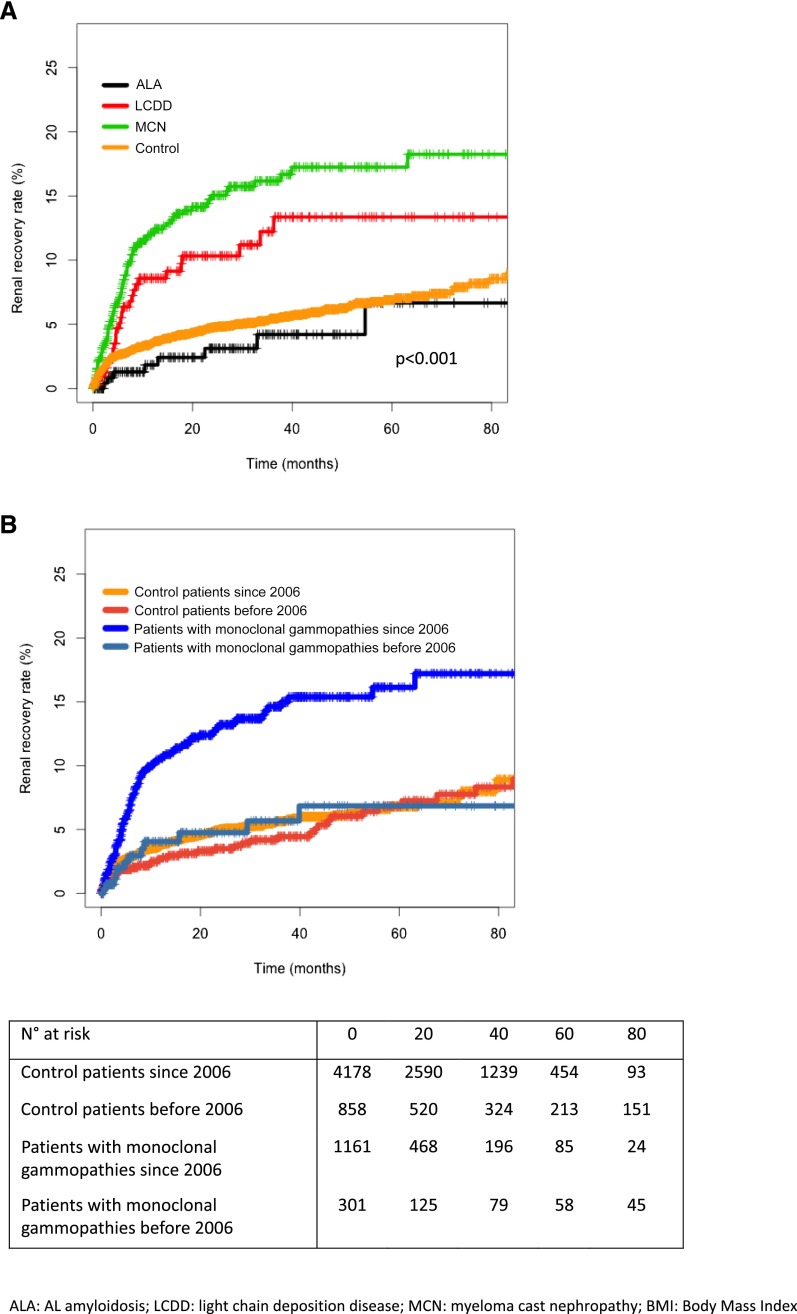
Renal recovery was reported in 133 (9.1%) patients (Table 2) after median dialysis duration of 5 months (IQR, 2–9). Renal recovery was more frequent in patients with MCN (11.3%) than in patients with LCDD (8.4%) and patients with ALA (3%; P<0.001) (Figure 3A). No patient stopped dialysis because of therapy withdrawal. Dialysis was eventually resumed in 20 (15%) of these patients after a median duration of 9 months (IQR, 4–19). Thirty-four (26%) patients died after a period of renal recovery, of which ten had resumed dialysis before dying.
Figure 3.
Renal recovery rates differ between monoclonal gammopathies and controls and has increased after 2006. Cumulative incidence of renal recovery in (A) patients with AL amyloidosis (ALA), light–chain deposition disease (LCDD), or myeloma cast nephropathy (MCN) and control patients on chronic dialysis and (B) patients with monoclonal gammopathies and control patients on chronic dialysis before or since January 1, 2006. Patients with monoclonal gammopathies: log–rank P value, P<0.001; control patients: log–rank P value, P=0.40).
Recovery rate was higher in patients with MG who had started dialysis since January 1, 2006 versus those who had started dialysis before January 1, 2006 (P<0.001), whereas no difference was observed in control patients between the two periods (Figure 3B).
Of note, baseline characteristics differed between patients who did and did not recover renal function (Table 3): patients who recovered were more likely to have started dialysis on a catheter in emergency. They were more likely to have diabetes, had a higher body mass index and serum albumin level, and had lower hemoglobin level.
Table 3.
Characteristics of patients with monoclonal gammopathy who did and did not recover renal function
| Characteristic | Nonrecovery (n=1329) | Recovery (n=133) | All Patients (n=1462) | P Value |
|---|---|---|---|---|
| Age (yr), mean±SD | 70.6±11.5 | 69.7±10.9 | 70.5±11.5 | <0.001 |
| Men (%) | 744 (56) | 71 (53.4) | 815 (55.7) | 0.63 |
| Catheter (%) | 943 (71) | 112 (84.2) | 1055 (72.2) | 0.004 |
| Missing | 87 (6.5) | 5 (3.7) | 92 (6.3) | |
| Dialysis start in emergency (%) | 593 (44.6) | 73 (54.8) | 666 (45.6) | 0.03 |
| Missing | 100 (7.5) | 9 (6.8) | 109 (7.5) | |
| First treatment modality (%) | ||||
| Hemodialysis | 1259 (94.7) | 130 (97.7) | 1389 (95) | 0.19 |
| Peritoneal dialysis | 70 (5.3) | 3 (2.3) | 73 (5) | |
| Diabetes (%) | 144(10.8) | 26 (19.5) | 170 (11.6) | 0.003 |
| Missing | 76 (5.7) | 10 (7.5) | 86 (5.9) | |
| High BP (%) | 674 (50.7) | 58 (43.6) | 732 (50.1) | 0.16 |
| Missing | 94 (7.1) | 11 (8.2) | 105 (7.2) | |
| Ever smoker (%) | ||||
| Never | 716 (53.9) | 62 (46.6) | 778 (53.2) | 0.48 |
| Current | 73 (5.5) | 5 (3.8) | 78 (5.3) | |
| Former | 205 (15.4) | 23 (17.3) | 228 (15.6) | |
| Missing | 335 (25.2) | 43 (32.3) | 378 (25.9) | |
| Heart failure (%) | 214 (16.1) | 18 (13.5) | 232 (15.9) | 0.52 |
| Missing | 107 (8.1) | 11 (8.3) | 118 (8.1) | |
| Peripheral vascular disease (%) | 80 (6) | 5 (3) | 85 (5.8) | 0.41 |
| Missing | 113 (8.5) | 14 (10.5) | 127 (8.7) | |
| Coronary artery disease (%) | 143 (10.8) | 15 (11.3) | 158 (10.8) | 0.96 |
| Missing | 104 (7.8) | 11 (8.3) | 115 (7.9) | |
| Cerebrovascular disease (%) | 73 (5.5) | 9 (6.8) | 82 (5.6) | 0.65 |
| Missing | 113 (8.5) | 13 (9.8) | 126 (8.6) | |
| Chronic lung disease (%) | 112 (8.4) | 5 (3.8) | 117 (8) | 0.08 |
| Missing | 109 (8.2) | 11 (8.3) | 120 (8.2) | |
| BMI (kg/m2), mean±SD | 23.8±4.4 | 24.1±4.3 | 23.9±4.4 | <0.001 |
| BMI<20 kg/m2 (%) | 152 (11.4) | 17 (12.8) | 169 (12) | 0.96 |
| Missing | 414 (31.2) | 35 (26.3) | 449 (31) | |
| Albumin (g/dl), mean±SD | 3.0±0.8 | 3.2±0.8 | 3.0±0.8 | <0.001 |
| Albumin <3.0 g/dl (%) | 332 (25) | 38 (28.6) | 370 (25.3) | 0.62 |
| Missing | 601 (45.2) | 43 (32.3) | 644 (44) | |
| Hemoglobin (g/dl), mean±SD | 9.6±1.9 | 9.2±1.6 | 9.6±1.9 | <0.001 |
| Missing (%) | 368 (27.7) | 28 (21) | 396 (27) | |
| Frailty (%) | 106 (8) | 5 (3.8) | 0.11 | |
| Missing | 258 (19.4) | 23 (17.3) | 281 (19.2) |
BMI, body mass index.
Incidence of KTR
Forty-six (3.1%) patients were registered on the KTR waiting list after a median of 15 months (IQR, 7–34), and 34 (2.3%) patients received a KTR after a median of 32.5 months (IQR, 18–55) on dialysis. Registration on the KTR waiting list was more frequent in patients with ALA (7.5%) than in patients with LCDD (4.5%) and patients with MCN (1.5%; P<0.001). KTR was also more frequent in patients with ALA (6.4%) than in patients with LCDD (3.3%) and patients with MCN (0.7%; P<0.001). Median follow-up after KTR was 32 months (IQR, 12–59). Only one patient lost KTR function after 9 months and died 8 months after she resumed dialysis.
Principal Causes of Death in Patients with MG on Chronic Dialysis
Main causes of death in patients with MG were malignancy in 338 (34.4%) patients, cardiovascular events in 177 (18%) patients, infectious diseases in 131 (13.3%) patients, and cachexia in 51 (5.2%) patients (Table 4). Cardiovascular events were more frequent in patients with ALA (30.6%) than in patients with LCDD (15.1%) and patients with MCN (15.4%; P<0.001), with more frequent sudden death (11.6% versus 5.5% and 7.1%, respectively) and CHF (6.9% versus 1.8% and 1.7%, respectively). Death caused by cachexia was also more frequent in patients with ALA (8.7%) than in patients with LCDD (3.2%) and patients with MCN (4.9%; P=0.05).
Risk Factors of Mortality in Patients with MG
Seven parameters were associated with mortality with univariate Kaplan–Meier survival analysis (Table 5): MCN group, HD initiation on a catheter, dialysis start in emergency, CHF, body mass index <20 kg/m2, frailty, and cardiac arrhythmia. Year of RRT initiation was not significantly associated with mortality in univariate Cox analysis (HR, 0.98%; 95% CI, 0.96 to 1.01; P=0.13).
Table 5.
Risk factors of mortality in patients with monoclonal gammopathy on chronic dialysis
| Characteristic | Median Survival (mo) or Hazard Ratio | 95% Confidence Interval | Log–Rank P Value |
|---|---|---|---|
| Univariate analysis | |||
| Men | 17.93 | 15.51 to 20.39 | 0.47 |
| Women | 18.33 | 15.87 to 21.7 | |
| ALA | 28.92 | 23.70 to 34.75 | Reference |
| LCDD | 18.39 | 14.85 to 24.10 | 0.15 |
| MCN | 16.00 | 12.52 to 18.33 | 0.001 |
| Hemodialysis | 18.33 | 16.36 to 20 | 0.65 |
| Peritoneal dialysis | 15.57 | 5.54 to 30.39 | |
| No catheter | 28.03 | 23.87 to 32.30 | <0.001 |
| Catheter | 16.49 | 13.93 to 18.82 | |
| No dialysis start in emergency | 20.23 | 18.30 to 25.28 | <0.01 |
| Dialysis start in emergency | 14.85 | 11.61 to 19.34 | |
| No chronic lung disease | 18.43 | 16.69 to 20.39 | 0.06 |
| Chronic lung disease | 16.23 | 9.87 to 21.70 | |
| No cerebrovascular disease | 18.07 | 16.22 to 20 | 0.96 |
| Cerebrovascular disease | 19.54 | 10.43 to 30.07 | |
| No congestive heart failure | 19.67 | 17.93 to 22.75 | <0.001 |
| Congestive heart failure | 10.16 | 8.16 to 12.82 | |
| No coronary artery disease | 18.59 | 16.69 to 20.59 | 0.10 |
| Coronary artery disease | 16.23 | 10.30 to 20.13 | |
| No peripheral vascular disease | 18.39 | 16.36 to 20.23 | 0.41 |
| Peripheral vascular disease | 15.87 | 9.02 to 22.62 | |
| No diabetes | 18.75 | 16.95 to 20.72 | 0.26 |
| Diabetes | 13.97 | 9.48 to 19.93 | |
| No high BP | 16.69 | 14.03 to 19.93 | 0.10 |
| High BP | 19.34 | 17.34 to 23.21 | |
| BMI>20 kg/m2 | 24.39 | 19.90 to 28.66 | 0.01 |
| BMI<20 kg/m2 | 16.62 | 11.21 to 23.61 | |
| Frailty | 5.15 | 4.07 to 6.82 | <0.001 |
| No frailty | 23.61 | 20.49 to 27.38 | |
| No cardiac arrhythmia | 18.72 | 16.49 to 20.72 | 0.05 |
| Cardiac arrhythmia | 17.11 | 10.49 to 21.70 | |
| Multivariate analysis | |||
| Age per yr | 1.03 | 1.02 to 1.03 | <0.001 |
| Frailty | 1.93 | 1.58 to 2.36 | <0.001 |
| Congestive heart failure | 1.54 | 1.23 to 1.93 | <0.001 |
| Catheter | 1.40 | 1.11 to 1.75 | 0.004 |
| Year of RRT initiation per yr | 0.95 | 0.91 to 0.99 | <0.01 |
| High BP | 0.80 | 0.67 to 0.97 | 0.02 |
ALA, immunoglobulin light chain amyloidosis; LCDD, light–chain deposition disease; MCN, myeloma cast nephropathy; BMI, body mass index.
Four risk factors were independently associated with mortality after multivariate logistic Cox regression analysis: age (HR per year increase, 1.03%; 95% CI, 1.02 to 1.03; P<0.001), frailty (HR, 1.93%; 95% CI, 1.58 to 2.36; P<0.001), CHF (HR, 1.54%; 95% CI, 1.23 to 1.93; P<0.001), and HD initiation on a catheter (HR, 1.40; 95% CI, 1.11 to 1.75; P=0.004). Two factors were independently associated with a lower mortality: year of RRT initiation (HR per year increase, 0.95%; 95% CI, 0.91 to 0.99; P<0.01) and high BP (HR, 0.80%; 95% CI, 0.67 to 0.97; P=0.02) (Table 5).
Discussion
This national cohort study shows that outcome of patients with MG on chronic dialysis is poor but improving over time, with an increased rate of renal recovery after January 1, 2006. Survival of patients with ALA was comparable with data from the Australia and New Zealand Dialysis and Transplant Registry, comprising 490 patients with ALA or amyloid A (AA) amyloidosis on chronic dialysis (16), and a smaller French cohort of patients with ALA (17). Survival was better (39 months) in a British cohort of 221 patients with ALA (10) but worse (11 months) in an Italian cohort of 59 patients (18). A mild increase in survival of patients with ALA on dialysis was reported by Gertz and coworkers (19,20) between 1992 (19) and 2009 (20).
Survival was lower in patients with MCN from this cohort, consistent with a Spanish cohort of 28 patients with MM incident on RRT in 1980–2000 (21) and the 10.8-month survival reported in the ERA-EDTA Registry of 2453 incident patients with MM in 1985–2005 (3). Survival did not differ between two time periods (1986–1990 versus 2001–2005) in this large European cohort. Mortality was higher in patients with MG than in controls in this study, such as in data from the US Renal Data System Registry (1992–1997) showing a 2-year mortality of 58% in patients with MM versus 31% in control patients on dialysis (5). A survival of 49 months was reported in an Italian cohort of 63 patients with LCDD and renal involvement, with no association of RRT requirement with mortality (16).
Malignancy was the first cause of death in our study like in the ERA-EDTA Study (3), and there were also similar rates of cardiovascular and infectious causes of death in both studies.
Independent risk factors of mortality were age, frailty, CHF, and dialysis initiation on a catheter. Frailty has already been described as an independent risk factor of mortality in chronic dialysis (22,23), and CHF is a major prognostic factor in patients with ALA (17). The surprising association of high BP at the initiation of dialysis with a reduced risk of death has been reported before in dialysis (24) and could be of particular relevance in patients with ALA, where low BP can be caused by CHF or dysautonomia. The year of RRT initiation was also associated with a lower risk of death, with the probability of death decreasing over the years when taking into account patients characteristics in multivariate analysis. This may be linked to global improvement of dialysis care and/or increasing use of more effective chemotherapies.
Renal recovery was not rare in patients with MG, especially patients with MCN, and was higher than in controls. It was more frequent after January 1, 2006, which could be related to the widening use of novel medications, including bortezomib. Indeed, patients with ESRD were excluded from the first studies on bortezomib in MM (25), but bortezomib became broadly used in patients with MM or ALA on dialysis in France after January 1, 2006. In 2007, Chanan-Khan et al. (26) reported a 75% response rate in 24 patients on dialysis treated between 2003 and 2005, among which 17% had a renal recovery. In acute MCN, renal recovery was observed after therapy including bortezomib in eight of ten patients requiring dialysis (27) in a Greek cohort and three of 14 patients in a retrospective Italian cohort (28). Recovery rates were lower in our study, probably because only patients with chronic ESRD were included in the REIN. The possible occurrence of late renal recovery, particularly in patients with MM, is noteworthy.
Registration on the KTR waiting list and KTR were rare in our cohort. Higher KTR rates have been reported in ALA (10.2% [29] and 9.4% [16]), with a shorter delay (27.6 months [29]), than in other cohorts. Mortality after KTR was low in our cohort, comparable with the 52% after 55 months observed in a British cohort (29). KTR was anecdotal in 1998 in patients with MG (30), and although it is now considered a therapeutic option in patients in remission, it remained exceptional in patients with MCN from our cohort. Risks of KTR in this population include recurrence of myeloma, monoclonal Ig–mediated graft dysfunction, and infections (31). Similarly, KTR rate in patients with LCDD was very low. A case series of seven patients with LCDD reported a median renal graft survival of 37.2 months, with two deaths caused by the progression of MM 3 months after KTR. Thus, benefit of KTR in patients with LCDD remains uncertain, especially in patients with MM (32).
We acknowledge certain limitations to this study, mainly related to the absence of available data on hematologic disease history and specific treatment in the REIN. Indeed, the REIN is the comprehensive national registry of all patients starting RRT in France, and no disease-specific data is recorded. Moreover, accurate coding of primary renal diagnose could not be verified retrospectively, because no renal biopsy data were available. Indeed, although the French thesaurus seems more comprehensive than the ERA-EDTA Study diagnostic codes (33,34), it might not have corresponded exactly to the evolving classification of MGs. Another pitfall is the possible overlap of MGs: there can be multiple causes of ESRD in a single patient with MM or ALA. Also, patients considered as having ESRD but recovering renal function during the follow-up had, in fact, prolonged AKI. However, such patients were included in the REIN similarly before and after January 1, 2006, and the increase in recovery rate after January 1, 2006 may reflect improvement of prolonged AKI recovery. Patients who recovered renal function were not systematically followed up in the REIN Registry after recovery, which is another limitation of this study, because only the outcome of patients with MG who remained on dialysis could be analyzed.
However, our study is the first to analyze together and then, compare between groups the characteristics and outcomes of patients on chronic dialysis with ALA, LCDD, or MCN. Completeness of the REIN Registry is the major strength of this study. Because of participation of all dialysis units in France, all patients on chronic dialysis caused by an MG were included. Another strength is the registration of easily assessable and meaningful clinical settings, like frailty. Last, the evaluation of renal recovery is of particular interest in patients with MG, and factors associated with renal recovery should be further investigated in future studies, especially regarding efficacy of novel chemotherapies.
Disclosures
None.
Acknowledgments
No financial support was received specifically for this study. REIN registry is funded by the French government. This study has been presented as an abstract at the American Society of Nephrology Kidney Week 2014 (Philadelphia, PA, November 11–16).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Phekoo KJ, Schey SA, Richards MA, Bevan DH, Bell S, Gillett D, Møller H; Consultant Haematologists, South Thames Haematology Specialist Committee: A population study to define the incidence and survival of multiple myeloma in a National Health Service Region in UK. Br J Haematol 127: 299–304, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Turesson I, Zettervall O, Cuzick J, Waldenstrom JG, Velez R: Comparison of trends in the incidence of multiple myeloma in Malmö, Sweden, and other countries, 1950–1979. N Engl J Med 310: 421–424, 1984 [DOI] [PubMed] [Google Scholar]
- 3.Tsakiris DJ, Stel VS, Finne P, Fraser E, Heaf J, de Meester J, Schmaldienst S, Dekker F, Verrina E, Jager KJ: Incidence and outcome of patients starting renal replacement therapy for end-stage renal disease due to multiple myeloma or light-chain deposit disease: An ERA-EDTA Registry study. Nephrol Dial Transplant 25: 1200–1206, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Haynes RJ, Read S, Collins GP, Darby SC, Winearls CG: Presentation and survival of patients with severe acute kidney injury and multiple myeloma: A 20-year experience from a single centre. Nephrol Dial Transplant 25: 419–426, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Abbott KC, Agodoa LY: Multiple myeloma and light chain-associated nephropathy at end-stage renal disease in the United States: Patient characteristics and survival. Clin Nephrol 56: 207–210, 2001 [PubMed] [Google Scholar]
- 6.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, San-Miguel JF, Bladé J, Boccadoro M, Cavenagh J, Dalton WS, Boral AL, Esseltine DL, Porter JB, Schenkein D, Anderson KC; Assessment of Proteasome Inhibition for Extending Remissions (APEX) Investigators: Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med 352: 2487–2498, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust JA, Greipp PR, Kyle RA, Gertz MA: Improved survival in multiple myeloma and the impact of novel therapies. Blood 111: 2516–2520, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comission de transparence de la république française: Avis de la comission sur le Velcade, 2004. Available at: www.has-sante.fr/portail/upload/docs/application/pdf/ct031654.pdf. Accessed October 13, 2004
- 9.Pinney JH, Smith CJ, Taube JB, Lachmann HJ, Venner CP, Gibbs SD, Dungu J, Banypersad SM, Wechalekar AD, Whelan CJ, Hawkins PN, Gillmore JD: Systemic amyloidosis in England: An epidemiological study. Br J Haematol 161: 525–532, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinney JH, Lachmann HJ, Bansi L, Wechalekar AD, Gilbertson JA, Rowczenio D, Sattianayagam PT, Gibbs SD, Orlandi E, Wassef NL, Bradwell AR, Hawkins PN, Gillmore JD: Outcome in renal AL amyloidosis after chemotherapy. J Clin Oncol 29: 674–681, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Ronco P, Plaisier E, Mougenot B, Aucouturier P: Immunoglobulin light (heavy)-chain deposition disease: From molecular medicine to pathophysiology-driven therapy. Clin J Am Soc Nephrol 1: 1342–1350, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Randall RE, Williamson WC, Jr., Mullinax F, Tung MY, Still WJS: Manifestations of systemic light chain deposition. Am J Med 60: 293–299, 1976 [DOI] [PubMed] [Google Scholar]
- 13.Ronco P: Disease classification: A pitfall of the ERA/EDTA registry? Nephrol Dial Transplant 25: 1022–1024, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Pozzi C, D’Amico M, Fogazzi GB, Curioni S, Ferrario F, Pasquali S, Quattrocchio G, Rollino C, Segagni S, Locatelli F: Light chain deposition disease with renal involvement: Clinical characteristics and prognostic factors. Am J Kidney Dis 42: 1154–1163, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Couchoud C, Stengel B, Landais P, Aldigier JC, de Cornelissen F, Dabot C, Maheut H, Joyeux V, Kessler M, Labeeuw M, Isnard H, Jacquelinet C: The renal epidemiology and information network (REIN): A new registry for end-stage renal disease in France. Nephrol Dial Transplant 21: 411–418, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Tang W, McDonald SP, Hawley CM, Badve SV, Boudville N, Brown FG, Clayton PA, Campbell SB, de Zoysa JR, Johnson DW: End-stage renal failure due to amyloidosis: Outcomes in 490 ANZDATA registry cases. Nephrol Dial Transplant 28: 455–461, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Bollée G, Guery B, Joly D, Snanoudj R, Terrier B, Allouache M, Mercadal L, Peraldi MN, Viron B, Fumeron C, Elie C, Fakhouri F: Presentation and outcome of patients with systemic amyloidosis undergoing dialysis. Clin J Am Soc Nephrol 3: 375–381, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergesio F, Ciciani AM, Manganaro M, Palladini G, Santostefano M, Brugnano R, Di Palma AM, Gallo M, Rosati A, Tosi PL, Salvadori M; Immunopathology Group of the Italian Society of Nephrology: Renal involvement in systemic amyloidosis: An Italian collaborative study on survival and renal outcome. Nephrol Dial Transplant 23: 941–951, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Gertz MA, Kyle RA, O'Fallon WM: Dialysis support of patients with primary systemic amyloidosis: A study of 211 patients. Arch Intern Med 152: 2245–2250, 1992 [PubMed] [Google Scholar]
- 20.Gertz MA, Leung N, Lacy MQ, Dispenzieri A, Zeldenrust SR, Hayman SR, Buadi FK, Dingli D, Greipp PR, Kumar SK, Lust JA, Rajkumar SV, Russell SJ, Witzig TE: Clinical outcome of immunoglobulin light chain amyloidosis affecting the kidney. Nephrol Dial Transplant 24: 3132–3137, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Martín Reyes G, Valera A, Frutos MA, Ramos B, Ordóñez V, López de Novales E: Survival of myeloma patients treated with dialysis. Nefrologia 23: 131–136, 2003 [PubMed] [Google Scholar]
- 22.Couchoud C, Labeeuw M, Moranne O, Allot V, Esnault V, Frimat L, Stengel B; French Renal Epidemiology and Information Network (REIN) registry: A clinical score to predict 6-month prognosis in elderly patients starting dialysis for end-stage renal disease. Nephrol Dial Transplant 24: 1553–1561, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romeu M, Couchoud C, Delarozière JC, Burtey S, Chiche L, Harlé JR, Gondouin B, Brunet P, Berland Y, Jourde-Chiche N: Survival of patients with ANCA-associated vasculitis on chronic dialysis: Data from the French REIN registry from 2002 to 2011. QJM 107: 545–555, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD: Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int 63: 793–808, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina M, Alexanian R, Siegel D, Orlowski RZ, Kuter D, Limentani SA, Lee S, Hideshima T, Esseltine DL, Kauffman M, Adams J, Schenkein DP, Anderson KC: A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med 348: 2609–2617, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Chanan-Khan AA, Kaufman JL, Mehta J, Richardson PG, Miller KC, Lonial S, Munshi NC, Schlossman R, Tariman J, Singhal S: Activity and safety of bortezomib in multiple myeloma patients with advanced renal failure: A multicenter retrospective study. Blood 109: 2604–2606, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Kastritis E, Anagnostopoulos A, Roussou M, Gika D, Matsouka C, Barmparousi D, Grapsa I, Psimenou E, Bamias A, Dimopoulos MA: Reversibility of renal failure in newly diagnosed multiple myeloma patients treated with high dose dexamethasone-containing regimens and the impact of novel agents. Haematologica 92: 546–549, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Morabito F, Gentile M, Ciolli S, Petrucci MT, Galimberti S, Mele G, Casulli AF, Mannina D, Piro E, Pinotti G, Palmieri S, Catalano L, Callea V, Offidani M, Musto P, Bringhen S, Baldini L, Tosi P, Di Raimondo F, Boccadoro M, Palumbo A, Cavo M: Safety and efficacy of bortezomib-based regimens for multiple myeloma patients with renal impairment: A retrospective study of Italian Myeloma Network GIMEMA. Eur J Haematol 84: 223–228, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Pinney JH, Lachmann HJ, Sattianayagam PT, Gibbs SD, Wechalekar AD, Venner CP, Whelan CJ, Gilbertson JA, Rowczenio D, Hawkins PN, Gillmore JD: Renal transplantation in systemic amyloidosis—importance of amyloid fibril type and precursor protein abundance. Am J Transplant 13: 433–441, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Montseny JJ, Kleinknecht D, Meyrier A, Vanhille P, Simon P, Pruna A, Eladari D: Long-term outcome according to renal histological lesions in 118 patients with monoclonal gammopathies. Nephrol Dial Transplant 13: 1438–1445, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Heher EC, Rennke HG, Laubach JP, Richardson PG: Kidney disease and multiple myeloma. Clin J Am Soc Nephrol 8: 2007–2017, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leung N, Lager DJ, Gertz MA, Wilson K, Kanakiriya S, Fervenza FC: Long-term outcome of renal transplantation in light-chain deposition disease. Am J Kidney Dis 43: 147–153, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Tsakiris DJ, Simpson K, Stel V, Wanner C, Jager KJ: Disease classification: A pitfall of the ERA/EDTA registry? Nephrol Dial Transplant 25: 2799, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Venkat-Raman G, Tomson CR, Gao Y, Cornet R, Stengel B, Gronhagen-Riska C, Reid C, Jacquelinet C, Schaeffner E, Boeschoten E, Casino F, Collart F, De Meester J, Zurriaga O, Kramar R, Jager KJ, Simpson K; ERA-EDTA Registry: New primary renal diagnosis codes for the ERA-EDTA. Nephrol Dial Transplant 27: 4414–4419, 2012 [DOI] [PMC free article] [PubMed]