Abstract
Background and objectives
Soluble TNF–like weak inducer of apoptosis (sTWEAK) is a proinflammatory cytokine belonging to the TNF superfamily. sTWEAK concentrations have been associated with the presence of CKD and cardiovascular disease (CVD). We hypothesized that sTWEAK levels may relate to a higher prevalence of atherosclerotic plaques, vascular calcification, and cardiovascular outcomes observed in patients with CKD.
Design, setting, participants, & measurements
A 4-year prospective, multicenter, longitudinal study was conducted in 1058 patients with CKD stages 3–5D (mean age =58±13 years old; 665 men) but without any history of CVD from the NEFRONA Study (a study design on the prevalence of surrogate markers of CVD). Ankle-brachial index and B-mode ultrasound were performed to detect the presence of carotid and/or femoral atherosclerotic plaques together with biochemical measurements and sTWEAK assessment. Patients were followed for cardiovascular outcomes (follow-up of 3.13±1.15 years).
Results
Patients with more advanced CKD had lower sTWEAK levels. sTWEAK concentrations were independently and negatively associated with carotid intima-media thickness. sTWEAK levels were lower in patients with carotid atherosclerotic plaques but not in those with femoral plaques. After adjustment by confounders, the odds ratio (OR) for presenting carotid atherosclerotic plaques in patients in the lowest versus highest tertile of sTWEAK was 4.18 (95% confidence interval [95% CI], 2.89 to 6.08; P<0.001). Furthermore, sTWEAK levels were lower in patients with calcified carotid atherosclerotic plaques. The OR for presenting calcified carotid plaques was 1.77 (95% CI, 1.06 to 2.93; P=0.02) after multivariable adjustment. After the follow-up, 41 fatal and 68 nonfatal cardiovascular events occurred. In a Cox model, after controlling for potential confounding factors, patients in the lowest tertile of sTWEAK concentrations had a higher risk of fatal and nonfatal cardiovascular events (hazard ratio [HR], 2.40; 95% CI, 1.33 to 4.33; P=0.004) and cardiovascular mortality (HR, 2.67; 95% CI, 1.05 to 6.76; P=0.04).
Conclusions
Low sTWEAK levels were associated with the presence of carotid atherosclerotic plaques in patients with CKD. Additionally, lower sTWEAK levels were associated with a higher risk of cardiovascular morbidity and mortality.
Keywords: cardiovascular disease; chronic kidney disease; cytokines; outcomes; sTWEAK; ankle brachial index; carotid intima-media thickness; humans; prospective studies; renal insufficiency, chronic; tumor necrosis factors
Introduction
CKD is an independent factor for several health outcomes, including cardiovascular disease (CVD) (1). Compared with the CVD mortality in the general population, patients with CKD die at accelerated rate, 15–30 times higher than subjects without CKD (2). The mechanisms for the elevated CVD risk in patients with CKD are complex and may implicate changes in both the vasculature and the heart. The presence of accelerated atherosclerosis in CKD could play a role in the higher cardiovascular (CV) mortality. Atherosclerosis is a multifactorial disease characterized by chronic inflammation and excessive cell proliferation. Unveiling the role of cytokines as inflammatory messengers provided a potential mechanism, whereby risk factors for atherosclerosis can alter arterial wall and produce a systemic response that favors atherothrombotic events (3). TNF–like weak inducer of apoptosis (TWEAK) participates through its sole receptor fibroblast growth factor–inducible 14 (Fn14) in several cellular responses associated with atherosclerosis (4). Experimental studies have shown a key role of the TWEAK-Fn14 axis in atherosclerotic plaque development, progression, and rupture (5–7). Moreover, specific gain- or loss-of-function phenotypes have shown that TWEAK participates in the development of experimental abdominal aortic aneurysms, stroke, myocardial infarction, and heart failure (8–11).
TWEAK can be proteolytically processed by furin, leading to the release of soluble TNF–like weak inducer of apoptosis (sTWEAK) (12). We initially showed that sTWEAK is secreted in lower amounts from human carotid atherosclerotic plaques than in healthy arteries, suggesting its potential role as a biomarker of atherosclerosis (13). After that, the association of sTWEAK with CVD or CVD-related diseases has been extensively studied. sTWEAK concentrations are diminished in patients with coronary artery disease (14), abdominal aortic aneurysm (15), systolic heart failure (16), type 2 diabetes (17), and CKD (18).
The NEFRONA Study was an observational, multicenter, prospective study designed to evaluate the prevalence and evolution of subclinical atheromatosis in patients with CKD (19). In this study, we wanted to confirm and extend the usefulness of sTWEAK as a biomarker of atherosclerosis in patients with CKD. We also tested the ability of sTWEAK concentrations to predict vascular calcification. Finally, we analyzed the association of sTWEAK with CV outcomes in the NEFRONA Study population.
Materials and Methods
Patients
The NEFRONA Study is a 4-year prospective multicenter cohort study aimed to assess the predictive value of noninvasive techniques for CVD events and mortality in patients with CKD (19). In that study, 2445 patients with CKD (patients 18–74 years of age were eligible if they had CKD stage 3 or higher as defined by current guidelines [eGFR<60 ml/min per 1.73 m2 estimated using the four–variable Modification of Diet in Renal Disease equation]) were enrolled from October of 2010 to June of 2012. Of this, 1058 patients with available serum samples were included in the substudy. Patients were enrolled within 69 Spanish primary care centers distributed in 38 different regions from Spain. The exclusion criteria were previous CV events, active infections (HIV or tuberculosis), pregnancy, having received any organ transplantation, and having a life expectancy of <1 year.
Primary outcomes were CVD events according to the International Classification of Diseases of the World Health Organization, which included unstable angina, myocardial infarction, transient ischemic attack, cerebrovascular accident, congestive heart failure, arrhythmia, peripheral artery disease or amputation for vascular disease, and aorta aneurism. At each visit, all-cause mortality and CV mortality were registered. All-cause mortality comprised infections, tumors, accidents, and uremia. CV mortality included myocardial infarction, arrhythmia, congestive heart failure, stroke, aneurism, mesenteric infarction, and sudden death.
Each physician responsible for the recruitment of patients with CKD accurately recorded CVD events and deaths. In the case of an out of hospital death, family members were interviewed by telephone to better ascertain the circumstances surrounding death.
Each local ethics committee approved the study. The authors adhered to the Declaration of Helsinki, and patients were included after providing informed consent.
Clinical and Biochemical Data
At recruitment, the patients were asked to complete a questionnaire, including clinical history of diabetes, hypertension, and dyslipidemia; CV risk factors; and medication use.
The ultrasound explorations (carotid and femoral) were performed according to a standardized protocol by three itinerant teams belonging to the Unit for Detection and Treatment of Atherothrombotic Diseases (UDETMA), Hospital Universitari Arnau de Vilanova (Lleida, Spain). The itinerant teams collected the anthropometric parameters and blood samples. Blood samples were processed immediately after extraction and storage at −20°C. After that, samples were sent and stored at −80°C within 24 hours at the centralized biobank of the Spanish Network for Nephrological Research.
Biochemical parameters were obtained from a routine fasting blood test. Serum sTWEAK concentrations were determined in duplicate with commercially available ELISA kits (Bender MedSystems, Vienna, Austria). The same investigator measured all samples in a blinded manner (Instituto de Investigación Sanitaria-Fundación Jiménez Díaz). The minimum detectable level of sTWEAK was 10 pg/ml. Intra- and interassay coefficients of variation were 7.3% and 8.5%, respectively.
Carotid and Femoral Ultrasound
B-mode ultrasound of the carotid/femoral arteries was performed using the Vivid BT09 Apparatus (GE Healthcare, Waukesha, WI) equipped with a 6- to 13-MHz broadband linear array probe. A single reader performed the analysis of the presence of atheromatous plaques in a blinded fashion using the semiautomatic software EchoPAC Dimension (GE Healthcare).
Ultrasound imaging was performed for the carotid and the common and superficial femoral arteries with the patients in a supine position. The presence of atheromatous plaques was defined as intima-media thickness ≥1.5 mm protruding into the lumen, which is the criterion given in the American Society of Echocardiography Consensus Statement (20) and the Mannheim Carotid Intima/Media Thickness Consensus (21). This criterion was used in the Atherosclerosis Risk in Communities Study (22) and the Framingham Offspring Study (23), two important studies showing the predictive value of atheromatous plaques in CV risk.
To assess the quality of the reading and the intraobserver reliability, a sample of 20 individuals was measured three to five times on different days. A κ-coefficient of one was obtained, indicating excellent intraobserver reliability. To assess the quality of the measurements of the intima-media thickness, a sample of 20 individuals was measured three to five times on different days, obtaining an intraclass correlation coefficient of 0.93. The analysis included both carotid and femoral arteries. To determine plaque calcification, a semiquantitative method previously described by de Bray et al. (24) was used.
The ankle-brachial index (ABI) was also measured. Limb BP was measured in brachial, posterior tibial, and dorsalis pedis arteries. ABI values were obtained by dividing BP in the four arteries of the lower limbs by the highest brachial BP, hence resulting in four ABI values. Ratios between 0.9 and 1.0 are considered normal, ratios <0.9 indicate a fall in distal blood flow, and ratios >1.4 reflect vascular wall stiffness. The most pathologic of the four ABI values was recorded.
Statistical Analyses
All of the statistical analyses were performed using the SPSS 11.0 (SPSS Inc., Chicago, IL) statistical package. Non–normally distributed variables were expressed as medians (ranges), and normally distributed variables were expressed as means±SDs. Between-group comparisons were assessed for nominal variables with the chi-squared test and the Kruskal–Wallis test. Spearman rank correlation was used to determine correlations between variables. Stepwise multivariate regression analysis was used to assess the predictors for ABI and carotid intima-media thickness (c-IMT) levels (non–normally distributed variables were log transformed for the statistical model). Time to event analysis of CV outcomes was done using the Cox proportional hazards model, including adjustment for potential confounding factors. Data are presented in the form of hazard ratios (HRs) and 95% confidence intervals (95% CIs). Statistical differences in c statistics were compared using the method by DeLong et al. (25); 95% CIs were calculated for each comparison. P value <0.05 was considered statistically significant.
Results
sTWEAK Concentrations in Patients with CKD
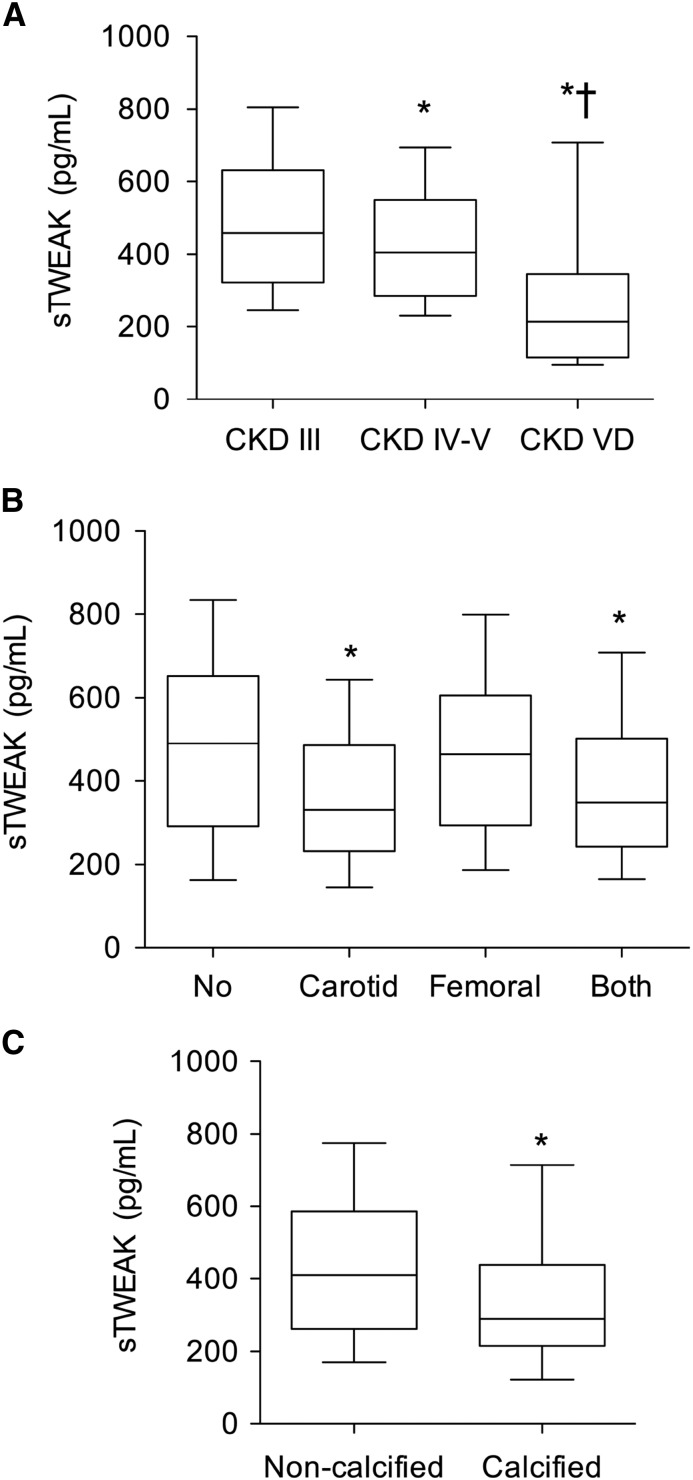
The demographic and clinical characteristics of the studied population are summarized in Table 1. There were significant differences among the different CKD stages regarding age, sex, body mass index, and CV risk factors. Patients with more advanced CKD had lower sTWEAK concentrations (Figure 1A) (P<0.001). The lowest sTWEAK levels were found in patients undergoing dialysis (Table 1). No differences were observed in the recovery percentages of sTWEAK between uremic and nonuremic sera (not shown), indicating that low sTWEAK levels observed in patients on hemodialysis were not related with their serum conditions.
Table 1.
Baseline demographic and biochemical parameters
| Parameter | All Patients, n=1058 | CKD Stage 3, n=435 | CKD Stages 4 and 5, n=377 | CKD Stage 5 Dialysis, n=246 | P Value |
|---|---|---|---|---|---|
| Age, yr | 58±13 | 61±11 | 58±12 | 53±14 | <0.001 |
| Men, % (N) | 62.8 (665) | 70.8 | 58.9 | 54.9 | <0.001 |
| BMI, kg/m2 | 28 (25–32) | 29 (26–32) | 28 (25–32) | 27 (23–32) | <0.001 |
| SBP, mmHg | 140 (129–156) | 142 (130–156) | 143 (130–159) | 137 (123–153) | <0.001 |
| DBP, mmHg | 81 (75–89) | 81 (75–89) | 81 (74–88) | 80 (70–90) | 0.10 |
| Cholesterol, mg/dl | 178±40 | 187±37 | 177±38 | 163±42 | <0.001 |
| LDL-cholesterol, mg/dl | 101±34 | 110±32 | 100±34 | 87±32 | <0.001 |
| HDL-cholesterol, mg/dl | 46 (38–57) | 47 (39–56) | 47 (37–59) | 44 (36–54) | 0.01 |
| Triglycerides, mg/dl | 126 (92–177) | 125 (96–175) | 127 (92–185) | 124 (82–176) | 0.81 |
| Glucose, mg/dl | 99 (88–118) | 103 (92–125) | 98 (89–112) | 91 (82–113) | <0.001 |
| hs-CRP, mg/L | 1.88 (0.92–4.7) | 1.83 (0.92–4.07) | 1.68 (0.82–4.22) | 2.44 (1.14–7.16) | 0.002 |
| eGFR, ml/min per 1.73 m2 | 26 (12–40) | 43 (36–50) | 21 (15–25) | 6.6 (5.2–9.1) | <0.001 |
| ABI | 1.04 (0.95–1.15) | 1.00 (0.93–1.12) | 1.04 (0.95–1.15) | 1.07 (0.96–1.25) | <0.001 |
| c-IMT, mm | 0.71 (0.62–0.82) | 0.75 (0.66–0.84) | 0.68 (0.59–0.81) | 0.69 (0.60–0.79) | <0.001 |
| Smoker, % (N) | 57.2 (605) | 60.9 (265) | 55.7 (210) | 52.8 (130) | 0.09 |
| Dyslipemia, % (N) | 64.6 (684) | 71.7 (312) | 69.0 (260) | 45.5 (112) | <0.001 |
| Medical treatment, % (N) | 57.0 (603) | 57.7 (251) | 67.1 (253) | 40.2 (99) | |
| Diabetes mellitus, % (N) | 29.0 (307) | 32.2 (140) | 30.0 (113) | 22.0 (54) | 0.02 |
| Medical treatment, % (N) | 27.9 (295) | 31.9 (139) | 28.6 (108) | 19.5 (48) | |
| Hypertension, % (N) | 92.1 (974) | 91.5 (398) | 96.0 (362) | 87.0 (214) | <0.001 |
| Medical treatment, % (N) | 89.2 (944) | 92.6 (403) | 95.8 (361) | 73.2 (180) | |
| Familial CVD, % (N) | 8.5 (90) | 9.4 (41) | 9.0 (34) | 6.1 (15) | 0.30 |
| sTWEAK, pg/ml | 389 (254–576) | 459 (323–631) | 405 (286–549) | 214 (115–346) | <0.001 |
Results are expressed as means±SDs or medians (interquartile ranges). BMI, body mass index; SBP, systolic BP; DBP, diastolic BP; hs-CRP, high–sensitivity C-reactive protein; ABI, ankle-brachial index; c-IMT, carotid intima-media thickness; CVD, cardiovascular disease; sTWEAK, soluble TNF–like weak inducer of apoptosis.
Figure 1.
Soluble TNF–like weak inducer of apoptosis (sTWEAK) levels, atherosclerotic burden, and vascular calcification in patients with CKD without any history of cardiovascular disease. (A) Box plots showing the difference in sTWEAK serum concentrations [median (IQR)] in parallel to stages of eGFR. P for trend <0.001. VD, CKD stage 5 dialysis. *P<0.001 versus CKD stage 3; †P<0.001 versus CKD stages 4 and 5. (B) Box plots showing sTWEAK levels (medians and interquartile ranges) according to the vascular territory affected. No plaques, n=329; carotid plaques, n=193; femoral plaques, n=116; both, n=420. *P<0.001 versus no plaques. (C) Box plots showing sTWEAK levels (medians and interquartile ranges) according to the presence of calcified carotid atherosclerotic plaques. Noncalcified carotid plaques, n=455; calcified carotid plaques, n=158. Whiskers represent 10th and 90th percentiles. *P<0.001 versus noncalcified carotid plaques.
No differences in sTWEAK concentrations were observed in patients with CKD with diabetes, hypertension, or dyslipemia compared with those without these CV risk factors. Current smokers (n=605) presented lower sTWEAK concentrations compared with nonsmokers (n=453; 372; interquartile range [IQR], 246–557 versus 412; IQR, 267–595 pg/ml; P=0.03). No differences were found according to the prescription of antihypertensive drugs, statins, or antidiabetic treatments.
Univariate associations of ABI and c-IMT are given in Table 2. ABI was negatively associated with eGFR, c-IMT, total cholesterol, LDL-cholesterol, and glucose concentrations. c-IMT was positively associated with age, body mass index, systolic BP, eGFR, triglycerides, glucose, and high–sensitivity C-reactive protein (hs-CRP) concentrations and negatively correlated with diastolic BP, HDL-cholesterol, ABI and sTWEAK levels. To clarify whether sTWEAK is an independent predictor of c-IMT, multiple lineal regression analyses were performed including variables that were statistically significant in the univariate analysis. Age (β=0.501; P<0.001), HDL-c (β=−0.128; P<0.001), glucose (β=0.066; P=0.01), eGFR (β=0.137; P<0.001), and systolic BP (β=0.086; P=0.03) as well as sTWEAK (β=−0.129; P<0.001) were independently associated with c-IMT.
Table 2.
Spearman correlation coefficients between carotid intima-media thickness, ankle-brachial index, soluble TNF–like weak inducer of apoptosis levels, and selected parameters in asymptomatic patients
| Parameter | c-IMT | ABI | sTWEAK | |||
|---|---|---|---|---|---|---|
| r | P Value | r | P Value | r | P Value | |
| Age, yr | 0.54 | <0.001 | −0.03 | 0.33 | −0.02 | 0.43 |
| BMI, kg/m2 | 0.21 | <0.001 | −0.05 | 0.07 | 0.03 | 0.29 |
| SBP, mmHg | 0.18 | <0.001 | −0.00 | 0.96 | −0.03 | 0.26 |
| DBP, mmHg | −0.07 | 0.02 | 0.03 | 0.39 | −0.04 | 0.17 |
| Cholesterol, mg/dl | −0.02 | 0.64 | −0.14 | <0.001 | 0.13 | <0.001 |
| HDL-cholesterol, mg/dl | −0.12 | <0.001 | −0.06 | 0.06 | 0.10 | 0.001 |
| LDL-cholesterol, mg/dl | 0.09 | 0.14 | −0.15 | <0.001 | 0.15 | <0.001 |
| Triglicerydes, mg/dl | 0.07 | 0.03 | −0.05 | 0.14 | −0.08 | 0.01 |
| Glucose, mg/dl | 0.22 | <0.001 | −0.11 | <0.001 | 0.01 | 0.88 |
| hs-CRP, mg/L | 0.13 | <0.001 | −0.04 | 0.21 | −0.04 | 0.23 |
| eGFR, ml/min per 1.73 m2 | 0.22 | <0.001 | −0.15 | <0.001 | 0.35 | <0.001 |
| c-IMT, mm | — | — | −0.12 | <0.001 | −0.10 | 0.002 |
| ABI | −0.18 | <0.001 | — | — | −0.05 | 0.08 |
| sTWEAK, pg/ml | −0.10 | 0.002 | −0.05 | 0.08 | — | — |
Variables’ non–normally distributed data were log transformed before analysis. – denotes that no value is reported in the statistical analysis. c-IMT, carotid intima-media thickness; ABI, ankle-brachial index; sTWEAK, soluble TNF–like weak inducer of apoptosis; BMI, body mass index; SBP, systolic BP; DBP, diastolic BP; hs-CRP, high–sensitivity C-reactive protein.
sTWEAK as a Predictor of Carotid Atherosclerotic Plaques
Patients with atherosclerotic plaques (n=729) showed lower sTWEAK concentrations compared with those without plaques (n=329; median =357; IQR, 244–517 versus 490; IQR, 296–652 pg/ml; P<0.001). When we analyzed sTWEAK serum levels according with the vascular territory affected (femoral or carotid arteries), only patients with carotid or both carotid and femoral plaques showed lower sTWEAK levels compared with those without atherosclerotic plaques (Figure 1B). sTWEAK concentrations were not significantly lower among patients with femoral atherosclerotic plaques than those without these lesions (Figure 1B).
Table 3 shows univariate regression logistic analysis to assess predictors of the presence or absence of carotid atherosclerotic plaques. Low sTWEAK levels were defined as sTWEAK concentration <33rd percentile (<289 pg/ml), and high sTWEAK levels were defined as its concentration >66th percentile (>465 pg/ml). After that, multivariable logistic regression analysis including only variables that were statistically significant in the univariate analysis was performed to assess predictors of the presence or absence of carotid atherosclerotic plaques. Older age, current smokers, and middle and lowest sTWEAK tertiles were independent predictors for the presence of carotid atherosclerotic plaques (Table 3). Patients with calcified carotid atherosclerotic plaques showed lower sTWEAK levels compared with patients in whom the plaques were not calcified (Figure 1C). Table 3 shows univariate regression logistic analysis to assess predictors of the presence or absence of calcified carotid atherosclerotic plaques. After that, multivariable logistic regression analysis was performed. Older age, eGFR, hs-CRP>3 mg/L, and lowest sTWEAK tertile were independent predictors for the presence of calcified carotid atherosclerotic plaques (Table 3).
Table 3.
Univariate and multivariate logistic analyses modeling the presence of carotid or calcified carotid atherosclerotic plaques in the studied population
| Parameter | Carotid Plaques | Calcified Carotid Plaques | ||||||
|---|---|---|---|---|---|---|---|---|
| Crude | Adjusted | Crude | Adjusted | |||||
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age, per 10 yr | 2.41 (2.12 to 2.74) | <0.001 | 2.60 (2.26 to 2.99) | <0.001 | 1.89 (1.56 to 2.29) | <0.001 | 1.39 (1.11 to 1.75) | 0.004 |
| Sex, men versus women | 1.61 (1.25 to 2.07) | <0.001 | 1.29 (0.93 to 1.80) | 0.13 | 1.01 (0.69 to 1.49) | 0.95 | — | — |
| Current smoker, yes versus no | 1.73 (1.35 to 2.21) | <0.001 | 1.93 (1.38 to 2.68) | <0.001 | 1.07 (0.73 to 1.55) | 0.74 | — | — |
| Hypertension, yes versus no | 1.57 (1.01 to 2.46) | 0.04 | 1.24 (0.71 to 2.15) | 0.45 | 1.69 (0.73 to 3.89) | 0.22 | — | — |
| Diabetes, yes versus no | 1.95 (1.47 to 2.58) | <0.001 | 1.30 (0.66 to 2.54) | 0.44 | 1.27 (0.87 to 1.84) | 0.22 | — | — |
| Hyperlipidemia, yes versus no | 1.22 (0.94 to 1.57) | 0.13 | — | — | 1.07 (0.73 to 1.58) | 0.72 | — | — |
| Familial CVD, yes versus no | 0.70 (0.46 to 1.57) | 0.11 | — | — | 1.05 (0.53 to 2.09) | 0.89 | — | — |
| eGFR, per 10 ml/min per 1.73 m2 | 0.98 (0.91 to 1.06) | 0.67 | — | — | 0.87 (0.79 to 0.97) | 0.02 | 0.88 (0.78 to 0.99) | 0.04 |
| hs-CRP, >3 mg/L | 1.24 (0.96 to 1.60) | 0.11 | — | — | 1.81 (1.26 to 2.62) | 0.001 | 1.69 (1.16 to 2.46) | <0.01 |
| sTWEAK, highest tertile, n=351 | 1.0 | — | 1.0 | — | 1.0 | — | 1.0 | — |
| sTWEAK, middle tertile, n=355 | 2.38 (1.76 to 3.22) | <0.001 | 2.39 (1.68 to 3.38) | <0.001 | 1.22 (0.74 to 2.05) | 0.43 | 1.23 (0.73 to 2.08) | 0.43 |
| sTWEAK, lowest tertile, n=352 | 3.22 (2.36 to 4.39) | <0.001 | 4.18 (2.89 to 6.08) | <0.001 | 1.86 (1.14 to 3.03) | 0.01 | 1.77 (1.06 to 2.93) | 0.02 |
| Antihypertensive drugs, yes versus no | 0.92 (0.62 to 1.37) | 0.70 | — | — | 0.75 (0.43 to 1.30) | 0.31 | — | — |
| Hypolipemic drugs, yes versus no | 1.22 (0.95 to 1.56) | 0.11 | — | — | 1.14 (0.79 to 1.65) | 0.49 | — | — |
| Oral antidiabetic drugs, yes versus no | 1.97 (1.26 to 3.08) | 0.003 | 0.99 (0.50 to 1.99) | 0.99 | 1.25 (0.73 to 2.14) | 0.41 | — | — |
| Insulin treatment, yes versus no | 1.66 (1.19 to 2.31) | 0.003 | 1.42 (0.71 to 2.81) | 0.32 | 1.19 (0.77 to 1.84) | 0.43 | — | — |
Multivariable logistic analysis included variables that were statistically significant in the univariate analysis. – denotes that no value is reported in the statistical analysis. OR, odds ratio; 95% CI, 95% confidence interval; CVD, cardiovascular disease; hs-CRP, high–sensitivity C-reactive protein; sTWEAK, soluble TNF–like weak inducer of apoptosis.
sTWEAK and CV Outcomes
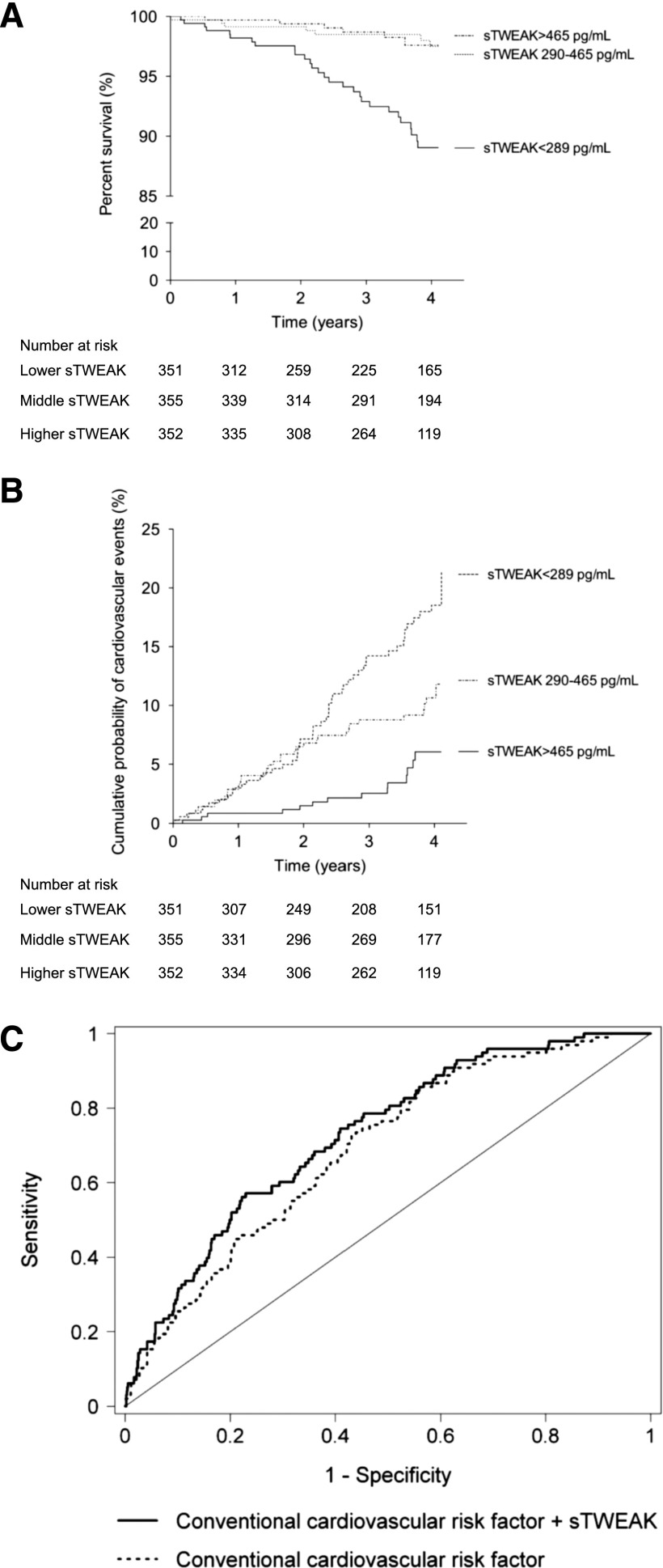
CV outcomes were determined from the day of examination onward, with a mean of follow-up of 3.13±1.15 years; 89 patients died, 41 (46%) of whom died because of CVD-related disease, such as myocardial infarction (n=11), stroke (n=6), sudden death (n=12), mesenteric infarction (n=7), or other CVD–related causes (n=5). sTWEAK levels were lower in patients suffering a fatal CV event compared with those free for fatal CV event (median =231; IQR, 174–354 versus 395; IQR, 261–577 pg/ml; P<0.001). Kaplan–Meier curves showed a significant association of lowest sTWEAK concentrations with worse survival (log rank =15.6; P<0.001) (Figure 2A).
Figure 2.
Effect of soluble TNF–like weak inducer of apoptosis (sTWEAK) on cardiovascular outcomes. (A) Kaplan–Meier curve of cardiovascular mortality risk in patients with CKD according to sTWEAK tertiles. (B) Kaplan–Meier plot of cumulative probability of a first major cardiovascular event when patients with CKD were grouped according to sTWEAK tertiles. Low sTWEAK levels were defined as sTWEAK concentration <33rd percentile (<289 pg/ml), and high sTWEAK levels were defined as its concentration >66th percentile (>465 pg/ml). (C) Receiver–operating characteristic curves for cardiovascular events prediction. Curves are on the basis of logistic regression models incorporating conventional risk variables (age, sex, smoking status, diabetes mellitus, hypertension, hyperlipidemia, and eGFR) with and without sTWEAK.
The predictors for CV mortality were studied by univariate and multivariate Cox analyses. In univariate Cox, diabetes, eGFR, being on dialysis, lowest sTWEAK tertile, and treatment with hypolipemic drugs and/or insulin were significant predictors of outcome (Table 4). Multivariate Cox was used to study the effect of variables that were statistically significant in the univariate analysis. After that, only the lowest sTWEAK tertile persisted as an independent predictor of CV death (Table 4).
Table 4.
Univariate and multivariate Cox regression analyses predicting cardiovascular outcomes and mortality
| Parameter | Major Cardiovascular Events | Cardiovascular Mortality | Total Mortality | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude | Adjusted | Crude | Adjusted | Crude | Adjusted | |||||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age, per 10 yr | 1.29 (1.08 to 1.56) | <0.01 | 1.27 (1.05 to 1.52) | 0.01 | 1.02 (0.99 to 1.05) | 0.11 | — | — | 1.41 (1.14 to 1.71) | 0.002 | 1.47 (1.19 to 1.81) | <0.001 |
| Sex, men versus women | 1.54 (1.02 to 2.34) | 0.04 | 1.77 (1.15 to 2.74) | 0.01 | 1.14 (0.60 to 2.18) | 0.69 | — | — | 1.22 (0.79 to 1.91) | 0.37 | — | — |
| Current smoker, yes versus no | 1.38 (0.66 to 4.01) | 0.10 | — | — | 1.18 (0.63 to 2.21) | 0.60 | — | — | 1.35 (0.87 to 2.08) | 0.18 | — | — |
| Hypertension, yes versus no | 1.63 (1.01 to 2.46) | 0.28 | — | — | 0.98 (0.30 to 3.18) | 0.98 | — | — | 0.61 (0.32 to 1.17) | 0.14 | — | — |
| Diabetes, yes versus no | 2.45 (1.69 to 3.58) | <0.001 | 2.05 (1.14 to 3.66) | 0.02 | 2.56 (1.39 to 4.73) | 0.003 | 2.40 (0.87 to 6.57) | 0.09 | 1.82 (1.19 to 2.77) | <0.01 | 1.46 (0.72 to 2.96) | 0.29 |
| Hyperlipidemia, yes versus no | 1.15 (0.76 to 1.74) | 0.50 | — | — | 0.80 (0.42 to 1.52) | 0.50 | — | — | 0.69 (0.45 to 1.05) | 0.61 | — | — |
| Familial CVD, yes versus no | 1.00 (0.51 to 1.97) | 0.99 | — | — | 1.21 (0.43 to 3.38) | 0.77 | — | — | 0.67 (0.27 to 1.65) | 0.38 | — | — |
| eGFR, per 10 ml/min per 1.73 m2 | 0.76 (0.67 to 0.86) | <0.001 | 0.86 (0.72 to 1.03) | 0.11 | 0.94 (0.92 to 0.97) | <0.001 | 0.77 (0.54 to 1.09) | 0.15 | 0.63 (0.54 to 0.74) | <0.001 | 0.76 (0.61 to 0.95) | 0.01 |
| Dialysis, yes versus no | 2.84 (1.91 to 4.23) | <0.001 | 1.79 (0.98 to 3.30) | 0.06 | 6.30 (3.39 to 11.68) | <0.001 | 2.07 (0.76 to 5.63) | 0.15 | 4.18 (2.74 to 6.37 | <0.001 | 1.75 (0.86 to 3.55) | 0.12 |
| hs-CRP, >3 mg/L | 1.74 (1.19 to 2.54) | 0.004 | 1.37 (0.92 to 2.02) | 0.12 | 1.50 (0.80 to 2.81) | 0.21 | — | — | 1.87 (1.23 to 2.85) | 0.003 | 1.33 (0.86 to 2.04) | 0.20 |
| sTWEAK highest tertile, n=351 | 1.0 | — | 1.0 | — | 1.0 | — | 1.0 | — | 1.0 | — | 1.0 | — |
| sTWEAK middle tertile, n=355 | 1.89 (1.05 to 3.38) | 0.03 | 1.94 (1.07 to 3.52) | 0.03 | 1.04 (0.35 to 3.11) | 0.94 | 1.05 (0.35 to 3.15) | 0.93 | 0.78 (0.43 to 1.43) | 0.43 | 0.75 (0.42 to 1.43) | 0.28 |
| sTWEAK, lowest tertile, n=352 | 3.74 (2.17 to 6.42) | <0.001 | 2.40 (1.33 to 4.33) | 0.004 | 4.92 (2.03 to 11.88) | <0.001 | 2.67 (1.05 to 6.76) | 0.04 | 2.12 (1.28 to 3.50) | 0.003 | 1.14 (0.66 to 1.99) | 0.63 |
| Antihypertensive drugs, yes versus no | 0.88 (0.45 to 1.74) | 0.72 | — | — | 1.07 (0.38 to 3.01) | 0.90 | — | — | 0.46 (0.27 to 0.79) | <0.01 | 0.78 (0.42 to 1.43) | 0.42 |
| Hypolipemic drugs, yes versus no | 1.12 (0.76 to 1.65) | 0.55 | — | — | 0.53 (0.29 to 0.99) | 0.04 | 0.61 (0.32 to 1.14) | 0.12 | 0.59 (0.39 to 0.89) | 0.01 | 0.58 (0.38 to 0.90) | 0.01 |
| Oral antidiabetic drugs, yes versus no | 0.93 (0.50 to 1.73) | 0.81 | — | — | 0.89 (0.32 to 2.50) | 0.82 | — | — | 0.49 (0.20 to 1.20) | 0.12 | — | — |
| Insulin treatment, yes versus no | 2.59 (1.75 to 3.85) | <0.001 | 1.22 (0.66 to 2.26) | 0.52 | 3.06 (1.63 to 5.73) | <0.001 | 1.25 (0.45 to 3.49) | 0.67 | 2.20 (1.41 to 3.45) | 0.001 | 1.25 (0.59 to 2.64) | 0.55 |
Multivariable Cox analysis included variables that were statistically significant in the univariate analysis. – denotes that no value is reported in the statistical analysis. HR, hazard ratio; 95% CI, 95% confidence interval; CVD, cardiovascular disease; hs-CRP, high–sensitivity C-reactive protein; sTWEAK, soluble TNF–like weak inducer of apoptosis.
The same analysis was done for total mortality. Total mortality was defined as mortality by any cause, including CV and non-CV death (cancer [n=21], infection [n=15], and others [n=12]). Crude analysis is shown in Table 4. After adjustment, only age, eGFR levels, and treatment with hypolipemic drugs persisted as predictors of total mortality (Table 4).
Because of the limited number of fatal CV events registered, we also analyzed the effect of sTWEAK concentrations on the prediction of CV events for a composite of fatal and nonfatal CV events (n=109). During the follow-up period, 68 additional nonfatal CV events were registered: transient ischemic attack (n=4), unstable angina (n=16), myocardial infarction (n=12), intermittent claudication (n=11), aortic aneurysm (n=2), cerebrovascular accident (n=16), and other CV event (n=7). sTWEAK concentrations were lower in patients with CKD suffering a nonfatal CV event compared with those without a CV event (median, 275; IQR, 191–428 versus 403; IQR, 263–586 pg/ml; P<0.001). Figure 2B displays the estimated cumulative evidence of major CV events. When patients with CKD were grouped according to sTWEAK tertiles, the probability of developing a CV event was higher in the lowest tertile of the sTWEAK concentrations group (HR, 3.74; 95% CI, 2.17 to 6.42; P<0.001). Cox proportional hazards model was performed to study the effect of traditional confounding factors on the probability of developing a CV event (Table 4). In univariate Cox, age, sex, diabetes, eGFR, being on dialysis, hs-CRP levels >3 mg/L, middle and lowest sTWEAK tertiles, and insulin treatment were associated with a high risk of developing a CV event (Table 4). Age, sex, diabetes, and middle and lowest sTWEAK tertiles persisted as independent predictors of CV events in multivariate Cox analysis (Table 4). Finally, to assess the clinical usefulness of sTWEAK in predicting CV outcomes in patients with CKD, we created multivariable regression models with and without sTWEAK. The model with conventional CV risk factors included age, sex, smoking status, diabetes, hypertension, and hyperlipidemia as well as eGFR. According to c statistic, the model including sTWEAK has shown a significant improvement from HR, 0.69; 95% CI, 0.64 to 0.74 to HR, 0.73; 95% CI, 0.68 to 0.78 in accuracy of CV events prediction (P=0.03) (Figure 2C).
Discussion
In this work, we investigated sTWEAK serum levels as predictors of CV outcomes in a CKD population with or without CV risk factors but without any history of CVD. sTWEAK levels were negatively and independently associated with c-IMT in our studied population. Furthermore, sTWEAK concentrations were an independent predictor for the presence of carotid and/or calcified atherosclerotic plaques. Finally, sTWEAK levels were significantly and independently related with CV outcomes in patients with CKD.
The population study included patients with CKD from several primary care centers in a daily clinical practice with or without concomitant medication with the aim to have a representative Spanish population. Previously (18) and in this study, patients with more advanced CKD had lower sTWEAK concentrations. Although we do not know why sTWEAK concentrations are particularly low in patients on hemodialysis, this could be related with two different mechanisms. Fn14 expression is almost undetectable in the vasculature and the kidney. However, under pathologic conditions, such as atherosclerosis and CKD, Fn14 expression is upregulated (26), favoring sTWEAK binding and retention in pathologic tissues. In addition, high CD163 concentrations have been observed in patients with severe CKD compared with controls (27). CD163 acts as a scavenger receptor for TWEAK. Both retention in the tissue by Fn14 and degradation by CD163 could be responsible for the diminished sTWEAK levels observed in patients on hemodialysis. However, this is a hypothesis that needs to be tested in future studies.
sTWEAK was negatively and independently associated with c-IMT in agreement with previous studies on asymptomatic subjects (13) and patients with CKD not on dialysis (28,29). However, positive association between sTWEAK and c-IMT has been reported in patients with transplants and CKD (30), which could be because of the presence of special therapy regimens used in patients with transplants and CKD.
An important finding from our study is the difference of sTWEAK concentrations observed depending on the vascular territory affected. Thus, sTWEAK was lower in patients with CKD with carotid plaques compared with those with femoral atherosclerotic plaques or without plaques. In agreement with previous data from subjects free from clinical CVD (31), sTWEAK concentrations were an independent predictor for the presence of carotid atherosclerotic plaques. Femoral plaques have increased fibrotic content compared with carotid atherosclerotic plaques, whereas carotid plaques are characterized by a higher inflammatory cell content than femoral arteries (32). Differences in sTWEAK concentrations between carotid and femoral plaques could be related to differences in cellular composition. More importantly, sTWEAK was also related to the presence of vascular calcification. Vascular calcification is a highly prevalent phenotype in patients with CKD, and it is both a risk factor and contributor to morbidity and mortality in patients with CKD (33). Interestingly, sTWEAK was an independent predictor of both CV mortality and first CV event in our population. These data are consistent with previous data from patients with stable heart failure (16) and patients with nondialysis CKD (34). In contrast, we have observed that sTWEAK was not associated with total mortality. These data could indicate that sTWEAK is a good biomarker for CV prognosis but not a good biomarker for other pathologies.
Strengths of this study are the large sample size of patients with CKD included in our study and the fact that the vascular exploration was performed by the same itinerant team, minimizing the variability associated with the techniques. The assessment of plaques presence also in the femoral territories adds additional information to the analysis. In addition, this is the first time that sTWEAK levels are associated with the presence of CV events and CV mortality in a general CKD population. However, some limitations of our study should be highlighted for a correct interpretation of the implications of our findings. There is an intentional bias; only patients with no history of CV events were included, because the study was aimed to primary prevention of CV events. This bias was necessary, but its consequences have to be considered when interpreting the results. A relatively low number of CV deaths was reported during the follow-up, which might limit the statistical power of our analysis. Finally, in very complex diseases, such as CVD, a single biomarker could not be sufficient to detect atherosclerotic plaques. sTWEAK levels could add information to known CV risk factors and contribute to a better prediction of CV events.
In conclusion, low sTWEAK serum levels were associated with the presence of carotid atherosclerotic plaques in patients with CKD. In addition, sTWEAK levels were significantly and independently related with CV outcomes in patients with CKD.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank the NEFRONA Study team (Eva Castro, Virtudes María, Teresa Molí, and Meritxell Soria), the NEFRONA Study investigators, and the Biobank of RedInRen for invaluable support.
This work was supported by Fondo de Investigaciones Sanitarias Programa I3-SNS (to L.M.B.-C.); Instituto de Salud Carlos III/Fondo Europeo de Desarrollo Regional grants PI13/00395, PS12/01770, PI13/01565, PI14/00386; Redes Temáticas de Investigación Cooperativa en Red grants RD12/0042/0038, RD12/0021/0026; and Biobancos RD09/0076/00101; and the Spanish Biomedical Research Centre in Diabetes and Associated Metabolic Disorders. The NEFRONA Study is funded by a research grant from AbbVie.
The NEFRONA Study investigators are listed in the Supplemental Material.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07900715/-/DCSupplemental.
References
- 1.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX: Chronic kidney disease and mortality risk: A systematic review. J Am Soc Nephrol 17: 2034–2047, 2006 [DOI] [PubMed] [Google Scholar]
- 2.de Jager DJ, Grootendorst DC, Jager KJ, van Dijk PC, Tomas LM, Ansell D, Collart F, Finne P, Heaf JG, De Meester J, Wetzels JF, Rosendaal FR, Dekker FW: Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 302: 1782–1789, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Ramji DP, Davies TS: Cytokines in atherosclerosis: Key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev 26: 673–685, 2015 [DOI] [PMC free article] [PubMed]
- 4.Blanco-Colio LM: TWEAK/Fn14 axis: A promising target for the treatment of cardiovascular disease. Front Immunol 5: 3, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sastre C, Fernández-Laso V, Madrigal-Matute J, Muñoz-García B, Moreno JA, Pastor-Vargas C, Llamas-Granda P, Burkly LC, Egido J, Martín-Ventura JL, Blanco-Colio LM: Genetic deletion or TWEAK blocking antibody administration reduce atherosclerosis and enhance plaque stability in mice. J Cell Mol Med 18: 721–734, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schapira K, Burkly LC, Zheng TS, Wu P, Groeneweg M, Rousch M, Kockx MM, Daemen MJ, Heeneman S: Fn14-Fc fusion protein regulates atherosclerosis in ApoE-/- mice and inhibits macrophage lipid uptake in vitro. Arterioscler Thromb Vasc Biol 29: 2021–2027, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Muñoz-García B, Moreno JA, López-Franco O, Sanz AB, Martín-Ventura JL, Blanco J, Jakubowski A, Burkly LC, Ortiz A, Egido J, Blanco-Colio LM: Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) enhances vascular and renal damage induced by hyperlipidemic diet in ApoE-knockout mice. Arterioscler Thromb Vasc Biol 29: 2061–2068, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Potrovita I, Zhang W, Burkly L, Hahm K, Lincecum J, Wang MZ, Maurer MH, Rossner M, Schneider A, Schwaninger M: Tumor necrosis factor-like weak inducer of apoptosis-induced neurodegeneration. J Neurosci 24: 8237–8244, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarín C, Fernández-Laso V, Sastre C, Madrigal-Matute J, Gómez M, Zaragoza C, Egido J, Burkly LC, Martín-Ventura JL, Blanco-Colio LM: Tumor necrosis factor-like weak inducer of apoptosis or Fn14 deficiency reduce elastase perfusion-induced aortic abdominal aneurysm in mice. J Am Heart Assoc 3: e000723, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain M, Jakubowski A, Cui L, Shi J, Su L, Bauer M, Guan J, Lim CC, Naito Y, Thompson JS, Sam F, Ambrose C, Parr M, Crowell T, Lincecum JM, Wang MZ, Hsu YM, Zheng TS, Michaelson JS, Liao R, Burkly LC: A novel role for tumor necrosis factor-like weak inducer of apoptosis (TWEAK) in the development of cardiac dysfunction and failure. Circulation 119: 2058–2068, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pachel C, Mathes D, Bayer B, Dienesch C, Wangorsch G, Heitzmann W, Lang I, Ardehali H, Ertl G, Dandekar T, Wajant H, Frantz S: Exogenous administration of a recombinant variant of TWEAK impairs healing after myocardial infarction by aggravation of inflammation. PLoS One 8: e78938, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chicheportiche Y, Bourdon PR, Xu H, Hsu YM, Scott H, Hession C, Garcia I, Browning JL: TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem 272: 32401–32410, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Blanco-Colio LM, Martín-Ventura JL, Muñóz-García B, Orbe J, Páramo JA, Michel JB, Ortiz A, Meilhac O, Egido J: Identification of soluble tumor necrosis factor-like weak inducer of apoptosis (sTWEAK) as a possible biomarker of subclinical atherosclerosis. Arterioscler Thromb Vasc Biol 27: 916–922, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Jelić-Ivanović Z, Bujisić N, Spasić S, Bogavac-Stanojević N, Spasojević-Kalimanovska V, Kotur-Stevuljević J: Circulating sTWEAK improves the prediction of coronary artery disease. Clin Biochem 42: 1381–1386, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Martín-Ventura JL, Lindholt JS, Moreno JA, Vega de Céniga M, Meilhac O, Michel JB, Egido J, Blanco-Colio LM: Soluble TWEAK plasma levels predict expansion of human abdominal aortic aneurysms. Atherosclerosis 214: 486–489, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Chorianopoulos E, Rosenberg M, Zugck C, Wolf J, Katus HA, Frey N: Decreased soluble TWEAK levels predict an adverse prognosis in patients with chronic stable heart failure. Eur J Heart Fail 11: 1050–1056, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Kralisch S, Ziegelmeier M, Bachmann A, Seeger J, Lössner U, Blüher M, Stumvoll M, Fasshauer M: Serum levels of the atherosclerosis biomarker sTWEAK are decreased in type 2 diabetes and end-stage renal disease. Atherosclerosis 199: 440–444, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Yilmaz MI, Carrero JJ, Ortiz A, Martín-Ventura JL, Sonmez A, Saglam M, Yaman H, Yenicesu M, Egido J, Blanco-Colio LM: Soluble TWEAK plasma levels as a novel biomarker of endothelial function in patients with chronic kidney disease. Clin J Am Soc Nephrol 4: 1716–1723, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Junyent M, Martínez M, Borràs M, Coll B, Valdivielso JM, Vidal T, Sarró F, Roig J, Craver L, Fernández E: Predicting cardiovascular disease morbidity and mortality in chronic kidney disease in Spain. The rationale and design of NEFRONA: A prospective, multicenter, observational cohort study. BMC Nephrol 11: 14, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS, American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine : Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. J Am Soc Echocardiogr 21: 93–111, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Desvarieux M, Ebrahim S, Fatar M, Hernandez Hernandez R, Kownator S, Prati P, Rundek T, Taylor A, Bornstein N, Csiba L, Vicaut E, Woo KS, Zannad F, Advisory Board of the 3rd Watching the Risk Symposium 2004, 13th European Stroke Conference : Mannheim intima-media thickness consensus. Cerebrovasc Dis 18: 346–349, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Nambi V, Chambless L, Folsom AR, He M, Hu Y, Mosley T, Volcik K, Boerwinkle E, Ballantyne CM: Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: The ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol 55: 1600–1607, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polak JF, Pencina MJ, Pencina KM, O’Donnell CJ, Wolf PA, D’Agostino RB, Sr.: Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med 365: 213–221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Bray JM, Baud JM, Delanoy P, Camuzat JP, Dehans V, Descamp-Le Chevoir J, Launay JR, Luizy F, Sentou Y, Cales P: Reproducibility in ultrasonic characterization of carotid plaques. Cerebrovasc Dis 8: 273–277, 1998 [DOI] [PubMed] [Google Scholar]
- 25.DeLong ER, DeLong DM, Clarke-Pearson DL: Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 44: 837–845, 1988 [PubMed] [Google Scholar]
- 26.Muñoz-García B, Martín-Ventura JL, Martínez E, Sánchez S, Hernández G, Ortega L, Ortiz A, Egido J, Blanco-Colio LM: Fn14 is upregulated in cytokine-stimulated vascular smooth muscle cells and is expressed in human carotid atherosclerotic plaques: Modulation by atorvastatin. Stroke 37: 2044–2053, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Axelsson J, Møller HJ, Witasp A, Qureshi AR, Carrero JJ, Heimbürger O, Bárány P, Alvestrand A, Lindholm B, Moestrup SK, Stenvinkel P: Changes in fat mass correlate with changes in soluble sCD163, a marker of mature macrophages, in patients with CKD. Am J Kidney Dis 48: 916–925, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Carrero JJ, Ortiz A, Qureshi AR, Martín-Ventura JL, Bárány P, Heimbürger O, Marrón B, Metry G, Snaedal S, Lindholm B, Egido J, Stenvinkel P, Blanco-Colio LM: Additive effects of soluble TWEAK and inflammation on mortality in hemodialysis patients. Clin J Am Soc Nephrol 4: 110–118, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassan SB, El-demery AB, Ahmed AI, Abukhalil RE: Soluble TWEAK and cardiovascular morbidity and mortality in chronic kidney disease patients. Arab J Nephrol Transplant 5: 27–32, 2012 [PubMed] [Google Scholar]
- 30.Turkmen K, Tonbul HZ, Erdur FM, Toker A, Biyik Z, Ozbiner H, Gaipov A, Gul EE, Kayrak M, Solak Y, Ozbek O, Turk S, Covic A: Soluble TWEAK independently predicts atherosclerosis in renal transplant patients. BMC Nephrol 14: 144, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernández-Laso V, Sastre C, Valdivielso JM, Fernández E, Martín-Ventura JL, Egido J, Blanco-Colio LM: Soluble TWEAK levels predict the presence of carotid atherosclerotic plaques in subjects free from clinical cardiovascular diseases. Atherosclerosis 239: 358–363, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Herisson F, Heymann MF, Chétiveaux M, Charrier C, Battaglia S, Pilet P, Rouillon T, Krempf M, Lemarchand P, Heymann D, Gouëffic Y: Carotid and femoral atherosclerotic plaques show different morphology. Atherosclerosis 216: 348–354, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA: Vascular calcifications as a marker of increased cardiovascular risk: A meta-analysis. Vasc Health Risk Manag 5: 185–197, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yilmaz MI, Sonmez A, Ortiz A, Saglam M, Kilic S, Eyileten T, Caglar K, Oguz Y, Vural A, Çakar M, Egido J, Altun B, Yenicesu M, Blanco-Colio LM, Carrero JJ: Soluble TWEAK and PTX3 in nondialysis CKD patients: Impact on endothelial dysfunction and cardiovascular outcomes. Clin J Am Soc Nephrol 6: 785–792, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.