Abstract
Background and objectives
Computed tomography (CT) measurements can distinguish between cortical and trabecular bone density in vivo. High-resolution CTs assess both bone volume and density in the same compartment, thus potentially yielding information regarding bone mineralization as well. The relationship between bone histomorphometric parameters of skeletal mineralization and bone density from microcomputed tomography (μCT) measurements of bone cores from patients on dialysis has not been assessed.
Design, setting, participants, & measurements
Bone cores from 68 patients with ESRD (age =13.9±0.5 years old; 50% men) and 14 controls (age =15.3±3.8 years old; 50% men) obtained as part of research protocols between 1983 and 2006 were analyzed by bone histomorphometry and μCT.
Results
Bone histomorphometric diagnoses in the patients were normal to high bone turnover in 76%, adynamic bone in 13%, and osteomalacia in 11%. Bone formation rate did not correlate with any μCT determinations. Bone volume measurements were highly correlated between bone histomorphometry and μCT (bone volume/tissue volume between the two techniques: r=0.70; P<0.001, trabecular thickness and trabecular separation: r=0.71; P<0.001, and r=0.56; P<0.001, respectively). Osteoid accumulation as determined by bone histomorphometry correlated inversely with bone mineral density as assessed by μCT (osteoid thickness: r=−0.32; P=0.01 and osteoid volume: r=−0.28; P=0.05). By multivariable analysis, the combination of bone mineral density and bone volume (as assessed by μCT) along with parathyroid hormone and calcium levels accounted for 38% of the variability in osteoid volume (by histomorphometry).
Conclusions
Measures of bone volume can be accurately assessed with μCT. Bone mineral density is lower in patients with excessive osteoid accumulation and higher in patients with adynamic, well mineralized bone. Thus, bone mineralization may be accurately assessed by μCT of bone biopsy cores. Additional studies are warranted to define the value of high-resolution CT in the prediction of bone mineralization in vivo.
Keywords: renal osteodystrophy; bone biopsy; micro CT; bone density; calcification, physiologic; child; humans; kidney failure, chronic; renal dialysis
Introduction
Bone disease (renal osteodystrophy) is a universal complication of advanced kidney disease, contributing to an increased fracture rate in adults (1) and fractures, bone deformities, bone pain, and growth failure in children (2). Because of the morbidity associated with bone disease, its assessment and treatment are of critical importance. Current guidelines recommend that bone turnover, mineralization, volume, strength, and growth be assessed in all adults and children with CKD, and the bone biopsy is the gold standard for the diagnosis of renal osteodystrophy (3,4). Because this is an invasive technique used primarily in the research setting, circulating biomarkers are often used to assess bone turnover, and estimates of bone volume and density are often obtained from imaging studies (5–7). To date, however, only one study has evaluated the relationship between imaging data and bone histomorphometry in patients on dialysis; in this study, differences in bone density, as assessed by in vivo quantitative computed tomography (QCT), were noted between adult patients on dialysis with high and low bone turnover (8). Since that time, newer microcomputed tomography (μCT) techniques have been developed that yield sensitive assessment of volume, trabecular volume, and density in bone samples. However, the correlation between histomorphometric parameters of bone turnover, mineralization, and computed tomography (CT) measurements of bone volume and density in bone cores from patients with advanced CKD remains unknown.
Thus, to assess the potential for μCT measurements to predict indices of skeletal mineralization in pediatric CKD, this study compared bone density parameters obtained by μCT scanning of bone biopsy cores with parameters of bone turnover, mineralization, and volume as assessed by bone histomorphometry.
Materials and Methods
Bone Cores
Transiliac bone biopsy cores obtained from pediatric patients with ESRD and control specimens from healthy individuals (9) were obtained from a deidentified repository maintained in the Division of Pediatric Nephrology at the University of California, Los Angeles (UCLA). Cores from patients on dialysis were obtained from research protocols performed at this institution between 1983 and 2006. Samples were chosen on the basis of bone histomorphometric diagnosis (vide infra): (1) normal to high bone turnover, (2) adynamic bone, and (3) osteomalacia. All study participants with ESRD had been treated with chronic cycling peritoneal dialysis before bone biopsy. No study participants had a history of metabolic bone disease other than renal osteodystrophy, none had undergone parathyroidectomy within the year preceding the biopsy, and none were treated with immunosuppressive therapy or recombinant human growth hormone within the year before the biopsy. Control cores were obtained from healthy children at the time of orthopedic surgery (9). All study participants had undergone double-tetracycline labeling before the bone biopsy. The study was approved by the Institutional Review Boards at UCLA and Veterans Affairs Sepulveda.
Biochemical Values
Biochemical values were obtained at the time of bone biopsy. Serum calcium, phosphorous, and alkaline phosphatase concentrations were determined by a Technicon Autoanalyzer (17;27). The coefficients of variation for each of these metabolites have been previously published and are as follows: calcium: 1.3% (10); phosphorus: <1.5% (11); and alkaline phosphatase: <10% (12). Parathyroid hormone (PTH) levels were determined by the UCLA Clinical Laboratory using an immunometric PTH assay by Nichols (San Clemente, CA), which detects the full–length PTH molecule [PTH(1–84)] as well as C-terminal fragments of the PTH molecule up to and including PTH(7–84). The intra- and interassay coefficients of variation for this assay were 5.2% and 9.0%, respectively (13).
Histomorphometry
Five-millimeter-diameter specimens of iliac crest were dehydrated in alcohol, cleared with xylene, and embedded in methyl methacrylate before analysis. Static histomorphometric parameters were evaluated in undecalcified 5-μm sections treated with Toluidine blue stain, and tetracycline labeling was assessed in unstained 10-μm sections. Primary bone histomorphometric parameters were characterized in trabecular bone under ×200 magnification using the OsteoMetrics System (OsteoMetrics, Decatur, GA) by a histomorphometrist (R.C.P.). Mineralized bone was defined by dark blue staining areas; pale-blue seams at least 1.5 μm in width were included in measurements of osteoid. Derived indices were calculated as described by Parfitt et al. (14). Specimens were classified according to the Turnover, Mineralization, and Volume classification system (4). Normal parameters of bone histomorphometry were defined from bone biopsies obtained from a control group of 31 pediatric patients with normal kidney function (mean age, 12.4±8.4 years old; 71% boys; 48% white; 26% Hispanic) who were undergoing elective orthopedic surgery (9).
μCT Analyses
Bone samples were subjected to μCT analysis with the Scanco μCT35 (Scanco Medical AG, Bruttisellen, Switzerland) by an investigator (D.S.B.) blinded as to bone histologic diagnosis and biochemical parameters. Bones were oriented vertically in a 12.3-mm sample holder, and scans were performed using medium-resolution settings with a source voltage of 70 E (kVp) and I of 114 μA. The images had a final element size of 12.5 μm. Two-dimensional images were analyzed using software supplied from Scanco Medical AG (Image Processing Language, version 5.6). Images were contoured to establish volumes of interest by visual examination of serial slices in all of the specimens. An optimum arbitrary threshold value of 230 (highlighting mineralized bone) was used uniformly for all specimens to quantify mineralized regions from surrounding unmineralized areas. Histomorphometric analysis of three-dimensional reconstructions was performed using Scanco Evaluation Script No. 5 (Bone Trabecular Morphometry) for three–dimensional image reconstruction and bone parameter determinations. Bone architecture measurements obtained included trabecular bone volume ratio (percentages), trabecular number (numbers per millimeter), trabecular thickness (micrometers), trabecular spacing (micrometers), connectivity (numbers per millimeter3), and trabecular and cortical bone mineral density (milligrams hydroxyapatite [HA] per centimeter3). Fourteen of the bone cores from which normal histomorphometric parameters were ascertained were of suitable quality for μCT and used as the normal control population for these measurements.
Statistical Analyses
Variables are reported as means±SDs or medians (interquartile ranges). Pearson correlation coefficients were used to express the relationship between bone histomorphometric and μCT parameters; all parameters evaluated by this method had normal distributions. Multivariable linear regression was performed to assess the ability of μCT, biochemical measurements, and the presence or absence of vitamin D therapy at the time of bone biopsy to predict bone histomorphometric measures of unmineralized osteoid; skewed values (specifically PTH levels and bone formation rates) were log transformed to normalize their distribution before statistical analysis. All statistical analyses were performed using SAS software (SAS Institute Inc., Cary, NC), and all tests were two sided. A probability of type 1 error <5% was considered statistically significant, and ordinary P values are reported.
Results
Sample and Patient Characteristics
Bone cores from 68 patients with ESRD and 14 controls were analyzed by bone histomorphometry and μCT. The 68 patients with ESRD were 13.9±4.1 years of age, and 50% were boys. Cores were obtained from individuals who had been on dialysis for 1.1 (0.6–1.8) years; 37% of patients were receiving vitamin D sterols at the time of the bone biopsy, and the remainder received no vitamin D sterols for at least 4 weeks before the bone biopsy. The 14 healthy controls were 15.3±14.2 years of age, and 50% were boys. Bone histomorphometric diagnoses in the patients were normal or high bone turnover: 76%; adynamic bone: 13%; and osteomalacia: 11%. Biochemical values obtained at bone biopsy from 68 patients are displayed in Table 1.
Table 1.
Biochemical parameters in patients on dialysis with normal to high bone turnover, pure osteomalacia, and adynamic bone disease
| Parameter | Dialysis: Normal to High Bone Turnover (n=52) | Dialysis: Pure Osteomalacia (n=7) | Dialysis: Adynamic Bone (n=9) |
|---|---|---|---|
| Calcium, mg/dl | 9.4±0.1 | 7.7±1.1 | 8.9±1.1 |
| Phosphorus, mg/dl | 6.0±0.1 | 7.0±1.0 | 6.2±0.7 |
| Alkaline phosphatase, IU/L | 337±38 | 127±23a | 538±145b |
| PTH (first generation IMA) | 736 (422, 1063) | 293 (289, 555) | 45 (21, 220)c |
Values are expressed as mean+SD, except PTH, which is expressed as median (interquartile range). PTH, parathyroid hormone; IMA, immunometric assay.
P<0.001 between osteomalacia and normal to high bone turnover.
P=0.04 between adynamic bone and osteomalacia.
P<0.001 between adynamic bone and normal to high bone turnover.
Bone Histomorphometry
Consistent with previous pediatric data (15), bone volume (bone volume/tissue volume) and trabecular thickness were normal or increased, and values did not differ between histologic subtypes of renal osteodystrophy. By contrast and consistent with the definition of the different lesions, osteoid accumulation and bone turnover differed between groups. In patients in the normal to high bone turnover group, bone formation rate was normal in 23 individuals and increased in 29 individuals, whereas osteoid volume was increased from the normal range in the majority. Osteoid volume was further increased in patients with osteomalacia, although bone formation was suppressed in these individuals. By contrast, both bone turnover and osteoid accumulation were decreased in patients with adynamic bone. By definition, osteoid accumulation as assessed by bone histomorphometry was higher in patients with osteomalacia than in those with adynamic bone (P<0.001 between groups) (Table 2).
Table 2.
Bone histomorphometric parameters in patients on dialysis with normal to high bone turnover, pure osteomalacia, and adynamic bone disease and healthy controls
| Parameter | Dialysis: Normal To High Bone Turnover (n=52) | Dialysis: Pure Osteomalacia (n=7) | Dialysis: Adynamic Bone (n=9) | Healthy Controls (n=14) |
|---|---|---|---|---|
| Bone volume (BV/TV), % | 30.1±1.1 | 29.1±3.5 | 28.0±1.0 | 21.4±1.5 |
| Trabecular thickness, μm | 167±5 | 145±15 | 137±13 | 141±14 |
| Trabecular separation, μm | 406±16 | 343±38 | 355±29 | 459±22 |
| Osteoid volume (OV/BV), % | 11.9±0.7 | 28.4±3.9 | 1.1±0.4a | 2.2±0.4 |
| Osteoid surface (OS/BS), % | 47.0±1.8 | 67.2±6.5 | 10.0±2.0a | 16.6±2.7 |
| Osteoid thickness, μm | 19.8±0.6 | 27.8±4.0 | 6.1±0.6a | 8.3±0.8 |
| Bone formation rate (BFR/BS), μm3/μm2 per year | 85.2 (51.7, 133.4) | 0 (0, 7.7) | 0.8 (0, 5.9) | 59.1 (23.0, 64.7) |
BV/TV, bone volume/tissue volume; OV/BV, osteoid volume/bone volume; OS/BS, osteoid surface/bone surface; BFR/BS, bone formation rate/bone surface.
P<0.001 between adynamic bone and osteomalacia for OV/BV, OS/BS, and osteoid thickness.
As previously described (15), serum PTH and alkaline phosphatase values correlated directly with bone formation rate (r=0.67; P<0.001 and r=0.38; P=0.004 for PTH and alkaline phosphatase, respectively) as well as with osteoid accumulation (osteoid thickness: r=0.54; P<0.001 and r=0.34; P<0.01, respectively), whereas serum calcium levels were inversely related to osteoid thickness (r=−0.43; P<0.001).
μCT Parameters and Their Relationship to Biochemical Parameters and Bone Histomorphometry
In contrast to histomorphometric measurements, μCT measurements of bone volume differed between histologic subgroups, with bone volume appearing lower in patients with osteomalacia than in those with adynamic bone disease (P=0.03 between groups). μCT measurements of bone mineral density also appeared lower in patients with osteomalacia than in those with adynamic bone (P=0.03 between groups) (Table 3), and values were inversely correlated with both serum PTH levels (r=−0.35; P<0.01) and alkaline phosphatase activity (r=−0.57; P<0.001).
Table 3.
Microcomputed tomography parameters in patients on dialysis with normal to high bone turnover, pure osteomalacia, and adynamic bone disease and healthy controls
| Parameter | Dialysis: Normal To High Bone Turnover (n=52) | Dialysis: Pure Osteomalacia (n=7) | Dialysis: Adynamic Bone (n=9) | Healthy Controls (n=14) |
|---|---|---|---|---|
| Bone volume (BV/TV), % | 32±1 | 23±3 | 29±2a | 20±2 |
| Connectivity density, no. per millimeter3 | 17.4±1.2 | 36.3±13.4 | 21. 3±2.7a | 16.5±2.2 |
| Trabecular no., no. per millimeter | 2.0±0.1 | 2.3±0.2 | 2.1±0.1 | 1.8±0.1 |
| Trabecular thickness, μm | 160±10 | 120±10 | 150±10 | 140±10 |
| Trabecular separation, μm | 500±20 | 480±50 | 460±40 | 550±30 |
| Bone mineral density, mg HA/cm | 814±7 | 784±15 | 821±8a | 792±9 |
BV/TV, bone volume/tissue volume; HA, hydroxyapatite.
P=0.03 between adynamic bone and osteomalacia for both bone volume and bone mineral density.
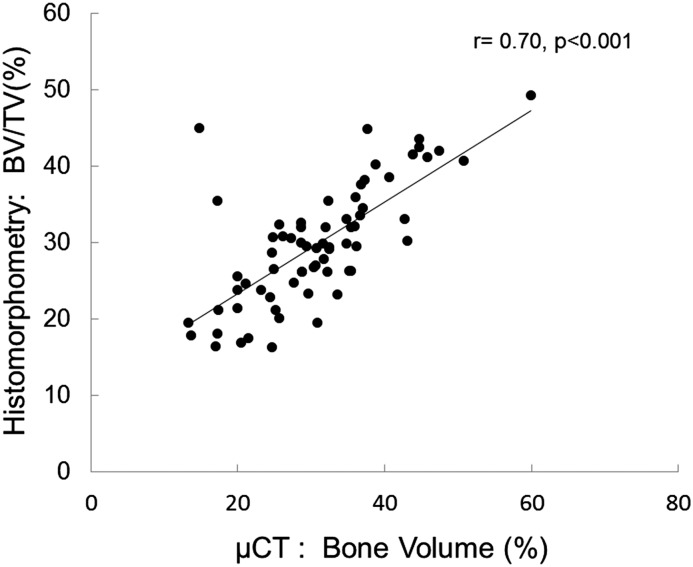
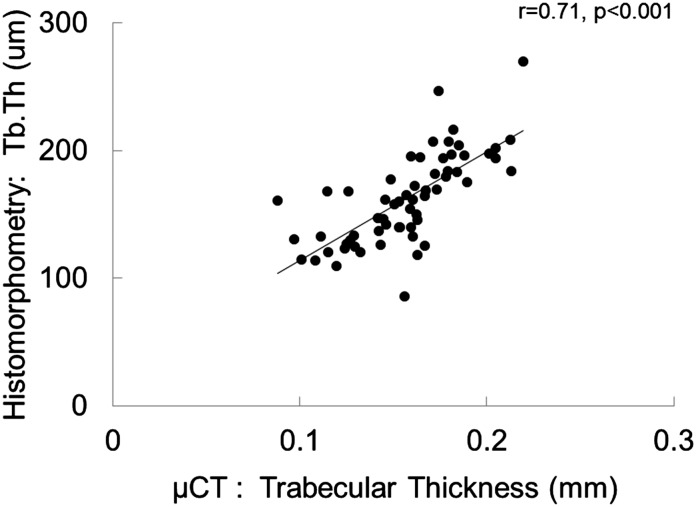
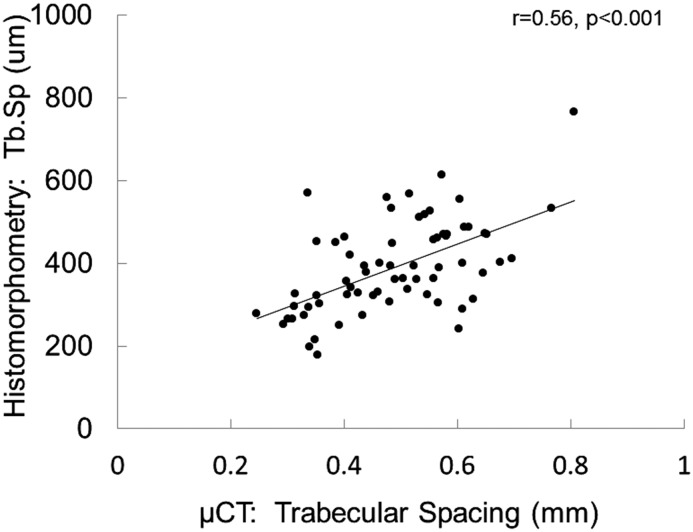
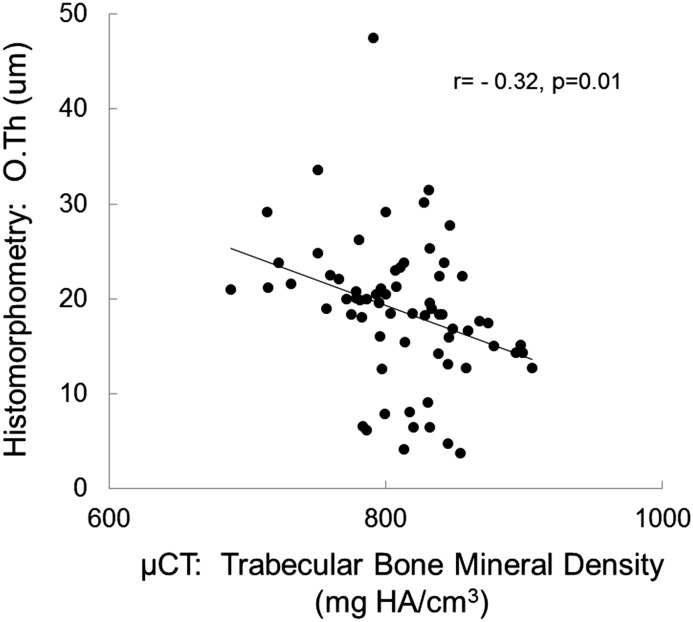
Although histomorphometric measurements of bone formation rate did not correlate with any μCT determinations, bone volume measurements were correlated between bone histomorphometry and μCT. The correlation coefficient between bone volume/tissue volume as assessed by the two techniques was r=0.70 (P<0.001) (Figure 1), whereas the correlation between measurements of trabecular thickness and trabecular separation were r=0.71 (P<0.001) (Figure 2) and r=0.56 (P<0.001) (Figure 3), respectively. Although the accumulation of unmineralized osteoid was not directly assessed by μCT, bone mineral density as assessed by μCT correlated inversely with osteoid accumulation as determined by bone histomorphometry (r=−0.32; P=0.01) (Figure 4) and osteoid volume/bone volume (r=−0.28; P=0.05). Correcting by μCT measures of bone volume bone mineral density predicted histomorphometric measurements of bone osteoid volume with an adjusted R2 of 0.15 (P=0.003). The addition of PTH and calcium to the model further improved the prediction of osteoid volume to a final adjusted R2 of 0.38. Considering whether the study participant was receiving vitamin D therapy at the time of bone biopsy did not change the predictive capability of this model.
Figure 1.
Correlation between bone volume measurements. Pearson correlation between measurements of bone volume (bone volume/tissue volume [BV/TV]) as assessed by bone histomorphometry versus microcomputed tomography (μCT).
Figure 2.
Correlation between trabecular thickness measurements. Pearson correlation between measurements of trabecular thickness (Tb.Th) as assessed by bone histomorphometry versus microcomputed tomography (μCT).
Figure 3.
Correlation between trabecular separation measurements. Pearson correlation between measurements of trabecular separation (Tb.Sp) as assessed by bone histomorphometry versus microcomputed tomography (μCT).
Figure 4.
Correlation between bone mineral density and osteoid accumulation. Pearson correlation between measurements of unmineralized osteoid accumulation (osteoid thickness [O.Th]) as assessed by bone histomorphometry and bone mineral density as assessed by microcomputed tomography (μCT). HA, hydroxyapatite.
Discussion
Assessment of bone turnover, mineralization, and volume is recommended for the diagnosis of the different subtypes of renal osteodytrophy in CKD (3,4), because all parameters likely contribute to bone fragility, deformities, and/or poor growth. Currently, the gold standard for the assessment of all three parameters continues to be the bone biopsy; however, to avoid the invasive nature of the bone biopsy, noninvasive techniques, such as pQCT, QCT, and dual–energy x-ray absorptiometry (DXA), are widely used for the evaluation of bone mass/bone volume. These techniques are not equivalent, because the predictive capability for bone volume varies markedly between techniques, and none to date have been evaluated in their ability to assess skeletal mineralization using bone histomorphometry as a standard. This study, in which bone histomorphometric measures of osteoid accumulation correlated with μCT measures of bone mineral density, suggests that skeletal mineralization, in addition to bone volume, may be assessable by high–resolution bone CT in bone cores from patients on dialysis.
Various investigators have evaluated the ability of imaging techniques to predict both bone composition and structure. Wehrli et al. (16) have performed many studies on the value of magnetic resonance imaging (MRI) in the assessment of bone mineralization in animals, with mineralization assessed by multiple techniques, including ash weight, 31P solution nuclear magnetic resonance, CT density, and water content. Wehrli et al. (16) have also evaluated the potential clinical use of MRI in 17 pediatric patients on dialysis (16); however, no comparison with histomorphometry was performed in this analysis, and assessment was limited to bone volume and structure. Misof et al. (17) also evaluated the relationship between cortical and cancellous mineralization parameters by backscatter electron microscopy in adults with normal kidney function; however, histomorphometric variables were likewise not reported in this analysis. Two studies in individuals with normal kidney function—one by Borah et al. (18) and one by Tamminen et al. (19)—did relate imaging measures to bone histomorphometry. Borah et al. (18) showed an improvement in the proportion of highly mineralized bone as assessed by μCT in adults in response to bisphosphonate therapy—an improvement that correlated with changes in bone formation (R2=0.79), and Tamminen et al. (19) showed that mineralization heterogeneity, as determined by backscatter electron microscopy, correlated with histomorphometric parameters and serum markers of bone turnover in pediatric patients with osteoporosis. Boling et al. (8) have also previously shown that bone density, as assessed by QCT, differed between adult patients on hemodialysis with low and high bone turnover. However, in each of these previous studies, it is important to consider that assessment of skeletal mineralization, independent of bone turnover, was not considered, and although bone turnover and skeletal mineralization tend to be highly correlated in individuals with normal kidney function, defects in skeletal mineralization may be independent of altered bone turnover in the pediatric CKD population (20–22).
A variety of biomarkers, including calcium, phosphorus, alkaline phosphatase, and PTH values, have all been used to assess bone quality in the pediatric CKD population. Despite much research and innovations in assay techniques over the years, however, these tests remain imperfect in their assessment capability. Current Kidney Disease Improving Global Outcomes guidelines suggest that PTH values within a very wide range (two to nine times the normal range) are associated with normal bone turnover, and current pediatric guidelines vary considerably according to region (Kidney Disease Outcomes Quality Initiative and European guidelines). Over the past decade, reassessment of biomarkers has suggested that the use of a combination of serum calcium, PTH, and alkaline phosphatase may aid the assessment of both bone turnover and skeletal mineralization and that a new biomarker, fibroblast growth factor 23, may predict skeletal mineralization. Although bone histomorphometry remains the gold standard for the assessment of bone turnover, mineralization, and volume in CKD, this analysis suggests that the addition of biochemical parameters to noninvasive imaging analysis improves the assessment of skeletal mineralization in pediatric patients with ESRD. Although this finding is promising, an R2 of 0.38 for the model as a whole—including μCT measures of bone volume, bone mineralization, serum calcium, and serum PTH levels—suggests that these factors together predict 38% of the variation in osteoid volume, leaving another 62% of the variation unrelated to these factors. Dialysis vintage may also affect bone structure and composition, and certain treatments, including vitamin D sterols (21) and growth hormone therapy (23), have been shown to disrupt the relationship between bone turnover and PTH. In this study of patients who were relatively new to dialysis, the addition of the presence of active vitamin D sterol therapy to the model did not improve prediction of osteoid accumulation. Circulating values of fibroblast growth factor 23 have also previously been associated with indices of skeletal mineralization in the pediatric dialysis population (20,24), and other markers of bone health, including circulating sclerostin levels and 25(OH) vitamin D stores, may affect skeletal mineralization; whether the addition of these parameters to traditional biochemical markers and μCT measurements can aid in the assessment of skeletal mineralization in the CKD population as a whole remains unknown.
This study consisted of a comparison between parameters of bone as assessed by bone histology and μCT measurements of bone biopsy specimens. Differences were noted within patients with respect to the amount of osteoid accumulation; however, future prospective studies are needed to assess the power of μCT in discriminating between normally and abnormally mineralized bone. It is also important to note that the μCT measurements in this study were obtained from bone biopsy specimens. Whether measurements obtained by μCT measurements directly in patients will yield similar results and whether measurements of bone density and structure at other sites will be related to bone histomorphometric parameters as assessed in the iliac crest are currently unknown. The benefit of CT scanning, relative to other imaging techniques, also remains an open question. Indeed, the use of CT scans—particularly on a repeated basis—in children is not without risk; cumulative doses of 50 mGy might almost triple the risk of leukemia, and doses of about 60 mGy might triple the risk of brain cancer (25). Other techniques, such as MRI, have no ionizing radiation; however, the relative ability of these two techniques to assess skeletal mineralization in bone cores, let alone in patients themselves, is unknown and warrants study.
In conclusion, these data confirm that measures of bone volume can be accurately assessed with μCT. Skeletal mineralization may also be assessed by μCT of bone biopsy cores, because bone mineral density was determined to be lower in patients with excessive osteoid accumulation and similar amounts of bone volume and higher in patients with adynamic, well mineralized bone. The potential value for noninvasive high–resolution CT assessment of bone in vivo to predict mineralization is unknown and warrants additional investigation.
Disclosures
None.
Acknowledgments
This work was supported, in part, by United States Public Health Services grants DK-67563, DK-35423, and DK-080984 and Clinical and Translational Science Institute grant UL1 TR-000124 and funds from the Children’s Discovery and Innovation Institute, the American Society of Nephrology Foundation’s Norman Siegel Research Scholar Award, and the Casey Lee Ball Foundation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Jamal SA, West SL, Nickolas TL: The clinical utility of FRAX to discriminate fracture status in men and women with chronic kidney disease. Osteoporos Int 25: 71–76, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Groothoff JW, Offringa M, Van Eck-Smit BL, Gruppen MP, Van De Kar NJ, Wolff ED, Lilien MR, Davin JC, Heymans HS, Dekker FW: Severe bone disease and low bone mineral density after juvenile renal failure. Kidney Int 63: 266–275, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Moe S, Drüeke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G, Kidney Disease: Improving Global Outcomes (KDIGO) : Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69: 1945–1953, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group: KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 113:S1-130, 2009 [DOI] [PubMed]
- 5.Tsampalieros A, Kalkwarf HJ, Wetzsteon RJ, Shults J, Zemel BS, Foster BJ, Foerster DL, Leonard MB: Changes in bone structure and the muscle-bone unit in children with chronic kidney disease. Kidney Int 83: 495–502, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wetzsteon RJ, Kalkwarf HJ, Shults J, Zemel BS, Foster BJ, Griffin L, Strife CF, Foerster DL, Jean-Pierre DK, Leonard MB: Volumetric bone mineral density and bone structure in childhood chronic kidney disease. J Bone Miner Res 26: 2235–2244, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lima EM, Goodman WG, Kuizon BD, Gales B, Emerick A, Goldin J, Salusky IB: Bone density measurements in pediatric patients with renal osteodystrophy. Pediatr Nephrol 18: 554–559, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Boling EP, Primavera C, Friedman G, King M, Bosserman L, Schulz EE, Goodman WG: Non-invasive measurements of bone mass in adult renal osteodystrophy. Bone 14: 409–413, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Sanchez CP, Salusky IB, Kuizon BD, Ramirez JA, Gales B, Ettenger RB, Goodman WG: Bone disease in children and adolescents undergoing successful renal transplantation. Kidney Int 53: 1358–1364, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Jacklyn CL, Freeman CD, Gray ME, MacAulay MA: Determination of serum calcium by use of an AutoAnalyzer I-II hybrid. Clin Chem 19: 516–520, 1973 [PubMed] [Google Scholar]
- 11.Amador E, Urban J: Simplified serum phosphorus analyses by continuous-flow ultraviolet spectrophotometry. Clin Chem 18: 601–604, 1972 [PubMed] [Google Scholar]
- 12.Proksch GJ, Bonderman DP, Griep JA: AutoAnalyzer assay for serum alkaline phosphatase activity, with sodium thymolphthalein monophosphate as substrate. Clin Chem 19: 103–105, 1973 [PubMed] [Google Scholar]
- 13.Aloia JF, Feuerman M, Yeh JK: Reference range for serum parathyroid hormone. Endocr Pract 12: 137–144, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR: Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2: 595–610, 1987 [DOI] [PubMed] [Google Scholar]
- 15.Bakkaloglu SA, Wesseling-Perry K, Pereira RC, Gales B, Wang HJ, Elashoff RM, Salusky IB: Value of the new bone classification system in pediatric renal osteodystrophy. Clin J Am Soc Nephrol 5: 1860–1866, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wehrli FW, Leonard MB, Saha PK, Gomberg BR: Quantitative high-resolution magnetic resonance imaging reveals structural implications of renal osteodystrophy on trabecular and cortical bone. J Magn Reson Imaging 20: 83–89, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Misof BM, Dempster DW, Zhou H, Roschger P, Fratzl-Zelman N, Fratzl P, Silverberg SJ, Shane E, Cohen A, Stein E, Nickolas TL, Recker RR, Lappe J, Bilezikian JP, Klaushofer K: Relationship of bone mineralization density distribution (BMDD) in cortical and cancellous bone within the iliac crest of healthy premenopausal women. Calcif Tissue Int 95: 332–339, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borah B, Ritman EL, Dufresne TE, Jorgensen SM, Liu S, Sacha J, Phipps RJ, Turner RT: The effect of risedronate on bone mineralization as measured by micro-computed tomography with synchrotron radiation: Correlation to histomorphometric indices of turnover. Bone 37: 1–9, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Tamminen IS, Misof BM, Roschger P, Mäyränpää MK, Turunen MJ, Isaksson H, Kröger H, Mäkitie O, Klaushofer K: Increased heterogeneity of bone matrix mineralization in pediatric patients prone to fractures: A biopsy study. J Bone Miner Res 29: 1110–1117, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Pereira RC, Jüppner H, Azucena-Serrano CE, Yadin O, Salusky IB, Wesseling-Perry K: Patterns of FGF-23, DMP1, and MEPE expression in patients with chronic kidney disease. Bone 45: 1161–1168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wesseling-Perry K, Pereira RC, Sahney S, Gales B, Wang HJ, Elashoff R, Jüppner H, Salusky IB: Calcitriol and doxercalciferol are equivalent in controlling bone turnover, suppressing parathyroid hormone, and increasing fibroblast growth factor-23 in secondary hyperparathyroidism. Kidney Int 79: 112–119, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Nawrot-Wawrzyniak K, Misof BM, Roschger P, Pańczyk-Tomaszewska M, Ziółkowska H, Klaushofer K, Fratzl-Zelman N: Changes in bone matrix mineralization after growth hormone treatment in children and adolescents with chronic kidney failure treated by dialysis: A paired biopsy study. Am J Kidney Dis 61: 767–777, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Bacchetta J, Wesseling-Perry K, Kuizon B, Pereira RC, Gales B, Wang HJ, Elashoff R, Salusky IB: The skeletal consequences of growth hormone therapy in dialyzed children: A randomized trial. Clin J Am Soc Nephrol 8: 824–832, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wesseling-Perry K, Pereira RC, Wang H, Elashoff RM, Sahney S, Gales B, Juppner H, Salusky IB: Relationship between plasma fibroblast growth factor-23 concentration and bone mineralization in children with renal failure on peritoneal dialysis. J Clin Endocrinol Metab 94: 511–517, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, Howe NL, Ronckers CM, Rajaraman P, Sir Craft AW, Parker L, Berrington de González A: Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: A retrospective cohort study. Lancet 380: 499–505, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]