Abstract
Background and objectives
In primary FSGS, calcineurin inhibitors have primarily been studied in patients deemed resistant to glucocorticoid therapy. Few data are available about their use early in the treatment of FSGS. We sought to estimate the association between choice of therapy and ESRD in primary FSGS.
Design, setting, participants, & measurements
We used an inception cohort of patients diagnosed with primary FSGS by kidney biopsy between 1980 and 2012. Factors associated with initiation of therapy were identified using logistic regression. Time–dependent Cox models were performed to compare time to ESRD between different therapies.
Results
In total, 458 patients were studied (173 treated with glucocorticoids alone, 90 treated with calcineurin inhibitors with or without glucocorticoids, 12 treated with other agents, and 183 not treated with immunosuppressives). Tip lesion variant, absence of severe renal dysfunction (eGFR≥30 ml/min per 1.73 m2), and hypoalbuminemia were associated with a higher likelihood of exposure to any immunosuppressive therapy. Only tip lesion was associated with initiation of glucocorticoids alone over calcineurin inhibitors. With adjusted Cox regression, immunosuppressive therapy with glucocorticoids and/or calcineurin inhibitors was associated with better renal survival than no immunosuppression (hazard ratio, 0.49; 95% confidence interval, 0.28 to 0.86). Calcineurin inhibitors with or without glucocorticoids were not significantly associated with a lower likelihood of ESRD compared with glucocorticoids alone (hazard ratio, 0.42; 95% confidence interval, 0.15 to 1.18).
Conclusions
The use of immunosuppressive therapy with calcineurin inhibitors and/or glucocorticoids as part of the early immunosuppressive regimen in primary FSGS was associated with improved renal outcome, but the superiority of calcineurin inhibitors over glucocorticoids alone remained unproven.
Keywords: focal segmental glomerulosclerosis, immunosuppression, ESRD, nephrotic syndrome, glomerular disease, calcineurin inhibitors, glucocorticoids, humans, immunosuppressive agents
Introduction
In adults, idiopathic FSGS represents the most common cause of primary nephrotic syndrome and the most common cause of ESRD related to glomerular disease (1,2). FSGS encompasses different histologic variants, which differ in their epidemiology, clinical course, and response to therapy (3).
Glucocorticoids have historically been used as a first-line therapy in FSGS on the basis of retrospective or uncontrolled prospective cohort studies (4–7); however, no randomized, controlled trials were performed to provide direct evidence of their efficacy in preserving renal function. Furthermore, FSGS variant was not taken into consideration in the choice of therapy in these studies (8). The major randomized, controlled trials in FSGS evaluated patients deemed steroid resistant and used remission of proteinuria as their primary end point (9,10). Although renal failure has been studied as a short–term secondary end point in primary FSGS clinical trials, calcineurin inhibitors (CNIs; tacrolimus or cyclosporin) have not been formally evaluated with respect to long–term renal survival (ESRD) as a primary outcome, and they were not compared with high-dose glucocorticoids in a head-to-head randomized, controlled trial.
This study was performed to determine the patient and disease characteristics associated with choice of therapy and estimate the association between choice of early therapy and renal outcome (ESRD) in primary idiopathic FSGS. This analysis tested the hypothesis that the use of CNI therapy is associated with a decrease in the likelihood of ESRD after controlling for other factors affecting renal survival.
Materials and Methods
Study Design and Population
All patients in the Glomerular Disease Collaborative Network (GDCN) with biopsy-proven FSGS diagnosed between 1980 and 2012 were considered for this inception cohort study. The GDCN FSGS registry enrolls patients coming from 600 participating physicians in >250 clinics in eight states. Patients with a known secondary cause of FSGS, such as HIV, hepatitis B or C, intravenous drug use, sickle cell disease, single kidney, reflux nephropathy, or other GN, and transplant recipients were excluded. Perihilar variant FSGS, believed to be an “adaptive response to nephron loss or glomerular hypertension” (11–16), was excluded. All biopsy specimens had a minimum of five glomeruli assessed by light microscopy. FSGS variant was determined using the biopsy report. Patients with any level of proteinuria were included in the study to examine the full spectrum of FSGS. All subjects provided written informed consent. This study was approved by the University of North Carolina’s Institutional Review Board in agreement with the Declaration of Helsinki.
Clinical Data and Definitions
Clinical and laboratory variables were extracted from medical records from the time of renal biopsy to the last available follow-up visit and/or initiation of RRT. Presence of arterial hypertension was defined as systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg for adults and systolic and/or diastolic BP ≥95th percentile for age, sex, and height for children (17). Quantification of proteinuria was on the basis of either spot urinary protein-to-creatinine ratio (Up/c) or 24-hour urine collection as reported in the medical record. Complete remission was defined as a reduction in proteinuria to ≤0.3 g/24 h (or 0.3 g/g on Up/c) with ≤25% increase in serum creatinine. Partial remission was defined as a reduction in proteinuria by ≥50% to a level <3.5 g/24 h (or 2.0 g/g on Up/c) and >0.3 g/24 h or 0.3 g/g with stable renal function. Information was also collected on immunosuppressive and antihypertensive therapy. The primary outcome was ESRD (eGFR<15 ml/min per 1.73 m2, dialysis, or transplantation). eGFR was calculated using the Modification of Diet in Renal Disease Study group equation (18) for adults and the Schwartz formula for children using height and serum creatinine (19).
Immunomodulatory therapy was classified into three groups: glucocorticoids alone, CNIs with or without glucocorticoids, and other immunosuppressive agents (e.g., azathioprine, mycophenolate mofetil, and cyclophosphamide). Exposure to therapy was defined as the first immunosuppressive treatment started after initial kidney biopsy, regardless of the duration between biopsy and beginning of immunosuppression. CNI therapy started within 3 months of glucocorticoid initiation was considered early therapy. Some patients were treated with a CNI and glucocorticoids simultaneously, with the latter given at any dosage. Patients who were given glucocorticoids alone at a high dose (1 mg/kg or ≥30 mg/d) were considered treated with glucocorticoids. CNIs at any dosage were considered as treatment with CNIs. Length of CNI therapy was defined as the interval during which the drug was prescribed, regardless of dosage. Length of glucocorticoid therapy was defined as the interval during which the drug was prescribed at high dose (1 mg/kg or ≥30 mg/d). Any treatment with angiotensin–converting enzyme inhibitor, angiotensin receptor blocker, or selective aldosterone blocker was defined as renin-angiotensin-aldosterone system inhibition.
Statistical Analyses
Normally distributed continuous variables were summarized as means±SDs and compared using t tests. Variables that were not normally distributed were summarized as medians (interquartile ranges [IQRs]) and compared with Mann–Whitney U tests. Chi-squared or Fisher exact tests were used to compare categorical variables regarding treatment and remission. A kernel density estimator was used to approximate density from observations on the length between renal biopsy and immunosuppression initiation.
Identifying Factors Associated with Choice of Therapy.
To identify potentially important confounders in the association between the choice of early immunomodulatory therapy and time to ESRD, factors associated with the initiation of treatment with an immunomodulatory agent at all (treated versus untreated) were investigated. Patients who were prescribed high-dose glucocorticoids (1 mg/kg or ≥30 mg/d), CNIs with or without glucocorticoids at any dosage, or any other immunomodulatory agent were considered treated, regardless of the duration of therapy. A logistic regression was performed to identify factors associated with being prescribed any immunosuppressive therapy (versus none).
Among those prescribed any immunosuppressive therapy, another logistic regression model was fitted to identify variables associated with having been prescribed CNIs (with or without glucocorticoids) compared with high-dose glucocorticoids alone. The 12 patients treated with various other immunomodulatory agents were excluded from this analysis.
Modeling the Association between Choice of Therapy and ESRD.
Cox proportional hazards models were constructed to assess the association between initiation of therapy and time to ESRD adjusted for potential confounders. Time zero was biopsy time. Time–dependent Cox models in which the primary exposure (glucocorticoids alone or CNIs with or without glucocorticoids) was allowed to change over time were used. The proportional hazards assumption was tested using goodness of fit testing (Schoenfeld residuals), log-log plots, and observed versus expected plots. Renin-angiotensin-aldosterone system inhibition was not included in models given that a high proportion of patients on immunosuppression was exposed (>80%). Cox survival models were also performed in adults only, and results are presented separately.
Both the Kaplan–Meier survival method and Cox adjusted survival curves were used to visually evaluate the relationship between treatment with immunosuppressive therapy and primary end point (ESRD). Nelson–Aalen adjusted survival curves were generated using the following pattern of covariates: mean values for age, baseline serum albumin, and eGFR among patients treated with glucocorticoids alone or CNIs with or without glucocorticoids; men; and not otherwise specified (NOS) variant.
Managing Missing Data.
Missing values for race (4.2%), baseline eGFR (2.8%), baseline 24-hour proteinuria (27.1%), baseline serum albumin (17.7%), edema (15.7%), and presence of hypertension at baseline (15.7%) were imputed using an iterative Markov chain Monte Carlo multiple imputation technique (20 imputations) (20). The primary exposure (immunosuppressive therapy or CNI treatment with or without glucocorticoids glucocorticoids) was not imputed in any analysis.
When proteinuria was measured using Up/c only without concomitant 24-hour excretion quantification, the missing 24-hour urine data were handled in two ways. First, missing values were imputed as described above. Second, missing 24-hour urine values were estimated from the Up/c values using 1:1 and 1:1.5 conversion factors (21). Cox regression models using both methods and both conversion factors were fitted and compared with the hazard ratio (HR) estimates.
Results for logistic regression models were expressed as odds ratios (ORs) with 95% confidence intervals (95% CIs), and results for Cox proportional hazards models were expressed as HRs with 95% CIs. Statistical analyses were performed using Stata 13 (StataCorp., College Station, TX). All authors had access to the primary data; L.-P.L. performed the statistical analysis.
Results
Patient Characteristics
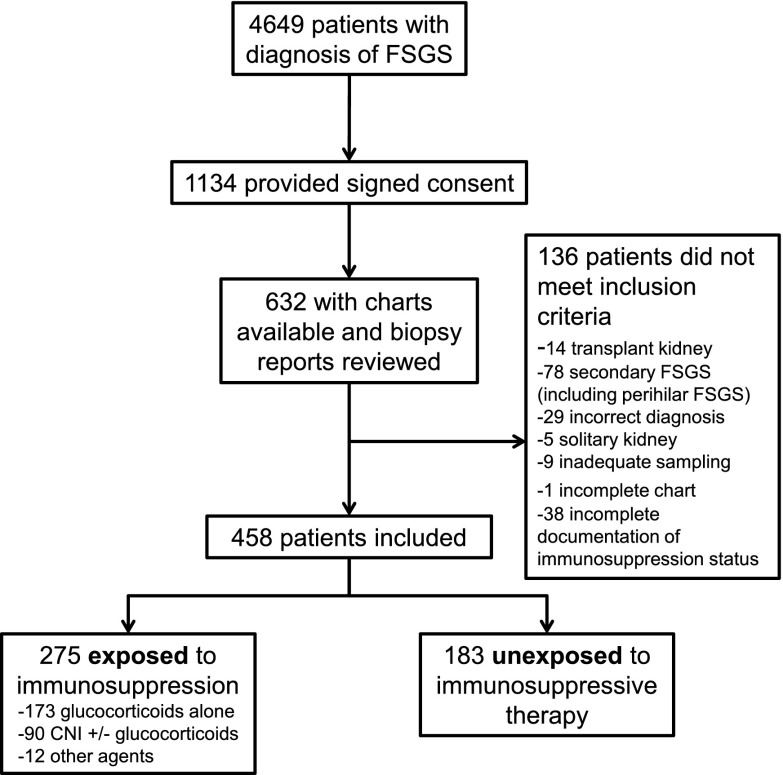
Of 458 patients (84 children) with biopsy-proven FSGS, 183 received no immunosuppressive therapy, 173 received glucocorticoids alone, 90 were treated with CNIs with or without glucocorticoids (Figure 1, Table 1), and 12 were treated with other immunomodulatory agents (six with mycophenolate mofetil/glucocorticoids, four with cyclophosphamide/glucocorticoids, one with azathioprine, and one with adalimumab). Patients who did not receive immunosuppressive therapy had lower median baseline eGFR (43.8 ml/min per 1.73 m2; IQR, 27.2–69.9 ml/min per 1.73 m2 versus 62.8 ml/min per 1.73 m2; IQR, 41.7–85.7 ml/min per 1.73 m2; P<0.001) and lower median baseline 24-hour proteinuria (3.8 g/d; IQR, 2.4–6.6 g/d versus 6.0 g/d; IQR, 3.5–12.0 g/d; P<0.001) than those who received immunosuppressive therapy.
Figure 1.
Study flow diagram. CNI, calcineurin inhibitor.
Table 1.
Baseline characteristics by immunosuppression status
| Variables | No Immunosuppression, n=183 | Immunosuppression,a n=275 | ||||
|---|---|---|---|---|---|---|
| Any Immunosuppression, n=275 | P Valueb | Glucocorticoids Alone, n=173 | CNIs with or without Glucocorticoids, n=90 | P Valuec | ||
| Age at biopsy (yr) | 48 (32–63) | 36 (18–55) | <0.001 | 41 (25–58) | 25 (13–47) | <0.001 |
| Children <18 yr old, % | 10.4 | 23.6 | 15.0 | 38.9 | ||
| Women, % | 48.1 | 49.1 | 0.83 | 45.7 | 55.6 | 0.13 |
| Black race, % | 45.1 | 45.9 | 0.87 | 41.5 | 55.6 | 0.03 |
| Missing, % | 5.5 | 3.3 | 5.2 | 0 | ||
| FSGS variant, % | <0.001 | <0.001 | ||||
| NOS | 83.6 | 65.1 | 61.9 | 71.1 | ||
| Tip | 5.5 | 19.6 | 27.2 | 5.6 | ||
| Collapsing | 10.9 | 15.3 | 11.0 | 23.3 | ||
| eGFR at biopsy (ml/min per 1.73 m2) | 43.8 (27.2–69.9) | 62.8 (41.7–85.7) | <0.001 | 61.9 (40.5–83.9) | 65.5 (46.5–88.2) | 0.39 |
| Missing, % | 1.6 | 2.5 | 1.2 | 3.3 | ||
| 24-h Proteinuria at biopsy (g/d) | 3.8 (2.4–6.6) | 6.0 (3.5–12.0) | <0.001 | 6.4 (3.8–12.0) | 5.5 (2.9–13.0) | 0.46 |
| Missing, % | 20.2 | 31.6 | 19.1 | 52.2 | ||
| Serum albumin at biopsy (mg/dl) | 3.5 (3–4) | 2.4 (1.7–3.3) | <0.001 | 2.5 (1.9–3.5) | 2.1 (1.5–2.9) | 0.01 |
| Missing, % | 19.7 | 16.4 | 15.6 | 16.7 | ||
| Hypertension at baseline, % | 67.3 | 64.7 | 0.60 | 68.4 | 55.4 | 0.78 |
| Missing, % | 11.5 | 18.6 | 10.4 | 27.8 | ||
| Edema at baseline, % | 45.5 | 68.3 | <0.001 | 68.9 | 70.6 | 0.07 |
| Missing, % | 21.9 | 11.6 | 12.7 | 5.6 | ||
Continuous variables are expressed as medians (interquartile ranges). CNI, calcineurin inhibitor; NOS, not otherwise specified.
Twelve patients were exposed to a variety of other immunosuppressive agents.
No immunosuppression versus any immunosuppression.
Glucocorticoids alone versus CNIs with or without glucocorticoids.
Immunosuppression
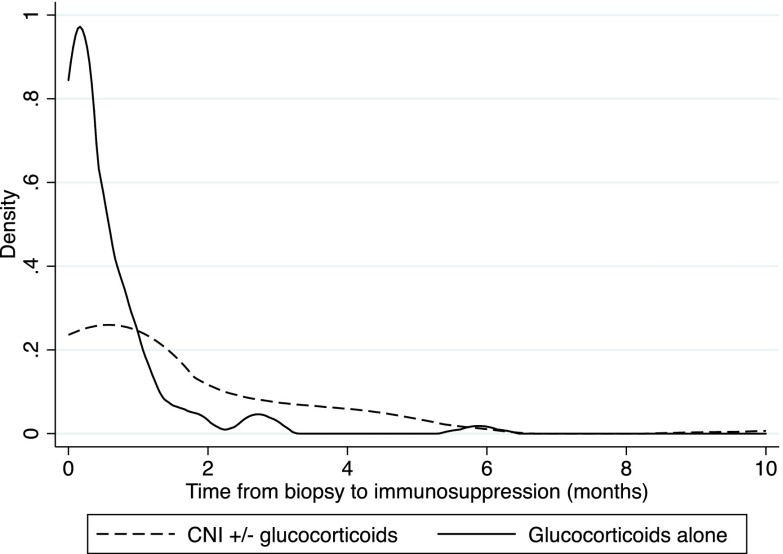
Median time to initiation of high-dose glucocorticoids after biopsy was 0.3 months (IQR, 0.03–0.8 months), and median time to initiation of CNI therapy after biopsy was 0.7 months (IQR, 0.1–3.0 months). Median duration of high–dose glucocorticoid therapy was 3.0 months (IQR, 1.5–5.9 months). Among those treated with CNIs, 75% started CNIs within 3 months after kidney biopsy (Figure 2), and only 28 (31.1%) patients were given CNIs alone. CNI treatment had a median duration of 19.6 months (IQR, 6.5–34.8 months).
Figure 2.
Probability density function of time length between renal biopsy and immunosuppression therapy. CNI, calcineurin inhibitor.
Factors Associated with Exposure to Immunosuppressive Therapy
The factors significantly associated with immunosuppressive treatment are summarized in Table 2. The odds of receiving immunosuppression were significantly lower among patients with eGFR<30 ml/min per 1.73 m2 at baseline than in those with an eGFR≥30 ml/min per 1.73 m2 (OR, 0.53; 95% CI, 0.29 to 0.99) and those with advancing age (OR, 0.82 for each 10-year increment; 95% CI, 0.74 to 1). Lower baseline serum albumin was associated with a higher likelihood of receiving immunosuppressive therapy (OR, 2.22 for each 1 g/dl decrease; 95% CI, 1.59 to 3.13). Patients with a tip lesion were three times more likely to be treated with immunosuppressives than those with the NOS FSGS variant (OR, 3.00; 95% CI, 1.23 to 7.32) (Supplemental Table 1).
Table 2.
Factors associated with treatment with immunosuppressive therapy
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Age at biopsy per 10-yr higher | 0.82 | 0.74 to 0.90 | 0.82 | 0.74 to 1.00 |
| Men | 0.96 | 0.66 to 1.40 | 1.28 | 0.77 to 2.13 |
| Black race | 1.02 | 0.70 to 1.50 | 0.87 | 0.50 to 1.50 |
| FSGS variant | ||||
| NOS | 1.00 | 1.00 | ||
| Tip | 4.62 | 2.27 to 9.37 | 3.00 | 1.23 to 7.32 |
| Collapsing | 1.79 | 1.01 to 3.19 | 1.19 | 0.53 to 2.68 |
| Baseline eGFR <30 ml/min per 1.73 m2 | 0.42 | 0.26 to 0.67 | 0.53 | 0.29 to 0.99 |
| Baseline proteinuria ≥3.5 g/d | 2.03 | 1.32 to 3.13 | 1.04 | 0.56 to 1.93 |
| Serum albumin at biopsy per 1 g/dl lower | 2.44 | 1.92 to 3.03 | 2.22 | 1.59 to 3.13 |
| Edema at baseline | 2.59 | 1.69 to 3.96 | 1.42 | 0.81 to 2.49 |
| Hypertension at baseline | 0.82 | 0.54 to 1.25 | 0.92 | 0.52 to 1.64 |
OR, odds ratio; 95% CI, 95% confidence interval; NOS, not otherwise specified.
Factors Associated with Choice of Immunosuppressive Therapy
The multivariable logistic regression model (including age, race, and baseline albuminemia and proteinuria) identified only histologic variant as independently associated with the odds of being treated with CNIs; compared with FSGS NOS, tip lesion FSGS was associated with significantly lower likelihood of treatment with CNI (OR, 0.17; 95% CI, 0.05 to 0.53).
Immunosuppressive Therapy and Time to ESRD
Any Immunosuppression Versus None.
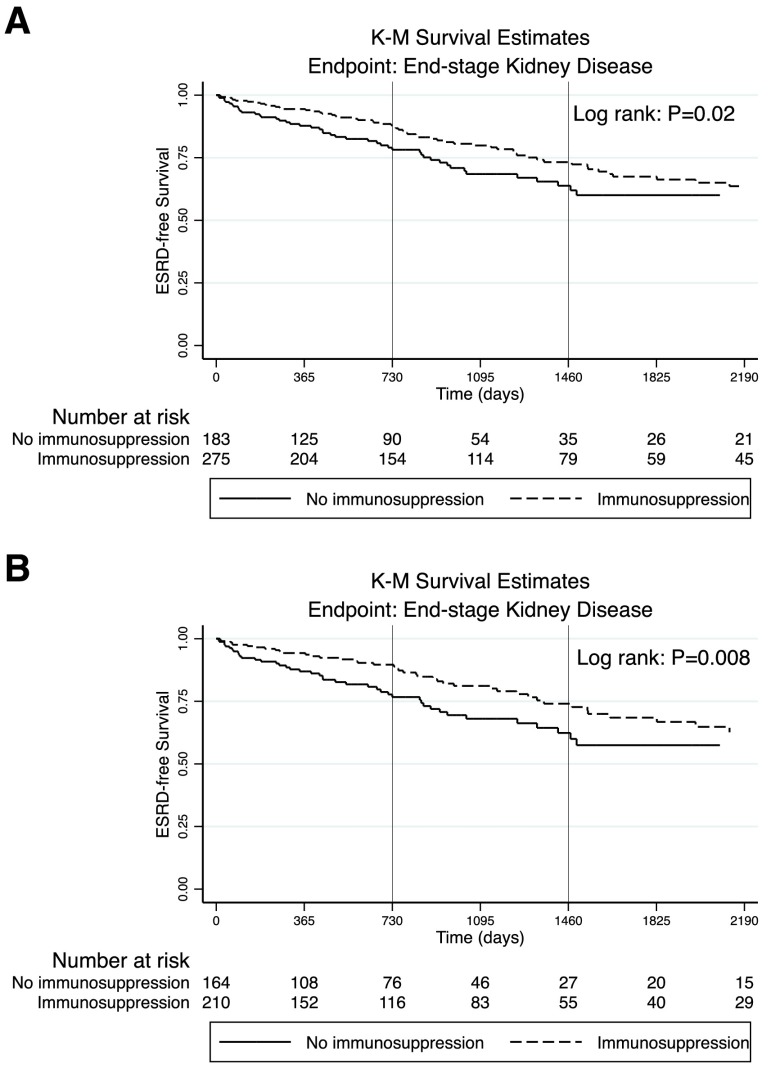
Exposure to any immunosuppressive therapy (versus none) was associated with better renal survival in both unadjusted and adjusted Cox survival models (Table 3). The crude cumulative probabilities of being ESRD free at 1, 2, and 5 years were 94.4% (204 at risk), 87.9% (154 at risk), and 67.4% (59 at risk) for those treated with any immunosuppressive therapy compared with 87.8% (125 at risk), 78.2% (90 at risk), and 60.0% (26 at risk) for patients not treated with immunosuppressive therapy (Figure 3A). Compared with no treatment, treatment with any immunosuppression was associated with significantly lower hazards of ESRD (HR, 0.49; 95% CI, 0.28 to 0.86). A baseline eGFR <30 ml/min per 1.73 m2 was associated with higher hazards of ESRD (HR, 4.28; 95% CI, 2.81 to 6.48). Among adults only, treatment with any immunosuppression was also associated with significantly lower hazards of ESRD (HR, 0.43; 95% CI, 0.21 to 0.86).
Table 3.
Factors associated with ESRD by immunosuppression status
| Variables | Hazard Ratio | 95% Confidence Interval |
|---|---|---|
| Unadjusted | ||
| Immunosuppression | ||
| None | 1 | |
| CNIs with or without glucocorticoids or glucocorticoids alone | 0.50 | 0.29 to 0.87 |
| Adjusteda | ||
| Immunosuppression | ||
| None | 1 | |
| CNIs with or without glucocorticoids or glucocorticoids alone | 0.49 | 0.28 to 0.86 |
| Age per 1-yr increase | 1.00 | 0.99 to 1.01 |
| Men | 1.15 | 0.76 to 1.73 |
| FSGS variant | ||
| NOS | 1 | |
| Tip | 0.21 | 0.09 to 0.48 |
| Collapsing | 1.71 | 0.99 to 2.95 |
| Baseline eGFR <30 ml/min per 1.73 m2 | 4.28 | 2.81 to 6.48 |
| Baseline proteinuria ≥3.5 g/d | 1.25 | 0.68 to 2.29 |
| Serum albumin at biopsy per 1 g/dl higher | 0.69 | 0.53 to 0.90 |
| Hypertension at baseline | 1.33 | 0.81 to 2.20 |
| Adjusted (adults only)b | ||
| Immunosuppression | ||
| None | 1 | |
| CNIs with or without glucocorticoids or glucocorticoids alone | 0.43 | 0.21 to 0.86 |
CNI, calcineurin inhibitor; NOS, not otherwise specified.
Cox regression adjusted for immunosuppression, age, sex, black race, FSGS variant, and baseline eGFR, proteinuria, serum albumin, and hypertension.
Cox regression in adult patients only adjusted for immunosuppression, age, sex, black race, FSGS variant, and baseline eGFR, proteinuria, serum albumin, and hypertension.
Figure 3.
Kaplan–Meier (K-M) curves of time to ESRD by immunosuppression status. (A) For all patients; (B) for adults only.
CNIs Versus Glucocorticoids Alone.
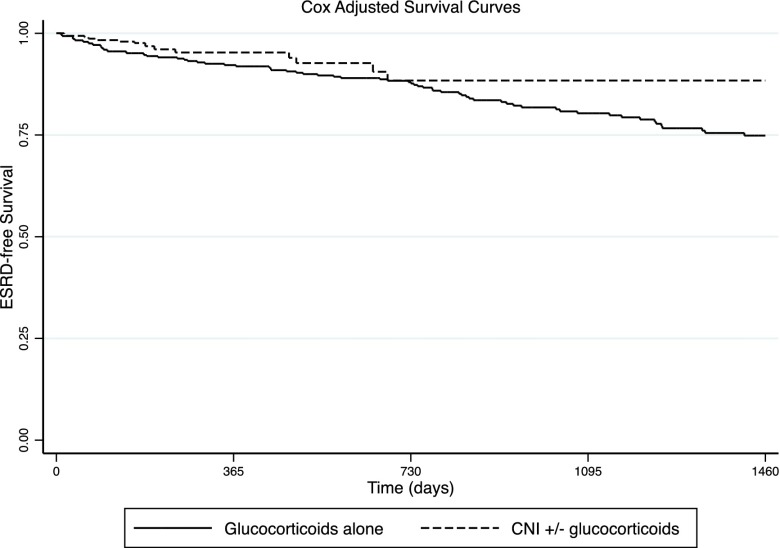
Compared with those treated with glucocorticoids alone, the adjusted risk of ESRD was not statistically significantly lower among those treated with CNIs (HR, 0.42; 95% CI, 0.15 to 1.18) (Table 4). Adjusted survival curves suggested a trend toward better long–term renal survival associated with treatment including CNIs than that with glucocorticoids alone (Figure 4). A baseline eGFR <30 ml/min per 1.73 m2 was also associated with higher hazards of ESRD (HR, 2.61; 95% CI, 1.37 to 4.96) among treated patients. An additional Cox regression model, including proteinuria at baseline in lieu of baseline albuminemia as a covariate, showed a similar nonstatistically significant lower risk of ESRD associated with CNIs compared with glucocorticoids alone (HR, 0.50; 95% CI, 0.18 to 1.44). In this model, each 1-g/d increment in baseline proteinuria was associated with a higher likelihood of ESRD (HR, 1.06; 95% CI, 1.02 to 1.11). Among treated adults only, treatment with CNIs with or without glucocorticoids was not associated with significantly lower hazards of ESRD (HR, 0.40; 95% CI, 0.09 to 1.90).
Table 4.
Factors associated with ESRD among treated patients
| Variables | Hazard Ratio | 95% Confidence Interval |
|---|---|---|
| Unadjusted | ||
| Immunosuppressive therapy | ||
| Glucocorticoids | 1.00 | |
| CNIs with or without glucocorticoids | 0.60 | 0.21 to 1.70 |
| Adjusteda | ||
| Immunosuppressive therapy | ||
| Glucocorticoids | 1.00 | |
| CNIs with or without glucocorticoids | 0.42 | 0.15 to 1.18 |
| Age per 1-yr increase | 1.00 | 0.99 to 1.02 |
| Men | 1.00 | 0.56 to 1.76 |
| FSGS variant | ||
| NOS | 1.00 | |
| Tip | 0.14 | 0.04 to 0.47 |
| Collapsing | 0.98 | 0.48 to 2.00 |
| Baseline eGFR <30 ml/min per 1.73 m2 | 2.61 | 1.37 to 4.96 |
| Serum albumin at biopsy per 1 g/dl higher | 0.55 | 0.39 to 0.78 |
| Adjusted (adults only)b | ||
| Immunosuppressive therapy | ||
| Glucocorticoids | 1.00 | |
| CNIs with or without glucocorticoids | 0.40 | 0.09 to 1.90 |
CNI, calcineurin inhibitor; NOS, not otherwise specified.
Cox regression adjusted for immunosuppression, age, sex, black race, FSGS variant, and baseline eGFR and serum albumin; baseline hypertension was not predictive of renal survival by univariate analysis.
Cox regression in adult patients only adjusted for immunosuppression, age, FSGS variant, and baseline eGFR and serum albumin.
Figure 4.
Predicted ESRD–free Nelson–Aalen curves: these curves are predicted for the adjusted Cox time–dependent model. Outcomes for the glucocorticoids alone and calcineurin inhibitors (CNIs) with or without glucocorticoids groups are computed at the mean (age, men, not otherwise specified variant, baseline serum albumin, and eGFR).
Sensitivity Analyses for Proteinuria at Baseline
Additional multivariable Cox regression models were constructed, in which missing 24-hour proteinuria data were converted from Up/c (using 1:1 and 1:1.5 conversion factors) rather than imputed. The adjusted HRs (time to ESRD) obtained from these models for treatment with immunosuppressive therapy were of similar magnitude and significance (HR, 0.47; 95% CI, 0.27 to 0.84 for 1:1 conversion; HR, 0.46; 95% CI, 0.26 to 0.83 for 1:1.5 conversion) to those estimated when missing 24-hour proteinuria values were imputed (HR, 0.49; 95% CI, 0.28 to 0.86).
Immunosuppressive Therapy and Remission
Among 163 patients with nephrotic-range proteinuria at baseline and documented proteinuria quantification during follow-up, 66 (40.5%) reached partial remission, and 36 (22.1%) reached complete remission. A high proportion of patients who were nephrotic (46.6%) had incomplete documentation of proteinuria during the course of disease. Exposure to immunosuppressive therapy was not significantly associated with remission in proteinuria, with 26 (39.4%) patients exposed to any immunosuppression reaching complete or partial remission compared with eight (28.6%) patients unexposed reaching complete or partial remission (P=0.32).
Discussion
Primary FSGS is a heterogeneous entity for which evidence of effective immunosuppressive therapy is limited. Current recommendations on the use of glucocorticoids as first-line treatment are on the basis of retrospective studies using a variety of regimens (4–7). However, high–dose glucocorticoid therapy is associated with a number of adverse effects (22). The use of CNIs in primary FSGS is on the basis of clinical trials in patients deemed resistant to glucocorticoid therapy (23). No clinical trial has assessed CNIs as first–line immunosuppressive therapy in primary FSGS or directly compared CNI treatment with glucocorticoids alone. This study describes the association between choice of early therapy (including CNIs) and renal survival (ESRD) in primary FSGS, while adjusting for the most important potential confounders in this relationship between treatment and renal outcome.
Several patient and disease characteristics were potentially associated with both initiation of therapy and renal outcome. To assure the inclusion of the most important ones in the analyses, factors associated with a decision to treat with immunosuppressives were first identified. The results showed that the initiation of immunosuppressive therapy was associated with FSGS variant, baseline renal function, and severity of nephrotic syndrome as indicated by the degree of hypoalbuminemia. Also, factors associated with the choice of early immunosuppressive therapy (glucocorticoids versus CNIs) were determined. This study identified only the presence of tip lesion as an independent factor favoring glucocorticoids alone over CNIs.
The association between exposure to any immunosuppressive therapy and ESRD was subsequently examined while considering important confounders. This study supports a role for immunosuppressive therapy with glucocorticoids and/or CNIs to positively influence renal survival. It also suggests that early treatment of patients with FSGS with CNIs combined with glucocorticoids may be associated with an improved renal survival compared with no immunosuppressive therapy.
The association between CNIs (with or without glucocorticoids) and time to ESRD among patients treated with immunosuppressive therapy was next assessed. Although not statistically significant, the results suggest that early introduction of immunosuppressives, including CNIs, may be associated with a lower likelihood of ESRD compared with treatment with glucocorticoids alone after controlling for variables known to affect renal outcome (HR, 0.42; 95% CI, 0.15 to 1.18).
Since the 1999 landmark study by Cattran et al. (9), which reported an increased likelihood of remission of proteinuria in steroid-resistant patients treated with CNIs, clinicians may have been more likely to prescribe CNIs as a first-choice treatment. Indeed, >10% of patients on immunosuppression in our cohort were exposed to CNIs alone. Although several patient and disease characteristics seemed to influence selection of the immunomodulatory agent on univariate analyses, a significant independent association was only found between tip lesion FSGS and glucocorticoid therapy alone. Tip lesion FSGS has been described to share common clinical features with minimal change disease and respond promptly to high-dose glucocorticoids (24).
This study supports a potential role for including CNIs in the early treatment of primary FSGS but is not able to prove superiority of CNIs with or without glucocorticoids over glucocorticoids alone. Combinations of immunosuppressive agents were frequently used, and few patients were exposed to CNIs alone (n=28). Because the majority of patients receiving CNIs were also treated with glucocorticoids, the ability to distinguish the benefits of CNIs alone from the combination of CNIs and high-dose glucocorticoids is limited because of lack of power.
The findings are consistent with previously reported clinical findings. Baseline level of proteinuria was previously shown to have prognostic significance (25,26). It showed that baseline proteinuria was also predictive of renal survival among patients treated with immunosuppressive therapy. It was not possible to examine the effect of remission in proteinuria on renal outcome because of incomplete documentation of proteinuria at fixed intervals. Moreover, incompleteness of data on follow-up proteinuria precluded the accurate description of the response to immunosuppressive therapy. As expected and previously shown, severe renal dysfunction at baseline (eGFR<30 ml/min per 1.73 m2) was predictive of poorer renal survival (27,28).
Severe hypoalbuminemia has been shown to be associated with complications of the nephrotic syndrome, such as thromboembolism (29–31). However, the value of hypoalbuminemia as a predictor of renal outcome in primary FSGS had not been previously described. The results of this analysis suggest that baseline serum albumin is associated with renal survival. However, the effect of recovery from severe hypoalbuminemia on renal survival has not been examined with this study design. It was also not possible to include both baseline albuminemia and proteinuria in the same Cox regression model when comparing CNIs (with or without glucocorticoids) with glucocorticoids alone because of the small number of renal failure events, resulting in power limitations.
This study has some limitations inherent to its retrospective nature. These include a lack of uniform treatment protocol and systematic laboratory tests at fixed intervals and variable follow-up intervals. As a result, some variables of interest had a significant proportion of missing values, impeding their use in statistical models with multiple imputations. This could lead to residual confounding. For example, presence of obesity (high body mass index) could influence the initiation of immunosuppressive therapy by favoring steroid-sparing agents to avoid glucocorticoid-induced diabetes and modify disease trajectory by adding a superimposed glomerular injury. For the balance of the variables, missing values were handled using multiple imputation, which provides less biased estimates than complete patient analysis (20). The survival model also has limitations by not perfectly adjusting for all potential confounders. Some patients, especially children, may have been exposed to immunosuppression before renal biopsy. The results show, however, similar finding when restricting the survival analyses to adults only. Unaccounted prebiopsy treatment with glucocorticoids alone is not likely to have influenced our results significantly, because children <10 years old at the time of renal biopsy accounted for only 5% of the total cohort.
Use of propensity score methods was considered to account for confounding by variables associated with clinical choice to initiate treatment (32). However, creation of a propensity score–matched cohort would have required the exclusion of numerous subjects from our analysis for those not paired, which would have considerably reduced already limited statistical power.
In conclusion, despite the fact that patients treated with immunosuppressives tended to have evidence of more severe nephrotic syndrome than those who were untreated, this study showed a significant association between treatment with immunosuppressive therapy and better renal survival. This observation could not, however, establish differences in efficacy between treatment modalities. Whether the early use of CNIs alone or in addition to glucocorticoids is of benefit in preserving renal function in patients with FSGS should be formally tested in a prospective randomized clinical trial.
Disclosures
None.
Supplementary Material
Acknowledgments
L.-P.L. received salary support from the Hôpital Maisonneuve-Rosemont Scholarship of Improvement Program, the Société Québécoise de Néphrologie, and the Department of Medicine, Université de Montréal.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Rethinking First-Line Immunosuppression for Idiopathic FSGS,” on pages 372–373.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07110615/-/DCSupplemental.
References
- 1.Haas M, Meehan SM, Karrison TG, Spargo BH: Changing etiologies of unexplained adult nephrotic syndrome: A comparison of renal biopsy findings from 1976-1979 and 1995-1997. Am J Kidney Dis 30: 621–631, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Kitiyakara C, Eggers P, Kopp JB: Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis 44: 815–825, 2004 [PubMed] [Google Scholar]
- 3.D’Agati VD, Kaskel FJ, Falk RJ: Focal segmental glomerulosclerosis. N Engl J Med 365: 2398–2411, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Korbet SM, Schwartz MM, Lewis EJ: Primary focal segmental glomerulosclerosis: Clinical course and response to therapy. Am J Kidney Dis 23: 773–783, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Banfi G, Moriggi M, Sabadini E, Fellin G, D’Amico G, Ponticelli C: The impact of prolonged immunosuppression on the outcome of idiopathic focal-segmental glomerulosclerosis with nephrotic syndrome in adults. A collaborative retrospective study. Clin Nephrol 36: 53–59, 1991 [PubMed] [Google Scholar]
- 6.Cattran DC, Rao P: Long-term outcome in children and adults with classic focal segmental glomerulosclerosis. Am J Kidney Dis 32: 72–79, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Rydel JJ, Korbet SM, Borok RZ, Schwartz MM: Focal segmental glomerular sclerosis in adults: Presentation, course, and response to treatment. Am J Kidney Dis 25: 534–542, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Braun N, Schmutzler F, Lange C, Perna A, Remuzzi G, Risler T, Willis NS: Immunosuppressive treatment for focal segmental glomerulosclerosis in adults. Cochrane Database Syst Rev 3: CD003233, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cattran DC, Appel GB, Hebert LA, Hunsicker LG, Pohl MA, Hoy WE, Maxwell DR, Kunis CL, North America Nephrotic Syndrome Study Group : A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. Kidney Int 56: 2220–2226, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Lieberman KV, Tejani A: A randomized double-blind placebo-controlled trial of cyclosporine in steroid-resistant idiopathic focal segmental glomerulosclerosis in children. J Am Soc Nephrol 7: 56–63, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Hodgin JB, Rasoulpour M, Markowitz GS, D’Agati VD: Very low birth weight is a risk factor for secondary focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 4: 71–76, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD: Obesity-related glomerulopathy: An emerging epidemic. Kidney Int 59: 1498–1509, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Herlitz LC, Markowitz GS, Farris AB, Schwimmer JA, Stokes MB, Kunis C, Colvin RB, D’Agati VD: Development of focal segmental glomerulosclerosis after anabolic steroid abuse. J Am Soc Nephrol 21: 163–172, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhathena DB, Julian BA, McMorrow RG, Baehler RW: Focal sclerosis of hypertrophied glomeruli in solitary functioning kidneys of humans. Am J Kidney Dis 5: 226–232, 1985 [DOI] [PubMed] [Google Scholar]
- 15.Harvey JM, Howie AJ, Lee SJ, Newbold KM, Adu D, Michael J, Beevers DG: Renal biopsy findings in hypertensive patients with proteinuria. Lancet 340: 1435–1436, 1992 [DOI] [PubMed] [Google Scholar]
- 16.D’Agati V: Pathologic classification of focal segmental glomerulosclerosis. Semin Nephrol 23: 117–134, 2003 [DOI] [PubMed] [Google Scholar]
- 17.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents : The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114[2 Suppl 4th Report]: 555–576, 2004 [PubMed] [Google Scholar]
- 18.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothman KJ, Greenland S, Lash TL: Modern Epidemiology, 3rd Ed., Philadelphia, Lippincott Williams & Wilkins, 2008 [Google Scholar]
- 21.Ginsberg JM, Chang BS, Matarese RA, Garella S: Use of single voided urine samples to estimate quantitative proteinuria. N Engl J Med 309: 1543–1546, 1983 [DOI] [PubMed] [Google Scholar]
- 22.Hoes JN, Jacobs JW, Verstappen SM, Bijlsma JW, Van der Heijden GJ: Adverse events of low- to medium-dose oral glucocorticoids in inflammatory diseases: A meta-analysis. Ann Rheum Dis 68: 1833–1838, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Anonymous: Chapter 6: Idiopathic focal segmental glomerulosclerosis in adults. Kidney Int Suppl (2011) 2: 181–185, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stokes MB, Markowitz GS, Lin J, Valeri AM, D’Agati VD: Glomerular tip lesion: A distinct entity within the minimal change disease/focal segmental glomerulosclerosis spectrum. Kidney Int 65: 1690–1702, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Chitalia VC, Wells JE, Robson RA, Searle M, Lynn KL: Predicting renal survival in primary focal glomerulosclerosis from the time of presentation. Kidney Int 56: 2236–2242, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Cameron JS, Turner DR, Ogg CS, Chantler C, Williams DG: The long-term prognosis of patients with focal segmental glomerulosclerosis. Clin Nephrol 10: 213–218, 1978 [PubMed] [Google Scholar]
- 27.Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC, Toronto Glomerulonephritis Registry Group : Focal and segmental glomerulosclerosis: Definition and relevance of a partial remission. J Am Soc Nephrol 16: 1061–1068, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Stokes MB, Valeri AM, Markowitz GS, D’Agati VD: Cellular focal segmental glomerulosclerosis: Clinical and pathologic features. Kidney Int 70: 1783–1792, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Lionaki S, Derebail VK, Hogan SL, Barbour S, Lee T, Hladunewich M, Greenwald A, Hu Y, Jennette CE, Jennette JC, Falk RJ, Cattran DC, Nachman PH, Reich HN: Venous thromboembolism in patients with membranous nephropathy. Clin J Am Soc Nephrol 7: 43–51, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macé C, Chugh SS: Nephrotic syndrome: Components, connections, and angiopoietin-like 4-related therapeutics. J Am Soc Nephrol 25: 2393–2398, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie J, Kiryluk K, Wang W, Wang Z, Guo S, Shen P, Ren H, Pan X, Chen X, Zhang W, Li X, Shi H, Li Y, Gharavi AG, Chen N: Predicting progression of IgA nephropathy: New clinical progression risk score. PLoS One 7: e38904, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Austin PC: A tutorial and case study in propensity score analysis: An application to estimating the effect of in-hospital smoking cessation counseling on mortality. Multivariate Behav Res 46: 119–151, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.