Abstract
Background and objectives
The prevalence of nephrolithiasis in the United States has increased substantially, but recent changes in incidence with respect to age, sex, and race are not well characterized. This study examined temporal trends in the annual incidence and cumulative risk of nephrolithiasis among children and adults living in South Carolina over a 16-year period.
Design, setting, participants, & measurements
We performed a population–based, repeated cross–sectional study using the US Census and South Carolina Medical Encounter data, which capture all emergency department visits, surgeries, and admissions in the state. The annual incidence of nephrolithiasis in South Carolina from 1997 to 2012 was estimated, and linear mixed models were used to estimate incidence rate ratios for age, sex, and racial groups. The cumulative risk of nephrolithiasis during childhood and over the lifetime was estimated for males and females in 1997 and 2012.
Results
Among an at-risk population of 4,625,364 people, 152,925 unique patients received emergency, inpatient, or surgical care for nephrolithiasis. Between 1997 and 2012, the mean annual incidence of nephrolithiasis increased 1% annually from 206 to 239 per 100,000 persons. Among age groups, the greatest increase was observed among 15–19 year olds, in whom incidence increased 26% per 5 years (incidence rate ratio, 1.26; 95% confidence interval, 1.22 to 1.29). Adjusting for age and race, incidence increased 15% per 5 years among females (incidence rate ratio, 1.15; 95% confidence interval, 1.14 to 1.16) but remained stable for males. The incidence among blacks increased 15% more per 5 years compared with whites (incidence rate ratio, 1.15; 95% confidence interval, 1.14 to 1.17). These changes in incidence resulted in doubling of the risk of nephrolithiasis during childhood and a 45% increase in the lifetime risk of nephrolithiasis for women over the study period.
Conclusions
The incidence of kidney stones has increased among young patients, particularly women, and blacks.
Keywords: kidney stones, children, adults, incidence, epidemiology, temporal trends, humans, nephrolithiasis, prevalence
Introduction
Kidney stone disease (nephrolithiasis) is a systemic metabolic disorder associated with increased risks of cardiovascular disease and CKD in adults and fracture in children and adults (1–3), and it has annual costs >$5.1 billion (4). The estimated prevalence of nephrolithiasis in the United States increased from 5.2% (1988–1994) to 8.8% (2007–2010) (5,6). Historically, the prevalence of nephrolithiasis has been highest among middle–aged, white men (5,6). However, over the last 25 years, there were smaller changes in the prevalence among men than women, and there have been reports of increasing frequency of nephrolithiasis among children and adolescents (7–10).
The populations at increasing risk for nephrolithiasis remain unclear, because previous studies ascertained only a history of kidney stones without describing the age of onset (5,6), and most prior studies included only children or adults (5–11) or had small sample sizes that precluded examination of interactions between age, sex, and race (12,13). Understanding changes in kidney stone incidence is critical to defining research priorities and developing strategies to improve health care delivery, particularly for those in whom nephrolithiasis was historically rare.
We performed a population-based study in South Carolina from 1997 to 2012 to describe nephrolithiasis incidence rates in different age, race, and sex groups and estimate the cumulative risk of nephrolithiasis during childhood and over the lifetime.
Materials and Methods
We performed a repeated cross–sectional study to estimate the annual incidence of nephrolithiasis among the population of South Carolina from January 1, 1997 to December 31, 2012. We used South Carolina Medical Encounter Data and Financial Reports, which contain individual patient–level data using encounter–level data elements, including unique patient identifiers; zip code of patient’s mailing address; dates of services; International Classification of Diseases, 9th revision (ICD-9) codes; and payer classification. The database, by law, captures all emergency department (ED) visits, surgeries, and hospital admissions for South Carolina’s population, including those who are uninsured or have government insurance. Data are sent by all health care facilities in the state on a monthly basis. By law, all data must be 99.9% accurate (valid codes) and 99.5% complete (nonmissing). Routine audits are performed to ensure compliance and accuracy (14).
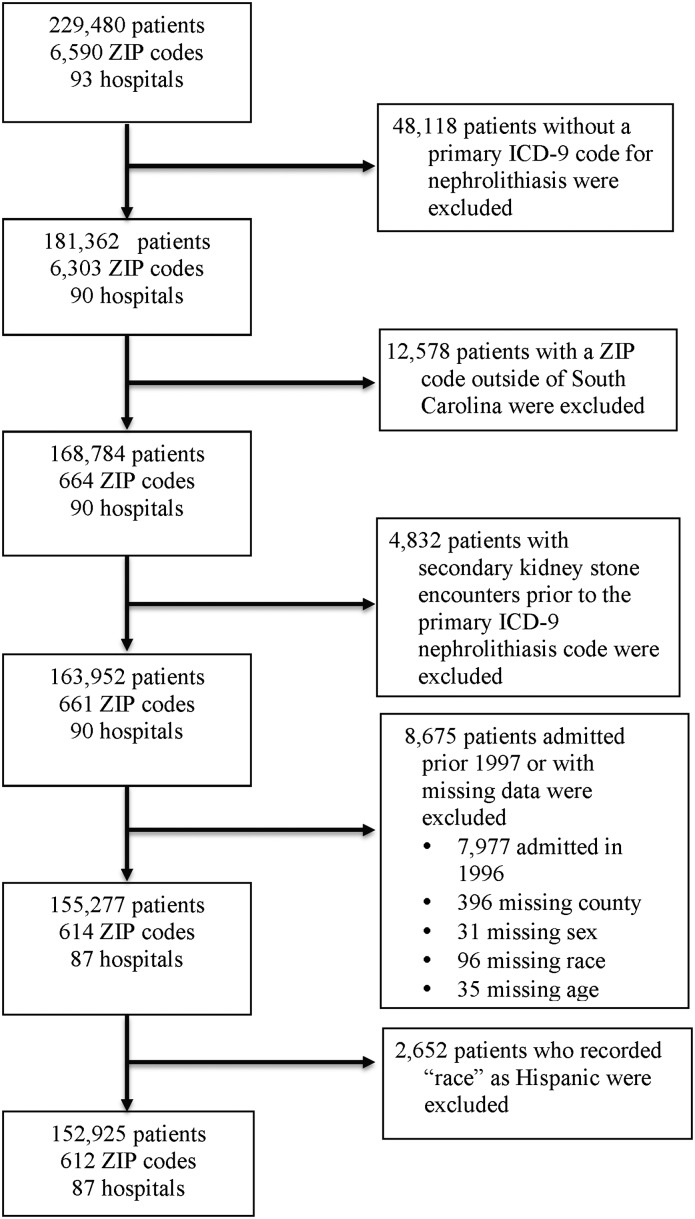
We included all patients who had encounters between 1997 and 2012 associated with primary ICD-9 diagnosis codes for nephrolithiasis. To reduce misclassification of prevalent stone diagnoses, we excluded patients who had secondary ICD-9 codes for nephrolithiasis before the primary code. Beginning in 1996, we used a 1-year lookback for secondary ICD-9 codes to identify and exclude prior stone diagnoses. We excluded patients with residential zip codes outside South Carolina, those with missing geographic or demographic data, and individuals self-identified as Hispanic (<2% of population) because of conflation of race and ethnicity in the dataset.
Kidney Stone Incidence
Kidney stone presentation was defined as ED, inpatient, or surgical care for a primary diagnosis of nephrolithiasis (Supplemental Table). These criteria likely capture nearly all clinically important stone events because of the acute nature of stone episodes that require emergency evaluation, hospital admission, and/or surgery (8,9,15). The first qualifying claim defined the date of presentation. Outcomes among unique individuals were limited to the earliest occurrence to avoid counting patients with recurrent stones multiple times.
Aggregated at the county level, patients who presented with kidney stones were combined with the corresponding age–, sex–, and race–specific populations from the 2000 and 2010 US Censuses using linear extrapolation for intercensus years (16). Annual incidences were calculated by dividing the number of unique patients with kidney stones by the at-risk population stratified by age, sex, and race. Because kidney stones in any given year are rare, we assumed that the whole population was at risk. We categorized age to reflect developmental phases of childhood and ranges in which the prevalence of nephrolithiasis is similar among adults (6). Native Hawaiian, Native American, Asian, and mixed race patients were classified as other because of small sample sizes and resultant unstable annual incidences among individual races.
Statistical Analyses
We used Bayesian generalized linear mixed models with random intercepts for county of residence to estimate incidence rate ratios (IRRs) of nephrolithiasis (17). We used six models to examine changes in nephrolithiasis incidence among age groups, sexes, and races across time. Model 1 included an interaction term between age and year to estimate changes in incidence within age groups adjusting for race and sex. Model 2 included an interaction term between sex and year to estimate changes in incidence among males and females adjusting for race and age. Model 3 included a three-way interaction between age, sex, and year to estimate sex differences in incidence rates by age group over time adjusting for race. Model 4 included an interaction term between race and year to estimate changes in incidence among racial groups adjusting for sex and age. Model 5 included a three-way interaction between race, sex, and year to estimate sex differences in incidence over time for different racial groups adjusting for age. Model 6 included a three-way interaction between age, race, and year to estimate racial differences in incidence rates by age group adjusting for sex. Models included all lower–order interaction terms and main effects for the variables included in the interaction terms. Statistical significance was defined by exclusion of zero in the posterior 95% confidence interval (95% CI).
The cumulative risk of nephrolithiasis, defined as the population-level risk of developing nephrolithiasis during a certain age span assuming no other causes of death, was calculated for females and males in 1997 and 2012 during childhood (birth to 19 years old) and over the lifetime (birth to 85 years old). The cumulative risk is
where cumulative rate is the summation of the age-specific rates from birth to the defined upper age limit (16).
We performed a sensitivity analysis including patients with secondary ICD-9 code(s) for nephrolithiasis before the qualifying primary code(s). Analyses were performed in R, version 3.1.2 and SAS 9.2 (SAS Institute Inc., Cary, NC). This study was deemed nonhuman subjects research by the local institutional review board and exempted from review.
Results
Kidney Stone Incidence
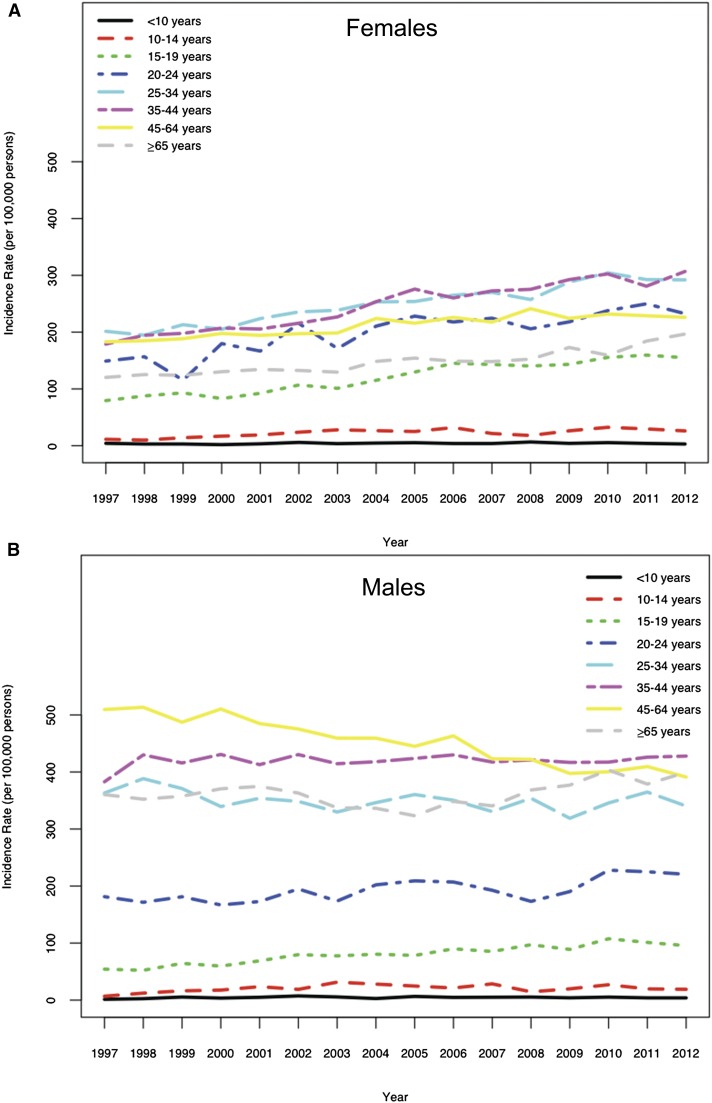
From 1997 to 2012, 152,925 children and adults received ED, inpatient, or surgical care for nephrolithiasis among a population of 4,625,364 people (Figure 1, Table 1). The observed mean annual incidence of nephrolithiasis from 1997 to 2012 was 226 per 100,000 persons. Unadjusted mean annual incidence was highest for 35–44 year olds (336 per 100,000 persons) and lowest for <10 year olds (four per 100,000 persons). From an unadjusted regression model (Supplemental Material), incidence increased an estimated 5% per 5 years (IRR, 1.05; 95% CI, 1.04 to 1.06) from 206 per 100,000 persons in 1997 to 239 per 100,000 persons in 2012. In any given year, adjusting for race, annual incidence was higher among females ages 10–24 years old than among males the same age. The annual incidence of nephrolithiasis was 52% higher among 15- to 19-year-old females than males the same age. Nephrolithiasis was more common among men beginning at 25 years old.
Figure 1.
Identification of patients with incident kidney stones living in South Carolina from 1997 to 2012. ICD-9, International Classification of Diseases, 9th revision.
Table 1.
Characteristics of patients with kidney stones and the population of South Carolina
| Characteristics | Patients with Kidney Stone (n=152,925) | Census 2000 (n=4,012,012) | Census 2010 (n= 4,625,364) |
|---|---|---|---|
| Male, n (%) | 93,887(61.4) | 1,948,929 (48.6) | 2,250,101 (48.6) |
| Urban, n (%) | 116,411 (76.1) | 2,427,124 (60.5) | 3,067,809 (66.3) |
| Race, n (%) | |||
| White | 129,989 (85.0) | 2,695,560 (67.2) | 3,060,000 (64.1) |
| Black | 18,464 (12.1) | 1,185,216 (29.5) | 1,279,998 (27.7) |
| Asian | 530 (0.3) | 36,014 (0.9) | 58,307 (1.3) |
| American Indian | 216 (0.1) | 13,718 (0.3) | 18,727 (0.4) |
| Other | 3726 (2.4) | 81,504 (2.0) | 196,105 (4.2) |
| Age group, yr, n (%) | |||
| <10 | 396 (0.3) | 549,922 (13.7) | 598,150 (12.9) |
| 10–14 | 1012 (0.7) | 290,479 (7.2) | 297,286 (6.4) |
| 15–19 | 5015 (3.3) | 295,377 (7.4) | 328,989 (7.1) |
| 20–24 | 9631 (6.3) | 281,714 (7.0) | 332,494 (7.2) |
| 25–34 | 27,580 (18.0) | 560,831 (14.0) | 592,056 (12.8) |
| 35–44 | 32,547 (21.3) | 625,124 (15.6) | 601,292 (13.0) |
| 45–64 | 55,638 (36.4) | 923,232 (23.0) | 1,243,223 (27.0) |
| ≥65 | 21,106 (13.8) | 485,333 (12.1) | 631,874 (13.6) |
| Insurance, n (%) | |||
| Public | 38,130 (24.9) | ||
| Private | 89,777 (58.7) | ||
| Self-pay | 24,073 (15.7) | ||
| No insurance | 945 (0.6) |
Changes in Kidney Stone Incidence by Age, Sex, and Race
For ease of interpretation, we present group-level changes in incidence over time (Figure 2, Tables 2–7). Associated model coefficients and 95% CIs are displayed in Supplemental Material. Effect modification was deemed statistically significant if zero was excluded from the 95% CIs of the interaction terms. Adjusting for sex and race, the incidence for 15–19 year olds increased an estimated 26% per 5 years (IRR, 1.26; 95% CI, 1.22 to 1.29) (Table 2), which was statistically significant compared with the 5-year increase for <10 year olds. Adjusting for age and race, the incidence of nephrolithiasis among females increased an estimated 15% per 5 years (IRR, 1.15; 95% CI, 1.14 to 1.16) and was stable among males (IRR, 0.99; 95% CI, 0.98 to 1.00) (Table 3). Moreover, differences in annual incidences between males and females decreased statistically significantly over the study period, which was evidenced by the negative coefficient for the sex-by-year interaction term in Supplemental Material. No statistically significant three–way interaction was observed between change over time, age, and sex adjusting for race (Table 4). However, a general pattern of sex differences in age–specific incidence rates was found. Kidney stone incidences increased 10%–28% per 5 years for females, with highest rates among 10–19 year olds. Incidence changed <5% per 5 years in men >25 years old (Table 4).
Figure 2.
Temporal trends of nephrolithiasis incidence. Annual incidence of nephrolithiasis among (A) females and (B) males in South Carolina from 1997 to 2012.
Table 2.
Adjusted estimates of group–level incidence rates of nephrolithiasis in South Carolina by age from 1997 to 2012: Model 1
| Model 1: Age Group (yr) | 5-yr Change in Kidney Stone Incidence (95% CI) |
|---|---|
| <10 | 1.09 (0.98 to 1.22) |
| 10–14 | 1.20 (1.12 to 1.28) |
| 15–19 | 1.26 (1.22 to 1.29) |
| 20–24 | 1.14 (1.11 to 1.17) |
| 25–34 | 1.07 (1.05 to 1.08) |
| 35–44 | 1.08 (1.07 to 1.09) |
| 45–64 | 0.98 (0.97 to 0.99) |
| ≥65 | 1.08 (1.07 to 1.10) |
Model 1 included an interaction term between age and year. All models with interactions additionally included each main effect, lower–order interaction terms, and a random intercept for county of residence. Models examining for age and sex effects were adjusted for race. 95% CI, 95% confidence interval.
Table 7.
Adjusted estimates of group–level incidence rates of nephrolithiasis in South Carolina by age and race from 1997 to 2012: Model 6
| Model 6: Age Group (yr) | 5-yr Change in Kidney Stone Incidence among Whites (95% Confidence Interval) | 5-yr Change in Kidney Stone Incidence among Blacks (95% Confidence Interval) | 5-yr Change in Kidney Stone Incidence among Other Races (95% Confidence Interval) |
|---|---|---|---|
| <10 | 1.11 (0.99 to 1.24) | 1.23 (0.89 to 1.69) | 0.53 (0.15 to 1.93) |
| 10–14 | 1.21 (1.13 to 1.29) | 1.15 (0.89 to 1.50) | 0.94 (0.51 to 1.72) |
| 15–19 | 1.25 (1.20 to 1.28) | 1.37 (1.23 to 1.52) | 1.70 (1.34 to 2.16) |
| 20–24 | 1.13 (1.10 to 1.15) | 1.30 (1.22 to 1.38) | 1.15 (1.02 to 1.31) |
| 25–34 | 1.05 (1.04 to 1.07) | 1.21 (1.16 to 1.26) | 1.13 (1.04 to 1.21) |
| 35–44 | 1.06 (1.05 to 1.07) | 1.20 (1.16 to 1.25) | 1.26 (1.18 to 1.36) |
| 45–64 | 0.95 (0.94 to 0.96) | 1.05 (1.02 to 1.07) | 1.63 (1.52 to 1.73) |
| ≥65 | 1.06 (1.04 to 1.07) | 1.17 (0.83 to 1.65) | 2.44 (1.68 to 3.53) |
Model 6 included an interaction term between race, age, and year. All models with interactions additionally included each main effect, lower–order interaction terms, and a random intercept for county of residence. Models examining for race effects were adjusted for age and sex.
Table 3.
Adjusted estimates of group–level incidence rates of nephrolithiasis in South Carolina by sex from 1997 to 2012: Model 2
| Model 2: Sex | 5-yr Change in Kidney Stone Incidence (95% Confidence Interval) |
|---|---|
| Females | 1.15 (1.14 to 1.16) |
| Males | 0.99 (0.98 to 1.00) |
Model 2 included an interaction term between sex and year. All models with interactions additionally included each main effect, lower–order interaction terms, and a random intercept for county of residence. Models examining for age and sex effects were adjusted for race.
Table 4.
Adjusted estimates of group–level incidence rates of nephrolithiasis in South Carolina by age and sex from 1997 to 2012: Model 3
| Model 3: Age Group (yr) | 5-yr Change in Kidney Stone Incidence among Females (95% Confidence Interval) | 5-yr Change in Kidney Stone Incidence among Males (95% Confidence Interval) |
|---|---|---|
| <10 | 1.10 (0.94 to 1.29) | 1.08 (0.93 to 1.25) |
| 10–14 | 1.27 (1.15 to 1.40) | 1.13 (1.03 to 1.24) |
| 15–19 | 1.28 (1.22 to 1.33) | 1.23 (1.18 to 1.29) |
| 20–24 | 1.19 (1.15 to 1.23) | 1.09 (1.06 to 1.13) |
| 25–34 | 1.17 (1.15 to 1.19) | 1.00 (0.98 to 1.02) |
| 35–44 | 1.19 (1.17 to 1.22) | 1.02 (1.00 to 1.03) |
| 45–64 | 1.10 (1.08 to 1.11) | 0.92 (0.91 to 0.93) |
| ≥65 | 1.16 (1.13 to 1.19) | 1.03 (1.02 to 1.05) |
Model 3 included an interaction term between age, sex, and year. All models with interactions additionally included each main effect, lower–order interaction terms, and a random intercept for county of residence. Models examining for age and sex effects were adjusted for race.
Adjusting for age and sex, the changes in kidney stone incidence over time were significantly modified by race (Table 5). The incidence increased an estimated 3% per 5 years among whites (IRR, 1.03; 95% CI, 1.02 to 1.03) compared with an estimated 15% increase among blacks (IRR, 1.15; 95% CI, 1.14 to 1.17) (Table 5). There was evidence of a three-way interaction between race, sex, and time adjusting for age (Table 6): the estimated changes in incidence over time were greater among black females than black males. Adjusting for sex, there was little evidence of a three-way interaction between race, age, and time (Table 7). However, changes in incidence over time were greater among blacks than whites in all age groups, with the exception of 10–14 year olds (Table 7).
Table 5.
Adjusted estimates of group–level incidence rates of nephrolithiasis in South Carolina by race from 1997 to 2012: Model 4
| Model 4: Race | 5-yr Change in Kidney Stone Incidence (95% Confidence Interval) |
|---|---|
| White | 1.03 (1.02 to 1.03) |
| Black | 1.15 (1.14 to 1.17) |
| Other | 1.43 (1.38 to 1.48) |
Model 4 included an interaction term between race and year. All models with interactions additionally included each main effect, lower–order interaction terms, and a random intercept for county of residence. Models examining for race effects were adjusted for age and sex.
Table 6.
Adjusted estimates of group–level incidence rates of nephrolithiasis in South Carolina by age and race from 1997 to 2012: Model 5
| Model 5: Race | 5-yr Change in Kidney Stone Incidence among Females (95% Confidence Interval) | 5-yr Change in Kidney Stone Incidence among Males (95% Confidence Interval) |
|---|---|---|
| White | 1.13 (1.12 to 1.14) | 0.97 (0.96 to 0.98) |
| Black | 1.21 (1.18 to 1.24) | 1.10 (1.08 to 1.13) |
| Other | 1.56 (1.47 to 1.66) | 1.36 (1.30 to 1.42) |
Model 5 included an interaction between race, sex, and year. All models with interactions additionally included each main effect, lower–order interaction terms, and a random intercept for county of residence. Models examining for race effects were adjusted for age and sex.
Cumulative Risk of Nephrolithiasis during Childhood and over the Lifetime
Between 1997 and 2012, the cumulative risk of nephrolithiasis during childhood increased similarly among girls (87%) and boys (90%), although the risk in 2012 was modest at 0.9% and 0.6% in girls and boys, respectively. The lifetime risk of nephrolithiasis among women increased 45% between 1997 (10.5%) and 2012 (15.2%) and remained stable among men at approximately 23% (Table 8).
Table 8.
Age–specific incidence rates and cumulative risks of nephrolithiasis among males and females during childhood and over a lifetime in 1997 and 2012
| Age Group (yr) | Length of Age Class (ti) | 1997 | 2012 | ||
|---|---|---|---|---|---|
| Age-Specific Rate per 100,000 (ai) | Age-Specific Ratea (Length of Age Class) per 100,000 (aiti) | Age-Specific Rate per 100,000 (ai) | Age-Specific Ratea (Length of Age Class) per 100,000 (aiti) | ||
| Females | |||||
| <10 | 10 | 4.23 | 42.26 | 3.00 | 30.03 |
| 10–14 | 5 | 11.30 | 56.52 | 26.09 | 130.43 |
| 15–19 | 5 | 79.43 | 397.17 | 154.91 | 774.53 |
| 20–24 | 5 | 149.04 | 745.18 | 232.49 | 1162.44 |
| 25–34 | 10 | 201.45 | 2014.50 | 292.04 | 2920.42 |
| 36–44 | 10 | 178.92 | 1789.18 | 306.80 | 3068.00 |
| 45–64 | 20 | 183.05 | 3661.05 | 226.10 | 4521.90 |
| ≥65a | 20 | 120.30 | 2406.03 | 196.59 | 3931.78 |
| Cumulative rateb per 100,000: 0–19 | 495.96 | 935.00 | |||
| Cumulative risk,c %: 0–19 | 0.49 | 0.93 | |||
| Cumulative rateb per 100,000: 0–85 | 11,111.90 | 16,539.53 | |||
| Lifetimea risk,c %: 0–85 | 10.52 | 15.24 | |||
| Males | |||||
| <10 | 10 | 1.45 | 14.54 | 3.89 | 38.95 |
| 10–14 | 5 | 6.81 | 34.04 | 18.96 | 94.79 |
| 15–19 | 5 | 54.27 | 271.37 | 95.49 | 477.46 |
| 20–24 | 5 | 181.36 | 906.82 | 220.61 | 1103.06 |
| 25–34 | 10 | 363.17 | 3631.71 | 340.32 | 3403.23 |
| 36–44 | 10 | 382.90 | 3828.98 | 427.88 | 4278.83 |
| 45–64 | 20 | 509.54 | 10,190.88 | 391.14 | 7822.88 |
| ≥65a | 20 | 360.37 | 7207.43 | 399.54 | 7990.82 |
| Cumulative rateb per 100,000: 0–19 | 319.95 | 611.19 | |||
| Cumulative risk,c %: 0–19 | 0.32 | 0.61 | |||
| Cumulative rateb per 100,000: 0–85 | 26,085.76 | 25,210.00 | |||
| Lifetimea risk,c %: 0–85 | 22.96 | 22.28 | |||
Assuming lifetime risk is from (0–85 years)i.
Cumulative rate =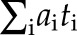 .
.
Cumulative risk =100×(1−exp(−cumulative rate/100,000)).
Sensitivity Analyses
Results including patients who may have had prevalent stones were consistent with and did not alter the statistical significance of our findings.
Discussion
This study examined temporal trends in the annual incidence of nephrolithiasis among the general population living in a large geographic area in the United States. The annual incidence of nephrolithiasis increased 16% between 1997 and 2012, which far outpaces the increase in obesity prevalence over a similar period (18,19). The greatest rates of increase were observed among adolescents, females, and blacks, which indicate that the historic distribution of nephrolithiasis is changing. These changes in incidence resulted in a doubling of the risk of nephrolithiasis during childhood for boys and girls and a 45% increase in the lifetime risk of nephrolithiasis for women over the study period.
Prior studies from the Rochester Epidemiology Project evaluated temporal trends among 248 patients who developed incident stones in Olmstead County, Minnesota between 1970 and 2000 (12,13). These prior studies expanded our knowledge of the changing incidence of nephrolithiasis, but small sample sizes, a population that is 86% white, and calculating incidence every 10 years limited their generalizability and granularity. Our study, which included 152,925 patients, adds to this prior work by evaluating the interaction between age, sex, and race on annual incidence rates of kidney stones among a larger and geographically distinct population.
Our results offer insight into the age groups that are contributing to the narrowing sex and race gap of nephrolithiasis in the United States (5,6,12,13). Incidence increased 23% per 5 years among boys ages 15–19 years old but remained stable among men. Among females, incidence increased in all age groups, with rates of 27%–28% per 5 years among girls ages 10–19 years old and slightly lower rates of 10%–19% per 5 years among older women. In the Olmstead County study, decennial incidence rates between 1970 and 2000 were highest among women ages 20–29 years old. The higher rates among adolescent girls that we observed in our study, which contained an additional 12 years of data, suggest that the age of onset of stone disease has continued to decrease over time. Now, adolescent girls are the group in which the risk of nephrolithiasis is increasing at the fastest rate. Additionally, the greatest increases in incidence among blacks were noted in younger patients: for blacks ages 15–44 years old, the incidence of nephrolithiasis increased 20%–37% per 5 years, which was higher than the rates among whites of the same age and sex.
The emergence of nephrolithiasis as a disease that begins in childhood is worrisome because there is limited evidence about how to best treat children with nephrolithiasis. Consequently, there is unwanted variability in evaluation and treatment practices for children with nephrolithiasis, including poor adherence to guidelines aimed at reducing radiation exposure among children with suspected nephrolithiasis and use of surgical interventions that are dependent more on local availability and ease of use than clinical indications (20–22). The rising incidence of nephrolithiasis among younger girls also has important public health implications. Controlling for comorbidities, medications, body mass index, and diet, nephrolithiasis is associated with an increased risk of CKD, cardiovascular disease, and fracture, with the greatest risks observed among younger women (1–3). Although these associations may not be causal, it is likely that the risks for and burden of these diseases will be greater for patients who develop nephrolithiasis earlier in life because of a longer lifetime over which kidney, heart, and bone diseases may develop.
Our data show areas needing additional research. Prior randomized trials of thiazides and citrate to reduce kidney stone recurrence have been conducted among adult populations of primarily white men (23,24). However, the efficacy of thiazides for hypertension is dependent on age and race, and black women have a higher urine pH than age–matched white women, raising questions about potential differences in the efficacy of these medications for treating nephrolithiasis among younger, nonwhite populations (25,26). New trials conducted among populations that reflect the contemporary incidence of nephrolithiasis are needed. Research is also needed to determine the factors that contribute to the differences in incidence that we observed. For example, finding that the sex gap for nephrolithiasis reverses during the transition from adolescence to adulthood can inform research on modifiable risk factors that are unique to particular populations. Possible factors include poor water intake, because dehydration is common and varies by age, sex, and race (27). Other ecologic trends in dietary habits that also vary by age, sex, and race, such as increasing sodium and decreasing calcium intake, are consistent with the increases in nephrolithiasis incidence that we observed and could contribute to differences in incidence between patient groups (28–32). However, our ability to examine for these differences is limited because of the lack of longitudinal cohorts that span childhood and adulthood. Expanding the National Health and Nutritional Examination Survey (NHANES) to include stone history for participants <21 years old will help identify modifiable risk factors that vary by age, sex, and race. Additionally, prospective cohort studies of patients who develop nephrolithiasis in childhood and are followed into adult life are needed to determine recurrence rates and metabolic risk factors for early onset of nephrolithiasis. This knowledge will facilitate targeted primary and secondary prevention strategies for the groups in which nephrolithiasis is increasing at the highest rates.
South Carolina is the largest of a few states that capture all surgery, inpatient admissions, and ED visits in an all–payer claims database and the only state that began collecting data in the 1990s. South Carolina Medical Encounter Data include unique patient identifiers and dated encounters for care received at every hospital in the state over a 16-year period. We were thus able to describe annual changes in the distribution and frequency of nephrolithiasis in the population, which are obscured in analyses using the NHANES and Healthcare Cost and Utilization Project databases and could not be examined in the Rochester Epidemiology Project because of insufficient sample sizes (5,6,33).
Nevertheless, our study has several limitations. First, our observations are on the basis of data from one state. It is possible that the absolute incidence of nephrolithiasis is higher in South Carolina than the rest of the United States because of the higher prevalence of nephrolithiasis in the Southeast (34,35). However, the trends that we observed are consistent with increases in nephrolithiasis frequency reported in prior studies from other areas in the United States (1,7,912,13,36). These results should not be generalized to other countries, such as Japan, where the incidence of nephrolithiasis has increased over time but not among younger patients (37).
Second, we cannot completely discount that changes in imaging use contributed to changes in nephrolithiasis incidence. However, detection bias is unlikely, because computed tomography use stabilized among young adults and decreased among children beginning in the 2000s (38,39). Additionally, although climate change may affect future nephrolithiasis prevalence (40) and hot and cold temperatures are associated with increased risk of stone presentation (35), any environmental changes over the study period would not substantively affect nephrolithiasis incidence. It is also likely that factors other than increasing prevalence of obesity and diabetes (41) account for the increasing incidence of nephrolithiasis that we observed, particularly because obesity may not be associated with increased risk for kidney stones among children or adolescents (42). It is more likely that dietary choices and behaviors associated with stone risk differ between the groups that we studied. However, our dataset did not contain dietary or clinical information that could help ascertain potential causes of the differential changes in nephrolithiasis incidence.
Third, we may have underestimated incidence because we did not capture asymptomatic nephrolithiasis or nephrocalcinosis or less severe presentations of stones that did not warrant admission, surgery, or an ED visit. However, the consistency of the age- and sex-specific incidences that we observed with the incidences reported for adults in the Health Professionals Follow-Up and Nurses Health Studies suggests that our outcome criteria were valid (43).
Fourth, recurrent stones may have been misclassified as incident stones. However, recurrent stones would be more likely to occur in older white men, who had the highest incidence of nephrolithiasis in any given year. The rising frequency among younger patients, women, and blacks argues against misclassification as an important source of bias in our findings.
Fifth, our results cannot be generalized to Hispanic patients, because we excluded Hispanics because of the conflation of race and ethnicity in the dataset. However, patients who self-identified as Hispanic represented <2% of the population who presented with nephrolithiasis. It is unlikely that the results would change substantively if we were able to classify by race and ethnicity.
In conclusion, the annual incidence of nephrolithiasis has increased among young patients, particularly girls, and also among blacks, showing a reversal of historical trends. Understanding modifiable risk factors that account for the age, sex, and race differences in incidence rates will facilitate targeted secondary prevention strategies, particularly for those groups in which nephrolithiasis were once rare but are now increasing.
Disclosures
None.
Supplementary Material
Acknowledgments
The study was supported by the Howard M. Snyder Endowed Chair in Pediatric Urology at the Children’s Hospital of Philadelphia and National Institutes of Health (NIH) Grants K23-DK106428 (to G.E.T.), K23-DK093556 (to M.D.), and K24-DK078737 (to S.F.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, and approval of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07610715/-/DCSupplemental.
References
- 1.Ferraro PM, Taylor EN, Eisner BH, Gambaro G, Rimm EB, Mukamal KJ, Curhan GC: History of kidney stones and the risk of coronary heart disease. JAMA 310: 408–415, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander RT, Hemmelgarn BR, Wiebe N, Bello A, Morgan C, Samuel S, Klarenbach SW, Curhan GC, Tonelli M, Alberta Kidney Disease Network : Kidney stones and kidney function loss: A cohort study. BMJ 345: e5287, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denburg MR, Leonard MB, Haynes K, Tuchman S, Tasian G, Shults J, Copelovitch L: Risk of fracture in urolithiasis: A population-based cohort study using the health improvement network. Clin J Am Soc Nephrol 9: 2133–2140, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saigal CS, Joyce G, Timilsina AR, Urologic Diseases in America Project : Direct and indirect costs of nephrolithiasis in an employed population: Opportunity for disease management? Kidney Int 68: 1808–1814, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC: Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Int 63: 1817–1823, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Scales CD, Jr., Smith AC, Hanley JM, Saigal CS, Urologic Diseases in America Project : Prevalence of kidney stones in the United States. Eur Urol 62: 160–165, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dwyer ME, Krambeck AE, Bergstralh EJ, Milliner DS, Lieske JC, Rule AD: Temporal trends in incidence of kidney stones among children: A 25-year population based study. J Urol 188: 247–252, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sas DJ, Hulsey TC, Shatat IF, Orak JK: Increasing incidence of kidney stones in children evaluated in the emergency department. J Pediatr 157: 132–137, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Bush NC, Xu L, Brown BJ, Holzer MS, Gingrich A, Schuler B, Tong L, Baker LA: Hospitalizations for pediatric stone disease in United States, 2002-2007. J Urol 183: 1151–1156, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Routh JC, Graham DA, Nelson CP: Epidemiological trends in pediatric urolithiasis at United States freestanding pediatric hospitals. J Urol 184: 1100–1104, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Scales CD, Jr., Curtis LH, Norris RD, Springhart WP, Sur RL, Schulman KA, Preminger GM: Changing gender prevalence of stone disease. J Urol 177: 979–982, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Lieske JC, Peña de la Vega LS, Slezak JM, Bergstralh EJ, Leibson CL, Ho KL, Gettman MT: Renal stone epidemiology in Rochester, Minnesota: An update. Kidney Int 69: 760–764, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Johnson CM, Wilson DM, O’Fallon WM, Malek RS, Kurland LT: Renal stone epidemiology: A 25-year study in Rochester, Minnesota. Kidney Int 16: 624–631, 1979 [DOI] [PubMed] [Google Scholar]
- 14.Council SCDO: Principles and Protocol for the Release of Health Care Data, 2014. Available at: http://rfa.sc.gov/files/DOC Principles and Protocols 072014.pdf. Accessed August 28, 2014
- 15.Ghani KR, Roghmann F, Sammon JD, Trudeau V, Sukumar S, Rahbar H, Kumar R, Karakiewicz PI, Peabody JO, Menon M, Sun M, Trinh QD: Emergency department visits in the United States for upper urinary tract stones: Trends in hospitalization and charges. J Urol 191: 90–96, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Day NE: Cumulative rates and cumulative risk. In: Cancer Incidence in Five Continents, edited by Muir C, Waterhouse J, Mack T, Powell J, Whelan S, Lyon, France, International Agency for Research on Cancer, 1987 [Google Scholar]
- 17.Laird NM, Ware JH: Random-effects models for longitudinal data. Biometrics 38: 963–974, 1982 [PubMed] [Google Scholar]
- 18.Flegal KM, Carroll MD, Kit BK, Ogden CL: Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 307: 491–497, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Ogden CL, Carroll MD, Kit BK, Flegal KM: Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA 307: 483–490, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tasian GE, Pulido JE, Keren R, Dick AW, Setodji CM, Hanley JM, Madison R, Saigal CS, Urologic Diseases in America Project : Use of and regional variation in initial CT imaging for kidney stones. Pediatrics 134: 909–915, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Routh JC, Graham DA, Nelson CP: Trends in imaging and surgical management of pediatric urolithiasis at American pediatric hospitals. J Urol 184[Suppl]: 1816–1822, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Wang HHS, Huang L, Routh JC, Nelson CP: Shock wave lithotripsy vs ureteroscopy: Variation in surgical management of kidney stones at freestanding children’s hospitals. J Urol 187: 1402–1407, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Ettinger B, Citron JT, Livermore B, Dolman LI: Chlorthalidone reduces calcium oxalate calculous recurrence but magnesium hydroxide does not. J Urol 139: 679–684, 1988 [DOI] [PubMed] [Google Scholar]
- 24.Ala-Opas M, Elomaa I, Porkka L, Alfthan O: Unprocessed bran and intermittent thiazide therapy in prevention of recurrent urinary calcium stones. Scand J Urol Nephrol 21: 311–314, 1987 [DOI] [PubMed] [Google Scholar]
- 25.Preston RA, Materson BJ, Reda DJ, Williams DW, Hamburger RJ, Cushman WC, Anderson RJ, Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents : Age-race subgroup compared with renin profile as predictors of blood pressure response to antihypertensive therapy. JAMA 280: 1168–1172, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Taylor EN, Curhan GC: Differences in 24-hour urine composition between black and white women. J Am Soc Nephrol 18: 654–659, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Kenney EL, Long MW, Cradock AL, Gortmaker SL: Prevalence of inadequate hydration among US children and disparities by gender and race/ethnicity: National Health and Nutrition Examination Survey, 2009-2012. Am J Public Health 105: e113–e118, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kant AK, Graubard BI: Contributors of water intake in US children and adolescents: Associations with dietary and meal characteristics--National Health and Nutrition Examination Survey 2005-2006. Am J Clin Nutr 92: 887–896, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wigertz K, Palacios C, Jackman LA, Martin BR, McCabe LD, McCabe GP, Peacock M, Pratt JH, Weaver CM: Racial differences in calcium retention in response to dietary salt in adolescent girls. Am J Clin Nutr 81: 845–850, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Clark MA, Fox MK: Nutritional quality of the diets of US public school children and the role of the school meal programs. J Am Diet Assoc 109[Suppl]: S44–S56, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Briefel RR, Johnson CL: Secular trends in dietary intake in the United States. Annu Rev Nutr 24: 401–431, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Moore LL, Singer MR, Qureshi MM, Bradlee ML, Daniels SR: Food group intake and micronutrient adequacy in adolescent girls. Nutrients 4: 1692–1708, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kusumi K, Becknell B, Schwaderer A: Trends in pediatric urolithiasis: Patient characteristics, associated diagnoses, and financial burden. Pediatr Nephrol 30: 805–810, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Soucie JM, Thun MJ, Coates RJ, McClellan W, Austin H: Demographic and geographic variability of kidney stones in the United States. Kidney Int 46: 893–899, 1994 [DOI] [PubMed] [Google Scholar]
- 35.Tasian GE, Pulido JE, Gasparrini A, Saigal CS, Horton BP, Landis JR, Madison R, Keren R, Urologic Diseases in America Project : Daily mean temperature and clinical kidney stone presentation in five U.S. metropolitan areas: A time-series analysis. Environ Health Perspect 122: 1081–1087, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strope SA, Wolf JS Jr., Hollenbeck BK: Changes in gender distribution of urinary stone disease. Urology 75: 543–546, 2010 [DOI] [PMC free article] [PubMed]
- 37.Yasui T, Iguchi M, Suzuki S, Kohri K: Prevalence and epidemiological characteristics of urolithiasis in Japan: National trends between 1965 and 2005. Urology 71: 209–213, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Kocher KE, Meurer WJ, Fazel R, Scott PA, Krumholz HM, Nallamothu BK: National trends in use of computed tomography in the emergency department. Ann Emerg Med 58: 452–462, 2011 [DOI] [PubMed]
- 39.Menoch MJ, Hirsh DA, Khan NS, Simon HK, Sturm JJ: Trends in computed tomography utilization in the pediatric emergency department. Pediatrics 129: e690–e697, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Brikowski TH, Lotan Y, Pearle MS: Climate-related increase in the prevalence of urolithiasis in the United States. Proc Natl Acad Sci U S A 105: 9841–9846, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP: The continuing epidemics of obesity and diabetes in the United States. JAMA 286: 1195–1200, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Kim SS, Luan X, Canning DA, Landis JR, Keren R: Association between body mass index and urolithiasis in children. J Urol 186[Suppl]: 1734–1739, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor EN, Stampfer MJ, Curhan GC: Obesity, weight gain, and the risk of kidney stones. JAMA 293: 455–462, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.