Abstract
Background and objectives
Atherosclerotic renal artery stenosis (ARAS) can reduce renal blood flow, tissue oxygenation, and GFR. In this study, we sought to examine associations between renal hemodynamics and tissue oxygenation with single-kidney function, pressor hormones, and inflammatory biomarkers in patients with unilateral ARAS undergoing medical therapy alone or stent revascularization.
Design, setting, participants, & measurements
Nonrandomized inpatient studies were performed in patients with unilateral ARAS (>60% occlusion) before and 3 months after revascularization (n=10) or medical therapy (n=20) or patients with essential hypertension (n=32) under identical conditions. The primary study outcome was change in single-kidney GFR. Individual kidney hemodynamics and volume were measured using multidetector computed tomography. Tissue oxygenation (using R2* as a measure of deoxyhemoglobin) was determined by blood oxygen level–dependent magnetic resonance imaging at 3 T. Renal vein neutrophil gelatinase–associated lipocalin (NGAL), monocyte chemoattractant protein-1 (MCP-1), and plasma renin activity were measured.
Results
Total GFR did not change over 3 months in either group, but the stenotic kidney (STK) GFR rose over time in the stent compared with the medical group (+2.2[−1.8 to 10.5] versus −5.3[−7.3 to −0.3] ml/min; P=0.03). Contralateral kidney (CLK) GFR declined in the stent group (43.6±19.7 to 36.6±19.5 ml/min; P=0.03). Fractional tissue hypoxia fell in the STK (fraction R2* >30/s: 22.1%±20% versus 14.9%±18.3%; P<0.01) after stenting. Renal vein biomarkers correlated with the degree of hypoxia in the STK: NGAL(r=0.3; P=0.01) and MCP-1(r=0.3; P=0.02; more so after stenting). Renal vein NGAL was inversely related to renal blood flow in the STK (r=−0.65; P<0.001). Biomarkers were highly correlated between STK and CLK, NGAL (r=0.94; P<0.001), and MCP-1 (r=0.96; P<0.001).
Conclusions
These results showed changes over time in single-kidney GFR that were not evident in parameters of total GFR. Furthermore, they delineate the relationship of measurable tissue hypoxia within the STK and markers of inflammation in human ARAS. Renal vein NGAL and MCP-1 indicated persistent interactions between the ischemic kidney and both CLK and systemic levels of inflammatory cytokines.
Keywords: renal artery stenosis, BOLD-MRI, renal tissue oxygenation, single kidney glomerular filtration rate, renin, renal biomarkers, renovascular hypertension
Introduction
Reduced renal blood flow (RBF) as a result of occlusive atherosclerotic renovascular disease produces complex downstream effects. For >80 years, reduced renal perfusion pressure has been linked to activation of pressor pathways, including the renin-angiotensin-aldosterone system, leading to secondary hypertension (1). Sustained activation of the renin-angiotensin-aldosterone system is most commonly associated with unilateral atherosclerotic renal artery stenosis (ARAS) affecting the stenotic kidney (STK), whereas the nonstenotic contralateral kidney (CLK) may have relatively suppressed renin release and be exposed to higher pressures and blood flows (2).
Recent prospective randomized, controlled trials fail to identify consistent recovery of overall kidney function, despite technically successful renal revascularization. In each of these trials, however, a cohort comprising 16%–22% of participants progressed to a renal end point associated with loss of function during follow-up periods between 3 and 4 years (3–5). Unilateral ARAS leads to not only hypoperfusion–dependent renin release and injury to the ipsilateral STK but also, functional changes within the nonstenotic CLK. Putative interactions between the STK and CLK affected by neurohormonal pathways are thought to produce dysregulation of the CLK, affecting sodium excretion, kidney hypertrophy, and persistent elevations of BP. Observational studies indicate structural consequences affecting both STK and CLK not easily explained by hemodynamics alone, suggesting functional interactions or crosstalk between the two (6). The sequence and reversibility of hemodynamic and cytokine pathways affecting STKs and CLKs and leading to loss of function in ARAS are incompletely understood. Despite higher blood flows and reduced renin release, the CLK seems to be affected by parallel signaling or interactions that mirror many of the processes in the STK. We observed that inflammatory cytokine levels fail to return to baseline, despite restoration of blood flow in patients undergoing renal revascularization (7).
In this study, we aimed to define the relationships between RBF, tissue oxygenation, and GFR of the affected STK and nonstenotic CLK by ARAS for groups of patients treated with either medical therapy alone or stent revascularization. In addition, we sought to examine the relationship between changes in RBF, tissue oxygenation, and renin release as they relate to neutrophil gelatinase–associated lipocalin (NGAL) and monocyte chemoattractant protein-1 (MCP-1) cytokine signals obtained from sampling the renal vein from both the STK and the CLK in human ARAS. We hypothesized that renal artery stenting would lead to recovery of single–kidney STK GFR compared with medical therapy alone. In addition, we hypothesized that these factors would be altered by changes in oxygenation and renin release associated with restoring patency of the renal artery to the STK. To address these issues, we studied patients with unilateral ARAS on two occasions separated by 3 months either on stable medical therapy or after technically successful renal revascularization.
Materials and Methods
Patient Enrollment and Selection
This is an observational study of physiologic parameters in nondiabetic white patients with ARAS (n=38). Cases were patients with ARAS who received revascularization, and controls were patients identified with essential hypertension (EH; n=32) or ARAS treated medically only. These patients were enrolled between January of 2008 and September of 2012. Eight patients with bilateral high–grade renal artery stenosis were excluded from this report. Ten patients with unilateral ARAS underwent subsequent revascularization for specific clinical indications (e.g., progressive decline of kidney function while on angiotensin–converting enzyme [ACE] inhibitor/angiotensin receptor blocker [ARB] therapy, resistant hypertension, or frequent heart failure exacerbation), whereas 20 patients continued medical therapy only. Patients with ARAS were included with arterial occlusion estimated at >60% using criteria used in the Cardiovascular Outcomes for Renal Atherosclerotic Lesions (CORAL) Trial as described previously (8). Informed written consent was obtained after approval by the Institutional Review Board at the Mayo Clinic in accordance with the Declaration of Helsinki. Hemodynamically significant stenosis was defined by elevated ultrasound velocities across stenotic lesions >200 cm/s (mean =345.2±154.8 cm/s) as previously described or the presence of parvus tardus (9,10). All patients received either an ACE inhibitor or ARB for hypertension during these studies.
Clinical Research Unit Protocol
A 3-day inpatient protocol was performed in the clinical research unit of St. Mary’s Hospital (Rochester, MN). Dietary intake was regulated at 150 mEq sodium. In brief, the first study day included measurement of GFR by iothalamate clearance (iothalamate meglumine; Conray) (11). BP was measured by automated oscillometric unit. The primary outcome parameter was single-kidney GFR determined by apportioning the measured iothalamate clearance by percentage of computed tomography–derived blood flow for each kidney (12).
On the second day, renal tissue oxygenation was measured using R2* maps as a reflection of deoxyhemoglobin using blood oxygen level–dependent (BOLD) magnetic resonance imaging performed on a GE Signa HDxt 3.0-T System (GE Medical Systems, Waukesha, WI) using a 12–channel torso–phased array coil (13) (details are in Supplemental Material) To determine the portion of measured kidney area in which tissue hypoxia was present, we defined fractional tissue hypoxia by measuring the percentage of voxels from the whole-kidney regions of interest with R2* values >30 seconds−1 (mainly represents the medulla) and taking the average of all available slices (14).
On the third day, the right femoral vein was cannulated with a 6-F sheath, and blood samples were drawn from the right and left renal veins and the infrarenal inferior vena cava with a 5-F Pigtail Cobra Catheter (Cook, Inc., Bloomington, IN) for measurement of pressor and inflammatory biomarkers. A 5-F pigtail catheter was advanced into the right atrium for central venous injection of contrast for transit time studies using multidetector computed tomography as described previously (9). Image analysis was performed using ANALYZE (Biomedical Imaging Resource Center, Mayo Clinic, Rochester, MN). Single–kidney and total blood flows were determined as the sum of medullary and cortical blood flows. Cortical and medullary volumes were calculated using the stereology module within ANALYZE. Regions of interest for the cortical and medullary regions were defined on each successive slice and subsequently, multiplied by slice width; these were then summed to obtain cortical, medullary, and total renal volume (12).
After the 3-day inpatient protocol, patients either continued medical therapy alone or underwent revascularization on the fourth day on the basis of clinical criteria. All patients with ARAS returned after 3 months to the clinical research unit to undergo the same studies.
Renin and Biomarkers Assays
Human plasma renin activity was measured by the radioimmunoassay kit from DiaSorin Inc. (catalog no. CA1533). Renal vein blood samples for NGAL were tested by ELISA according to the manufacturer’s protocol (catalog no. KIT 036; BioPorto Diagnostics). MCP-1 was measured by the Luminex Multiplex Assay Kit (catalog no. MPXHCYTO-60K; Millipore) (details are in Supplemental Material).
Statistical Analyses
The primary outcome parameter was intergroup single–kidney GFR.
Results were expressed using mean values and SDs or medians (interquartile ranges) as appropriate or using percentages for categorical variables. The analysis was done on the statistical software JMP, version 9.0 (JMP Statistical Discovery, Cary, NC). Comparisons among all three groups were performed with one-way ANOVA using ranks. The independent groups of STK stented/CLK versus STK/CLK medically treated were performed using two–sample t tests with unequal variance (or the Wilcoxon rank sum test for skewed data) and a chi-squared or Fisher exact test for categorical variables as appropriate. Comparisons before and after intervention or medical therapy alone on the STK or he CLK within the same patient were performed using paired tests (or Wilcoxon signed rank tests for skewed data). Intergroup comparisons of STK and CLK parameter differences were tested using Wilcoxon rank sum tests and unpaired t tests as appropriate. Correlation analysis was used to test associations between fractional hypoxia, inflammatory biomarkers, RBF, and GFR. A P value <0.05 was considered statistically significant.
Results
Demographics
Thirty-two patients with EH and 30 patients with unilateral ARAS completed the protocol for this study: 10 patients underwent stent revascularization after the first study, and 20 patients were treated medically. The demographic and clinical features of these patients are summarized in Table 1. Age was similar in all groups along with most biochemical and urine parameters. Number of men, baseline weight, systemic BP, and serum creatinine were higher in patients with stented ARAS as was the number of antihypertensive drugs.
Table 1.
Clinical, laboratory, and demographic data from patients with essential hypertension and unilateral atherosclerotic renal artery stenosis: Stent-treated and medically treated groups
| Parameter | EH, n=32 | Stent, n=10 | Medical, n=20 | Overall P Value | P Value Stent Versus Medical | P Value Stent Versus EH | P Value EH Versus Medical |
|---|---|---|---|---|---|---|---|
| Age, yr | 63.1±16.3 | 65.1±9.4 | 67.5±8.3 | 0.84 | 0.64 | 0.98 | 0.60 |
| Women (%) | 13 (41) | 1 (10) | 12 (60) | 0.02 | 0.02 | 0.20 | 0.25 |
| Weight, kg | 78.2±15.7 | 92.8±16.4 | 74.7±16.8 | 0.02 | 0.01 | 0.01 | 0.37 |
| BMI, kg/m2 | 27.4±4.3 | 29.7±4.2 | 26.9±3.7 | 0.26 | 0.14 | 0.13 | 0.96 |
| SBP, mmHg | 135±19 | 143.6±21.6 | 133.8±19.3 | 0.41 | 0.22 | 0.24 | 0.77 |
| DBP, mmHg | 71.2±12.3 | 69.6±7.9 | 67.3±8.2 | 0.38 | 0.39 | 0.68 | 0.19 |
| Creatinine, mg/dl | 1.0±0.26 | 1.5±0.4 | 1.1±0.3 | <0.001 | 0.02 | <0.001 | 0.12 |
| eGFR ml/min per 1.73 m2 | 77.8±20.7 | 53.4±20.1 | 63.6±21.3 | <0.01 | 0.43 | 0.02 | 0.08 |
| Statin, N (%) | 16 (50) | 5 (50) | 14 (70) | 0.30 | 0.42 | >0.99 | 0.24 |
| ACE/ARB, yes/no | 31/1 | 10/0 | 20/0 | >0.99 | >0.99 | >0.99 | >0.99 |
| No. of anti-HTN agents | 2.9±1.1 | 3.5±1.5 | 2.6±1.3 | 0.22 | 0.23 | 0.49 | 0.59 |
| Total cholesterol, mg/dl | 182.2±30.4 | 189.9±38.8 | 172.3±31.7 | 0.37 | 0.27 | 0.47 | 0.27 |
| 24-h Albuminuria, mga | 19 (13–29) | 17.5 (8.5–52) | 17.5 (4.2–23.5) | 0.60 | 0.76 | >0.99 | 0.61 |
| 24-h Proteinuria, mg | 89.9±74.9 | 239.1±474.6 | 86.9±60.8 | 0.44 | 0.57 | 0.46 | 0.91 |
| 24-h Na urine, mmol | 172.4±43.5 | 152.3±61.4 | 162.7±65.9 | 0.45 | 0.84 | 0.43 | 0.24 |
Values represent means±SDs. EH, essential hypertension; BMI, body mass index; SBP, systolic BP; DBP, diastolic BP; ACE/ARB, angiotensin-converting enzyme/angiotensin receptor blocker; HTN, hypertensive; Na, sodium.
Median (interquartile range) reported because of skewed data.
Single-Kidney GFR and RBF
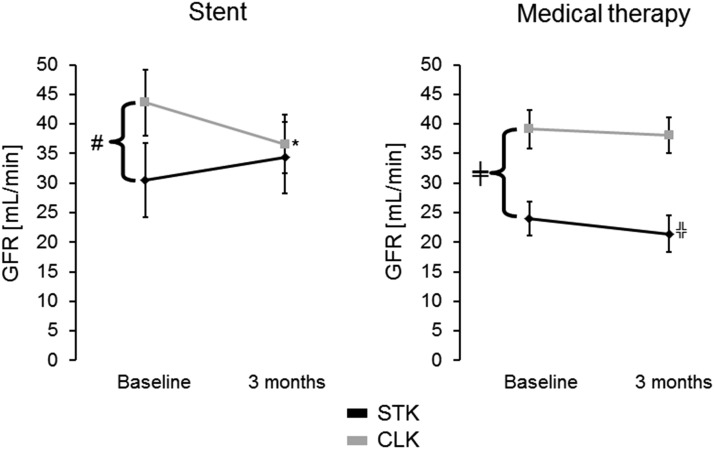
Hemodynamic measurements for individual kidneys are summarized in Tables 2–4. Differences over time between baseline and 3 months were apparent between measurements for the STK between the stent group and the medical group (Table 2). The primary outcome, difference in single-kidney GFR in the STK, persisted after multivariate adjustment for sex, weight, and creatinine. These results corresponded to parallel changes over time between the stent and medical groups regarding measurements of RBF and volume of the STK (Table 2). Changes in these outcome parameters did not differ in intergroup comparisons for the CLK (Table 2). In intragroup comparisons, GFR, RBF, and volume were lower in STK than CLK in both the stent and medical groups at baseline (Table 3). This difference was no longer evident in the stent group after 3 months (Table 4). A decrease of CLK GFR was noted in the stent group but not in the medical group at 3 months follow-up (Figure 1, left panel). Total iothalamate GFR remained unchanged over time (Tables 3 and 4). On the contrary, serum creatinine decreased in the stent group (1.5±0.4–1.2±0.3 mg/dl; P=0.01) over 3 months follow-up but remained unchanged in the medical therapy group (1.1±0.3–1.1±0.3 mg/dl; P=0.28).
Table 2.
Parameter differences at baseline and 3 months in the stent and medical therapy groups
| Parameter | Stent: Difference of Means 3 mo Versus Baseline STK, n=10 | Medical: Difference of Means 3 mo Versus Baseline STK, n=20 | P Value | Stent: Difference of Means 3 mo Versus Baseline CLK, n=10 | Medical: Difference of Means 3 mo Versus Baseline CLK, n=20 | P Value |
|---|---|---|---|---|---|---|
| CT single–kidney volume total, cm3 | 14.7±24.8 | −4±0.49 | <0.01 | −14.3±30.9 | −4.5±9.9 | 0.49 |
| Single–kidney blood flow, ml/min | 66.9±106.6 | −34.1±74.9 | <0.01 | −43.2±162 | −22.1±86.4 | 0.30 |
| Single-kidney GFR, ml/mina | 2.2 (−1.8 to 10.5) | −5.3 (−7.3 to −0.3) | 0.03 | −6.7 (−12 to −1.1) | 1.2 (−9.2 to 6.9) | 0.11 |
| Single-kidney R2*, 1/s | −2.3±4.6 | 0.4±2.9 | 0.16 | −0.8±2.3 | −0.4±2.7 | 0.20 |
| Cortex R2*, 1/s | −2.5±4.2 | −1±3.8 | 0.54 | 1±2.4 | 0.8±2.8 | 0.75 |
| Fractional hypoxia, % R2* >30% | −7.7±8 | 0.6±11.8 | 0.12 | −0.07±6.9 | −0.9±12.3 | 0.88 |
| Single–kidney vein PRA, ng/ml per 1 ha | −6.2 (−14.7 to 6.2) | −0.34 (−4.1 to 2.3) | 0.03 | −2.73 (−6.9 to −0.5) | −0.08 (−4.5 to 1.9) | 0.07 |
| Stent, n=10 | Medical, n=20 | P Value | ||||
| Systolic BP, mmHga | −7 (−30.7 to −1.7) | 7.5 (−16.2 to 13.5) | 0.10 | |||
| Diastolic BP, mmHga | −3.5 (−10.5 to 2.5) | −2 (−8 to 9.7) | 0.34 | |||
Data are differences of means±SDs. STK, stenotic kidney; CLK, contralateral kidney; CT, computed tomography; PRA, plasma renin activity.
Median (interquartile range) reported because of skewed data.
Table 4.
Measurements of individual kidney volume, hemodynamics, oxygenation, GFR, and overall iothalamate clearance after revascularization or medical therapy alone
| Parameter | 3 mo | P Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stent STK, n=10 | Stent CLK, n=10 | P Value Stent | Medical STK, n=20 | Medical CLK, n=20 | P Value Medical | 3 mo Versus Baseline Stent STK | 3 mo Versus Baseline Stent CLK | 3 mo Versus Baseline Medical STK | 3 mo Versus Baseline Medical CLK | |
| CT single–kidney volume total, cm3 | 148.1±43.6 | 151.8±51.8 | 0.80 | 93.5±37.4a | 144.8±42.7 | <0.001 | 0.09 | 0.20 | 0.01 | 0.06 |
| Single–kidney blood flow, ml/min | 320.8±175.3 | 334.7±202.1 | 0.80 | 191.4±130.7b | 367.9±183.6 | <0.001 | 0.08 | 0.40 | 0.06 | 0.30 |
| Single-kidney GFR, ml/min | 34.3±15.7 | 36.6±19.5 | 0.70 | 21.4±13.4c | 38.1±13.2 | <0.001 | 0.20 | 0.03 | 0.06 | 0.70 |
| Single-kidney R2*, 1/s | 22.4±4.1 | 21.5±2 | 0.46 | 22.2±3.9 | 21.3±2.5 | 0.35 | 0.03 | 0.30 | 0.70 | 0.50 |
| Cortex R2*, 1/s | 19.8±3.1 | 19.2±3 | 0.30 | 20.7±5 | 18.9±2.3 | 0.20 | 0.10 | 0.20 | 0.70 | 0.20 |
| Fractional hypoxia, % R2* >30% | 14.9±18.3 | 10.7±7.1 | 0.40 | 15.2±17.2 | 10.8±6.8 | 0.20 | <0.01 | 0.90 | 0.30 | 0.80 |
| P Value Stent Versus Medical Group | Stent Group 3 mo Versus Baseline | Medical Group 3 mo Versus Baseline | ||||
|---|---|---|---|---|---|---|
| Patient total iothalamate Cl, ml/min | 70.9±32.1 | 60.9±23 | 0.50 | 0.20 | 0.50 |
Data are means±SDs. STK, stenotic kidney; CLK, contralateral kidney; CT, computed tomography; Cl, clearance.
P value =0.004 medical STK versus stent STK at 3 months.
P value =0.01 medical STK versus stent STK at 3 months.
P value =0.03 medical STK versus stent STK at 3 months.
Table 3.
Measurements of individual kidney volume, hemodynamics, oxygenation, GFR, and overall iothalamate clearance before revascularization or medical therapy alone
| Parameter | Baseline | P Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stent STK, n=10 | Stent CLK, n=10 | P Value Stent | Medical STK, n=20 | Medical CLK, n=20 | P Value Medical | EH, n=32 | Stent STK Versus Medical STK | EH Versus Stent STK | EH Versus Medical STK | |
| CT single–kidney volume total, cm3 | 133.4±43.3 | 166.1±50 | 0.02 | 97.5±39.4 | 149.3±44 | <0.001 | 141.4±43.7 | 0.12 | 0.58 | 0.002 |
| Single–kidney blood flow, ml/min | 253±123.3 | 377±159 | 0.02 | 227.6±149.7 | 349.9±151.4 | <0.001 | 399.2±174.2 | 0.66 | 0.04 | <0.001 |
| Single-kidney GFR, ml/min | 30.5±17.7 | 43.6±19.7 | 0.02 | 24±12 | 39.1±14 | 0.03 | 44.3±13.6 | 0.60 | 0.04 | <0.001 |
| Single-kidney R2*, 1/s | 24.7±6.2 | 20.9±2.1 | 0.04 | 21.6±3.6 | 20.8±3 | 0.08 | 21±2.4 | 0.40 | 0.15 | 0.99 |
| Cortex R2*, 1/s | 21.6±6 | 18.4±3.2 | 0.01 | 19.8±3.9 | 18±3 | 0.02 | 18.5±2.7 | 0.52 | 0.09 | 0.39 |
| Fractional hypoxia, % R2* >30% | 22.1±20 | 10.8±6 | 0.02 | 14.1±12.6 | 10.6±12.3 | 0.04 | 9.6±7 | 0.26 | 0.03 | 0.19 |
| Stent Versus Medical | EH Versus Stent | EH Versus Medical | ||||||
|---|---|---|---|---|---|---|---|---|
| Patient total iothalamate Cl, ml/min | 74±34.8 | 62.6±21.8 | 87.9±24.3 | 0.80 | 0.30 | 0.003 |
Data are means±SDs. STK, stenotic kidney; CLK, contralateral kidney; EH, essential hypertension; CT, computed tomography; Cl, clearance.
Figure 1.
Contralateral kidney (CLK) GFR drops after revascularization in atherosclerotic renal artery stenosis (ARAS) patients and remains the same in the medical group. *P=0.03 versus baseline; ╬P=0.06 versus baseline; #P=0.02 CLK versus stenotic kidney (STK) within the group for stent; ╪P=0.03 CLK versus STK within the medical group.
Tissue Oxygenation
Tissue oxygenation defined by both cortical and whole–kidney slice R2* values and fractional hypoxia (percentage R2* >30/s) are summarized in Tables 2–4. Representative coronal BOLD images (R2* parametric maps) are shown in Figure 2. Cortical R2* and fractional tissue hypoxia were higher in the STK than the CLK to similar degrees in both the stent and medical therapy groups. Both stent and medical therapy STK values were higher than those observed in the EH group. At 3 months follow-up, single-kidney R2* and fractional hypoxia decreased in the stent group (fraction R2* >30/s: from 22.1%±20% to 14.9%±18.3%; P<0.01) but remained unchanged in the medical group. Mean parameter differences regarding tissue oxygenation over time did not differ between the stent and the medical therapy group (Table 2). Levels for baseline fractional hypoxia correlated inversely with GFR and RBF in the STK (Spearman rank correlation coefficient; r=−0.3; P=0.01 and r=−0.3; P=0.02, respectively). No correlations were evident between RBF, GFR, and R2* levels for the CLK at any time point.
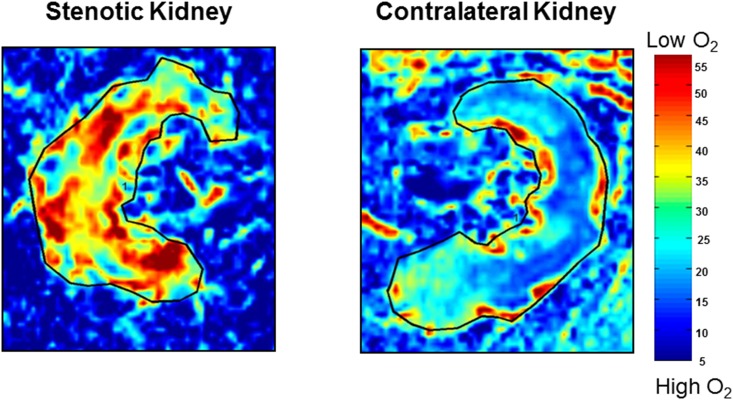
Figure 2.
Oxygenation levels are lower in the stenotic kidney as compared to the nonstenotic contralateral kidney: Representative coronal blood oxygen level-dependent images. The R2* map (reflecting the level of deoxyhemoglobin) of the stenotic kidney shows a hypoxic cortical zone and widespread areas of elevated deoxyhemoglobin in the medullary segments (red). The unaffected contralateral kidney depicts the R2* map with a lower (blue) cortical zone and more gradual development of deeper medullary areas of hypoxia, and it is close to the appearance of a normal nonatherosclerotic renal artery stenosis kidney.
Renin Levels and BP
Renal vein renin levels were elevated in both ARAS groups compared with the EH group and were higher from ARAS STK compared with ARAS CLK (18.5±15.2 versus14.6±13 ng/ml per hour, respectively; P=0.01). Renin activity from the STK and the CLK and inferior vena cava of the stented group decreased 3 months after revascularization to levels not different from those in the EH group (Tables 5 and 6), whereas renin activity levels in the medical therapy group remained unchanged (Table 2). Systolic BP also fell from 143.6±21.6 to 130.2±14.5 mmHg (P=0.04) at 3 months in the stent group without change in the number of antihypertensive drugs (Tables 5 and 6). However, the intergroup change in BP was not different between the stent and medical groups (Table 2).
Table 5.
Plasma renin activity determinations and BP in patients with atherosclerotic renal artery stenosis at baseline after revascularization or medical therapy
| Parameter | Baseline | P Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stent STK, n=10 | Stent CLK, n=10 | P Value Stent | Medical STK, n=20 | Medical CLK, n=20 | P Value Medical | EH, n=32 | Stent Versus Medical STK | EH Versus Stent STK | EH Versus Medical STK | |
| Single–kidney vein PRA, ng/ml per 1 h | 16.6±10.1 | 13.2±8.9 | 0.06 | 19.5±17.4 | 15.3±14.7 | <0.01 | 8.9±9.6 | 0.80 | 0.04 | 0.02 |
| P Value Stent Versus Medical Group | Stent Versus Medical Group | EH Versus Stent Group | EH Versus Medical Group | |||||
|---|---|---|---|---|---|---|---|---|
| PRA IVC, ng/ml per 1 h | 11.1±8.1 | 14.1±14 | 0.80 | 7.8±9.1 | 0.90 | 0.30 | 0.30 | |
| SBP, mmHg | 143.6±21.6 | 133.8±19.3 | 0.20 | 134.9±18.9 | 0.40 | 0.50 | 0.90 | |
| DBP, mmHg | 69.6±7.9 | 67.3±8.2 | 0.40 | 71.2±12.3 | 0.70 | 0.90 | 0.40 |
Data are means±SDs. STK, stenotic kidney; CLK, contralateral kidney; EH, essential hypertension; PRA, plasma renin activity; IVC, inferior vena cava; SBP, systolic BP; DBP, diastolic BP.
Table 6.
Plasma renin activity determinations and BP in patients with atherosclerotic renal artery stenosis at 3 months after revascularization or medical therapy
| Parameter | 3 mo | P Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stent STK, n=10 | Stent CLK, n=10 | P Value | Medical STK, n=20 | Medical CLK, n=20 | P Value | 3 mo Versus Baseline Stent STK | 3 mo Versus Baseline Stent CLK | 3 mo Versus Baseline Medical STK | 3 mo Versus Baseline Medical CLK | |
| Single–kidney vein PRA, ng/ml per 1 h | 9.3±10.6 | 9.3±9.3 | 0.90 | 19.2±18.4 | 15.7±14.1 | 0.03 | 0.01 | 0.02 | 0.80 | 0.90 |
| P Value Stent Versus Medical Group | 3 mo Versus Baseline Stent Group | 3 mo Versus Baseline Medical Group | ||||
|---|---|---|---|---|---|---|
| PRA IVC, ng/ml per 1 h | 6.7±8.3 | 14.6±14.6 | 0.30 | 0.01 | 0.70 | |
| SBP, mmHg | 130.2±14.5 | 133.6±19.1 | 0.90 | 0.04 | 0.90 | |
| DBP, mmHg | 64.9±10.3 | 67.1±12.2 | 0.60 | 0.08 | 0.90 |
Data are means±SDs. STK, stenotic kidney; CLK, contralateral kidney; PRA, plasma renin activity; IVC, inferior vena cava; SBP, systolic BP; DBP, diastolic BP.
NGAL and MCP-1 from the Renal Veins
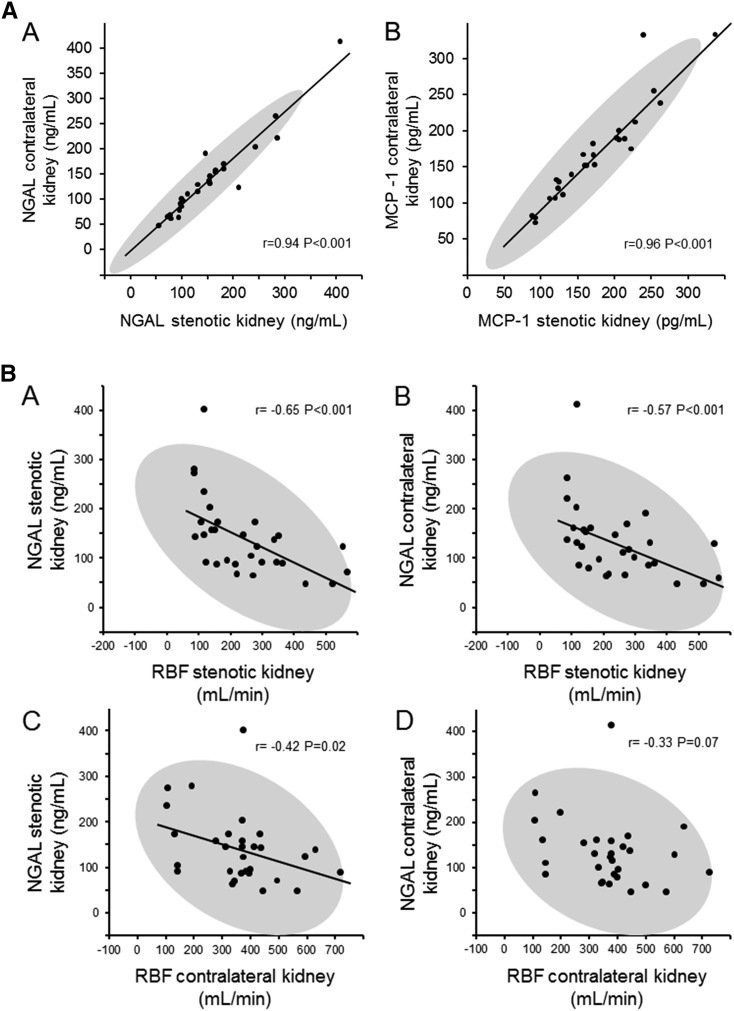
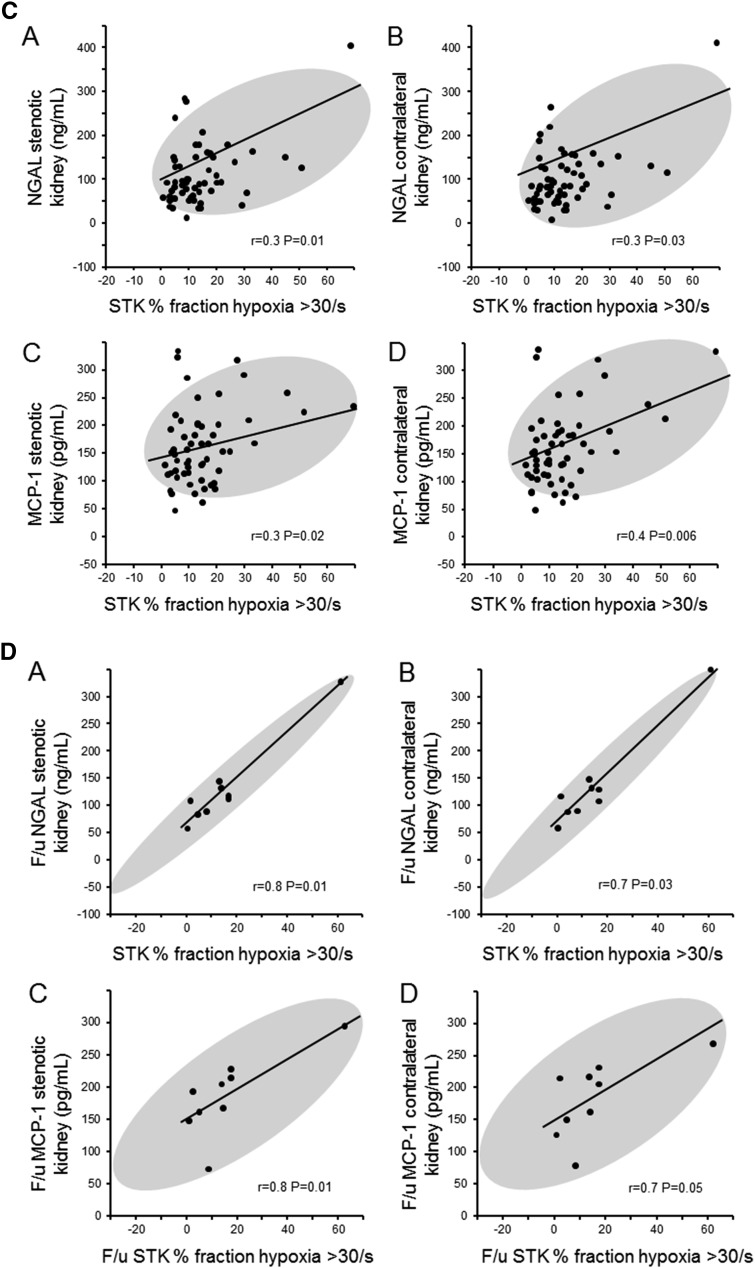
Renal vein basal levels of NGAL were elevated at baseline in both ARAS STK (144.1±77.7 ng/ml) and CLK (136.1±74.5 ng/ml) compared with in patients with EH (69.7±34.3 ng/ml; P<0.001 versus ARAS STK and CLK). Average levels did not change after 3 months for either treatment group. Renal vein NGAL and MCP-1 levels from the CLK were highly associated with the levels of NGAL (r=0.94; P<0.001) and MCP-1 (r=0.96; P<0.001), respectively, obtained from the STK (Figure 3A). Furthermore, renal vein levels of NGAL from the STK and CLK at baseline correlated inversely with STK RBF (Figure 3B [A and B]).These correlations were not present for renal vein MCP-1 (data not shown). In all groups, the levels of baseline renal vein NGAL and MCP-1 correlated directly with levels of hypoxia from the STK (Figure 3C) but not from the CLK. Three months after revscularization, correlations between NGAL and MCP-1 levels and measures of overall fractional hypoxia became even stronger (Figure 3D).
Figure 3.
Renal vein inflammatory biomarkers correlations between contralateral and stenotic kidneys. (A) Renal vein inflammatory biomarkers (neutrophil gelatinase-associated lipocalin [NGAL], [A] and monocyte chemoattractant protein-1 [MCP-1], [B]) were highly correlated between stenotic (STK) and unaffected contralateral kidneys (CLK). (B) Renal vein NGAL in both stenotic kidney, (A and C) and contralateral kidney, (B and D) correlates inversely with renal blood flow (RBF) in the STK (A and B) and CLK (C). (C) Renal vein NGAL in the stenotic kidney (A) and contralateral kidney (B) and renal vein MCP-1 in the STK (C) and CLK (D) correlated with fractional hypoxia in the STK. (D) After revascularization, there is a stronger correlation of renal vein NGAL in the stenotic kidney (A) and contralateral kidney (B) and renal vein MCP-1 in the stenotic kidney (C) and contralateral kidney, (D) with fractional hypoxia in the STK. F/u, follow-up.
Discussion
The results of this study show parameter differences over time in both RBF and GFR in the revascularized STK compared with medical therapy for unilateral ARAS. Overall, GFR remained unchanged, with a shift in the relative contribution of the STK and the CLK to the total GFR. Despite measurable increases in RBF, reduction in renin release, and partial reduction of fractional hypoxia in the STK within the stent group, overall parameter difference in tissue hypoxia over 3 months did not differ from that of the medically treated group, possibly indicating residual microvascular impairment after stenting. Importantly, levels of renal venous inflammatory biomarkers correlated with the degree of hypoxia affecting the STK both before and after stenting.
Patients treated with medical therapy alone maintained intragroup hemodynamic differences between the STK and the CLK throughout the study period. By contrast, CLK GFR dropped considerably 3 months after STK revascularization in the stent group. This phenomenon partly may explain the limited improvement of overall iothalamate GFR (from both kidneys) observed in patients with unilateral ARAS after revascularization because of reversal of the compensation from the CLK in unilateral ARAS. This shift in single-kidney dynamics after stenting extends previous observations of compensatory hyperfiltration in renovascular disease (15,16) and the adaptation of the remaining kidney after unilateral nephrectomy (17,18).
Despite baseline demographic differences between the stented and medical groups, such as sex and body weight, the baseline iothalamate GFR, tissue hypoxia, and RBF were not different between the groups at baseline. Differences between stent and medical groups in terms of STK RBF and GFR became more evident 3 months after revascularization, a difference that persisted after adjusting for baseline differences in sex, weight, and GFR. The group subjected to revascularization on the basis of clinical indications showed a reduction in serum creatinine level and a small but positive STK GFR change over time compared with the medical group. Levels of kidney function (eGFR) (Table 1) were generally comparable with those in the CORAL Trial but somewhat higher than those in Angioplasty and Stenting for Renal Artery Lesions Trial, possibly because of the focus on unilateral ARAS.
Our results further identify, for the first time, relationships between reductions in single–kidney blood flow and oxygenation in ARAS to the activation of inflammatory pathways related to NGAL and MCP-1 in humans. Reductions in blood flow in ARAS STKs and associated tissue hypoxia (both cortical R2* and fractional hypoxia >30/s BOLD magnetic resonance imaging) were related to higher levels of NGAL. Renal venous inflammatory markers derived from the STK were highly related to those obtained from the CLK as well. These data underscore the systemic nature of inflammation related to renal hypoxia and possible interactions between kidneys affected by unilateral ARAS in humans.
Levels of NGAL and MCP-1 failed to return to normal with intensive antihypertensive medical therapy or restoration of blood flow. Previous studies have shown that inflammatory markers are elevated in patients with ARAS compared with in patients with EH (7,19,20). Transjugular biopsies obtained from poststenotic human ARAS kidneys identify widespread tissue activation of TGF and accumulation of tissue macrophages (21). Our results are consistent with active interactions between the two kidneys in human ARAS as reflected by the high correlation between levels of inflammatory markers in the CLK and STK. In experimental models, activation of endothelin, oxidative stress pathways, and pressure injury have been implicated in these signaling events (22–24). Our studies suggest a role for reduced blood flow and tissue hypoxia related to NGAL and MCP-1, possibly related to inflammatory injury. Remarkably, the correlation between inflammatory markers and residual hypoxia was stronger 3 months after revascularization, despite some restoration of RBF and attenuated hypoxia. We speculate that this might be related to ongoing microvascular limitations of blood flow within the poststenotic kidney.
These data further underscore the conceptual transition in ARAS from a hemodynamic condition to one associated with ongoing tissue injury and inflammation that may not respond to restoring large-vessel patency alone. They emphasize the importance of pursuing novel strategies to reverse inflammatory and profibrotic injury in ARAS as we have proposed (25).
Although NGAL correlates with kidney injury in some settings, some evidence suggests that NGAL may protect renal tubular cells (26). In a hypoxia-reperfusion model of renal tubular epithelial cells, NGAL seems to favorably influence the balance of pro- and antiapoptotic factors toward survival (27). Moreover, exogenous NGAL was found to reduce renal tubular cell apoptosis and protect renal function in rats subjected to ischemia-reperfusion injury (28). Thus, sustained increases in circulating NGAL in our patients may represent an active endogenous response improving tubular regeneration and limiting renal injury in these patients.
One limitation of our study is the lack of systemic levels of NGAL pre- and poststenting. Previous studies have shown that systemic plasma NGAL blood levels are increased in patients with ARAS compared with those in patients with EH and healthy volunteers (20). In patients with carotid atherosclerosis, plasma NGAL concentration is associated with patient age, hypertension, and GFR (29). All of our patients had evident atherosclerotic disease and hypertension, and the medical versus stent groups had similar GFR. We cannot exclude a role for ischemic processes in the STK activating systemic mechanisms that are reflected in renal vein levels indirectly.
Limiting these studies to patients with unilateral renovascular disease allowed direct comparison of renal vein renin levels in kidneys with compromised and normal blood flow. Interpretation of both peripheral and/or renal vein renin levels is highly affected by sampling conditions (e.g., volume status, sodium intake, and drug therapy). All patients received ACE/ARB therapy and diuretics during BOLD imaging, and this may explain the high levels of plasma renin activity and limited lateralization in ARAS, which in general, is expected in this population (2,30). We found that, 3 months after revascularization, peripheral and renal vein renin levels had fallen more than those in the medical group to levels similar to those in the EH group.
It might be considered a limitation that our control group comprised patients with EH rather than truly healthy individuals. Furthermore, this was a nonrandomized study that excluded patients with diabetes.
In summary, results of this study show the change over time in single-kidney GFR that is not evident in parameters of total GFR. Also, these studies further underscore the relationships between impaired tissue oxygenation and reduced blood flow and renal vein levels of NGAL and MCP-1 in unilateral human ARAS. Residual levels of STK fractional tissue hypoxia continued to be associated with NGAL and MCP-1 levels 3 months later. These data support interactions between the STK and the CLK and systemic inflammation.
Disclosures
None.
Supplementary Material
Acknowledgments
The projects described were supported by Award R01 DK100081 from the National Institute for Diabetes, Digestive and Kidney Diseases and National Institutes of Health/National Center for Research Resources Clinical and Translational Science Award grant UL1 RR024150.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK or the NIH.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03620415/-/DCSupplemental.
References
- 1.Goldblatt H, Lynch J, Hanzal RF, Summerville WW: Studies on experimental hypertension: I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med 59: 347–379, 1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaughan ED, Jr., Bühler FR, Laragh JH, Sealey JE, Baer L, Bard RH: Renovascular hypertension: Renin measurements to indicate hypersecretion and contralateral suppression, estimate renal plasma flow, and score for surgical curability. Am J Med 55: 402–414, 1973 [DOI] [PubMed] [Google Scholar]
- 3.Bax L, Woittiez AJ, Kouwenberg HJ, Mali WP, Buskens E, Beek FJ, Braam B, Huysmans FT, Schultze Kool LJ, Rutten MJ, Doorenbos CJ, Aarts JC, Rabelink TJ, Plouin PF, Raynaud A, van Montfrans GA, Reekers JA, van den Meiracker AH, Pattynama PM, van de Ven PJ, Vroegindeweij D, Kroon AA, de Haan MW, Postma CT, Beutler JJ: Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: A randomized trial. Ann Intern Med 150: 840–848, 2009 [DOI] [PubMed]
- 4.Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, Baigent C, Carr S, Chalmers N, Eadington D, Hamilton G, Lipkin G, Nicholson A, Scoble J, ASTRAL Investigators : Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 361: 1953–1962, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, Cohen DJ, Matsumoto AH, Steffes M, Jaff MR, Prince MR, Lewis EF, Tuttle KR, Shapiro JI, Rundback JH, Massaro JM, D’Agostino RB, Sr., Dworkin LD, CORAL Investigators : Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 370: 13–22, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juncos LA, Chandrashekar KB, Lopez-Ruiz AF, Juncos LI, editors: Interaction between Stenotic and Contralateral Kidneys: Unique Features of Each in Unilateral Disease, 1st Ed., London, Springer-Verlag, 2014 [Google Scholar]
- 7.Saad A, Herrmann SM, Crane J, Glockner JF, McKusick MA, Misra S, Eirin A, Ebrahimi B, Lerman LO, Textor SC: Stent revascularization restores cortical blood flow and reverses tissue hypoxia in atherosclerotic renal artery stenosis but fails to reverse inflammatory pathways or glomerular filtration rate. Circ Cardiovasc Interv 6: 428–435, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy TP, Cooper CJ, Dworkin LD, Henrich WL, Rundback JH, Matsumoto AH, Jamerson KA, D’Agostino RB: The Cardiovascular Outcomes with Renal Atherosclerotic Lesions (CORAL) study: Rationale and methods. J Vasc Interv Radiol 16: 1295–1300, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Gloviczki ML, Glockner JF, Lerman LO, McKusick MA, Misra S, Grande JP, Textor SC: Preserved oxygenation despite reduced blood flow in poststenotic kidneys in human atherosclerotic renal artery stenosis. Hypertension 55: 961–966, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chi YW, White CJ, Thornton S, Milani RV: Ultrasound velocity criteria for renal in-stent restenosis. J Vasc Surg 50: 119–123, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Wilson DM, Bergert JH, Larson TS, Liedtke RR: GFR determined by nonradiolabeled iothalamate using capillary electrophoresis. Am J Kidney Dis 30: 646–652, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Krier JD, Ritman EL, Bajzer Z, Romero JC, Lerman A, Lerman LO: Noninvasive measurement of concurrent single-kidney perfusion, glomerular filtration, and tubular function. Am J Physiol Renal Physiol 281: F630–F638, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Gloviczki ML, Glockner J, Gomez SI, Romero JC, Lerman LO, McKusick M, Textor SC: Comparison of 1.5 and 3 T BOLD MR to study oxygenation of kidney cortex and medulla in human renovascular disease. Invest Radiol 44: 566–571, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saad A, Crane J, Glockner JF, Herrmann SM, Friedman H, Ebrahimi B, Lerman LO, Textor SC: Human renovascular disease: Estimating fractional tissue hypoxia to analyze blood oxygen level-dependent MR. Radiology 268: 770–778, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossignol P, Chatellier G, Azizi M, Plouin PF: Proteinuria in renal artery occlusion is related to active renin concentration and contralateral kidney size. J Hypertens 20: 139–144, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Ebrahimi B, Rihal N, Woollard JR, Krier JD, Eirin A, Lerman LO: Assessment of renal artery stenosis using intravoxel incoherent motion diffusion-weighted magnetic resonance imaging analysis. Invest Radiol 49: 640–646, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeon HG, Lee SR, Joo DJ, Oh YT, Kim MS, Kim YS, Yang SC, Han WK: Predictors of kidney volume change and delayed kidney function recovery after donor nephrectomy. J Urol 184: 1057–1063, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Taner T, Iqbal CW, Textor SC, Stegall MD, Ishitani MB: Compensatory hypertrophy of the remaining kidney in medically complex living kidney donors over the long term. Transplantation 99: 555–559, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Eirin A, Gloviczki ML, Tang H, Gössl M, Jordan KL, Woollard JR, Lerman A, Grande JP, Textor SC, Lerman LO: Inflammatory and injury signals released from the post-stenotic human kidney. Eur Heart J 34: 540–548a, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eirin A, Gloviczki ML, Tang H, Rule AD, Woollard JR, Lerman A, Textor SC, Lerman LO: Chronic renovascular hypertension is associated with elevated levels of neutrophil gelatinase-associated lipocalin. Nephrol Dial Transplant 27: 4153–4161, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gloviczki ML, Keddis MT, Garovic VD, Friedman H, Herrmann S, McKusick MA, Misra S, Grande JP, Lerman LO, Textor SC: TGF expression and macrophage accumulation in atherosclerotic renal artery stenosis. Clin J Am Soc Nephrol 8: 546–553, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chade AR, Best PJ, Rodriguez-Porcel M, Herrmann J, Zhu X, Sawamura T, Napoli C, Lerman A, Lerman LO: Endothelin-1 receptor blockade prevents renal injury in experimental hypercholesterolemia. Kidney Int 64: 962–969, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Zhu XY, Chade AR, Krier JD, Daghini E, Lavi R, Guglielmotti A, Lerman A, Lerman LO: The chemokine monocyte chemoattractant protein-1 contributes to renal dysfunction in swine renovascular hypertension. J Hypertens 27: 2063–2073, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lerman LO, Textor SC, Grande JP: Mechanisms of tissue injury in renal artery stenosis: Ischemia and beyond. Prog Cardiovasc Dis 52: 196–203, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Textor SC, Lerman LO: Paradigm shifts in atherosclerotic renovascular disease: Where are we now? J Am Soc Nephrol 26: 2074–2080, 2015 [DOI] [PMC free article] [PubMed]
- 26.Tong Z, Wu X, Ovcharenko D, Zhu J, Chen CS, Kehrer JP: Neutrophil gelatinase-associated lipocalin as a survival factor. Biochem J 391: 441–448, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zang X, Zheng F, Hong HJ, Jiang Y, Song Y, Xia Y: Neutrophil gelatinase-associated lipocalin protects renal tubular epithelial cells in hypoxia-reperfusion by reducing apoptosis. Int Urol Nephrol 46: 1673–1679, 2014 [DOI] [PubMed] [Google Scholar]
- 28.An S, Zang X, Yuan W, Zhuge Y, Yu Q: Neutrophil gelatinase-associated lipocalin (NGAL) may play a protective role against rats ischemia/reperfusion renal injury via inhibiting tubular epithelial cell apoptosis. Ren Fail 35: 143–149, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Giaginis C, Zira A, Katsargyris A, Klonaris C, Theocharis S: Clinical implication of plasma neutrophil gelatinase-associated lipocalin (NGAL) concentrations in patients with advanced carotid atherosclerosis. Clin Chem Lab Med 48: 1035–1041, 2010 [DOI] [PubMed]
- 30.Sealey JE, Bühler FR, Laragh JH, Vaughan ED, Jr.: The physiology of renin secretion in essential hypertension. Estimation of renin secretion rate and renal plasma flow from peripheral and renal vein renin levels. Am J Med 55: 391–401, 1973 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.