ABSTRACT
Simian hemorrhagic fever (SHF) is a highly lethal disease in captive macaques. Three distinct arteriviruses are known etiological agents of past SHF epizootics, but only one, simian hemorrhagic fever virus (SHFV), has been isolated in cell culture. The natural reservoir(s) of the three viruses have yet to be identified, but African nonhuman primates are suspected. Eleven additional divergent simian arteriviruses have been detected recently in diverse and apparently healthy African cercopithecid monkeys. Here, we report the successful isolation in MARC-145 cell culture of one of these viruses, Kibale red colobus virus 1 (KRCV-1), from serum of a naturally infected red colobus (Procolobus [Piliocolobus] rufomitratus tephrosceles) sampled in Kibale National Park, Uganda. Intramuscular (i.m.) injection of KRCV-1 into four cynomolgus macaques (Macaca fascicularis) resulted in a self-limiting nonlethal disease characterized by depressive behavioral changes, disturbance in coagulation parameters, and liver enzyme elevations. In contrast, i.m. injection of SHFV resulted in typical lethal SHF characterized by mild fever, lethargy, lymphoid depletion, lymphoid and hepatocellular necrosis, low platelet counts, increased liver enzyme concentrations, coagulation abnormalities, and increasing viral loads. As hypothesized based on the genetic and presumed antigenic distance between KRCV-1 and SHFV, all four macaques that had survived KRCV-1 injection died of SHF after subsequent SHFV injection, indicating a lack of protective heterotypic immunity. Our data indicate that SHF is a disease of macaques that in all likelihood can be caused by a number of distinct simian arteriviruses, although with different severity depending on the specific arterivirus involved. Consequently, we recommend that current screening procedures for SHFV in primate-holding facilities be modified to detect all known simian arteriviruses.
IMPORTANCE
Outbreaks of simian hemorrhagic fever (SHF) have devastated captive Asian macaque colonies in the past. SHF is caused by at least three viruses of the family Arteriviridae: simian hemorrhagic fever virus (SHFV), simian hemorrhagic encephalitis virus (SHEV), and Pebjah virus (PBJV). Nine additional distant relatives of these three viruses were recently discovered in apparently healthy African nonhuman primates. We hypothesized that all simian arteriviruses are potential causes of SHF. To test this hypothesis, we inoculated cynomolgus macaques with a highly divergent simian arterivirus (Kibale red colobus virus 1 [KRCV-1]) from a wild Ugandan red colobus. Despite being only distantly related to red colobuses, all of the macaques developed disease. In contrast to SHFV-infected animals, KRCV-1-infected animals survived after a mild disease presentation. Our study advances the understanding of an important primate disease. Furthermore, our data indicate a need to include the full diversity of simian arteriviruses in nonhuman primate SHF screening assays.
INTRODUCTION
In 1964, two nearly simultaneous outbreaks of a novel and almost uniformly lethal viral hemorrhagic fever occurred among captive Asian macaques at primate-holding facilities at the Institute of Experimental Pathology and Therapy in Sukhumi, Georgian Soviet Socialist Republic, Soviet Union, and at the National Institutes of Health, Primate Quarantine Unit in Bethesda, MD (1–4). The affected macaques in both outbreaks had been acquired from the same exporter in India and housed together with African cercopithecids, including baboons, grivets, and/or patas monkeys (2, 5). The novel disease, which is currently thought to affect only Asian macaques of diverse species, was named simian hemorrhagic fever (SHF). Since 1964, some 15 additional SHF outbreaks may have occurred in Austria, the United Kingdom, the United States, and Soviet Union; the latest one occurred in 1989 in the United States (see reference 6). Diagnosis of SHF in all cases was made based on clinical presentation, epizootiology, and limited serology, most often without virus isolation.
Macaques suffering from SHF typically present with inappetence, cyanosis, fever, dehydration, marked depression, diarrhea, dyspnea, mild facial edema, lymphadenopathy, weight loss, and splenomegaly. These signs occur together with hemorrhagic manifestations, such as epistaxis, hematomas, hematuria, melena, periocular hemorrhages, and petechiae, in part due to disseminated intravascular coagulation. Central nervous system (CNS) involvement, as indicated by somnolence, tremors, ataxia, weakened tone or pareses of hind limbs, meningism, and epileptoid convulsions, has been observed during some outbreaks (e.g., at Sukhumi in 1964 and at Davis in 1967) but not during other outbreaks (e.g., at Bethesda in 1964 and at Sukhumi in 1967). The clinical parameters for SHF are lymphopenia accompanied by neutrophilia with a left shift, depressed thrombocyte counts to severe thrombocytopenia, induction of proinflammatory cytokines, proteinuria, increased prothrombin and partial thromboplastin times and increased D-dimer concentrations with a lack of compensatory fibrinolytic activity, and decreased hematocrit. Macaques suffering from SHF generally die after a sharp temperature decrease 7 to 15 days after infection. Pathologically, SHF is characterized by limited focal necroses in the liver and adrenal glands, punctate hemorrhages in the lungs and gastrointestinal mucous membranes and typical hemorrhages at the gastroduodenal junction, and swelling of neuronal bodies and dissipation of Nissl substance in the brain (4, 7–13).
Until recently, the etiological agent of SHF was thought to be a single virus, simian hemorrhagic fever virus (SHFV) (Nidovirales: Arteriviridae: Arterivirus), which was isolated in cell culture from clinical samples during the 1964 Bethesda epizootic (14). However, variations in the disease course during temporally distinct SHF epizootics (most notably, the presence or absence of severe CNS involvement) (4, 7–13) and serological investigations (15, 16) suggest that SHF may be multicausal. Unfortunately, with the exception of the original SHFV isolate, all other virus isolates had been destroyed. However, in 2015, genomic sequencing studies performed on the few preserved tissue samples from past SHF outbreaks revealed that at least the 1964 Sukhumi outbreak and an outbreak in Alamogordo, NM, in 1989 were caused by two previously uncharacterized arteriviruses, now called simian hemorrhagic encephalitis virus (SHEV) and Pebjah virus (PBJV). Both viruses are only distantly related to each other and to SHFV (6). The natural hosts of all three viruses remain to be determined.
PBJV, SHEV, and SHFV are not the only known simian arteriviruses. Since 2011, nine additional viruses have been discovered by deep sequencing RNA in sera from apparently healthy African cercopithecid primates (17–20). The genomes of all simian arteriviruses are characterized by the presence of 3 to 4 open reading frames that are absent in the genomes of the other known, nonsimian arteriviruses (see reference 6).
We hypothesize that all simian arteriviruses establish subclinical infections in their African cercopithecid hosts and that many of them may cause SHF after accidental or deliberate introduction into Asian macaques. To test our hypothesis directly, we isolated Kibale red colobus virus 1 (KRCV-1), which was originally discovered in 2011 in serum of a wild red colobus (Procolobus [Piliocolobus] rufomitratus tephrosceles) in Uganda’s Kibale National Park (19). Then, we experimentally infected cynomolgus macaques (Macaca fascicularis) intramuscularly (i.m.) with KRCV-1 in parallel with SHFV controls. As expected, we observed the typical lethal disease course of SHF in SHFV-infected macaques. In contrast, KRCV-1-infected macaques suffered from a self-limiting nonlethal disease that nevertheless had SHF-like characteristics, such as increased liver enzyme and D-dimer concentrations. To evaluate whether KRCV-1 infection provides cross protection against SHFV, all KRCV-1 infection survivors were exposed to SHFV. All animals developed severe and fatal SHF, indicating an absence of cross protection. Our data indicate that SHF is a disease with a potentially wide spectrum of severity that in all likelihood can be caused by a broad diversity of simian arteriviruses.
RESULTS
KRCV-1 grows in MARC-145 cells and produces SHFV-like particles.
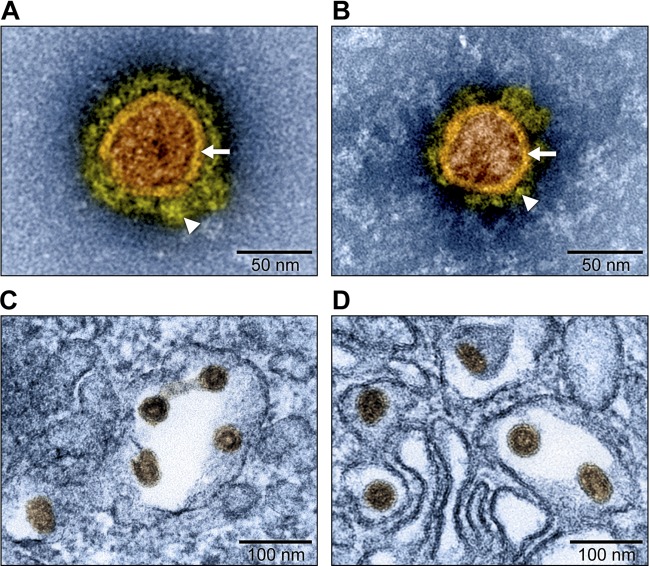
Currently, only one simian arterivirus, SHFV, has repeatedly been cultured in cell culture. Interestingly, the in vitro cell tropism of SHFV is extremely limited, as the virus can only be cultured thus far in the immortalized grivet (Chlorocebus aethiops) kidney MA-104 cell line and its various subclones (such as MARC-145) and in various primary nonhuman primate cell lines (14, 21). Attempts to isolate KRCV-1 from sera of subclinically infected red colobuses at first failed in all cell lines tested (data not shown). To increase the likelihood of virus isolation, we inoculated rhesus monkey bronchoalveolar lavage (BAL) fluid leukocytes with diluted red colobus sera, followed by blind passages on MARC-145 cells. MARC-145 cell supernatant was then inoculated onto a variety of vertebrate cell lines, which were observed for cytopathic effect (CPE) and tested for the presence of viral nucleic acids using quantitative reverse transcription PCR (qRT-PCR). A signal indicative of KRCV-1 replication was only obtained with MARC-145 cells. Transmission electron microscopy (TEM) was used to compare KRCV-1 particle morphology to SHFV particle morphology using infected MARC-145 (KRCV-1) or MA-104 cells (SHFV) and concentrated cell culture supernatants. As expected, KRCV-1 particles largely resembled those produced by SHFV; KRCV-1 particles are enveloped, spherical, with an approximate diameter of 60 nm, and contain an isometric nucleocapsid. Evenly dispersed surface projections embedded in the envelope were clearly visible (Fig. 1). These results are comparable to those observed for SHFV and demonstrate that KRCV-1 can be propagated in vitro.
FIG 1 .
Electron micrographs of KRCV-1 and SHFV particles (artificially colored). Grivet kidney MARC-145 and MA-104 cells were infected with Kibale red colobus virus 1 (KRCV-1) and simian hemorrhagic fever virus (SHFV), respectively. (A) Electron micrograph of a negatively stained KRCV-1 particle from direct-pelleted supernatant. (B) Electron micrograph of a negatively stained SHFV particle from direct-pelleted supernatant. Samples were stained with 1.0% phosphotungstic acid. Note viral envelope (arrows) and envelope fringe proteins (arrowheads). (C) Electron micrograph of KRCV-1 particles in infected cells. (D) Electron micrograph of SHFV particles in infected cells.
KRCV-1 produces a mild illness in cynomolgus macaques that does not confer immunity to SHFV infection.
Two groups of four cynomolgus macaques were injected i.m. in parallel with 1,000 PFU of SHFV and 109 genome copies of KRCV-1, respectively, and observed postexposure for the development of clinical signs characteristic of SHF. KRCV-1 was expanded on rhesus monkey peripheral blood mononuclear cells (PBMCs). Survival postexposure for all experimental groups is summarized in Fig. 2. The onset of clinical signs was rapid and consistent for both groups of animals, occurring at days 2 to 3 postexposure. Animals exposed to either virus developed a reduction in appetite, dehydration, and depression with associated recumbence and/or prostration. The SHFV-infected animals developed a prominent disease characterized by severely depressed responsiveness, activity, and appetite, mild to moderate dehydration, and frequent recumbence that included prostration. All SHFV-infected animals succumbed to disease, with a range of 4 to 8 days and a median time to death of 7 days.
FIG 2 .
Kaplan-Meier survival curves for macaques experimentally infected with simian arteriviruses. Cynomolgus macaques were injected with 1,000 PFU of simian hemorrhagic fever virus (SHFV, green line) or 109 genome copies of Kibale red colobus virus 1 (KRCV-1, blue line). Animals that survived KRCV-1 infection were injected with SHFV to test for cross protection (orange line).
Conversely, KRCV-1-exposed animals exhibited mild depression of activity, a moderate reduction in appetite, and mild dehydration. While the KRCV-1-exposed animals exhibited recumbent postures, they remained responsive for the duration of the study. The overt clinical signs observed among all animals are summarized in Fig. 3B. Animals suffering from KRCV-1-induced illness completely recovered within 2.5 weeks of exposure.
FIG 3 .
Viral loads in macaques experimentally infected with simian arteriviruses. (A) Viral loads in cynomolgus macaques infected with simian hemorrhagic fever virus (SHFV, green line), Kibale red colobus virus 1 (KRCV-1, blue line), and KRCV-1 survivors infected with SHFV (orange line) were determined by qRT-PCR. vRNA, viral RNA. (B) Overt clinical signs of infected macaques over the study duration. DPE, days postexposure.
Injection of the survivors of KRCV-1 infection with SHFV resulted in lethal disease nearly identical to that seen in KRCV-1-naive animals infected with SHFV (Fig. 2 and 3B). Log rank survival analysis of naive and KRCV-1-convalescent animals exposed to SHFV demonstrated no statistical difference (P = 0.196). However, animals that had previously cleared KRCV-1 infection presented with neurological signs, such as minor body tremors and piloerection, which had not been observed in SHFV-infected control animals (Fig. 3B).
In all three groups (SHFV infection, KRCV-1 infection, and KRCV-1 infection followed by SHFV infection), the onset of clinical signs occurred when viremia increased markedly, as determined by qRT-PCR (Fig. 3A and B). High viral loads (108 to 1010 genome copies/ml of serum starting at day 3 postexposure) were sustained in SHFV-infected macaques until time of death. In KRCV-1-infected macaques, viremia reached ≈107 genome copies/ml serum at day 3, peaked at day 5, fell gradually on days 6 to 8, and then rapidly decreased after day 8 postexposure. The genome copy concentrations dropped below detectable limits by day 28. This drop in viremia coincided with clinical recovery of the animals. Infection of these convalescent animals with SHFV resulted in viral loads similar to those seen in KRCV-1-naive animals (peak loads of 1010 genome copies/ml of serum) in correspondence with disease progression. The day-3 viral loads of both naive animals and KRCV-1-convalescent animals infected with SHFV differed significantly from those of KRCV-1-infected animals (P = 0.0007 and P < 0.0001, respectively). At the same time, KRCV-1 genome copies could not be detected in these animals by the time of SHFV inoculation (day 71) or on subsequent days (days 74, 76, 77, and 78), suggesting that KRCV-1 infection had truly cleared. Together, these data demonstrate that cynomolgus macaques can be infected with KRCV-1, KRCV-1 replication can be maintained in the animals for more than 3 weeks, and KRCV-1 is pathogenic in cynomolgus macaques. Furthermore, these data indicate that surviving KRCV-1 infection does not provide protection from subsequent SHFV infection and disease.
SHFV infection of cynomolgus macaques results in typical SHF pathology.
Necropsies and histological examinations were performed on all animals that succumbed to SHFV infection (those infected with SHFV only and those infected with KRCV-1 followed by SHFV). The gross findings were consistent with SHF as described previously (7–9, 11) and included congestion, hemorrhage, and necrosis of the gastrointestinal tract, renal capsule, subcutis, and lungs (Table 1). Remarkable histological findings included lymphoid depletion and necroses in the spleen and peripheral lymph nodes associated with infiltrating macrophages and fibrin deposition. Hepatocellular degeneration was a consistent finding in all animals, with some instances of necrosis characterized by swelling and vacuolization of hepatocytes. Renal changes were inconsistent and mild when noted, typically consisting of tubular epithelial degeneration. Effects on the pulmonary system included both congestion and edema in some animals.
TABLE 1 .
Incidence of pathology in cynomolgus macaques infected with SHFV or with KRCV-1 followed by SHFV
| Type of observations, organ system | Finding(s) (no. of animals with finding/total no. in group) in: |
|
|---|---|---|
| KRCV-1-naive, SHFV-infected animals | KRCV-1-infected then SHFV-infected animals | |
| Macroscopic observations | ||
| Neurological | Meningeal congestion (2/4) | Meningeal edema (1/4) |
| Cardiopulmonary | Pericardial edema (1/4), pulmonary congestion (2/4) | Pericardial edema (1/4), pulmonary congestion (1/4) |
| Gastrointestinal | Duodenal congestion (2/4), jejunal congestion (2/4), ileal congestion (1/4) | Significant pathology not observed |
| Hepatic | Discoloration (1/4), congestion (2/4) | Significant pathology not observed |
| Hemolymphatic | Spleen congestion (2/4), peripheral lymphadenopathy (1/4), tracheobronchial lymphadenopathy (1/4) | Mesenteric lymphadenopathy (2/4), peripheral lymphadenopathy (2/4), tracheobronchial lymphadenopathy (1/4) |
| Integumentary | Dermal hemorrhage (1/4) | Dermal hemorrhage (2/4) |
| Adrenal | Hemorrhage (1/4) | Significant pathology not observed |
| Reproductive | Testicular congestion (1/4) | Testicular edema (2/4) |
| Histological observations | ||
| Pulmonary | Congestion (moderate in 3/4), edema (moderate in 1/4), lymphocytic/lymphohistiocytic inflammation (moderate in 1/4) | Congestion (mild in 1/4), edema (moderate in 1/4), lymphocytic/lymphohistiocytic inflammation (mild to moderate in 2/4) |
| Hepatic | Hepatocellular degeneration (mild to moderate in 4/4), hepatocellular necrosis (minimal in 1/4) | Hepatocellular degeneration (minimal to moderate in 4/4), hepatocellular necrosis (minimal in 1/4) |
| Hemolymphatic: | ||
| Spleen | Depletion (mild to moderate in 2/4), lymphoid degeneration and/or necrosis (minimal to moderate in 3/4) | Depletion (mild to moderate in 4/4), lymphoid necrosis (minimal to moderate in 3/4) |
| Lymph nodes: | ||
| Axillary | Depletion (minimal to mild in 2/4), necrosis (minimal to moderate in 3/4), histiocytosis (moderate in 1/4) | Depletion (mild in 4/4), necrosis (minimal to mild in 3/4), histiocytosis (mild in 4/4) |
| Inguinal | Depletion (moderate in 3/4), necrosis (minimal to mild in 2/4), histiocytosis (mild to moderate in 2/4) | Depletion (mild to moderate in 4/4), necrosis (mild 3/4), histiocytosis (mild to moderate in 3/4) |
| Mesenteric | Depletion (minimal to moderate in 3/4), necrosis (minimal to mild in 4/4), histiocytosis (mild to moderate in 2/4) | Depletion (minimal to mild in 4/4), necrosis (mild to moderate in 4/4), histiocytosis (mild to moderate in 4/4) |
| Tracheobronchial | Necrosis (moderate in 2/2) | Depletion (minimal to mild in 3/4), necrosis (minimal to mild in 3/4), histiocytosis (mild in 2/4) |
| Mandibular | Depletion (minimal to moderate in 3/3), necrosis (minimal to moderate in 2/3), histiocytosis (mild in 2/3) | Depletion (minimal to moderate in 4/4), necrosis (mild to moderate in 4/4), histiocytosis (minimal to moderate in 4/4) |
| Renal (kidney) | Lymphocytic/lymphohistiocytic inflammation (mild in 1/4) | Lymphocytic/lymphohistiocytic inflammation (mild in 2/4) |
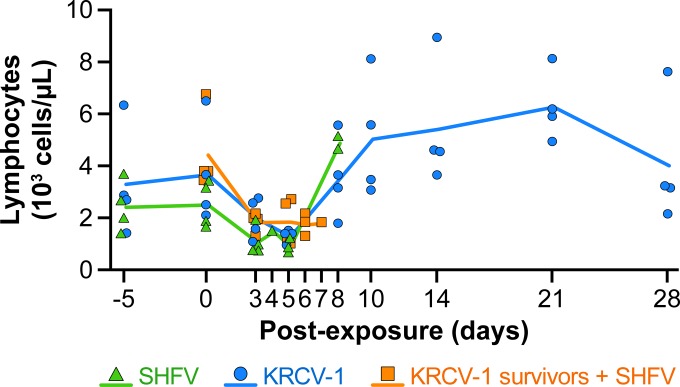
KRCV-1-infected cynomolgus macaques experience mild alterations in hematological and biochemical parameters without coagulopathy characteristic of SHF.
A modest reduction in absolute lymphocyte counts was observed in SHFV- and KRCV-1-infected macaques and KRCV-1 survivors infected with SHFV as early as day 3 postexposure (Fig. 4). Lymphocyte counts rebounded in animals surviving beyond day 7 postexposure to counts higher than those measured at baseline (days −5 and 0). The total lymphocyte counts were highest in the macaques that survived KRCV-1 infection.
FIG 4 .
Lymphocyte counts in macaques experimentally infected with simian arteriviruses. Average lymphocytes counts of cynomolgus macaques infected with simian hemorrhagic fever virus (SHFV, green line) or Kibale red colobus virus 1 (KRCV-1, blue line) and of KRCV-1 survivors infected with SHFV (orange line) were determined by analyzing blood samples. The values for individual animals are shown by symbols.
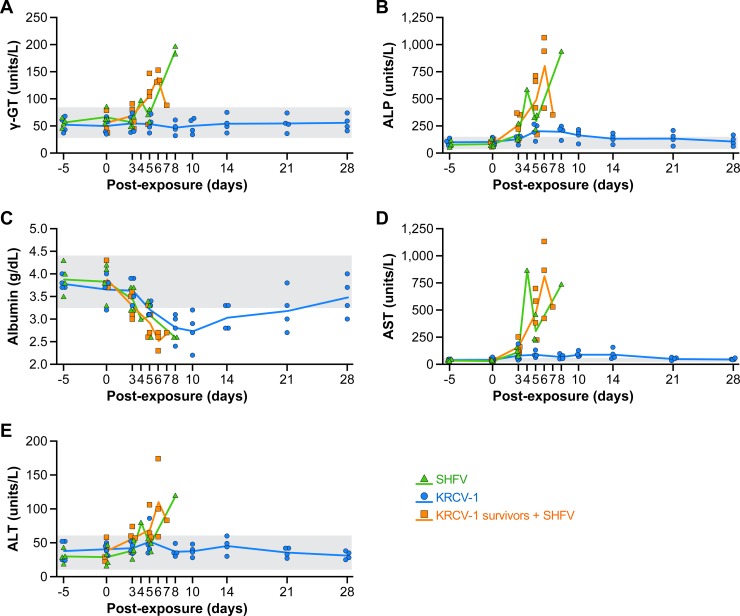
Comprehensive metabolic panels were performed throughout the study course to evaluate the effects of simian arterivirus infection on liver function (Fig. 5). SHFV infection (with or without prior KRCV-1 infection) generally led to higher serum concentrations of γ-glutamyltransferase (γ-GT), alanine transaminase (ALT), and in particular, aspartate transaminase (AST), and alkaline phosphatase (ALP) than in animals infected only with KRCV-1. Hypoalbuminemia was observed in all KRCV-1-infected animals. Whereas the maximum concentrations of serum enzymes and the drop in serum albumin are clinically relevant compared to individual baseline values, no definitive statistical comparison could be made between groups, as measurements were not consistently made at the same time points postexposure for all animals.
FIG 5 .
Effect of simian arterivirus infections on liver function. Sera from cynomolgus macaques infected with simian hemorrhagic fever virus (SHFV, green lines) or Kibale red colobus virus 1 (KRCV-1, blue lines) and from KRCV-1 survivors infected with SHFV (orange lines) were analyzed using a Piccolo point-of-care blood analyzer with the comprehensive metabolic panel disc for parameters including γ-glutamyltransferase (γ-GT) (A), alkaline phosphatase (ALP) (B), albumin (C), aspartate transaminase (AST) (D), and alanine transaminase (ALT) (E). Normal ranges were calculated as 2 standard deviations above and below pre-exposure averages of l baseline concentration from each animal (grey shading).
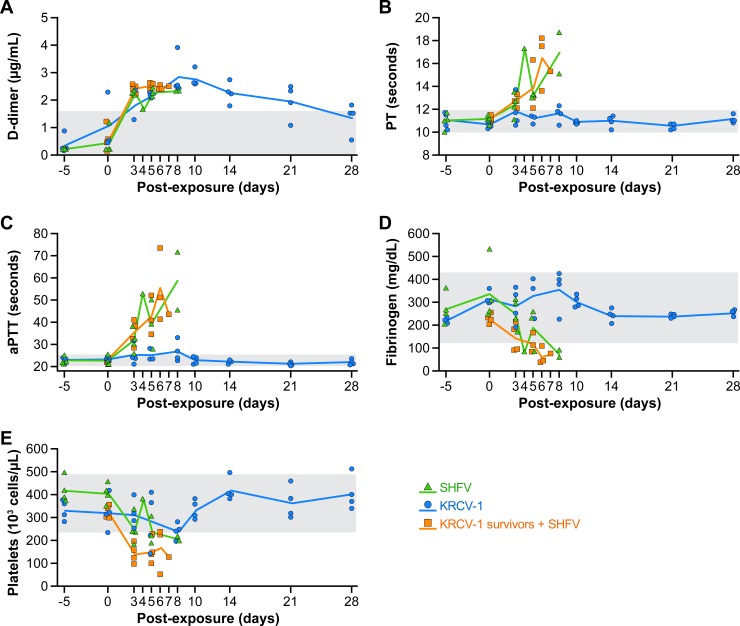
Next, we evaluated the development and severity of coagulopathy, a hallmark of SHF (7–9), including the characteristic disseminated intravascular coagulation (DIC). Fibrinogen and D-dimer concentrations were measured in serum samples to assess the consumption of fibrin precursors and generation of fibrin downstream degradation products, respectively. Platelet counts, prothrombin times (PT), and activated partial thromboplastin times (aPTT) were measured to evaluate functional changes in blood clotting (Fig. 6). With the exception of D-dimers, a contrast was found between SHFV- and KRCV-1-infected macaques. Specifically, SHFV-infected animals had increased D-dimer concentrations that coincided with a reduction in fibrinogen and increased PT and aPTT. Platelet counts declined over the course of disease, consistent with clinical observations. While KRCV-1 infection induced comparable increased concentrations of D-dimers, the fibrinogen concentrations and platelet counts stayed largely within baseline limits. Interestingly, though they were not as prominent as in SHFV-infected animals, increased aPTT and platelet counts could be measured in several KRCV-1-infected animals. One of these animals also presented with a day 8 PT exceeding the upper limit of the normal range. As with serum chemistries, statistical comparisons of trends were impossible due to the small number of animals and frequency of sampling. Together, these results confirm that KRCV-1 infection of cynomolgus macaques results in hematological and biochemical changes characteristic of a mild disease that has some features of SHF.
FIG 6 .
Functional changes in blood clotting parameters in cynomolgus macaques infected with simian arteriviruses. Sera/blood from cynomolgus macaques infected with simian hemorrhagic fever virus (SHFV, green lines), Kibale red colobus virus 1 (KRCV-1, blue lines), and KRCV-1 survivors infected with SHFV (orange lines) were analyzed for the following parameters: D-dimer concentrations (A); prothrombin times (PT) (B); activated partial thromboplastin times (aPTT) (C); fibrinogen concentrations (D); and platelet counts (E). Normal ranges were calculated as 2 standard deviations above and below pre-exposure averages of baselines from all animals (grey shading).
DISCUSSION
Simian hemorrhagic fever (SHF) is an almost uniformly lethal disease of macaques. Consequently, the possibility of an outbreak of this disease is a significant concern in macaque-holding facilities. Until recently, SHF was thought to be caused by infection with a single simian arterivirus, simian hemorrhagic fever virus (SHFV) (22). After we and others discovered numerous novel simian arteriviruses in apparently healthy African cercopithecids (17–20), we postulated that SHF may also be caused by accidental introduction of these viruses into macaque colonies. Indeed, our recent genomic surveillance studies revealed that at least two SHF outbreaks were not due to SHFV infection, as previously thought, but instead were due to infection with two previously unknown and distinct simian arteriviruses, Pebjah virus (PBJV) and simian hemorrhagic encephalitis virus (SHEV), respectively (6). However, other than SHFV, none of the other recently discovered simian arteriviruses had yet been cultured. To evaluate whether cercopithecid non-SHFV arteriviruses are able to cause disease in macaques, we isolated one of these viruses, Kibale red colobus virus 1 (KRCV-1), in cell culture. Using infected cells and cell culture supernatants, we demonstrated that KRCV-1 produces cellular changes and virions morphologically similar to those produced by SHFV and other arteriviruses (23–25).
The natural host reservoir of SHFV remains unknown, but most other known simian arteriviruses infect cercopithecine primates. KRCV-1, on the other hand, was found to infect red colobuses (Colobinae) (19). Given the high degree of genomic divergence of KRCV-1 from SHFV (50% at the nucleotide level [19]) and the fact that colobines and cercopithecines (which include macaques) are only very distantly related (26), we considered KRCV-1 an ideal candidate for the evaluation of our hypothesis that distinct simian arteriviruses could be pathogenic for Asian macaques. We therefore sought to evaluate whether exposure of cynomolgus macaques to KRCV-1 would result in clinical illness. We used SHFV exposure as a comparative control based on the well-characterized disease induced in macaques (4, 7–13).
As expected, SHFV-infected animals succumbed to a fulminant and fatal disease, consistent with previous reports (4, 7–13). Confirming our hypothesis, KRCV-1 proved to be pathogenic for macaques. However, in contrast to SHFV, KRCV-1 exposure only induced a mild and self-limiting disease. Similar to SHFV-infected macaques, KRCV-1-infected animals exhibited depression with associated recumbence, but in contrast to SHFV-infected animals, they remained responsive to study personnel. Reduced appetite and dehydration were observed but were less prominent than in SHFV-infected controls. The lymphocyte counts in KRCV-1-infected animals declined early in disease, similarly to SHFV-infected controls. In contrast to SHFV infection, KRCV-1 infection led to liver enzyme concentration increases that were only transient and unlikely to be clinically significant, although mild effects on liver function could not be excluded. Perhaps the survival of KRCV-1-infected animals can be attributed to the fact that they did not develop coagulopathy consistent with disseminated intravascular coagulation, as is typical for SHFV-infected macaques. D-dimer concentration increases, observed in both SHFV- and KRCV-1-infected animals, may be partially attributable to mild suppression of liver function that is responsible for clearance.
Alternatively, the relatively mild disease course in KRCV-1-infected macaques could be a result of dosing. We are unclear at this point whether a dose of KRCV-1 different (lower or higher) from the dose we used (109 genome/copies) might not lead to a disease course reminiscent of that caused by SHFV. The dose of KRCV-1 had to be picked in the absence of a cell line in which KRCV-1 can be quantified by plaque assay, without available data on the relationship between SHFV or KRCV-1 particle counts, genome copies, and PFU, and in the absence of SHFV- or KRCV-1-specific antibodies for fluorescence-based quantitation assays. In addition to raising and validating antibodies, in vitro screening studies will have to be performed to identify physiologically relevant (macaque macrophage-like) KRCV-1- and SHFV-susceptible cell types that can be cytopathically infected to compare the infection kinetics and determine the multiplicities of infection of both viruses side-by-side.
To evaluate whether previous KRCV-1 infection provides protection from SHFV infection, the KRCV-1 infection survivors were injected with SHFV within 60 days of disease resolution. As expected due to the significant genetic divergence and presumed antigenic dissimilarity of SHFV and KRCV-1, previous exposure to KRCV-1 provided no protection against subsequent SHFV infection. The disease progression and severity observed in these animals did not differ from those in KRCV-1-naive animals injected with SHFV, except that KRCV-1 infection survivors uniformly developed signs of neurological disease after SHFV infection, including body tremors and piloerection. Histologically, half of the KRCV-1 naive, SHFV-infected animals and a single KRCV-1-infected animal showed meningeal congestion or edema, respectively, suggesting neurological involvement in both groups. Macaques affected during historical SHF outbreaks sometimes presented with neurological signs. We speculate that, in addition to the possibility that etiological agents of SHF lead to different presentations with respect to neurological involvement, previous exposure to related simian arteriviruses may elicit altered disease phenotype(s).
Our data indicate that genetically distinct simian arteriviruses may cause diseases that differ substantially in severity. The fact that KRCV-1, a virus of colobine primates, proved to be pathogenic in cercopithecine macaques suggests that, in all likelihood, most or all simian arteriviruses can at least infect, if not cause disease, in macaques. This conclusion underscores the need for primate facilities to update their PCR- or serology-based pathogen screening methods to include all simian arteriviruses, rather than just SHFV. Furthermore, the existence of related simian arteriviruses with distinct pathological consequences for macaques provides an intriguing opportunity for understanding the molecular and immunological determinants of SHF pathogenesis and, by extrapolation, possibly those of other viral hemorrhagic fevers.
MATERIALS AND METHODS
Cells and viruses.
Simian hemorrhagic fever virus (SHFV) variant NIH LVR42-0/M6941 was obtained in 2011 from the American Type Culture Collection, Manassas, VA (ATCC VR-533). The SHFV animal exposure stock was grown in grivet (Chlorocebus aethiops) fetal kidney MA-104 C-1 cells (ATCC CRL-2378.1) as previously described (27) using Eagle’s minimal essential medium (EMEM; Lonza, Walkersville, MD) supplemented with 10% heat-inactivated fetal bovine serum (FBS; SAFC Biosciences, Lenexa, KS) at 37°C in a humidified 5% CO2 atmosphere. The genomic sequence of the prepared passage 3 virus stock was determined experimentally by deep sequencing (28) and was validated against the prototype sequence (GenBank AF180391.2).
Kibale red colobus virus 1 (KRCV-1) was isolated at the Wisconsin National Primate Research Center (WNPRC) in Madison, WI, from serum obtained from an apparently healthy but infected wild Kibale red colobus monkey (Procolobus [Piliocolobus] rufomitratus tephrosceles, animal RC01) (18). Primary leukocytes from bronchoalveolar lavage (BAL) fluid were obtained from a rhesus monkey (Macaca mulatta) by filtering BAL fluid through a 70-µm filter, followed by centrifugation at 530 × g for 5 min. BAL fluid leukocytes were resuspended in RPMI 1640 medium containing 10% fetal calf serum (FCS) (R10), and 100,000 leukocytes were plated out in each of 10 wells of a 96-well plate in a final volume of 180 µl. Next, 9 fourfold serial dilutions of serum from RC01 were prepared by starting with 40 µl of serum diluted into 120 µl of R10. Following dilution, 20 µl of each dilution was added to the appropriate wells of a 96-well plate containing 100,000 BAL fluid leukocytes in 180 µl of R10. Starting at 3 days postinoculation, cytopathic effects (CPE; continuous cell lysis) were observed in wells inoculated with serum diluted 1:256 to 1:4,096. Fifty microliters of BAL fluid leukocyte culture supernatant from day 3 postinoculation was used to inoculate nearly confluent MARC-145 cells in a 6-well plate in 2 ml of minimal essential medium supplemented with 10% FCS (MEM10). These cultures were sampled regularly for virus production by removal of 200 µl of supernatant and replacement with fresh medium. After 18 days in culture, filamentous material accumulated in the cytoplasm of MARC-145 cells inoculated with BAL fluid supernatant. After 26 days in culture, 50 µl of MARC-145 culture supernatant was used to inoculate a new MARC-145 culture in a 6-well plate containing 2 ml of MEM10. CPE appeared after 7 days in this passage. Fifty microliters was again passaged onto a final MARC-145 culture in 2 ml of MEM10. In this culture, CPE appeared after 4 days postinoculation. All cultures were incubated at 37°C and 5% CO2. Fifty microliters of supernatant from the final MARC-145 cell culture was used to inoculate rhesus monkey peripheral blood mononuclear cells (PBMCs) by spinoculation. One million PBMCs were suspended in 0.45 ml of R10 with 10% FCS and 10 ng/ml of macrophage colony-stimulating factor (M-CSF), mixed with 50 µl of MARC-145 cell supernatant, and spun at 1,200 × g at 4°C for 1 h. Inoculated cells were then resuspended and transferred to a tissue culture plate with an additional 0.5 ml of R10 containing 10 ng/ml of M-CSF. Cells were incubated at 37°C and 5% CO2 for 3 days, at which point supernatant was collected. One hundred fifty microliters of this supernatant was again spinoculated (as described above) onto 1 million rhesus monkey PBMCs cultured in R10. On day 3 postinoculation of this culture, supernatant was collected and passaged for a third time onto PBMCs. For the final expansion of KRCV-1, 23 µl of PBMC passage 2 supernatant (2 × 107 viral RNA copies/ml) was spinoculated onto each of 14 aliquots of 1 million rhesus monkey PBMCs per tube (in 500 µl of R10). Once resuspended, the spinoculated cells were added to a culture containing an additional 90 million PBMCs at a concentration of 2 million cells/ml in R10. On day 3, the entire culture was harvested, cells were pelleted, the supernatant was filtered through a 0.2-µm filter, and the resulting stock was cryopreserved in 1-ml aliquots.
SHFV and KRCV-1 animal exposure stocks were judged sterile after negative blood agar streaks and the absence of mycoplasma with Mycosensor (Agilent Technologies, Santa Clara, CA), and detection of endotoxins in the normal range using the limulus test (Endosafe-PTS; Charles River, Wilmington, MA). In addition, inoculation of grivet Vero E6 (ATCC CRL-1586) cells under MA-104 growth conditions (SHFV and KRCV-1 only grow in MA-104 or MA-104-derived cells) with such exposure stocks did not result in CPE. The titers of SHFV stocks were determined by plaque assay on MA-104 cells as previously described (9, 27). KRCV-1 quantification was performed only by quantitative reverse transcriptase PCR (qRT-PCR), since KRCV-1 did not cause CPE in MA-104 or MARC-145 cells. Briefly, viral RNA was reverse transcribed and quantified using the SuperScript III one-step qRT-PCR system (Invitrogen, Carlsbad, CA) on a LightCycler 480 (Roche, Indianapolis, IN). Reverse transcription was carried out as follows: 37°C for 15 min, 50°C for 30 min, a 2-min activation at 95°C, and 50 cycles of amplification of 95°C for 15 s and 60°C for 1 min. The reaction mixture contained MgSO4 at a final concentration of 3.0 mM, 150 ng of random primers (Promega), amplification primers at a concentration of 600 nM (5′-ACACGGCTACCCTTACTCC-3′ and 5′-TCGAGGTTAARCGGTTGAGA-3′), and probe (5′-Quasar-670-TTCTGGTCCTCTTGCGAAGGC-black hole quencher 2 [BHQ2]-3′) at a concentration of 100 nM.
Electron microscopy.
Electron microscopy was performed as previously described (27). MARC-145 cells were infected with KRCV-1 particles for 20 days at 37°C. For negative-staining analysis, cell culture supernatant was collected, clarified, and direct pelleted by ultracentrifugation. The resulting pellets were preserved and inactivated in 1.0% paraformaldehyde (E.M. Sciences, Warrington, PA) prior to resuspending in Millonig’s sodium phosphate buffer (Tousimis Research, Rockville, MD). A 2.0-µl aliquot of concentrated supernatant was mounted on 300-mesh copper grids and stained with 1.0% phosphotungstic acid (E.M. Sciences). For conventional thin-section microscopic evaluation, infected MARC-145 cells and direct-pelleted viral supernatants were preserved and inactivated for 2 h in 2.5% glutaraldehyde (E.M. Sciences) and 2.0% paraformaldehyde (E.M. Sciences) in Millonig’s sodium phosphate buffer. Samples were scraped and pelleted, washed repeatedly in Millonig’s buffer, and incubated for 2 h in 1.0% osmium tetroxide. Following rinsing steps in ultrapure water and en bloc staining with 2.0% uranyl acetate, the samples were dehydrated in a series of graded ethanols and infiltrated and embedded in DER-736 plastic resin. Embedded blocks were sectioned using a Reichert-Jung Ultracut E ultramicrotome (Reichert Technologies, Depew, NY). Sections 50 to 70 nm in thickness were collected on 200-mesh copper grids and poststained with Reynold’s lead citrate. Samples were examined in a Tecnai spirit twin transmission electron microscope (TEM; FEI, Hillsboro, OR), operating at 80 kV.
Inoculations.
Eight male cynomolgus macaques (Macaca fascicularis), 7 to 9 years old and weighing 5.95 to 8.7 kg, were obtained from WNPRC. Prior to study inclusion, all animals were serologically screened prior to facility entry for simian immunodeficiency virus (SIV), simian T-lymphotropic virus (STLV), and simian retrovirus infection; the animals were negative. The animals were tested multiple times and were negative for Mycobacterium tuberculosis infection. The animals also underwent physical exams and routine bloodwork and were confirmed appropriate for study assignment by veterinarians at the Integrated Research Facility at Frederick, MD (IRF-Frederick).
Virus inocula were prepared by serial dilution in sterile phosphate-buffered saline (pH 7.4) to achieve the target concentration in a 1-ml volume and injected i.m. in the quadriceps of the right leg of each anesthetized animal in dorsal recumbency. The animals each received a target dose of 1,000 PFU of SHFV (equivalent to 106 genome copies) or 109 genome copies of KRCV-1. The animals that survived KRCV-1 infection were injected with 1,000 PFU of SHFV. Inoculated animals were monitored on a daily basis for clinical signs and received periodic physical exams that included blood draws and urinalysis. Experimental animals were evaluated for study endpoint criteria based on a 10-point clinical score scale (0 = normal) divided into five broad categories (A, overall clinical appearance and signs of hemorrhage; B, respiratory rate, mucous membrane color, and dyspnea; C, recumbency; D, nonresponsiveness; and E, core temperature of anesthetized animal). A score of 10 points in any of the five categories or lower scores that added up to 10 points in multiple categories required immediate euthanasia.
Cynomolgus macaques were housed in a biosafety level 4 (BSL-4) containment facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). Experimental procedures were approved by the National Institute of Allergy and Infectious Diseases (NIAID), Division of Clinical Research (DCR), Animal Care and Use Committee (ACUC), and were in compliance with the Animal Welfare Act regulations (29), Public Health Service policy (30), and the Guide for the Care and Use of Laboratory Animals recommendations (31).
Serology and clinical chemistry.
Complete blood counts, including leukocyte differentials (CBC/diff), were determined from blood samples collected in EDTA-coated blood tubes and analyzed using a Sysmex XT2000V (Sysmex America, Mundelein, IL). Biochemical analyses of serum samples collected in red-top Vacutainers were performed using the Roche COBAS Integra 400 plus analyzer (Roche Diagnostics, Indianapolis, IN). For coagulation studies, blood was collected in 3.2% sodium citrate-coated vacutainers and processed according to the manufacturer’s recommendations. Plasma from the citrate-coated tubes was analyzed on a STA Compact analyzer (Diagnostica Stago, Parsippany, NJ) for activated partial thromboplastin time (aPTT) and prothrombin time (PT) and for fibrinogen and D-dimer concentrations.
Necropsy.
Necropsies were performed after euthanasia (7 animals) or after animals were found dead in their cages (1 animal). Tissue samples were collected from all major organs for histopathological analysis and determination of viral loads. Following fixation in 10% neutral buffered formalin, tissues were trimmed into 5-mm-thick sections and placed in cassettes. The tissues were then dehydrated through a series of graded alcohols, cleared in an organic solvent, and infiltrated with molten paraffin by using the Sakura Tissue Tek VIP tissue processor (VIP 6-a1; Sakura). After processing, tissues were placed into molds and embedded in paraffin (Paraplast Plus; McCormick). The resulting blocks were sectioned to 4 to 6 µm thickness using a rotary microtome and floated on a 48°C water bath before placement on Superfrost plus gold slides (catalog number 15-188-48; Fisher). The sections were then baked for 20 min at 60°C, and a hematoxylin-and-eosin (H&E) stain was applied using the Leica automated staining system (ST5020 multistainer; Leica). Stained slides were then examined via standard light microscopy.
Statistical analysis.
Survival differences between animal groups were determined using a log rank test. Significant differences in log10 viral load between animal groups were assessed using the unpaired two sample t test at day 3 postexposure.
ACKNOWLEDGMENTS
The content of this publication does not necessarily reflect the views or policies of the U.S. Department of Health and Human Services or the institutions and companies affiliated with the authors.
We thank Cassandra Lyons, Cindy Allan, and Ricky Adams of the IRF-Frederick for experimental support and are grateful to Laura Bollinger and Jiro Wada of the IRF-Frederick for critically editing the manuscript and creating figures, respectively.
Funding Statement
This work was funded in part through Battelle Memorial Institute’s prime contract with the US National Institute of Allergy and Infectious Diseases (NIAID) under Contract No. HHSN272200700016I. J.C.J. performed this work as an employee of Battelle Memorial Institute. Subcontractors to Battelle Memorial Institute who performed this work are: O.R., R.B., D.R.R., L.H., employees of Charles River Laboratories; V.W-J., J.G.B, Y.C., H.B.S., and J.H.K, employees of Tunnell Government Services, Inc.; M.C., an employee of Lovelace Respiratory Research Institute; and E.Z. and S.M., employees of MRIGlobal Research Institute. This study was also funded, in part, by the NIAID Division of Intramural Research (R.F.J., P.B.J.). The work of M.L., J.T.W., L.H.M., A.M.W., O.C., A.L.B., T.L.G., D.H.O’C., and T.C.F. was supported in part by the Wisconsin National Primate Research Center Base Grant from the National Center for Research Resources (P51 RR000167) and the Office of Research Infrastructure Programs (P51 OD011106) of the National Institutes of Health (NIH); NIH grant R01AI116382; NIH grant TW009237 as part of the joint NIH-NSF Ecology of Infectious Disease program, grant R01 AI077376; and by the Office of Research Infrastructure Programs (ORIP) grant P51OD011106. A.L.B. was additionally supported via a National Research Service Award (NRSA) through the Microbes in Health and Disease (MHD) training program at the University of Wisconsin (T32 AI55397) and University of Wisconsin’s Medical Scientist Training Program (MSTP) grant T32 GM008692.
Footnotes
Citation Wahl-Jensen V, Johnson JC, Lauck M, Weinfurter JT, Moncla LH, Weiler AM, Charlier O, Rojas O, Byrum R, Ragland DR, Huzella L, Zommer E, Cohen M, Bernbaum JG, Caì Y, Sanford HB, Mazur S, Johnson RF, Qin J, Palacios GF, Bailey AL, Jahrling PB, Goldberg TL, O’Connor DH, Friedrich TC, Kuhn JH. 2016. Divergent simian arteriviruses cause simian hemorrhagic fever of different severity in macaques. mBio 7(1):e02009-15. doi:10.1128/mBio.02009-15.
REFERENCES
- 1.Lapin BA, Pekerman SM, Yakovleva LA, Dzhikidze EK, Shevtsova ZV, Kuksova MI, Dan’ko LV, Krylova RI, Akbroit E, Agrba VZ. 1967. Hemorrhagic fever in monkeys. Vopr Virusol 12:168–173. (In Russian.). [PubMed] [Google Scholar]
- 2.Palmer AE, Allen AM, Tauraso NM, Shelokov A. 1968. Simian hemorrhagic fever. I. Clinical and epizootiologic aspects of an outbreak among quarantined monkeys. Am J Trop Med Hyg 17:404–412. [PubMed] [Google Scholar]
- 3.Shevtsova ZV. 1967. Study of the etiology of hemorrhagic fever in monkeys. Vopr Virusol 12:47–51. (In Russian.) [PubMed] [Google Scholar]
- 4.Shevtsova ZV, Kuksova MI, Dzhikidze EK, Krylova RI, Dan’ko LV. 1966. Experimental studies of hemorrhagic fever of monkeys, p 146–150. In Lapin BA. (ed), Biology and pathology of monkeys, studies of human diseases in experiments on monkeys. Materials of Symposium in Sukhumi, October 17–22 Academy of Medical Sciences of the USSR, Institute of Experimental Pathology and Therapy, Tbilisi, Georgian SSR, USSR. (In Russian.) [Google Scholar]
- 5.Shevtsova ZV. 1969. A further study of simian hemorrhagic fever virus. Vopr Virusol 14:604–607. (In Russian.) [PubMed] [Google Scholar]
- 6.Lauck M, Alkhovsky SV, Bào Y, Bailey AL, Shevtsova ZV, Shchetinin AM, Vishnevskaya TV, Lackemeyer MG, Postnikova E, Mazur S, Wada J, Radoshitzky SR, Friedrich TC, Lapin BA, Deriabin PG, Jahrling PB, Goldberg TL, O’Connor DH, Kuhn JH. 2015. Historical outbreaks of simian hemorrhagic fever in captive macaques were caused by distinct arteriviruses. J Virol 89:8082–8087. doi: 10.1128/JVI.01046-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen AM, Palmer AE, Tauraso NM, Shelokov A. 1968. Simian hemorrhagic fever. II. Studies in pathology. Am J Trop Med Hyg 17:413–421. [DOI] [PubMed] [Google Scholar]
- 8.Vatter HA, Donaldson EF, Huynh J, Rawlings S, Manoharan M, Legasse A, Planer S, Dickerson MF, Lewis AD, Colgin LM, Axthelm MK, Pecotte JK, Baric RS, Wong SW, Brinton MA. 2015. A simian hemorrhagic fever virus isolate from persistently infected baboons efficiently induces hemorrhagic fever disease in Japanese macaques. Virology 474:186–198. doi: 10.1016/j.virol.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson RF, Dodd LE, Yellayi S, Gu W, Cann JA, Jett C, Bernbaum JG, Ragland DR, St Claire M, Byrum R, Paragas J, Blaney JE, Jahrling PB. 2011. Simian hemorrhagic fever virus infection of rhesus macaques as a model of viral hemorrhagic fever: clinical characterization and risk factors for severe disease. Virology 421:129–140. doi: 10.1016/j.virol.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krylova RI, Shevtsova ZV. 1969. Pathomorphology of experimental hemorrhagic fever in monkeys. Arkh Patol 31:65–69. (In Russian.) [PubMed] [Google Scholar]
- 11.Abildgaard C, Harrison J, Espana C, Spangler W, Gribble D. 1975. Simian hemorrhagic fever: studies of coagulation and pathology. Am J Trop Med Hyg 24:537–544. [DOI] [PubMed] [Google Scholar]
- 12.Giddens WE Jr, Mayer LA, Jessup D, Schmer G. 1978. Studies on simian hemorrhagic fever, p 75–77. In Chivers DJ, Ford EHR (ed), Recent advances in primatology, vol 4: Medicine. Academic Press, New York, NY. [Google Scholar]
- 13.Renquist D. 1990. Outbreak of simian hemorrhagic fever. J Med Primatol 19:77–79. [PubMed] [Google Scholar]
- 14.Tauraso NM, Shelokov A, Palmer AE, Allen AM. 1968. Simian hemorrhagic fever. 3. Isolation and characterization of a viral agent. Am J Trop Med Hyg 17:422–431. [PubMed] [Google Scholar]
- 15.Lapin BA, Shevtsova ZV. 1971. On the identity of two simian hemorrhagic fever virus strains (Sukhumi and NIH). Z Versuchstierkd 13:21–23. [PubMed] [Google Scholar]
- 16.Tauraso NM, Shelokov A, Allen AM, Palmer AE, Aulisio CG. 1968. Epizootic of simian haemorrhagic fever. Nature 218:876–877. doi: 10.1038/218876a0. [DOI] [Google Scholar]
- 17.Bailey AL, Lauck M, Sibley SD, Pecotte J, Rice K, Weny G, Tumukunde A, Hyeroba D, Greene J, Correll M, Gleicher M, Friedrich TC, Jahrling PB, Kuhn JH, Goldberg TL, Rogers J, O’Connor DH. 2014. Two novel simian arteriviruses in captive and wild baboons (Papio spp.). J Virol 88:13231–13239. doi: 10.1128/JVI.02203-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailey AL, Lauck M, Weiler A, Sibley SD, Dinis JM, Bergman Z, Nelson CW, Correll M, Gleicher M, Hyeroba D, Tumukunde A, Weny G, Chapman C, Kuhn JH, Hughes AL, Friedrich TC, Goldberg TL, O’Connor DH. 2014. High genetic diversity and adaptive potential of two simian hemorrhagic fever viruses in a wild primate population. PLoS One 9:e90714. doi: 10.1371/journal.pone.0090714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lauck M, Hyeroba D, Tumukunde A, Weny G, Lank SM, Chapman CA, O’Connor DH, Friedrich TC, Goldberg TL. 2011. Novel, divergent simian hemorrhagic fever viruses in a wild Ugandan red colobus monkey discovered using direct pyrosequencing. PLoS One 6:e19056. doi: 10.1371/journal.pone.0019056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lauck M, Sibley SD, Hyeroba D, Tumukunde A, Weny G, Chapman CA, Ting N, Switzer WM, Kuhn JH, Friedrich TC, O’Connor DH, Goldberg TL. 2013. Exceptional simian hemorrhagic fever virus diversity in a wild African primate community. J Virol 87:688–691. doi: 10.1128/JVI.02433-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myers MG, Vincent MM, Hensen SA, Tauraso NM. 1972. Problems in the laboratory isolation of simian hemorrhagic fever viruses and isolation of the agent responsible for the Sussex/69 epizootic. Appl Microbiol 24:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lapin BA, Shevtsova ZV. 2015. To the 50th anniversary of the discovery of the simian hemorrhagic fever and SHF virus. Vopr Virusol 60:5–11. (In Russian.) [PubMed] [Google Scholar]
- 23.Knoops K, Bárcena M, Limpens RW, Koster AJ, Mommaas AM, Snijder EJ. 2012. Ultrastructural characterization of arterivirus replication structures: reshaping the endoplasmic reticulum to accommodate viral RNA synthesis. J Virol 86:2474–2487. doi: 10.1128/JVI.06677-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedersen KW, van der Meer Y, Roos N, Snijder EJ. 1999. Open reading frame 1a-encoded subunits of the arterivirus replicase induce endoplasmic reticulum-derived double-membrane vesicles which carry the viral replication complex. J Virol 73:2016–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood O, Tauraso N, Liebhaber H. 1970. Electron microscopic study of tissue cultures infected with simian haemorrhagic fever virus. J Gen Virol 7:129–136. doi: 10.1099/0022-1317-7-2-129. [DOI] [PubMed] [Google Scholar]
- 26.Perelman P, Johnson WE, Roos C, Seuánez HN, Horvath JE, Moreira MA, Kessing B, Pontius J, Roelke M, Rumpler Y, Schneider MP, Silva A, O’Brien SJ, Pecon-Slattery J. 2011. A molecular phylogeny of living primates. PLoS Genet 7:e1001342. doi: 10.1371/journal.pgen.1001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caì Y, Postnikova EN, Bernbaum JG, Yú SQ, Mazur S, Deiuliis NM, Radoshitzky SR, Lackemeyer MG, McCluskey A, Robinson PJ, Haucke V, Wahl-Jensen V, Bailey AL, Lauck M, Friedrich TC, O’Connor DH, Goldberg TL, Jahrling PB, Kuhn JH. 2015. Simian hemorrhagic fever virus cell entry is dependent on CD163 and uses a clathrin-mediated endocytosis-like pathway. J Virol 89:844–856. doi: 10.1128/JVI.02697-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lauck M, Palacios G, Wiley MR, Li Y, Fāng Y, Lackemeyer MG, Caì Y, Bailey AL, Postnikova E, Radoshitzky SR, Johnson RF, Alkhovsky SV, Deriabin PG, Friedrich TC, Goldberg TL, Jahrling PB, O’Connor DH, Kuhn JH. 2014. Genome sequences of simian hemorrhagic fever virus variant NIH LVR42-0/M6941 isolates (Arteriviridae: Arterivirus). Genome Announc 2:e00978-14. doi: 10.1128/genomeA.00978-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.United States Department of Agriculture. 2013. USDA Animal Welfare Act https://www.gpo.gov/fdsys/pkg/USCODE-2013-title7/pdf/USCODE-2013-title7-chap54.pdf.
- 30.National Institutes of Health 2015. Public Health Service policy on humane care and use of laboratory animals Office of; Laboratory: Animal Welfare, National Institutes of Health, Bethesda, MD. http://grants.nih.gov/grants/olaw/references/phspolicylabanimals.pdf. [Google Scholar]
- 31.National Research Council of the National Academies 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC: https://www.aaalac.org/resources/Guide_2011.pdf [Google Scholar]