Abstract
Background
The World Health Organization recommends malaria to be confirmed by either microscopy or a rapid diagnostic test (RDT) before treatment. The correct use of RDTs in resource-limited settings facilitates basing treatment onto a confirmed diagnosis; contributes to speeding up considering a correct alternative diagnosis, and prevents overprescription of anti-malarial drugs, reduces costs and avoids unnecessary exposure to adverse drug effects. This review aims to evaluate health workers’ compliance to RDT results and factors contributing to compliance.
Methods
A PROSPERO-registered systematic review was conducted to evaluate health workers’ compliance to RDTs in sub-Saharan Africa, following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Studies published up to November 2015 were searched without language restrictions in Medline/Ovid, Embase, Cochrane Central Register of Controlled Trials, Web of Science, LILACS, Biosis Previews and the African Index Medicus. The primary outcome was health workers treating patients according to the RDT results obtained.
Results
The literature search identified 474 reports; 14 studies were eligible and included in the quantitative analysis. From the meta-analysis, health workers’ overall compliance in terms of initiating treatment or not in accordance with the respective RDT results was 83 % (95 % CI 80–86 %). Compliance to positive and negative results was 97 % (95 % CI 94–99 %) and 78 % (95 % CI 66–89 %), respectively. Community health workers had higher compliance rates to negative test results than clinicians. Patient expectations, work experience, scepticism of results, health workers’ cadres and perceived effectiveness of the test, influenced compliance.
Conclusions
With regard to published data, compliance to RDT appears to be generally fair in sub-Saharan Africa; compliance to negative results will need to improve to prevent mismanagement of patients and overprescribing of anti-malarial drugs. Improving diagnostic capacity for other febrile illnesses and developing local evidence-based guidelines may help improve compliance and management of negative RDT results.
Trial registration: CRD42015016151 (PROSPERO)
Electronic supplementary material
The online version of this article (doi:10.1186/s12936-016-1218-5) contains supplementary material, which is available to authorized users.
Keywords: Malaria, Rapid diagnostic test (RDT), Health workers, Sub-Saharan Africa, Plasmodium falciparum, Clinical decision making, Adherence, Compliance
Background
Plasmodium falciparum malaria is estimated to have caused 528,000 deaths and 163 million clinical episodes in sub-Saharan Africa in 2013 [1]. Early diagnosis and treatment with appropriate anti-malarial drugs can prevent severe illness and lethal outcome [2, 3]. Artemisinin-based combination therapy (ACT) is currently recommended for the treatment of uncomplicated malaria caused by P. falciparum [3, 4] and is increasingly used for non-falciparum malaria [5]. Effective case-management of malaria consists of an efficacious treatment, prompt access to treatment and diagnosis, provider compliance to treatment guidelines, and patient adherence to medication [3, 6] (Fig. 1).
Fig. 1.
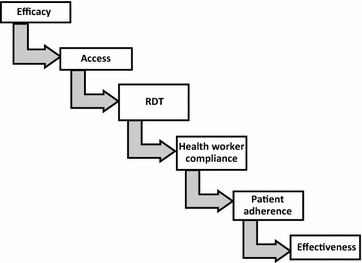
Pathway of health systems effectiveness of malaria diagnosis and treatment. (Adapted from MalERA consultative group) [6]
Presumptive diagnosis and treatment of malaria based on symptoms leads to over- diagnosis of malaria and missed diagnosis for patients without malaria [7, 8]. The World Health Organization (WHO) recommends that any suspected malaria case in any epidemiological setting should be parasitologically-confirmed by either microscopy or rapid diagnostic test (RDT) before treatment [3]. Lack of trained personnel, equipment [9], and reagents for microscopy in most remote rural areas in Africa [10, 11] with high malaria burden makes the RDT the most practically suitable tool to confirm a malaria diagnosis [12, 13]. RDTs are immunochromatographic test kits which confirm the presence of malaria parasites in suspected patients by detecting one or a combination of the following three Plasmodium antigens: Plasmodium histidine-rich protein (HRP) 2 (pHRP-2) for P. falciparum or a ‘pan-specific’ aldolase to detect other species, such as P. vivax or Plasmodium lactate dehydrogenase (LDH) variants (pLDH) (with clonality specific to the various Plasmodium species infecting humans) [14, 15].
The use of malaria RDT can reduce over-prescribing of anti-malarial drugs (AMD). Studies have shown that in most endemic countries in sub-Saharan Africa, health workers of different cadres do not comply with malaria RDTs; they prescribe AMDs to patients with RDT negative results [13, 16–18]. This has implications on resources for patient, family members and health system since some drug combinations are relatively expensive [19–21]. Non-compliance to malaria negative results by prescribing AMDs neglects underlying cause of fever and expose patients unnecessarily to adverse effects; underlying infections, such as sepsis, pneumonia and meningitis [22–25], present as malaria clinically but are not routinely investigated [26] and may not be treated [10, 27].
To treat malaria effectively, to reduce costs and avoid unnecessary exposure to drug adverse effects, there is a need to correctly diagnose and comply with malaria treatment guidelines or clinical decision algorithms. Health workers (HWs) need to use the correct treatment based on the RDT results.
This systematic review examines data available on HWs compliance to RDT results in sub-Saharan Africa, and investigates factors associated with compliance to results (HW treating patients according to the RDT result). The primary outcome is the percentage of HWs compliant to overall, positive or negative, test results.
Methods
This systematic review was registered in advance in the International prospective register of systematic reviews (PROSPERO; registration number CRD42015016151) which included pre-specified the objectives and inclusion criteria [28].
An experienced information specialist (RS) conducted a search without language or time restrictions in the online electronic databases Ovid Medline, Ovid Embase, Cochrane Central Register of Controlled Trials, CINAHL Plus with Full Text, African Index Medicus, and African Journals Online (AJOL). The search used both free text words and medical subject headings for ‘malaria’, ‘RDT’, ‘health worker’ and ‘compliance’. The search was conducted on 3 March 2015 and updated on 12 November 2015. Studies reporting on malaria suspected patients of any age presenting to HWs of any cadre in sub-Saharan Africa were searched. The intervention was the use of a WHO recommended RDT kit for parasitological confirmation of a malaria diagnosis (a list of WHO recommended RDTs is available online [29]).
Bibliographies of relevant studies retrieved from the studies were checked for additional publications. The search strategy is described in Additional file 1. EndNote X7.4 (Thomson Reuters) was used to manage, de-duplicate and screen the references for eligibility. The inclusion criteria were: studies were conducted in sub-Saharan Africa; RDTs were used to diagnose malaria in symptomatic patients; the RDTs used were WHO-recommended; absolute numbers of RDT result adherence as primary or secondary outcome were reported. Exclusion criteria were: studies using RDT for active case finding and population screening; conference abstracts; no absolute numbers were reported; studies outside sub-Saharan Africa. Eligibility assessment of studies was performed independently in a blinded, standardized way by two reviewers (ANK and BJV). Titles and abstracts were screened first, and the two reviewers screened and selected relevant full-text articles. ANK extracted quantitative data based on the pre-specified criteria into an excel sheet (Additional file 2); factors associated with compliance were also extracted into the same sheet. All the quantitative data was independently checked by BJV. Data extracted included author name, year of publication, place of study, transmission setting, type of RDT, cadre and number of HW, age of patients, number of test results, RDT positives treated and RDT negatives not treated. Both qualitative and quantitative factors were also extracted from included studies which reported them. The risk of bias of studies was not assessed because of the diversity of the study designs included.
The primary outcome measure was proportions in percentage of RDT results with appropriate AMD prescription disaggregated to positive and negative results adherence. Appropriate treatment was defined as AMDs prescribed to RDT positive and AMD not prescribed to RDT negative patients (Fig. 2). Formulae for these calculations are included in Additional file 3. STATA version 13 (StataCorp, College Station, TX, USA) was used to calculate the pooled estimate of proportions appropriately treated overall and negative and positive compliance using random effects. Random effects analysis was used after an initial fixed effect analysis had I2 above 50 %, suggesting heterogeneity. Pooled estimates were also stratified by health personnel cadre, age of patients and malaria transmission setting. A qualitative synthesis of factors contributing to compliance was also reported for the included studies.
Fig. 2.
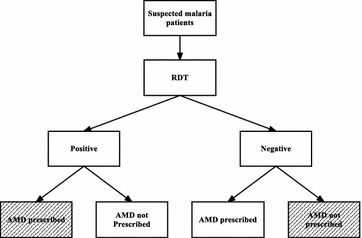
Patient pathway for malaria diagnosis and treatment. The shaded areas represent appropriate management (RDT rapid diagnostic test, AMD anti-malarial drug)
Results
Study selection
The total number of articles after removing duplicates was 474 (Fig. 3). After screening title and abstracts for eligibility, 75 full-text articles were examined for eligibility; 14 studies were included in the quantitative analysis [7, 16–18, 30–39]. Five of the studies reported on factors associated with compliance to RDT results and were included in the summary of associated factors [7, 17, 30, 31, 36].
Fig. 3.
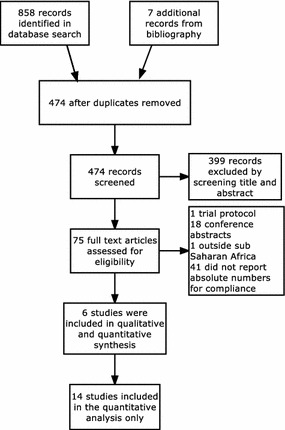
Study selection flow (PRISMA)
There were five study designs (Table 1): one randomized control trial [31], four observational [32–34, 38], four cross-sectional [17, 18, 36, 37], four cluster randomized trials [7, 16, 30, 35] and pre-post intervention study [39]. RDT adherence was a secondary outcome in five out of the 14 studies [17, 31, 35, 37, 39].
Table 1.
Characteristics of studies included
| Authors | Year | Country | Study setting | Study design | HW cadre | Number of HWs | Age of study participants | RDT | Sample size |
|---|---|---|---|---|---|---|---|---|---|
| Bisoffi [31] | 2009 | Burkina Faso | Stable malaria with seasonal transmission | RCT | Nurses | NR | >6 months | Paracheck Pf | 1050 |
| Masanja [33] | 2010 | Tanzania | Holoendemic | Observational | Clinicians | 99 | >5 years | ParaHIT | 10,650 |
| Bottieau [32] | 2013 | Mozambique | Perennial transmission with seasonal peaks | Observational | Clinicians | NR | All | Paracheck Pf; ICT malaria Pf; SD Bioline Pf | 1385 |
| Manyando [38] | 2014 | Zambia | Both low and high transmission | Observational | Clinicians | NR | <5 years | ICT malaria Pf | 1492 |
| Chinkhumba [37] | 2010 | Malawi | Stable malaria with seasonal peak | Cross sectional | Clinicians and nurses | NR | >5 years | ICT malaria pf; SD Bioline; Paracheck Pf; First Response | 1390 |
| Uzochukwu [36] | 2011 | Nigeria | High transmission | Cross sectional | Clinicians, nurses and CHW | 32 | All | ICT malaria Pf | 280 |
| Mubi [17] | 2013 | Tanzania | Perennial transmission | Cross sectional | Clinicians and nurses | 20 | >3 months | NR | 105 |
| Shakely [18] | 2013 | Zanzibar | Low transmission | Cross sectional | Clinicians and nurses | 33 | All | Paracheck Pf | 3889 |
| Batwala [30] | 2011 | Uganda | Both low and high transmission | CRT | Clinical officers and nurses | 30 | All | Paracheck Pf | 44,565 |
| Mukanga [35] | 2012 | Ghana, Uganda | Seasonal | CRT | CHW | 44 | 4–59 months | Paracheck Pf; ICT malaria Pf | 1559 |
| Mbacham [16] | 2014 | Cameroon | NR | CRT | Clinicians | 198 | All | SD Bioline | 1194 |
| Bastiaens [39] | 2011 | Tanzania | NR | Before and after | Clinical officers | NR | Below 10 year olds | ICT malaria Pf; Paracheck Pf | 501 |
| Mbonye [7] | 2015 | Uganda | Perennial transmission | CRT | DSV | 10 | All | First response | 8073 |
| Mukanga [34] | 2011 | Uganda | High transmission | Observational | CHW | 14 | Under 5 years | NR | 182 |
CHW community health worker, CRT cluster randomized trial, DSV drug shop vendor, NR Not reported, RCT randomized control trial
Health workers’ compliance to malaria results
A pooled meta-analysis using random effects (Fig. 4) for the 14 studies [7, 16–18, 30, 31, 33–40] shows an overall compliance of 83 % (95 % CI 80–86 %); I2 = 99.9 %, Z = 54.35, p < 0.001. Appropriate malaria treatment based on RDT results (Table 2) was as low as 39.7 % in a Zambian study [38] to as high as 99.9 % in Zanzibar [18]. The pooled meta-analysis result using random effects for RDT positives prescribed AMDs (Fig. 5) was 97 % (95 % CI 94–99 %); I2 = 99.2 %, Z = 37.31, p < 0.001. The proportion of positive RDT results prescribed AMDs ranged from 72.1 to 100 %. 12 studies reported appropriate prescription of AMDs to RDT positive patients above 93 %; six of these studies had 100 % RDT positive compliance (Table 2).
Fig. 4.
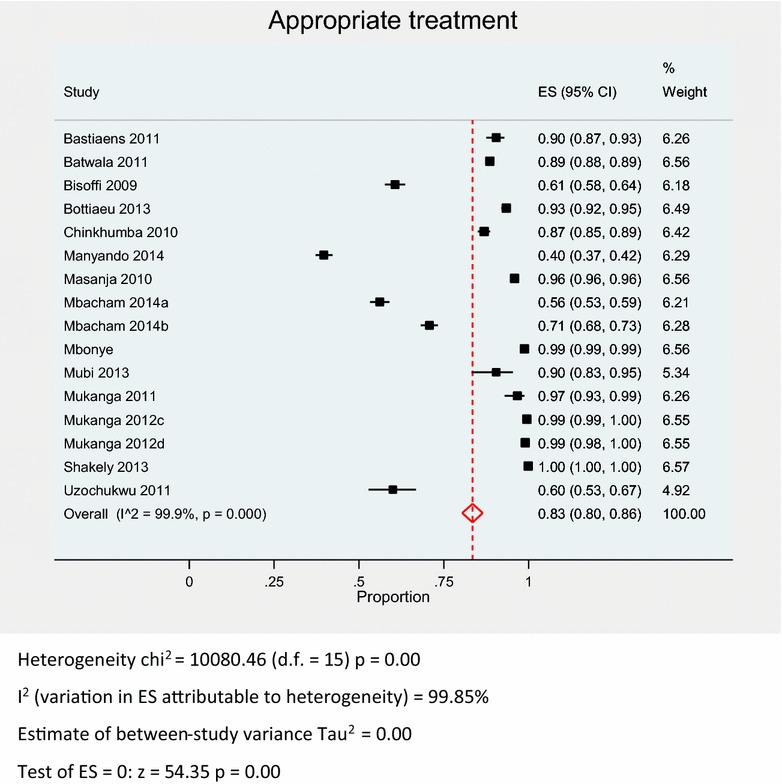
Pooled meta-analysis of overall compliance to RDT results
Table 2.
Appropriate treatment overall, RDT positive and RDT negative results
| Study design | Authors | Country | Health personnel cadre |
Appropriate treatment (%) |
Positives treated (%) |
Negatives not treated (%) |
|---|---|---|---|---|---|---|
| RCT | Bisoffi | Burkina Faso | Nurses | 60.7 | 97.7 | 19.0 |
| Observational | Masanja | Tanzania | Clinicians | 95.9 | 95.8 | 96.0 |
| Bottiaeua | Mozambique | Clinicians | 93.4 | 95.1 | 92.8 | |
| Mukanga | Uganda | CHW | 97.8 | 98.6 | 95.2 | |
| Manyando | Zambia | Clinicians | 39.7 | 93.9 | 31.4 | |
| Cross sectional | Chinkhumba | Malawi | Clinicians and nurses | 86.9 | 98.0 | 57.9 |
| Uzochukwu | Nigeria | Clinicians, nurses and CHW | 60.0 | 100.0 | 25.9 | |
| Mubi | Tanzania | Clinicians and nurses | 90.5 | 100.0 | 86.5 | |
| Shakely | Zanzibar | Clinicians and nurses | 99.9 | 100.0 | 99.9 | |
| CRT | Batwala | Uganda | Clinical officers and nurses | 88.5 | 100.0 | 76.6 |
| Mukangab | Ghana | CHW | 99.5 | 100.0 | 96.7 | |
| Mukangab | Uganda | CHW | 99.0 | 99.9 | 92.4 | |
| Mbachamc | Cameroon | Clinicians | 56.1 | 72.1 | 48.1 | |
| Mbachamd | Cameroon | Clinicians | 70.8 | 72.9 | 69.4 | |
| Mbonye | Uganda | DSV | 98.8 | 99.0 | 98.5 | |
| Before and after | Bastiaens | Tanzania | Clinical officers | 90.4 | 100.0 | 90.0 |
CHW community health worker, DSV drug shop vendors
a Excludes missing data
b Excludes Burkina Faso results
c Basic training
d Enhanced training
Fig. 5.
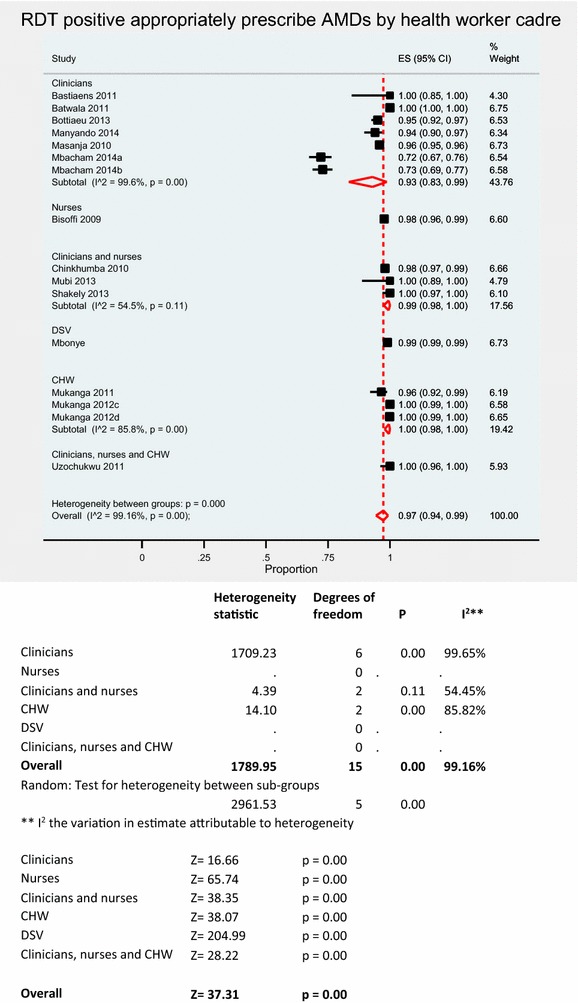
Pooled meta-analysis of RDT positive results appropriately prescribed AMDs stratified by HW cadre
Pooled meta-analysis using random effects for RDT negative patients not prescribed AMDs (Fig. 6) was 78 % (95 % CI 66–89 %); I2 = 99.8 %, Z = 14.60, p < 0.001. The proportion of RDT negative patients appropriately not prescribed and AMD was between 19.0–99.9 % (Table 2). Five studies [16, 31, 36–38] reported less than 60 % compliance to RDT negative results.
Fig. 6.
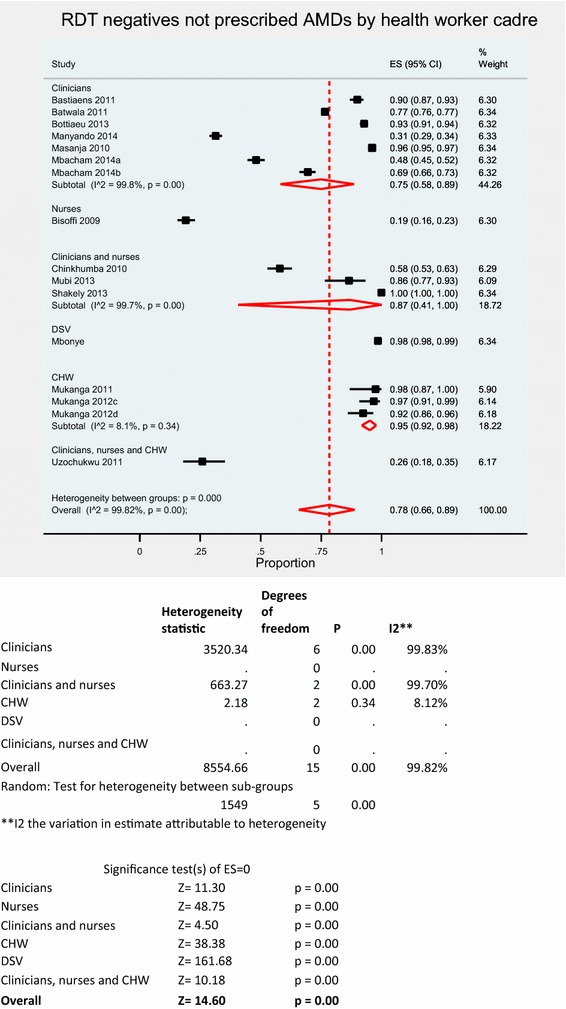
RDT negative results not prescribed AMD stratified by HW
Community health workers (CHWs) had the highest adherence to negative results (Fig. 6) with a random effects pooled proportion of 95 % (95 % CI 92–98 %); I2 = 86.4 %, Z = 23.26, p < 0.001 than clinicians with a pooled proportion of 75 % (95 % CI 58–89 %); I2 = 99.8 %, Z = 11.30, p < 0.001. There were no differences in compliance when stratified by patient age or transmission setting in pooled meta-analyses.
Compliance factors
Six out of the 14 studies included [7, 17, 30, 31, 36, 38] reported quantitative or qualitative assessment of factors associated with compliance to RDT. Uzochokwu et al. [36] reported that HWs adhered to RDT positive results, as they believed they were more reliable in confirming a malaria diagnosis than presumptive diagnosis or microscopy. Bisoffi et al. [31] compared prescribing behaviour of HWs in the dry compared to rainy seasons and reported improved RDT negative results compliance during the dry season; alternative diagnoses were also made in the dry than the rainy season.
Manyando et al. [38] reported no association between prescribing of AMD to negative RDTs in children under five, and fever in a Zambian study. There was also no association between community health worker (CHW) or socio-demographic characteristics and classification of malaria based on RDT in a bivariate analysis in Uganda [34]. In one study though, 70 % (14/20) of the respondents (HW) believed that RDTs gave inaccurate/false negative results for malaria [17]. Persistence of symptoms and patient pressure and demand were other factors reported to contribute to inappropriate AMD prescription in RDT negative cases [7, 17, 41]. HWs would end up prescribing AMDs in these cases to satisfy patients and maintain their reputation. Some HW reported that RDT negative patients improved when they were prescribed AMDs.
Discussion
This is the first systematic review and meta-analysis evaluating the proportion of health workers’ compliance with RDT results. Overall the compliance is fair. However, it also confirms that compliance to RDT negative results compared to positive results was generally low among HWs.
Diagnostic accuracy of RDT for both falciparum and non-falciparum malaria is high [14, 15]; sensitivity of up to 99.5 % and specificity of up to 90.6 % compared to microscopy for P. falciparum [14]. Community health workers can appropriately diagnose and treat malaria using RDT in resource limited settings [13]. The use of RDT to guide treatment reduces AMD prescription especially where health workers adhere to results [42].
The results show a high proportion of HWs prescribe appropriate treatment based on RDT results. A proportion of patients still remain over- or under-treated, despite policy change of administering ACT to parasitological confirmed cases only. Approximately 17 % of RDT negative patients are inappropriately prescribed AMDs. This estimate, extrapolated to sub-Saharan Africa means hundreds of thousands of patients are inappropriately diagnosed for malaria and prescribed AMD drugs unnecessarily; unnecessary (=incorrect) AMD prescription leads to drug wastage, unnecessary exposure to drug adverse effects and an increased risk of drug resistance development for current AMDs [43]. Where underlying infection is not treated, the patient’s illness prolongs and worsens; the patient or guardian makes multiple visits to seek health services, lose productivity time or income and leads to school absenteeism for school-going children [21] leading to a vicious cycle of poverty and malaria [44].
Lower cadres of HW showed more compliance to RDT results than trained HWs. The high adherence is likely due to trust in RDT result for confirming malaria diagnosis. Trained HWs on the other hand may trust clinical symptoms and past experience more than RDT result [17, 45].
Factors associated with HW compliance from qualitative studies include knowledge of alternative diagnosis, fever during the dry season and a trust in RDT result [30, 31, 46]. Trust may be increased by improving diagnostic capacity for other common febrile illnesses, and by developing evidence informed guidelines for treatment of symptomatic RDT negative patients. Such guidelines may not apply in non-endemic areas and therefore should be specific to particular settings.
Knowledge of alternative diagnosis is related to the level of training and experience of HW [47]. HWs reported they likely made alternative diagnosis during the dry season when malaria transmission is perceived lower in febrile children with negative RDT result compared to the wet season when transmission peaks. For febrile patients, alternative diagnoses were made during the dry season while more patients were treated for malaria during the wet season in one study [31].
Qualitative studies report pressure on prescribers to satisfy patient expectations as one factor, which contributes to non-compliance of RDT negative results [44, 48]. Chandler et al. [49] reported patient psychology and prescriber reputation as other factors influencing non-compliance to of HWs to negative RDT.
Interventions to improve compliance have not been successful, although they led to a decrease in ACT prescriptions in particular. Some HWs prescribed a non-recommended AMD in malaria negative patients [16, 50].
In cases of patients demanding AMDs, community sensitisation on RDTs was reported to improve patient satisfaction [7]. At facility level, involvement of patient in discussing malaria results also improved patient satisfaction and reduced patient demand for AMDs [51].
Notably, few studies were available which quantified HW’s compliance to malaria RDT results, and even less studies investigated the factors contributing to compliance. Understanding these factors can help design effective strategies to improve compliance of anti-malarial drugs. Chandler et al. [49] describe a systematic method of designing an intervention in Tanzania; formative research would be key in designing such an intervention. However, interventions are context-specific and may not be applicable to all settings, and for all HWs. It is essential to investigate factors contributing to non-compliance in specific cadres and settings, exploring impact in a context specific manner before designing and implementing interventions.
Although ideal for rural areas in Africa, RDT kits inherently are not 100 % sensitive and specific [14, 42]. Clinically diagnosed malaria and positive malaria test may be due to other underlying causes of the fever [27]. Crump et al. reported only 1.6 % of 820 patients with fever or history of fever actually had malaria infection in a Tanzanian prospective cohort study; bacterial and fungal bloodstream infections were responsible for 9.8 and 2.9 % of the fever, respectively. Resource limited settings lack diagnostic equipment and capacity for some diseases. Diagnostic accuracy of RDTs can be affected further by low and extremely high parasite densities [52, 53], patient-intrinsic factors such as rheumatoid factor positivity [54], user factors such as result interpretation and performance of the test, and environmental storage conditions including high temperatures. It is, therefore, possible, though infrequent, for malaria-infected patients to have a false positive (leading to not-indicated treatment) or more importantly, false negative result, and hence miss malaria treatment if WHO malaria treatment guidelines are followed. A more robust and highly specific test may be useful to rule out malaria. False positives, where malaria parasitaemia is not the cause of the illness (in endemic areas) lead to neglecting of other febrile illnesses.
Multidisciplinary research to explore, measure and design interventions for increasing compliance to RDT results in different settings in Africa need to be conducted. More innovation in diagnosis of common febrile illnesses in malaria endemic regions needs to be available. There is sparse data on prevalence of other non-malaria febrile illnesses in most malaria endemic regions of Africa.
The meta-analysis may have overestimated compliance: studies evaluating diagnostic tests generally report higher compliance when assessed in the study setting compared to a non-study setting. Most studies reported higher compliance to positive results compared to negative results.
A limitation for the results in the review is that risk of bias and publication bias were not assessed for the studies included; the quality of evidence therefore cannot be reported.
Conclusion
HWs compliance to RDT is fair; compliance to positive RDT results is generally higher compared to negative RDT results. Over-treatment of malaria is still a major problem in sub-Saharan Africa. Both HW and patient factors contribute to inappropriate prescribing of AMDs to RDT negative patients; interventions to improve compliance should target both patients and HWs. Treatment guidelines should be developed for other causes of fever informed by local context and research. Multidisciplinary research will improve compliance of HWs to RDT results.
Authors’ contributions
ANK, KSP and MVV conceived the review. ANK and BJV developed the study design and the outline of the report. ANK and RS searched the scientific literature. ANK and BJV screened the search results. ANK prepared the first draft of the report and performed the statistical analyses. BJV checked all the quantitative data. BJV, RS, KSP, MGP and MVV contributed to the outline of the report. All authors contributed to the writing, and approved of the final manuscript version. All authors read and approved the final manuscript.
Acknowledgements
This study was supported by the Dioraphte Foundation, The Netherlands. We thank the reviewer for his/her thorough and excellent review.
Competing interests
The authors declare that they have no competing interests.
Abbreviations
- ACT
artemisinin-based combination therapy
- AL
artemether-lumefantrine
- AMC
Academic Medical Center
- AMDs
anti-malarial drugs
- CERMEL
Centre de Recherches Médicales de Lambaréné
- CI
confidence interval
- CHW
community health worker
- HW
health worker
- RDT
rapid diagnostic test
- WHO
World Health Organization
Additional files
10.1186/s12936-016-1218-5 Search strategy.
10.1186/s12936-016-1218-5 Data extraction form.
10.1186/s12936-016-1218-5 Formulae for appropriate treatment.
Contributor Information
Alinune N. Kabaghe, Email: akabaghe@medcol.mw
Benjamin J. Visser, Email: b.j.visser@amc.uva.nl
Rene Spijker, Email: r.spijker@amc.uva.nl.
Kamija S. Phiri, Email: kamijaphiri@gmail.com
Martin P. Grobusch, Email: m.p.grobusch@amc.uva.nl
Michèle van Vugt, Email: m.vanvugt@amc.uva.nl.
References
- 1.WHO . World malaria report: 2014. Geneva: World Health Organization; 2014. [Google Scholar]
- 2.Grobusch MP, Kremsner PG. Uncomplicated malaria. Curr Top Microbiol Immunol. 2005;295:83–104. [PubMed] [Google Scholar]
- 3.WHO . Guidelines for the treatment of malaria. 3. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]
- 4.Visser BJ, van Vugt M, Grobusch MP. Malaria: an update on current chemotherapy. Expert Opin Pharmacother. 2014;15:2219–2254. doi: 10.1517/14656566.2014.944499. [DOI] [PubMed] [Google Scholar]
- 5.Visser BJ, Wieten RW, Kroon D, Nagel IM, Belard S, van Vugt M, et al. Efficacy and safety of artemisinin combination therapy (ACT) for non-falciparum malaria: a systematic review. Malar J. 2014;13:463. doi: 10.1186/1475-2875-13-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.malERA Consultative Group on Health Systems and Operational Research A research agenda for malaria eradication: health systems and operational research. PLoS Med. 2011;8:e1000397. doi: 10.1371/journal.pmed.1000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mbonye AK, Magnussen P, Lal S, Hansen KS, Cundill B, Chandler C, et al. A cluster randomised trial introducing rapid diagnostic tests into registered drug shops in Uganda: impact on appropriate treatment of malaria. PLoS One. 2015;10:e0129545. doi: 10.1371/journal.pone.0129545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinhardt LC, Chinkhumba J, Wolkon A, Luka M, Luhanga M, Sande J, et al. Patient-, health worker-, and health facility-level determinants of correct malaria case management at publicly funded health facilities in Malawi: results from a nationally representative health facility survey. Malar J. 2014;13:64. doi: 10.1186/1475-2875-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiseman V, Ezeoke O, Nwala E, Mangham LJ, Cundill B, Enemuo J, et al. A cost-effectiveness analysis of provider and community interventions to improve the treatment of uncomplicated malaria in Nigeria: study protocol for a randomized controlled trial. Trials. 2012;13:81. doi: 10.1186/1745-6215-13-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nankabirwa J, Zurovac D, Njogu JN, Rwakimari JB, Counihan H, Snow RW, et al. Malaria misdiagnosis in Uganda–implications for policy change. Malar J. 2009;8:66. doi: 10.1186/1475-2875-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahama-Maro J, D’Acremont V, Mtasiwa D, Genton B, Lengeler C. Low quality of routine microscopy for malaria at different levels of the health system in Dar es Salaam. Malar J. 2011;10:332. doi: 10.1186/1475-2875-10-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO. World Malaria Report: 2013. Geneva: World Health Organization; 2013.
- 13.Ruizendaal E, Dierickx S, Peeters Grietens K, Schallig HD, Pagnoni F, Mens PF. Success or failure of critical steps in community case management of malaria with rapid diagnostic tests: a systematic review. Malar J. 2014;13:229. doi: 10.1186/1475-2875-13-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abba K, Deeks JJ, Olliaro P, Naing CM, Jackson SM, Takwoingi Y, et al. Rapid diagnostic tests for diagnosing uncomplicated P. falciparum malaria in endemic countries. Cochrane Database Syst Rev. 2011;7:CD008122. doi: 10.1002/14651858.CD008122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abba K, Kirkham AJ, Olliaro PL, Deeks JJ, Donegan S, Garner P, et al. Rapid diagnostic tests for diagnosing uncomplicated non-falciparum or Plasmodium vivax malaria in endemic countries. Cochrane Database Syst Rev. 2014;12:CD011431. doi: 10.1002/14651858.CD011431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mbacham WF, Mangham-Jefferies L, Cundill B, Achonduh OA, Chandler CIR, Ambebila JN, et al. Basic or enhanced clinician training to improve adherence to malaria treatment guidelines: a cluster-randomised trial in two areas of Cameroon. Lancet Glob Health. 2014;2:e346–e358. doi: 10.1016/S2214-109X(14)70201-3. [DOI] [PubMed] [Google Scholar]
- 17.Mubi M, Kakoko D, Ngasala B, Premji Z, Peterson S, Bjorkman A, et al. Malaria diagnosis and treatment practices following introduction of rapid diagnostic tests in Kibaha district, coast region, Tanzania. Malar J. 2013;12:293. doi: 10.1186/1475-2875-12-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shakely D, Elfving K, Aydin-Schmidt B, Msellem MI, Morris U, Omar R, et al. The usefulness of rapid diagnostic tests in the new context of low malaria transmission in Zanzibar. PLoS One. 2013;8:e72912. doi: 10.1371/journal.pone.0072912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shillcutt SD, Morel CM, Coleman PG, Mills AJ, Goodman CA. Cost-effectiveness of malaria diagnosis in sub-Saharan Africa: the role of rapid diagnostic tests in rural settings with high Plasmodium falciparum transmission. Geneva: World Health Organization; 2006. p. 173. http://www.who.int/malaria/publications/atoz/malaria-subsaharan-africa/en/. Accessed 3 Mar 2016.
- 20.Shillcutt S, Morel C, Goodman C, Coleman P, Bell D, Whitty CJ, et al. Cost-effectiveness of malaria diagnostic methods in sub-Saharan Africa in an era of combination therapy. Bull World Health Organ. 2008;86:101–110. doi: 10.2471/BLT.07.042259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amexo M, Tolhurst R, Barnish G, Bates I. Malaria misdiagnosis: effects on the poor and vulnerable. Lancet. 2004;364:1896–1898. doi: 10.1016/S0140-6736(04)17446-1. [DOI] [PubMed] [Google Scholar]
- 22.Akpede GO, Sykes RM. Malaria with bacteraemia in acutely febrile preschool children without localizing signs: coincidence or association/complication? J Trop Med Hyg. 1993;96:146–150. [PubMed] [Google Scholar]
- 23.Berkley JA, Maitland K, Mwangi I, Ngetsa C, Mwarumba S, Lowe BS, et al. Use of clinical syndromes to target antibiotic prescribing in seriously ill children in malaria endemic area: observational study. BMJ. 2005;330:995. doi: 10.1136/bmj.38408.471991.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molyneux E, Walsh A, Phiri A, Molyneux M. Acute bacterial meningitis in children admitted to the Queen Elizabeth Central Hospital, Blantyre, Malawi in 1996–97. Trop Med Int Health. 1998;3:610–618. doi: 10.1046/j.1365-3156.1998.00278.x. [DOI] [PubMed] [Google Scholar]
- 25.English M, Punt J, Mwangi I, McHugh K, Marsh K. Clinical overlap between malaria and severe pneumonia in Africa children in hospital. Trans R Soc Trop Med Hyg. 1996;90:658–662. doi: 10.1016/S0035-9203(96)90423-X. [DOI] [PubMed] [Google Scholar]
- 26.Nichols C, Cruz Espinoza LM, von Kalckreuth V, Aaby P, Ahmed El Tayeb M, Ali M, et al. Bloodstream infections and frequency of pretreatment associated with age and hospitalization status in sub-Saharan Africa. Clin Infect Dis. 2015;61(Suppl 4):S372–S379. doi: 10.1093/cid/civ730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koram KA, Molyneux ME. When Is “Malaria” Malaria? The different burdens of malaria infection, malaria disease, and malaria-like illnesses. Am J Trop Med Hyg. 2007;77:1–5. [PubMed] [Google Scholar]
- 28.Health workers’ compliance to Rapid Diagnostic Tests (RDTs) to guide malaria treatment: protocol for systematic review and meta-analysis. http://www.crd.york.ac.uk/PROSPERO/DisplayPDF.php?ID=CRD42015016151. Accessed 3 Mar 2016. [DOI] [PMC free article] [PubMed]
- 29.Information note on recommended selection criteria for procurement of malaria rapid diagnostic tests. http://www.who.int/malaria/publications/atoz/rdt_selection_criteria/en/. Accessed 3 Mar 2015.
- 30.Batwala V, Magnussen P, Nuwaha F. Comparative feasibility of implementing rapid diagnostic test and microscopy for parasitological diagnosis of malaria in Uganda. Malar J. 2011;10:373. doi: 10.1186/1475-2875-10-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bisoffi Z, Sirima BS, Angheben A, Lodesani C, Gobbi F, Tinto H, et al. Rapid malaria diagnostic tests vs. clinical management of malaria in rural Burkina Faso: safety and effect on clinical decisions. A randomized trial. Trop Med Int Health. 2009;14:491–498. doi: 10.1111/j.1365-3156.2009.02246.x. [DOI] [PubMed] [Google Scholar]
- 32.Bottieau E, Gillet P, De Weggheleire A, Scheirlinck A, Stokx J, Mosse CDD, et al. Treatment practices in patients with suspected malaria in provincial hospital of Tete, Mozambique. Tran R Soc Trop Med Hyg. 2013;107:176–182. doi: 10.1093/trstmh/trs012. [DOI] [PubMed] [Google Scholar]
- 33.Masanja MI, McMorrow M, Kahigwa E, Kachur SP, McElroy PD. Health workers’ use of malaria rapid diagnostic tests (RDTS) to guide clinical decision making in rural dispensaries, Tanzania. Am J Trop Med Hyg. 2010;83:1238–1241. doi: 10.4269/ajtmh.2010.10-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukanga D, Babirye R, Peterson S, Pariyo GW, Ojiambo G, Tibenderana JK, et al. Can lay community health workers be trained to use diagnostics to distinguish and treat malaria and pneumonia in children? Lessons from rural Uganda. Trop Med Int Health. 2011;16:1234–1242. doi: 10.1111/j.1365-3156.2011.02831.x. [DOI] [PubMed] [Google Scholar]
- 35.Mukanga D, Tiono AB, Anyorigiya T, Kallander K, Konate AT, Oduro AR, et al. Integrated community case management of fever in children under five using rapid diagnostic tests and respiratory rate counting: a multi-country cluster randomized trial. Am J Trop Med Hyg. 2012;87:21–29. doi: 10.4269/ajtmh.2012.11-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uzochukwu BSC, Onwujekwe E, Ezuma NN, Ezeoke OP, Ajuba MO, Sibeudu FT. Improving rational treatment of malaria: perceptions and influence of RDTs on prescribing behaviour of health workers in Southeast Nigeria. PLoS One. 2011;6:e14627. doi: 10.1371/journal.pone.0014627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chinkhumba J, Skarbinski J, Chilima B, Campbell C, Ewing V, San Joaquin M, et al. Comparative field performance and adherence to test results of four malaria rapid diagnostic tests among febrile patients more than five years of age in Blantyre, Malawi. Malar J. 2010;9:209. doi: 10.1186/1475-2875-9-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manyando C, Njunju EM, Chileshe J, Siziya S, Shiff C. Rapid diagnostic tests for malaria and health workers’ adherence to test results at health facilities in Zambia. Malar J. 2014;13:166. doi: 10.1186/1475-2875-13-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bastiaens GJ, Schaftenaar E, Ndaro A, Keuter M, Bousema T, Shekalaghe SA. Malaria diagnostic testing and treatment practices in three different Plasmodium falciparum transmission settings in Tanzania: before and after a government policy change. Malar J. 2011;10:76. doi: 10.1186/1475-2875-10-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Briggs M, Chinkhumba J, Bauleni A, Sande J, Luhanga M, Dodoli W, et al. Nationwide health facility survey of severe malaria case management-Malawi 2012. ASTMH 62nd Annual Meeting, November 13–17, Washington, DC, Poster 211; 2013.
- 41.Brasseur P, Badiane M, Cisse M, Vaillant M, Olliaro PL. Changes in malaria prevalence and health provider’s behavior towards fever with the introduction of act and RDT at peripheral health centre level in Southwestern Senegal (2000–2011). ASTMH 61st Annual Meeting, November 11–15, Atlanta, Poster 900; 2012.
- 42.Odaga J, Sinclair D, Lokong JA, Donegan S, Hopkins H, Garner P. Rapid diagnostic tests versus clinical diagnosis for managing people with fever in malaria endemic settings. Cochrane Database Syst Rev. 2014;4:CD008998. doi: 10.1002/14651858.CD008998.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bloland PB. Drug resistance in malaria. Geneva: World Health Organization; 2001. p. 15. http://www.who.int/csr/resources/publications/drugresist/malaria.pdf?ua=1. Accessed 3 Mar 2016.
- 44.Sachs J, Malaney P. The economic and social burden of malaria. Nature. 2002;415:680–685. doi: 10.1038/415680a. [DOI] [PubMed] [Google Scholar]
- 45.Diggle E, Asgary R, Gore-Langton G, Nahashon E, Mungai J, Harrison R, et al. Perceptions of malaria and acceptance of rapid diagnostic tests and related treatment practises among community members and health care providers in Greater Garissa, North Eastern Province, Kenya. Malar J. 2014;13:502. doi: 10.1186/1475-2875-13-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bisoffi Z, Gobbi F, Van Den Ende J. Rapid diagnostic tests for malaria. BMJ. 2014;348:3846. doi: 10.1136/bmj.g3846. [DOI] [PubMed] [Google Scholar]
- 47.Selemani M, Masanja IM, Kajungu D, Amuri M, Njozi M, Khatib RA, et al. Health worker factors associated with prescribing of artemisinin combination therapy for uncomplicated malaria in rural Tanzania. Malar J. 2013;12:334. doi: 10.1186/1475-2875-12-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Achonduh OA, Mbacham WF, Mangham-Jefferies L, Cundill B, Chandler C, Pamen-Ngako J, et al. Designing and implementing interventions to change clinicians’ practice in the management of uncomplicated malaria: lessons from Cameroon. Malar J. 2014;13:204. doi: 10.1186/1475-2875-13-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chandler CI, Meta J, Ponzo C, Nasuwa F, Kessy J, Mbakilwa H, et al. The development of effective behaviour change interventions to support the use of malaria rapid diagnostic tests by Tanzanian clinicians. Implement Sci. 2014;9:83. doi: 10.1186/1748-5908-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skarbinski J, Ouma PO, Causer LM, Kariuki SK, Barnwell JW, Alaii JA, et al. Effect of malaria rapid diagnostic tests on the management of uncomplicated malaria with artemether-lumefantrine in Kenya: a cluster randomized trial. Am J Trop Med Hyg. 2009;80:919–926. [PubMed] [Google Scholar]
- 51.Chandler CI, Mwangi R, Mbakilwa H, Olomi R, Whitty CJ, Reyburn H. Malaria overdiagnosis: is patient pressure the problem? Health Policy Plan. 2008;23:170–178. doi: 10.1093/heapol/czm046. [DOI] [PubMed] [Google Scholar]
- 52.Gillet P, Mori M, Van Esbroeck M, Van den Ende J, Jacobs J. Assessment of the prozone effect in malaria rapid diagnostic tests. Malar J. 2009;8:271. doi: 10.1186/1475-2875-8-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gillet P, Scheirlinck A, Stokx J, De Weggheleire A, Chauque HS, Canhanga OD, et al. Prozone in malaria rapid diagnostics tests: how many cases are missed? Malar J. 2011;10:166. doi: 10.1186/1475-2875-10-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grobusch MP, Alpermann U, Schwenke S, Jelinek T, Warhurst DC. False-positive rapid tests for malaria in patients with rheumatoid factor. Lancet. 1999;353:297. doi: 10.1016/S0140-6736(05)74930-8. [DOI] [PubMed] [Google Scholar]


