Abstract
Background:
Dengue fever (DF) is a viral hemorrhagic fever causing severe morbidity and mortality in affected patients.
Aims:
The purpose of our study was to evaluate the changing trends in radiological findings in DF, to find if ultrasound is useful in the diagnosis of DF during an epidemic in absence of serological tests, and also to investigate the effects of DF in pregnancy.
Materials and Methods:
A prospective study was conducted in 2013 comprising of 400 patients who were serologically positive for dengue. Out of these, radiological investigations were conducted for 107 patients who were analyzed.
Results:
Out of the 107 patients, 85 patients underwent ultrasound, 12 computed tomography (CT) scans of brain or paranasal sinuses, and 21 chest radiography. The maximum numbers of patients (79%) were in the age group of 20-50 years. The most common ultrasound finding was hepatomegaly that was seen in 62% of the patients. Other findings were splenomegaly (45%), gallbladder (GB) wall edema (45%), right-sided pleural effusion (37%), bilateral pleural effusion (22%), and ascites (36%). Out of 10 pregnant patients, 5 had oligohydramnios, 2 had intrauterine growth restriction, 2 had intrauterine fetal demise, and 5 had a normal antenatal ultrasound.
Conclusion:
Ultrasound findings of hepatosplenomegaly, GB wall edema, right-sided or bilateral pleural effusion, and ascites in patients presenting with signs and symptoms of DF during an epidemic are virtually diagnostic of DF. There have been recent changing trends with hepatosplenomegaly being the more common manifestation, in comparison to ascites and GB wall edema. DF also has catastrophic effects in pregnancy such as oligohydramnios and intrauterine fetal demise.
Keywords: Dengue fever (DF), hepatosplenomegaly, oligohydramnios, gallbladder (GB) wall edema, ultrasound
Introduction
Dengue is a mosquito-borne viral infection that in recent years has become a major international public health problem.[1] It is caused by one of the four serotypes of the dengue virus belonging to the family Flaviviridae. The virus serotypes are closely related but antigenically distinct. Dengue infections can result in a wide spectrum of disease severity ranging from an influenza-like illness (dengue fever; DF) to the life-threatening dengue hemorrhagic fever or dengue shock syndrome. The disease is now endemic in more than 100 tropical and subtropical countries. The World Health Organization (WHO) estimates that there may be 50 million dengue infections worldwide every year.[2] The resurgence of dengue has been observed in India and dengue outbreaks have been frequently reported from different parts of the country in both urban and rural populations.[3]
Clinically dengue manifests with acute onset of fever, severe headache, retro-ocular pain, and pain involving the muscles and joints. Hemorrhagic diathesis and thrombocytopenia with concurrent hemoconcentration is a constant finding.[1]
We conducted a prospective study from June 2013 to October 2013 during the dengue epidemic of northern India. The purpose of our study was to evaluate the changing trends in radiological findings in DF; to find whether ultrasound of the abdomen is an important adjunct to clinical and laboratory profile in diagnosing DF, if ultrasound is useful in diagnosis of the disease during an epidemic, in absence of serological tests; and also to investigate the effects of DF in pregnancy.
Materials and Methods
After obtaining formal approval from institutional review board, a prospective study was conducted between June 2013 and October 2013, comprising of 400 patients who were serologically positive for dengue. Informed consent was obtained from the patients before conducting any radiological examination. Out of these, radiological investigations were conducted for 107 patients including ultrasound scanning of both abdomen and thorax or computed tomography (CT) scan of brain or chest radiography or a combination of above investigations. The remaining 293 patients who did not undergo any radiological investigations were excluded from the study.
Serological testing was performed in the Department of Microbiology using the dengue card test that was a rapid solid-phase immune-chromatographic test for the qualitative detection of dengue nonstructural protein 1 (NS1) antigen and the differential detection of immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies to dengue virus in human serum/plasma.
All ultrasound examinations were performed with an ultrasound machine (Medison or Siemens) using both linear and convex probes. The scans were performed by three sonographers with more than 5 years of experience each in performing sonography. Thoracic scanning was done in either sitting or supine posture. Both the pleural spaces were evaluated through an intercostal and subcostal approach. Pleural effusion was evaluated with both convex and linear probes so that even minimal pleural effusions were not missed. Abdominal scanning was done after 4-6 h of fasting to allow better distension of gallbladder (GB). Subjects who had GB wall thickness >3 mm as measured on ultrasound were identified as positive for GB wall edema.
CT scans were carried out using a 128-slice Philips Brilliance multidetector CT scan machine (Amsterdam, the Netherlands). Radiography was done using a 800 mAs x-ray machine.
The data were tabulated using Microsoft Excel sheet and all the necessary calculations were made.
Results
Out of the 400 serologically positive dengue patients, 107 patients underwent radiological investigations for various reasons. Eighty-five patients underwent ultrasound, 11 patients underwent CT scan of brain, 1 patient underwent CT scan of paranasal sinuses, and 21 patients underwent chest radiography. There was a slight male predominance (46 males and 39 females). The youngest patient was 1 year old and the eldest patient was 82 years old. Most of the patients (79%) were in the age group of 20-50 years.
Hepatomegaly was seen in 53 patients. The average liver size in patients with hepatomegaly was 16.2 cm. Only nine patients showed altered echo pattern of the liver. Thirty-nine patients showed splenomegaly with an average spleen size of 13.0 cm. GB wall edema was seen in 38 patients [Figure 1]. Right-sided pleural effusion was seen in 32 patients [Figure 2]. Bilateral pleural effusion was seen in 19 patients [Figure 3]. Only two patients showed only left-sided pleural effusion. Ascites was seen in 31 patients [Figure 4]. No sonological abnormality was detected in 13 patients [Figure 5].
Figure 1.
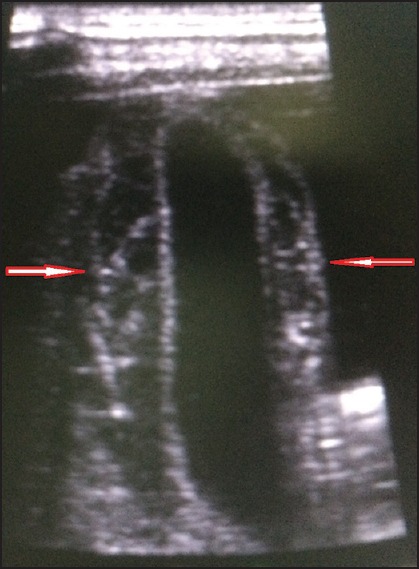
Ultrasound image showing gallbladder wall edema
Figure 2.
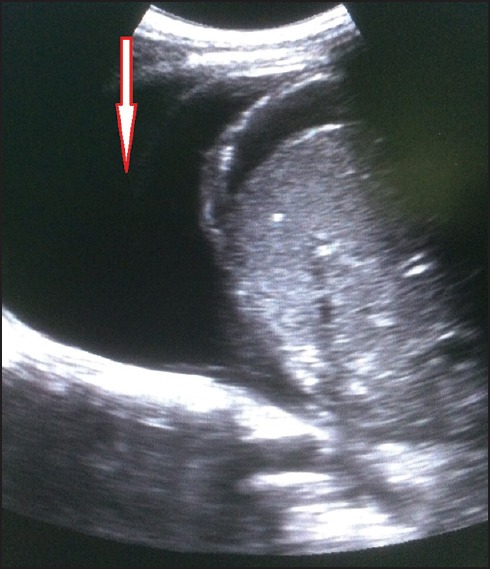
Ultrasound image showing right-sided pleural effusion
Figure 3.
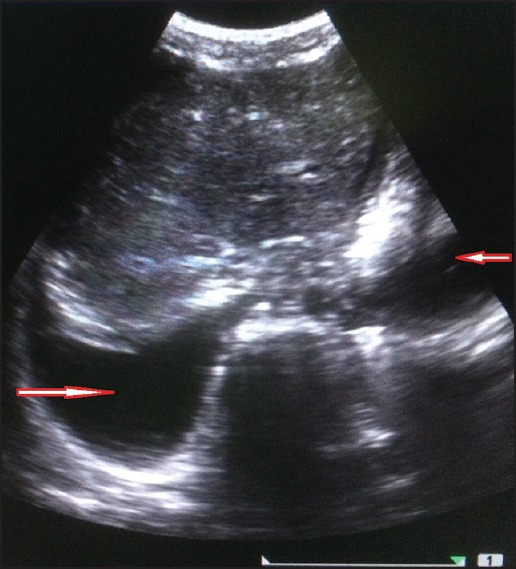
Ultrasound image showing bilateral pleural effusion
Figure 4.
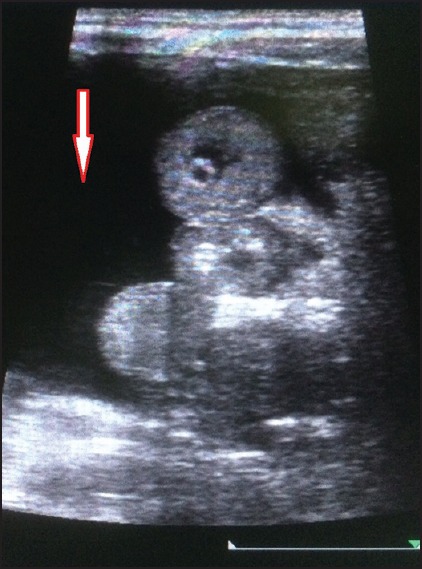
Ultrasound image showing ascites
Figure 5.
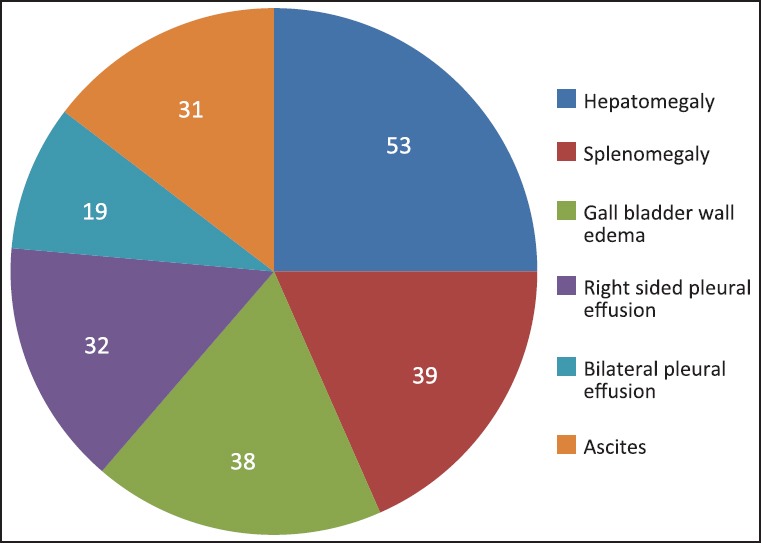
Pie chart showing distribution of various ultrasound findings
Repeat ultrasound was done in four patients, one on day 2, one on day 4, and two on day 5. Three of them showed findings same as first ultrasound and one of them done on day 5 showed evolution of findings in the form of hepatosplenomegaly, ascites, and bilateral pleural effusion.
Ten patients in our study were pregnant who underwent an obstetric ultrasound. Five of them had oligohydramnios, two had intrauterine growth restriction, and five had a normal antenatal ultrasound. Two intrauterine fetal deaths were encountered.
Nine of the 21 chest radiographs were normal. Only one of the radiographs showed only pleural effusion that was bilateral. Others showed a mixture of findings of Koch's and consolidation.
Seven of the 11 patients who underwent CT brain were normal. Two patients had calcified granulomas, one had and infarct, and communicating hydrocephalus was seen in one patient.
CT scan of paranasal sinuses was done for the evaluation of nasal bleed, whose exact source could not be determined.
Discussion
Most common etiological agents of acute febrile illness in India are dengue, malaria, typhoid, and enteric fever as seen in previous studies[4,5,6,7,8,9] that can be ruled out by appropriate clinical, laboratory, and radiological methods.
Dengue virus is a small single-stranded RNA virus comprising four distinct serotypes. These closely related serotypes of the dengue virus belong to the genus Flavivirus, family Flaviviridae. Various serotypes of the dengue virus are transmitted to humans through the bites of infected Aedes mosquitoes, principally Aedes aegypti.[10]
The increase of DF is due to uncontrolled population growth and urbanization in the absence of appropriate water management, global spread of dengue strains via travel and trade, and erosion of vector control programs.[4] After an incubation period of 4-10 days, infection by any of the four virus serotypes can produce a wide spectrum of illness, although most infections are asymptomatic or subclinical. Primary infection is thought to induce lifelong protective immunity to the infecting serotype. Individuals suffering an infection are protected from clinical illness with a different serotype within 2-3 months of the primary infection but with no long-term cross-protective immunity. Plasma leakage, hemoconcentration, and abnormalities in hemostasis characterize severe dengue. Hemorrhage may be a consequence of the thrombocytopenia and associated platelet dysfunction or disseminated intravascular coagulation.[10]
Efficient and accurate diagnosis of dengue is of primary importance for clinical care, surveillance activities, outbreak control, pathogenesis, academic research, vaccine development, and clinical trials. Laboratory diagnosis methods for confirming dengue virus infection may involve detection of the virus, of the viral nucleic acid, of the antigens or antibodies, or a combination of these techniques.[10] The major diagnostic methods available presently are viral culture, viral nucleic acid detection by recombinant polymerase chain reaction, and serological tests such as IgM capture enzyme-linked immunosorbent assay (ELISA).[11] After the onset of illness, the virus can be detected in serum, plasma, circulating blood cells, and other tissues for 4-5 days. During the early stages of the disease, virus isolation, nucleic acid or antigen detection can be used to diagnose the infection that gives a definitive diagnosis of dengue. At the end of the acute phase of infection, serology is the method of choice for the diagnosis.[10] Hemagglutination inhibition antibodies usually appear at detectable level by day 5 to day 6 of febrile illness resulting in delayed diagnosis of DF is often delayed owing to time taken for availability of results.[1]
The aim of our study was to evaluate the radiological findings in DF, to find whether ultrasound of the abdomen is an important adjunct to clinical and laboratory profile in diagnosing DF and further if ultrasound is useful in the diagnosis of the disease during an epidemic, in absence of serological tests.
In our study, we found that there was a very slight male preponderance with 54% males and 46% females. A whopping 67 patients (79%) were in the age group of 20-50 years that constitutes the major workforce in the society reflecting the far-reaching consequences of this disease. Similar findings have been found in the previous study conducted by Santhosh et al.[12] with maximum patients in their study being found in the age group of 20-39 years. However, the percentage of patients in this age group was lesser in their study (44%) as compared to ours (79%).
Very few previous studies have been conducted for the evaluation of radiological findings in DF. One of the earliest studies conducted by Venkata Sai et al.[1] showed that, in an epidemic of dengue, ultrasound features of thickened GB wall, pleural effusion, and ascites should strongly favor the diagnosis of DF. A recent study conducted by Santhosh et al.[12] showed that sonographic features of thickened GB wall, pleural effusion (bilateral or right side), ascites, and hepatosplenomegaly should definitely support the diagnosis of DF in patients presenting with fever and associated symptoms, particularly during an epidemic.
The commonest ultrasound finding in our study was hepatomegaly that was seen in 53 patients (62%) with mean liver size of approximately 16.2 cm. The next common finding in our study was splenomegaly seen in 39 patients (45%) with an average spleen size of 13.0 cm indicating mild splenomegaly as ultrasound feature of DF. Javed et al. found hepatomegaly in 35.5% patients and splenomegaly in 28.9% patients.[13] Our findings are hence in contrast to previously conducted studies most of which have shown GB edema and ascites as the commonest finding on ultrasound. This difference could be due to a different antigenic strain of dengue virus.
Mild splenomegaly has previously been adopted as a criterion for identifying patients at high risk for developing hypovolemic shock (dengue shock syndrome) with a specificity of 92%.[8]
Altered liver echotexture was seen in only nine patients, which has been seen in previous studies and has been attributed to intraparenchymal and subcapsular hemorrhages.[14] However, altered liver echotexture has not been a consistent ultrasound finding of DF as it has not been found in all the studies.[1]
Only 38 patients (45%) showed GB wall edema in our study. Thickened GB wall was first reported as a finding of DF by Pramuljo et al.[15] It has been found in a lot of studies to be a consistent and common ultrasound finding of DF. Venkata Sai et al.[1] had found it in 100% patients in their study as the most common initial ultrasound finding. In fact, it has been propagated to be used in children as a reliable criterion to predict the onset of severe dengue hemorrhage fever.[16] However, we found it in only 45% of the patients in our study, reflecting again a change in pattern of DF.
Right-sided pleural effusion was seen in 32 patients (37%). Bilateral pleural effusion was seen in 19 patients (22%). Only two patients showed left-sided pleural effusion. Pleural effusion (either only right-sided or bilateral) has also been seen in previous studies as one of the common findings of DF with isolated left-sided pleural effusion being exceedingly rare.[17]
Ascites was seen in 31 patients (36%) in our study which was seen commonly in previous studies.[17] In fact, it has been found to be the commonest ultrasound finding in some studies.[17] However, in our study we found ascites in less than half of the patients, although we used both linear and convex ultrasound probes for the evaluation of the same.
Repeat ultrasound was done in four patients, three of them showed findings same as first ultrasound and one of them showed evolution of findings. In the study conducted by Venkata Sai et al.,[1] on follow-up scan (days 5-7), the incidence of hepatosplenomegaly and right-sided pleural effusion increased, GB wall edema persisted in all the patients and new findings including ascites and left-sided pleural effusion were noted.
Out of the 10 pregnant patients in our study, 5 of them had oligohydramnios, 2 had intrauterine growth restriction, and 5 had a normal antenatal ultrasound. Two intrauterine fetal deaths were encountered. In the study conducted by Sampath et al.,[18] 2 out of 15 patients had an intrauterine fetal demise. Oligohydramnios has only been reported rarely as an outcome of DF in pregnancy.[19] In our study, half of the patients had oligohydramnios, which could be attributed to loss of fluid due to capillary leakage. However, more studies with larger sample size are needed to confirm the effects of DF in pregnancy.
Chest radiographs showed normal findings in 9 out of the 21 patients. Pleural effusion was visible in only one of the radiographs in which it was bilateral. This could be due to a small amount of effusion that might not be visible on radiography. Others showed a mixture of findings of Koch's and consolidation.
CT brain was normal in 7 of the 11 patients. Calcified granulomas were seen in two patients, infarct in one and communicating hydrocephalus in another patient. Neurological manifestations have been infrequently reported as clinical consequences of dengue infection in the form of meningoencephalitis in severe DF.[20]
CT scan of paranasal sinuses was done for the evaluation of nasal bleed, whose exact source could not be determined.
Conclusion
In conclusion, ultrasound findings of hepatosplenomegaly, GB wall edema, right-sided or bilateral pleural effusion, and ascites in a patient presenting with signs and symptoms of DF during an epidemic are virtually diagnostic of DF. Hence, ultrasound being a cost-effective, less time-consuming, and easily available modality can easily be used to diagnose DF during an epidemic and can help to start treatment before the antigen assays are available. We have also concluded that there have been recent changing trends in the ultrasound manifestations of DF with hepatosplenomegaly being the more common manifestation, in comparison to findings of ascites and GB wall edema, which could be due to antigenic variation in dengue virus. DF also has catastrophic effects in pregnancy with oligohydramnios and even intrauterine fetal demise occurring in pregnant patients with DF. However, more studies with larger sample size are needed to further investigate the effect of DF in pregnancy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Venkata Sai PM, Dev B, Krishnan R. Role of ultrasound in dengue fever. Br J Radiol. 2005;78:416–8. doi: 10.1259/bjr/54704044. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed MM. Clinical profile of dengue fever infection in King Abdul Aziz University Hospital Saudi Arabia. J Infect Dev Ctries. 2010;4:503–10. doi: 10.3855/jidc.1038. [DOI] [PubMed] [Google Scholar]
- 3.Karoli R, Fatima J, Siddiqi Z, Kazmi KI, Sultania AR. Clinical profile of dengue infection at a teaching hospital in North India. J Infect Dev Ctries. 2012;6:551–4. doi: 10.3855/jidc.2010. [DOI] [PubMed] [Google Scholar]
- 4.Weekly epidemiological record No 24, 16th June. 2000:193–6. [Google Scholar]
- 5.Mittal G, Ahmad S, Agarwal RK, Dhar M, Mittal M, Sharma S. Aetiologies of acute undifferentiated febrile illness in adult patients – An experience from a tertiary care hospital in Northern India. J Clin Diagn Res. 2015;9:DC22–4. doi: 10.7860/JCDR/2015/11168.6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joshi R, Colford JM, Jr, Reingold AL, Kalantri S. Nonmalarial acute undifferentiated fever in a rural hospital in central India: Diagnostic uncertainty and overtreatment with antimalarial agents. Am J Trop Med Hyg. 2008;78:393–9. [PubMed] [Google Scholar]
- 7.Thangarasu S, Natarajan P, Rajavelu P, Rajagopalan A, Seelinger Devey JS. A protocol for the emergency department management of acute undifferentiated febrile illness in India. Int J Emerg Med. 2011;4:57. doi: 10.1186/1865-1380-4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chrispal A, Boorugu H, Gopinath KG, Chandy S, Prakash JA, Thomas EM, et al. Acute undifferentiated febrile illness in adult hospitalized patients: The disease spectrum and diagnostic predictors — an experience from a tertiary care hospital in South India. Trop Doct. 2010;40:230–4. doi: 10.1258/td.2010.100132. [DOI] [PubMed] [Google Scholar]
- 9.Singh R, Singh SP, Ahmad N. A study of etiological pattern in an epidemic of acute febrile illness during monsoon in a tertiary health care institute of Uttarakhand, India. J Clin Diagn Res. 2014;8:MC01–03. doi: 10.7860/JCDR/2014/8965.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dengue guidelines for diagnosis, treatment, prevention and control. A joint publication of the World Health Organization (WHO) and the Special Programme for Research and Training in Tropical Diseases (TDR) 2009 [Google Scholar]
- 11.Mahapatra D, Sarangi G, Mahapatra A, Paty BP, Das P, Chayani N. NS1 antigen capture ELISA an effective method for diagnosis of early dengue infection - Report of an outbreak at Angul district, Odisha, India. J Clin Diagn Res. 2014;8:DC08–10. doi: 10.7860/JCDR/2014/8589.4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santhosh VR, Patil PG, Srinath MG, Kumar A, Jain A, Archana M. Sonography in the diagnosis and assessment of dengue fever. J Clinical Imaging Sci. 2014;4:14. doi: 10.4103/2156-7514.129260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asghar J, Farooq K. Radiological appearance and their significance in the management of dengue hemorrhagic fever. Pak J Med Health Sci. 2011;5:685–92. [Google Scholar]
- 14.Joshi P, Rathnam VG, Sharma S. USG findings in dengue haemorrhagic fever — Our experience in the recent epidemic. Ind J Radiol Imag. 1997;7:189–92. [Google Scholar]
- 15.Pramuljo HS, Harun SR. Ultrasound findings in dengue haemorrhagic fever. Pediatr Radiol. 1991;21:100–2. doi: 10.1007/BF02015615. [DOI] [PubMed] [Google Scholar]
- 16.Setiawan MW, Samsi TK, Pool TN, Sugianto D, Wulur H. Gallbladder wall thickening in dengue hemorrhagic fever: An ultrasonographic study. J Clin Ultrasound. 1995;23:357–62. doi: 10.1002/jcu.1870230605. [DOI] [PubMed] [Google Scholar]
- 17.Motla M, Manaktala S, Gupta V, Aggarwal M, Bhoi SK, Aggarwal P, et al. Sonographic evidence of ascites, pleura-pericardial effusion and gallbladder wall edema for dengue fever. Prehosp Disaster Med. 2011;26:335–41. doi: 10.1017/S1049023X11006637. [DOI] [PubMed] [Google Scholar]
- 18.Kariyawasam S, Senanayake H. Dengue infections during pregnancy: Case series from a tertiary care hospital in Sri Lanka. J Infect Dev Ctries. 2010;4:767–75. doi: 10.3855/jidc.908. [DOI] [PubMed] [Google Scholar]
- 19.Rosado León R, Muñoz Rodríguez MR, Soler Huerta E, Parissi Crivelli A, Méndez Machado GF. Dengue fever during pregnancy. Cases report. Ginecol Obstet Mex. 2007;75:687–90. [PubMed] [Google Scholar]
- 20.Goswami RP, Mukherjee A, Biswas T, Karmakar PS, Ghosh A. Two cases of dengue meningitis: A rare first presentation. J Infect Dev Ctries. 2012;6:208–11. doi: 10.3855/jidc.2241. [DOI] [PubMed] [Google Scholar]


