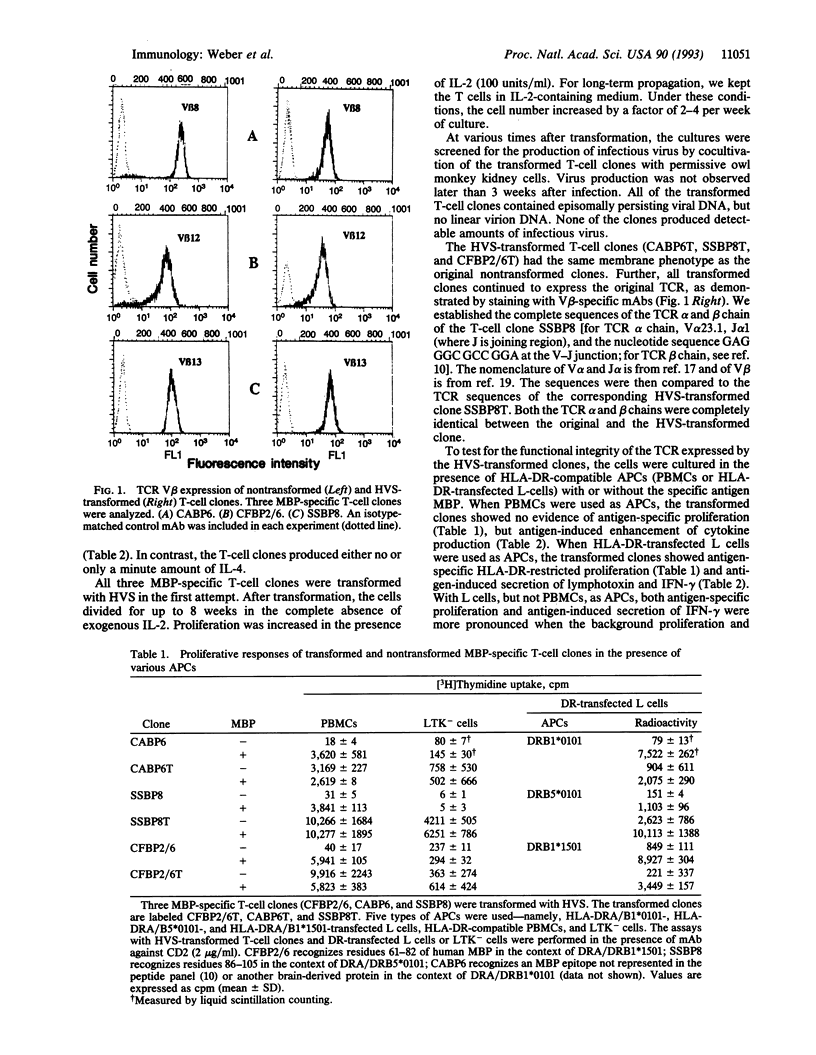
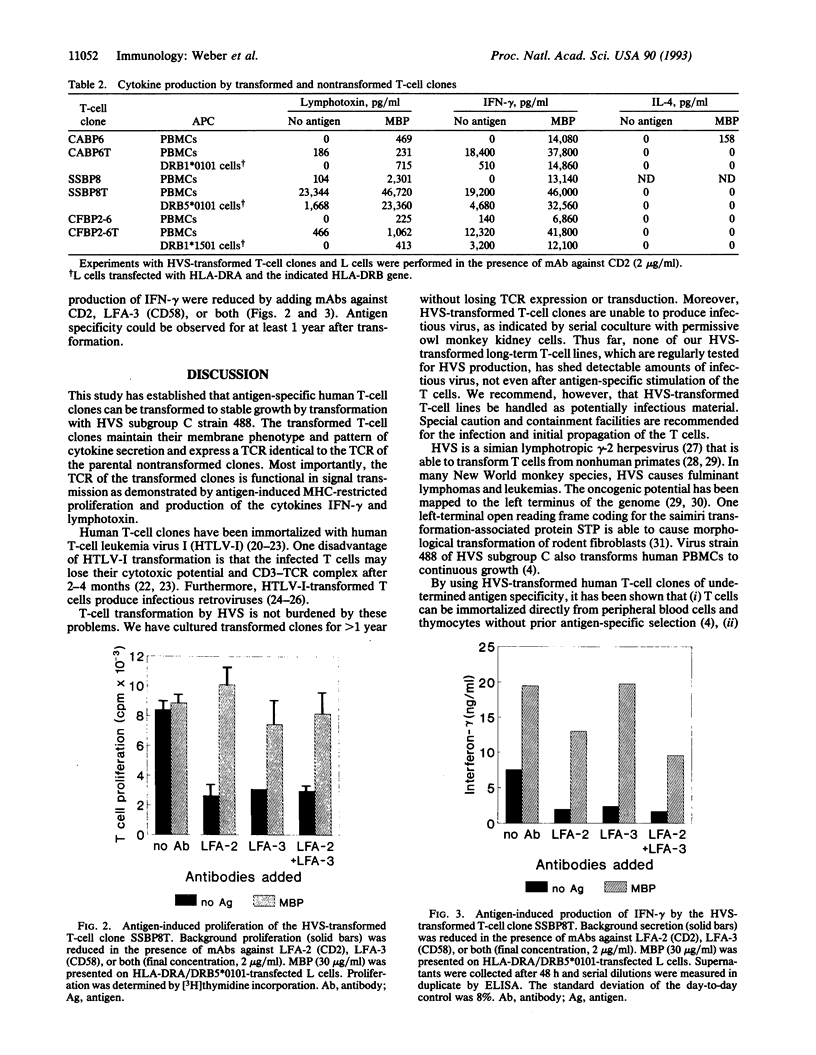
Abstract
Herpesvirus saimiri has recently been shown to immortalize human T cells. It was unknown, however, whether Herpesvirus saimiri transformation affects T-cell receptor (TCR) expression and signal transduction. In the present study, we have transformed CD4+ human T-cell clones specific for human myelin basic protein. The transformed T cells were grown in interleukin 2 and divided in the absence of antigen and antigen-presenting cells. They retained the membrane phenotype of activated T cells and secreted the cytokines interferon gamma and lymphotoxin, but interleukin 4 was not detected. Further, the transformed T cells continued to express the original TCR as demonstrated by TCR variable-region-V beta-specific monoclonal antibodies and TCR sequencing. Antigen-specific recognition and signal transduction by the TCR were demonstrated by myelin-basic-protein-induced HLA-DR-restricted secretion of interferon gamma and lymphotoxin and by myelin-basic-protein-specific proliferation. Antigen specificity and reactivity have been maintained for > 1 year after transformation. Transformation with Herpesvirus saimiri now allows the production of virtually unlimited numbers of (auto)antigen-specific T cells expressing functional TCR and a stable membrane phenotype. This technology will facilitate studies of the pathogenesis of putative autoimmune diseases, such as multiple sclerosis, and may be of help in TCR-targeted immunotherapy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Nun A., Wekerle H., Cohen I. R. The rapid isolation of clonable antigen-specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. Eur J Immunol. 1981 Mar;11(3):195–199. doi: 10.1002/eji.1830110307. [DOI] [PubMed] [Google Scholar]
- Biesinger B., Müller-Fleckenstein I., Simmer B., Lang G., Wittmann S., Platzer E., Desrosiers R. C., Fleckenstein B. Stable growth transformation of human T lymphocytes by herpesvirus saimiri. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3116–3119. doi: 10.1073/pnas.89.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesinger B., Trimble J. J., Desrosiers R. C., Fleckenstein B. The divergence between two oncogenic Herpesvirus saimiri strains in a genomic region related to the transforming phenotype. Virology. 1990 Jun;176(2):505–514. doi: 10.1016/0042-6822(90)90020-r. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C., Silva D. P., Waldron L. M., Letvin N. L. Nononcogenic deletion mutants of herpesvirus saimiri are defective for in vitro immortalization. J Virol. 1986 Feb;57(2):701–705. doi: 10.1128/jvi.57.2.701-705.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faller D. V., Crimmins M. A., Mentzer S. J. Human T-cell leukemia virus type I infection of CD4+ or CD8+ cytotoxic T-cell clones results in immortalization with retention of antigen specificity. J Virol. 1988 Aug;62(8):2942–2950. doi: 10.1128/jvi.62.8.2942-2950.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferradini L., Roman-Roman S., Azocar J., Michalaki H., Triebel F., Hercend T. Studies on the human T cell receptor alpha/beta variable region genes. II. Identification of four additional V beta subfamilies. Eur J Immunol. 1991 Apr;21(4):935–942. doi: 10.1002/eji.1830210412. [DOI] [PubMed] [Google Scholar]
- Gardella T., Medveczky P., Sairenji T., Mulder C. Detection of circular and linear herpesvirus DNA molecules in mammalian cells by gel electrophoresis. J Virol. 1984 Apr;50(1):248–254. doi: 10.1128/jvi.50.1.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohlfeld R. Neurological autoimmune disease and the trimolecular complex of T-lymphocytes. Ann Neurol. 1989 Jun;25(6):531–538. doi: 10.1002/ana.410250602. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Toyka K. V., Heininger K., Grosse-Wilde H., Kalies I. Autoimmune human T lymphocytes specific for acetylcholine receptor. Nature. 1984 Jul 19;310(5974):244–246. doi: 10.1038/310244a0. [DOI] [PubMed] [Google Scholar]
- Inatsuki A., Yasukawa M., Kobayashi Y. Functional alterations of herpes simplex virus-specific CD4+ multifunctional T cell clones following infection with human T lymphotropic virus type I. J Immunol. 1989 Aug 15;143(4):1327–1333. [PubMed] [Google Scholar]
- Jung J. U., Trimble J. J., King N. W., Biesinger B., Fleckenstein B. W., Desrosiers R. C. Identification of transforming genes of subgroup A and C strains of Herpesvirus saimiri. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7051–7055. doi: 10.1073/pnas.88.16.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R., McFarland H. F., McFarlin D. E. Immunological aspects of demyelinating diseases. Annu Rev Immunol. 1992;10:153–187. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- McFarlin D. E., McFarland H. F. Multiple sclerosis (first of two parts). N Engl J Med. 1982 Nov 4;307(19):1183–1188. doi: 10.1056/NEJM198211043071905. [DOI] [PubMed] [Google Scholar]
- Medveczky P., Szomolanyi E., Desrosiers R. C., Mulder C. Classification of herpesvirus saimiri into three groups based on extreme variation in a DNA region required for oncogenicity. J Virol. 1984 Dec;52(3):938–944. doi: 10.1128/jvi.52.3.938-944.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinl E., Weber F., Drexler K., Morelle C., Ott M., Saruhan-Direskeneli G., Goebels N., Ertl B., Jechart G., Giegerich G. Myelin basic protein-specific T lymphocyte repertoire in multiple sclerosis. Complexity of the response and dominance of nested epitopes due to recruitment of multiple T cell clones. J Clin Invest. 1993 Dec;92(6):2633–2643. doi: 10.1172/JCI116879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuya H., Guo H. G., Cossman J., Megson M., Reitz M. S., Jr, Broder S. Functional properties of antigen-specific T cells infected by human T-cell leukemia-lymphoma virus (HTLV-I). Science. 1984 Sep 28;225(4669):1484–1486. doi: 10.1126/science.6206569. [DOI] [PubMed] [Google Scholar]
- Mittrücker H. W., Müller-Fleckenstein I., Fleckenstein B., Fleischer B. CD2-mediated autocrine growth of herpes virus saimiri-transformed human T lymphocytes. J Exp Med. 1992 Sep 1;176(3):909–913. doi: 10.1084/jem.176.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi I., Kubonishi I., Yoshimoto S., Akagi T., Ohtsuki Y., Shiraishi Y., Nagata K., Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981 Dec 24;294(5843):770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- Murthy S. C., Trimble J. J., Desrosiers R. C. Deletion mutants of herpesvirus saimiri define an open reading frame necessary for transformation. J Virol. 1989 Aug;63(8):3307–3314. doi: 10.1128/jvi.63.8.3307-3314.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pette M., Fujita K., Wilkinson D., Altmann D. M., Trowsdale J., Giegerich G., Hinkkanen A., Epplen J. T., Kappos L., Wekerle H. Myelin autoreactivity in multiple sclerosis: recognition of myelin basic protein in the context of HLA-DR2 products by T lymphocytes of multiple-sclerosis patients and healthy donors. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7968–7972. doi: 10.1073/pnas.87.20.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Lange-Wantzin G., Sarin P. S., Mann D., Gallo R. C. Transformation of human umbilical cord blood T cells by human T-cell leukemia/lymphoma virus. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5402–5406. doi: 10.1073/pnas.80.17.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saruhan-Direskeneli G., Weber F., Meinl E., Pette M., Giegerich G., Hinkkanen A., Epplen J. T., Hohlfeld R., Wekerle H. Human T cell autoimmunity against myelin basic protein: CD4+ cells recognizing epitopes of the T cell receptor beta chain from a myelin basic protein-specific T cell clone. Eur J Immunol. 1993 Feb;23(2):530–536. doi: 10.1002/eji.1830230235. [DOI] [PubMed] [Google Scholar]
- Schirm S., Müller I., Desrosiers R. C., Fleckenstein B. Herpesvirus saimiri DNA in a lymphoid cell line established by in vitro transformation. J Virol. 1984 Mar;49(3):938–946. doi: 10.1128/jvi.49.3.938-946.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu Y., Wege H., Straus A., Ott M., Bannwarth W., Lanchbury J., Panayi G., Steinmetz M. The T-cell-receptor repertoire in the synovial fluid of a patient with rheumatoid arthritis is polyclonal. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8534–8538. doi: 10.1073/pnas.88.19.8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekerle H. Myelin specific, autoaggressive T cell clones in the normal immune repertoire: their nature and their regulation. Int Rev Immunol. 1992;9(3):231–241. doi: 10.3109/08830189209061793. [DOI] [PubMed] [Google Scholar]
- Wilkinson D., de Vries R. R., Madrigal J. A., Lock C. B., Morgenstern J. P., Trowsdale J., Altmann D. M. Analysis of HLA-DR glycoproteins by DNA-mediated gene transfer. Definition of DR2 beta gene products and antigen presentation to T cell clones from leprosy patients. J Exp Med. 1988 Apr 1;167(4):1442–1458. doi: 10.1084/jem.167.4.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraith D. C., McDevitt H. O., Steinman L., Acha-Orbea H. T cell recognition as the target for immune intervention in autoimmune disease. Cell. 1989 Jun 2;57(5):709–715. doi: 10.1016/0092-8674(89)90786-1. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig K. W., Weiner H. L., Hafler D. A. T-cell recognition of myelin basic protein. Immunol Today. 1991 Aug;12(8):277–282. doi: 10.1016/0167-5699(91)90126-E. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Okada M., Koyanagi Y., Kannagi M., Hinuma Y. Transformation of human leukocytes by cocultivation with an adult T cell leukemia virus producer cell line. Science. 1982 Aug 20;217(4561):737–739. doi: 10.1126/science.6980467. [DOI] [PubMed] [Google Scholar]
- Yssel H., de Waal Malefyt R., Duc Dodon M. D., Blanchard D., Gazzolo L., de Vries J. E., Spits H. Human T cell leukemia/lymphoma virus type I infection of a CD4+ proliferative/cytotoxic T cell clone progresses in at least two distinct phases based on changes in function and phenotype of the infected cells. J Immunol. 1989 Apr 1;142(7):2279–2289. [PubMed] [Google Scholar]
- Zamvil S. S., Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]



