Abstract
Background:
Alcohol and hypertriglyceridemia (HTG) are among the most common causes of acute pancreatitis (AP) after gallstones. However, differences in severity at the time of presentation and outcomes have not been well-studied.
Objective:
The aim of this study is to assess the differences between severity at presentation and outcomes of AP of hypertriglyceridemic and alcoholic origins.
Materials and Methods:
A retrospective review of 177 patients who were discharged with diagnosis of AP was performed. Severity at presentation was identified by the presence of systemic inflammatory response syndrome, bedside index for severity in AP (BISAP) score, and Balthazar index. Outcomes were measured by the length of stay, intensive care unit care, surgical intervention, and mortality.
Results:
We found 147 patients with alcoholic pancreatitis and 30 patients with hypertriglyceridemic pancreatitis. A larger percentage of hypertriglyceridemic pancreatitis patients (23.33%) had a BISAP score of ≥2 compared to the alcoholic group (12.24%). Only 32.65% of the patients with alcoholic pancreatitis but 60% of the patients with hypertriglyceridemic pancreatitis had the presence of systemic inflammatory response syndrome (SIRS) at admission (P = 0.0067). There were 73.34% hypertriglyceridemic pancreatits patients and only 40.28% alcoholic pancreatitis patients with Balthazar index C or greater, suggesting a higher disease burden at admission for hypertriglyceridemic pancreatitis patients (P = 0.0047). There was a statistically significant difference in the relative number of hypertriglyceridemic and alcoholic pancreatitis patients receiving intensive care (P = 0.00030) and in receiving surgical interventions related to pancreatitis (P = 0.016).
Conclusion:
Our study found that patients with hypertriglyceridemic pancreatitis have a greater severity of disease and they experience less favorable outcomes than patients with alcoholic pancreatitis.
Keywords: Alcoholic pancreatitis, hypertriglyceridemic pancreatitis, outcome, severity
Introduction
Hypertriglyceridemia (HTG) and alcoholism are among the most common causes of acute pancreatitis (AP) after gallstones. Elevated triglyceride levels, especially >1,000 mg/dL, are a well-recognized risk factor for the development of AP.[1] Animal studies have shown that infusion of triglycerides causes hyperamylasemia, inducing pancreatic edema and hemorrhage in canines.[2] The mainstay of treatment for AP remains supportive. Occasionally, treatment of hypertriglyceridemic acute pancreatitis (HTGP) differs from the management of acute alcoholic pancreatitis (AAP) by the use of heparin or insulin infusion and plasmapheresis.[3,4] It has been shown that HTG aggravates the severity of AP and it is also associated with higher mortality.[5] Elevated triglyceride levels have been shown to increase the severity and mortality in biliary pancreatitis.[6] In a study by Navarro,[7] HTGP patients had more severe disease and more complications than patients with biliary pancreatitis. Heavy alcohol intake is also a risk factor for the development of HTG and it has been suggested that alcohol use be stopped in patients with concurrent HTG.[8] However, in our knowledge there has been no study in the medical literature, which has specifically evaluated if patients with HTGP have a higher disease burden, worse outcomes, and higher treatment costs when compared to patients with AAP.
The purpose of this study was to determine if there was a difference in the severity of disease at the time of presentation as well as to determine the relative outcomes and treatment charges of patients with HTGP and AAP.
Materials and Methods
Study design
A retrospective cohort study of AP patients was conducted to compare the severity of the disease at the time of presentation and outcomes between HTG- and alcohol-induced pancreatitis.
Patient population
A retrospective review of the electronic medical records of all consecutive patients who were discharged from the Medical Center, Navicent Health, Macon, Georgia, the United States with a principal diagnosis of AP from January 2009 to June 2015 was performed. The Medical Center, Navicent Health is a tertiary care teaching hospital and is the second largest hospital in the state of Georgia. The study was approved by the joint Institutional Review Board (IRB) of the Medical Center and Mercer University. Patients in the study population were identified by the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) code 577.0. Patients who were <18 years or pregnant and who stayed less than 1 day at the hospital were excluded from the analysis. Patients who were transferred from other acute care hospitals with the diagnosis of pancreatitis were also excluded. If a patient had multiple admissions during the study period, only the first admission of those patients was included in the study as the index admission. The diagnosis of AP was again confirmed with revised Atlanta Criteria.[9] The causes of pancreatitis were determined and patients with AAP and HTGP were identified and included in the study. Patients were identified to have AAP if no other obvious cause of pancreatitis other than alcohol was found, and if alcohol was charted as the cause of pancreatitis. Patients with AAP who also had gallstones on imaging studies were excluded from the analysis. HTGP patients were identified if patients’ triglyceride levels were >1,000 mg/dL and no other obvious cause of pancreatitis was found. HTGP patients with alcohol use and gallstones on imaging were excluded from analysis. The demographic, clinical, laboratorial, and radiological data for all the eligible patients was also collected. If a patient had fibrosis or calcification in the pancreas on imaging studies suggesting chronic pancreatitis, he/she was excluded from analysis.
The clinical severity and outcome parameters
The following clinical severity admission parameters were recorded: Bedside index for severity in AP (BISAP) score,[10] systemic inflammatory response syndrome (SIRS), and Balthazar computed tomography (CT) scan grade.[11,12] BISAP score consists of: Blood urea nitrogen (BUN) >25 mg/dL, impaired mental status evidenced by disorientation or disturbance in mental status, presence of the SIRS, age >60 years, and pleural effusion on chest x-ray or CT scan. SIRS was defined by the presence of ≥2 of the following: Pulse >90 beats/min, respiration >20 breaths/min or partial pressure of carbon dioxide (PaCO2) <32 mmHg, temperature >100.4°F or <96.8°F, and white blood cell count >12,000 or <4,000 cells per mm3. Balthazar grading depends on the appearance of the pancreas on CT scan; it is graded from A to E with Grade A representing normal CT, Grade B representing focal or diffuse enlargement of the pancreas, Grade C representing pancreatic gland abnormalities and peripancreatic inflammation, Grade D representing fluid collection in a single location and the worst grade being Grade E, representing two or more collections and/or gas bubbles in or adjacent to the pancreas.
Outcomes were defined by the median length of stay (in days), need for intensive care unit (ICU) management, local pancreatic complications, need for surgical intervention in relation to pancreatitis, and mortality. Comorbidities were defined as the preexisting disease or conditions in addition to AP, and Charlson comorbidity index[13] modified with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9, CM) codes was used to assess the effect of comorbidities on severity and outcome.
Statistical analysis
Categorical variables were analyzed with the chi-square test or Fisher's exact test, and continuous variables were analyzed with the Wilcoxon rank-sum test. Two-tailed tests were performed, and a P value less than 0.05 was used to determine statistical significance. Statistical Analysis System (SAS) version 9.4 (SAS Institute, Cary, North Carolina, USA) was used for all analyses.
Results
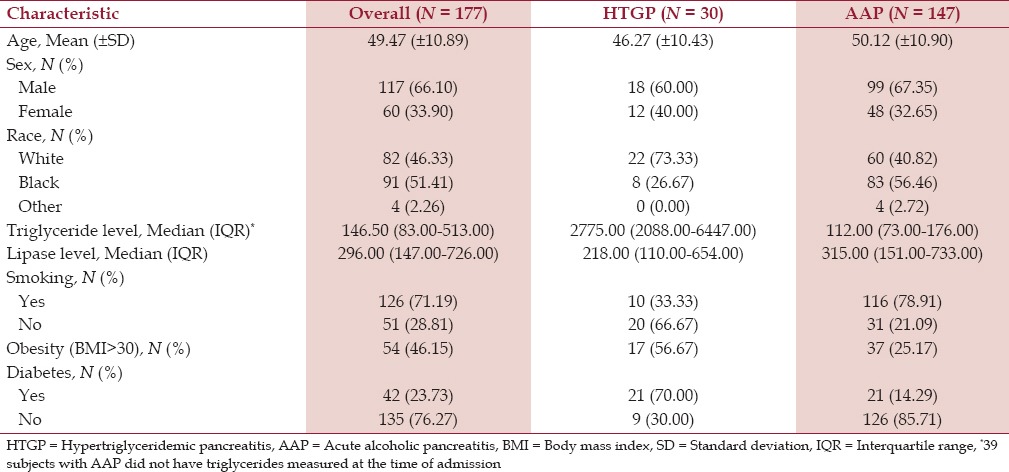
There were a total of 177 patients included in the study, out of which 147 (83.05%) had a final principal diagnosis of AAP and 30 (16.95%) of HTGP. The majority of the patients were males in both categories. The mean age in the HTGP group was 46.27 (±10.43) years while it was 50.12 (±10.90) years in the AAP group. The HTGP group had more obese patients (body mass index >30) than AAP patients. Similarly, diabetes was more prevalent in HTGP patients (70%) than in AAP patients (14.29%) [Table 1].
Table 1.
Characteristics of acute pancreatitis study patients
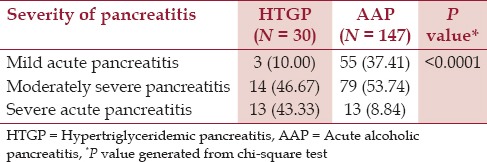
As per the modified Atlanta Criteria, 43.33% of HTGP patients had severe pancreatitis compared to only 8.84% of AAP patients [Figure 1 and Table 2].
Figure 1.
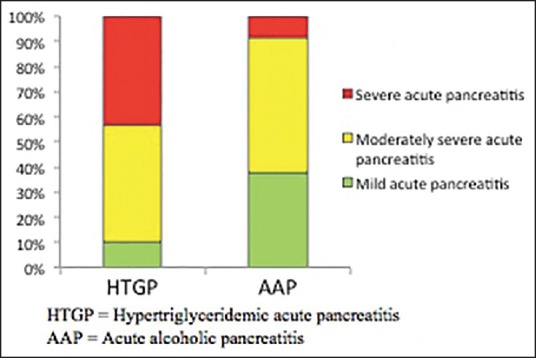
Revised Atlanta Criteria by the pancreatitis group HTGP = Hypertriglyceridemic pancreatitis, AAP = Acute alcoholic pancreatitis
Table 2.
Severity of pancreatitis as per modified Atlanta Criteria
Seven (23.33%) HTGP patients had BISAP score of 2 or more compared to 18 (12.24%) AAP patients who had a BISAP score of 2 or more. Only 32.65% of the patients in the AAP group versus 60% of the patients in the HTGP group had SIRS at the time of admission (P = 0.0067) [Table 3]. There were 73.34% HTGP patients and only 40.28% AAP patients with Balthazar index of C or greater, suggesting a higher disease burden at the time of admission for HTGP patients (P = 0.0047) [Table 3 and Figure 2].
Table 3.
Severity score comparisons between the patients with HTGP and AAP
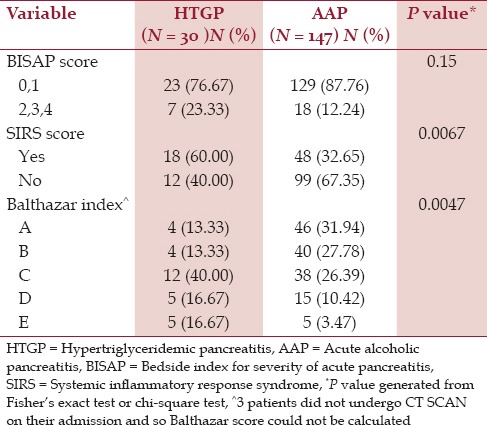
Figure 2.
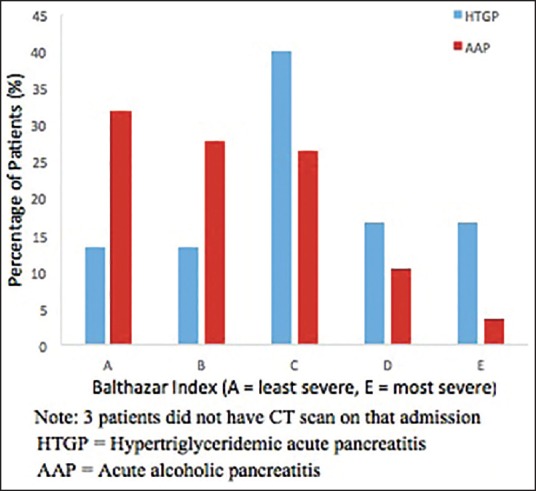
Balthazar index by the pancreatitis group HTGP = Hypertriglyceridemic pancreatitis, AAP = Acute alcoholic pancreatitis
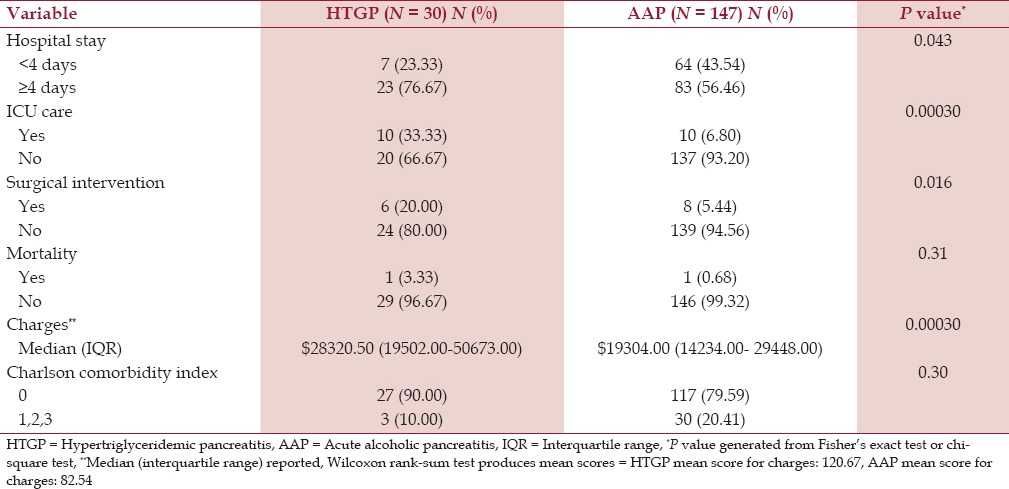
The median length of stay was 4 days, and a significantly higher number of patients in the HTGP group (76.67%) stayed for more than 4 days than patients with AAP (56.46%) (P = 0.043). There was a statistically significant difference in receiving ICU care (P = 0.0003) and in surgical interventions related to pancreatitis (P = 0.016). Only one patient died in each group. Hospital charges were significantly higher in the HTGP group with a median of $28320.50 compared to $19304.00 in the AAP group (P = 0.0003). The two groups were not statistically different with regard to the Charlson comorbidity index modified with ICD-9 codes but a greater percentage of AAP patients had a score greater than 0 (20.41%) compared to the HTGP patients (10%) [Table 4]. All patients with HTGP were treated with intravenous insulin infusion, with the exception of two patients who were treated with plasmapheresis.
Table 4.
Outcome comparisons between the patients with HTGP and AAP
Discussion
In our study, we hypothesized that there are differences in severity of disease and outcomes between HTGP and AAP patients. We found that HTGP patients had higher markers of severe pancreatitis than their alcoholic counterparts; HTGP patients also experienced worse outcomes.
It has been suggested that the clinical course of HTGP is no different from other causes of pancreatitis.[14] Balachandra et al.[15] demonstrated that HTG is not a risk factor in outcomes of pancreatitis and serum lipid levels do not modulate the severity of pancreatitis. In other studies, HTG has been found to be associated with a higher severity of AP of any cause. In one study, AP patients with HTG (>500 mg/dL) had higher 24 h Acute Physiology and Chronic Health Evaluation II (APACHE II) scores, and higher APACHE II scores are associated with more systemic complications and higher mortality.[5] In some of the cases, the authors have treated high levels of triglycerides (>1000 mg/dL) to specifically prevent the development of AP.[16,17,18]
Definition and classification of AP were updated in 2012 (Revised Atlanta Criteria).[9] We used these criteria to identify the patients with AP in our study. These revised criteria classify the severity of pancreatitis into mild, moderately severe, and severe AP to identify the patients who need early aggressive management and specialist referral. In our study, we found that about 43% of the cases with HTGP versus only about 9% of the cases with AAP had acute severe pancreatitis, showing that patients with HTGP may need early aggressive management and specialist referral.
The Balthazar CT severity index has been found to be better than Ranson's criteria and APACHE II scoring system in predicting the outcome of AP.[19,20] Abnormal triglyceride levels have been found to be an independent risk factor for CT severity grading in AP of any cause in one study.[21] Interestingly, all but three patients underwent CT scans during their index admission in our study cohort, which we think could have been because of their first AP episode although it is not recommended. In our study, we found more patients with HTGP had Balthazar index of C or greater, suggesting increased pancreatic inflammation and fluid collections in these patients than in patients with AAP.
The BISAP score was first proposed by Wu et al.,[10] and it was found to be an accurate score for risk stratification in patients with AP, with prognostic accuracy similar to APACHE II.[22] It has been found to be a reliable indicator for the early identification of patients with unfavorable outcomes.[23] In our study, we found that more patients with HTGP (23.33%) had a higher BISAP score (>2) than patients with AAP (12.24%) and this difference was statistically significant. Similarly, more than half of the patients with HTGP (60%) had SIRS at the time of admission versus only about one-third of the patients with AAP (32.65%).
We also demonstrated that patients with HTGP had a higher length of hospital stay and needed more ICU care than AAP patients, which increased the charges associated with the treatment. This could be due to the fact that HTGP is sometimes treated with modalities other than conservative management. HTGP has also been occasionally treated with use of plasmapheresis, heparin, and insulin infusion. Our study found that 28 patients were treated with intravenous insulin infusion and only two patients received plasmapheresis. None of the patients received heparin infusion for the treatment of HTGP. This could also account for the higher charges associated with the treatment of HTGP, which were demonstrated in our study.
This study has implications for clinical practice as it shows that patients with HTGP have more severe disease; therefore, these patients need close clinical observation at the time of admission. Patients with HTGP also needed more ICU management, indicating an increasingly complicated course of the disease. Despite these clinical implications, there were some limitations in our study. It was a retrospective study with a limited number of patients and no long-term outcome data were available. Patients with first admission during the study period (index admission) were included to avoid recurrent or chronic pancreatitis but there could have been study cases whose first admission was prior to the study period.
Conclusion
In conclusion, our study found that patients with hypertriglyceridemic pancreatitis have clinically more severe pancreatitis, higher disease burden, and worse outcomes than patients with AAP. Treatment of hypertriglyceridemic pancreatitis was found to be costlier than AAP. Further, larger prospective studies should be performed to confirm our results.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We would like to gratefully acknowledge Elizabeth May, MD for her critical review and helpful comments to improve this manuscript.
References
- 1.Tsuang W, Navaneethan U, Ruiz L, Palascak JB, Gelrud A. Hypertriglyceridemic pancreatitis: Presentation and management. Am J Gastroenterol. 2009;104:984–91. doi: 10.1038/ajg.2009.27. [DOI] [PubMed] [Google Scholar]
- 2.Saharia P, Margolis S, Zuidema GD, Cameron JL. Acute pancreatitis with hyperlipemia: Studies with an isolated perfused canine pancreas. Surgery. 1977;82:60–7. [PubMed] [Google Scholar]
- 3.Stefanutti C, Labbadia G, Morozzi C. Severe hypertriglyceridemia-related acute pancreatitis. Ther Apher Dial. 2013;17:130–7. doi: 10.1111/1744-9987.12008. [DOI] [PubMed] [Google Scholar]
- 4.Scherer J, Singh VP, Pitchumoni CS, Yadav D. Issues in hypertriglyceridemic pancreatitis: An update. J Clin Gastroenterol. 2014;48:195–203. doi: 10.1097/01.mcg.0000436438.60145.5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng LH, Xue P, Xia Q, Yang XN, Wan MH. Effect of admission hypertriglyceridemia on the episodes of severe acute pancreatitis. World J Gastroenterol. 2008;14:4558–61. doi: 10.3748/wjg.14.4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng L, Luo Z, Xiang K, Ren J, Huang Z, Tang L, et al. Clinical significance of serum triglyceride elevation at early stage of acute biliary pancreatitis. BMC Gastroenterol. 2015;15:19. doi: 10.1186/s12876-015-0254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navarro S, Cubiella J, Feu F, Zambón D, Fernández-Cruz L, Ros E. Hypertriglyceridemic acute pancreatitis. Is its clinical course different from lithiasic acute pancreatitis? Med Clin (Barc) 2004;123:567–70. doi: 10.1016/s0025-7753(04)74599-6. [DOI] [PubMed] [Google Scholar]
- 8.Klop B, do Rego AT, Cabezas MC. Alcohol and plasma triglycerides. Curr Opin Lipidol. 2013;24:321–6. doi: 10.1097/MOL.0b013e3283606845. [DOI] [PubMed] [Google Scholar]
- 9.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis – 2012: Revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–11. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 10.Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: A large population-based study. Gut. 2008;57:1698–703. doi: 10.1136/gut.2008.152702. [DOI] [PubMed] [Google Scholar]
- 11.Balthazar EJ, Ranson JH, Naidich DP, Megibow AJ, Caccavale R, Cooper MM. Acute pancreatitis: Prognostic value of CT. Radiology. 1985;156:767–72. doi: 10.1148/radiology.156.3.4023241. [DOI] [PubMed] [Google Scholar]
- 12.Balthazar EJ. Acute pancreatitis: Assessment of severity with clinical and CT evaluation. Radiology. 2002;223:603–13. doi: 10.1148/radiol.2233010680. [DOI] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Yadav D, Pitchumoni CS. Issues in hyperlipidemic pancreatitis. J Clin Gastroenterol. 2003;36:54–62. doi: 10.1097/00004836-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Balachandra S, Virlos IT, King NK, Siriwardana HP, France MW, Siriwardena AK. Hyperlipidaemia and outcome in acute pancreatitis. Int J Clin Pract. 2006;60:156–9. doi: 10.1111/j.1742-1241.2005.00645.x. [DOI] [PubMed] [Google Scholar]
- 16.Costantini N, Mameli A, Marongiu F. Plasmapheresis for preventing complication of hypertriglyceridemia: A case report and review of literature. Am J Ther. 2016;23:e288–91. doi: 10.1097/MJT.0000000000000079. [DOI] [PubMed] [Google Scholar]
- 17.Piolot A, Nadler F, Cavallero E, Coquard JL, Jacotot B. Prevention of recurrent acute pancreatitis in patients with severe hypertriglyceridemia: Value of regular plasmapheresis. Pancreas. 1996;13:96–9. doi: 10.1097/00006676-199607000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Chatzicostas C, Roussomoustakaki M, Vardas E, Romanos J, Kouroumalis EA. Balthazar computed tomography severity index is superior to Ranson criteria and APACHE II and III scoring systems in predicting acute pancreatitis outcome. J Clin Gastroenterol. 2003;36:253–60. doi: 10.1097/00004836-200303000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Leung TK, Lee CM, Lin SY, Chen HC, Wang HJ, Shen LK, et al. Balthazar computed tomography severity index is superior to Ranson criteria and APACHE II scoring system in predicting acute pancreatitis outcome. World J Gastroenterol. 2005;11:6049–52. doi: 10.3748/wjg.v11.i38.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen CH, Dai CY, Hou NJ, Chen SC, Chuang WL, Yu ML. Etiology, severity and recurrence of acute pancreatitis in southern Taiwan. J Formos Med Assoc. 2006;105:550–5. doi: 10.1016/S0929-6646(09)60149-2. [DOI] [PubMed] [Google Scholar]
- 21.Papachristou GI, Muddana V, Yadav D, O’Connell M, Sanders MK, Slivka A, et al. Comparison of BISAP, Ranson's, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol. 2010;105:435–42. doi: 10.1038/ajg.2009.622. [DOI] [PubMed] [Google Scholar]
- 22.Senapati D, Debata PK, Jenasamant SS, Nayak AK, Gowda S M, Swain NN. A prospective study of the Bedside Index for Severity in Acute Pancreatitis (BISAP) score in acute pancreatitis: An Indian perspective. Pancreatology. 2014;14:335–9. doi: 10.1016/j.pan.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Gao W, Yang HX, Ma CE. The value of BISAP score for predicting mortality and severity in acute pancreatitis: A systematic review and meta-analysis. PLoS One. 2015;10:e0130412. doi: 10.1371/journal.pone.0142025. [DOI] [PMC free article] [PubMed] [Google Scholar]


