Abstract
Background:
If postoperative acute pain remains unrelieved, it may result in significant morbidity and mortality. Preemptive analgesic initiated before surgery offers premature analgesia even before exposure to an initial noxious stimulus bestowing effective postoperative analgesia. In developed countries, it is regularly practiced as a part of well-defined protocol. In our country however, only a few centers practice it and that too irregularly and with undefined protocol. Few studies support preemptive analgesic efficacy of novel antiepileptic agent gabapentin. Though lamotrigine is a proven analgesic in animal models of chronic pain and clinical studies of gabapentin-resistant neuropathic pain, a literature search revealed scarce data on its preemptive analgesic efficacy.
Aims:
The present study is designed to study the preemptive analgesic efficacy of lamotrigine in comparison with diclofenac sodium in postoperative pain control.
Materials and Methods:
This randomized clinical trial included 90 patients of both sexes, between 18 years and 70 years undergoing major surgeries. Patients were randomly allocated into placebo, control, and test groups and received the respective treatment 30 min before the induction of anesthesia. Aldrete score and pain score were recorded using visual analog scale (VAS), facial rating scale (FRS), and behavioral rating scale (BRS) at awakening and at 1 h, 2 h, 4 h, 6 h, and 24 h. Postoperative rescue analgesic consumption for 24 h was recorded.
Results:
Significantly higher pain scores were observed in the placebo group postoperatively for 2 h on all pain scales (P < 0.05), whereas in the control group it was significantly higher at 1 h (P < 0.05). The test group patients were more comfortable throughout the study and postoperative analgesic requirement was significantly less (P < 0.05).
Conclusions:
The study recommends the use of single oral dose lamotrigine as preemptive analgesic for effective postoperative pain control.
Keywords: Modified Aldrete scoring system, behavioral rating scale (BRS), facial rating scale (FRS), preemptive analgesia, visual analog scale (VAS)
Introduction
Transmission of pain signals evoked by tissue damage leads to sensitization of the peripheral and central pain pathways.[1] Preemptive analgesia is defined as a treatment that is initiated before surgery in order to avert the sensitization stirred up by the incisional and inflammatory injuries occurring during surgery and in the early postoperative period.[2]
The concept of preemptive analgesia has gained popularity and previous studies have demonstrated the value of preemptive effect of some drugs such as opioid, local anesthetics, and nonsteroidal anti-inflammatory drugs.[3] Surgical procedures, even skin incisions, may result in this initial sensitization. These observations on the genesis and perception of pain led to the concept that analgesia administered before an initial noxious stimulus may be more effective than the same dose given later.[1,3]
Preemptive analgesic efficacy of gabapentin has been demonstrated earlier in a few studies.[4,5,6] Lamotrigine had demonstrated analgesic effects in animal models of neuropathic pain including painful diabetic neuropathy and gabapentin-resistant neuropathic pain. They are also reported to be effective in several forms of neuropathic pain in clinical studies.[7,8]
This class of drugs works via three major mechanisms, i.e., potentiation of gamma-aminobutyric acid (GABA) transmission, reduction of glutamate-mediated excitatory transmission, and blockade of voltage-activated ion channels. The later mechanism of action, in particular, is responsible for the success of the newer generation of antiepileptic drugs such as lamotrigine, gabapentin, and topiramate, which have all been shown to be effective in animal models of neuropathic pain.[7] Although these novel antiepileptic agents share the same mechanism for their efficacy in neuropathic pain, unlike gabapentin the preemptive analgesic efficacy of lamotrigine has not been extensively studied and our experience about these drugs in acute postoperative pain management is still limited.
Our literature review showed that there were no adequate previous studies to compare the preemptive analgesic effect of lamotrigine with established preemptive analgesics such as diclofenac sodium in patients who underwent major surgeries. Hence, we designed the present study to compare the preemptive analgesic efficacy of oral lamotrigine 100 mg with oral diclofenac sodium 100 mg in patients eligible for surgery under spinal anesthesia.
Materials and Methods
It was a randomized, double-blind, placebo-controlled clinical trial in which three parallel arms study group design was used. After getting approval from the Institutional Ethics Committee [Ref. Smt. Kashibai Navale Medical College and General Hospital (SKNMC) No/Ethics/App/228/2014] and written informed consent, the patients were randomly assigned to one of the study groups. Randomization was done with the help of OpenEpi software version 2.3 (Andrew G. Dean and Kevin M. Sullivan, Atlanta, GA, USA).
In this single dose pharmacodynamic study, 90 postoperative cases of both sexes between 18 years and 70 years and those operated under spinal anesthesia in surgical departments such as orthopedic/obstetrics-gynecology and surgery were included. Patients with diabetes, ischemic heart disease, stroke, malignancy, psychiatric diseases, pregnancy and lactation, and American Society of Anesthesiologists (ASA) III and IV patients were excluded. This study was performed in accordance with the Declaration of Helsinki.
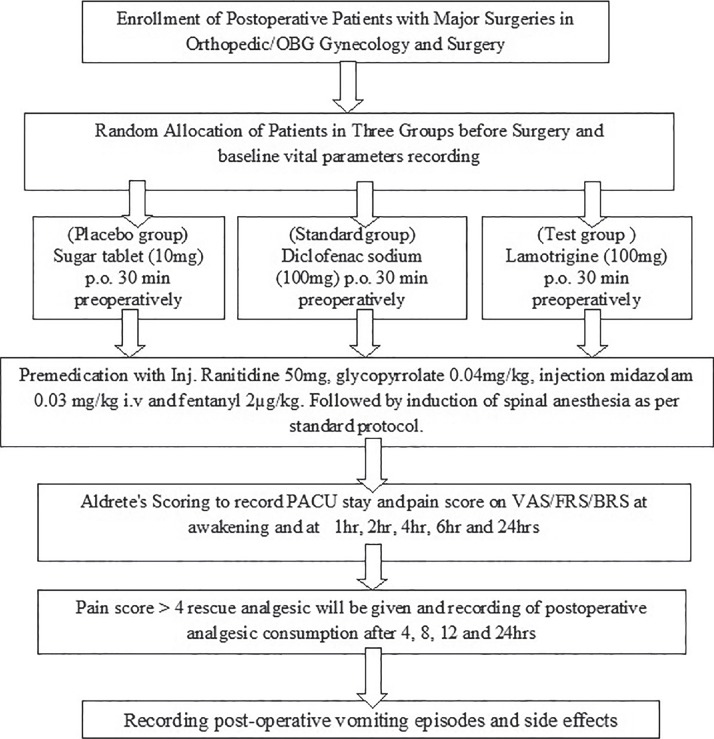
Premedication with injection ranitidine 50 mg, glycopyrrolate 0.04 mg/kg, injection midazolam 0.03 mg/kg, and fentanyl 2 μg/kg intravenous (IV) was followed by spinal anesthesia, performed in the right lateral position, with 26G Quincke needle (Becton, Dickinson and Company) at L3-4 interspace by a standard technique. After free flow of cerebrospinal fluid from each patient after the standard procedure of spinal anesthesia, each patient received 3 mL (15 mg) of 0.5% hyperbaric bupivacaine. The respective drug, i.e., sugar tablet 10 mg (placebo group), diclofenac 100 mg (control group), and lamotrigine 100 mg (test group) was given orally by one investigator 30 min prior to the induction of anesthesia and follow-up was done at awakening and at 1 h, 2 h, 4 h, 6 h, and 24 h by another investigator who was blinded. The baseline and postoperative periodic, i.e., (1 h, 2 h, 4 h, and 6 h) vital parameters such as pulse rate, systolic blood pressure (BP), diastolic BP, and peripheral capillary oxygen saturation (SpO2) of all the patients were recorded. Postoperative level of analgesia was noted on visual analog scale (VAS),[9] facial rating scale (FRS), and behavioral rating scale (BRS),[10] at awakening, and at 1 h, 2 h, 4 h, 6 h, and 24 h; when pain score noted was >4 on the VAS,[9,11] rescue analgesic diclofenac 50-100 mg was given and postoperative analgesic consumption for 24 h was recorded. Maximum dose of diclofenac was limited to 300 mg in 24 h. The duration of postoperative anesthesia care unit (PACU) stay was recorded by using modified Aldrete scoring system; patients with score >9 were shifted to the ward.[12] Postoperative vomiting episodes and side effects were also recorded [Figure 1].
Figure 1.
Study flowchart
Statistical analysis
The sample size was calculated by using OpenEpi statistical software, expecting a mean difference of pain score on VAS of 2 and standard deviation (SD) of 2 at an α-error of 5%, power of 80, and a 1:1:1 ratio between the test, control, and placebo groups; the minimum required sample size was 28:28:28, rounded up to 30 patients in each group.
The data were analyzed using analysis of variance followed by post hoc Tukey's statistical test; Student's t-test was used for comparison of the means of continuous variables and normally distributed data and Mann–Whitney U test (nonparametric test) using OpenEpi (version 2.3) and SciStatCalc version 1.3, 2013, Alijah Ahmed, software, respectively. P < 0.05 was considered significant.
Results
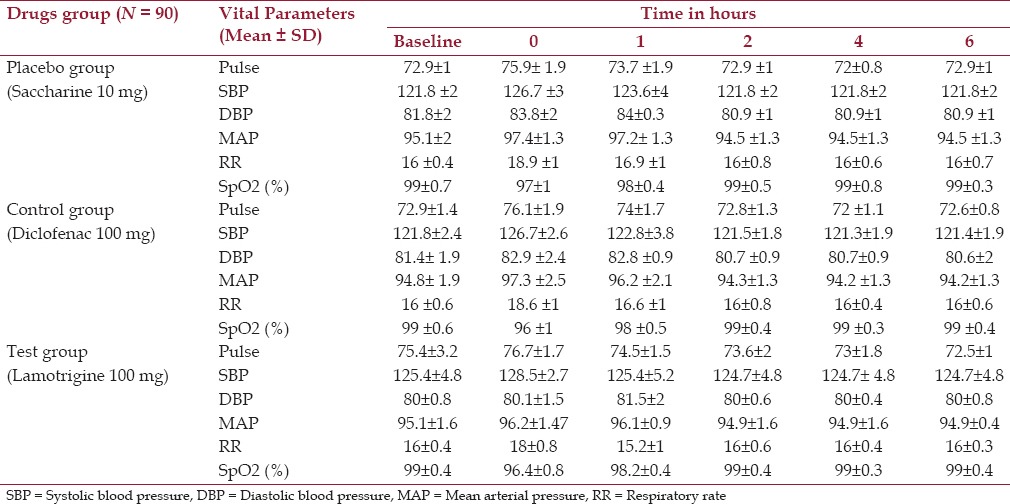
In this randomized, double-blind, placebo-controlled study, we have assessed the preemptive analgesic efficacy of a single oral dose of lamotrigine (100 mg) on postoperative pain management. The demographic profiles of these patients were also studied and are presented in Table 1. There were 58 males and 32 females (mean age: 46.8 years) enrolled in this study. The effect of preemptive analgesia on postoperative vital parameters showed no significant difference among the groups, as presented in Table 2.
Table 1.
Baseline demographic profile of the patients undergoing surgeries
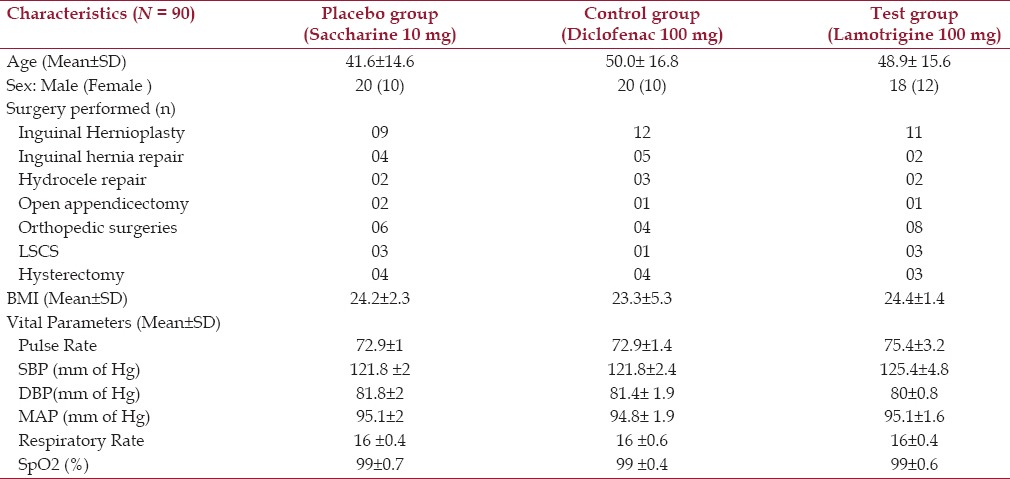
Table 2.
Effect on postoperative vital parameters (Pulse, BP, MAP, RR and SpO2%)
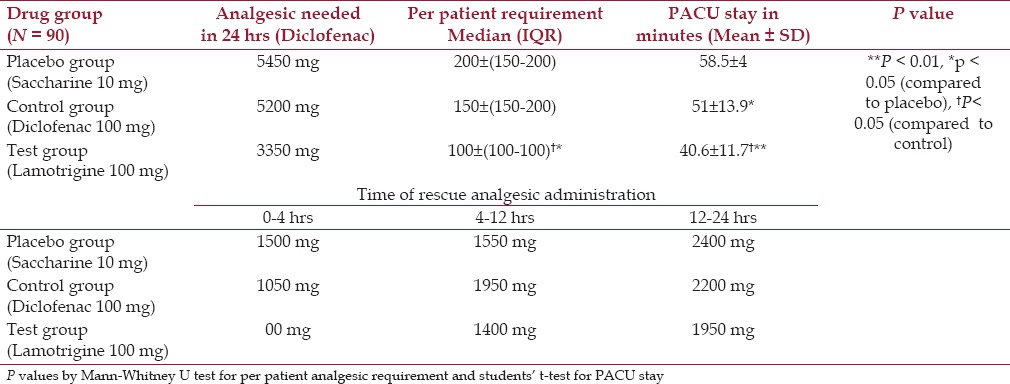
From the results of this study, it becomes evident that the preemptive lamotrigine administration yields an encouraging outcome. The significantly higher pain score was observed in the placebo group on all the three scales at 1 h and further till 2 h (P < 0.05) compared to the test group while it was recorded to be significantly higher on VAS and BRS for 1 h (P < 0.05) compared to the control group. Pain scores among the test and control groups were comparable except on VAS till 2 h and FRS for 1 h, which were found to be significant (P < 0.05) in the control group [Table 3]. Patients with placebo, the control group as well as the test group were more or less comfortable during the study; however, test group patients were more comfortable throughout the study as observed with pain scales and adverse effects in 24 h [Table 3]. Analgesic requirement was significantly more with the placebo group and control group as compared to the test group (P < 0.05), whereas PACU stay was also recorded to be significantly less in test group patients compared to the placebo group (P < 0.001) and control group (P < 0.05) [Table 4].
Table 3.
Effect on postoperative VAS, facial and behavioral pain score
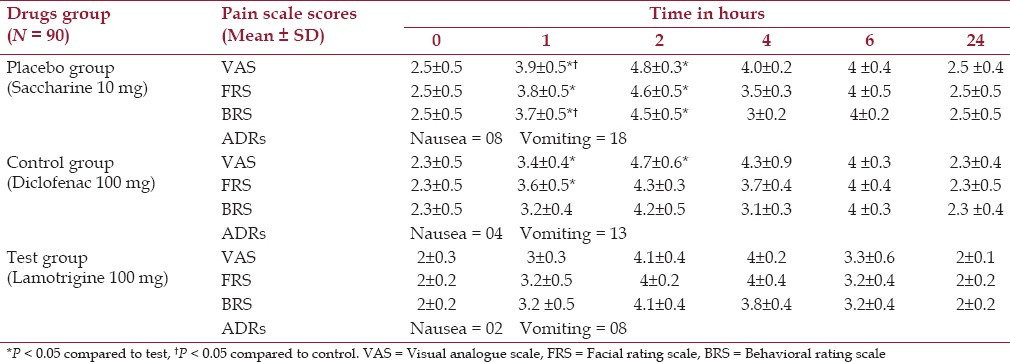
Table 4.
Postoperative rescue analgesic requirement and PACU stay in study subjects
Discussion
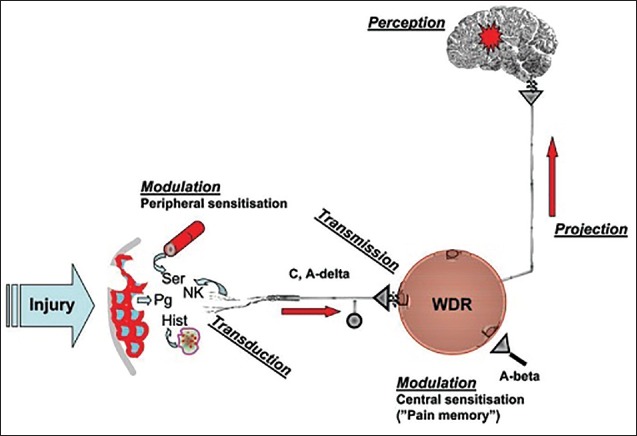
Algesia or pain is an unpleasant sensation usually stirred up by an external or internal noxious stimulus. These noxious stimuli are detected by the free endings of peripheral primary afferent neurons, altogether called nociceptors. At the site of the stimulus the nociceptors act as transducers, converting chemical, mechanical, or thermal energy to electrical activity, which is then conducted to the dorsal horn of the central nervous system (CNS) [Figure 2]. The myelinated Aδ nociceptors are specialized in detecting mechanical and thermal injuries, triggering a rapid sharp pain response, termed “first pain.” The unmyelinated C nociceptors respond to strong mechanical, thermal, and/or chemical stimuli, and mediate delayed burning pain response, termed “second pain.”[2]
Figure 2.
Tissue damage initiated alterations of the peripheral and central pain pathways outlasting the stimuli that trigger a “pain memory”
In dorsal horn, the pain signals are transmitted from the nociceptors to secondary nociceptive neurons. Nociceptive-specific (NS) neurons respond only to pain signals in Aδ and C nociceptors, whereas wide dynamic range (WDR) neurons respond to both nonnociceptive impulses in Aβ fibers (e.g., touch) and nociceptive impulses in Aδ and C nociceptors [Figure 2]. The activity of these neurons is determined by the convergence of excitatory and inhibitory inputs from peripheral nerve fibers, local circuit excitatory and inhibitory neurons, and descending inputs from supraspinal sites.[2]
Pain signals from damaged tissue are not transmitted to the CNS through “hard-wired” pathways. In contrast, nociceptive signals, once initiated, launch a cascade of alterations in the somatosensory system, with increased responsiveness of both peripheral and central pain pathways. These alterations increase the response to subsequent stimuli and thus, cause pain amplification.[1] The underlying cellular mechanism is long-term potentiation (LTP) of synaptic strength, a mechanism also described to be the basis of pain memory formation.[13] LTP at the first nociceptive synapse is regarded as a cellular model of hyperalgesia induced by noxious stimulation. As only anesthesia without additional analgesia is not sufficient to protect the spinal cord from intraoperative noxious input, LTP in spinal nociceptive pathways may heighten acute postoperative pain.[14]
Preemptive analgesia is a treatment that is initiated before the surgical procedure in order to “preempt” or prevent neurophysiological and biochemical consequences of a noxious input to the CNS provoked by the procedure.[15] It means that the concept of preemptive analgesia is not to prevent pain but to prevent CNS alterations leading to pain amplification following first pain experience and development of chronic pain syndromes.[16] Considering this “protective” effect on the nociceptive pathways, preemptive analgesia has the potential to be more effective than a similar analgesic treatment initiated after surgery. Consequently, immediate postoperative pain may be reduced and the development of chronic pain may be prevented.[17]
Diclofenac sodium is an established preemptive analgesic with extensively studied preemptive analgesic efficacy in earlier studies.[18,19,20] In the present placebo controlled study, it was used as control. Preemptive analgesic efficacy of lamotrigine was studied in comparison with placebo and control. Results of the present study are strongly suggestive of preemptive analgesic efficacy of lamotrigine, as patients were more comfortable throughout the study as evident from significantly lesser pain score, PACU stay, and postoperative analgesic requirement in the lamotrigine-treated group. These results are in agreement with a few clinical studies that mention novel antiepileptics such as gabapentin impart preemptive analgesic effect.[4,5,6] However, we could not come across any study with lamotrigine preemptive analgesia. Hence, the results of the present study may be a fruitful accumulation in the data favoring the use of novel antiepileptics for preemptive analgesia.
Lamotrigine inhibits the voltage-gated sodium and calcium channels. Inhibition of the voltage-gated sodium channel stabilizes the presynaptic neuronal membrane, thus preventing the release of excitatory neurotransmitters such as glutamate and inhibiting sustained repetitive neuronal firing. These merits of lamotrigine are suggestive of its antinociceptive properties.[7] Lamotrigine has weak 5-hydroxytryptamine-3 (5-HT3) receptor inhibitory action as well.[21] Lamotrigine-mediated blockade of supraspinal 5-HT3 receptors in the spinal descending pronociceptive pathway[22] may also have contributed to its antinociceptive potential. Superior preemptive analgesic efficacy of lamotrigine compared to diclofenac can be explained on the basis of these multimodal actions.
The limitation of this study is that the preemptive analgesic efficacy was studied only in patients operated under spinal anesthesia; patients operated under general anesthesia as well need to be studied. Furthermore, we have evaluated the preemptive analgesic efficacy of a single oral dose of only 100 mg lamotrigine; the single highest safe dose needs to be evaluated to offer maximal postoperative pain control through practicing preemptive analgesia.
Conclusions
In the light of results of the present study, preemptive use of novel antiepileptics such as lamotrigine is strongly recommended to prevent CNS sensitization leading to pain amplification by the incisional and inflammatory injuries occurring during surgery. Immediate postoperative pain may be reduced and the development of chronic pain may be prevented with effective use of preemptive analgesic. However, more studies need to be conducted to strengthen the findings of our study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors are thankful to Dr. A. V. Bhore, Dean of SKNMC and Dr. R. W. Naphade, Head of the Department (HOD) Anesthesia for providing the facilities to perform the experiments of this work.
References
- 1.Dahl JB, Møiniche S. Pre-emptive analgesia. Br Med Bull. 2004;71:13–27. doi: 10.1093/bmb/ldh030. [DOI] [PubMed] [Google Scholar]
- 2.Mishra AK, Afzal M, Mookerjee SS, Bandyopadhyay KH, Paul A. Pre-emptive analgesia: Recent trends and evidences. Indian J Pain. 2013;27:114–20. [Google Scholar]
- 3.Kashefi P, Honarmand A, Safavi M. Effects of preemptive analgesia with celecoxib or acetaminophen on postoperative pain relief following lower extremity orthopedic surgery. Adv Biomed Res. 2012;1:66. doi: 10.4103/2277-9175.100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandey CK, Priye S, Singh S, Singh U, Singh RB, Singh PK. Preemptive use of Gabapentin significantly decreases postoperative pain and rescue analgesic requirements in laparoscopic cholecystectomy. Can J Anaesth. 2004;51:358–63. doi: 10.1007/BF03018240. [DOI] [PubMed] [Google Scholar]
- 5.Parikh HG, Dash SK, Upasani CB. Study of the effect of oral gabapentin used as preemptive analgesia to attenuate post-operative pain in patients undergoing abdominal surgery under general anesthesia. Saudi J Anaesth. 2010;4:137–41. doi: 10.4103/1658-354X.71409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grover VK, Mathew PJ, Yaddanapudi S, Sehgal S. A single dose of preoperative gabapentin for pain reduction and requirement of morphine after total mastectomy and axillary dissection: Randomized placebo-controlled double-blind trial. J Postgrad Med. 2009;55:257–60. doi: 10.4103/0022-3859.58928. [DOI] [PubMed] [Google Scholar]
- 7.Paudel KR, Bhattacharya S, Rauniar G, Das B. Comparison of antinociceptive effect of the antiepileptic drug gabapentin to that of various dosage combinations of gabapentin with lamotrigine and topiramate in mice and rats. J Neurosci Rural Pract. 2011;2:130–6. doi: 10.4103/0976-3147.83577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vinik AI, Tuchman M, Safirstein B, Corder C, Kirby L, Wilks K, et al. Lamotrigine for treatment of pain associated with diabetic neuropathy: Results of two randomized, double-blind, placebo-controlled studies. Pain. 2007;128:169–79. doi: 10.1016/j.pain.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 9.Panah Khahi M, Yaghooti AA, Marashi SH, Nadjafi A. Effect of pre-emptive gabapentin on postoperative pain following lower extremity orthopedic surgery under spinal anesthesia. Singapore Med J. 2011;52:879–82. [PubMed] [Google Scholar]
- 10.Cohen LL, Lemanek K, Blount RL, Dahlquist LM, Lim CS, Palermo TM, et al. Evidence-based assessment of pediatric pain. J Pediatr Psychol. 2008;33:939–57. doi: 10.1093/jpepsy/jsm103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khoromi S, Patsalides A, Parada S, Salehi V, Meegan JM, Max MB. Topiramate in chronic lumbar radicular pain. J Pain. 2005;6:829–36. doi: 10.1016/j.jpain.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Cohen LB, Delegge MH, Aisenberg J, Brill JV, Inadomi JM, Kochman ML, et al. AGA Institute review of endoscopic sedation. Gastroenterology. 2007;133:675–701. doi: 10.1053/j.gastro.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Drdla R, Sandkühler J. Long-term potentiation at C-fibre synapses by low-level presynaptic activity in vivo . Mol Pain. 2008;4:18. doi: 10.1186/1744-8069-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruscheweyh R, Wilder-Smith O, Drdla R, Liu XG, Sandkühler J. Long-term potentiation in spinal nociceptive pathways as a novel target for pain therapy. Mol Pain. 2011;7:20. doi: 10.1186/1744-8069-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kissin I. Preemptive analgesia. Anaesthesiology. 2000;93:1138–43. doi: 10.1097/00000542-200010000-00040. [DOI] [PubMed] [Google Scholar]
- 16.Werner M, Søholm L, Rotbøll-Nielsen P, Kehlet H. Does an acute pain service improve postoperative outcome? Anesth Analg. 2002;95:1361–72. doi: 10.1097/00000539-200211000-00049. [DOI] [PubMed] [Google Scholar]
- 17.Woolf CJ, Chong MS. Preemptive analgesia — Treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362–79. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
- 18.Campbell WI, Kendrick R, Patterson C. Intravenous diclofenac sodium. Does its administration before operation suppress postoperative pain? Anaesthesia. 1990;45:763–6. doi: 10.1111/j.1365-2044.1990.tb14450.x. [DOI] [PubMed] [Google Scholar]
- 19.Canbay O, Karakas O, Celebi N, Peker L, Coskun F, Aypar U. The preemptive use of diclofenac sodium in combination with ketamine and remifentanil does not enhance postoperative analgesia after laparoscopic gynecological procedures. Saudi Med J. 2006;27:642–5. [PubMed] [Google Scholar]
- 20.Goel P, Kothari S, Gupta N, Kumar A, Chaturvedi SK. Pre-emptive analgesia with iv paracetamol and iv diclofenac sodium in patients undergoing various surgical procedures: A comparative study. Int J Biol Med Res. 2013;4:3294–300. [Google Scholar]
- 21.Thomas SP, Nandhra HS, Jayaraman A. Systematic review of lamotrigine augmentation of treatment resistant unipolar depression (TRD) J Ment Health. 2010;19:168–75. doi: 10.3109/09638230903469269. [DOI] [PubMed] [Google Scholar]
- 22.Bhosale UA, Khobragade R, Naik C, Yegnanarayan R, Kale J. Randomized, double-blind, placebo-controlled study to investigate the pharmacodynamic interaction of 5-HT3 antagonist ondansetron and paracetamol in postoperative patients operated in an ENT department under local anesthesia. J Basic Clin Physiol Pharmacol. 2015;26:217–22. doi: 10.1515/jbcpp-2014-0070. [DOI] [PubMed] [Google Scholar]