Abstract
STUDY QUESTION
Are perinatal outcomes improved in singleton pregnancies resulting from fresh embryo transfers performed following unstimulated/natural cycle IVF (NCIVF) compared with stimulated IVF?
SUMMARY ANSWER
Infants conceived by unstimulated/NCIVF have a lower risk of being low birthweight than infants conceived by stimulated IVF; however, this risk did not remain significant after adjusting for gestation age.
WHAT IS ALREADY KNOWN
Previous studies have shown that infants born after modified NCIVF have a higher average birthweight and are less likely to be low birthweight than those infants conceived with conventional stimulated IVF.
STUDY DESIGN, SIZE AND DURATION
Retrospective cohort study of singleton live births in non-smoking women undergoing fresh IVF–embryo transfer cycles from 2007 to 2013 in a single IVF center. The women were stratified by stimulated (n = 174) or unstimulated (n = 190) IVF exposure status. Unstimulated/NCIVF is defined as IVF without the use of exogenous gonadotrophins, and only includes the use of HCG to time oocyte retrieval.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Demographic data including maternal age, BMI, infertility diagnosis and IVF cycle characteristics were collected. The perinatal outcomes used for comparison between the two study groups were length of gestation, birthweight, preterm delivery, very preterm delivery, low birthweight, small for gestational age and large for gestational age.
MAIN RESULTS AND ROLE OF CHANCE
Although women in the NCIVF group were older than those in the stimulated group (35.0 versus 34.2 years, P < 0.05), parity and history of prior ART cycles were comparable between the groups. The mean birthweight was significantly higher in the NCIVF group by 163 g than in the stimulated group (3436 ± 420 g versus 3273 ± 574 g, P < 0.05). Consistent with this finding, there were also less low birthweight (<2500 g) infants in the NCIVF group versus stimulated group (1 versus 8.6%, P < 0.005). The reduction in risk for low birthweight in the NCIVF group remained significant after adjustment for maternal age, infertility diagnosis, ICSI, number of embryos transferred and blastocyst transfer (odds ratio (OR) 0.07; 95% CI 0.014–0.35). As NCIVF group had less preterm infants, additional adjustment for gestational age was performed and this showed a tendency towards lower risk of low birthweight in NCIVF (OR 0.11; 95% CI 0.01–1.0). While gestational age at delivery was comparable between the groups, both preterm births (<37 weeks gestation) (31 versus 42%, P < 0.05) and very preterm births (<32 weeks gestation) (0.52 versus 6.3%, P < 0.005) were significantly reduced in the NCIVF group. However, after adjustment for potential confounders, the reduction in risk of preterm and very preterm delivery associated with the NCIVF group was no longer significant (OR 1.1; 95% CI 0.48–2.5).
LIMITATIONS, REASONS FOR CAUTION
Limitations of this study are the retrospective nature of the data collection and the lack of information about parental characteristics associated with birthweight.
WIDER IMPLICATIONS OF THE FINDINGS
The improved perinatal outcomes following successful unstimulated/NCIVF suggest that this treatment should be considered as a viable option for infertile couples. NCIVF could reduce potential adverse perinatal outcomes such as low birthweight related to fresh embryo transfers performed following ovarian stimulation. The etiology of the improved perinatal outcomes following NCIVF needs to be explored further to determine if the improvement is derived from endometrial factors versus follicular/oocyte factors.
STUDY FUNDING/COMPETING INTERESTS
The study was supported by the following grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NICHD K12HD047018 (W.M.), NICHD K12HD001271 (L.A.K.). The authors have no competing interests.
Keywords: unstimulated, stimulated, natural cycle, in vitro fertilization, obstetric, neonatal, outcomes, preterm birth, low birthweight
Introduction
The era of Assisted Reproductive Technologies (ART) started over 35 years ago with the in vitro conception and subsequent birth of Louise Brown. Since Louise's birth, ART has rapidly evolved to incorporate ovulation induction, ICSI, extended embryo culture, preimplantation diagnosis (PGD) and cryopreservation of embryos and oocytes. It is estimated that 1–3% of children born in developed countries are conceived through ART. In the most recent world report by the International Committee for Monitoring ART over 237 000 infants were born worldwide in a single year (Sullivan et al., 2013). The vast majority of ART babies are healthy, however, in recent years, health concerns about ART conceived children have surfaced. Several large epidemiological studies have found that ART conceived infants have an increased risk of adverse perinatal outcomes such as preterm delivery and low birthweight, and that this risk is present even in singleton pregnancies (Schieve et al., 2002; Jackson et al., 2004; Poikkeus et al., 2007; Declercq et al., 2015).
The underlying etiologies of these adverse perinatal outcomes are largely unknown, but contributing factors include the subfertility of the couple, the use of hormonal stimulation and the use of various ART techniques (Kondapalli and Perales-Puchalt, 2013; Pinborg et al., 2013).
Louise Brown was conceived through natural cycle IVF (NCIVF). Although ART can succeed without ovarian stimulation, it was introduced to facilitate the success of laparoscopic oocyte retrieval. In fact, the recruitment of more than one ovarian follicle may no longer be necessary to achieve acceptable pregnancy rates. For example live birth rates for NCIVF can vary between 15.2 and 35–50% (Allersma et al., 2013; Gordon et al., 2013). The major advantages of unstimulated cycles are the avoidance of exogenous gonadotrophin stimulation, which minimizes the side effects of controlled ovarian stimulation, and except in rare cases, leads to transfer of only a single embryo, reducing multiple gestation rates. In addition, there is evidence that infants conceived after minimal stimulation IVF may have better perinatal outcomes with respect to birthweight (Pelinck et al., 2010). These investigators found a mean birthweight difference of 134 g between infants conceived through minimal stimulation IVF (n = 84) compared with those conceived following conventional IVF (n = 106). However, the study had a small sample size. Since then, another study examined the pregnancy and perinatal outcomes following unstimulated (or NCIVF) compared with conventional stimulated IVF. These authors used the Japanese ART registry that provided a much larger cohort of infants, and this study showed an increased risk of low birthweight in the stimulated IVF group (n = 6336; OR 1.72, CI 1.17–2.62) compared with the NCIVF group (n = 610) (Nakashima et al., 2013). Both of these studies suggest an increase in birthweight and lower likelihood of low birthweight in infants after conception with NCIVF.
The objective of our study was to compare births following fresh embryo transfer in NCIVF versus stimulated IVF in order to examine the role that ovarian stimulation might play in perinatal outcomes.
Materials and Methods
Ethical approval
The study was submitted for independent institutional review board for approval to carry out a retrospective analysis of our patient medical records and deemed exempt.
Patients
All treatments were performed at a private fertility center between 2007 and 2013. All patients under age of 40 years were considered eligible for either stimulated or NCIVF cycles regardless of a history of failed IVF cycles, the results of ovarian reserve testing or the need for TESE with ICSI. All patients with regular menstrual cycles seen at our practice are offered both NCIVF and conventional IVF cycles with full financial disclosures; however, the final decision on treatment modality is based on patients' preference. All fresh/non-donor/non-PGD/PGS ART cycles of women <40 years carried out in our center during the study period were reviewed. In brief, only singleton live births were included in the final analysis study. Patients were excluded from the study if they were smokers or their smoking status was unknown or if the initial pregnancy ultrasound revealed a multiple gestation even if subsequent studies demonstrated only a singleton pregnancy (vanishing twin). In addition, patients were excluded if their chart lacked important data such as diagnosis type, demographics data etc. (incomplete chart) (Fig. 1).
Figure 1.
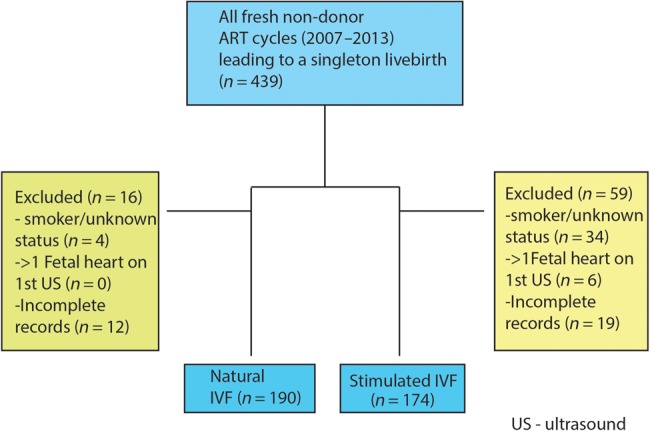
Flowchart describing the selection criteria of our study cohort. ART, assisted reproduction technology; US, ultrasound.
NCIVF was performed as previously described (DiMattina et al., 2014). All patients had regular menstrual cycles 23–36 days in length. Neither ovarian stimulation nor GnRH antagonists were used. In brief, the patient's cycle was monitored beginning on Day 2 or Day 3 of their menstrual cycle and again on Day 7 using transvaginal ultrasound and serum estradiol (E2), progesterone (P4) and LH testing (Immulite 2000, Siemens, Erlangen, Germany) until follicle maturity. Final follicular maturation was induced with 10 000 units of HCG (Pregnyl, Organon, Roseland, NJ, USA). Since 2008 the criteria for follicular maturity has been a mean follicular diameter of ≥ 15 mm and a serum E2 level of >100 pg/ml. Oocyte retrieval occurred 34 h later using transvaginal ultrasound-guided aspiration using a 17-gauge single channel IVF aspiration needle (Cook EchoTip Norfolk aspiration needle, Cook Medical, Bloomington, Indiana) with multiple flushing of the follicle (up to 12 flushes) using modified human tubal fluid (HTF) medium (Irvine Scientific, Irvine, CA, USA) until the oocyte was recovered. ICSI was performed in over 90% of NCIVF cycles with the exception being couples with proven fertility or who declined. Initially, the majority of embryo transfers were performed on Day 3. After 2009, the center adopted a blastocyst transfer policy. All patients received luteal support using oral estradiol valerate 2 mg and progesterone vaginal suppositories 100 mg bid beginning on the day of oocyte retrieval. Ongoing clinical pregnancies were defined as a pregnancy showing an intrauterine gestational sac with normal fetal cardiac activity by transvaginal ultrasonography beyond 6 weeks of gestation. All deliveries were confirmed with the patient.
Conventional IVF cycles were performed using standard long luteal, microdose Lupron flare or GnRH antagonist protocols. Follicular development and estradiol levels were monitored and HCG trigger was performed when two follicles measuring ≥18 mm mean diameter were noted. Oocyte retrieval was performed 36 h after HCG trigger. ICSI was performed for male factor or unexplained infertility.
The gestational age and birthweight were obtained by follow up with the patient as per Society of Assisted Reproductive Technologies (SARTs) reporting guidelines. Pregnancy complications are not required as part of SART reporting data and therefore this is not routinely collected in our medical records. Small for gestational age was defined as <10th percentile for gestational age using a standardized birthweight to gestational age reference chart for US infants (Talge et al., 2014).
Statistical analysis
An a priori sample size calculation indicated that 270 participants (135 per group) were required for this study. The sample size calculations were based on the published estimates of mean birthweight in fresh and frozen embryo transfers (Nakashima et al., 2013), an estimated difference of 150 g in mean birthweight between stimulated and NCIVF, Type I error of 0.05, power of 90% and equal number of exposed to unexposed. Individual comparisons between demographic and clinical characteristics of the participants based on exposure group were evaluated by Student's t test or Mann–Whitney U test as appropriate for continuous variables and χ2 test for categorical variables. Birthweight was the primary outcome of interest. Secondary outcomes included preterm delivery, very preterm delivery, low birthweight and small for gestational age large for gestational age. Multivariable logistic regression models were employed to evaluate the associations between variables of interest and clinical pregnancy and live birth while adjusting for potential confounders. Data analysis was conducted using STATA version 13.1 (Stata Corp, College Station, TX, USA). Statistical significance was interpreted as P-value <0.05.
Results
Women in the NCIVF group were slightly older and had a higher mean FSH level than women in the stimulated IVF group (Table I). While the prevalence of endometriosis was higher in the NCIVF group, PCOS was more common among women undergoing stimulated IVF. As expected, the mean number of retrieved oocytes (11.3 versus 1.1, P < 0.001) and two pronuclear (2pn) stage embryos (6.2 versus 1.0, P < 0.001) was significantly higher in conventional IVF cycles than in NCIVF cycles (Table II). Patients undergoing stimulated IVF also had significantly more embryos transferred and were more likely to undergo Day 5 blastocyst transfer.
Table I.
Baseline patient characteristics.
| Stimulated IVF (n = 174) | Natural cycle IVF (n = 190) | P-Value | |
|---|---|---|---|
| Maternal age (years) | 34.2 ± 3.8 | 35.0 ± 4.0 | 0.04 |
| Maternal age groups | |||
| <35 | 101 (58.0) | 93 (48.7) | 0.38 |
| 35–37 | 36 (20.7) | 43 (22.5) | |
| 38–40 | 29 (16.7) | 39 (20.4) | |
| 41–42 | 6 (3.4) | 12 (6.3) | |
| >42 | 2 (1.1) | 4 (2.1) | |
| Prior full-term birth | 119 (32) | 81 (58) | 0.07 |
| ≥1 previous pregnancy | 69 (39.7) | 84 (44.2) | 0.44 |
| BMI (kg/m2) | 23.4 ± 3.7 | 23.3 ± 4.4 | 0.24 |
| Max Day 3 FSH (IU) | 6.3 ± 1.9 | 7.4 ± 3.4 | 0.001 |
| Infertility diagnosis (n, %) | |||
| Male factor | 86 (73.5) | 92 (73.0) | 0.93 |
| Endometriosis | 2 (3.4) | 11 (15.9) | 0.02 |
| Polycystic ovary syndrome | 19 (16.4) | 1 (0.9) | <0.001 |
| DOR | 41 (45.0) | 60 (52.6) | 0.31 |
| Tubal | 26 (32.9) | 34 (34.7) | 0.80 |
| Uterine | 6 (9.7) | 5 (6.0) | 0.41 |
| Unexplained | 23 (31.5) | 22 (22.4) | 0.18 |
Values are mean ± SD or n (%).
DOR, diminished ovarian reserve.
Table II.
IVF cycle outcomes, stimulated IVF versus natural cycle IVF.
| Stimulated IVF (n = 174) | Natural cycle IVF (n = 190) | P-Value | |
|---|---|---|---|
| Ovarian suppression protocol: | N/A | N/A | |
| Long dose Lupron | 105 (77.8) | ||
| Microdose Lupron Flare | 37 (42.5) | ||
| GnRH Antagonist | 21 (15.0) | ||
| Total FSH dose (IU) | 2412 ± 1461 | ||
| Days to retrieval | 12.9 ± 2.7 | 11.0 ± 3.0 | <0.001 |
| No. oocytes retrieved | 11.3 ± 5.5 | 1.1 ± 0.69 | <0.001 |
| No. two pronucleate oocytes | 6.2 ± 4.4 | 1.0 ± 0.28 | <0.001 |
| Failed fertilization (n, %) | 18 (10.3) | 6 (3.1) | 0.003 |
| ICSI | 144 (82.8) | 178 (93.2) | <0.001 |
| Assisted hatching | 73 (46.8) | 98 (53.3) | 0.23 |
| Day of embryo transfer | 4.8 ± 0.73 | 4.4 ± 0.94 | <0.001 |
| Number of embryos transferred | 1.7 ± 0.79 | 1.0 ± 0.16 | <0.001 |
| Blastocyst transfer | 129 (83.2) | 112 (60.9) | <0.001 |
.Values are mean ± SD or n (%).
The overall mean birthweight was significantly different between the two study groups (Table III). The birthweight of infants conceived with NCIVF was 163 g greater than the birthweight of those infants conceived with conventional IVF. There was no significant difference in small for gestational age or large for gestational age (LGA > 4000 g) infants between the two groups. Interestingly, there were significantly fewer low birthweight infants in the NCIVF group (1%) than the conventional IVF group (8.6%). Furthermore, even after adjustment for maternal age, infertility diagnosis, ICSI fertilization, blastocyst transfer and number of embryos transferred, the association of NCIVF with a reduction in risk of low birthweight remained significant (OR 0.07; 95% CI 0.014–0.35). When also adjusted for gestational age, there is a tendency towards significance (OR 0.11; 95% CI 0.01–1.0).
Table III.
Pregnancy and neonatal outcomes.
| Stimulated IVF (n = 174) | Natural cycle IVF (n = 190) | P-Value | |
|---|---|---|---|
| Duration of pregnancy (weeks) | 37.1 ± 2.2 | 37.4 ± 1.3 | 0.52 |
| Preterm delivery (<37 weeks) | 73 (42.0) | 59 (31.5) | 0.03 |
| Very preterm delivery (<32 weeks) | 11 (6.3) | 1 (0.52) | 0.002 |
| Birthweight (g) | 3273 ± 574 | 3436 ± 420 | 0.01 |
| Low birthweight (<2500 g) | 15 (8.6) | 2 (1.0) | <0.001 |
| Small for gestational age (<10 percentile) | 3 (1.7) | 1 (0.53) | 0.27 |
| Large for gestational age (>4000 g) | 13 (7.5) | 15 (7.9) | 0.88 |
| Infant gender (female) | 89 (52.2) | 98 (52.6) | 0.94 |
Values are mean ± SD or n (%).
The mean gestational age at delivery was similar between the two study groups (Table III). However, following stratification of the data, pregnancies resulting from NCIVF had both significantly fewer overall preterm deliveries (31.5 versus 42%) and fewer very preterm deliveries (0.52 versus 6.3%) than those resulting from conventional IVF. Stratified age at delivery data are shown in Supplementary data, Table SI. Although the use of NCIVF was associated with a lower risk of preterm delivery in univariate analysis (P = 0.03), following adjustment for maternal age, infertility diagnosis, blastocyst transfer and number of embryos transferred, this association was no longer significant (OR 1.1; 95% CI 0.48–2.5). A similar result was obtained if the number of embryos transferred and blastocyst transfer (which are strongly correlated with stimulation) are removed from the model.
We conclude that our study shows that the method of stimulation affects the birthweight of the infants and that infants conceived by NCIVF are at lower risk for being low birthweight than infants conceived by conventional stimulated IVF.
Discussion
This is the first US study to investigate the pregnancy and perinatal outcomes of women undergoing NCIVF compared with stimulated IVF. Previously, a European group (Pelinck et al., 2010) studied the effect of minimal ovarian stimulation on perinatal outcomes. That study found a modest effect on birthweight between the conventionally stimulated and minimally stimulated group. However, patients in the minimal stimulation group were started on an antagonist once follicular dominance was established and were supplemented with FSH until trigger. The failure of that study to detect a significant difference between the two populations could have been related to the use of minimal stimulation as opposed to unstimulated or NCIVF. A large Japanese registry cohort study demonstrated that infants conceived with NCIVF had a decreased risk of low birthweight compared with stimulated IVF infants at term (Nakashima et al., 2013).
In the present study, ovarian stimulation was not employed. HCG was given to trigger final oocyte maturation in order to time retrieval. Our study revealed both a significant increase in mean birthweight and a reduced risk of low birthweight in NCIVF conceived infants as opposed to those conceived with stimulated IVF. However, after adjustment for gestational age, this reduction in risk of low birthweight tends towards significance. Our findings are consistent with the tendency previously noted by the Japanese study that demonstrated fewer low birthweight infants in the NCIVF group even after multivariate adjustment. These findings suggest that ovarian stimulation impacts the birthweight of infants conceived with ART.
In addition, our findings suggest that there is a reduction in risk of preterm delivery with NCIVF; however, on multivariate analysis this reduction in risk was not significant. However, our study was not powered to detect a difference in the incidence of preterm delivery and therefore, future studies will be needed to explore this possible association.
In agreement with previous studies, we demonstrated overall better pregnancy and perinatal outcomes in NCIVF pregnancies compared with those arising from traditional stimulated cycle IVF. These improvements in pregnancy and perinatal outcome may be a direct result of the more physiological estradiol and progesterone levels during the follicular growth phase and at the time of implantation in unstimulated IVF cycles. Oocyte quality and/or the endometrial milieu may be improved in NCIVF cycles as suggested by the higher fertilization and implantation rates (DiMattina et al., 2014). The supernumerary oocytes recruited with stimulated IVF may be of poorer quality compared with the single follicle/oocyte selected in a natural cycle. We have shown previously that NCIVF attenuates the natural decline in pregnancy and live birth rates seen in women over 35 years old (DiMattina et al., 2014) again suggesting that oocyte quality in NCIVF cycles may be superior to stimulated cycles. The follicular fluid hormonal milieu differs markedly between NCIVF and stimulated cycle IVF, which may represent a possible mechanism for changes in the follicle leading to suboptimal oocyte quality and possibly embryonic development (von Wolff et al., 2014).
Improved perinatal outcomes in NCIVF may also result from the more physiologic peri-implantation estrogen levels. Several studies have shown improved perinatal outcomes when fresh versus frozen embryo transfer cycles are compared (Kalra et al., 2011). More recently, a study using SART CORS (Clinical Reporting System) data found that an increasing number of oocytes retrieved in autologous cycles was associated with decrease in mean birthweight and increase in low birthweight in singleton pregnancies with double embryo transfer (Baker et al., 2015). The authors also reported that this tendency was not seen in donor egg IVF cycles further supporting the concept of an abnormal endometrial hormonal milieu contributing to adverse perinatal outcomes.
The strengths of our study include that it is the first study in the USA to compare the pregnancy and perinatal outcomes from NCIVF and conventional IVF cycles with an adequate sample size for statistical analysis. Additionally, we further restricted the analysis to singleton pregnancies and mothers who are non-smokers. In order to account for the potential adverse impact of a ‘vanishing twin’, we further restricted our analysis to pregnancies with a single fetal heartbeat at first ultrasound. Since our study is a single center study confounders such as differences in stimulation protocols and ART laboratory practices were eliminated as opposed to those studies relying upon a large registry database.
Weaknesses of our study include that it is a retrospective cohort study. Furthermore, since NCIVF can only be offered to patients with regular menstrual cycles it is not surprising that there are several potential confounders in our study. As expected, the stimulated IVF group includes more patients with PCOS while the NCIVF group has more patients with endometriosis. In a recent study, patients with endometriosis did not show any difference in adverse perinatal outcomes with IVF and are therefore unlikely to be a confounder in our study (Stern et al., 2015). Pregnancy in PCOS patients can be complicated by gestational diabetes and pre-eclampsia which could lead to macrosomia and preterm delivery, respectively. On the other hand, the patients undergoing NCIVF were older and advanced maternal age can lead to an increase in pregnancy-related complications such as intrauterine growth restriction and pre-eclampsia both of which would lead to reduced birthweight and early delivery. Another confounder was the increased incidence of blastocyst transfers in the conventional IVF group. Extended embryo culture has been shown in some studies to adversely affect pregnancy and perinatal outcomes (Kallen et al., 2010; Kalra et al., 2012; Oron et al., 2014). However, in a recent study comparing single blastocyst and cleavage stage transfers, no difference in perinatal outcome was found (Oron et al., 2014). We performed multivariate analysis to account for these confounders in our statistical model. In addition, as a result of the limited size of our cohort, stratification of low birthweight at term and preterm could not be performed. A further limitation is that we lack information about parental characteristics, e.g. height, ethnicity that are associated with birthweight.
In this observational study, ovarian stimulation was associated with more adverse pregnancy and perinatal outcomes following IVF treatment as compared with IVF treatment without ovarian stimulation. We believe that clinicians should consider NCIVF as a reasonable option for infertility patients with regular menstrual cycles and further research is needed to explore whether the improved outcomes seen in this study are the result of the differences in the endometrium or in the follicular hormone milieu.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
W.M.: study design, execution and manuscript preparation. L.A.K.: study design, statistical analysis and critical discussion. G.C., M.P., J.G. and M.D.: data collection, manuscript preparation and critical discussion.
Funding
The study was supported by the following grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NICHD K12HD047018 (W.M.) and NICHD K12HD001271 (L.A.K.).
Conflict of interest
None declared.
Supplementary Material
References
- Allersma T, Farquhar C, Cantineau AE. Natural cycle in vitro fertilisation (IVF) for subfertile couples. Cochrane Database Syst Rev 2013;8:CD010550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker VL, Brown MB, Luke B, Conrad KP. Association of number of retrieved oocytes with live birth rate and birth weight: an analysis of 231,815 cycles of in vitro fertilization. Fertil Steril 2015;103:931–938, e932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declercq E, Luke B, Belanoff C, Cabral H, Diop H, Gopal D, Hoang L, Kotelchuck M, Stern JE, Hornstein MD. Perinatal outcomes associated with assisted reproductive technology: the Massachusetts Outcomes Study of Assisted Reproductive Technologies (MOSART). Fertil Steril 2015;103:888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMattina M, Gordon JD, Botes A, Celia G, Payson M, Graves-Herring J. Follicular and estradiol parameters that improve success with natural cycle in vitro fertilization. J Reprod Med 2014;59:267–273. [PubMed] [Google Scholar]
- Gordon JD, DiMattina M, Reh A, Botes A, Celia G, Payson M. Utilization and success rates of unstimulated in vitro fertilization in the United States: an analysis of the Society for Assisted Reproductive Technology database. Fertil Steril 2013;100:392–395. [DOI] [PubMed] [Google Scholar]
- Jackson RA, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol 2004;103:551–563. [DOI] [PubMed] [Google Scholar]
- Kallen B, Finnstrom O, Lindam A, Nilsson E, Nygren KG, Olausson PO. Blastocyst versus cleavage stage transfer in in vitro fertilization: differences in neonatal outcome? Fertil Steril 2010;94:1680–1683. [DOI] [PubMed] [Google Scholar]
- Kalra SK, Ratcliffe SJ, Coutifaris C, Molinaro T, Barnhart KT. Ovarian stimulation and low birth weight in newborns conceived through in vitro fertilization. Obstet Gynecol 2011;118:863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra SK, Ratcliffe SJ, Barnhart KT, Coutifaris C. Extended embryo culture and an increased risk of preterm delivery. Obstet Gynecol 2012;120:69–75. [DOI] [PubMed] [Google Scholar]
- Kondapalli LA, Perales-Puchalt A. Low birth weight: is it related to assisted reproductive technology or underlying infertility? Fertil Steril 2013;99:303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima A, Araki R, Tani H, Ishihara O, Kuwahara A, Irahara M, Yoshimura Y, Kuramoto T, Saito H, Nakaza A et al. Implications of assisted reproductive technologies on term singleton birth weight: an analysis of 25,777 children in the national assisted reproduction registry of Japan. Fertil Steril 2013;99:450–455. [DOI] [PubMed] [Google Scholar]
- Oron G, Sokal-Arnon T, Son WY, Demirtas E, Buckett W, Zeadna A, Holzer H, Tulandi T. Extended embryo culture is not associated with increased adverse obstetric or perinatal outcome. Am J Obstet Gynecol 2014;211:165, e161–e167. [DOI] [PubMed] [Google Scholar]
- Pelinck MJ, Keizer MH, Hoek A, Simons AH, Schelling K, Middelburg K, Heineman MJ. Perinatal outcome in singletons after modified natural cycle IVF and standard IVF with ovarian stimulation. Eur J Obstet Gynecol Reprod Biol 2010;148:56–61. [DOI] [PubMed] [Google Scholar]
- Pinborg A, Wennerholm UB, Romundstad LB, Loft A, Aittomaki K, Soderstrom-Anttila V, Nygren KG, Hazekamp J, Bergh C. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum Reprod Update 2013;19:87–104. [DOI] [PubMed] [Google Scholar]
- Poikkeus P, Gissler M, Unkila-Kallio L, Hyden-Granskog C, Tiitinen A. Obstetric and neonatal outcome after single embryo transfer. Hum Reprod 2007;22:1073–1079. [DOI] [PubMed] [Google Scholar]
- Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS. Low and very low birth weight in infants conceived with use of assisted reproductive technology. N Engl J Med 2002;346:731–737. [DOI] [PubMed] [Google Scholar]
- Stern JE, Luke B, Tobias M, Gopal D, Hornstein MD, Diop H. Adverse pregnancy and birth outcomes associated with underlying diagnosis with and without assisted reproductive technology treatment. Fertil Steril 2015;103:1438–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EA, Zegers-Hochschild F, Mansour R, Ishihara O, de Mouzon J, Nygren KG, Adamson GD. International Committee for Monitoring Assisted Reproductive Technologies (ICMART) world report: assisted reproductive technology 2004. Hum Reprod 2013;28:1375–1390. [DOI] [PubMed] [Google Scholar]
- Talge NM, Mudd LM, Sikorskii A, Basso O. United States birth weight reference corrected for implausible gestational age estimates. Pediatrics 2014;133:844–853. [DOI] [PubMed] [Google Scholar]
- von Wolff M, Kollmann Z, Fluck CE, Stute P, Marti U, Weiss B, Bersinger NA. Gonadotrophin stimulation for in vitro fertilization significantly alters the hormone milieu in follicular fluid: a comparative study between natural cycle IVF and conventional IVF. Hum Reprod 2014;29:1049–1057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


